OraSure Technologies, Inc. Jefferies 2015 Global Healthcare Conference
|
|
|
- Tiffany Wiggins
- 7 years ago
- Views:
Transcription
1 OraSure Technologies, Inc. Jefferies 2015 Global Healthcare Conference
2 Forward-Looking Statements These slides and the associated presentation contain certain forward-looking statements, including statements with respect to revenues, earnings, technology, new products, product performance, markets, clinical development, regulatory filings and approvals, and business plans. Factors affecting these statements include, but are not limited to, the ability to develop new technology, technology changes, ability to fund research and development, required regulatory approvals, product performance and market acceptance of products. Please see the Company s SEC filings, including its registration statements, and the Company s most recent Form 10-K and Form 10-Q, for a more detailed description of specific factors that may cause actual results or events to differ materially from those described in the forwardlooking statements. The Company undertakes not duty to update these statements. 2
3 Investment Summary Industry leader in rapid point-of-care infectious disease testing and DNA/RNA sample collection, stabilization and preparation products Multiple growth opportunities: Market leading rapid HCV test further bolstered by copromotion agreement with AbbVie Highly regarded molecular diagnostic sample collection devices Portfolio of approved products addressing attractive global markets Strong balance sheet with ~ $90M in cash, no debt Expected profitability for full year
4 OraQuick HCV Addressing a Significant Unmet Need 170 million people infected globally, 4-5 million people infected in U.S. The majority of HCV infection remains undiagnosed Newly approved drugs, and those in the pipeline, are expected to drive demand for increased diagnoses and increase the number of patients initiating therapy Availability of a rapid, non-instrumented POC test will increase opportunities for diagnosis through increased testing outside of laboratory settings 4
5 Most Americans Living with HCV Were Born During Baby-boomers account for approximately 2.7 M ( m) HCV+ adults Prevalence five fold higher than others (3.29% vs. 0.55%) ¹ ² 44% did not report an HCV risk ¹ Smith, et al. American Association for the Study of Liver Disease Liver Meeting, San Francisco, CA Armstrong, et al. Ann Int Med
6 OraQuick HCV Rapid HCV Antibody Test The first and only FDA-approved, CLIA-waived rapid HCV test HHS issued Hepatitis Action Plan in May 2011, updated in 2014 CDC issued new screening guidelines to test all baby-boomers (born ), 80 million Americans Testing all baby-boomers is estimated to be a $1B revenue opportunity in the U.S. for HCV diagnostic testing USPSTF and CMS recommend reimbursement for baby-boomer screening 6
7 AbbVie Co-Promotion Agreement AbbVie and OraSure are co-promoting the OraQuick HCV Rapid Test in the U.S. OraSure has granted AbbVie exclusive promotional rights in certain markets. AbbVie will pay OraSure up to $75.0 million through contract period expiring in 2019 OraSure captures all revenues from the sale of OraQuick HCV Rapid Tests in all markets OraSure can earn $3.5 million to $55.5 million per annum upon achievement of certain performance based metrics starting in
8 DNA Genotek Product Overview DNA Genotek s powerful platforms have been leveraged to create multiple product line extensions Human Genetics Infectious Disease Genetics Animal & Livestock Genetics Oragene Dx collection device is the first and only saliva DNA collection and stabilization device to receive 510(k) clearance. 8
9 DNA Genotek Oral Sample Collection Anytime, anywhere sample collection Easy, reliable and non-invasive Ambient temperature stability Captures high quality DNA and RNA Scalable 9
10 2014 Sales Mix Commercial/Academic mix ~ 61%/39% Commercial revenues are predominately in North America 10
11 Oragene Helping Drive Results 11
12 DNA Genotek R&D Pipeline Stabilization and sample prep reagents to improve recoverability of Tuberculosis DNA for Molecular Diagnostic testing Microbiome stabilization reagents and transport devices Stabilization and preservation reagent systems for blood fraction storage at ambient temperatures 12
13 Ebola Opportunity High level of interest in fast tracking development of a rapid Ebola antigen test Active discussions with government agencies on accelerated deployment path Significant progress made in establishing feasibility of rapid test on OraQuick platform Focus on both blood and oral fluid Working to secure funding of project Concurrent evaluation of sustainable business proposition 13
14 OraQuick Advance Rapid HIV-1/2 Antibody Test OraQuick In-Home HIV Test The first FDA-approved, CLIA-waived oral fluid rapid HIV-1/2 test. Highly accurate test results in 20 minutes. Market leader in Rapid HIV testing in U.S. Professional market test used by diverse customers, e.g., hospitals, public health facilities, physician offices, jails, medical clinics. Approved and marketed in over 50 countries. Over 30 million sold worldwide. First and only FDA approved at-home HIV test for sale directly to consumers in the overthe-counter (OTC) market. OTC Distribution Strategy broadly available at retail and online: Implementing new promotional strategy expected to improve returns on invested capital. Recently received CE mark for HIV OTC test in Europe. 14
15 Summary Approved products address substantial opportunities in point-of-care testing and molecular diagnostics New margin expansion drivers Diversified revenue stream Strong capital structure to support growth Expected profitability for the full year
Overview of CLIA-Waived Rapid HIV Tests and the HIV Rapid
 Overview of CLIA-Waived Rapid HIV Tests and the HIV Rapid Testing Algorithm (RTA) Project Jacqueline Rurangirwa, MPH Office of AIDS Programs and Policy Planning and Research Division HIV Prevention Planning
Overview of CLIA-Waived Rapid HIV Tests and the HIV Rapid Testing Algorithm (RTA) Project Jacqueline Rurangirwa, MPH Office of AIDS Programs and Policy Planning and Research Division HIV Prevention Planning
Abbott Diagnostics. Durable Growth Business
 Abbott Diagnostics Durable Growth Business Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation Reform Act
Abbott Diagnostics Durable Growth Business Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation Reform Act
Diagnosis of HIV-1 Infection. Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University
 Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
Diagnosis of HIV-1 Infection Estelle Piwowar-Manning HPTN Central Laboratory The Johns Hopkins University Tests Used to Diagnose HIV-1 Infection HIV antibody (today s topic) HIV p24 antigen HIV DNA HIV
BUILDING A BRIGHTER FUTURE... FOR A BETTER TOMORROW. OraSure Technologies, Inc. 2007 ANNUAL REPORT
 BUILDING A BRIGHTER FUTURE... FOR A BETTER TOMORROW OraSure Technologies, Inc. 2007 ANNUAL REPORT To Our Stockholders I am extremely pleased to report that 2007 was an excellent year for our Company on
BUILDING A BRIGHTER FUTURE... FOR A BETTER TOMORROW OraSure Technologies, Inc. 2007 ANNUAL REPORT To Our Stockholders I am extremely pleased to report that 2007 was an excellent year for our Company on
Coding and Billing. Commonly Asked Questions. Physician Office Reimbursement Guideline Q1. A1. Q2. A2.
