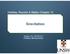
Figure 1. Visible spectrum of atomic hydrogen.
|
|
|
- Bathsheba Berry
- 7 years ago
- Views:
Transcription
1 Chapter 7 THE HYDROGEN ATOM; ATOMIC ORBITALS Atomic Spectra When gaseous hydrogen in a glass tube is excited by a 5-volt electrical discharge, four lines are observed in the visible part of the emission spectrum: red at nm, blue-green at nm, blue violet at nm and violet at 41.2 nm: Figure 1. Visible spectrum of atomic hydrogen. Other series of lines have been observed in the ultraviolet and infrared regions. Rydberg (189) found that all the lines of the atomic hydrogen spectrum could be fitted to a single formula 1 λ = R ( 1 n n 2 2 ), n 1 = 1,2,3..., n 2 > n 1 (1) where R, known as the Rydberg constant, has the value 19,677 cm 1 for hydrogen. The reciprocal of wavelength, in units of cm 1, is in general use by spectroscopists. This unit is also designated wavenumbers, since it represents the number of wavelengths per cm. The Balmer series of spectral lines in the visible region, shown in Fig. 1, correspond to the values n 1 = 2, n 2 = 3,4, 5 and 6. The lines with n 1 = 1 in the ultraviolet make up the Lyman series. The line with n 2 = 2, designated the Lyman alpha, has the longest wavelength (lowest wavenumber) in this series, with 1/λ = cm 1 or λ = nm. Other atomic species have line spectra, which can be used as a fingerprint to identify the element. However, no atom other than hydrogen has a simple relation analogous to (1) for its spectral frequencies. Bohr in 1913 proposed that all atomic spectral lines arise from transitions between discrete energy levels, giving a photon such that E = hν = hc λ (2) 1
2 This is called the Bohr frequency condition. We now understand that the atomic transition energy E is equal to the energy of a photon, as proposed earlier by Planck and Einstein. The Bohr Atom The nuclear model proposed by Rutherford in 1911 pictures the atom as a heavy, positively-charged nucleus, around which much lighter, negativelycharged electrons circulate, much like planets in the Solar system. This model is however completely untenable from the standpoint of classical electromagnetic theory, for an accelerating electron (circular motion represents an acceleration) should radiate away its energy. In fact, a hydrogen atom should exist for no longer than sec, time enough for the electron s death spiral into the nucleus. This is one of the worst quantitative predictions in the history of physics. It has been called the Hindenberg disaster on an atomic level. (Recall that the Hindenberg, a hydrogen-filled dirigible, crashed and burned in a famous disaster in 1937.) Bohr sought to avoid an atomic catastrophe by proposing that certain orbits of the electron around the nucleus could be exempted from classical electrodynamics and remain stable. The Bohr model was quantitatively successful for the hydrogen atom, as we shall now show. We recall that the attraction between two opposite charges, such as the electron and proton, is given by Coulomb s law F = e2 r 2 (gaussian units) e2 4πɛ r 2 (SI units) (3) We prefer to use the gaussian system in applications to atomic phenomena. Since the Coulomb attraction is a central force (dependent only on r), the potential energy is related by dv (r) F = dr (4) We find therefore, for the mutual potential energy of a proton and electron, V (r) = e2 r (5) 2
3 Bohr considered an electron in a circular orbit of radius r around the proton. To remain in this orbit, the electron must be experiencing a centripetal acceleration a = v2 (6) r where v is the speed of the electron. Using (4) and (6) in Newton s second law, we find e 2 r 2 = mv2 r where m is the mass of the electron. For simplicity, we assume that the proton mass is infinite (actually m p 1836m e ) so that the proton s position remains fixed. We will later correct for this approximation by introducing reduced mass. The energy of the hydrogen atom is the sum of the kinetic and potential energies: (7) E = T + V = 1 2 mv2 e2 r (8) Using Eq (7), we see that T = 1 2 V and E = 1 2V = T (9) This is the form of the virial theorem for a force law varying as r 2. Note that the energy of a bound atom is negative, since it is lower than the energy of the separated electron and proton, which is taken to be zero. For further progress, we need some restriction on the possible values of r or v. This is where we can introduce the quantization of angular momentum L = r p. Since p is perpendicular to r, we can write simply Using (9), we find also that L = rp = mvr (1) r = L2 me 2 (11) We introduce angular momentum quantization, writing L = n h, n = 1, 2... (12) 3
4 excluding n =, since the electron would then not be in a circular orbit. The allowed orbital radii are then given by where r n = n 2 a (13) a h2 me 2 = m =.529 Å (14) which is known as the Bohr radius. The corresponding energy is E n = e2 2a n 2 = me4 2 h 2 n = 1, 2... (15) n2, Rydberg s formula (1) can now be deduced from the Bohr model. We have hc λ = E n 2 E n1 = 2π2 me 4 ( 1 h 2 n 2 1 ) 1 n 2 (16) 2 and the Rydbeg constant can be identified as R = 2π2 me 4 h 3 c 19,737 cm 1 (17) The slight discrepency with the experimental value for hydrogen (19,677) is due to the finite proton mass. This will be corrected later. The Bohr model can be readily extended to hydrogenlike ions, systems in which a single electron orbits a nucleus of arbitrary atomic number Z. Thus Z = 1 for hydrogen, Z = 2 for He +, Z = 3 for Li ++, and so on. The Coulomb potential (5) generalizes to the radius of the orbit (13) becomes V (r) = Ze2 r, (18) and the energy (15) becomes r n = n2 a Z (19) E n = Z2 e 2 2a n 2 (2) 4
5 De Broglie s proposal that electrons can have wavelike properties was actually inspired by the Bohr atomic model. Since L = rp = n h = nh 2π (21) we find 2πr = nh p = nλ (22) Therefore, each allowed orbit traces out an integral number of de Broglie wavelengths. Wilson (1915) and Sommerfeld (1916) generalized Bohr s formula for the allowed orbits to pdr = nh, n = 1,2... (23) The Sommerfeld-Wilson quantum conditions (23) reduce to Bohr s results for circular orbits, but allow, in addition, elliptical orbits along which the momentum p is variable. According to Kepler s first law of planetary motion, the orbits of planets are ellipses with the Sun at one focus. Fig. 2 shows the generalization of the Bohr theory for hydrogen, including the elliptical orbits. The lowest energy state n = 1 is still a circular orbit. But n = 2 allows an elliptical orbit in addition to the circular one; n = 3 has three possible orbits, and so on. The energy still depends on n alone, so that the elliptical orbits represent degenerate states. Atomic spectroscopy shows in fact that energy levels with n > 1 consist of multiple states, as implied by the splitting of atomic lines by an electric field (Stark effect) or a magnetic field (Zeeman effect). Some of these generalized orbits are drawn schematically in Fig. 2. 5
