Special Report: Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders
|
|
|
- Winifred Wood
- 7 years ago
- Views:
Transcription
1 Technology Evaluation Center Special Report: Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders Assessment Program Volume 28, No. 3 August 2013 Executive Summary Background There are different clinical diagnostic scenarios for which traditional Sanger DNA sequencing technology is clinically available. Its success as a diagnostic tool depends on the accuracy and completeness of the differential diagnosis, as well as the number and locations of mutations responsible for the observed disease. Single-gene disorders are diseases that result from a mutation in one gene that has a large effect on phenotype. A clinical focus of this Special Report is the scenario of an undiagnosed, suspected single-gene disorder that features multiple congenital anomalies. Disorders (often early developmental) that present with multiple anomalies suggest a genetic etiology. They are often clinically difficult to diagnose due to nonspecific presentation, potentially overlapping differential diagnoses (clinical heterogeneity) or a rare disorder, and lack of a clear diagnostic testing path. The search for a clinical diagnosis may result in a long (months to years) diagnostic odyssey comprising several specialist consults and many different individual molecular, cytogenetic and biochemical tests, as well as other types of diagnostic procedures. A relatively unbiased sequencing application that examines most genes without requiring prior knowledge of potential diagnoses would be advantageous. Single-gene disorders are inherited in various patterns (e.g., autosomal dominant, autosomal recessive, X-linked) and are affected by penetrance (proportion of individuals with the disease-related mutation who have the disease phenotype at all) and by expressivity (variable phenotypes that can occur in different individuals with the same genetic mutation). Disorders arising from a mutation in a single gene may be caused by exactly the same mutation in all individuals with the condition, or may be caused by any one of many different mutations in the same gene (allelic heterogeneity). In contrast, a large proportion of heritable disorders are genetically heterogeneous; clinically indistinguishable or overlapping phenotypes may be caused by mutations in different genes (locus heterogeneity). The number of genes involved can range from just a few to hundreds. For many of these genetically heterogeneous disorders, the full complement of causal genes is not yet known. BlueCross BlueShield Association An Association of Independent Blue Cross and Blue Shield Plans Determining genetic causality for disease and establishing a molecular diagnosis in clinical practice is challenging; in recent years, molecular technologies have strikingly increased gene discovery and understanding the causes of single-gene disorders among patients with suspected, but previously undiagnosed, genetic disorders. The value of molecular diagnosis in these patients can be viewed from a clinical perspective similar to other diagnostic tests; it is also commonly addressed from perspectives of clinical and personal utility, as well as a societal view. A molecular diagnosis can: confirm a suspected or established clinical diagnosis; inform prognosis; aid in selecting treatment, surveillance or preventive options; reveal mode of inheritance; identify carrier/risk status of family members; and guide research regarding new therapy or patient management. Available guidelines are currently focused on molecular diagnosis, distinguishing among phenotypically overlapping possible conditions, and on referral for genetic consultation. NOTICE OF PURPOSE: TEC Assessments are scientific opinions, provided solely for informational purposes. TEC Assessments should not be construed to suggest that the Blue Cross Blue Shield Association, Kaiser Permanente Medical Care Program or the TEC Program recommends, advocates, requires, encourages, or discourages any particular treatment, procedure, or service; any particular course of treatment, procedure, or service; or the payment or non-payment of the technology or technologies evaluated Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 1
2 Technology Evaluation Center Sanger sequencing is currently the gold standard for molecular diagnosis of unknown point mutations in known genes (usually limited to the coding regions and intron/exon splice sites). However, when the gene in question is very large or when there is substantial locus heterogeneity, comprehensive Sanger sequencing may become unacceptably burdensome. For rare conditions, this may preclude laboratories from offering such testing, consequently making genetic testing unavailable for diagnostic purposes. Recently, next-generation sequencing technologies have become more accessible in terms of cost, analytic validity, and rapidity. Exome sequencing has the capacity to determine in a single assay an individual s exomic variation profile, limited to most of the protein coding sequence of an individual (approximately 85%), composed of about 20,000 genes, 180,000 exons (protein-coding segments of a gene), and constituting approximately 1% of the whole genome. It is believed that the exome contains about 85% of heritable disease-causing mutations. In 2009, the first proof-ofprinciple study was published in which the authors developed an exome sequencing method and applied it to samples from unrelated individuals all known to have the same Mendelian disorder, for which the gene was already established. Mutations in the known gene were identified as uniquely common to all patients. Less than 3 years later, a MEDLINE search on Mendelian disorders using exome sequencing identified publications on over 100 diseases and more than 100 newly identified genes. Exome sequencing has the advantages of speed and efficiency relative to Sanger sequencing of multiple genes, delivering results in weeks to months. After candidate variants can be sufficiently reduced in number (from an initial 20 30,000 per individual exome), targeted Sanger sequencing may be used to confirm the presence or absence of the potential variant(s) in the individual being diagnosed, and in affected and unaffected family members. In this way it is relatively straightforward to perform exome sequencing as a clinical service for new patients to help determine a clinical diagnosis and identify causal variants in known genes. Exome sequencing, relying on next-generation sequencing technologies, is not without challenges and limitations. In the human exome, about 1 in every 1,000 nucleotides will vary. Computer algorithms can eliminate most that are presumed irrelevant to the condition of concern. The final steps of variant filtering involve manual research and review. This requires expertise including knowledge of the patient s phenotype and family history, extremely current knowledge and exploration of published variant-disease associations, and judgment, all of which can contribute to variability in the final variant interpretation. Reproducibility at the level of final manual variant interpretation has not been fully characterized; comparisons of exome capture platforms using detection of known medically relevant variants as the sequencing result for assessment indicate small differences in detection, gaps in capture platforms, as well as regions of poor coverage that are unlikely to benefit from improved capture or greater depth of sequencing. Tools to improve the computational and variant analysis pipeline are available and under development. Like Sanger sequencing, next-generation ( Next-Gen or NGS ) sequencing cannot detect large deletions or duplications of DNA or nucleotide repeats that can cause disease. Error rates due to uneven sequencing coverage, gaps in exon capture prior to sequencing, difficulties with narrowing the large initial number of variants to manageable numbers without losing likely candidate mutations, poorly annotated variant databases, lack of standardized procedures, and identifying mutations in unrelated genes or unknown genes are all issues. Detailed guidance from regulatory or professional organizations is under development, and the variability contributed by the different platforms and procedures used by clinical laboratories offering exome sequencing as a clinical service is unknown. There are also ethical questions about reporting incidental findings, such as identifying medically relevant mutations in genes unrelated to the diagnostic question, sex chromosome abnormalities and non-paternity when family studies are performed Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited.