 Coding and Billing oorasure Technologies is pleased to provide you information on billing and reimbursement for HCV testing with the OraQuick HCV Rapid Antibody Test. Correctly identifying services delivered
Coding and Billing oorasure Technologies is pleased to provide you information on billing and reimbursement for HCV testing with the OraQuick HCV Rapid Antibody Test. Correctly identifying services delivered
Abbott to Acquire Alere, Becoming Leader in Point of Care Testing and Significantly Advancing Global Diagnostics Presence
 News Release Abbott to Acquire Alere, Becoming Leader in Point of Care Testing and Significantly Advancing Global Diagnostics Presence ABBOTT GAINS LEADERSHIP IN THE $5.5 BILLION POINT OF CARE SEGMENT,
News Release Abbott to Acquire Alere, Becoming Leader in Point of Care Testing and Significantly Advancing Global Diagnostics Presence ABBOTT GAINS LEADERSHIP IN THE $5.5 BILLION POINT OF CARE SEGMENT,
3/25/2014. April 3, 2014. Dennison MM, et al. Ann Intern Med. 2014;160:293 300.
 April 3, 2014 3.6 million persons ever infected; 2.7 million chronic infections 1 Up to 75% unaware of status Transmitted through percutaneous exposure to infected blood Injection drug use (IDU) is the
April 3, 2014 3.6 million persons ever infected; 2.7 million chronic infections 1 Up to 75% unaware of status Transmitted through percutaneous exposure to infected blood Injection drug use (IDU) is the
Testing the 1945-1965 Birth Cohort for HCV at BIDMC/ CareGroup
 Testing the 1945-1965 Birth Cohort for HCV at BIDMC/ CareGroup Camilla S. Graham, MD, MPH Division of Infectious Diseases Beth Israel Deaconess Medical Center Boston, MA, USA BIDMC/CareGroup Experience
Testing the 1945-1965 Birth Cohort for HCV at BIDMC/ CareGroup Camilla S. Graham, MD, MPH Division of Infectious Diseases Beth Israel Deaconess Medical Center Boston, MA, USA BIDMC/CareGroup Experience
Coding and Billing for HIV Services in Healthcare Facilities
 P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
P a g e 1 Coding and Billing for HIV Services in Healthcare Facilities The Hawai i State Department of Health STD/AIDS Prevention Branch is pleased to provide you information on billing and reimbursement
Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019. February 2015
 Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019 February 2015 INTELAB CORPORATION MARKET AND TECHNOLOGY REPORTS Report 530 Copyright 2015 Worldwide Markets and Emerging
Worldwide Markets and Emerging Technologies for Point-of-Care Testing, 2013-2019 February 2015 INTELAB CORPORATION MARKET AND TECHNOLOGY REPORTS Report 530 Copyright 2015 Worldwide Markets and Emerging
HIV/AIDS: General Information & Testing in the Emergency Department
 What Is HIV? HIV/AIDS: General Information & Testing in the Emergency Department HIV is the common name for the Human Immunodeficiency Virus. HIV is a retrovirus. This means it can enter the body s own
What Is HIV? HIV/AIDS: General Information & Testing in the Emergency Department HIV is the common name for the Human Immunodeficiency Virus. HIV is a retrovirus. This means it can enter the body s own
Craig Hallum Conference Investor Presentation
 Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
Using Substance Abuse Prevention and Treatment (SAPT) Block Grant HIV Set- Aside Funds for Integrated Services
 Using Substance Abuse Prevention and Treatment (SAPT) Block Grant HIV Set- Aside Funds for Integrated Services ADP Training Conference, Sacramento, August 21, 2012 Rachel McLean, MPH, CA Dept. of Public
Using Substance Abuse Prevention and Treatment (SAPT) Block Grant HIV Set- Aside Funds for Integrated Services ADP Training Conference, Sacramento, August 21, 2012 Rachel McLean, MPH, CA Dept. of Public
A Ministry of the Archdiocese of Galveston-Houston A United Way Agency
 A Ministry of the Archdiocese of Galveston-Houston A United Way Agency Integrated Multidsciplinary Approach to Adapt Routine HIV Screening in a Safety Net Clinic Setting Sherri D. Onyiego MD, PhD Baylor
A Ministry of the Archdiocese of Galveston-Houston A United Way Agency Integrated Multidsciplinary Approach to Adapt Routine HIV Screening in a Safety Net Clinic Setting Sherri D. Onyiego MD, PhD Baylor
Background Information
 Background Information r One About NAT Testing NAT testing has been widely embraced by laboratories throughout the world as a means to identify CT cases because of its high degree of accuracy and reliability
Background Information r One About NAT Testing NAT testing has been widely embraced by laboratories throughout the world as a means to identify CT cases because of its high degree of accuracy and reliability
Corporate Overview. April 2014 OTCQB: LCDX
 Corporate Overview April 2014 OTCQB: LCDX Company Snapshot biopsy Provides non-invasive, point-of-care cellular imaging technologies VivaScope systems can improve patient outcomes while reducing costs
Corporate Overview April 2014 OTCQB: LCDX Company Snapshot biopsy Provides non-invasive, point-of-care cellular imaging technologies VivaScope systems can improve patient outcomes while reducing costs
Types of HIV test. Antigen - Any substance (such as an immunogen or a hapten) foreign to the body that stimulates an immune system response.
