CALIFORNIA controlled substance prescription pads FAQ s
|
|
|
- Gervais Merritt
- 7 years ago
- Views:
Transcription
1 CALIFORNIA controlled substance prescription pads FAQ s Q: Where can I find the regulations for the controlled substance prescription pads for the state of California? A: Q: Can you ship to a PO Box? A: Wilmer uses UPS to ship their orders and UPS does not deliver to PO Boxes. The state of California will not allow shipments to a PO Box, as they feel this is not a safe place to leave controlled substance prescriptions pads unattended. All shipments must be signed for, as we need to pull this information and keep it in our files. UPS provides this with their on-line tracking site. Q: Where can I ship the order of prescription pads? A: As a California Security Printer, we must follow the guidelines set forth by the state. Our orders must ship to the physicians address on file for their state license or DEA license number. Q: What if the physician I am ordering for doesn t want their order shipped to that address, or it is a PO Box? A: The Physician must update their address with the state or DEA. They can do this online. We can then take their confirmation print out and print one order. Any future orders must be looked up in the DEA database and verified that the new address is in there before we process the order. To update their state license, they can go online to and log in to update their information. To update their DEA number, they can go online to Q: I have a new physician with an out-of-state license number. What do I do? A: The physician has 30 days to report their move to California with the state board and update their information. See the response above for more information regarding placing orders. Q: I have a physician who does not have a DEA number. Can I order the controlled substance prescription pads without it? A: If the physician does not have a DEA number they cannot write prescriptions for controlled substances, therefore they would not need the controlled substance prescription pads. You may order one of our standard prescription pads or our standard bond paper. Q: Can I order prescription pads if the physicians license or DEA number has expired?
2 A: We cannot print controlled substance prescriptions if either of their license numbers has expired. If it s expired, they no longer have the permissions to write prescriptions. Q: What options does my customer have when ordering CA controlled substance scripts? A: There are three scenarios for imprinting prescription forms: 1. One physician is using the prescription pad/book/laser sheet and their name, category of licensure, state license number and DEA number is imprinted on the script. 2. Two or more physicians are using the prescription pad/book/laser sheets and each of them have their name, category of licensure, state license number and DEA number imprinted on the scripts along with a check box by their name. This must be checked at the time the prescription is written to specify which physician is prescribing. 3. The institutional style(facility must qualify as one) allows for many to use the same sheets, but a designated prescriber must have their name, category of licensure, state license number and DEA number imprinted on the script, along with the facilities name, address, and Department of Health Services license number. The designated prescriber must maintain a record of distribution of the scripts that includes the name, category of licensure, state license number, DEA number and the quantity of institution scripts issued to each physician within the facility. These records must be maintained for three years in an easily retrievable format, in case of audit. The prescribing physician must print, stamp or handwrite their name, state license number, category of licensure and DEA number on the script at the time of prescribing. Q: Does my customer qualify as a licensed health care facility so that they may use the institutional style forms discussed above? A: A licensed health care facility means a facility that has been licensed pursuant to Article 1 (commencing with section 1250) of Chapter 2 of Division 2 of the California Health and Safety Code. Q: We work out of a hospital and can print prescriptions with our software. Can t we add the imprinting and numbering ourselves to the laser Rx sheets? A: For controlled substance prescriptions, the security printer must apply all security features and meet all guidelines set forth by the state of California, which includes imprinting the physician(s), designated physician, or facilities name, address and DEA number and numbering on the pad before it leaves the facility.
3 Q: Can the pre-printed prescriber information be added at the hospital with our software? A: See response above for more information. The designated prescriber must be on the prescription script before it leaves the facility. At the time you print the prescription on the laser Rx sheet, you would need to add the prescribing physician s name, license number and DEA number. If all the physicians using the paper are listed with checkboxes on the sheet upon leaving our facility, you must then check the box next to the prescribing physician. Q: Do the laser Rx sheets need to show the six quantity check boxes on the script? A: No. They are exempt from this security feature, as it is very difficult to have the software match up to the boxes. However, they must meet all other guidelines, including designating the number of units and refills, a check box indicating the prescribers orders not to substitute and the area to fill in the number of prescriptions on the script. Q: Is there a limit to the number of prescriptions that can be on the script? A: Not in any formal law, however the Department of Justice would like it limited to five prescriptions on a script, or the information gets too convoluted. Q: If my customer has several offices, can they have all the addresses printed on the scripts? Multiple addresses for a prescriber may be listed on the script. There should be a check box next to each location to identify the address where the patient was seen. Q: Can we use Wilmer s standard laser Rx sheets to print our controlled substance prescriptions on? A: The stock items PRESLBL s or PRESLGN s can be used for non-controlled California prescriptions, however, controlled substance prescriptions must have certain security features that our standard line does not have. (i.e. a watermark on the back of the prescription which says California Security Prescription ) We now offer two California laser Rx sheets that meet the Health and Safety Code regulations. (PRESCALBLI top script, or PRESCABLBLI bottom script)we currently do not offer an 8-1/2 x 11 sheet, but are looking into adding it some time in early Q: Can we use your standard thermal prescription rolls to print our controlled substance prescriptions on? A: Please see above response. Q: Does a resident physician need their supervising physicians name pre-printed on the script?
4 A: No. They have graduated from medical school and are able to write prescriptions. Since most, at this time, are in their residency to learn a specialty, a physician must supervisor their daily activities. Q: Does a resident physician need to have their information pre-printed on the prescription script? A: If they are writing controlled substances, yes. They must follow the rules as all other physicians. (i.e. the supervising physician cannot order a large amount of prescription pads with their information on there for the resident(s) to write their name on it at the time of prescribing. The residents information must be preprinted on the script) Q: Does a nurse practitioner or certified midwife need to have their information pre-printed on the prescription script? It cannot be stamped or hand-written. Q: Does a nurse practitioner or certified midwife need their supervising physicians name pre-printed on the script? A: No. Q: Does a physician assistant need to have their information pre-printed on the prescription script? It cannot be stamped or hand-written. Q: Does a physician assistant need their supervising physicians name pre-printed on the script? The law requires that a physician s assistant is authorized to write controlled substance prescriptions based on the authority granted to them by their supervising physician and DEA registration. The script must have the physician assistants name, category of licensure, DEA number and state license number preprinted. It must also have their supervising physician s name, category of licensure, DEA number and state license number pre-printed on the script. Q: Does a dentist need to have a DEA number? A: If they are writing controlled substances, yes. Q: Does an optometrist need a DEA number? A: If they are writing schedule III controlled substances, yes. Q: Does a veterinarian need to follow the guidelines for controlled substances?
