Summary of the risk management plan (RMP) for Simbrinza (brinzolamide/brimonidine tartrate)
|
|
|
- Prosper Owens
- 7 years ago
- Views:
Transcription
1 EMA/339377/2014 Summary of the risk management plan (RMP) for Simbrinza (brinzolamide/brimonidine tartrate) This is a summary of the risk management plan (RMP) for Simbrinza, which details the measures to be taken in order to ensure that Simbrinza is used as safely as possible. For more information on RMP summaries, see here. This RMP summary should be read in conjunction with the EPAR summary and the product information for Simbrinza, which can be found on Simbrinza s EPAR page. Overview of disease epidemiology Simbrinza is used to reduce intra-ocular pressure (pressure inside the eye) in adults with ocular hypertension (high intra-ocular pressure) or in those with an eye condition known as open-angle glaucoma. Glaucoma as well as other causes of high pressure in the eye increase the risk of damage to the retina and the optic nerve that sends signals from the eye to the brain. This can result in serious vision loss and even blindness. Glaucoma is the second leading cause of blindness and is the leading cause of irreversible visual loss. Treating glaucoma and ocular hypertension by reducing the pressure inside the eye has been demonstrated to protect against further damage to the optic nerve. It has been estimated that by the year 2020 there will be almost 80 million people in the world that are affected by either open-angle glaucoma or angle-closure glaucoma. The majority of these people will have open-angle glaucoma, which is the most common type of glaucoma among Europeans or Africans. Risk factors for open-angle glaucoma include increased age, African ethnicity, family history, increased pressure inside the eye, myopia (short-sightedness), and decreased corneal thickness (the cornea is the transparent layer in front of the eye that covers the pupil and iris). Blindness in both eyes from glaucoma is expected to affect more than 11 million people worldwide by Summary of treatment benefits Simbrinza contains two active substances brinzolamide and brimonidine tartrate and is used when treatment with one single agent does not lower eye pressure enough. A clinical study involving 560 patients showed that Simbrinza reduced intra-ocular pressure (IOP) more effectively than either brinzolamide or brimonidine alone. Another clinical study showed that the effect of Simbrinza is comparable to the effect of brinzolamide and brimonidine added together. Unknowns relating to treatment benefits Simbrinza has not been studied in patients under 18 years and in pregnant or breastfeeding women. Simbrinza has also not been studied in patients with severe liver or kidney impairment, cardiovascular Page 1/5
2 disease, and those with other forms of glaucoma or eye disease or taking antidepressants. The benefit of Simbrinza in these patients is unknown. Elderly patients were included in the main clinical trials and no differences were observed between them and other adult patients. Summary of safety concerns Important identified risks Risk Preventability Hypersensitivity/ eye allergy/ anaphylaxis (severe allergic reaction)/ severe skin reactions Central nervous system depression in children Heart and circulatory disorders Corneal oedema (swelling of the thin, transparent Eye allergy has been reported when using brimonidine alone and may be delayed for up to 15 months after treatment is started and may be associated with a loss of control of intraocular pressure. Skin reactions and other more serious allergic reactions might also occur. Symptoms of central nervous system depression like slow heart rate, shallow breathing or cessation of breathing, hypotension (low blood pressure), low body temperature, low heart rate, drowsiness, sleepiness and even coma have been reported in the literature in association with brimonidine eye drops in children. Children younger than 6 years and weighing less than 20 kg may be at greatest risk. Brimonidine can cause fatigue (tiredness) and reduce blood pressure at rest. There have been isolated and reversible cases of corneal oedema reported in the literature with the use Patients should be asked about any previous history of eye-drop allergies or dry eye and should be monitored for early symptoms like conjunctivitis, red eyes, eyelid swelling, redness around the eye and breathing difficulties. Simbrinza should not be used in patients with allergies or hypersensitivity to any of the active substances, to other medicines of the same class, or to any of the excipients (inactive ingredients) Simbrinza must not be used in newborns and infants under the age of 2 years and is not recommended in children or adolescents. These effects can be prevented by prescribing Simbrinza with caution in patients with severe or unstable and uncontrolled heart or circulatory disease, cerebral or coronary insufficiency (insufficient blood supply to the brain or heart), tendency to develop pale or blue fingers, dizziness or faintness on standing up, and small blood vessels that become inflamed and swollen. This effect can be prevented by appropriate selection of patients, avoiding use in high risk patients, such Page 2/5