3 Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders Objective The objective of this Special Report is to compare Sanger sequencing and next-generation sequencing clinical molecular methods for diagnosing disorders that are caused by mutations in a single gene, and the potential uses of exome sequencing for molecular diagnosis. We first describe exome sequencing using next-generation sequencing and compare it with Sanger sequencing. Second, three clinical scenarios caused by single-gene disorders where exome sequencing might be applied are described. Finally, we review evidence related to its clinical use in evaluating patients with undiagnosed suspected genetic disorders accompanied by multiple anomalies focusing on diagnostic yield and potential impact on patient outcomes. Search Strategy MEDLINE was searched via PubMed using the search string ( Sequence Analysis, DNA [MeSH] OR ((DNA OR gene) AND ( sequence analysis OR sequencing))) AND (( Mutation [MeSH]) OR ( Polymorphism, Single Nucleotide [MeSH] OR Polymorphism, Genetic [MeSH] ) OR mutation* OR polymorphism*) AND ( Exome [MeSH] OR exom*), through March A search performed July 2013 identified no studies that would affect conclusions. Selection Criteria Studies of clinical diagnosis included for review met the following criteria: n Patients were described as having an undiagnosed suspected genetic disorder accompanied by multiple anomalies n Studies employed exome sequencing by next-generation technology n Purpose of study appeared to be focused on patient(s) and on improving diagnostic accuracy and possible therapy, or on molecular method as a way to improve diagnosis n Patients selected for exome sequencing were primary, not secondary to purpose of study n Actual disorder investigated was secondary to purpose of study n Conclusions focused on patients and at least on improved methods of clinical diagnosis (if not on treatment outcomes) Main Results The diagnostic yield of exome sequencing in the 6 larger patient series (n>10; each study sequenced 12 to 118 exomes) varied from 10% to 54%. Results were more often successful in the 21 small individual/family studies (n 10; each study sequenced 1 to 10 exomes). Since these studies were largely positive or negative on the basis of the index case, and few negative results were found in this group of studies, selective reporting of positive results could have occurred. Beyond diagnostic yield, occasional anecdotal reports were identified of clinical benefit following molecular diagnosis by exome sequencing. Such potential benefits included a revision of the original diagnosis; a few instances of treatments initiated or discontinued as a result of the known functional consequences of the mutation, and genetic counseling to the affected patient and/or family members. However, no systematic study of clinical outcomes was identified. Studies of exome sequencing compared with traditional Sanger sequencing generally agree that exome sequencing is more efficient and more likely to result in a diagnosis. One study reported a sensitivity of 98.3% for detecting previously identified mutations, as well as benign variants. Particular limitations noted were gaps in coverage of the exome and difficulty interpreting variants. Author s Conclusions and Comment Exome sequencing using next-generation technology has recently become available as a laboratory-developed diagnostic clinical laboratory test. A major indication for use is molecular diagnosis of patients with suspected genetic disorders. These patients may be left without a satisfactory clinical diagnosis of their disorder despite a lengthy diagnostic odyssey involving a variety of traditional molecular and other conventional diagnostic tests. For some patients, exome sequencing obtained after initial diagnostic evaluation (that may include other genetic testing) has failed may avoid the diagnostic odyssey and return a likely causal variant. Currently, the diagnostic yield appears 2013 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 3
4 Technology Evaluation Center to be no greater than 50% and possibly less for patients with suspected genetic disorder accompanied by multiple anomalies. Medical management decisions, including initiation of new treatment or discontinuing inappropriate treatment, may result for only a subset of those diagnosed. Reproductive decisions for parents considering an additional pregnancy may be informed by determining the mode of inheritance. Appropriate use of exome sequencing requires considerable genetic, clinical, and genetic counseling expertise. Contents Objective 5 Background 5 Methods 17 Discussion 24 Conclusions 25 References 27 Review 17 Published in cooperation with Kaiser Foundation Health Plan and Southern California Permanente Medical Group. TEC Staff Contributors Lead Author Margaret Piper, Ph.D., M.P.H.; Co-Author Mark D. Grant M.D., M.P.H.; TEC Executive Director Naomi Aronson, Ph.D.; TEC Director, Technology Assessments Mark D. Grant, M.D., M.P.H.; Director, Clinical Science Services Kathleen M. Ziegler, Pharm.D.; Research/Editorial Staff Claudia J. Bonnell, B.S.N., M.L.S.; Kimberly L. Hines, M.S. Acknowledgments Staff would like to acknowledge the contributions of Scott McLean, M.D., to the research and development of this Special Report. Blue Cross and Blue Shield Association Medical Advisory Panel Trent T. Haywood, M.D., J.D. Chairman, Senior Vice President, Clinical Affairs/Medical Director, Blue Cross and Blue Shield Association; Steven N. Goodman, M.D., M.H.S., Ph.D. Scientific Advisor, Dean for Clinical and Translational Research, Stanford University School of Medicine, Professor, Departments of Medicine, Health Research and Policy; Mark A. Hlatky, M.D. Scientific Advisor, Professor of Health Research and Policy and of Medicine (Cardiovascular Medicine), Stanford University School of Medicine. Panel Members Peter C. Albertsen, M.D., Professor, Chief of Urology, and Residency Program Director, University of Connecticut Health Center; Sarah T. Corley, M.D., F.A.C.P., Chief Medical Officer, NexGen Healthcare Information Systems, Inc. American College of Physicians Appointee; Helen Darling, M.A., President, National Business Group on Health; Josef E. Fischer, M.D., F.A.C.S., William V. McDermott Professor of Surgery, Harvard Medical School American College of Surgeons Appointee; I. Craig Henderson, M.D., Adjunct Professor of Medicine, University of California, San Francisco; Jo Carol Hiatt, M.D., M.B.A., F.A.C.S., Chair, Inter-Regional New Technology Committee, Kaiser Permanente; Saira A. Jan, M.S., Pharm.D., Associate Clinical Professor, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Residency Director and Director of Clinical Programs Pharmacy Management, Horizon Blue Cross and Blue Shield of New Jersey; Thomas Kowalski, R.Ph., Clinical Pharmacy Director, Blue Cross Blue Shield of Massachusetts; Lawrence Hong Lee, M.D., M.B.A., F.A.C.P., Vice President and Executive Medical Director for Quality and Provider Relations, Blue Cross and Blue Shield of Minnesota; Bernard Lo, M.D., Professor of Medicine and Director, Program in Medical Ethics, University of California, San Francisco; Randall E. Marcus, M.D., Charles H. Herndon Professor and Chairman, Department of Orthopaedic Surgery, Case Western Reserve University School of Medicine; Barbara J. McNeil, M.D., Ph.D., Ridley Watts Professor and Head of Health Care Policy, Harvard Medical School, Professor of Radiology, Brigham and Women s Hospital; William R. Phillips, M.D., M.P.H., Clinical Professor of Family Medicine, University of Washington American Academy of Family Physicians Appointee; Richard Rainey, M.D., Medical Director, Regence BlueShield of Idaho; Rita F. Redberg, M.D., M.Sc., F.A.C.C., Professor of Medicine and Director, Women s Cardiovascular Services, University of California San Francisco; Maren T. Scheuner, M.D., M.P.H., F.A.C.M.G., Chief, Medical Genetics, VA Greater Los Angeles Healthcare System; Associate Clinical Professor, Department of Medicine, David Geffen School of Medicine at UCLA, Affiliate Natural Scientist, RAND Corporation; J. Sanford Schwartz, M.D., F.A.C.P., Leon Hess Professor of Medicine and Health Management & Economics, School of Medicine and The Wharton School, University of Pennsylvania. CONFIDENTIAL: This document contains proprietary information that is intended solely for Blue Cross and Blue Shield Plans and other subscribers to the TEC Program. The contents of this document are not to be provided in any manner to any other parties without the express written consent of the Blue Cross and Blue Shield Association Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited.