5 A: Yes, they must follow the same guidelines for controlled substance prescriptions as a physician who sees humans would. The prescription must also have a spot for the owners name, animals name and the animals date of birth. Q: What are considered controlled substances? A: Any substances that fall into schedule II, III, IV and V are considered controlled substances and must follow these guidelines. Schedule I drugs are not used for medicinal purposes. Q: Where can I find a list of the controlled substances and the drug schedule? A: The federal government lists controlled substances on their website. Q: Are there any exceptions to this? Schedule III, IV and V can be orally prescribed or faxed to the pharmacy. Schedule II can only be faxed in an emergency case, however the prescription must be hand-written and postmarked within seven days, or if the hand-written prescription is presented at the pharmacy before dispensing. Q: Can a prescriber write a prescription for non-controlled substances on a controlled substances script? Q: Can a prescriber write a prescription for non-controlled substances and controlled substances on the same script? Q: Is a federal controlled substance registration number the same thing as a DEA registration number? For more information about guidelines for security printers see the following link:
How To Write A Prescription In California
 Competitive PR4 PHONE & FAX 916-760-4477 2443 FAIR OAKS BLVD. #322, SACRAMENTO, CA 95825 WWW.CPR4RX.COM CPR4RX@CPR4RX.COM CA CONTROLLED SUBSTANCE RX REFERENCE GUIDE May 2013 Table of Contents Requirements
Competitive PR4 PHONE & FAX 916-760-4477 2443 FAIR OAKS BLVD. #322, SACRAMENTO, CA 95825 WWW.CPR4RX.COM CPR4RX@CPR4RX.COM CA CONTROLLED SUBSTANCE RX REFERENCE GUIDE May 2013 Table of Contents Requirements
DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY
 DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY Remote Pharmacy Owner Name: DBA Name: Address: Telephone Number: Fax Number:
DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY Remote Pharmacy Owner Name: DBA Name: Address: Telephone Number: Fax Number:
RULES OF THE TENNESSEE BOARD OF NURSING CHAPTER 1000-04 ADVANCED PRACTICE NURSES AND CERTIFICATES OF FITNESS TO PRESCRIBE TABLE OF CONTENTS
 RULES OF THE TENNESSEE BOARD OF NURSING R 1000-04 ADVANCED PRACTICE NURSES AND CERTIFICATES TABLE OF CONTENTS 1000-04-.01 Purpose and Scope 1000-04-.07 Processing of Applications 1000-04-.02 Definitions
RULES OF THE TENNESSEE BOARD OF NURSING R 1000-04 ADVANCED PRACTICE NURSES AND CERTIFICATES TABLE OF CONTENTS 1000-04-.01 Purpose and Scope 1000-04-.07 Processing of Applications 1000-04-.02 Definitions
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES
 FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: April 2015 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: April 2015 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
314.042 License to practice as an advanced practice registered nurse -- Application -- Renewal -- Reinstatement -- Use of "APRN" -- Prescriptive
 314.042 License to practice as an advanced practice registered nurse -- Application -- Renewal -- Reinstatement -- Use of "APRN" -- Prescriptive authority under CAPA-NS and CAPA-CS -- Exemption from CAPA-NS
314.042 License to practice as an advanced practice registered nurse -- Application -- Renewal -- Reinstatement -- Use of "APRN" -- Prescriptive authority under CAPA-NS and CAPA-CS -- Exemption from CAPA-NS
Pharmacy Operations. Assisting the Pharmacist. Pharmacy Technician Training Systems Passassured, LLC
 Pharmacy Operations Assisting the Pharmacist Pharmacy Technician Training Systems Passassured, LLC Pharmacy Operations, Assisting the Pharmacist PassAssured's Pharmacy Technician Training Program Pharmacy
Pharmacy Operations Assisting the Pharmacist Pharmacy Technician Training Systems Passassured, LLC Pharmacy Operations, Assisting the Pharmacist PassAssured's Pharmacy Technician Training Program Pharmacy
Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy
 Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy Legislative changes PDMP program changes 503B outsourcing facilities Rules changes Ph800 review Ph300 Ph400 Ph700 Current issues
Michael D. Bullek BSP Rph. Commissioner, New Hampshire Board of Pharmacy Legislative changes PDMP program changes 503B outsourcing facilities Rules changes Ph800 review Ph300 Ph400 Ph700 Current issues
EPCS FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES. Revised: January 2016
 FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: January 2016 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: January 2016 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES
 FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: October 2014 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
FREQUENTLY ASKED QUESTIONS FOR ELECTRONIC PRESCRIBING OF CONTROLLED SUBSTANCES EPCS Revised: October 2014 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic
CURES Prescription Drug Monitoring Program
 California Department of Justice CURES Prescription Drug Monitoring Program February, 2013 The mission of the California Prescription Drug Monitoring Program (PDMP) is to eliminate pharmaceutical drug
California Department of Justice CURES Prescription Drug Monitoring Program February, 2013 The mission of the California Prescription Drug Monitoring Program (PDMP) is to eliminate pharmaceutical drug
NH Laws / Rules Regarding Limited Retail Drug Distributors
 NH Laws / Rules Regarding Limited Retail Drug Distributors 318:1, VII-a. "Limited retail drug distributor'' means a distributor of legend devices or medical gases delivered directly to the consumer pursuant
NH Laws / Rules Regarding Limited Retail Drug Distributors 318:1, VII-a. "Limited retail drug distributor'' means a distributor of legend devices or medical gases delivered directly to the consumer pursuant
Exceptions to the Rule: A Pharmacy Law Presentation. Objectives DISCLAIMER 10/16/2015
 Exceptions to the Rule: A Pharmacy Law Presentation Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify basic legal frameworks that govern the
Exceptions to the Rule: A Pharmacy Law Presentation Eric Roath, Pharm.D. Director of Professional Practice Michigan Pharmacists Association Objectives 1. Identify basic legal frameworks that govern the
POINT OF CLARIFICATION
 Follow Up Questions and Answers from the Preparing for the Medicare Part D Requirements for e-prescribing in Long-Term Care Webinar Of June 5 and 12, 2014 NOTE: All answers regarding what is allowed, or