3 Risk Preventability covering over the eye) and/or corneal decompensation Metabolic acidosis (increase acid levels in blood) and/or kidney impairment of brinzolamide applied directly onto the eye. Carbonic anhydrase inhibitors (medicines that work in a similar way to brinzolamide) have been reported to cause metabolic acidosis in patients with kidney function impairment. as those with sensitive corneas, those who wear contact lenses, those who suffer from diabetes or corneal diseases, and those who have a history of eye surgery. Simbrinza should not be used in patients with acidosis and/or with severe kidney impairment or in patients with conditions which can lead to acidosis (e.g patients with kidney disease and elderly patients with reduced kidney function). Important potential risks Risk Damage to the corneal epithelium due to use of eye drops containing preservatives Patients with open-angle glaucoma and ocular hypertension are usually required to use eye drops for life. The presence of a preservative in eye drops increases the risk of both adverse effects on the corneal surface and the possibility of allergic reactions. The extent of the damage depends on, among other factors, the type of eye drops used, and the frequency and duration of use. Accidental overdose/ingestion in children Several reports of serious adverse effects following accidental ingestion of brimonidine by children have been published. After an accidental overdose, the children experienced symptoms of central nervous system depression like slow heart rate, shallow breathing or cessation of breathing, hypotension, low body temperature, low heart rate, drowsiness, sleepiness, and even coma, and all of them required admission to intensive care. They completely recovered after intensive care, mostly within 6-24 hours. Children younger than 6 years and weighing less than 20 kg are at greatest risk. Missing information Risk Use in pregnant women There are no or limited data from the use of Simbrinza in pregnant women. Animal studies have shown that Brinzolamide may interfere with normal fetal development, following systemic administration. Oral administration of brimonidine did not indicate direct harmful effects on reproduction. In animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Page 3/5
4 Risk Use by breastfeeding mothers Long-term safety of Simbrinza No studies have been carried out in breastfeeding women. It is not known whether Simbrinza is excreted in human milk. In animal studies, minimal levels of brinzolamide were detected in breast milk following oral administration. Most glaucoma patients receive long-term medical treatment. It is known that long-term eye drop use may cause damage to the eye surface, mainly due to the preservatives in the eye drops. Brimonidine can also cause eye allergy that may take months to occur but with symptoms generally similar to those of an immediate allergic reaction. Summary of risk minimisation measures by safety concern All medicines have a summary of product characteristics (SmPC) which provides physicians, pharmacists and other healthcare professionals with details on how to use the medicine, and also describes the risks and recommendations for minimising them. Information for patients is available in lay language in the package leaflet. The measures listed in these documents are known as routine risk minimisation measures. The SmPC and the package leaflet are part of the medicine s product information. The product information for Simbrinza can be found on Simbrinza s EPAR page. This medicine has no additional risk minimisation measures. Planned post-authorisation development plan List of studies in post-authorisation development plan Study/activity (including study number) Objectives Safety concerns /efficacy issue addressed Status Planned date for submission of (interim and) final results M Evaluate the additional IOP lowering effect with Simbrinza when used as an adjunct to travoprost (thrice daily dosing) Efficacy of Simbrinza in an adjunctive setting with a prostaglandin analogue (PGA) Started Planned Q M Evaluate the additional IOP lowering effect with Simbrinza when used as an adjunct to a PGA (thrice daily dosing) Efficacy of Simbrinza in an adjunctive setting with a PGA Started Planned Q M Evaluate the 24-hour intra-ocular pressure lowering of Simbrinza Nocturnal intraocular pressure control Started Planned Q Page 4/5
5 Study/activity (including study number) Objectives Safety concerns /efficacy issue addressed Status Planned date for submission of (interim and) final results (thrice daily dosing) Studies which are a condition of the marketing authorisation None of the above studies are conditions of the marketing authorisation. Summary of changes to the risk management plan over time Not applicable. This summary was last updated in Page 5/5
Summary of the risk management plan (RMP) for Ionsys (fentanyl)
 EMA/764409/2015 Summary of the risk management plan (RMP) for Ionsys (fentanyl) This is a summary of the risk management plan (RMP) for Ionsys, which details the measures to be taken in order to ensure
EMA/764409/2015 Summary of the risk management plan (RMP) for Ionsys (fentanyl) This is a summary of the risk management plan (RMP) for Ionsys, which details the measures to be taken in order to ensure
Summary of the risk management plan (RMP) for Rasagiline ratiopharm (rasagiline)