 Types of HIV test There are two basic categories of HIV test 4 th generation and 3 rd generation. Each individual test differs as to what it tests (whether antibodies and/or p24 antigens), how it tests
Types of HIV test There are two basic categories of HIV test 4 th generation and 3 rd generation. Each individual test differs as to what it tests (whether antibodies and/or p24 antigens), how it tests
Case Finding for Hepatitis B and Hepatitis C
 Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Case Finding for Hepatitis B and Hepatitis C John W. Ward, M.D. Division of Viral Hepatitis Centers for Disease Control and Prevention Atlanta, Georgia, USA Division of Viral Hepatitis National Center
Strategies to Improve the HCV Continuum of Care:
 Strategies to Improve the HCV Continuum of Care: Best Practices in Testing, Linkage to Care & Treatment Ronald O. Valdiserri, M.D., M.P.H. Deputy Assistant Secretary for Health, Infectious Diseases June
Strategies to Improve the HCV Continuum of Care: Best Practices in Testing, Linkage to Care & Treatment Ronald O. Valdiserri, M.D., M.P.H. Deputy Assistant Secretary for Health, Infectious Diseases June
Notes. Complete childhood vaccination course (CCV) CCV and DTP booster as adolescent/adult within last 10 years
 Student Immunisation Record School of Nursing, Midwifery and Social Work Section 1: Information for students enrolled in Nursing and Midwifery programs Students enrolled in programs offered by our School
Student Immunisation Record School of Nursing, Midwifery and Social Work Section 1: Information for students enrolled in Nursing and Midwifery programs Students enrolled in programs offered by our School
Clinical Laboratory Improvement Amendments (CLIA) How to Obtain a CLIA Certificate of Waiver
 Clinical Laboratory Improvement Amendments (CLIA) How to Obtain a CLIA Certificate of Waiver When is a CLIA Certificate of Waiver Required? NOTE: Congress passed the Clinical Laboratory Improvement Amendments
Clinical Laboratory Improvement Amendments (CLIA) How to Obtain a CLIA Certificate of Waiver When is a CLIA Certificate of Waiver Required? NOTE: Congress passed the Clinical Laboratory Improvement Amendments
Coding guide for routine HIV testing in health care settings
 Coding guide f routine HIV testing in health care settings Background In September of 2006, CDC issued recommendations f Human immunodeficiency virus (HIV) testing in health care settings. The Revised
Coding guide f routine HIV testing in health care settings Background In September of 2006, CDC issued recommendations f Human immunodeficiency virus (HIV) testing in health care settings. The Revised
Preface. TTY: (888) 232-6348 or cdcinfo@cdc.gov. Hepatitis C Counseling and Testing, contact: 800-CDC-INFO (800-232-4636)
 Preface The purpose of this CDC Hepatitis C Counseling and Testing manual is to provide guidance for hepatitis C counseling and testing of individuals born during 1945 1965. The guide was used in draft
Preface The purpose of this CDC Hepatitis C Counseling and Testing manual is to provide guidance for hepatitis C counseling and testing of individuals born during 1945 1965. The guide was used in draft
Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988
 Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988 U.S. Department of Health and Human Services Centers for Disease
Quality Assurance Guidelines for Testing Using Rapid HIV Antibody Tests Waived Under the Clinical Laboratory Improvement Amendments of 1988 U.S. Department of Health and Human Services Centers for Disease
A Cost Effective Way to De Risk Biomarker Clinical Trials: Early Development Considerations
 A Cost Effective Way to De Risk Biomarker Clinical Trials: Early Development Considerations Ce3, Inc. and Insight Genetics, Inc. Oncology Forum July 15, 2015 Agenda Introductions Definitions Regulations
A Cost Effective Way to De Risk Biomarker Clinical Trials: Early Development Considerations Ce3, Inc. and Insight Genetics, Inc. Oncology Forum July 15, 2015 Agenda Introductions Definitions Regulations
In this study, the DCV+ASV regimen had low rates of discontinuation (5%) due to adverse events, and low rates of serious adverse events (5.
