Methylprednisolone aceponate 0.1% ointment/cream. 1 g cream or ointment contains 1 mg (0.1%) methylprednisolone aceponate.
|
|
|
- Gerard Scott
- 7 years ago
- Views:
Transcription
1 ADVANTAN Methylprednisolone aceponate 0.1% ointment/cream Presentation 1 g cream or ointment contains 1 mg (0.1%) methylprednisolone aceponate. Uses Actions After topical application, ADVANTAN suppresses inflammatory and allergic skin reactions as well as reactions associated with hyperproliferation, leading to regression of the objective symptoms (erythema, edema, infiltration, lichenification) and the subjective complaints (itching, burning, pain). On application of methylprednisolone aceponate in topically effective dosage, the systemic effect is minimal in both man and animals. After large-area treatment of patients with skin disorders, the plasma cortisol values remain within the normal range, circadian cortisol rhythm is maintained and no reduction of cortisol has been ascertained in 24-hour urine. As for all other glucocorticoids, so far the mechanism of action of methylprednisolone aceponate is not completely understood. It is known that methylprednisolone aceponate itself binds to the intracellular glucocorticoid receptor and this is especially true of the principal metabolite 6 -methyl - prednisolone -17-propionate, which is formed after cleavage in the skin. The steroid receptor complex binds to certain regions of DNA, thereby triggering a series of biological effects. The understanding of the mechanism of the anti-inflammatory action is more precise. Binding of the steroid receptor complex results in induction of macrocortin synthesis. Macrocortin inhibits the release of arachidonic acid and thus the formation of inflammation mediators such as prostaglandins and leukotrienes. The immunosuppressive action of glucocorticoids can be explained by inhibition of cytokine synthesis and an antimitotic effect, which so far is not well understood. Inhibition of the synthesis of vasodilating prostaglandins or potentiation of the vasoconstrictive effect of epinephrine finally results in the vasoconstrictive activity of glucocorticoids. The respective bases are of major importance to the therapeutic effect of the ADVANTAN formulations. 1
2 ADVANTAN cream As a low-fat formulation with a high water content, ADVANTAN cream is particularly suitable for acute and subacute weeping stages of eczema, for very greasy skin and for use on exposed or hairy parts of the body. ADVANTAN ointment Skin conditions which are neither weeping nor very dry require a base with balanced proportions of fat and water. ADVANTAN ointment is suitable for dry, fissured, scaly or hyperkeratinised skin areas. It should not be used in areas such as axilla, groin or skin folds. ADVANTAN ointment makes the skin slightly greasy without retaining warmth and fluid. Of the three formulations, ADVANTAN ointment has the widest field of use. Pharmacokinetics Methylprednisolone aceponate (MPA) becomes available in the skin from all formulations (cream, ointment). The concentration in the stratum corneum and living skin decreases from outside to inside. Methylprednisolone aceponate is hydrolyzed in the epidermis and dermis to the main metabolite 6 -methylprednisolone-17-propionate which binds more firmly to the corticoid receptor an indication of "bioactivation" in the skin. The degree of percutaneous absorption depends on the state of the skin, the formulation and the conditions of application (open/occlusion). Studies in juvenile and adult patients with neurodermatitis and psoriasis have shown that the percutaneous absorption on open application was only slightly ( 2.5%) greater than the percutaneous absorption in volunteers with normal skin ( %). When the horny layer is removed before the application, the corticoid levels in the skin are about three times higher than after application to intact skin. After reaching the systemic circulation, the primary hydrolysis product of MPA, 6 methylprednisolone-17-propionate, is quickly conjugated with glucuronic acid and inactivated as a result. The metabolites of MPA (main metabolite: 6 -methylprednisolone-17- propionate-21- glucuronide) are eliminated primarily via the kidneys with a half-life of about 16 hours. Following i.v. administration, excretion of the 14 C- labeled substances with the urine and feces was complete within 7 days. No accumulation of substance or metabolites takes place in the body. Indications Atopic dermatitis (endogenous eczema, neurodermatitis), contact eczema, degenerative, dyshidrotic, vulgar eczema, eczema in children. 2