Follow Up Questions and Answers from the Preparing for the Medicare Part D Requirements for e-prescribing in Long-Term Care Webinar Of June 5 and 12, 2014 NOTE: All answers regarding what is allowed, or
COLORADO MEDICAL BOARD RULES AND REGULATIONS REGARDING THE PHYSICIAN S ROLE IN PRESCRIPTIVE AUTHORITY FOR ADVANCED PRACTICE NURSES
 Rule 950 3 CCR 713-37 COLORADO MEDICAL BOARD RULES AND REGULATIONS REGARDING THE PHYSICIAN S ROLE IN PRESCRIPTIVE AUTHORITY FOR ADVANCED PRACTICE NURSES Basis: The authority for the promulgation of these
Rule 950 3 CCR 713-37 COLORADO MEDICAL BOARD RULES AND REGULATIONS REGARDING THE PHYSICIAN S ROLE IN PRESCRIPTIVE AUTHORITY FOR ADVANCED PRACTICE NURSES Basis: The authority for the promulgation of these
COUNTY OF LOS ANGELES DEPARTMENT OF MENTAL HEALTH PSYCHIATRIC MENTAL HEALTH NURSE PRACTITIONER STANDARDIZED PROCEDURES
 COUNTY OF LOS ANGELES DEPARTMENT OF MENTAL HEALTH PSYCHIATRIC MENTAL HEALTH NURSE PRACTITIONER STANDARDIZED August 2010 Page 2 TABLE OF CONTENTS I. Development, Review and Approval of Psychiatric Mental
COUNTY OF LOS ANGELES DEPARTMENT OF MENTAL HEALTH PSYCHIATRIC MENTAL HEALTH NURSE PRACTITIONER STANDARDIZED August 2010 Page 2 TABLE OF CONTENTS I. Development, Review and Approval of Psychiatric Mental
Thank you for choosing Rcopia, the award-winning e-prescribing system from DrFirst! This packet contains all the registration forms needed to get
 Thank you for choosing Rcopia, the award-winning e-prescribing system from DrFirst! This packet contains all the registration forms needed to get your practice up-and-running with Rcopia. Registration
Thank you for choosing Rcopia, the award-winning e-prescribing system from DrFirst! This packet contains all the registration forms needed to get your practice up-and-running with Rcopia. Registration
3.Prescriber DEA: 4.Board Registration Number: 5.Business Tel:
 Commonwealth of Massachusetts Department of Public Health, Office of Prescription Monitoring and Drug Control 99 Chauncy Street, Boston, MA 02111 Tel: 617-753-7310 Email: mapmp.dph@state.ma.us MA ONLINE
Commonwealth of Massachusetts Department of Public Health, Office of Prescription Monitoring and Drug Control 99 Chauncy Street, Boston, MA 02111 Tel: 617-753-7310 Email: mapmp.dph@state.ma.us MA ONLINE
INSTRUCTIONS FOR USE: OA-RX
 P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
Implementation of Florida s PDMP. Rebecca Poston Program Manager
 Implementation of Florida s PDMP Rebecca Poston Program Manager Disclaimer I have no relevant financial relationships or commercial interest in the content presented in this program. Learning Objectives:
Implementation of Florida s PDMP Rebecca Poston Program Manager Disclaimer I have no relevant financial relationships or commercial interest in the content presented in this program. Learning Objectives:
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL
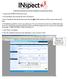 GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS
 DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
Manual of Instructions
 NEW YORK STATE DEPARTMENT OF HEALTH OFFICIAL NEW YORK STATE PRESCRIPTION PROGRAM ELECTRONIC DATA TRANSMISSION Manual of Instructions New York State Department of Health Bureau of Narcotic Enforcement 433
NEW YORK STATE DEPARTMENT OF HEALTH OFFICIAL NEW YORK STATE PRESCRIPTION PROGRAM ELECTRONIC DATA TRANSMISSION Manual of Instructions New York State Department of Health Bureau of Narcotic Enforcement 433
UTCVM PHARMACY STANDARD OPERATING PROCEDURES
 UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
UTCVM PHARMACY STANDARD OPERATING PROCEDURES Updated: 4/5/2004 I. General Procedures A. Hours: The Pharmacy will be open Monday through Friday, 8:00AM to 6:00PM; Saturday 8:00AM to 1:00PM. The Pharmacy
Medicaid Tamper-Resistant Prescription Requirements FAQs for Pharmacists
 Medicaid Tamper-Resistant Prescription Requirements FAQs for Pharmacists On April 1, 2008, all hand-written or computer-generated/printed Medicaid prescriptions were required to have at least one approved
Medicaid Tamper-Resistant Prescription Requirements FAQs for Pharmacists On April 1, 2008, all hand-written or computer-generated/printed Medicaid prescriptions were required to have at least one approved
Controlled II Substance Rules & Regulations. Controlled II Substance Rules & Regulations
 North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
E-Prescribing of Controlled Substances (EPCS) New York State Board for Podiatry
 E-Prescribing of Controlled Substances (EPCS) As of March 27, 2015 it will be mandatory for practitioners, excluding veterinarians, to issue electronic prescriptions for controlled and non-controlled substances.
E-Prescribing of Controlled Substances (EPCS) As of March 27, 2015 it will be mandatory for practitioners, excluding veterinarians, to issue electronic prescriptions for controlled and non-controlled substances.
11/26/2012. Implementation of Florida s PDMP. Disclosure
 Implementation of Florida s PDMP Rebecca R. Poston Program Manager December 1, 2012 Disclosure I have no vested interest in or affiliation with any corporate organizations offering financial support or
Implementation of Florida s PDMP Rebecca R. Poston Program Manager December 1, 2012 Disclosure I have no vested interest in or affiliation with any corporate organizations offering financial support or
PROPOSED REGULATION OF THE STATE BOARD OF NURSING. LCB File No. R114-13
 PROPOSED REGULATION OF THE STATE BOARD OF NURSING LCB File No. R114-13 AUTHORITY: 1-4 and 6-19, NRS 632.120 and 632.237; 5, NRS 632.120 and 632.345. A REGULATION relating to nursing; Section 1. Chapter
PROPOSED REGULATION OF THE STATE BOARD OF NURSING LCB File No. R114-13 AUTHORITY: 1-4 and 6-19, NRS 632.120 and 632.237; 5, NRS 632.120 and 632.345. A REGULATION relating to nursing; Section 1. Chapter
- 1 - First Time Pharmacy Managers (Revised 02/02/2011)
 State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
HOUSE OF REPRESENTATIVES STAFF ANALYSIS REFERENCE ACTION ANALYST STAFF DIRECTOR. 1) Health Care Mitchell Collins 2) 3) 4) 5)
 HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: HB 103 Medicinal Drug Prescriptions SPONSOR(S): Quinones TIED BILLS: IDEN./SIM. BILLS: SB 132 (i) REFERENCE ACTION ANALYST STAFF DIRECTOR 1) Health Care
HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: HB 103 Medicinal Drug Prescriptions SPONSOR(S): Quinones TIED BILLS: IDEN./SIM. BILLS: SB 132 (i) REFERENCE ACTION ANALYST STAFF DIRECTOR 1) Health Care
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS for the Revised: February 2014 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic Online PMP Q: What is
FREQUENTLY ASKED QUESTIONS for the Revised: February 2014 NEW YORK STATE DEPARTMENT OF HEALTH Bureau of Narcotic Enforcement 1-866-811-7957 www.health.ny.gov/professionals/narcotic Online PMP Q: What is
CHAPTER 61-03-02 CONSULTING PHARMACIST REGULATIONS FOR LONG-TERM CARE FACILITIES (SKILLED, INTERMEDIATE, AND BASIC CARE)
 CHAPTER 61-03-02 CONSULTING PHARMACIST REGULATIONS FOR LONG-TERM CARE FACILITIES (SKILLED, INTERMEDIATE, AND BASIC CARE) Section 61-03-02-01 Definitions 61-03-02-02 Absence of Provider or Consulting Pharmacist
CHAPTER 61-03-02 CONSULTING PHARMACIST REGULATIONS FOR LONG-TERM CARE FACILITIES (SKILLED, INTERMEDIATE, AND BASIC CARE) Section 61-03-02-01 Definitions 61-03-02-02 Absence of Provider or Consulting Pharmacist
Manitoba Prescribing Practices Program Pharmacist Questions and Answers
 College of Pharmacists of Manitoba 200 Tache Avenue, Winnipeg, Manitoba R2H 1A7 Phone (204) 233-1411 Fax: (204) 237-3468 E-mail: info@cphm.ca Website: www.cphm.ca Manitoba Prescribing Practices Program
College of Pharmacists of Manitoba 200 Tache Avenue, Winnipeg, Manitoba R2H 1A7 Phone (204) 233-1411 Fax: (204) 237-3468 E-mail: info@cphm.ca Website: www.cphm.ca Manitoba Prescribing Practices Program
Prescription Drug Monitoring Program. A Powerful Tool
 Prescription Drug Monitoring Program A Powerful Tool September 16, 2015 No reportable financial interest. Controlled Substance Utilization Review and Evaluation System >16,000 Prescription Painkiller Overdose
Prescription Drug Monitoring Program A Powerful Tool September 16, 2015 No reportable financial interest. Controlled Substance Utilization Review and Evaluation System >16,000 Prescription Painkiller Overdose
16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain
 16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain a limited drug permit as described in Section 61-11-14
16.19.10.11 PUBLIC HEALTH CLINICS: A. CLINIC LICENSURE: (1) All clinics where dangerous drugs are administered, distributed or dispensed shall obtain a limited drug permit as described in Section 61-11-14
Ohio Legislative Service Commission
 Ohio Legislative Service Commission Bill Analysis Lisa Musielewicz S.B. 228 129th General Assembly (As Introduced) Sens. Burke, Lehner, Schiavoni, Tavares, Schaffer BILL SUMMARY Authorizes a certified
Ohio Legislative Service Commission Bill Analysis Lisa Musielewicz S.B. 228 129th General Assembly (As Introduced) Sens. Burke, Lehner, Schiavoni, Tavares, Schaffer BILL SUMMARY Authorizes a certified
Pharmacy Program Pre-Test
 Last Name: Pharmacy Program Pre-Test * For each question, put a check mark for the one option that you think is correct. 1. A pharmacist receives a security prescription from a known local medical group
Last Name: Pharmacy Program Pre-Test * For each question, put a check mark for the one option that you think is correct. 1. A pharmacist receives a security prescription from a known local medical group
EMR DOCUMENTATION LYNX. Instructor Script
 EMR DOCUMENTATION LYNX Instructor Script Table of Contents TABLE OF CONTENTS INFORMATION SECURITY AND CONFIDENTIALITY... 4 OVERVIEW... 5 LEARNING OBJECTIVES... 5 TIPS AND TRICKS... 5 SOLUTION ICONS...
EMR DOCUMENTATION LYNX Instructor Script Table of Contents TABLE OF CONTENTS INFORMATION SECURITY AND CONFIDENTIALITY... 4 OVERVIEW... 5 LEARNING OBJECTIVES... 5 TIPS AND TRICKS... 5 SOLUTION ICONS...
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY. LCB File No. R047-15. September 15, 2015
 PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
BEFORE THE BOARD OF PHARMACY DEPARTMENT OF CONSUMER AFFAIRS STATE OF CALIFORNIA PARTIES JURISDICTION
 EDMUND G. BROWN JR. Attorney General of California KAREN B. CHAPPELLE Supervising Deputy Attorney General CHRISTINA V. TUSAN Deputy Attorney General State Bar No. 0 00 So. Spring Street, Suite Los Angeles,
EDMUND G. BROWN JR. Attorney General of California KAREN B. CHAPPELLE Supervising Deputy Attorney General CHRISTINA V. TUSAN Deputy Attorney General State Bar No. 0 00 So. Spring Street, Suite Los Angeles,
Massachusetts Department of Public Health (MDPH) Prescription Monitoring Program (MA PMP) and Drug Control Program (DCP) April 8, 2014
 MA PMP Pharmacy Data Entry and Data Submitter s FAQ Utilizing ASAP 4.2 Supplement to the MA PMP Pharmacy Data Entry and Data Submitter s Guide Utilizing ASAP 4.2 Massachusetts Department of Public Health
MA PMP Pharmacy Data Entry and Data Submitter s FAQ Utilizing ASAP 4.2 Supplement to the MA PMP Pharmacy Data Entry and Data Submitter s Guide Utilizing ASAP 4.2 Massachusetts Department of Public Health
Alaska Prescription Drug Monitoring Program
 Alaska 1. What is a PDMP? PDMP stands for. This is a commonly used term for the programs implemented by various states to monitor the dispensing of controlled substances within their borders. For this
Alaska 1. What is a PDMP? PDMP stands for. This is a commonly used term for the programs implemented by various states to monitor the dispensing of controlled substances within their borders. For this
EMR 1, / 1, 2008 1, 2008. CMS
 Dear Prescriber, This letter contains critically important information regarding compliance with the Medicaid Tamper Resistant Requirements. As of October 1, all fee-for-service Medicaid prescriptions
Dear Prescriber, This letter contains critically important information regarding compliance with the Medicaid Tamper Resistant Requirements. As of October 1, all fee-for-service Medicaid prescriptions
Electronic Prescribing In New York State
 Electronic Prescribing In New York State Joshua S. Vinciguerra, Director Bureau of Narcotic Enforcement October 28, 2015 October 28, 2015 2 Overview Review the problem & e-prescribing mandate Describe
Electronic Prescribing In New York State Joshua S. Vinciguerra, Director Bureau of Narcotic Enforcement October 28, 2015 October 28, 2015 2 Overview Review the problem & e-prescribing mandate Describe
PHYSICIAN ASSISTANT NOTIFICATION OF CHANGE
 State of Utah DIVISION OF OCCUPATIONAL & PROFESSIONAL LICENSING 160 East 300 South, P.O. Box 146741 Salt Lake City, Utah 84114-6741 Telephone (801) 530-6628 www.dopl.utah.gov PHYSICIAN ASSISTANT NOTIFICATION