 BMS Submits First All-Oral, Interferon-Free and Ribavirin-Free Treatment Regimen for Regulatory Review in Japan for Patients with Chronic Hepatitis C Infection An overall SVR 24 rate of 84.7 percent was
BMS Submits First All-Oral, Interferon-Free and Ribavirin-Free Treatment Regimen for Regulatory Review in Japan for Patients with Chronic Hepatitis C Infection An overall SVR 24 rate of 84.7 percent was
Hepatitis C. Eliot Godofsky, MD University Hepatitis Center Bradenton, FL
 Hepatitis C Eliot Godofsky, MD University Hepatitis Center Bradenton, FL Recent Advances in Hepatitis C Appreciation that many patients are undiagnosed Improved screening to identify infected persons Assessment
Hepatitis C Eliot Godofsky, MD University Hepatitis Center Bradenton, FL Recent Advances in Hepatitis C Appreciation that many patients are undiagnosed Improved screening to identify infected persons Assessment
Hepatitis C Infections in Oregon September 2014
 Public Health Division Hepatitis C Infections in Oregon September 214 Chronic HCV in Oregon Since 25, when positive laboratory results for HCV infection became reportable in Oregon, 47,252 persons with
Public Health Division Hepatitis C Infections in Oregon September 214 Chronic HCV in Oregon Since 25, when positive laboratory results for HCV infection became reportable in Oregon, 47,252 persons with
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
Managing Bloodborne Pathogens Exposures
 Managing Bloodborne Pathogens Exposures House Staff Orientation 2015 Phillip F. Bressoud, MD, FACP Associate Professor of Medicine and Executive Director Campus Health Services University of Louisville
Managing Bloodborne Pathogens Exposures House Staff Orientation 2015 Phillip F. Bressoud, MD, FACP Associate Professor of Medicine and Executive Director Campus Health Services University of Louisville
Offering Testing for Hepatitis B and C in Primary Care
 Offering Testing for Hepatitis B and C in Primary Care Presentation 3 January 2014 Quality Education for a Healthier Scotland 1 Learning Outcomes Participants will be able to:- Undertake a pre-test discussion
Offering Testing for Hepatitis B and C in Primary Care Presentation 3 January 2014 Quality Education for a Healthier Scotland 1 Learning Outcomes Participants will be able to:- Undertake a pre-test discussion
AMAG to Acquire Cord Blood Registry
 to Acquire Cord Blood Registry June 29, Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 o 617.498.3300 A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE
to Acquire Cord Blood Registry June 29, Pharmaceuticals, Inc. 1100 Winter Street Waltham, MA 02451 o 617.498.3300 A SPECIALTY PHARMACEUTICAL COMPANY DEDICATED TO BRINGING TO MARKET THERAPIES THAT IMPROVE
Overview of the BCBSRI Prescription Management Program
 Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm
 Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm November 2013 Introduction In March 2010, the Centers for Disease Control and Prevention (CDC) and the Association of Public
Suggested Reporting Language for the HIV Laboratory Diagnostic Testing Algorithm November 2013 Introduction In March 2010, the Centers for Disease Control and Prevention (CDC) and the Association of Public
National Health Burden of CLD in Italy
 National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
Testing Oral Presence of
 Issues in Brief: Oral Fluid Testing for HIV Antibodies February 2013 Testing Oral Fluid for the Presence of HIV Antibodies 2013 Status Report HIV testing is primarily done using blood-based specimens (fingerstick
Issues in Brief: Oral Fluid Testing for HIV Antibodies February 2013 Testing Oral Fluid for the Presence of HIV Antibodies 2013 Status Report HIV testing is primarily done using blood-based specimens (fingerstick
THE VIRAL HEPATITIS CONGRESS 2015. 10 12 September 2015, Kap Europa, Frankfurt, Germany THE VIRAL HEPATITIS CONGRESS. Scientific Programme
 THE VIRAL HEPATITIS CONGRESS THE VIRAL HEPATITIS CONGRESS 2015 10 12 September 2015, Kap Europa, Frankfurt, Germany Scientific Programme PRE-CONGRESS WORKSHOP SPONSOR ACHILLION Achillion is seeking to
THE VIRAL HEPATITIS CONGRESS THE VIRAL HEPATITIS CONGRESS 2015 10 12 September 2015, Kap Europa, Frankfurt, Germany Scientific Programme PRE-CONGRESS WORKSHOP SPONSOR ACHILLION Achillion is seeking to
COMMUNICABLE DISEASE
 Public Health Activities & Services Inventory Technical Notes COMMUNICABLE DISEASE CLINICAL SERVICES, SURVEILLANCE AND CONTROL In 2014, decision was made to adopt number of national public health activities
Public Health Activities & Services Inventory Technical Notes COMMUNICABLE DISEASE CLINICAL SERVICES, SURVEILLANCE AND CONTROL In 2014, decision was made to adopt number of national public health activities
Great Basin Reports 2015 Second Quarter Results and Business Update
 Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update
 August 10, 2015 Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update DUBLIN, IRELAND -- (Marketwired) -- 08/10/15 -- Theravance Biopharma, Inc. (NASDAQ:
August 10, 2015 Theravance Biopharma, Inc. Reports Second Quarter 2015 Financial Results and Provides Business Update DUBLIN, IRELAND -- (Marketwired) -- 08/10/15 -- Theravance Biopharma, Inc. (NASDAQ:
BG MEDICINE. Corporate Presentation February 2013
 BG MEDICINE Corporate Presentation February 2013 CONFIDENTIAL Oct 9, 2007 Forward-Looking Statements This presentation contains forward-looking statements regarding future events or the future financial
BG MEDICINE Corporate Presentation February 2013 CONFIDENTIAL Oct 9, 2007 Forward-Looking Statements This presentation contains forward-looking statements regarding future events or the future financial