 EMA/744222/2014 Summary of the risk management plan (RMP) for Rasagiline ratiopharm (rasagiline) This is a summary of the risk management plan (RMP) for Rasagiline ratiopharm, which details the measures
EMA/744222/2014 Summary of the risk management plan (RMP) for Rasagiline ratiopharm (rasagiline) This is a summary of the risk management plan (RMP) for Rasagiline ratiopharm, which details the measures
Summary of the risk management plan (RMP) for Cerdelga (eliglustat)
 EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
Summary of the risk management plan (RMP) for Aripiprazole Pharmathen (aripiprazole)
 EMA/303592/2015 Summary of the risk management plan (RMP) for Aripiprazole Pharmathen (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Pharmathen, which details the measures
EMA/303592/2015 Summary of the risk management plan (RMP) for Aripiprazole Pharmathen (aripiprazole) This is a summary of the risk management plan (RMP) for Aripiprazole Pharmathen, which details the measures
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide)
 EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
Summary of the risk management plan (RMP) for Ofev (nintedanib)
 EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000
 UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
GLAUCOMA. American Academy of Ophthalmology
 GLAUCOMA American Academy of Ophthalmology What is glaucoma? Glaucoma is a disease of the optic nerve, which is the part of the eye that carries the images we see to the brain. The optic nerve is made
GLAUCOMA American Academy of Ophthalmology What is glaucoma? Glaucoma is a disease of the optic nerve, which is the part of the eye that carries the images we see to the brain. The optic nerve is made
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Summary of the risk management plan (RMP) for Accofil (filgrastim)
 EMA/475472/2014 Summary of the risk management plan (RMP) for Accofil (filgrastim) This is a summary of the risk management plan (RMP) for Accofil, which details the measures to be taken in order to ensure
EMA/475472/2014 Summary of the risk management plan (RMP) for Accofil (filgrastim) This is a summary of the risk management plan (RMP) for Accofil, which details the measures to be taken in order to ensure
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
Eye Diseases. 1995-2014, The Patient Education Institute, Inc. www.x-plain.com otf30101 Last reviewed: 05/21/2014 1
 Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Eye Diseases Introduction Some eye problems are minor and fleeting. But some lead to a permanent loss of vision. There are many diseases that can affect the eyes. The symptoms of eye diseases vary widely,
Risk Management Plan
 Risk Management Plan Active substance: Drospirenone/ethinylestradiol Version number: 4.0 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Oral contraception Effective control
Risk Management Plan Active substance: Drospirenone/ethinylestradiol Version number: 4.0 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Oral contraception Effective control
Treatments for Open-Angle Glaucoma. A Review of the Research for Adults
 Treatments for Open-Angle Glaucoma A Review of the Research for Adults Is This Information Right for Me? Yes, this information is for you if: Your eye doctor has said that you have open-angle glaucoma,
Treatments for Open-Angle Glaucoma A Review of the Research for Adults Is This Information Right for Me? Yes, this information is for you if: Your eye doctor has said that you have open-angle glaucoma,
Summary of the risk management plan (RMP) for Sirturo (bedaquiline)
 EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
EMA/16634/2014 Summary of the risk management plan (RMP) for Sirturo (bedaquiline) This is a summary of the risk management plan (RMP) for Sirturo, which details the measures to be taken in order to ensure
MEDICATION GUIDE. PROCRIT (PRO KRIT) (epoetin alfa)
 MEDICATION GUIDE PROCRIT (PROKRIT) (epoetin alfa) Read this Medication Guide: before you start PROCRIT. if you are told by your healthcare provider that there is new information about PROCRIT. if you are
MEDICATION GUIDE PROCRIT (PROKRIT) (epoetin alfa) Read this Medication Guide: before you start PROCRIT. if you are told by your healthcare provider that there is new information about PROCRIT. if you are
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
Trileptal (Oxcarbazepine)
 Brand and Generic Names: Trileptal Tablets: 150mg, 300mg, 600mg Liquid Suspension: 300mg/5mL Generic name: oxcarbazepine What is Trileptal and what does it treat? Trileptal (Oxcarbazepine) Oxcarbazepine
Brand and Generic Names: Trileptal Tablets: 150mg, 300mg, 600mg Liquid Suspension: 300mg/5mL Generic name: oxcarbazepine What is Trileptal and what does it treat? Trileptal (Oxcarbazepine) Oxcarbazepine
1 What Anapen is and what it is used for?
 PACKAGE LEAFLET: INFORMATION FOR THE USER Anapen 500 micrograms in 0.3 ml solution for injection (pre-filled syringe) Adrenaline (Epinephrine) Auto-Injector Read all of this leaflet carefully before you
PACKAGE LEAFLET: INFORMATION FOR THE USER Anapen 500 micrograms in 0.3 ml solution for injection (pre-filled syringe) Adrenaline (Epinephrine) Auto-Injector Read all of this leaflet carefully before you
Glaucoma. OET: Reading Part A. Reading Sub-test. Complete the following summary using the information in the four texts provided.