State of Utah DIVISION OF OCCUPATIONAL & PROFESSIONAL LICENSING 160 East 300 South, P.O. Box 146741 Salt Lake City, Utah 84114-6741 Telephone (801) 530-6628 www.dopl.utah.gov PHYSICIAN ASSISTANT NOTIFICATION
MUNICIPAL REGULATIONS for CLINICAL NURSE SPECIALISTS
 MUNICIPAL REGULATIONS for CLINICAL NURSE SPECIALISTS CHAPTER 60 CLINICAL NURSE SPECIALIST Secs. 6000 Applicability 6001 General Requirements 6002 Term of Certificate 6003 Renewal of Certificate 6004 Educational
MUNICIPAL REGULATIONS for CLINICAL NURSE SPECIALISTS CHAPTER 60 CLINICAL NURSE SPECIALIST Secs. 6000 Applicability 6001 General Requirements 6002 Term of Certificate 6003 Renewal of Certificate 6004 Educational
TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM
 TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM Section 1. Authority 8-1 Section 2. Transmission of Information Regarding Dispensing of Controlled Substances to Certain Persons 8-1 Section
TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM Section 1. Authority 8-1 Section 2. Transmission of Information Regarding Dispensing of Controlled Substances to Certain Persons 8-1 Section
MUNICIPAL REGULATIONS for NURSE PRACTITIONERS
 MUNICIPAL REGULATIONS for NURSE PRACTITIONERS CHAPTER 59 NURSE-PRACTITIONERS Secs. 5900 Applicability 5901 General Requirements 5902 Term of Certificate 5903 Renewal of Certificate 5904 Educational Requirements
MUNICIPAL REGULATIONS for NURSE PRACTITIONERS CHAPTER 59 NURSE-PRACTITIONERS Secs. 5900 Applicability 5901 General Requirements 5902 Term of Certificate 5903 Renewal of Certificate 5904 Educational Requirements
E-Signature. The Pharmacy Perspective
 E-Signature The Pharmacy Perspective Prescriptions arrive at a pharmacy today... Written by a prescriber ( not always on a prescription pad). Do not always know the prescriber s s handwriting legibility
E-Signature The Pharmacy Perspective Prescriptions arrive at a pharmacy today... Written by a prescriber ( not always on a prescription pad). Do not always know the prescriber s s handwriting legibility
34-21-82. Joint Committee Appointment, terms of office, office of chairperson, and meetings
 ARTICLE 5 ADVANCED PRACTICE NURSING 34-21-80. Declaration of Legislature 34-21-81. Definitions 34-21-82. Joint Committee Appointment, terms of office, office of chairperson, and meetings 34-21-83. State
ARTICLE 5 ADVANCED PRACTICE NURSING 34-21-80. Declaration of Legislature 34-21-81. Definitions 34-21-82. Joint Committee Appointment, terms of office, office of chairperson, and meetings 34-21-83. State
SCOPE OF PRACTICE FOR ARNPS
 FLORIDA'S PROHIBITION ON ADVANCED REGISTERED NURSE PRACTITIONERS (ARNPs) PRESCRIBING CONTROLLED SUBSTANCES AND THE REQUIREMENTS TO REPORT SUCH ACTIVITY Florida is one of the two states that does not allow
FLORIDA'S PROHIBITION ON ADVANCED REGISTERED NURSE PRACTITIONERS (ARNPs) PRESCRIBING CONTROLLED SUBSTANCES AND THE REQUIREMENTS TO REPORT SUCH ACTIVITY Florida is one of the two states that does not allow
E-Prescribing FAQ s for Independent Pharmacies Table of Contents
 Table of Contents How do I work with my software vendor to enable my pharmacy for e-prescribing via the Surescripts network?...2 Why must I report issues directly through my software vendor and not Surescripts?...2
Table of Contents How do I work with my software vendor to enable my pharmacy for e-prescribing via the Surescripts network?...2 Why must I report issues directly through my software vendor and not Surescripts?...2
How to Utilize the Security Portal to Access PDMP (User Guide for Practitioners, Pharmacists, CRNPs, Physician Assistants, Law Enforcement, and CNMs)
 How to Utilize the Security Portal to Access PDMP (User Guide for Practitioners, Pharmacists, CRNPs, Physician Assistants, Law Enforcement, and CNMs) July 30, 2014 Version 2 Software Release Version 3.4.11
How to Utilize the Security Portal to Access PDMP (User Guide for Practitioners, Pharmacists, CRNPs, Physician Assistants, Law Enforcement, and CNMs) July 30, 2014 Version 2 Software Release Version 3.4.11
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE April 8, 2011 EFFECTIVE DATE April 8, 2011 MEDICAL ASSISTANCE BULLETIN NUMBER 03-11-01, 09-11-02, 14-11-01, 18-11-01 24-11-03, 27-11-02, 31-11-02, 33-11-02 SUBJECT Electronic Prescribing Internet-based
ISSUE DATE April 8, 2011 EFFECTIVE DATE April 8, 2011 MEDICAL ASSISTANCE BULLETIN NUMBER 03-11-01, 09-11-02, 14-11-01, 18-11-01 24-11-03, 27-11-02, 31-11-02, 33-11-02 SUBJECT Electronic Prescribing Internet-based
MUNICIPAL REGULATIONS FOR NURSE- MIDWIVES
 MUNICIPAL REGULATIONS FOR NURSE- MIDWIVES CHAPTER 58 NURSE-MIDWIVES Secs. 5800 Applicability 5801 General Requirement 5802 Term of Certificate 5803 Renewal of Certificate 5804 Educational and Experience
MUNICIPAL REGULATIONS FOR NURSE- MIDWIVES CHAPTER 58 NURSE-MIDWIVES Secs. 5800 Applicability 5801 General Requirement 5802 Term of Certificate 5803 Renewal of Certificate 5804 Educational and Experience
What Every Practitioner Needs to Know About Controlled Substance Prescribing
 What Every Practitioner Needs to Know About Controlled Substance Prescribing New York State Department of Health Use of Controlled Substances Controlled substances can be effective in the treatment of
What Every Practitioner Needs to Know About Controlled Substance Prescribing New York State Department of Health Use of Controlled Substances Controlled substances can be effective in the treatment of
Delegation of Services Agreements Change in Regulations
 Delegation of Services Agreements Change in Regulations Title 16, Division 13.8, Article 4, section 1399.540 was amended to include several requirements for the delegation of medical services to a physician
Delegation of Services Agreements Change in Regulations Title 16, Division 13.8, Article 4, section 1399.540 was amended to include several requirements for the delegation of medical services to a physician
Prepared By: The Professional Staff of the Committee on Health Policy REVISED:
 BILL: SB 152 The Florida Senate BILL ANALYSIS AND FISCAL IMPACT STATEMENT (This document is based on the provisions contained in the legislation as of the latest date listed below.) Prepared By: The Professional
BILL: SB 152 The Florida Senate BILL ANALYSIS AND FISCAL IMPACT STATEMENT (This document is based on the provisions contained in the legislation as of the latest date listed below.) Prepared By: The Professional
SAMPLE WRITTEN SUPERVISION AGREEMENT