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K. GEOVAX LABS, INC. (Exact name of registrant as specified in its charter)
 SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event reported):
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event reported):
Hepatitis C. Screening, Diagnosis and Linkage to Care
 Hepatitis C Screening, Diagnosis and Linkage to Care Diagnosis If your hepatitis C antibody test is reactive, a second test will be needed to diagnose and determine if you are currently infected. Screening
Hepatitis C Screening, Diagnosis and Linkage to Care Diagnosis If your hepatitis C antibody test is reactive, a second test will be needed to diagnose and determine if you are currently infected. Screening
UNIVERSITY OF KENTUCKY HEALTH CARE COLLEGES POLICY ON EDUCATIONAL EXPOSURE TO BLOOD BORNE PATHOGENS
 I. Purpose and Definition UNIVERSITY OF KENTUCKY HEALTH CARE COLLEGES POLICY ON EDUCATIONAL EXPOSURE TO BLOOD BORNE PATHOGENS The purpose of this policy is to delineate the management of incidents of exposure
I. Purpose and Definition UNIVERSITY OF KENTUCKY HEALTH CARE COLLEGES POLICY ON EDUCATIONAL EXPOSURE TO BLOOD BORNE PATHOGENS The purpose of this policy is to delineate the management of incidents of exposure
Viral Safety of Plasma-Derived Products
 Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
FEDERAL BUREAU OF PRISONS REPORT ON INFECTIOUS DISEASE MANAGEMENT
 FEDERAL BUREAU OF PRISONS REPORT ON INFECTIOUS DISEASE MANAGEMENT What is the purpose of this report? The purpose of this report is to present the administrative policies and clinical guidelines for the
FEDERAL BUREAU OF PRISONS REPORT ON INFECTIOUS DISEASE MANAGEMENT What is the purpose of this report? The purpose of this report is to present the administrative policies and clinical guidelines for the
Changing Concept of FMD diagnostics: from Central to Local. Aniket Sanyal Project Directorate on FMD Mukteswar, India
 Changing Concept of FMD diagnostics: from Central to Local Aniket Sanyal Project Directorate on FMD Mukteswar, India OBJECTIVES OF DIAGNOSIS IN THE FIELD/LOCAL 1. To arrive at quick diagnosis 2. To implement
Changing Concept of FMD diagnostics: from Central to Local Aniket Sanyal Project Directorate on FMD Mukteswar, India OBJECTIVES OF DIAGNOSIS IN THE FIELD/LOCAL 1. To arrive at quick diagnosis 2. To implement
Guidelines for Viral Hepatitis CTR Services
 Guidelines for Viral Hepatitis CTR Services During the 2007 North Dakota Legislative Assembly, legislation that called for the creation of a viral hepatitis program was introduced and approved. The North
Guidelines for Viral Hepatitis CTR Services During the 2007 North Dakota Legislative Assembly, legislation that called for the creation of a viral hepatitis program was introduced and approved. The North
in hiv diagnostics the role of phls
 Issues in Brief: HIV Diagnostics UPDATE Association of Public Health Laboratories August 2011 Conference calls Focus on New trends in hiv diagnostics the role of phls In February 2011, the Association
Issues in Brief: HIV Diagnostics UPDATE Association of Public Health Laboratories August 2011 Conference calls Focus on New trends in hiv diagnostics the role of phls In February 2011, the Association
Prospects for Vaccines against Hepatitis C Viruses. T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS
 Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Prospects for Vaccines against Hepatitis C Viruses T. Jake Liang. M.D. Liver Diseases Branch NIDDK, NIH, HHS HCV Vaccine Prevention strategies Protective immunity Barriers and solutions Vaccine candidates
Introduction. Background
 INFORMATION DRIVES SOUND ANALYSIS, INSIGHT PHARMACY BENEFIT ADVISORY Introduction According to the Centers for Disease Control and Prevention (CDC), the rate of new hepatitis C virus (HCV) infections in
INFORMATION DRIVES SOUND ANALYSIS, INSIGHT PHARMACY BENEFIT ADVISORY Introduction According to the Centers for Disease Control and Prevention (CDC), the rate of new hepatitis C virus (HCV) infections in
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice
 Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
U.S. Clinical Laboratory and Pathology Testing 2013-2015:
 U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
Hepatitis C in Colorado 2007 Surveillance Report Cases of Acute and Chronic Hepatitis C in Colorado
 Hepatitis C in Colorado 2007 Surveillance Report Cases of Acute and Chronic Hepatitis C in Colorado Note: This report is published by the Viral Hepatitis Program (VHP), Disease Control and Environmental
Hepatitis C in Colorado 2007 Surveillance Report Cases of Acute and Chronic Hepatitis C in Colorado Note: This report is published by the Viral Hepatitis Program (VHP), Disease Control and Environmental
HBV DNA < monitoring interferon Rx
 Hepatitis B Virus Suspected acute hepatitis >>Order: Acute Unknown hepatitis screen Suspected chronic hepatitis >>Order: Chronic unknown hepatitis screen Acute HBV or Delayed Anti HBs response after acute
Hepatitis B Virus Suspected acute hepatitis >>Order: Acute Unknown hepatitis screen Suspected chronic hepatitis >>Order: Chronic unknown hepatitis screen Acute HBV or Delayed Anti HBs response after acute
New All Oral Therapy for Chronic Hepatitis C Virus (HCV): A Cost-Benefit Analysis
 New All Oral Therapy for Chronic Hepatitis C Virus (HCV): A Cost-Benefit Analysis Jennifer Orsi, MPH 4 th International Conference on Viral Hepatitis March 17, 2014 2014 Walgreen Co. All rights reserved.
New All Oral Therapy for Chronic Hepatitis C Virus (HCV): A Cost-Benefit Analysis Jennifer Orsi, MPH 4 th International Conference on Viral Hepatitis March 17, 2014 2014 Walgreen Co. All rights reserved.