6 n=3 n=2 n=1 Figure 2. Bohr-Sommerfeld orbits for n = 1,2,3 (not to scale). The Bohr model was an important first step in the historical development of quantum mechanics. It introduced the quantization of atomic energy levels and gave quantitative agreement with the atomic hydrogen spectrum. With the Sommerfeld-Wilson generalization, it accounted as well for the degeneracy of hydrogen energy levels. Although the Bohr model was able to sidestep the atomic Hindenberg disaster, it cannot avoid what we might call the Heisenberg disaster. By this we mean that the assumption of well-defined electronic orbits around a nucleus is completely contrary to the basic premises of quantum mechanics. Another flaw in the Bohr picture is that the angular momenta are all too large by one unit, for example, the ground state actually has zero orbital angular momentum (rather than h). Quantum Mechanics of Hydrogenlike Atoms In contrast to the particle in a box and the harmonic oscillator, the hydrogen atom is a real physical system that can be treated exactly by quantum mechanics. in addition to their inherent significance, these solutions suggest prototypes for atomic orbitals used in approximate treatments of complex atoms and molecules. For an electron in the field of a nucleus of charge +Ze, the Schrödinger equation can be written } { h2 2m 2 Ze2 ψ(r) = E ψ(r) (24) r It is convenient to introduce atomic units in which length is measured in 6
7 bohrs: and energy in hartrees: a = h2 me 2 = m 1 bohr e 2 a = J = ev 1 hartree Electron volts (ev) are a convenient unit for atomic energies. One ev is defined as the energy an electron gains when accelerated across a potential difference of 1 volt. The ground state of the hydrogen atom has an energy of 1/2 hartree or ev. Conversion to atomic units is equivalent to setting h = e = m = 1 in all formulas containing these constants. Rewriting the Schrödinger equation in atomic units, we have { Z } ψ(r) = E ψ(r) (25) r Since the potential energy is spherically symmetrical (a function of r alone), it is obviously advantageous to treat this problem in spherical polar coordinates r,θ,φ. Expressing the Laplacian operator in these coordinates [cf. Eq (6-2)], 1 2 { 1 r 2 r r2 r + 1 r 2 sinθ θ sinθ θ + 1 r 2 sin 2 θ 2 } φ 2 ψ(r, θ, φ) Z r ψ(r, θ, φ) = E ψ(r, θ, φ) (26) Eq (6-33) shows that the second and third terms in the Laplacian represent the angular momentum operator ˆL 2. Clearly, Eq (26) will have separable solutions of the form ψ(r, θ, φ) = R(r) Y lm (θ,φ) (27) 7
8 Substituting (27) into (26) and using the angular momentum eigenvalue equation (6-34), we obtain an ordinary differential equation for the radial function R(r): { 1 2r 2 d dr r2 d dr + l(l + 1) 2r 2 Z r } R(r) = E R(r) (28) Note that in the domain of the variable r, the angular momentum contribution l(l + 1)/2r 2 acts as an effective addition to the potential energy. It can be identified with centrifugal force, which pulls the electron outward, in opposition to the Coulomb attraction. Carrying out the successive differentiations in (29) and simplifying, we obtain 1 2 R (r) + 1 [ Z r R (r) + r l(l + 1) 2r 2 ] + E R(r) = (29) another second-order linear differential equation with non-constant coefficients. It is again useful to explore the asymptotic solutions to (29), as r. In the asymptotic approximation, R (r) 2 E R(r) (3) having noted that the energy E is negative for bound states. Solutions to (3) are R(r) const e ± 2 E r (31) We reject the positive exponential on physical grounds, since R(r) as r, in violation of the requirement that the wavefunction must be finite everywhere. Choosing the negative exponental and setting E = Z 2 /2, the ground state energy in the Bohr theory (in atomic units), we obtain R(r) const e Zr (32) It turns out, very fortunately, that this asymptotic approximation is also an exact solution of the Schrödinger equation (29) with l =, just what happened for the harmonic-oscillator problem in Chap. 5. The solutions to Eq (29), designated R nl (r), are labelled by n, known as the principal 8
9 quantum number, as well as by the angular momentum l, which is a parameter in the radial equation. The solution (32) corresponds to R 1 (r). This should be normalized according to the condition A useful definite integral is [R 1 (r)] 2 r 2 dr = 1 (33) r n e αr dr = n! α n+1 (34) The normalized radial function is thereby given by R 1 (r) = 2Z 3/2 e Zr (35) Since this function is nodeless, we identify it with the ground state of the hydrogenlike atom. Multipyling (35) by the spherical harmonic Y = 1/ 4π, we obtain the total wavefunction (27) ψ 1 (r) = ( ) Z 3 1/2 e Zr (36) π This is conventionally designated as the 1s function ψ 1s (r). Integrals in spherical-polar coordinates over a spherically-symmetrical integrand can be significantly simplified. We can do the reduction π 2π f(r) r 2 sinθ dr dθ dφ = f(r) 4πr 2 dr (37) since integration over θ and φ gives 4π, the total solid angle of a sphere. The normalization of the 1s wavefunction can thus be written as [ψ 1s (r)] 2 4πr 2 dr = 1 (38) 9
10 Hydrogen Atom Ground State There are a number of different ways of representing hydrogen-atom wavefunctions graphically. We will illustrate some of these for the 1s ground state. In atomic units, ψ 1s (r) = 1 e r (39) π is a decreasing exponential function of a single variable r, and is simply plotted in Fig s (r) s (r) r/bohr Figure 3. Wavefunctions for 1s and 2s orbitals for atomic hydrogen. The 2s-function (scaled by a factor of 2) has a node at r = 2 bohr. Fig. 4 gives a somewhat more pictorial representation, a three-dimensional contour plot of ψ 1s (r) as a function of x and y in the x, y-plane. Figure 4. Contour map of 1s orbital in the x, y-plane. 1
11 According to Born s interpretation of the wavefunction, the probability per unit volume of finding the electron at the point (r,θ,φ) is equal to the square of the normalized wavefunction ρ 1s (r) = [ψ 1s (r)] 2 = 1 π e 2r (4) This is represented in Fig. 5 by a scatter plot describing a possible sequence of observations of the electron position. Although results of individual measurements are not predictable, a statistical pattern does emerge after a sufficiently large number of measurements. Figure 5. Scatter plot of electron position measurements in hydrogen 1s orbital. The probability density is normalized such that ρ 1s (r) 4πr 2 dr = 1 (41) In some ways ρ(r) does not provide the best description of the electron distribution, since the region around r =, where the wavefunction has its largest values, is a relatively small fraction of the volume accessible to the electron. Larger radii r represent larger physical regions since, in spherical polar coordinates, a value of r is associated with a shell of volume 4πr 2 dr. A more significant measure is therefore the radial distribution function D 1s (r) = 4πr 2 [ψ 1s (r)] 2 (42) which represents the probability density within the entire shell of radius r, normalized such that D 1s (r) dr = 1 (43) 11