5 Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders Objective The objective of this Special Report is to compare Sanger sequencing and next-generation sequencing clinical molecular methods for diagnosing disorders that are caused by mutations in a single gene, and the potential uses of exome sequencing for molecular diagnosis. We first describe exome sequencing using next-generation sequencing and compare it with Sanger sequencing. Second, three clinical scenarios caused by single-gene disorders where exome sequencing might be useful are described. Finally, we review evidence related to use of exome sequencing in evaluating patients with undiagnosed suspected genetic disorders accompanied by multiple anomalies focusing on diagnostic yield and potential impact on patient outcomes. Background Definitions and Epidemiology Single-gene disorders are the result of a mutation in one gene that has a large effect on phenotype. Mutations may be inherited or may arise de novo in parental gametes during meiosis. 1 Such disorders are often classified as rare or orphan diseases because they affect only small populations. There is no universal definition of a rare disease; a 1984 amendment to the 1983 Orphan Drug Act defines a rare disease as a disease or condition that affects fewer than 200,000 people in the U.S. This definition was repeated in the Rare Disease Act of 2002 that established the (already existing) National Institutes of Health Office of Rare Disease Research in statute. The number of rare diseases has been estimated at 5,000 to 8,000 (IOM, 2010), and is rapidly growing. The prevalence of specific single-gene disorders may vary among different populations that are defined ethnically and geographically. Collectively, an estimated 25 to 30 million people in the United States are believed to have a rare disease (NIH Office of Rare Diseases Research, AboutUs.aspx). Single-gene disorders may be expressed in utero, early during postnatal development, or across the childhood and adult age spectrum. We consider different clinical diagnostic scenarios for which traditional, Sanger sequencing technology is already clinically available, but its success as a diagnostic tool depends on the accuracy and completeness of the differential diagnosis (e.g., skill and experience of ordering clinician). Clinical scenarios where nextgeneration sequencing might be particularly useful include 2 : 1. A suspected single-gene disorder that features multiple congenital anomalies. Disorders (often early developmental) that present with an unusual constellation of findings or multiple congenital anomalies, suggest a genetic etiology, and may be exceedingly difficult to diagnose clinically due to nonspecific presentation or potentially overlapping differential diagnoses (clinical heterogeneity). The search for a clinical diagnosis may result in a long (potentially months to years) diagnostic odyssey comprising several specialist consults and many different individual molecular, cytogenetic and biochemical tests, as well as other types of diagnostic procedures. For example, the Undiagnosed Diseases Program at the National Institutes of Health enrolls and evaluates patients with conditions that have long eluded diagnosis ( info.nih.gov/resources.aspx?pageid=31). Arriving at a molecular diagnosis may inform management decisions, determine risk for family members, and can provide information for reproductive decision making of the patient or a patient s parents. 2. A known single-gene disorder with locus heterogeneity and the need for subtype identification. A mutation in one of several different genes may cause the disorder. Molecular diagnostic testing is not usually necessary for diagnosis but may be useful in some cases for confirmation or for identifying subtypes of the disorder with different symptoms and requiring different management decisions. It may also determine risk 1 The presence of a mutation found in an individual but not the parents may also be due to post-conception mutations that have attained a high level of somatic mosaicism (i.e., a mixture of cells with and without the new mutation). This happens either because the mutations occurred at an early stage of embryonic development or because the cells with the variant had better survival or increased proliferation. 2 In addition to these scenarios where traditional sequencing is available, there are others where exome sequencing may also be used for a suspected single-gene disorder where Sanger sequencing is not available because the responsible gene is not known or suspected Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 5
6 Technology Evaluation Center for family members and provide helpful information for reproductive decisionmaking. An example is congenital disorders of glycosylation (CDG), a large group of inherited metabolic disorders affecting several steps of the protein glycosylation pathway. There are numerous subtypes depending on the affected gene. Subtypes have been described in only a few individuals, limiting understanding of the phenotype, and the range of phenotypic expression across subtypes is broad. For some subtypes, the differential diagnosis may overlap with other disorders such as mitochondrial disease or congenital muscular dystrophies. The diagnostic test for all subtypes of CDG is transferrin isoform analysis; however, while the specific enzymes are known, enzymatic assays are not generally available. To identify specific subtypes, molecular diagnosis is necessary. Traditional diagnostic sequencing of each of the known associated genes is available to identify variants for most subtypes and may be helpful for those few subtypes where treatment other than supportive treatment is available to alleviate symptoms. The most likely subtypes must be prioritized by clinical indications and sequencing tests selected accordingly. 3. A suspected single-gene disorder that features a common disease (e.g., cancer) with locus heterogeneity. Knowledge of the genetic diagnosis may influence clinical management, including decisions regarding surveillance and prevention; may determine risk for family members; and may provide helpful information for reproductive decisionmaking. An example is a patient with a personal and family history of early onset breast cancer. This scenario may be encountered with an inherited mutation in one of several known genes (e.g., BRCA1, BRCA2, PALB2, PTEN, TP53, STK11, CHEK2, ATM) or unknown genes. Sanger sequencing can evaluate mutations in each of these genes one by one. This typically begins with the BRCA1/2 genes due to the relatively higher prevalence of mutations in these genes. However, because not all genes associated with inherited breast cancer have been identified, some families may exhaust the known list of associated genes without identifying a family mutation. As suggested by the examples, traditional sequencing can be highly informative, but frequently is limited in scope and requires knowing the most likely diagnosis and the genes associated with that diagnosis. An unbiased sequencing application that examines the exome without requiring prior knowledge would offer some advantages in all three scenarios. The use of next-generation sequencing to identify potential causative variants in the exome is an unbiased sequencing application that does not require prior clinical knowledge of the diagnosis or of the genes contributing to the diagnosis to direct test selection. This Special Report addresses these scenarios while focusing on evidence for clinical utility in the first clinical scenario, i.e., suspected singlegene disorders accompanied by multiple congenital anomalies. Patterns of Inheritance. The inheritance patterns of single-gene disorders resemble those originally described by Mendel: n Inherited disorders are autosomal if they are encoded by genes on one of the 22 pairs of autosomes (non-sex chromosomes). n Inherited disorders are X-linked if encoded by a gene on the X chromosome. n Dominant conditions are those expressed in heterozygotes, i.e., individuals with one copy of a mutant allele and one copy of a normal (wild-type) allele. n Recessive conditions are clinically manifest only in individuals homozygous for the mutant allele (or individuals said to be compound heterozygotes because they carry two different mutant alleles), i.e., carrying a double dose of an abnormal gene, or hemizygous for an X-linked recessive condition. A non-mendelian inheritance pattern is that of mitochondrial inheritance. Mitochondria are normal cellular organelles located in the cytoplasm and are involved in energy production. Mitochondrial genes are inherited only from the mother because they are contained in the cytoplasm of the ovum. The presence of causative gene variants that are not found in the parents can be due to de novo mutations that occurred in the parental gametes during meiosis. Such mutations are then heritable only if the individual with the disorder has children. Other explanations for mutations not found in the parents include nonpaternity and parental germline mosaicism Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited.
7 Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders Inheritance patterns can sometimes be determined by analyzing the family history or pedigree, i.e., tracking the individuals who do and do not express disease within the context of their family relationships. Discerning such patterns is important for making a genetic diagnosis, genetic counseling for reproductive risks, carrier testing, and identifying at-risk relatives of an individual with disease. However, inheritance patterns can be complicated by reduced penetrance (not all individuals with the diseaserelated mutation will develop a disease phenotype) and by variable expressivity (variable phenotypes that can occur in different individuals with the same genetic mutation). Genetic Heterogeneity. Disorders known to be caused by mutations in a single gene may be caused by exactly the same mutation in all individuals with the condition. For example, sickle cell disease, an autosomal recessive disorder, is caused by a specific and invariant point mutation in the gene for the beta-globin chain of hemoglobin, resulting in the amino acid glutamic acid to be replaced with valine at the sixth position (Lazarus and Schmaier, 2011). Cystic fibrosis, however, can be caused by any one of over 1,500 mutations in the gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR) protein; this is allelic heterogeneity (Bobadilla et al. 2002). Cystic fibrosis is also an autosomal recessive disorder and can result from the same mutation in each copy of the CFTR gene (homozygous alleles) or different mutations in each gene copy (compound heterozygote). The various mutations have a range of effects on the CFTR protein resulting in a spectrum of mild to severe disease. In contrast, a large proportion of single-gene disorders are genetically heterogeneous: clinically indistinguishable or overlapping phenotypes may be caused by mutations in different genes (also termed locus heterogeneity ). The number of associated genes can range from just a few to hundreds. For many of these genetically heterogeneous disorders, the full complement of causal genes is unknown. Molecular Diagnosis. Given the complexity of genetic diseases, determining causation for disease and establishing a molecular diagnosis in clinical practice is challenging. The contribution of modern genomic technologies to gene discovery and molecular diagnosis of singlegene disorders has been phenomenal. In 1982, fewer than 5 disorders had known molecular genetic