 Glaucoma Reading Sub-test TIME LIMIT: 15 MINUTES Complete the following summary using the information in the four texts provided. You do not need to read each text from beginning to end to complete the
Glaucoma Reading Sub-test TIME LIMIT: 15 MINUTES Complete the following summary using the information in the four texts provided. You do not need to read each text from beginning to end to complete the
MEDICATION GUIDE ACTOPLUS MET (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets
 MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
MEDICATION GUIDE (ak-tō-plus-met) (pioglitazone hydrochloride and metformin hydrochloride) tablets Read this Medication Guide carefully before you start taking and each time you get a refill. There may
LASIK. What is LASIK? Eye Words to Know. Who is a good candidate for LASIK?
 2014 2015 LASIK What is LASIK? LASIK (laser in situ keratomileusis) is a type of refractive surgery. This kind of surgery uses a laser to treat vision problems caused by refractive errors. You have a refractive
2014 2015 LASIK What is LASIK? LASIK (laser in situ keratomileusis) is a type of refractive surgery. This kind of surgery uses a laser to treat vision problems caused by refractive errors. You have a refractive
Package leaflet : information for the user. Dilute Adrenaline/Epinephrine Injection 1:10,000 adrenaline (epinephrine) (as acid tartrate) 0.
 Package leaflet : information for the user Dilute Adrenaline/Epinephrine Injection 1:10,000 adrenaline (epinephrine) (as acid tartrate) 0.1mg per ml Because of your condition it may not be possible for
Package leaflet : information for the user Dilute Adrenaline/Epinephrine Injection 1:10,000 adrenaline (epinephrine) (as acid tartrate) 0.1mg per ml Because of your condition it may not be possible for
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Hunter syndrome is a rare genetic disease which mainly affects males of all ethnicities. The incidence rate ranges from 0.6 to
Zomig Nasal Spray. Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION
 Zomig Nasal Spray Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some of the common questions people ask about It does not contain all
Zomig Nasal Spray Zolmitriptan 5 mg Nasal Spray Solution. CONSUMER MEDICINE INFORMATION What is in this leaflet This leaflet answers some of the common questions people ask about It does not contain all
Low Blood Pressure. This reference summary explains low blood pressure and how it can be prevented and controlled.
 Low Blood Pressure Introduction Low blood pressure, or hypotension, is when your blood pressure reading is 90/60 or lower. Some people have low blood pressure all of the time. In other people, blood pressure
Low Blood Pressure Introduction Low blood pressure, or hypotension, is when your blood pressure reading is 90/60 or lower. Some people have low blood pressure all of the time. In other people, blood pressure
PACKAGE LEAFLET: INFORMATION FOR THE USER. Dalacin C 150 mg Capsules. clindamycin hydrochloride. Dalacin C 150mg Capsules clindamycin hydrochloride
 PACKAGE LEAFLET: INFORMATION FOR THE USER PFIZER Dalacin C 150 mg Capsules clindamycin hydrochloride Dalacin C 150mg Capsules clindamycin hydrochloride PFIZER Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER PFIZER Dalacin C 150 mg Capsules clindamycin hydrochloride Dalacin C 150mg Capsules clindamycin hydrochloride PFIZER Read all of this leaflet carefully before
STRATTERA (Stra-TAIR-a)
 1 PV 5859 AMP MEDICATION GUIDE STRATTERA (Stra-TAIR-a) (atomoxetine) Capsules Read the Medication Guide that comes with STRATTERA before you or your child starts taking it and each time you get a refill.
1 PV 5859 AMP MEDICATION GUIDE STRATTERA (Stra-TAIR-a) (atomoxetine) Capsules Read the Medication Guide that comes with STRATTERA before you or your child starts taking it and each time you get a refill.