12 The functions ρ 1s (r) and D 1s (r) are both shown in Fig. 6. Remarkably, the 1s RDF has its maximum at r = a, equal to the radius of the first Bohr orbit. D(r) (r) r/bohr Atomic Orbitals Figure 6. Density ρ(r) and radial distribution function D(r) for hydrogen 1s orbital. The general solution for R nl (r) has a rather complicated form which we give without proof: R nl (r) = N nl ρ l L 2l+1 n+l (ρ) e ρ/2 ρ 2Zr n (44) Here L α β is an associated Laguerre polynomial and N nl, a normalizing constant. The angular momentum quantum number l is by convention designated by a code: s for l =, p for l = 1, d for l = 2, f for l = 3, g for l = 4, and so on. The first four letters come from an old classification scheme for atomic spectral lines: sharp, principal, diffuse and fundamental. Although these designations have long since outlived their original significance, they 12
13 remain in general use. The solutions of the hydrogenic Schrödinger equation in spherical polar coordinates can now be written in full ψ nlm (r,θ,φ) = R nl (r)y lm (θ, φ) n = 1,2... l =, 1...n 1 m =, ±1,±2... ± l (45) where Y lm are the spherical harmonics tabulated in Chap. 6. Table 1 below enumerates all the hydrogenic functions we will actually need. These are ψ 2s = 1 2 2π ψ 1s = 1 π e r ( 1 r ) 2 ψ 2pz = 1 4 2π z e r/2 ψ 2px, ψ 2py e r/2 analogous 1 ψ 3s = 81 3π (27 18r + 2r2 ) e r/3 2 ψ 3pz = 81 (6 r) z e r/3 π ψ 3px, ψ 3py analogous 1 ψ 3dz 2 = 81 6π (3z2 r 2 ) e r/3 2 ψ 3dzx = 81 zx e r/3 π ψ 3dyz, ψ 3dxy analogous ψ 3dx 2 y 2 = 1 81 π (x2 y 2 ) e r/3 Table 1 Real hydrogenic functions in atomic units. 13
14 called hydrogenic atomic orbitals, in anticipation of their later applications to the structure of atoms and molecules. The energy levels for a hydrogenic system are given by E n = Z2 2n 2 hartrees (46) and depends on the principal quantum number alone. Considering all the allowed values of l and m, the level E n has a degeneracy of n 2. Fig. 7 shows an energy level diagram for hydrogen (Z = 1). For E, the energy is a continuum, since the electron is in fact a free particle. The continuum represents states of an electon and proton in interaction, but not bound into a stable atom. Fig. 7 also shows some of the transitions which make up the Lyman series in the ultraviolet and the Balmer series in the visible region. E = E = E = -3.4 Lyman Balmer E = ev Figure 7. Energy levels of atomic hydrogen. The ns orbitals are all spherically symmetrical, being associated with a constant angular factor, the spherical harmonic Y = 1/ 4π. They have n 1 radial nodes spherical shells on which the wavefunction equals zero. The 1s ground state is nodeless and the number of nodes increases with energy, in a pattern now familiar from our study of the particle-in-a-box and harmonic oscillator. The 2s orbital, with its radial node at r = 2 bohr, is also shown in Fig
15 p- and d-orbitals The lowest-energy solutions deviating from spherical symmetry are the 2porbitals. Using Eqs (44), (45) and the l = 1 spherical harmonics, we find three degenerate eigenfunctions: and ψ 21 (r, θ, φ) = 1 4 2π r e r/2 cosθ (47) ψ 21±1 (r, θ, φ) = 1 4 2π r e r/2 sinθ e ±iφ (48) The function ψ 21 is real and contains the factor r cosθ, which is equal to the cartesian variable z. In chemical applications, this is designated as a 2p z orbital: ψ 2pz = 1 4 2π z e r/2 (49) A contour plot is shown in Fig. 8. Note that this function is cylindricallysymmetrical about the z-axis with a node in the x, y-plane. The ψ 21±1 are complex functions and not as easy to represent graphically. Their angular dependence is that of the spherical harmonics Y 1±1, shown in Fig As noted in Chap. 4, any linear combination of degenerate eigenfunctions is an equally-valid alternative eigenfunction. Making use of the Euler formulas for sine and cosine cosφ = eiφ + e iφ 2 and sinφ = eiφ e iφ 2i (5) and noting that the combinations sinθ cosφ and sinθ sinφ correspond to the cartesian variables x and y, respectively, we can define the alternative 2p orbitals ψ 2px = 1 (ψ 21 1 ψ 211 ) = π xe r/2 (51) and ψ 2py = i 2 (ψ ψ 211 ) = 1 4 2π y e r/2 (52) 15
16 Clearly, these have the same shape as the 2pz-orbital, but are oriented along the x- and y-axes, respectively. The threefold degeneracy of the p-orbitals is very clearly shown by the geometric equivalence the functions 2px, 2py and 2pz, which is not obvious for the spherical harmonics. The functions listed in Table 1 are, in fact, the real forms for all atomic orbitals, which are more useful in chemical applications. All higher p-orbitals have analogous functional forms xf(r), yf(r) and zf(r) and are likewise 3-fold degenerate. Figure 8. Contour plot of 2p z orbital. Negative values are shown in red. Scale units in bohrs. The orbital ψ 32 is, like ψ 21, a real function. It is known in chemistry as the d z 2-orbital and can be expressed as a cartesian factor times a function of r: ψ 3dz 2 = ψ 32 = (3z 2 r 2 )f(r) (53) A contour plot is shown in Fig. 9. This function is also cylindrically symmetric about the z-axis with two angular nodes the conical surfaces with 3z 2 r 2 =. The remaining four 3d orbitals are complex functions containing the spherical harmonics Y 2±1 and Y 2±2 pictured in Fig We can 16
17 again construct real functions from linear combinations, the result being four geometrically equivalent four-leaf clover functions with two perpendicular planar nodes. These orbitals are designated d x2 y 2, d xy, d zx and d yz. Two of them are shown in Fig. 9. The d z 2 orbital has a different shape. However, it can be expressed in terms of two non-standard d-orbitals, d z2 x 2 and d y 2 z 2. The latter functions, along with d x 2 y2 add to zero and thus constitute a linearly dependent set. Two combinations of these three functions can be chosen as independent eigenfunctions. Summary d z 2 d x 2 y 2 d xy Figure 9. Contour plots of 3d orbitals. The atomic orbitals listed in Table 1 are illustrated in Fig. 1. Blue and yellow indicate, respectively, positive and negative regions of the wavefunctions (the radial nodes of the 2s and 3s orbitals are obscured). These pictures are intended as stylized representations of atomic orbitals and should not be interpreted as quantitatively accurate. 17
18 Figure 1. Hydrogenic atomic orbitals. 18
19 The electron charge distribution in an orbital ψ nlm (r) is given by ρ(r) = ψ nlm (r) 2 (54) which for the s-orbitals is a function of r alone. The radial distribution function can be defined, even for orbitals containing angular dependence, by D nl (r) = r 2 [R nl (r)] 2 (55) This represents the electron density in a shell of radius r, including all values of the angular variables θ,φ. Fig. 11 shows plots of the RDF for the first few hydrogen orbitals. D(r) 3d 3p 3s r/bohr D(r) 1s 2p 2s r/bohr Figure 11. Some radial distribution functions. 19
Review of the isotope effect in the hydrogen spectrum
 Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Review of the isotope effect in the hydrogen spectrum 1 Balmer and Rydberg Formulas By the middle of the 19th century it was well established that atoms emitted light at discrete wavelengths. This is in
Chapter 18: The Structure of the Atom
 Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Chapter 18: The Structure of the Atom 1. For most elements, an atom has A. no neutrons in the nucleus. B. more protons than electrons. C. less neutrons than electrons. D. just as many electrons as protons.