Blood, Plasma, and Cellular Blood Components INTRODUCTION
 Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
(SAMPLE COPY, NOT FOR RESALE)
 TriMark Publications May 2016 Volume: TMRMDIDT16-0501 MOLECULAR DIAGNOSTICS IN INFECTIOUS DISEASE TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
TriMark Publications May 2016 Volume: TMRMDIDT16-0501 MOLECULAR DIAGNOSTICS IN INFECTIOUS DISEASE TESTING (SAMPLE COPY, NOT FOR RESALE) Trends, Industry Participants, Product Overviews and Market Drivers
CAREERS IN BIOMEDICAL SCIENCE & THE IBMS. Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire
 CAREERS IN BIOMEDICAL SCIENCE & THE IBMS Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire What is a biomedical scientist? Biomedical scientists carry out investigations
CAREERS IN BIOMEDICAL SCIENCE & THE IBMS Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire What is a biomedical scientist? Biomedical scientists carry out investigations
Expanding Access through Pharmacy-Based Point-of-Care Testing
 Expanding Access through Pharmacy-Based Point-of-Care Testing Donald G. Klepser, PhD Associate Professor, Department of Pharmacy Practice University of Nebraska Medical Center Doug Read, Pharm.D. Director,
Expanding Access through Pharmacy-Based Point-of-Care Testing Donald G. Klepser, PhD Associate Professor, Department of Pharmacy Practice University of Nebraska Medical Center Doug Read, Pharm.D. Director,
Product: Point-of-care immunoassay system yielding rapid labquality quantitative blood test results with unprecedented ease-ofuse.
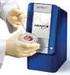 Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
Tuberculosis (TB) Screening Guidelines for Substance Use Disorder Treatment Programs in California
 Tuberculosis (TB) Screening Guidelines for Substance Use Disorder Treatment Programs in California 1 of 7 Table of Contents Preface 2 TB Symptoms and TB History 2 Initial Screening 2 Follow-Up Screening
Tuberculosis (TB) Screening Guidelines for Substance Use Disorder Treatment Programs in California 1 of 7 Table of Contents Preface 2 TB Symptoms and TB History 2 Initial Screening 2 Follow-Up Screening
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
Viral Hepatitis. 2009 APHL survey report
 Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
The Evolution of a Point of Care Company From R & D to Commercial Launch
 The Evolution of a Point of Care Company From R & D to Commercial Launch Karen Hedine, President Micronics, Inc. : Background Founded 1996 Technology platform : laminar flow diffusionbased microfluidics
The Evolution of a Point of Care Company From R & D to Commercial Launch Karen Hedine, President Micronics, Inc. : Background Founded 1996 Technology platform : laminar flow diffusionbased microfluidics
Overview of the BCBSRI Prescription Management Program
 Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
Definitions Overview of the BCBSRI Prescription Management Program DISPENSING GUIDELINES mean: the prescription order or refill must be limited to the quantities authorized by your doctor not to exceed
Why Disruptive Innovations Matter in Laboratory Diagnostics
 Article: S. Nam.. Clin Chem 2015;61:935-937. http://www.clinchem.org/content/61/7/935.extract Guest: Spencer Nam is a Research Fellow specializing in healthcare at the Clayton Christensen Institute for
Article: S. Nam.. Clin Chem 2015;61:935-937. http://www.clinchem.org/content/61/7/935.extract Guest: Spencer Nam is a Research Fellow specializing in healthcare at the Clayton Christensen Institute for
Credit Suisse Healthcare Conference
 Credit Suisse Healthcare Conference November 14, 2013 Safe Harbor Statement 2 Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: To the extent any statements made in this
Credit Suisse Healthcare Conference November 14, 2013 Safe Harbor Statement 2 Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: To the extent any statements made in this
Understanding the HIV Care Continuum
 Understanding the HIV Care Continuum Overview Recent scientific advances have shown that antiretroviral therapy (ART) not only preserves the health of people living with HIV, but also dramatically lowers
Understanding the HIV Care Continuum Overview Recent scientific advances have shown that antiretroviral therapy (ART) not only preserves the health of people living with HIV, but also dramatically lowers
Patient Education CONTENTS. Introduction... 12.2
 CONTENTS Introduction... 12.2 Purpose... 12.2 General Guidelines... 12.3 Language and Comprehension Barriers... 12.4 Education Topics... 12.5 Medical Diagnosis... 12.5 Contact Investigation... 12.6 Isolation...
CONTENTS Introduction... 12.2 Purpose... 12.2 General Guidelines... 12.3 Language and Comprehension Barriers... 12.4 Education Topics... 12.5 Medical Diagnosis... 12.5 Contact Investigation... 12.6 Isolation...