causes (Ginsburg 2011). By 1990 the number had increased to about 150, and by 2011 was over 3,000. Since then new, confirmed gene-disease associations (and descriptions of new syndromes with molecular diagnoses) have been published at a rapid rate. However, for many single-gene developmental disorders a causative gene remains to be discovered. The value of molecular diagnosis in patients with undiagnosed suspected genetic disorders can be considered from a clinical perspective, similar to other diagnostic tests, but is also commonly addressed from a patient, family, and societal perspective. The following are possible uses of molecular diagnosis; not all apply to every single-gene disorder, particularly where treatment is not yet available. Anecdotal examples can be found for each, but the overall contribution of each type of outcome to the total picture of heritable disorders is not available. n Confirm a suspected or clinically established genetic disease diagnosis n Determine prognosis n Aid in selecting patient treatment, and risk-appropriate surveillance and prevention options n Determine carrier/risk status of family members n Determine reproductive risk for the patient with a genetic diagnosis, or for couples with an affected child who are considering an additional pregnancy n Guide research regarding new therapy or patient management As an example, Miller et al. (2011) note that for patients with Charcot-Marie-Tooth (CMT) disease, which to date is associated with causative mutations in over 50 genes/loci accounting for only a portion of affected patients, genetic testing may be performed for several possible reasons: identifying the inheritance pattern of their CMT, making family planning decisions, and obtaining knowledge about the cause and natural history of their form of CMT. Several guidelines address molecular diagnosis, in the context of the genetic testing methods available at the time the guidelines were written and published; Table 1 lists examples (note that all but the American College of Medical Genetics and Genomics guideline address targeted testing for specific disorders 2013 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 7
8 Technology Evaluation Center or syndromes). Consistent themes are that molecular diagnosis is important for classifying disorders and distinguishing among overlapping conditions; in some cases is necessary for confirming a diagnosis; and supplies important information for genetic counseling. When a disease-causing gene(s) is established, assays based, for example, on polymerase chain reaction technology can be designed to specifically detect known mutations for clinical diagnosis. When many different point mutations in a gene are possible, Sanger sequencing, the current gold standard for detecting unknown point mutations, can be employed to determine the entire sequence of the coding and intron/exon splice sites of gene regions where mutations are most likely to be found. However, when genes are large and mutations are possible in many or all exons (proteincoding regions of the gene), and when there is genetic (locus) heterogeneity, comprehensive Sanger sequencing may be prohibitively laborious and costly. Currently available clinical assays designed for molecular diagnosis may be incomplete due to genetic heterogeneity and unknown causative genes, or because only a portion of the known genes and mutations can be efficiently tested using conventional molecular methods. Recently, next-generation sequencing technologies have become more accessible in terms of cost and speed; it is a technology that has been adopted by a growing number of molecular genetic clinical laboratories. Next-generation sequencing platforms produced by different companies use different technologies, but in general are high throughput, producing thousands or millions of sequences at the same time. These methods offer increased sequencing capacity while decreasing some of the labor and cost associated with Sanger sequencing. However, time and costs for interpretation are increased. Nextgeneration sequencing can be used to sequence the genome of an individual, or selected portions of the genome (e.g., a targeted panel that includes all genes known to be related to a particular condition or group of disorders such as the inherited cardiomyopathies; this eliminates the challenge of dealing with mutations found in unknown genes). Genome sequencing has the capacity to determine an individual s complete genomic variation profile in a single experiment (Gilissen et al. 2011). However, genome sequencing is currently limited in terms of throughput, data analysis, and cost efficiency, although rapid improvements continue. Exome Sequencing. Exome sequencing consists of similar methods as genome sequencing, except for an extra step involving exome capture, which limits sequencing to the protein coding regions of the genome, composed of about 20,000 genes, 180,000 exons (protein-coding segments of a gene), and constituting approximately 1% of the whole genome. It is believed that the exome contains about 85% of heritable disease-causing mutations (Choi et al. 2009). Prior to determining the sequence of an individual s exome, the exome must first be physically captured from the sample by various available methods. Capture designs usually include all protein coding sequences, flanking splice sequences, and untranslated regions that could contain damaging variants. Different sources (e.g. the National Center for Biotechnology Information Consensus Coding DNA Sequence) may be used as the reference gene set for the design template. Thus, there is variability across capture methods (Fuchs et al. 2012). After an individual s exome is sequenced, a search for the disease-causing mutation is performed by comparing the sequencing data with a human genome reference, resulting in a list of all non-reference variants. Typically, 20 30,000 variants result for each exome sequence (Robinson et al. 2011). Variants are first screened according to various parameters, e.g., depth of coverage (a minimum of 10 sequencing reads per base are needed for a basic level of reliability of the variant call; recommended numbers of reads are typically much higher and vary depending on the specific application). The next step is to distinguish between normal variation in the general population and possible disease-causing variants using a variety of publicly available databases. Variants predicted to be benign are computationally removed. Once the variant list is sufficiently reduced to a relatively small number, the remaining variants can be combined with information known about the disease to determine if the list includes potential variants that are known or suspected to be deleterious (e.g., nonsense or frameshift mutations) and that explain the patient phenotype Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited.
9 2013 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 9 Table 1. Examples of Guidelines/Policies that Address Molecular Testing in Hereditary Disorders Guideline Source Year Focus of Guideline Impact of Genetic Testing American College 2012 Clinical applications of Exome sequencing should be considered for clinical diagnosis of Medical Genetics genomic sequencing of an affected individual when: and Genomics a genetic etiology is likely but targeted testing is not available (ACMG, 2012) patient s disorder has high genetic heterogeneity targeted testing resulted in no diagnosis a fetus has a likely genetic disorder and targeted tests are negative; turnaround time should be considered American Academy 2004 Diagnostic assessment Differentiate between cerebral palsy and other neurometabolic of Neurology of the child with cerebral disorders that may share symptomology during early years; (Ashwal et al. 2004) palsy some non-cerebral palsy disorders may benefit from early diagnosis and available treatment (e.g., glutaric aciduria) American Academy 2009 Evaluation of the child Microcephaly has been associated with numerous genetic of Neurology with microcephaly etiologies; genetic etiologies have been reported in 15 to (Ashwal et al. 2009) 53% of cases using targeted testing; currently available data likely underestimate the contribution of genetic testing to the diagnostic evaluation European Federation of 2010 Molecular diagnosis Hereditary ataxias must be distinguished from non-hereditary Neurological Societies of ataxias and spastic forms; different forms have different modes of inheritance (Gasser et al. 2010) paraplegias and are clinically overlapping, making them hard to diagnose without molecular testing; hereditary spastic paraplegia syndromes are also clinically heterogeneous and are classified based on mode of inheritance and genetic linkage European Federation of 2010 Molecular diagnosis Channelopathies: molecular testing may aid diagnosis but Neurological Societies of channelopathies, cannot be considered routine due to the large number of (Burgunder et al. 2010) epilepsies, migraine, genes and mutations; stroke and dementias Cerebrovascular diseases: targeted tests suggested to help resolve specific clinical situations; Familial hemiplegic migraine: confirm diagnosis by sequencing most commonly associated gene; Alzheimer s disease: useful for genetic counseling in early onset autosomal dominant form of disease Type of Genetic Testing/ Algorithm Referenced Policy specifically references exome sequencing (and also whole genome sequencing for same indications) None specified Targeted testing may be considered to determine etiology; as the yield of screening tests (not including exome sequencing) is unknown, no recommendations can be made Targeted testing for specific syndromes, in some cases depending on clinical presentation and the results of other laboratory tests Targeted testing for specific syndromes, as recommended in clinical context Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders
10 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. Table 1. Examples of Guidelines/Policies that Address Molecular Testing in Hereditary Disorders (cont d) Guideline Source Year Focus of Guideline Impact of Genetic Testing European Federation of 2011 Diagnosis and treatment Diagnosis is clinical; genetic testing can aid in classification, Neurological Societies of primary dystonias which is important for appropriate management, prognostic (Albanese et al. 2011) information, and genetic counseling European Federation of 2009 Molecular diagnosis of Diagnostic workup follows a stepwise procedure: Neurological Societies mitochondrial disorders 1) initiate comprehensive family history and clinical review; (Finsterer et al. 2009) 2) determine syndromic vs. non-syndromic form and inheritance pattern; 3) results of 1 and 2 determine recommended genetic tests for likely syndromic forms European Federation of 2010 Diagnosis and Mutation testing in the DMD gene is necessary to confirm Neurological Societies management of Duchenne diagnosis, even when absence of dystrophin protein expression (Bushby et al. 2010) muscular dystrophy on muscle biopsy has been shown; results provide information required for genetic counselling, prenatal diagnosis, and consideration for future mutation-specific therapies European Federation of 2007 Diagnosis and Detection of an associated mutation is the standard of Neurological Societies management of limb girdle diagnosis and necessary to be able to offer carrier or presymptomatic (Norwood et al. 2007) muscular dystrophies testing to other family members Mutation detection for the rarer types of LGMD may only be available on a research basis. May change counseling advice e.g., if differential includes Becker muscular dystrophy American Academy 2009 Evaluation of distal Genetic testing should be conducted for the accurate diagnosis of Neurology symmetric polyneuropathy and classification of hereditary neuropathies the genetic (England et al. 2009) testing profile should be guided by the clinical phenotype, inheritance pattern, and electrodiagnostic (EDX) features Type of Genetic Testing/ Algorithm Referenced Targeted testing for some specific forms of dystonia is available, as recommended in clinical context Various types of genetic tests depending on differential diagnosis; many genes/ types of mutations are possible Deletion/duplication analysis followed by gene sequencing if negative; many and different types of mutations are possible Targeted testing for specific syndromes, per clinical differential diagnosis Targeted testing per decision algorithm Technology Evaluation Center