Experiment #12: The Bohr Atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes, Holder, and Variac Flashlight
 Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
Experiment #12: The Bohr Atom Purpose: To observe the visible spectrum of hydrogen and helium and verify the Bohr model of the hydrogen atom. Equipment: Spectroscope Hydrogen and Helium Gas Discharge Tubes,
Name Date Class ELECTRONS IN ATOMS. Standard Curriculum Core content Extension topics
 13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
13 ELECTRONS IN ATOMS Conceptual Curriculum Concrete concepts More abstract concepts or math/problem-solving Standard Curriculum Core content Extension topics Honors Curriculum Core honors content Options
5.61 Fall 2012 Lecture #19 page 1
 5.6 Fall 0 Lecture #9 page HYDROGEN ATOM Consider an arbitrary potential U(r) that only depends on the distance between two particles from the origin. We can write the Hamiltonian simply ħ + Ur ( ) H =
5.6 Fall 0 Lecture #9 page HYDROGEN ATOM Consider an arbitrary potential U(r) that only depends on the distance between two particles from the origin. We can write the Hamiltonian simply ħ + Ur ( ) H =
Atomic Structure: Chapter Problems
 Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Atomic Structure: Chapter Problems Bohr Model Class Work 1. Describe the nuclear model of the atom. 2. Explain the problems with the nuclear model of the atom. 3. According to Niels Bohr, what does n stand
Photons. ConcepTest 27.1. 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy. Which has more energy, a photon of:
 ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
ConcepTest 27.1 Photons Which has more energy, a photon of: 1) red light 2) yellow light 3) green light 4) blue light 5) all have the same energy 400 nm 500 nm 600 nm 700 nm ConcepTest 27.1 Photons Which
PHY4604 Introduction to Quantum Mechanics Fall 2004 Practice Test 3 November 22, 2004
 PHY464 Introduction to Quantum Mechanics Fall 4 Practice Test 3 November, 4 These problems are similar but not identical to the actual test. One or two parts will actually show up.. Short answer. (a) Recall
PHY464 Introduction to Quantum Mechanics Fall 4 Practice Test 3 November, 4 These problems are similar but not identical to the actual test. One or two parts will actually show up.. Short answer. (a) Recall
Light as a Wave. The Nature of Light. EM Radiation Spectrum. EM Radiation Spectrum. Electromagnetic Radiation
 The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
The Nature of Light Light and other forms of radiation carry information to us from distance astronomical objects Visible light is a subset of a huge spectrum of electromagnetic radiation Maxwell pioneered
ATOMIC SPECTRA. Apparatus: Optical spectrometer, spectral tubes, power supply, incandescent lamp, bottles of dyed water, elevating jack or block.
 1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS
 DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
DO PHYSICS ONLINE FROM QUANTA TO QUARKS QUANTUM (WAVE) MECHANICS Quantum Mechanics or wave mechanics is the best mathematical theory used today to describe and predict the behaviour of particles and waves.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES. PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points
 TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
TIME OF COMPLETION NAME SOLUTION DEPARTMENT OF NATURAL SCIENCES PHYS 3650, Exam 2 Section 1 Version 1 October 31, 2005 Total Weight: 100 points 1. Check your examination for completeness prior to starting.
Wave Function, ψ. Chapter 28 Atomic Physics. The Heisenberg Uncertainty Principle. Line Spectrum
 Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
Wave Function, ψ Chapter 28 Atomic Physics The Hydrogen Atom The Bohr Model Electron Waves in the Atom The value of Ψ 2 for a particular object at a certain place and time is proportional to the probability
Electron Orbits. Binding Energy. centrifugal force: electrostatic force: stability criterion: kinetic energy of the electron on its orbit:
 Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Electron Orbits In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. Consider the simple example of an atom with a nucleus of
Bohr's Theory of the Hydrogen Atom
 OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
OpenStax-CNX module: m42596 1 Bohr's Theory of the Hydrogen Atom OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Describe
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW
 CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
CHAPTER 9 ATOMIC STRUCTURE AND THE PERIODIC LAW Quantum mechanics can account for the periodic structure of the elements, by any measure a major conceptual accomplishment for any theory. Although accurate
Classical Angular Momentum. The Physics of Rotational Motion.
 3. Angular Momentum States. We now employ the vector model to enumerate the possible number of spin angular momentum states for several commonly encountered situations in photochemistry. We shall give
3. Angular Momentum States. We now employ the vector model to enumerate the possible number of spin angular momentum states for several commonly encountered situations in photochemistry. We shall give
Multi-electron atoms
 Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
Multi-electron atoms Today: Using hydrogen as a model. The Periodic Table HWK 13 available online. Please fill out the online participation survey. Worth 10points on HWK 13. Final Exam is Monday, Dec.
13- What is the maximum number of electrons that can occupy the subshell 3d? a) 1 b) 3 c) 5 d) 2
 Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
Assignment 06 A 1- What is the energy in joules of an electron undergoing a transition from n = 3 to n = 5 in a Bohr hydrogen atom? a) -3.48 x 10-17 J b) 2.18 x 10-19 J c) 1.55 x 10-19 J d) -2.56 x 10-19
2, 8, 20, 28, 50, 82, 126.
 Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
Chapter 5 Nuclear Shell Model 5.1 Magic Numbers The binding energies predicted by the Liquid Drop Model underestimate the actual binding energies of magic nuclei for which either the number of neutrons
Atoms Absorb & Emit Light
 Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Atoms Absorb & Emit Light Spectra The wavelength of the light that an element emits or absorbs is its fingerprint. Atoms emit and absorb light First Test is Thurs, Feb 1 st About 30 multiple choice questions
Flame Tests & Electron Configuration
 Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
Flame Tests & Electron Configuration INTRODUCTION Many elements produce colors in the flame when heated. The origin of this phenomenon lies in the arrangement, or configuration of the electrons in the
How To Understand Light And Color
 PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
PRACTICE EXAM IV P202 SPRING 2004 1. In two separate double slit experiments, an interference pattern is observed on a screen. In the first experiment, violet light (λ = 754 nm) is used and a second-order
Chapter 7. Electron Structure of the Atom. Chapter 7 Topics
 Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Chapter 7 Electron Structure of the Atom Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Chapter 7 Topics 1. Electromagnetic radiation 2. The Bohr model of
Atomic Structure Ron Robertson
 Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Atomic Structure Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\atomicstructuretrans.doc I. What is Light? Debate in 1600's: Since waves or particles can transfer energy, what is
Chemistry 102 Summary June 24 th. Properties of Light
 Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Chemistry 102 Summary June 24 th Properties of Light - Energy travels through space in the form of electromagnetic radiation (EMR). - Examples of types of EMR: radio waves, x-rays, microwaves, visible
Basic Nuclear Concepts
 Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Section 7: In this section, we present a basic description of atomic nuclei, the stored energy contained within them, their occurrence and stability Basic Nuclear Concepts EARLY DISCOVERIES [see also Section
Quantum Mechanics and Atomic Structure 1
 Quantum Mechanics and Atomic Structure 1 INTRODUCTION The word atom is derived from the Greek word, atomos, which means uncut or indivisible. It was Dalton (1808) who established that elementary constituents
Quantum Mechanics and Atomic Structure 1 INTRODUCTION The word atom is derived from the Greek word, atomos, which means uncut or indivisible. It was Dalton (1808) who established that elementary constituents
The Phenomenon of Photoelectric Emission:
 The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
The Photoelectric Effect. The Wave particle duality of light Light, like any other E.M.R (electromagnetic radiation) has got a dual nature. That is there are experiments that prove that it is made up of
AP* Atomic Structure & Periodicity Free Response Questions KEY page 1
 AP* Atomic Structure & Periodicity ree Response Questions KEY page 1 1980 a) points 1s s p 6 3s 3p 6 4s 3d 10 4p 3 b) points for the two electrons in the 4s: 4, 0, 0, +1/ and 4, 0, 0, - 1/ for the three
AP* Atomic Structure & Periodicity ree Response Questions KEY page 1 1980 a) points 1s s p 6 3s 3p 6 4s 3d 10 4p 3 b) points for the two electrons in the 4s: 4, 0, 0, +1/ and 4, 0, 0, - 1/ for the three
FLAP P11.2 The quantum harmonic oscillator
 F L E X I B L E L E A R N I N G A P P R O A C H T O P H Y S I C S Module P. Opening items. Module introduction. Fast track questions.3 Ready to study? The harmonic oscillator. Classical description of
F L E X I B L E L E A R N I N G A P P R O A C H T O P H Y S I C S Module P. Opening items. Module introduction. Fast track questions.3 Ready to study? The harmonic oscillator. Classical description of
GRID AND PRISM SPECTROMETERS
 FYSA230/2 GRID AND PRISM SPECTROMETERS 1. Introduction Electromagnetic radiation (e.g. visible light) experiences reflection, refraction, interference and diffraction phenomena when entering and passing
FYSA230/2 GRID AND PRISM SPECTROMETERS 1. Introduction Electromagnetic radiation (e.g. visible light) experiences reflection, refraction, interference and diffraction phenomena when entering and passing
3. Electronic Spectroscopy of Molecules I - Absorption Spectroscopy
 3. Electronic Spectroscopy of Molecules I - Absorption Spectroscopy 3.1. Vibrational coarse structure of electronic spectra. The Born Oppenheimer Approximation introduced in the last chapter can be extended
3. Electronic Spectroscopy of Molecules I - Absorption Spectroscopy 3.1. Vibrational coarse structure of electronic spectra. The Born Oppenheimer Approximation introduced in the last chapter can be extended
From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation?
 From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? From lowest energy to highest energy, which of the following correctly
From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? From lowest energy to highest energy, which of the following correctly
5. The Nature of Light. Does Light Travel Infinitely Fast? EMR Travels At Finite Speed. EMR: Electric & Magnetic Waves
 5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
5. The Nature of Light Light travels in vacuum at 3.0. 10 8 m/s Light is one form of electromagnetic radiation Continuous radiation: Based on temperature Wien s Law & the Stefan-Boltzmann Law Light has
Infrared Spectroscopy: Theory
 u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
u Chapter 15 Infrared Spectroscopy: Theory An important tool of the organic chemist is Infrared Spectroscopy, or IR. IR spectra are acquired on a special instrument, called an IR spectrometer. IR is used
Arrangement of Electrons in Atoms
 CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
CHAPTER 4 PRE-TEST Arrangement of Electrons in Atoms In the space provided, write the letter of the term that best completes each sentence or best answers each question. 1. Which of the following orbital
CHEM 1411 Chapter 5 Homework Answers
 1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
1 CHEM 1411 Chapter 5 Homework Answers 1. Which statement regarding the gold foil experiment is false? (a) It was performed by Rutherford and his research group early in the 20 th century. (b) Most of
2. Spin Chemistry and the Vector Model
 2. Spin Chemistry and the Vector Model The story of magnetic resonance spectroscopy and intersystem crossing is essentially a choreography of the twisting motion which causes reorientation or rephasing
2. Spin Chemistry and the Vector Model The story of magnetic resonance spectroscopy and intersystem crossing is essentially a choreography of the twisting motion which causes reorientation or rephasing
CHAPTER 13 MOLECULAR SPECTROSCOPY
 CHAPTER 13 MOLECULAR SPECTROSCOPY Our most detailed knowledge of atomic and molecular structure has been obtained from spectroscopy study of the emission, absorption and scattering of electromagnetic radiation
CHAPTER 13 MOLECULAR SPECTROSCOPY Our most detailed knowledge of atomic and molecular structure has been obtained from spectroscopy study of the emission, absorption and scattering of electromagnetic radiation
5.61 Physical Chemistry 25 Helium Atom page 1 HELIUM ATOM
 5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
5.6 Physical Chemistry 5 Helium Atom page HELIUM ATOM Now that we have treated the Hydrogen like atoms in some detail, we now proceed to discuss the next simplest system: the Helium atom. In this situation,
The Two-Body Problem
 The Two-Body Problem Abstract In my short essay on Kepler s laws of planetary motion and Newton s law of universal gravitation, the trajectory of one massive object near another was shown to be a conic
The Two-Body Problem Abstract In my short essay on Kepler s laws of planetary motion and Newton s law of universal gravitation, the trajectory of one massive object near another was shown to be a conic
Chapter 28 Atomic Physics
 614 Chapter 28 Atomic Physics GOALS After you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms and use it in an
614 Chapter 28 Atomic Physics GOALS After you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms and use it in an
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Free Electron Fermi Gas (Kittel Ch. 6)
 Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Free Electron Fermi Gas (Kittel Ch. 6) Role of Electrons in Solids Electrons are responsible for binding of crystals -- they are the glue that hold the nuclei together Types of binding (see next slide)
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total
 Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
Chemistry 2 Chapter 13: Electrons in Atoms Please do not write on the test Use an answer sheet! 1 point/problem 45 points total 1. Calculate the energy in joules of a photon of red light that has a frequency
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS
 PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
PHOTOELECTRIC EFFECT AND DUAL NATURE OF MATTER AND RADIATIONS 1. Photons 2. Photoelectric Effect 3. Experimental Set-up to study Photoelectric Effect 4. Effect of Intensity, Frequency, Potential on P.E.