Glaucoma. Quick reference guide. Issue date: April 2009. Diagnosis and management of chronic open angle glaucoma and ocular hypertension
 Issue date: April 2009 Glaucoma Diagnosis and management of chronic open angle glaucoma and ocular hypertension Developed by the National Collaborating Centre for Acute Care About this booklet This is
Issue date: April 2009 Glaucoma Diagnosis and management of chronic open angle glaucoma and ocular hypertension Developed by the National Collaborating Centre for Acute Care About this booklet This is
Color Vision Defects - Color Blindness
 Color Vision Defects - Color Blindness Introduction A color vision defect causes a person to see colors differently than most people. Color vision defects are sometimes called color blindness. There are
Color Vision Defects - Color Blindness Introduction A color vision defect causes a person to see colors differently than most people. Color vision defects are sometimes called color blindness. There are
Prochlorperazine 3 mg Buccal Tablets (PROCHLORPERAZINE MALEATE)
 Package leaflet: Information for the user Prochlorperazine 3 mg Buccal Tablets (PROCHLORPERAZINE MALEATE) Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the user Prochlorperazine 3 mg Buccal Tablets (PROCHLORPERAZINE MALEATE) Read all of this leaflet carefully before you start taking this medicine because it contains important
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PATIENT INFORMATION LEAFLET. Calcium Sandoz Syrup calcium glubionate and calcium lactobionate
 PATIENT INFORMATION LEAFLET Calcium Sandoz Syrup calcium glubionate and calcium lactobionate Read all of this leaflet carefully before you start taking this medicine Keep this leaflet. You may need to
PATIENT INFORMATION LEAFLET Calcium Sandoz Syrup calcium glubionate and calcium lactobionate Read all of this leaflet carefully before you start taking this medicine Keep this leaflet. You may need to
The Family Library. Understanding Diabetes
 The Family Library Understanding Diabetes What is Diabetes? Diabetes is caused when the body has a problem in making or using insulin. Insulin is a hormone secreted by the pancreas and is needed for the
The Family Library Understanding Diabetes What is Diabetes? Diabetes is caused when the body has a problem in making or using insulin. Insulin is a hormone secreted by the pancreas and is needed for the
Elements for a public summary. VI.2.1 Overview of disease epidemiology. VI.2.2 Summary of treatment benefits
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
Perfalgan 10 mg/ml, solution for infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (HCl) STEROP 0,8mg/1ml. Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
used to treat inflammation, corneal injury and bacterial infections in the external part of the eye.
 TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules
 Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
H1N1 Flu Vaccine Available to All Virginia Beach City Public Schools Students
 V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291
 Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 13 Summary of Product Characteristics
Memantine hydrochloride 20 mg film-coated tablets PL 17907/0291 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 13 Summary of Product Characteristics
2 What you need to know before you have Ampiclox
 Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Medication Guide KLONOPIN (KLON-oh-pin) (clonazepam) Tablets
 Medication Guide KLONOPIN (KLON-oh-pin) (clonazepam) Tablets Read this Medication Guide before you start taking KLONOPIN and each time you get a refill. There may be new information. This information does
Medication Guide KLONOPIN (KLON-oh-pin) (clonazepam) Tablets Read this Medication Guide before you start taking KLONOPIN and each time you get a refill. There may be new information. This information does
PATIENT INFORMATION BOOKLET
 (060110) VISIONCARE S IMPLANTABLE MINIATURE TELESCOPE ( BY DR. ISAAC LIPSHITZ ) AN INTRAOCULAR TELESCOPE FOR TREATING SEVERE TO PROFOUND VISION IMPAIRMENT DUE TO BILATERAL END-STAGE AGE-RELATED MACULAR
(060110) VISIONCARE S IMPLANTABLE MINIATURE TELESCOPE ( BY DR. ISAAC LIPSHITZ ) AN INTRAOCULAR TELESCOPE FOR TREATING SEVERE TO PROFOUND VISION IMPAIRMENT DUE TO BILATERAL END-STAGE AGE-RELATED MACULAR
Seeing Beyond the Symptoms
 Seeing Beyond the Symptoms Cataracts are one of the leading causes of vision impairment in the United States. 1 However, because cataracts form slowly and over a long period of time, many people suffer
Seeing Beyond the Symptoms Cataracts are one of the leading causes of vision impairment in the United States. 1 However, because cataracts form slowly and over a long period of time, many people suffer
Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets
 Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets Read this Medication Guide before you start taking Plavix and each time you get a refill. There may be new information. This Medication
Medication Guide Plavix (PLAV-iks) (clopidogrel bisulfate) tablets Read this Medication Guide before you start taking Plavix and each time you get a refill. There may be new information. This Medication
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
1. What Xylocaine with adrenaline is and what it is used for
 Package leaflet: Information for the user Xylocaine 1% and 2% with adrenaline (epinephrine) 1:200,000 Solution for Injection lidocaine, adrenaline (epinephrine) Read all of this leaflet carefully before
Package leaflet: Information for the user Xylocaine 1% and 2% with adrenaline (epinephrine) 1:200,000 Solution for Injection lidocaine, adrenaline (epinephrine) Read all of this leaflet carefully before
Eye Injuries. The Eyes The eyes are sophisticated organs. They collect light and focus it on the back of the eye, allowing us to see.
 Eye Injuries Introduction The design of your face helps protect your eyes from injury. But injuries can still damage your eyes. Sometimes injuries are severe enough that you could lose your vision. Most
Eye Injuries Introduction The design of your face helps protect your eyes from injury. But injuries can still damage your eyes. Sometimes injuries are severe enough that you could lose your vision. Most
Share the important information in this Medication Guide with members of your household.
 MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
MEDICATION GUIDE BUPRENORPHINE (BUE-pre-NOR-feen) Sublingual Tablets, CIII IMPORTANT: Keep buprenorphine sublingual tablets in a secure place away from children. Accidental use by a child is a medical
There may be no symptoms at first. Eye problems can. You can help prevent eye problems. Just because you have
 Keeping your eyes healthy when you have diabetes Oregon Diabetes Resource Bank Handouts to help people with diabetes If you have diabetes, here are things you need to know: 1 2 3 Having diabetes makes
Keeping your eyes healthy when you have diabetes Oregon Diabetes Resource Bank Handouts to help people with diabetes If you have diabetes, here are things you need to know: 1 2 3 Having diabetes makes
Ask your healthcare provider about LONG-ACTING AVEED (testosterone undecanoate) AVEED TESTOSTERONE INJECTION 5 SHOTS A YEAR. Not an actual patient.
 Ask your healthcare provider about LONG-ACTING AVEED (testosterone undecanoate) AVEED TESTOSTERONE INJECTION 5 SHOTS A YEAR AFTER THE FIRST MONTH OF THERAPY Not an actual patient. CONSUMERS What is the
Ask your healthcare provider about LONG-ACTING AVEED (testosterone undecanoate) AVEED TESTOSTERONE INJECTION 5 SHOTS A YEAR AFTER THE FIRST MONTH OF THERAPY Not an actual patient. CONSUMERS What is the
Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU
 FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
Cataracts. Cataract and Primary Eye Care Service...215-928-3041. Main Number...215-928-3000. Physician Referral...1-877-AT-WILLS 1-877-289-4557
 Main Number...215-928-3000 Physician Referral...1-877-AT-WILLS 1-877-289-4557 Emergency Service...215-503-8080 Cataract and Primary Eye Care Service...215-928-3041 Retina Service... 215-928-3300 Cataract
Main Number...215-928-3000 Physician Referral...1-877-AT-WILLS 1-877-289-4557 Emergency Service...215-503-8080 Cataract and Primary Eye Care Service...215-928-3041 Retina Service... 215-928-3300 Cataract
ELEMENTS FOR A PUBLIC SUMMARY. Overview of disease epidemiology. Summary of treatment benefits
 VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
MEASURING AND RECORDING BLOOD PRESSURE
 MEASURING AND RECORDING BLOOD PRESSURE INTRODUCTION The blood pressure, along with the body temperature, pulse, and respirations, is one of the vital signs. These measurements are used to quickly, easily,
MEASURING AND RECORDING BLOOD PRESSURE INTRODUCTION The blood pressure, along with the body temperature, pulse, and respirations, is one of the vital signs. These measurements are used to quickly, easily,
PACKAGE LEAFLET: INFORMATION FOR THE USER. /.../ 2.5 mg orodispersible tablets. Desloratadine
 PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER /.../ 2.5 mg orodispersible tablets Desloratadine Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet.
PACKAGE LEAFLET PACKAGE LEAFLET: INFORMATION FOR THE USER /.../ 2.5 mg orodispersible tablets Desloratadine Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet.
Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)]
![Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)]](/thumbs/27/12172665.jpg) EMA/14365/2014 Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Overview of disease epidemiology Diphtheria
EMA/14365/2014 Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Overview of disease epidemiology Diphtheria
Vitreo-Retinal and Macular Degeneration Frequently Asked Questions
 Vitreo-Retinal and Macular Degeneration Frequently Asked Questions What is a Vitreo-Retinal specialist? Retinal specialists are eye physicians and surgeons who focus on diseases in the back of the eye
Vitreo-Retinal and Macular Degeneration Frequently Asked Questions What is a Vitreo-Retinal specialist? Retinal specialists are eye physicians and surgeons who focus on diseases in the back of the eye
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium)
 MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
It is important that you tell your family and the people closest to you of this increased sensitivity to opioids and the risk of overdose.
 MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
MEDICATION GUIDE VIVITROL (viv-i-trol) (naltrexone for extended-release injectable suspension) Read this Medication Guide before you start receiving VIVITROL injections and each time you receive an injection.
Pregnancy and Substance Abuse
 Pregnancy and Substance Abuse Introduction When you are pregnant, you are not just "eating for two." You also breathe and drink for two, so it is important to carefully consider what you put into your
Pregnancy and Substance Abuse Introduction When you are pregnant, you are not just "eating for two." You also breathe and drink for two, so it is important to carefully consider what you put into your
Do not take Neulasta if you have had a serious allergic reaction to human G-CSFs such as pegfilgrastim or filgrastim products.