11 Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders In 2009, Ng et al. (2009) published a proof of principle study in which the authors developed next-generation sequencing methods for targeted sequencing of all protein-coding regions of the genome (i.e., the exome ). Exome sequencing was applied to samples from 4 unrelated individuals known to have Freeman- Sheldon syndrome, for which the associated gene was already known. The method identified variants in the known gene as uniquely common to the 4 patients. A second proof of principle study (Choi et al. 2009) used exome sequencing to identify a variant in the SLC26A3 gene, known to be associated with congenital chloride diarrhea, in an infant originally suspected to have a renal defect such as Bartter syndrome. Targeted sequencing of the gene in additional patients with suspect Bartter syndrome but no related gene variants found SLC26A3 variants and the diagnoses were subsequently revised to congenital chloride diarrhea, illustrating the unbiased nature of exome sequencing investigations. Exome sequencing has the advantages of speed and efficiency relative to Sanger sequencing, delivering results in weeks to months. If candidate variants can be sufficiently reduced in number, targeted Sanger sequencing may be used to confirm the presence or absence of the potential variant(s) in the individual being diagnosed. In addition, the variant call may be influenced by how the variant segregates in affected and unaffected family members (e.g., if a heterozygous mutation is not found in either parent, the variant is assumed de novo and more likely causative). In this way it is relatively straightforward to perform exome sequencing as a clinical service for patients to determine a molecular diagnosis by identifying causal variants in known genes (Lyon and Wang, 2012). Table 2 compares Sanger and nextgeneration sequencing in different clinical settings showing some of the major advantages and disadvantages of each. Table 3 reviews limitations of both methods. If no likely causal variants in genes known to be associated with the condition are identified, some clinical laboratories offer gene discovery as a clinical service, requiring exome sequencing of additional family members. The variant list for the affected individual can be compared with sequencing information from key individuals from the same family. For example, in the case of an autosomal recessive disorder, the affected individual must be homozygous or compound heterozygous for the causal variants, each of which occurs only once in each parent. For autosomal dominant conditions, if neither parent carries the mutation and there is no evidence of non-paternity, then there is evidence for a de novo mutation that is more likely deleterious. In this way, candidate gene variants can be rapidly reduced to one or a few candidates for further investigation. The number of patients needed to resolve potential causative variants depends on the pattern of inheritance. For example, because the number of possible causative variants is much higher in autosomal dominant disorders, more family members may be needed to determine co-segregation of a variant with the phenotype (Fuchs et al. 2012). Limitations of Exome Sequencing. Similar to Sanger sequencing, exome sequencing is not without limitations. For example, causative variants that occur in non-coding regulatory regions are not detected (Lyon and Wang, 2012) and certain types of mutations are not detected (large deletions, duplications, rearrangements, nucleotide repeats, and epigenetic changes). Exome capture methods may not capture each exon in every gene and insufficient depth of coverage in some regions can mask a diseasecausing variant (McDonald et al. 2012). Due to these and other detection limitations (see Table 3), the total proportion of gene variants that will be missed by current methods may be as high as 15-20% (Fuchs et al. 2012). Additionally, public databases containing information on putative disease-causing mutations are incomplete and may have high error rates (Bell et al. 2011) requiring manual curation; associations for some mutations in the database may not be causal. Improvements in shared databases are ongoing. Finally, next-generation sequencing has not yet been reviewed by the U.S. Food and Drug Administration, and only general technical guidelines have been discussed by a few organizations (Gargis et al. 2012; Schrijver et al. 2012). The College of American Pathologists Laboratory Accreditation Program has revised their molecular pathology checklist to include a dedicated section on next-generation sequencing as of July 31, Several laboratories 3 See CAP press release available at tlets%2fcontentviewer%2fshow&_windowlabel=cntvwrptlt&cntvwrptlt%7bactionform.contentreference%7d=media_ resources%2fnewsrel_checklist_next_gene.html&_state=maximized&_pagelabel=cntvwr 2013 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 11
12 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. Table 2. Comparison of Standard Sanger Sequencing vs. Next-generation Sequencing Sequencing Technology Sanger Next Generation Gold standard for detecting unknown point Yes Not yet mutations in coding and exon/exon splice sites Maximum feasible size of clinical sequencing unit Single-gene exon(s), single gene (if not excessively large) Exome (whole genome likely, in future) Ability to identify a previously established causal variant in a single gene Relatively efficient; if a small number of known mutations account for all known disease association, if there are Inefficient for most circumstances of a known causal variant; single-gene assays in development e.g. for cystic fibrosis common, ancestry-specific mutations, or if there is a known familial mutation Ability to identify a causal variant for a suspected single-gene disorder that features a common disease with locus heterogeneity, need for subtype identification, or identify a causal variant Requires accurate and complete differential diagnosis with knowledge of causative genes. Genes known to be associated with the disorder are sequenced one at a time in order of likelihood of harboring a mutation, based on prevalence and clinical findings. Currently available clinical tests may not address all mutations or all genes known to be associated with heterogeneous disorders, and clinical tests may not be available for known genes. Unknown genes will be missed. The exome or a panel of genes known to be associated with the disorder can all be done in a single sequencing assay; public databases help filter variants and identify previously established causal variants. Targeted sequencing of family members for variant may be needed in follow-up; if known variant not identified, may provide initial steps in discovery of new variant or new gene-disease association. If gene panel is performed, then unknown genes will be missed. Technology Evaluation Center
13 2013 Blue Cross and Blue Shield Association. Reproduction without prior authorization is prohibited. 13 Table 2. Comparison of Standard Sanger Sequencing vs. Next-generation Sequencing (cont d) Sequencing Technology Sanger Next Generation Major limitations (see also Limitations of exome sequencing, following) Sequence may contain gaps due to biases in cloning amplification step Sequence likely to contain gaps due to uneven efficiency of exome capture and amplification Structural variation (e.g. large deletions, duplications and rearrangements) not detected Nucleotide repeat disorders not detected Epigenetic changes not detected One or a few variants of uncertain significance may be detected Reduced efficiency/cost-effectiveness when full sequences of large single genes or full or partial sequences of several genes are required; Sanger sequencing of full exome cost prohibitive Structural variation (e.g. large deletions, duplications and rearrangements) not detected Nucleotide repeat disorders not detected Epigenetic changes not detected Thousands of variants of uncertain significance will be detected More efficient/cost effective when sequences of several genes or of whole exome are required; and when several patients batched Identifying deleterious variants (mutations) in unknown genes (i.e., genes that have not yet been characterized) Incidental findings of medically relevant/actionable variants; sex chromosomal abnormalities; and non-paternity if family studies are performed to interpret variants Exome Sequencing for Clinical Diagnosis of Patients with Suspected Genetic Disorders
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics
 Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES
 DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
Genetics Lecture Notes 7.03 2005. Lectures 1 2
 Genetics Lecture Notes 7.03 2005 Lectures 1 2 Lecture 1 We will begin this course with the question: What is a gene? This question will take us four lectures to answer because there are actually several
Genetics Lecture Notes 7.03 2005 Lectures 1 2 Lecture 1 We will begin this course with the question: What is a gene? This question will take us four lectures to answer because there are actually several
Delivering the power of the world s most successful genomics platform
 Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Information leaflet. Centrum voor Medische Genetica. Version 1/20150504 Design by Ben Caljon, UZ Brussel. Universitair Ziekenhuis Brussel
 Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
CHAPTER 15 THE CHROMOSOMAL BASIS OF INHERITANCE. Section B: Sex Chromosomes
 CHAPTER 15 THE CHROMOSOMAL BASIS OF INHERITANCE Section B: Sex Chromosomes 1. The chromosomal basis of sex varies with the organism 2. Sex-linked genes have unique patterns of inheritance 1. The chromosomal
CHAPTER 15 THE CHROMOSOMAL BASIS OF INHERITANCE Section B: Sex Chromosomes 1. The chromosomal basis of sex varies with the organism 2. Sex-linked genes have unique patterns of inheritance 1. The chromosomal
Genetic Mutations Cause Many Birth Defects:
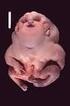 Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics
 G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS
 INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
INTRODUCTION TO THE UK CURRICULUM IN CLINICAL GENETICS Clinical Geneticists work in multidisciplinary regional genetic centres in the UK, in close collaboration with laboratory scientists, clinical co-workers
Just the Facts: A Basic Introduction to the Science Underlying NCBI Resources
 1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
This fact sheet describes how genes affect our health when they follow a well understood pattern of genetic inheritance known as autosomal recessive.