Theory of electrons and positrons
 P AUL A. M. DIRAC Theory of electrons and positrons Nobel Lecture, December 12, 1933 Matter has been found by experimental physicists to be made up of small particles of various kinds, the particles of
P AUL A. M. DIRAC Theory of electrons and positrons Nobel Lecture, December 12, 1933 Matter has been found by experimental physicists to be made up of small particles of various kinds, the particles of
Chapter 2. Quantum Theory
 Chapter 2 Quantum Theory 2.0 Introduction 2.6 Orbital Shapes, Signs, and Sizes 2.1 The Nature of Light 2.7 Electron Configurations 2.2 Quantization 2.8 Quantum Theory and the Periodic Table 2.3 Bohr Model
Chapter 2 Quantum Theory 2.0 Introduction 2.6 Orbital Shapes, Signs, and Sizes 2.1 The Nature of Light 2.7 Electron Configurations 2.2 Quantization 2.8 Quantum Theory and the Periodic Table 2.3 Bohr Model
Atomic Calculations. 2.1 Composition of the Atom. number of protons + number of neutrons = mass number
 2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
2.1 Composition of the Atom Atomic Calculations number of protons + number of neutrons = mass number number of neutrons = mass number - number of protons number of protons = number of electrons IF positive
Determination of Molecular Structure by MOLECULAR SPECTROSCOPY
 Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
Level 3 Achievement Scale
 Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 1: Atoms Level 3 Achievement Scale Can state the key results of the experiments associated with Dalton, Rutherford, Thomson, Chadwick, and Bohr and what this lead each to conclude. Can explain that
Unit 2: Chemical Bonding and Organic Chemistry
 Chemistry AP Unit : Chemical Bonding and Organic Chemistry Unit : Chemical Bonding and Organic Chemistry Chapter 7: Atomic Structure and Periodicity 7.1: Electromagnetic Radiation Electromagnetic (EM)
Chemistry AP Unit : Chemical Bonding and Organic Chemistry Unit : Chemical Bonding and Organic Chemistry Chapter 7: Atomic Structure and Periodicity 7.1: Electromagnetic Radiation Electromagnetic (EM)
Objectives. PAM1014 Introduction to Radiation Physics. Constituents of Atoms. Atoms. Atoms. Atoms. Basic Atomic Theory
 PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
PAM1014 Introduction to Radiation Physics Basic Atomic Theory Objectives Introduce and Molecules The periodic Table Electronic Energy Levels Atomic excitation & de-excitation Ionisation Molecules Constituents
APPLIED MATHEMATICS ADVANCED LEVEL
 APPLIED MATHEMATICS ADVANCED LEVEL INTRODUCTION This syllabus serves to examine candidates knowledge and skills in introductory mathematical and statistical methods, and their applications. For applications
APPLIED MATHEMATICS ADVANCED LEVEL INTRODUCTION This syllabus serves to examine candidates knowledge and skills in introductory mathematical and statistical methods, and their applications. For applications
Experiment #5: Qualitative Absorption Spectroscopy
 Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Experiment #5: Qualitative Absorption Spectroscopy One of the most important areas in the field of analytical chemistry is that of spectroscopy. In general terms, spectroscopy deals with the interactions
Sample Exercise 6.1 Concepts of Wavelength and Frequency
 Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
CHEM6085: Density Functional Theory Lecture 2. Hamiltonian operators for molecules
 CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
CHEM6085: Density Functional Theory Lecture 2 Hamiltonian operators for molecules C.-K. Skylaris 1 The (time-independent) Schrödinger equation is an eigenvalue equation operator for property A eigenfunction
Notes: Most of the material in this chapter is taken from Young and Freedman, Chap. 13.
 Chapter 5. Gravitation Notes: Most of the material in this chapter is taken from Young and Freedman, Chap. 13. 5.1 Newton s Law of Gravitation We have already studied the effects of gravity through the
Chapter 5. Gravitation Notes: Most of the material in this chapter is taken from Young and Freedman, Chap. 13. 5.1 Newton s Law of Gravitation We have already studied the effects of gravity through the
Precession of spin and Precession of a top
 6. Classical Precession of the Angular Momentum Vector A classical bar magnet (Figure 11) may lie motionless at a certain orientation in a magnetic field. However, if the bar magnet possesses angular momentum,
6. Classical Precession of the Angular Momentum Vector A classical bar magnet (Figure 11) may lie motionless at a certain orientation in a magnetic field. However, if the bar magnet possesses angular momentum,
2m dx 2 = Eψ(x) (1) Total derivatives can be used since there is but one independent variable. The equation simplifies to. ψ (x) + k 2 ψ(x) = 0 (2)
 CHAPTER 3 QUANTUM MECHANICS OF SOME SIMPLE SYSTEMS The Free Particle The simplest system in quantum mechanics has the potential energy V equal to zero everywhere. This is called a free particle since it
CHAPTER 3 QUANTUM MECHANICS OF SOME SIMPLE SYSTEMS The Free Particle The simplest system in quantum mechanics has the potential energy V equal to zero everywhere. This is called a free particle since it
DYNAMICS OF GALAXIES
 DYNAMICS OF GALAXIES 2. and stellar orbits Piet van der Kruit Kapteyn Astronomical Institute University of Groningen the Netherlands Winter 2008/9 and stellar orbits Contents Range of timescales Two-body
DYNAMICS OF GALAXIES 2. and stellar orbits Piet van der Kruit Kapteyn Astronomical Institute University of Groningen the Netherlands Winter 2008/9 and stellar orbits Contents Range of timescales Two-body
Halliday, Resnick & Walker Chapter 13. Gravitation. Physics 1A PHYS1121 Professor Michael Burton
 Halliday, Resnick & Walker Chapter 13 Gravitation Physics 1A PHYS1121 Professor Michael Burton II_A2: Planetary Orbits in the Solar System + Galaxy Interactions (You Tube) 21 seconds 13-1 Newton's Law
Halliday, Resnick & Walker Chapter 13 Gravitation Physics 1A PHYS1121 Professor Michael Burton II_A2: Planetary Orbits in the Solar System + Galaxy Interactions (You Tube) 21 seconds 13-1 Newton's Law
Notes on Elastic and Inelastic Collisions
 Notes on Elastic and Inelastic Collisions In any collision of 2 bodies, their net momentus conserved. That is, the net momentum vector of the bodies just after the collision is the same as it was just
Notes on Elastic and Inelastic Collisions In any collision of 2 bodies, their net momentus conserved. That is, the net momentum vector of the bodies just after the collision is the same as it was just
Solar Energy. Outline. Solar radiation. What is light?-- Electromagnetic Radiation. Light - Electromagnetic wave spectrum. Electromagnetic Radiation
 Outline MAE 493R/593V- Renewable Energy Devices Solar Energy Electromagnetic wave Solar spectrum Solar global radiation Solar thermal energy Solar thermal collectors Solar thermal power plants Photovoltaics
Outline MAE 493R/593V- Renewable Energy Devices Solar Energy Electromagnetic wave Solar spectrum Solar global radiation Solar thermal energy Solar thermal collectors Solar thermal power plants Photovoltaics