3 Dosage and Administration ADVANTAN is for EXTERNAL TOPICAL USE ONLY and NOT FOR OPTHALMIC USE. In general, the ADVANTAN formulation appropriate to the skin condition is applied thinly once per day to the diseased areas of skin. In general, the duration of use should not exceed 12 weeks in adults and 4 weeks in children. Contraindications ADVANTAN is contraindicated in most viral diseases (eg vaccinia, varicella/herpes zoster) and when tuberculous or syphilitic processes and post-vaccination skin reactions are present in the area to be treated. If rosacea, acne vulgaris or perioral dermatitis are present, ADVANTAN must not be applied to the face. Hypersensitivity to the active substance or to any of its excipients. Warnings and Precautions ADVANTAN should not be allowed to come into contact with the eyes when being applied to the face. Additional specific therapy is required in bacterially infected skin diseases and/or in fungal infections. Any spread of infection may require withdrawal of topical corticosteroid therapy. If the skin dries out excessively under protracted use of ADVANTAN cream, a switch should be made to ADVANTAN ointment, a formulation which has a higher fat content. If signs of hypersensitivity develop ADVANTAN should be discontinued and appropriate treatment instituted. Any of the side effects that have been reported following systemic use of corticosteroids, including adrenal suppression, may also occur with topical corticosteroids, especially in infants and children. ADVANTAN is a potent steroid formulation, as with other corticosteroids of this type the possibility of hypothalmic-pituitary-adrenal (HPA) axis suppression resulting from percutaneous absorption of methylprednisolone must be considered when initiating or reviewing therapy. However, to date, no impairment of adrenocortical function has been observed when used on large areas (40-60 % of the skin surface) or even occlusive treatment with ADVANTAN for up to 12 weeks in adults or 4 weeks in children. 3
4 Nevertheless, for the treatment of large areas duration of use should be kept as brief as possible. Systemic absorption of topical corticosteroids will be increased if extensive body surface areas are treated or if the occlusive technique is used. Suitable precautions should be taken under these conditions or when long-term use is anticipated, particularly in infants and children. Pediatric patients may demonstrate greater susceptibility to topical corticosteroid- induced HPA axis suppression and Cushing s syndrome than adults because of a larger skin surface area to bodyweight ratio. Use of topical corticosteriods in children should be limited to the least amount required for therapeutic effect. Chronic corticosteroid therapy may interfere with the growth and development of children. Local atrophy, telangiectasia and striae may occur after prolonged treatment or excessive application. Treatment should be discontinued if symptoms such as cutaneous atrophy occur. Prolonged use on flexures and in intertriginous areas is undesirable. ADVANTAN cream or ointment should not be used around the eyes. The use of topical corticosteroids on the face can exacerbate rosacea and lead to peri-orofacial dermatitis. Patients should be warned against using ADVANTAN on the face except on medical advice and any use on the face should be restricted to short periods. As known from systemic corticoids, glaucoma may also develop from using local corticoids (e.g. after large-dosed or extensive application over a prolonged period, occlusive dressing techniques, or application to the skin around the eyes). Preclinical safety data In systemic tolerance studies following repeated subcutaneous and dermal administration MPA showed the action profile of a typical glucocorticoid. It can be concluded from these results that following therapeutic use of ADVANTAN no side-effects other than those typical of glucocorticoids are to be expected even under extreme conditions such as application over a large surface area and/or occlusion. Specific tumorigenicity studies using MPA have not been carried out. Knowledge concerning the structure, the pharmacological effect mechanism and the results from systemic tolerance studies with long-term administration do not indicate any increase in the risk of tumor occurrence. As systemically effective immunosuppressive exposure is not reached with dermal application of ADVANTAN under the recommended conditions of use, no influence on the occurrence of tumors is to be expected. Neither in vitro investigations for detection of gene mutations on bacteria and mammalian cells nor in vitro and in vivo investigations for detection of chromosome and gene mutations gave any indication of a genotoxic potential of MPA. 4
5 Animal studies with MPA have shown embryolethal defects in rats dosed subcutaneously during the period of organogenesis at doses greater than 1mg/kg/day and in rabbits following dermal application at doses greater than 0.25mg/kg/day. No teratogenic effects were observed in rabbits, but in rats the incidences of ventricular septal defects and of cleft palate were increased at subcutaneous doses greater than 1 and 10 mg/kg/day. Similar embryolethal and teratogenic effects have been found with other corticosteroids and while not considered relevant to humans, particular care should be taken when prescribing ADVANTAN during pregnancy. In investigations into the local tolerance of MPA and ADVANTAN formulations on the skin and the mucosa, no findings other than the topical side-effects known for glucocorticoids were recorded. MPA showed no sensitizing potential on the skin of the guinea-pig. Effects on the ability to drive and use machines There is no effect. Pregnancy and