A Leader in Medication Management and Patient Safety. March 17, 2015
 A Leader in Medication Management and Patient Safety March 17, 2015 Disclaimers This slide presentation contains certain estimates and other forward-looking statements (as defined under Federal securities
A Leader in Medication Management and Patient Safety March 17, 2015 Disclaimers This slide presentation contains certain estimates and other forward-looking statements (as defined under Federal securities
CDC TB Testing Guidelines and Recent Literature Update
 Pocket Guide QuantiFERON -TB Gold CDC TB Testing Guidelines and Recent Literature Update Using IGRAs for TB screening in your patients June 2010 A full copy of the US Centers for Disease Control and Prevention
Pocket Guide QuantiFERON -TB Gold CDC TB Testing Guidelines and Recent Literature Update Using IGRAs for TB screening in your patients June 2010 A full copy of the US Centers for Disease Control and Prevention
AFFITECH and XOMA Sign Antibody Collaboration and Cross-License Agreement
 FOR IMMEDIATE RELEASE Ref 05AFF05 Contacts for Affitech: Contacts for XOMA Affitech (Norway): Investor Inquiries Dr. Martin Welschof Ellen M Martin Chief Executive Officer Kureczka/Martin Associates Phone:
FOR IMMEDIATE RELEASE Ref 05AFF05 Contacts for Affitech: Contacts for XOMA Affitech (Norway): Investor Inquiries Dr. Martin Welschof Ellen M Martin Chief Executive Officer Kureczka/Martin Associates Phone:
Ebola: Teaching Points for Nurse Educators
 Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
The Prize Fund for HIV/AIDS
 The Prize Fund for HIV/AIDS A New Paradigm for Supporting Sustainable Innovation and Access to New Drugs for AIDS: De-Linking Markets for Products from Markets for Innovation May 26, 2011 Introduction
The Prize Fund for HIV/AIDS A New Paradigm for Supporting Sustainable Innovation and Access to New Drugs for AIDS: De-Linking Markets for Products from Markets for Innovation May 26, 2011 Introduction
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND
 EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
Targeted HIV Testing & Enhanced Testing Technologies. HIV Prevention Section Bureau of HIV/AIDS
 Targeted HIV Testing & Enhanced Testing Technologies HIV Prevention Section Bureau of HIV/AIDS May 2012 1 Typing a Question in the Chat Box Type question in here 2 Completing the Webinar Evaluation (opened
Targeted HIV Testing & Enhanced Testing Technologies HIV Prevention Section Bureau of HIV/AIDS May 2012 1 Typing a Question in the Chat Box Type question in here 2 Completing the Webinar Evaluation (opened
Tuberculosis OUR MISSION THE OPPORTUNITY
 Tuberculosis OUR MISSION Guided by the belief that every life has equal value, the Bill & Melinda Gates Foundation works to help all people lead healthy, productive lives. Our Global Health Program is
Tuberculosis OUR MISSION Guided by the belief that every life has equal value, the Bill & Melinda Gates Foundation works to help all people lead healthy, productive lives. Our Global Health Program is
STEP-BY-STEP INSTRUCTIONS FOR INVESTIGATIONAL USE. Rapid HCV Antibody Test FOR ORAQUICK RAPID HCV ANTIBODY TEST
 Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965
 Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965 MMWR August 17, 2012 Prepared by : The National Viral Hepatitis Technical Assistance Center
Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965 MMWR August 17, 2012 Prepared by : The National Viral Hepatitis Technical Assistance Center
Developing Innovative Therapeutics for People with Orphan Liver Disease
 Developing Innovative Therapeutics for People with Orphan Liver Disease PIPELINE PROGRESS AND FIRST QUARTER 2015 EARNINGS UPDATE NASDAQ: OCRX Forward-Looking Statements Certain statements in this presentation
Developing Innovative Therapeutics for People with Orphan Liver Disease PIPELINE PROGRESS AND FIRST QUARTER 2015 EARNINGS UPDATE NASDAQ: OCRX Forward-Looking Statements Certain statements in this presentation
MedMira Inc. Management s Discussion & Analysis For the three month period ended October 31, 2011
 MedMira Inc. Management s Discussion & Analysis For the three month period ended Introduction The following Management s Discussion & Analysis (MD&A) for the three months ended has been prepared to help
MedMira Inc. Management s Discussion & Analysis For the three month period ended Introduction The following Management s Discussion & Analysis (MD&A) for the three months ended has been prepared to help
Dania Palanker Senior Health Policy Advisor National Women s Law Center December 5, 2012
 National Family Planning and Reproductive Health Association ACA Implementation: Health Insurance Exchanges Dania Palanker Senior Health Policy Advisor National Women s Law Center December 5, 2012 Why
National Family Planning and Reproductive Health Association ACA Implementation: Health Insurance Exchanges Dania Palanker Senior Health Policy Advisor National Women s Law Center December 5, 2012 Why
Guidelines for TB Blood Testing. Minnesota Department of Health TB Prevention and Control Program June 2011
 Guidelines for TB Blood Testing Minnesota Department of Health TB Prevention and Control Program June 2011 Outline Interferon-Gamma Release Assays aka TB blood tests 1. What are they? 2. What are the current
Guidelines for TB Blood Testing Minnesota Department of Health TB Prevention and Control Program June 2011 Outline Interferon-Gamma Release Assays aka TB blood tests 1. What are they? 2. What are the current
Chapter 8 Community Tuberculosis Control
 Chapter 8 Community Tuberculosis Control Table of Contents Chapter Objectives.... 227 Introduction.... 229 Roles and Responsibilities of the Public Health Sector Providers.... 229 Roles and Responsibilities
Chapter 8 Community Tuberculosis Control Table of Contents Chapter Objectives.... 227 Introduction.... 229 Roles and Responsibilities of the Public Health Sector Providers.... 229 Roles and Responsibilities
STANLEY BLACK & DECKER. Don Allan Senior Vice President & CFO Raymond James 36th Annual Institutional Investors Conference Monday, March 2, 2015
 STANLEY BLACK & DECKER Don Allan Senior Vice President & CFO Monday, March 2, 2015 Cautionary Statements This presentation contains forward-looking statements, that is, statements that address future,