Assessment Plan for Learning Outcomes for BA/BS in Physics
 Department of Physics and Astronomy Goals and Learning Outcomes 1. Students know basic physics principles [BS, BA, MS] 1.1 Students can demonstrate an understanding of Newton s laws 1.2 Students can demonstrate
Department of Physics and Astronomy Goals and Learning Outcomes 1. Students know basic physics principles [BS, BA, MS] 1.1 Students can demonstrate an understanding of Newton s laws 1.2 Students can demonstrate
Lecture 3: Optical Properties of Bulk and Nano. 5 nm
 Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Lecture 3: Optical Properties of Bulk and Nano 5 nm The Previous Lecture Origin frequency dependence of χ in real materials Lorentz model (harmonic oscillator model) 0 e - n( ) n' n '' n ' = 1 + Nucleus
Measurement of Charge-to-Mass (e/m) Ratio for the Electron
 Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Measurement of Charge-to-Mass (e/m) Ratio for the Electron Experiment objectives: measure the ratio of the electron charge-to-mass ratio e/m by studying the electron trajectories in a uniform magnetic
Orbital Dynamics with Maple (sll --- v1.0, February 2012)
 Orbital Dynamics with Maple (sll --- v1.0, February 2012) Kepler s Laws of Orbital Motion Orbital theory is one of the great triumphs mathematical astronomy. The first understanding of orbits was published
Orbital Dynamics with Maple (sll --- v1.0, February 2012) Kepler s Laws of Orbital Motion Orbital theory is one of the great triumphs mathematical astronomy. The first understanding of orbits was published
The Advanced Placement Examination in Chemistry. Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010
 The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
The Advanced Placement Examination in Chemistry Part I Multiple Choice Questions Part II Free Response Questions Selected Questions from1970 to 2010 Atomic Theory and Periodicity Part I 1984 1. Which of
State of Stress at Point
 State of Stress at Point Einstein Notation The basic idea of Einstein notation is that a covector and a vector can form a scalar: This is typically written as an explicit sum: According to this convention,
State of Stress at Point Einstein Notation The basic idea of Einstein notation is that a covector and a vector can form a scalar: This is typically written as an explicit sum: According to this convention,
Bohr model of hydrogen
 Chapter 3 Bohr model of hydrogen Figure 3.1: Democritus The atomic theory of matter has a long history, in some ways all the way back to the ancient Greeks (Democritus - ca. 400 BCE - suggested that all
Chapter 3 Bohr model of hydrogen Figure 3.1: Democritus The atomic theory of matter has a long history, in some ways all the way back to the ancient Greeks (Democritus - ca. 400 BCE - suggested that all
Exam 2 Practice Problems Part 2 Solutions
 Problem 1: Short Questions MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics 8. Exam Practice Problems Part Solutions (a) Can a constant magnetic field set into motion an electron, which is initially
Problem 1: Short Questions MASSACHUSETTS INSTITUTE OF TECHNOLOGY Department of Physics 8. Exam Practice Problems Part Solutions (a) Can a constant magnetic field set into motion an electron, which is initially
Module 3 : Molecular Spectroscopy Lecture 13 : Rotational and Vibrational Spectroscopy
 Module 3 : Molecular Spectroscopy Lecture 13 : Rotational and Vibrational Spectroscopy Objectives After studying this lecture, you will be able to Calculate the bond lengths of diatomics from the value
Module 3 : Molecular Spectroscopy Lecture 13 : Rotational and Vibrational Spectroscopy Objectives After studying this lecture, you will be able to Calculate the bond lengths of diatomics from the value
Physics 9e/Cutnell. correlated to the. College Board AP Physics 1 Course Objectives
 Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physics 9e/Cutnell correlated to the College Board AP Physics 1 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring
Physical Principle of Formation and Essence of Radio Waves
 Physical Principle of Formation and Essence of Radio Waves Anatoli Bedritsky Abstract. This article opens physical phenomena which occur at the formation of the radio waves, and opens the essence of the
Physical Principle of Formation and Essence of Radio Waves Anatoli Bedritsky Abstract. This article opens physical phenomena which occur at the formation of the radio waves, and opens the essence of the
Chapter 22: Electric Flux and Gauss s Law
 22.1 ntroduction We have seen in chapter 21 that determining the electric field of a continuous charge distribution can become very complicated for some charge distributions. t would be desirable if we
22.1 ntroduction We have seen in chapter 21 that determining the electric field of a continuous charge distribution can become very complicated for some charge distributions. t would be desirable if we
Differential Relations for Fluid Flow. Acceleration field of a fluid. The differential equation of mass conservation
 Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Differential Relations for Fluid Flow In this approach, we apply our four basic conservation laws to an infinitesimally small control volume. The differential approach provides point by point details of
Vector surface area Differentials in an OCS
 Calculus and Coordinate systems EE 311 - Lecture 17 1. Calculus and coordinate systems 2. Cartesian system 3. Cylindrical system 4. Spherical system In electromagnetics, we will often need to perform integrals
Calculus and Coordinate systems EE 311 - Lecture 17 1. Calculus and coordinate systems 2. Cartesian system 3. Cylindrical system 4. Spherical system In electromagnetics, we will often need to perform integrals
Physics 53. Gravity. Nature and Nature's law lay hid in night: God said, "Let Newton be!" and all was light. Alexander Pope
 Physics 53 Gravity Nature and Nature's law lay hid in night: God said, "Let Newton be!" and all was light. Alexander Pope Kepler s laws Explanations of the motion of the celestial bodies sun, moon, planets
Physics 53 Gravity Nature and Nature's law lay hid in night: God said, "Let Newton be!" and all was light. Alexander Pope Kepler s laws Explanations of the motion of the celestial bodies sun, moon, planets
Chapter 6 Circular Motion
 Chapter 6 Circular Motion 6.1 Introduction... 1 6.2 Cylindrical Coordinate System... 2 6.2.1 Unit Vectors... 3 6.2.2 Infinitesimal Line, Area, and Volume Elements in Cylindrical Coordinates... 4 Example
Chapter 6 Circular Motion 6.1 Introduction... 1 6.2 Cylindrical Coordinate System... 2 6.2.1 Unit Vectors... 3 6.2.2 Infinitesimal Line, Area, and Volume Elements in Cylindrical Coordinates... 4 Example
1. Units of a magnetic field might be: A. C m/s B. C s/m C. C/kg D. kg/c s E. N/C m ans: D
 Chapter 28: MAGNETIC FIELDS 1 Units of a magnetic field might be: A C m/s B C s/m C C/kg D kg/c s E N/C m 2 In the formula F = q v B: A F must be perpendicular to v but not necessarily to B B F must be