 11111 This fact sheet describes how genes affect our health when they follow a well understood pattern of genetic inheritance known as autosomal recessive. In summary Genes contain the instructions for
11111 This fact sheet describes how genes affect our health when they follow a well understood pattern of genetic inheritance known as autosomal recessive. In summary Genes contain the instructions for
Lecture 3: Mutations
 Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
REQUEST FOR IMAGe SYNDROME TESTING
 REQUEST FOR IMAGe SYNDROME TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
REQUEST FOR IMAGe SYNDROME TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives
 What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
What Is Genetic Counseling? Helping individuals and families understand how genetics affects their health and lives What does the career involve? Explore family histories to identify risks Reducing risks
Gene mutation and molecular medicine Chapter 15
 Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Gene mutation and molecular medicine Chapter 15 Lecture Objectives What Are Mutations? How Are DNA Molecules and Mutations Analyzed? How Do Defective Proteins Lead to Diseases? What DNA Changes Lead to
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs)
 Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program
 Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program Introduction: Cystic fibrosis (CF) is an inherited chronic disease that affects the lungs and
Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program Introduction: Cystic fibrosis (CF) is an inherited chronic disease that affects the lungs and
Preimplantation Genetic Diagnosis. Evaluation for single gene disorders
 Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Preimplantation Genetic Diagnosis Evaluation for single gene disorders What is Preimplantation Genetic Diagnosis? Preimplantation genetic diagnosis or PGD is a technology that allows genetic testing of
Human Genome Organization: An Update. Genome Organization: An Update
 Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Human Genome Organization: An Update Genome Organization: An Update Highlights of Human Genome Project Timetable Proposed in 1990 as 3 billion dollar joint venture between DOE and NIH with 15 year completion
Leading Genomics. Diagnostic. Discove. Collab. harma. Shanghai Cambridge, MA Reykjavik
 Leading Genomics Diagnostic harma Discove Collab Shanghai Cambridge, MA Reykjavik Global leadership for using the genome to create better medicine WuXi NextCODE provides a uniquely proven and integrated
Leading Genomics Diagnostic harma Discove Collab Shanghai Cambridge, MA Reykjavik Global leadership for using the genome to create better medicine WuXi NextCODE provides a uniquely proven and integrated
UNIT 13 (OPTION) Genetic Abnormalities
 Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Umm AL Qura University MUTATIONS. Dr Neda M Bogari
 Umm AL Qura University MUTATIONS Dr Neda M Bogari CONTACTS www.bogari.net http://web.me.com/bogari/bogari.net/ From DNA to Mutations MUTATION Definition: Permanent change in nucleotide sequence. It can
Umm AL Qura University MUTATIONS Dr Neda M Bogari CONTACTS www.bogari.net http://web.me.com/bogari/bogari.net/ From DNA to Mutations MUTATION Definition: Permanent change in nucleotide sequence. It can
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger
 The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
The Developing Person Through the Life Span 8e by Kathleen Stassen Berger Chapter 3 Heredity and Environment PowerPoint Slides developed by Martin Wolfger and Michael James Ivy Tech Community College-Bloomington
Becker Muscular Dystrophy
 Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Muscular Dystrophy A Case Study of Positional Cloning Described by Benjamin Duchenne (1868) X-linked recessive disease causing severe muscular degeneration. 100 % penetrance X d Y affected male Frequency
Patient Information. Ordering Physician Information. Indication for Testing (REQUIRED)
 EPILEPSY EXOME CLINICAL CHECKLIST REQUIRED Please check all clinical features that apply, and use the additional space provided at the bottom of the form if needed Patient Information Name: Last First
EPILEPSY EXOME CLINICAL CHECKLIST REQUIRED Please check all clinical features that apply, and use the additional space provided at the bottom of the form if needed Patient Information Name: Last First
Chromosomes, Mapping, and the Meiosis Inheritance Connection
 Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Genetic Testing for Duchenne and Becker Muscular Dystrophy
 Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
European registered Clinical Laboratory Geneticist (ErCLG) Core curriculum
 (February 2015; updated from paper issued by the European Society of Human Genetics Ad hoc committee for the accreditation of clinical laboratory geneticists, published in February 2012) Speciality Profile
(February 2015; updated from paper issued by the European Society of Human Genetics Ad hoc committee for the accreditation of clinical laboratory geneticists, published in February 2012) Speciality Profile
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Genetic diagnostics the gateway to personalized medicine
 Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
Genetics Review for USMLE (Part 2)
 Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Genetics Module B, Anchor 3
 Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
Genetics Module B, Anchor 3 Key Concepts: - An individual s characteristics are determines by factors that are passed from one parental generation to the next. - During gamete formation, the alleles for
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
The NeurOmics team at a recent project meeting
 Introduction Welcome to the NeurOmics project newsletter. This is the second edition and comes after the project has been underway for just over a year. This means that whilst we still have lots of work
Introduction Welcome to the NeurOmics project newsletter. This is the second edition and comes after the project has been underway for just over a year. This means that whilst we still have lots of work
A leader in the development and application of information technology to prevent and treat disease.
 A leader in the development and application of information technology to prevent and treat disease. About MOLECULAR HEALTH Molecular Health was founded in 2004 with the vision of changing healthcare. Today
A leader in the development and application of information technology to prevent and treat disease. About MOLECULAR HEALTH Molecular Health was founded in 2004 with the vision of changing healthcare. Today
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
Heredity. Sarah crosses a homozygous white flower and a homozygous purple flower. The cross results in all purple flowers.
 Heredity 1. Sarah is doing an experiment on pea plants. She is studying the color of the pea plants. Sarah has noticed that many pea plants have purple flowers and many have white flowers. Sarah crosses
Heredity 1. Sarah is doing an experiment on pea plants. She is studying the color of the pea plants. Sarah has noticed that many pea plants have purple flowers and many have white flowers. Sarah crosses
Handheld Radiofrequency Spectroscopy for Intraoperative Margin Assessment During Breast-Conserving Surgery
 Handheld Radiofrequency Spectroscopy for Intraoperative Margin Assessment During Breast-Conserving Surgery Assessment Program Volume 28, No. 4 August 2013 Executive Summary Background Breast-conserving
Handheld Radiofrequency Spectroscopy for Intraoperative Margin Assessment During Breast-Conserving Surgery Assessment Program Volume 28, No. 4 August 2013 Executive Summary Background Breast-conserving
Genetic Counseling and Testing: Cancer Genetics
 KAISER PERMANENTE HAWAII CLINICAL PRACTICE GUIDELINE Genetic Counseling and Testing: Cancer Genetics QUALITY COMMITTEE ADOPTION DATE: October 2015 LAST REVIEW DATE: September 2015 NEXT SCHEDULED REVIEW
KAISER PERMANENTE HAWAII CLINICAL PRACTICE GUIDELINE Genetic Counseling and Testing: Cancer Genetics QUALITY COMMITTEE ADOPTION DATE: October 2015 LAST REVIEW DATE: September 2015 NEXT SCHEDULED REVIEW
About The Causes of Hearing Loss
 About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
Delivering the Promise of Genetic and Genomic Medicine. From Research to Clinical Care:
 A m e r i c a n C o l l e g e o f M e d i c a l G e n e t i c s a n d G e n o m i c s Tr a n s l a t i n g G e n e s I n t o H e a l t h From Research to Clinical Care: Delivering the Promise of Genetic
A m e r i c a n C o l l e g e o f M e d i c a l G e n e t i c s a n d G e n o m i c s Tr a n s l a t i n g G e n e s I n t o H e a l t h From Research to Clinical Care: Delivering the Promise of Genetic
The following chapter is called "Preimplantation Genetic Diagnosis (PGD)".
 Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
Slide 1 Welcome to chapter 9. The following chapter is called "Preimplantation Genetic Diagnosis (PGD)". The author is Dr. Maria Lalioti. Slide 2 The learning objectives of this chapter are: To learn the
FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies
 FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies Raymond A. Huml, MS, DVM, RAC Executive Director, Head, Global Biosimilars Business Development and Strategic Planning, Quintiles
FSH Society s 2014 Biennial FSHD Connect Meeting: Natural History Studies Raymond A. Huml, MS, DVM, RAC Executive Director, Head, Global Biosimilars Business Development and Strategic Planning, Quintiles
1 Mutation and Genetic Change
 CHAPTER 14 1 Mutation and Genetic Change SECTION Genes in Action KEY IDEAS As you read this section, keep these questions in mind: What is the origin of genetic differences among organisms? What kinds
CHAPTER 14 1 Mutation and Genetic Change SECTION Genes in Action KEY IDEAS As you read this section, keep these questions in mind: What is the origin of genetic differences among organisms? What kinds
BioBoot Camp Genetics
 BioBoot Camp Genetics BIO.B.1.2.1 Describe how the process of DNA replication results in the transmission and/or conservation of genetic information DNA Replication is the process of DNA being copied before
BioBoot Camp Genetics BIO.B.1.2.1 Describe how the process of DNA replication results in the transmission and/or conservation of genetic information DNA Replication is the process of DNA being copied before
The National Institute of Genomic Medicine (INMEGEN) was
 Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency
 Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
Targeted. sequencing solutions. Accurate, scalable, fast TARGETED
 Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Targeted TARGETED Sequencing sequencing solutions Accurate, scalable, fast Sequencing for every lab, every budget, every application Ion Torrent semiconductor sequencing Ion Torrent technology has pioneered
Gene Mapping Techniques
 Gene Mapping Techniques OBJECTIVES By the end of this session the student should be able to: Define genetic linkage and recombinant frequency State how genetic distance may be estimated State how restriction
Gene Mapping Techniques OBJECTIVES By the end of this session the student should be able to: Define genetic linkage and recombinant frequency State how genetic distance may be estimated State how restriction
Common Cancers & Hereditary Syndromes
 Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
Common Cancers & Hereditary Syndromes Elizabeth Hoodfar, MS, LCGC Regional Cancer Genetics Coordinator Kaiser Permanente Northern California Detect clinical characteristics of hereditary cancer syndromes.
MUSCULAR DYSTROPHY GENETICS AND TESTING
 MUSCULAR DYSTROPHY GENETICS AND TESTING Information to help you make an informed choice about testing. MUSCULAR DYSTROPHY 111 BOUNDARY ROAD NORTH MELBOURNE 3051 AUSTRALIA +61 3 9320 9555 MDA.ORG.AU Genetic
MUSCULAR DYSTROPHY GENETICS AND TESTING Information to help you make an informed choice about testing. MUSCULAR DYSTROPHY 111 BOUNDARY ROAD NORTH MELBOURNE 3051 AUSTRALIA +61 3 9320 9555 MDA.ORG.AU Genetic
Fact Sheet 14 EPIGENETICS
 This fact sheet describes epigenetics which refers to factors that can influence the way our genes are expressed in the cells of our body. In summary Epigenetics is a phenomenon that affects the way cells
This fact sheet describes epigenetics which refers to factors that can influence the way our genes are expressed in the cells of our body. In summary Epigenetics is a phenomenon that affects the way cells
Chapter 4 Pedigree Analysis in Human Genetics. Chapter 4 Human Heredity by Michael Cummings 2006 Brooks/Cole-Thomson Learning
 Chapter 4 Pedigree Analysis in Human Genetics Mendelian Inheritance in Humans Pigmentation Gene and Albinism Fig. 3.14 Two Genes Fig. 3.15 The Inheritance of Human Traits Difficulties Long generation time
Chapter 4 Pedigree Analysis in Human Genetics Mendelian Inheritance in Humans Pigmentation Gene and Albinism Fig. 3.14 Two Genes Fig. 3.15 The Inheritance of Human Traits Difficulties Long generation time
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP
 GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
GENETIC CONSIDERATIONS IN CANCER TREATMENT AND SURVIVORSHIP WHO IS AT HIGH RISK OF HEREDITARY CANCER? Hereditary Cancer accounts for a small proportion of all cancer or approximately 5-10% THE DEVELOPMENT
Biological Sciences Initiative. Human Genome
 Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
Biological Sciences Initiative HHMI Human Genome Introduction In 2000, researchers from around the world published a draft sequence of the entire genome. 20 labs from 6 countries worked on the sequence.
Name: 4. A typical phenotypic ratio for a dihybrid cross is a) 9:1 b) 3:4 c) 9:3:3:1 d) 1:2:1:2:1 e) 6:3:3:6
 Name: Multiple-choice section Choose the answer which best completes each of the following statements or answers the following questions and so make your tutor happy! 1. Which of the following conclusions
Name: Multiple-choice section Choose the answer which best completes each of the following statements or answers the following questions and so make your tutor happy! 1. Which of the following conclusions
Executive summary. Current prenatal screening
 Executive summary Health Council of the Netherlands. NIPT: dynamics and ethics of prenatal screening. The Hague: Health Council of the Netherlands, 2013; publication no. 2013/34. In recent years, new tests
Executive summary Health Council of the Netherlands. NIPT: dynamics and ethics of prenatal screening. The Hague: Health Council of the Netherlands, 2013; publication no. 2013/34. In recent years, new tests
Bio EOC Topics for Cell Reproduction: Bio EOC Questions for Cell Reproduction:
 Bio EOC Topics for Cell Reproduction: Asexual vs. sexual reproduction Mitosis steps, diagrams, purpose o Interphase, Prophase, Metaphase, Anaphase, Telophase, Cytokinesis Meiosis steps, diagrams, purpose
Bio EOC Topics for Cell Reproduction: Asexual vs. sexual reproduction Mitosis steps, diagrams, purpose o Interphase, Prophase, Metaphase, Anaphase, Telophase, Cytokinesis Meiosis steps, diagrams, purpose
MUTATION, DNA REPAIR AND CANCER
 MUTATION, DNA REPAIR AND CANCER 1 Mutation A heritable change in the genetic material Essential to the continuity of life Source of variation for natural selection New mutations are more likely to be harmful
MUTATION, DNA REPAIR AND CANCER 1 Mutation A heritable change in the genetic material Essential to the continuity of life Source of variation for natural selection New mutations are more likely to be harmful
The correct answer is c A. Answer a is incorrect. The white-eye gene must be recessive since heterozygous females have red eyes.
 1. Why is the white-eye phenotype always observed in males carrying the white-eye allele? a. Because the trait is dominant b. Because the trait is recessive c. Because the allele is located on the X chromosome
1. Why is the white-eye phenotype always observed in males carrying the white-eye allele? a. Because the trait is dominant b. Because the trait is recessive c. Because the allele is located on the X chromosome
Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators
 Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators Introduction The Outcome Indicators are an adjunct to the Essential Nursing Competencies and Curricula
Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators Introduction The Outcome Indicators are an adjunct to the Essential Nursing Competencies and Curricula
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES. Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD
 LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
LEUKODYSTROPHY GENETICS AND REPRODUCTIVE OPTIONS FOR AFFECTED FAMILIES Leila Jamal, ScM Kennedy Krieger Institute, Baltimore MD 2 Outline Genetics 101: Basic Concepts and Myth Busting Inheritance Patterns
The RCGP Curriculum: Clinical Modules
 The RCGP Curriculum: Clinical Modules Version approved 18 May 2015 for implementation from 5 August 2015 3.02 Genetics in Primary Care Summary It has been estimated that at least one in ten of the patients
The RCGP Curriculum: Clinical Modules Version approved 18 May 2015 for implementation from 5 August 2015 3.02 Genetics in Primary Care Summary It has been estimated that at least one in ten of the patients
Preimplantation Genetic Diagnosis (PGD) in Western Australia
 Preimplantation Genetic Diagnosis (PGD) in Western Australia Human somatic cells have 46 chromosomes each, made up of the 23 chromosomes provided by the egg and the sperm cell from each parent. Each chromosome
Preimplantation Genetic Diagnosis (PGD) in Western Australia Human somatic cells have 46 chromosomes each, made up of the 23 chromosomes provided by the egg and the sperm cell from each parent. Each chromosome
Genetics 1. Defective enzyme that does not make melanin. Very pale skin and hair color (albino)
 Genetics 1 We all know that children tend to resemble their parents. Parents and their children tend to have similar appearance because children inherit genes from their parents and these genes influence
Genetics 1 We all know that children tend to resemble their parents. Parents and their children tend to have similar appearance because children inherit genes from their parents and these genes influence
BI122 Introduction to Human Genetics, Fall 2014
 BI122 Introduction to Human Genetics, Fall 2014 Course Overview We will explore 1) the genetic and molecular basis of heredity and inherited traits, 2) how genetics & genomics reveals an understanding
BI122 Introduction to Human Genetics, Fall 2014 Course Overview We will explore 1) the genetic and molecular basis of heredity and inherited traits, 2) how genetics & genomics reveals an understanding
Forensic DNA Testing Terminology
 Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR
 TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR Written by Danika Bannasch DVM PhD; Professor Department of Population Health and Reproduction, School of Veterinary Medicine, University of California-Davis
TWO NEW DNA BASED TESTS AVAILABLE FOR THE NSDTR Written by Danika Bannasch DVM PhD; Professor Department of Population Health and Reproduction, School of Veterinary Medicine, University of California-Davis
Rigid spine syndrome (RSS) (Congenital muscular dystrophy with rigidity of the spine, including RSMD1)
 Rigid spine syndrome (RSS) (Congenital muscular dystrophy with rigidity of the spine, including RSMD1) What is RSMD1? The congenital muscular dystrophies are a group of conditions which share early presentation
Rigid spine syndrome (RSS) (Congenital muscular dystrophy with rigidity of the spine, including RSMD1) What is RSMD1? The congenital muscular dystrophies are a group of conditions which share early presentation
Gene Therapy and Genetic Counseling. Chapter 20
 Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
Gene Therapy and Genetic Counseling Chapter 20 What is Gene Therapy? Treating a disease by replacing, manipulating or supplementing a gene The act of changing an individual s DNA sequence to fix a non-functional
PRACTICE PROBLEMS - PEDIGREES AND PROBABILITIES
 PRACTICE PROBLEMS - PEDIGREES AND PROBABILITIES 1. Margaret has just learned that she has adult polycystic kidney disease. Her mother also has the disease, as did her maternal grandfather and his younger
PRACTICE PROBLEMS - PEDIGREES AND PROBABILITIES 1. Margaret has just learned that she has adult polycystic kidney disease. Her mother also has the disease, as did her maternal grandfather and his younger
2011 One Voice Advocacy Summit Agenda February 13 15 th, 2011 Washington, DC ADVOCACY TRAINING
 ADVOCACY TRAINING Sunday, February 13 12:00 PM 1:00 PM Registration for Advocacy Conference/Duchenne Summit Washington Marriott (Nearest Metro Station: Dupont Circle Red Line; Foggy Bottom - Orange and
ADVOCACY TRAINING Sunday, February 13 12:00 PM 1:00 PM Registration for Advocacy Conference/Duchenne Summit Washington Marriott (Nearest Metro Station: Dupont Circle Red Line; Foggy Bottom - Orange and
Genetic Technology. Name: Class: Date: Multiple Choice Identify the choice that best completes the statement or answers the question.