lactation Use in Pregnancy Animal experimental studies with glucocorticoidsteroids have shown reproductive toxicity (refer to the Warnings and Precautions section of this leaflet). A number of epidemiological studies suggest that there could be increased risk of oral clefts among new-borns of women who were treated with systemic glucocorticosteroids during the first trimester of pregnancy. Oral clefts are a rare disorder and if systemic glucocorticosteroids are teratogenic, these may account for an increase of only one or two cases per 1000 women treated while pregnant. Data concerning topical glucocorticoidsteroid use during pregnancy are insufficient, however, a lower risk might be expected since systemic availability of topically applied glucocorticosteroids is very low. Reduced placental and birth weight have been recorded in animals and humans after long-term treatment with topical corticosteroids. Since the possibility of suppression of the adrenal cortex in the newborn baby after longterm treatment must be considered, the needs of the mother must be carefully weighed against the risk to the foetus when prescribing these drugs. Maternal pulmonary oedema has been reported, with tocolysis and fluid overload. As a general rule, topical preparations containing corticoids should not be applied during the first trimester of pregnancy. The clinical indication for treatment with ADVANTAN must be carefully reviewed and the benefits weighed against the risks in pregnant and lactating women. In particular, treatment of large areas or prolonged use (greater than 4 weeks) must be avoided. 5
6 Use in Lactation Nursing mothers should not be treated on the breasts. Adverse Effects Local concomitant symptoms such as itching, burning, erythema or vesiculation may occur in isolated cases under treatment with ADVANTAN. In clinical studies 5.4% of patients treated with ADVANTAN cream, and 4.7% of patients treated with ADVANTAN ointment experience such symptoms. Withdrawals associated with adverse events occurred in less than 1.5 % patients. The following reactions may occur when topical preparations containing corticoids are applied to large areas of the body (about 10% and more) or for prolonged periods of time (more than 4 weeks), particularly when an occlusive dressing is used: local symptoms such as atrophy of the skin, telangiectasia, striae, acneform changes of the skin and systemic effects of the corticoid due to absorption. During the clinical investigation, none of these side effects occurred under ADVANTAN on treatment up to 12 weeks (adults) and 4 weeks (children). As with other corticoids for topical application, the following side effects may occur in rare cases: Folliculitis, hypertrichosis, perioral dermatitis, skin discolouration, allergic skin reactions to any of the ingredients of the formulations. Interactions None so far known. Overdosage Symptoms of intoxication Results from acute toxicity studies do not indicate that any risk of acute intoxication is to be expected following a single dermal application of an overdose (application over a large area under conditions favorable to absorption) or inadvertent oral ingestion. Pharmaceutical Precautions 6
7 ADVANTAN cream/ointment Shelf life: 3 years Special precautions for storage: Store below 25 C Medicine Classification Prescription Medicine Package Quantities Tubes containing 15g. Tubes made of pure aluminum, interior wall coated with epoxy resin, and with a polyester-based external coating, fold seal ring is made of polyamide-based heat sealable material. The screw cap is made of high density polyethylene. Further Information List of excipients Cream: decyl oleate, glycerol monostearate %, cetostearyl alcohol, hard fat, caprylic-capric-stearic acid triglyceride (Softisan 378), polyoxyl-40- stearate, glycerol 85 %, disodium edetatem, benzyl alcohol, butyl hydroxytoluene, purified water. Ointment: beeswax (white), paraffin, liquid, Dehymuls E, paraffin (white soft), purified water Nature and contents of the container Ointment: Tubes of pure aluminium, interior wall coated with epoxy resin, and with a polyester-based external coating, fold seal ring is made of polyamide based heat sealable material. The screw cap is made of high density polyethylene. Instructions for use/handling Store all drugs properly and keep them out of reach of children. Name and Address Sponsor CSL Biotherapies (NZ) Limited 666 Great South Road 7
8 Penrose, Auckland 6, NEW ZEALAND Telephone: Date of Preparation 12 February
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
NERIDERM Cream, Ointment, Fatty Ointment
 PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
PRODUCT INFORMATION OINTMENT, CREAM & LOTION
 PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT
 NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis
 For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
PRODUCT MONOGRAPH. HydroVal. (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid
 PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
PRODUCT INFORMATION. Silver sulfadiazine is a sulfonamide and has broad antimicrobial activity against both Grampositive and Gram-negative organisms.
 PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
TOPICAL THERAPY. ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity
 TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET BUTACORT Budesonide (50 µg & 100 µg per actuation) Aqueous Nasal Suspension Presentation Budesonide aqueous spray 50µg and 100µg per metered actuation. 50 µg/actuation: An almost
NEW ZEALAND DATA SHEET BUTACORT Budesonide (50 µg & 100 µg per actuation) Aqueous Nasal Suspension Presentation Budesonide aqueous spray 50µg and 100µg per metered actuation. 50 µg/actuation: An almost
Hydrozole Cream Hydrocortisone (microfine) 1% w/w and Clotrimazole 1% w/w
 CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
DATA SHEET. Fluticasone propionate Inhaler (CFC-Free) (50, 125 or 250 micrograms per actuation). Qualitative and quantitative composition
 DATA SHEET FLIXOTIDE Inhaler Fluticasone propionate Inhaler (CFC-Free) (50, 125 or 250 micrograms per actuation). Qualitative and quantitative composition FLIXOTIDE 50 Inhaler is a pressurised metered-dose
DATA SHEET FLIXOTIDE Inhaler Fluticasone propionate Inhaler (CFC-Free) (50, 125 or 250 micrograms per actuation). Qualitative and quantitative composition FLIXOTIDE 50 Inhaler is a pressurised metered-dose
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON AVENA SATIVA L., FRUCTUS
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
PARACETAMOL REXIDOL. 600 mg Tablet. Analgesic-Antipyretic. Paracetamol 600 mg
 (Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
(Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Treatment of diseases affecting the kidney using steroids
 Treatment of diseases affecting the kidney using steroids Hope Building Renal 0161 206 5223 All Rights Reserved 2015. Document for issue as handout. This information has been written by the medical and
Treatment of diseases affecting the kidney using steroids Hope Building Renal 0161 206 5223 All Rights Reserved 2015. Document for issue as handout. This information has been written by the medical and
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
FLAMAZINE CREAM 1.0% w/w
 PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665
 Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Professor Andrew Wright,
 Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
VOLTAREN OPHTHA EYE DROPS
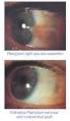 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
4.1 Objectives of Clinical Trial Assessment
 L1 4.1 Objectives of Clinical Trial Assessment Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office of Clinical
L1 4.1 Objectives of Clinical Trial Assessment Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office of Clinical
CONTRAINDICATIONS. None (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178
 Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
PRODUCT INFORMATION FLIXOTIDE JUNIOR INHALER, (CFC-FREE) INHALER, JUNIOR (CFC-FREE) INHALER, ACCUHALER AND JUNIOR ACCUHALER
 PRODUCT INFORMATION FLIXOTIDE JUNIOR INHALER, (CFC-FREE) INHALER, JUNIOR (CFC-FREE) INHALER, ACCUHALER AND JUNIOR ACCUHALER AUSTRALIAN APPROVED NAME: Fluticasone propionate STRUCTURE: CHEMICAL NAME: S-Fluoromethyl
PRODUCT INFORMATION FLIXOTIDE JUNIOR INHALER, (CFC-FREE) INHALER, JUNIOR (CFC-FREE) INHALER, ACCUHALER AND JUNIOR ACCUHALER AUSTRALIAN APPROVED NAME: Fluticasone propionate STRUCTURE: CHEMICAL NAME: S-Fluoromethyl
Testosterone propionate, phenylpropionate, isocaproate and decanoate. Please read this leaflet carefully before you start using SUSTANON 250.
 SUSTANON 250 Testosterone propionate, phenylpropionate, isocaproate and decanoate What is in this leaflet Please read this leaflet carefully before you start using SUSTANON 250. This leaflet answers some
SUSTANON 250 Testosterone propionate, phenylpropionate, isocaproate and decanoate What is in this leaflet Please read this leaflet carefully before you start using SUSTANON 250. This leaflet answers some
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol
 [GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
[GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
SYNACTHEN i.m./i.v. tetracosactide hexaacetate
 New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN
 FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
Guidance for Industry
 Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
What Alcohol Does to the Body. Chapter 25 Lesson 2
 What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
4 Clinical Particulars
 SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS
 Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: info.uk@dechra.com
Dechra Veterinary Products Limited (A business unit of Dechra Pharmaceuticals PLC) Sansaw Business Park Hadnall, Shrewsbury Shropshire SY4 4AS Tel: 01939 211200 Fax: 01939 211201 Email: info.uk@dechra.com
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/35/17235501.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection
 SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given SYNACTHEN
SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given SYNACTHEN
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (WELL-ESTABLISHED MEDICINAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
VOLTAREN OPHTHA EYE DROPS
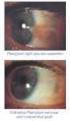 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER. 500 mg, film-coated tablet Active substance: metformin hydrochloride
 P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Diazepam Tablets BP 2mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Diazepam BP 2.00mg 3 PHARMACEUTICAL FORM Tablet 4. CLINICAL PARTICULARS
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Diazepam Tablets BP 2mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Diazepam BP 2.00mg 3 PHARMACEUTICAL FORM Tablet 4. CLINICAL PARTICULARS
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Steroids. What are steroids?
 Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
Community herbal monograph on Commiphora molmol Engler, gummi-resina
 12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