STANLEY BLACK & DECKER Don Allan Senior Vice President & CFO Monday, March 2, 2015 Cautionary Statements This presentation contains forward-looking statements, that is, statements that address future,
PIPC: Hepatitis Roundtable Summary and Recommendations on Dissemination and Implementation of Clinical Evidence
 PIPC: Hepatitis Roundtable Summary and Recommendations on Dissemination and Implementation of Clinical Evidence On May 8, 2014, the Partnership to Improve Patient Care (PIPC) convened a Roundtable of experts
PIPC: Hepatitis Roundtable Summary and Recommendations on Dissemination and Implementation of Clinical Evidence On May 8, 2014, the Partnership to Improve Patient Care (PIPC) convened a Roundtable of experts
PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL
 Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Pennsylvania PREPARED BY THE CENTER FOR HEALTH LAW AND POLICY INNOVATION OF HARVARD LAW SCHOOL Pennsylvania Hepatitis C Virus (HCV) in Pennsylvania Prevalence + + Although state-wide data were unavailable,
Acquisition of. Special Investor Presentation
 Acquisition of Special Investor Presentation October 21, 2015 Forward-looking Statements This presentation contains statements which constitute forward-looking statements within the meaning of Section
Acquisition of Special Investor Presentation October 21, 2015 Forward-looking Statements This presentation contains statements which constitute forward-looking statements within the meaning of Section
HIV/AIDS Care: The Diagnosis Code Series 2. Prepared By: Stacey L. Murphy, MPA, RHIA, CPC AHIMA Approved ICD-10-CM/ICD-10-CM Trainer
 HIV/AIDS Care: The Diagnosis Code Series 2 Prepared By: Stacey L. Murphy, MPA, RHIA, CPC AHIMA Approved ICD-10-CM/ICD-10-CM Trainer Learning Outcomes Identify and explain the difference between ICD-9-CM
HIV/AIDS Care: The Diagnosis Code Series 2 Prepared By: Stacey L. Murphy, MPA, RHIA, CPC AHIMA Approved ICD-10-CM/ICD-10-CM Trainer Learning Outcomes Identify and explain the difference between ICD-9-CM
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy
 New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
Comprehensive Medical Waste and Unused Medication Management Solutions Provider NASDAQ: SMED
 Comprehensive Medical Waste and Unused Medication Management Solutions Provider NASDAQ: SMED INVESTOR PRESENTATION NOVEMBER Safe Harbor These slides contain (and the accompanying oral discussion will contain)
Comprehensive Medical Waste and Unused Medication Management Solutions Provider NASDAQ: SMED INVESTOR PRESENTATION NOVEMBER Safe Harbor These slides contain (and the accompanying oral discussion will contain)
Press release Regulated information
 IBA TRADING UPDATE - THIRD QUARTER 2014 Louvain-La-Neuve, Belgium, 13 November 2014 - IBA (Ion Beam Applications S.A., EURONEXT), the world s leading provider of proton therapy solutions for the treatment
IBA TRADING UPDATE - THIRD QUARTER 2014 Louvain-La-Neuve, Belgium, 13 November 2014 - IBA (Ion Beam Applications S.A., EURONEXT), the world s leading provider of proton therapy solutions for the treatment
A LEADING GLOBAL HEALTH CARE GROUP. Frankfurt Stock Exchange (DAX30): FRE US ADR program (OTC): FSNUY wwww.fresenius.com/investors
 A LEADING GLOBAL HEALTH CARE GROUP Frankfurt Stock Exchange (DAX30): FRE US ADR program (OTC): FSNUY wwww.fresenius.com/investors SAFE HARBOR STATEMENT This presentation contains forward-looking statements
A LEADING GLOBAL HEALTH CARE GROUP Frankfurt Stock Exchange (DAX30): FRE US ADR program (OTC): FSNUY wwww.fresenius.com/investors SAFE HARBOR STATEMENT This presentation contains forward-looking statements
How Does a Doctor Test for AIDS?
 Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Urinalysis Compliance Tools. POCC Webinar January 19, 2011 Dr. Susan Selgren
 Urinalysis Compliance Tools POCC Webinar January 19, 2011 Dr. Susan Selgren Learning Objectives Be able to review and improve upon a laboratory plan for compliance including: Competency Documentation Proficiency
Urinalysis Compliance Tools POCC Webinar January 19, 2011 Dr. Susan Selgren Learning Objectives Be able to review and improve upon a laboratory plan for compliance including: Competency Documentation Proficiency
Quarterly cash flow and activities report 30 June 2015
 Quarterly cash flow and activities report 30 June 2015 Genetic Signatures (ASX: GSS) is pleased to report on its activities for the quarter ended 30 June 2015. Highlights: Continued growth in sales of
Quarterly cash flow and activities report 30 June 2015 Genetic Signatures (ASX: GSS) is pleased to report on its activities for the quarter ended 30 June 2015. Highlights: Continued growth in sales of
J.P. Morgan Aviation, Transportation & Industrials Conference
 A Diversified Technology Company J.P. Morgan Aviation, Transportation & Industrials Conference March 3, 2015 Safe Harbor Statement The information provided in this presentation contains forward-looking
A Diversified Technology Company J.P. Morgan Aviation, Transportation & Industrials Conference March 3, 2015 Safe Harbor Statement The information provided in this presentation contains forward-looking
Health Alert Communications Communication in Life Sciences
 Sorry, the document you sought has been removed. However, the attached pages may be of some interest. Otherwise, for more information, please visit our web site or the links to Amazon.com provided below:
Sorry, the document you sought has been removed. However, the attached pages may be of some interest. Otherwise, for more information, please visit our web site or the links to Amazon.com provided below:
Implementing HCV Screening into Federally Qualified Health Centers. Donna Brian, PhD, CRNP Catelyn, Coyle MEd
 Implementing HCV Screening into Federally Qualified Health Centers Donna Brian, PhD, CRNP Catelyn, Coyle MEd Background Public Health Management Corporation (PHMC): Public health Institute located in Philadelphia
Implementing HCV Screening into Federally Qualified Health Centers Donna Brian, PhD, CRNP Catelyn, Coyle MEd Background Public Health Management Corporation (PHMC): Public health Institute located in Philadelphia