 Name: Class: Date: Genetic Technology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. An application of using DNA technology to help environmental scientists
Name: Class: Date: Genetic Technology Multiple Choice Identify the choice that best completes the statement or answers the question. 1. An application of using DNA technology to help environmental scientists
Hereditary Breast Cancer Panels. High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel
 P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
P A T I E N T G U I D E Hereditary Breast Cancer Panels High Risk Hereditary Breast Cancer Panel Hereditary Breast/Ovarian/Endometrial Cancer Panel B a y l o r M i r a c a G e n e t i c s L a b o r a t
Genomic Medicine Education Initiatives of the College of American Pathologists
 Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Genomic Medicine Education Initiatives of the College of American Pathologists Debra G.B. Leonard, MD, PhD, FCAP Chair, Personalized Healthcare Committee, CAP Professor of Pathology, Weill Cornell Medical
Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation
 IG O Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation KD Carsten Bergmann carsten.bergmann@bioscientia.de carsten.bergmann@uniklinik-freiburg.de Controversies Conference on ADPKD
IG O Preimplantation Genetic Diagnosis (PGD) and Childhood Diagnostic Evaluation KD Carsten Bergmann carsten.bergmann@bioscientia.de carsten.bergmann@uniklinik-freiburg.de Controversies Conference on ADPKD
The Human Genome Project
 The Human Genome Project Brief History of the Human Genome Project Physical Chromosome Maps Genetic (or Linkage) Maps DNA Markers Sequencing and Annotating Genomic DNA What Have We learned from the HGP?
The Human Genome Project Brief History of the Human Genome Project Physical Chromosome Maps Genetic (or Linkage) Maps DNA Markers Sequencing and Annotating Genomic DNA What Have We learned from the HGP?
Muscular Dystrophy. By. Tina Strauss
 Muscular Dystrophy By. Tina Strauss Story Outline for Presentation on Muscular Dystrophy What is Muscular Dystrophy? Signs & Symptoms Types When to seek medical attention? Screening and Diagnosis Treatment
Muscular Dystrophy By. Tina Strauss Story Outline for Presentation on Muscular Dystrophy What is Muscular Dystrophy? Signs & Symptoms Types When to seek medical attention? Screening and Diagnosis Treatment
MEDICAL GENETICS GENERAL OBJECTIVE SPECIFIC OBJECTIVES
 SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
SUBJECT MEDICAL GENETICS CREDITS Total: 4.5 Theory 2.5 Practical 2 GENERAL OBJECTIVE To provide students with terminology and knowledge from the field of human genetics that will enable them to understand
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Genetic Testing: Scientific Background for Policymakers
 Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
ITT Advanced Medical Technologies - A Programmer's Overview
 ITT Advanced Medical Technologies (Ileri Tip Teknolojileri) ITT Advanced Medical Technologies (Ileri Tip Teknolojileri) is a biotechnology company (SME) established in Turkey. Its activity area is research,
ITT Advanced Medical Technologies (Ileri Tip Teknolojileri) ITT Advanced Medical Technologies (Ileri Tip Teknolojileri) is a biotechnology company (SME) established in Turkey. Its activity area is research,
BRCA in Men. Mary B. Daly,M.D.,Ph.D. June 25, 2010
 BRCA in Men Mary B. Daly,M.D.,Ph.D. June 25, 2010 BRCA in Men Inheritance patterns of BRCA1/2 Cancer Risks for men with BRCA1/2 mutations Risk management recommendations for men with BRCA1/2 mutations
BRCA in Men Mary B. Daly,M.D.,Ph.D. June 25, 2010 BRCA in Men Inheritance patterns of BRCA1/2 Cancer Risks for men with BRCA1/2 mutations Risk management recommendations for men with BRCA1/2 mutations
CHROMOSOMES AND INHERITANCE
 SECTION 12-1 REVIEW CHROMOSOMES AND INHERITANCE VOCABULARY REVIEW Distinguish between the terms in each of the following pairs of terms. 1. sex chromosome, autosome 2. germ-cell mutation, somatic-cell
SECTION 12-1 REVIEW CHROMOSOMES AND INHERITANCE VOCABULARY REVIEW Distinguish between the terms in each of the following pairs of terms. 1. sex chromosome, autosome 2. germ-cell mutation, somatic-cell
Genetic Mutations. Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes.
 Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Insurance. Chapter 7. Introduction
 65 Chapter 7 Insurance Introduction 7.1 The subject of genetic screening in relation to insurance is not new. In 1935 R A Fisher addressed the International Congress of Life Assurance Medicine on the topic,
65 Chapter 7 Insurance Introduction 7.1 The subject of genetic screening in relation to insurance is not new. In 1935 R A Fisher addressed the International Congress of Life Assurance Medicine on the topic,
Sequencing and microarrays for genome analysis: complementary rather than competing?
 Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Sequencing and microarrays for genome analysis: complementary rather than competing? Simon Hughes, Richard Capper, Sandra Lam and Nicole Sparkes Introduction The human genome is comprised of more than
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Integration of Genetic and Familial Data into. Electronic Medical Records and Healthcare Processes
 Integration of Genetic and Familial Data into Electronic Medical Records and Healthcare Processes By Thomas Kmiecik and Dale Sanders February 2, 2009 Introduction Although our health is certainly impacted
Integration of Genetic and Familial Data into Electronic Medical Records and Healthcare Processes By Thomas Kmiecik and Dale Sanders February 2, 2009 Introduction Although our health is certainly impacted
The iq&a interactive Medical Intelligence Zone BRCA Gene Testing
 The iq&a interactive Medical Intelligence Zone BRCA Gene Testing for Breast Cancer Focus on Guideline-Directed BRCA-1 and BRCA-2 Diagnostic Testing and Evidence-Based Management of Breast Cancer A Year
The iq&a interactive Medical Intelligence Zone BRCA Gene Testing for Breast Cancer Focus on Guideline-Directed BRCA-1 and BRCA-2 Diagnostic Testing and Evidence-Based Management of Breast Cancer A Year
Medicare Coverage of Genomic Testing
 Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Mendelian and Non-Mendelian Heredity Grade Ten
 Ohio Standards Connection: Life Sciences Benchmark C Explain the genetic mechanisms and molecular basis of inheritance. Indicator 6 Explain that a unit of hereditary information is called a gene, and genes
Ohio Standards Connection: Life Sciences Benchmark C Explain the genetic mechanisms and molecular basis of inheritance. Indicator 6 Explain that a unit of hereditary information is called a gene, and genes