 Patient Information Neulasta (nu-las-tah) (pegfilgrastim) injection Single-Dose Prefilled Syringe What is Neulasta? Neulasta is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is
Patient Information Neulasta (nu-las-tah) (pegfilgrastim) injection Single-Dose Prefilled Syringe What is Neulasta? Neulasta is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is
New Zealand Consumer Medicine Information. It does not take the place of talking to your doctor or pharmacist.
 New Zealand Consumer Medicine Information Antabuse Disulfiram 200mg tablets (die-sul-fear-am) What is in this leaflet? This leaflet answers some common questions about Antabuse. It does not contain all
New Zealand Consumer Medicine Information Antabuse Disulfiram 200mg tablets (die-sul-fear-am) What is in this leaflet? This leaflet answers some common questions about Antabuse. It does not contain all
MEDICATION GUIDE. Tranxene* (TRAN-zeen) T-TAB (clorazepate dipotassium) tablets
 MEDICATION GUIDE Tranxene* (TRAN-zeen) T-TAB (clorazepate dipotassium) tablets Read this Medication Guide before you start taking TRANXENE and each time you get a refill. There may be new information.
MEDICATION GUIDE Tranxene* (TRAN-zeen) T-TAB (clorazepate dipotassium) tablets Read this Medication Guide before you start taking TRANXENE and each time you get a refill. There may be new information.
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets
 MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets
 MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets Read this Medication Guide carefully before you start taking KOMBIGLYZE XR and each time you get
MEDICATION GUIDE KOMBIGLYZE XR (kom-be-glyze X-R) (saxagliptin and metformin HCl extended-release) tablets Read this Medication Guide carefully before you start taking KOMBIGLYZE XR and each time you get
Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O.
 Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O. Kurt N. Woeller, DO, is an osteopathic physician specializing in natural medicine. Dr. Woeller began his study of natural medicine
Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O. Kurt N. Woeller, DO, is an osteopathic physician specializing in natural medicine. Dr. Woeller began his study of natural medicine
Remeron (mirtazapine)
 Remeron (mirtazapine) FDA ALERT [07/2005] Suicidal Thoughts or Actions in Children and Adults Patients with depression or other mental illnesses often think about or attempt suicide. Closely watch anyone
Remeron (mirtazapine) FDA ALERT [07/2005] Suicidal Thoughts or Actions in Children and Adults Patients with depression or other mental illnesses often think about or attempt suicide. Closely watch anyone
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
MEDICATION GUIDE STELARA
 MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
Package leaflet: Information for the patient. Laxido Orange, powder for oral solution
 Package leaflet: Information for the patient Laxido Orange, powder for oral solution Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Package leaflet: Information for the patient Laxido Orange, powder for oral solution Read all of this leaflet carefully before you start taking this medicine because it contains important information for
How To Safely Use Aripiprazole
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bipolar I Disorder Reported prevalence rates for bipolar I disorder differ due to local variations in psychiatric practice, variations
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Bipolar I Disorder Reported prevalence rates for bipolar I disorder differ due to local variations in psychiatric practice, variations
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
Human Normal Immunoglobulin Solution for Intravenous Infusion.
 CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
SYNACTHEN i.m./i.v. tetracosactide hexaacetate
 New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS
 Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Descemet s Stripping Endothelial Keratoplasty (DSEK)
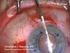 Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
Descemet s Stripping Endothelial Keratoplasty (DSEK) Your doctor has decided that you will benefit from a corneal transplant operation. This handout will explain your options to you. It explains the differences
How To Know If You Can See Without Glasses Or Contact Lense After Lasik
 The LASIK experience I WHO CAN HAVE LASIK? To be eligible for LASIK you should be at least 21 years of age, have healthy eyes and be in good general health. Your vision should not have deteriorated significantly
The LASIK experience I WHO CAN HAVE LASIK? To be eligible for LASIK you should be at least 21 years of age, have healthy eyes and be in good general health. Your vision should not have deteriorated significantly
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole
 PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
MEDICATION GUIDE mitoxantrone (mito-xan-trone) for injection concentrate
 MEDICATION GUIDE mitoxantrone (mito-xan-trone) for injection concentrate Read this Medication Guide before you start receiving mitoxantrone and each time you receive mitoxantrone. There may be new information.
MEDICATION GUIDE mitoxantrone (mito-xan-trone) for injection concentrate Read this Medication Guide before you start receiving mitoxantrone and each time you receive mitoxantrone. There may be new information.