Chapter 28: MAGNETIC FIELDS 1 Units of a magnetic field might be: A C m/s B C s/m C C/kg D kg/c s E N/C m 2 In the formula F = q v B: A F must be perpendicular to v but not necessarily to B B F must be
Quantum Mechanics. Dr. N.S. Manton. Michælmas Term 1996. 1 Introduction 1
 Quantum Mechanics Dr. N.S. Manton Michælmas Term 1996 Contents 1 Introduction 1 The Schrödinger Equation 1.1 Probabilistic Interpretation of ψ...................................1.1 Probability Flux and
Quantum Mechanics Dr. N.S. Manton Michælmas Term 1996 Contents 1 Introduction 1 The Schrödinger Equation 1.1 Probabilistic Interpretation of ψ...................................1.1 Probability Flux and
Bohr Model Calculations for Atoms and Ions
 Bohr Model Calculations for Atoms and Ions Frank Riou Department of Chemistry College of St. nedict St. Johnʹs University St. Joseph, MN 56374 Abstract A debroglie Bohr model is described that can be used
Bohr Model Calculations for Atoms and Ions Frank Riou Department of Chemistry College of St. nedict St. Johnʹs University St. Joseph, MN 56374 Abstract A debroglie Bohr model is described that can be used
Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE
 Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE Problems: 1-3, 13-15, 19, 23-25, 31-32, 43, 45-46, 49c, 50a, 50b, 57c, 58 (b,c,d), 61-62, 69, 71-74, 77-88, 91-94 9.5 LIGHT: Electromagnetic Radiation
Chapter 9: ELECTRONS IN ATOMS AND THE PERIODIC TABLE Problems: 1-3, 13-15, 19, 23-25, 31-32, 43, 45-46, 49c, 50a, 50b, 57c, 58 (b,c,d), 61-62, 69, 71-74, 77-88, 91-94 9.5 LIGHT: Electromagnetic Radiation
Orbits of the Lennard-Jones Potential
 Orbits of the Lennard-Jones Potential Prashanth S. Venkataram July 28, 2012 1 Introduction The Lennard-Jones potential describes weak interactions between neutral atoms and molecules. Unlike the potentials
Orbits of the Lennard-Jones Potential Prashanth S. Venkataram July 28, 2012 1 Introduction The Lennard-Jones potential describes weak interactions between neutral atoms and molecules. Unlike the potentials
Binary Stars. Kepler s Laws of Orbital Motion
 Binary Stars Kepler s Laws of Orbital Motion Kepler s Three Laws of orbital motion result from the solution to the equation of motion for bodies moving under the influence of a central 1/r 2 force gravity.
Binary Stars Kepler s Laws of Orbital Motion Kepler s Three Laws of orbital motion result from the solution to the equation of motion for bodies moving under the influence of a central 1/r 2 force gravity.
THE BOHR QUANTUM MODEL
 THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
THE BOHR QUANTUM MODEL INTRODUCTION When light from a low-pressure gas is subject to an electric discharge, a discrete line spectrum is emitted. When light from such a low-pressure gas is examined with
Copyright 2011 Casa Software Ltd. www.casaxps.com
 Table of Contents Variable Forces and Differential Equations... 2 Differential Equations... 3 Second Order Linear Differential Equations with Constant Coefficients... 6 Reduction of Differential Equations
Table of Contents Variable Forces and Differential Equations... 2 Differential Equations... 3 Second Order Linear Differential Equations with Constant Coefficients... 6 Reduction of Differential Equations
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Practice Questions - Chapter 7 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following represents an impossible set of
Halliday, Resnick & Walker Chapter 13. Gravitation. Physics 1A PHYS1121 Professor Michael Burton
 Halliday, Resnick & Walker Chapter 13 Gravitation Physics 1A PHYS1121 Professor Michael Burton II_A2: Planetary Orbits in the Solar System + Galaxy Interactions (You Tube) 21 seconds 13-1 Newton's Law
Halliday, Resnick & Walker Chapter 13 Gravitation Physics 1A PHYS1121 Professor Michael Burton II_A2: Planetary Orbits in the Solar System + Galaxy Interactions (You Tube) 21 seconds 13-1 Newton's Law
Symmetric Stretch: allows molecule to move through space
 BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
Chem 1A Exam 2 Review Problems
 Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
Chem 1A Exam 2 Review Problems 1. At 0.967 atm, the height of mercury in a barometer is 0.735 m. If the mercury were replaced with water, what height of water (in meters) would be supported at this pressure?
INFRARED SPECTROSCOPY (IR)
 INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
Simple Laser-Induced Fluorescence Setup to Explore Molecular Spectroscopy. Abstract
 Simple Laser-Induced Fluorescence Setup to Explore Molecular Spectroscopy S. B. Bayram and M.D. Freamat Miami University, Department of Physics, Oxford, OH 45056 (Dated: July 23, 2012) Abstract We will
Simple Laser-Induced Fluorescence Setup to Explore Molecular Spectroscopy S. B. Bayram and M.D. Freamat Miami University, Department of Physics, Oxford, OH 45056 (Dated: July 23, 2012) Abstract We will
Chapters 21-29. Magnetic Force. for a moving charge. F=BQvsinΘ. F=BIlsinΘ. for a current
 Chapters 21-29 Chapter 21:45,63 Chapter 22:25,49 Chapter 23:35,38,53,55,58,59 Chapter 24:17,18,20,42,43,44,50,52,53.59,63 Chapter 26:27,33,34,39,54 Chapter 27:17,18,34,43,50,51,53,56 Chapter 28: 10,11,28,47,52
Chapters 21-29 Chapter 21:45,63 Chapter 22:25,49 Chapter 23:35,38,53,55,58,59 Chapter 24:17,18,20,42,43,44,50,52,53.59,63 Chapter 26:27,33,34,39,54 Chapter 27:17,18,34,43,50,51,53,56 Chapter 28: 10,11,28,47,52
2. Molecular stucture/basic
 2. Molecular stucture/basic spectroscopy The electromagnetic spectrum Spectral region for atomic and molecular spectroscopy E. Hecht (2nd Ed.) Optics, Addison-Wesley Publishing Company,1987 Spectral regions
2. Molecular stucture/basic spectroscopy The electromagnetic spectrum Spectral region for atomic and molecular spectroscopy E. Hecht (2nd Ed.) Optics, Addison-Wesley Publishing Company,1987 Spectral regions
PHYSICS PAPER 1 (THEORY)
 PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
PHYSICS PAPER 1 (THEORY) (Three hours) (Candidates are allowed additional 15 minutes for only reading the paper. They must NOT start writing during this time.) ---------------------------------------------------------------------------------------------------------------------
NEW YORK STATE TEACHER CERTIFICATION EXAMINATIONS
 NEW YORK STATE TEACHER CERTIFICATION EXAMINATIONS TEST DESIGN AND FRAMEWORK September 2014 Authorized for Distribution by the New York State Education Department This test design and framework document
NEW YORK STATE TEACHER CERTIFICATION EXAMINATIONS TEST DESIGN AND FRAMEWORK September 2014 Authorized for Distribution by the New York State Education Department This test design and framework document
Group Theory and Chemistry
 Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
Group Theory and Chemistry Outline: Raman and infra-red spectroscopy Symmetry operations Point Groups and Schoenflies symbols Function space and matrix representation Reducible and irreducible representation