 A. Physician Assistant Information 1. Name: SAMPLE WRITTEN SUPERVISION AGREEMENT 2. Illinois PA License Number: Illinois Mid-Level Practitioner Controlled Substance License Number: Federal Mid-Level Practitioner
A. Physician Assistant Information 1. Name: SAMPLE WRITTEN SUPERVISION AGREEMENT 2. Illinois PA License Number: Illinois Mid-Level Practitioner Controlled Substance License Number: Federal Mid-Level Practitioner
LCB File No. R118-13. December 4, 2013
 PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R118-13 December 4, 2013 EXPLANATION Matter in italics is new; mailer in brackets Inmitted nmtealj is material to be omitted. AUTHORITY:
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R118-13 December 4, 2013 EXPLANATION Matter in italics is new; mailer in brackets Inmitted nmtealj is material to be omitted. AUTHORITY:
eprescribe FAQs General Application FAQs
 Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
Topics in Pharmacy Technician and Intern Laws for Ohio Pharmacists
 Topics in Pharmacy Technician and Intern Laws for Ohio Pharmacists By: Donald L. Sullivan, R.Ph., Ph.D. Program Number: 036-367-10-001-H03 C.E.U.s: 0.1 Contact Hours: 1 hour Release Date: 02/23/2010 Expiration
Topics in Pharmacy Technician and Intern Laws for Ohio Pharmacists By: Donald L. Sullivan, R.Ph., Ph.D. Program Number: 036-367-10-001-H03 C.E.U.s: 0.1 Contact Hours: 1 hour Release Date: 02/23/2010 Expiration
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS Section 1. Authority.... 1 Section 2. Definitions.... 1 Section 3. Qualifications and Requirements for Pharmacy Technicians and Pharmacy Technicians-in-Training....
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS TABLE OF CONTENTS Section 1. Authority.... 1 Section 2. Definitions.... 1 Section 3. Qualifications and Requirements for Pharmacy Technicians and Pharmacy Technicians-in-Training....
2013 -- H 5230 S T A T E O F R H O D E I S L A N D
 ======= LC00 ======= 01 -- H 0 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO HEALTH AND SAFETY - THE RETURN OR EXCHANGE OF DRUGS ACT Introduced
======= LC00 ======= 01 -- H 0 S T A T E O F R H O D E I S L A N D IN GENERAL ASSEMBLY JANUARY SESSION, A.D. 01 A N A C T RELATING TO HEALTH AND SAFETY - THE RETURN OR EXCHANGE OF DRUGS ACT Introduced
CHAPTER 27 THE SCOPE OF PROFESSIONAL NURSING PRACTICE AND ARNP AND CNM PROTOCOLS
 I. INTRODUCTION CHAPTER 27 THE SCOPE OF PROFESSIONAL NURSING PRACTICE AND ARNP AND CNM PROTOCOLS Advance registered nurse practitioners (ARNPs) and clinical nurse practitioners (CNPs) have their scope
I. INTRODUCTION CHAPTER 27 THE SCOPE OF PROFESSIONAL NURSING PRACTICE AND ARNP AND CNM PROTOCOLS Advance registered nurse practitioners (ARNPs) and clinical nurse practitioners (CNPs) have their scope
(128th General Assembly) (Amended Substitute Senate Bill Number 89) AN ACT
 (128th General Assembly) (Amended Substitute Senate Bill Number 89) AN ACT To amend sections 4723.01, 4723.06, 4723.48, 4723.482, and 4723.50; to amend, for the purpose of adopting new section numbers
(128th General Assembly) (Amended Substitute Senate Bill Number 89) AN ACT To amend sections 4723.01, 4723.06, 4723.48, 4723.482, and 4723.50; to amend, for the purpose of adopting new section numbers
APRN Practice Facts and Background Information about APRN Independent Practice February 2012
 APRN Practice Facts and Background Information about APRN Independent Practice February 2012 Under Federal law 38 USC 7402(b), the Department of Veterans Affairs (VA) is authorized to establish licensure
APRN Practice Facts and Background Information about APRN Independent Practice February 2012 Under Federal law 38 USC 7402(b), the Department of Veterans Affairs (VA) is authorized to establish licensure
IAC 10/5/11 Pharmacy[657] Ch 40, p.1 CHAPTER 40 TECH-CHECK-TECH PROGRAMS
![IAC 10/5/11 Pharmacy[657] Ch 40, p.1 CHAPTER 40 TECH-CHECK-TECH PROGRAMS IAC 10/5/11 Pharmacy[657] Ch 40, p.1 CHAPTER 40 TECH-CHECK-TECH PROGRAMS](/thumbs/25/6867725.jpg) IAC 10/5/11 Pharmacy[657] Ch 40, p.1 CHAPTER 40 TECH-CHECK-TECH PROGRAMS 657 40.1(155A) Purpose and scope. The board may authorize a hospital pharmacy to participate in a tech-check-tech program. The board
IAC 10/5/11 Pharmacy[657] Ch 40, p.1 CHAPTER 40 TECH-CHECK-TECH PROGRAMS 657 40.1(155A) Purpose and scope. The board may authorize a hospital pharmacy to participate in a tech-check-tech program. The board
Table of Contents. User Request for Access... 3. User Login... 3. Upload a Data File... 4
 Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
ALABAMA BOARD OF MEDICAL EXAMINERS ADMINISTRATIVE CODE CHAPTER 540-X-8 ADVANCED PRACTICE NURSES: COLLABORATIVE PRACTICE TABLE OF CONTENTS
 Medical Examiners Chapter 540-X-8 ALABAMA BOARD OF MEDICAL EXAMINERS ADMINISTRATIVE CODE CHAPTER 540-X-8 ADVANCED PRACTICE NURSES: COLLABORATIVE PRACTICE TABLE OF CONTENTS 540-X-8-.01 540-X-8-.02 540-X-8-.03
Medical Examiners Chapter 540-X-8 ALABAMA BOARD OF MEDICAL EXAMINERS ADMINISTRATIVE CODE CHAPTER 540-X-8 ADVANCED PRACTICE NURSES: COLLABORATIVE PRACTICE TABLE OF CONTENTS 540-X-8-.01 540-X-8-.02 540-X-8-.03
ACTION ITEM COMPLETED TAB 44
 H.B. 1 H.B. 751 Provisions of the H.B. 1 Appropriations Act The base appropriation for TSBP for FY2016-2017 is approximately $234,000 more than the base appropriation the previous biennium. The majority
H.B. 1 H.B. 751 Provisions of the H.B. 1 Appropriations Act The base appropriation for TSBP for FY2016-2017 is approximately $234,000 more than the base appropriation the previous biennium. The majority
ARKANSAS PRODUCTS: PRODUCT EXEMPTIONS: SALES LIMITS: SALES RESTRICTIONS:
 ARKANSAS PRODUCTS: Any product containing ephedrine, pseudoephedrine, or phenylpropanolamine or any of their salts, isomers, or salts of isomers, alone or in a mixture. (A.C.A. 5-64-212) PRODUCT EXEMPTIONS:
ARKANSAS PRODUCTS: Any product containing ephedrine, pseudoephedrine, or phenylpropanolamine or any of their salts, isomers, or salts of isomers, alone or in a mixture. (A.C.A. 5-64-212) PRODUCT EXEMPTIONS:
COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS
 Rule 400 3 CCR 713-7 COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS BASIS. The authority for promulgation of Rule 400 ( these Rules ) by the Colorado
Rule 400 3 CCR 713-7 COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS BASIS. The authority for promulgation of Rule 400 ( these Rules ) by the Colorado
NOTE: In the event that the seal is accidentally broken, the narcotic may be wasted via narcotic wastage procedures.
 HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
State of Vermont Office of Vermont Health Access (OVHA) Pharmacy Benefit Management Program
 Dear Prescriber: State of Vermont Office of Vermont Health Access (OVHA) Pharmacy Benefit Management Program Phase II: Federal Medicaid Law Regarding Tamper-Resistant Prescription Drug Pads October 1,
Dear Prescriber: State of Vermont Office of Vermont Health Access (OVHA) Pharmacy Benefit Management Program Phase II: Federal Medicaid Law Regarding Tamper-Resistant Prescription Drug Pads October 1,
Enroll in Interconnect
 Enroll in Interconnect Enrollment Form Checklist In this packet, you will find all of the necessary forms to enroll your patients in Interconnect and give them access to a full suite of support services
Enroll in Interconnect Enrollment Form Checklist In this packet, you will find all of the necessary forms to enroll your patients in Interconnect and give them access to a full suite of support services
COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS (PAs) INTRODUCTION
 Rule 400 3 CCR 713-7 COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS (PAs) INTRODUCTION BASIS. The authority for promulgation of Rule 400 ( these Rules
Rule 400 3 CCR 713-7 COLORADO MEDICAL BOARD RULES AND REGULATIONS FOR LICENSURE OF AND PRACTICE BY PHYSICIAN ASSISTANTS (PAs) INTRODUCTION BASIS. The authority for promulgation of Rule 400 ( these Rules
MIDWIFERY JOINT COMMITTEE STATE OF NORTH CAROLINA
 MIDWIFERY JOINT COMMITTEE STATE OF NORTH CAROLINA APPLICATION FOR APPROVAL AS A CERTIFIED NURSE-MIDWIFE GENERAL INFORMATION 1. BEFORE COMPLETING APPLICATION, photocopy blank forms for future use. 2. Initial
MIDWIFERY JOINT COMMITTEE STATE OF NORTH CAROLINA APPLICATION FOR APPROVAL AS A CERTIFIED NURSE-MIDWIFE GENERAL INFORMATION 1. BEFORE COMPLETING APPLICATION, photocopy blank forms for future use. 2. Initial
DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS
 Title 17 District of Columbia Municipal Regulations DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS Effective CHAPTER 99 PHARMACY TECHNICIANS Secs. 9900 General Provisions 9901 Term
Title 17 District of Columbia Municipal Regulations DISTRICT OF COLUMBIA MUNICIPAL REGULATIONS FOR PHARMACY TECHNICIANS Effective CHAPTER 99 PHARMACY TECHNICIANS Secs. 9900 General Provisions 9901 Term
Why should I report issues directly through my pharmacy management system vendor and not Surescripts?
 E-Prescribing FAQ Table of Contents p.4 How do I work with my pharmacy management system vendor to enable my pharmacy for e-prescribing via the Surescripts network? Why should I report issues directly
E-Prescribing FAQ Table of Contents p.4 How do I work with my pharmacy management system vendor to enable my pharmacy for e-prescribing via the Surescripts network? Why should I report issues directly
WellDyneRx Mail Service General Questions and Answers
 WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
PMP AWAR X E. User Support Manual V 1.2
 PMP AWAR X E User Support Manual V 1.2 04/09/2014 1 Contents 1 What Is a Requestor?... 3 2 Registration... 3 2.1 Registration Process... 3 2.2 Registering as a Delegate... 7 2.3 Email Verification... 11
PMP AWAR X E User Support Manual V 1.2 04/09/2014 1 Contents 1 What Is a Requestor?... 3 2 Registration... 3 2.1 Registration Process... 3 2.2 Registering as a Delegate... 7 2.3 Email Verification... 11
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
Date Submitted: July 20, 2000 Date Reviewed: May 31, 2005 January 17, 2006 March 17, 2009 Subject: Administration of Medication
 POLICY SOMERSET COUNTY BOARD OF EDUCATION 1. PURPOSE Date Submitted: July 20, 2000 Date Reviewed: May 31, 2005 January 17, 2006 March 17, 2009 Subject: Administration of Medication Number: 600-32 Date
POLICY SOMERSET COUNTY BOARD OF EDUCATION 1. PURPOSE Date Submitted: July 20, 2000 Date Reviewed: May 31, 2005 January 17, 2006 March 17, 2009 Subject: Administration of Medication Number: 600-32 Date
Adopted Rule - 11/19/14 1
 Adopted Rule - 11/19/14 1 CHAPTER Ph 800 PHARMACY TECHNICIANS Statutory Authority: RSA 318:5-a, X, XI Adopt Ph 801 & Ph 802, previously effective 7-25-01 (Doc. # 7535) and expired on 7-25-09, to read as
Adopted Rule - 11/19/14 1 CHAPTER Ph 800 PHARMACY TECHNICIANS Statutory Authority: RSA 318:5-a, X, XI Adopt Ph 801 & Ph 802, previously effective 7-25-01 (Doc. # 7535) and expired on 7-25-09, to read as
ADVANCED PRACTICE NURSING WRITTEN COLLABORATIVE AGREEMENT
 ADVANCED PRACTICE NURSING WRITTEN COLLABORATIVE AGREEMENT A. ADVANCED PRACTICE NURSE INFORMATION 1. NAME: 2. ILLINOIS RN LICENSE NUMBER: ILLINOIS APN LICENSE NUMBER: ILLINOIS MID-LEVEL PRACTIONER LICENSE
ADVANCED PRACTICE NURSING WRITTEN COLLABORATIVE AGREEMENT A. ADVANCED PRACTICE NURSE INFORMATION 1. NAME: 2. ILLINOIS RN LICENSE NUMBER: ILLINOIS APN LICENSE NUMBER: ILLINOIS MID-LEVEL PRACTIONER LICENSE
Session 3 THIS INITIATIVE IS BEING SUPPORTED BY A SPONSORSHIP FROM PFIZER
 Session 3 THIS INITIATIVE IS BEING SUPPORTED BY A SPONSORSHIP FROM PFIZER Disclosure The Immunization Action Coalition has been responsible for all aspects of content development for the enclosed presentation
Session 3 THIS INITIATIVE IS BEING SUPPORTED BY A SPONSORSHIP FROM PFIZER Disclosure The Immunization Action Coalition has been responsible for all aspects of content development for the enclosed presentation
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. (a) "Pharmacy Technician-in-training" means an individual
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population
 Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
ARKANSAS DEPARTMENT OF HEALTH
 ARKANSAS DEPARTMENT OF HEALTH PHARMACY SERVICES AND DRUG CONTROL BRANCH RULES AND REGULATIONS PERTAINING TO CONTROLLED SUBSTANCES December 1, 2014 I, James Myatt, P.D., Director, Pharmacy Services and
ARKANSAS DEPARTMENT OF HEALTH PHARMACY SERVICES AND DRUG CONTROL BRANCH RULES AND REGULATIONS PERTAINING TO CONTROLLED SUBSTANCES December 1, 2014 I, James Myatt, P.D., Director, Pharmacy Services and
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS. These regulations are promulgated as authorized by the Act.
 CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. Direct supervision means that a licensed pharmacist
CHAPTER 10 PHARMACY TECHNICIAN REGULATIONS Section 1. Authority. These regulations are promulgated as authorized by the Act. Section 2. Definitions. Direct supervision means that a licensed pharmacist
03 PHARMACY TECHNICIANS
 03 PHARMACY TECHNICIANS 03-00 PHARMACY TECHNICIANS REGISTRATION/PERMIT REQUIRED 03-00-0001 DEFINITIONS: A. PHARMACY TECHNICIAN: This term refers to those individuals identified as Pharmacist Assistants
03 PHARMACY TECHNICIANS 03-00 PHARMACY TECHNICIANS REGISTRATION/PERMIT REQUIRED 03-00-0001 DEFINITIONS: A. PHARMACY TECHNICIAN: This term refers to those individuals identified as Pharmacist Assistants
5) A legible copy of your diploma or official transcript from a VIBNL approved undergraduate nursing program.
 GOVERNMENT OF THE UNITED STATES VIRGIN ISLANDS -----O----- DEPARTMENT OF HEALTH Virgin Islands Board of Nurse Licensure P.O. Box 304247 Tel: (340) 776-7397 St. Thomas, Virgin Islands 00803 Fax: (340) 777-4003
GOVERNMENT OF THE UNITED STATES VIRGIN ISLANDS -----O----- DEPARTMENT OF HEALTH Virgin Islands Board of Nurse Licensure P.O. Box 304247 Tel: (340) 776-7397 St. Thomas, Virgin Islands 00803 Fax: (340) 777-4003
CHAPTER 101 (HB 500)
 AN ACT relating to crime victims. CHAPTER 101 1 CHAPTER 101 (HB 500) Be it enacted by the General Assembly of the Commonwealth of Kentucky: Section 1. KRS 216B.400 is amended to read as follows: (1) Where
AN ACT relating to crime victims. CHAPTER 101 1 CHAPTER 101 (HB 500) Be it enacted by the General Assembly of the Commonwealth of Kentucky: Section 1. KRS 216B.400 is amended to read as follows: (1) Where
ALABAMA BOARD OF NURSING ADMINISTRATIVE CODE CHAPTER 610-X-5 ADVANCED PRACTICE NURSING COLLABORATIVE PRACTICE TABLE OF CONTENTS
 ALABAMA BOARD OF NURSING ADMINISTRATIVE CODE CHAPTER 610-X-5 ADVANCED PRACTICE NURSING COLLABORATIVE PRACTICE TABLE OF CONTENTS 610-X-5-.01 610-X-5-.02 610-X-5-.03 610-X-5-.04 610-X-5-.05 610-X-5-.06 610-X-5-.07
ALABAMA BOARD OF NURSING ADMINISTRATIVE CODE CHAPTER 610-X-5 ADVANCED PRACTICE NURSING COLLABORATIVE PRACTICE TABLE OF CONTENTS 610-X-5-.01 610-X-5-.02 610-X-5-.03 610-X-5-.04 610-X-5-.05 610-X-5-.06 610-X-5-.07
State of Utah Department of Commerce Division of Occupational and Professional Licensing
 State of Utah Department of Commerce Division of Occupational and Professional Licensing Official Use Only Number: Date Approved/Denied: Approved/Denied By: Retired Volunteer Health Care Practitioner APPLICANT
State of Utah Department of Commerce Division of Occupational and Professional Licensing Official Use Only Number: Date Approved/Denied: Approved/Denied By: Retired Volunteer Health Care Practitioner APPLICANT
LexisNexis Public Access Portal: www.lexisnexis.com/njoal
 PLEASE READ Rules and regulations of the Division of Consumer Affairs, the boards and committees in, and other units of, the Division are codified in Title 13 of the New Jersey Administrative Code, published
PLEASE READ Rules and regulations of the Division of Consumer Affairs, the boards and committees in, and other units of, the Division are codified in Title 13 of the New Jersey Administrative Code, published
ARIZONA STATE SENATE Fifty-Second Legislature, Second Regular Session
 Assigned to HHS FOR COMMITTEE ARIZONA STATE SENATE Fifty-Second Legislature, Second Regular Session FACT SHEET FOR registered nurses; advanced practice Purpose Modifies statutes related to the licensing
Assigned to HHS FOR COMMITTEE ARIZONA STATE SENATE Fifty-Second Legislature, Second Regular Session FACT SHEET FOR registered nurses; advanced practice Purpose Modifies statutes related to the licensing
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS What is the Nova Scotia Drug Information System? The Nova Scotia Drug Information System is one of the clinical components of the Electronic Health Record in Nova Scotia (SHARE).
FREQUENTLY ASKED QUESTIONS What is the Nova Scotia Drug Information System? The Nova Scotia Drug Information System is one of the clinical components of the Electronic Health Record in Nova Scotia (SHARE).
it s easy to let your biases political, intellectual, or otherwise color your view of the world.
 THINK LIKE A BOARD Our Mission The mission of the Ohio Board of Nursing is to actively safeguard the health of the public through the effective regulation of nursing care. it s easy to let your biases
THINK LIKE A BOARD Our Mission The mission of the Ohio Board of Nursing is to actively safeguard the health of the public through the effective regulation of nursing care. it s easy to let your biases