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. sacubitril/valsartan film-coated tablets
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr ENTRESTO TM sacubitril/valsartan film-coated tablets Read this carefully before you start taking ENTRESTO TM and
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr ENTRESTO TM sacubitril/valsartan film-coated tablets Read this carefully before you start taking ENTRESTO TM and
MEDICATION GUIDE. These serious side effects are described below:
 MEDICATION GUIDE LYRICA (LEER-i-kah) (pregabalin) Capsules and Oral Solution, CV Read this Medication Guide before you start taking LYRICA and each time you get a refill. There may be new information.
MEDICATION GUIDE LYRICA (LEER-i-kah) (pregabalin) Capsules and Oral Solution, CV Read this Medication Guide before you start taking LYRICA and each time you get a refill. There may be new information.
MEDICATION GUIDE. What is Morphine Sulfate Oral Solution?
 MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
MEDICATION GUIDE Morphine Sulfate (mor-pheen) (CII) Oral Solution IMPORTANT: Keep Morphine Sulfate Oral Solution in a safe place away from children. Accidental use by a child is a medical emergency and
A list of all medications you are taking also include any vitamins, supplements, over-the-counter medicines, or herbal products
 This is your customized insulin discussion guide to bring to your next doctor s visit. WHAT TO BRING TO YOUR NEXT CHECK UP A record of your recent blood sugar readings A list of all medications you are
This is your customized insulin discussion guide to bring to your next doctor s visit. WHAT TO BRING TO YOUR NEXT CHECK UP A record of your recent blood sugar readings A list of all medications you are
Laser Vision Correction
 How will Laser Vision Correction affect my Lifestyle? Your Guide to Laser Vision Correction The Gift of Better Vision A few things to note after your surgery. As you enjoy your new-and-improved eyesight,
How will Laser Vision Correction affect my Lifestyle? Your Guide to Laser Vision Correction The Gift of Better Vision A few things to note after your surgery. As you enjoy your new-and-improved eyesight,
Package leaflet: Information for the patient Sativex Oromucosal Spray. (delta-9-tetrahydrocannabinol and cannabidiol)
 Package leaflet: Information for the patient Sativex Oromucosal Spray (delta-9-tetrahydrocannabinol and cannabidiol) Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient Sativex Oromucosal Spray (delta-9-tetrahydrocannabinol and cannabidiol) Read all of this leaflet carefully before you start using this medicine because it contains
Now that your Doctor has prescribed Livial for you
 Now that your Doctor has prescribed Livial for you This educational brochure is only for use by patients prescribed LIVIAL The Menopause The term menopause refers to the very last menstrual period a woman
Now that your Doctor has prescribed Livial for you This educational brochure is only for use by patients prescribed LIVIAL The Menopause The term menopause refers to the very last menstrual period a woman
Vibramycin Capsules Doxycycline hyclate capsules USP. Vibra-Tabs Film Coated Tablets Doxycycline hyclate tablets USP
 32 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Vibramycin Capsules Doxycycline hyclate capsules USP Vibra-Tabs Film Coated Tablets Doxycycline hyclate tablets USP
32 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Vibramycin Capsules Doxycycline hyclate capsules USP Vibra-Tabs Film Coated Tablets Doxycycline hyclate tablets USP
MEDICATION GUIDE SUBOXONE (Sub-OX-own) (buprenorphine and naloxone) Sublingual Film for sublingual or buccal administration (CIII)
 MEDICATION GUIDE SUBOXONE (Sub-OX-own) (buprenorphine and naloxone) Sublingual Film for sublingual or buccal administration (CIII) IMPORTANT: Keep SUBOXONE in a secure place away from children. Accidental
MEDICATION GUIDE SUBOXONE (Sub-OX-own) (buprenorphine and naloxone) Sublingual Film for sublingual or buccal administration (CIII) IMPORTANT: Keep SUBOXONE in a secure place away from children. Accidental
MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules. What is the most important information I should know about POMALYST?
 MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules What is the most important information I should know about POMALYST? Before you begin taking POMALYST, you must read and agree to all of
MEDICATION GUIDE POMALYST (POM-uh-list) (pomalidomide) capsules What is the most important information I should know about POMALYST? Before you begin taking POMALYST, you must read and agree to all of
Humulin (HU-mu-lin) R
 1 PATIENT INFORMATION Humulin (HU-mu-lin) R Regular U-500 (Concentrated) insulin human injection, USP (rdna origin) Read the Patient Information that comes with Humulin R U-500 before you start taking
1 PATIENT INFORMATION Humulin (HU-mu-lin) R Regular U-500 (Concentrated) insulin human injection, USP (rdna origin) Read the Patient Information that comes with Humulin R U-500 before you start taking
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
