NOVO NORDISK A/S. Hypertension Liver disorders (e.g. liver adenoma) Diabetes mellitus with or without vascular involvement
|
|
|
- Emory Nash
- 7 years ago
- Views:
Transcription
1 08-15 Vagifem NOVO NORDISK A/S Each Vagifem film-coated vaginal tablet contains estradiol hemihydrate equivalent to estradiol 25 micrograms. The tablet core contains: Hypromellose, lactose monohydrate, maize starch, magnesium stearate. The film-coating contains: Hypromellose, macrogol The vaginal tablets are marked Novo 279. Each white tablet is contained in a disposable single-use There are 15 applicators with vaginal tablets in each box. Pharmaco-therapeutic group Oestrogen preparation (sex hormone). Manufacturer Novo Nordisk A/S, Novo Allé, 2880 Bagsvaerd, Denmark Indications Vagifem is indicated for the treatment of atrophic vaginitis due to oestrogen deficiency. The experience of treating women older than 65 years is limited. Contraindications Known, past or suspected breast cancer Known or suspected oestrogendependent malignant tumours (e.g. endometrial cancer) Undiagnosed genital bleeding Untreated endometrial hyperplasia Previous idiopathic or current venous thromboembolism (deep venous thrombosis, pulmonary embolism) Known hypersensitivity to the active substances or to any of the excipients Porphyria. Special warnings and special precautions for use For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually and HRT should only be continued as long as the benefit outweighs the risk. Medical examination/follow-up Before initiating or reinstituting hormone therapy, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contraindications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised which changes in their breasts should be reported to their doctor or nurse. Investigations including mammography should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual. Conditions which need supervision If any of the following conditions are present, have occurred previously, and/or have been aggravated during pregnancy or previous hormone treatment, the patient should be closely supervised. It should be taken into account that these conditions may recur or be aggravated during systemic oestrogen treatment, in particular: Leiomyoma (uterine fibroids) or endometriosis A history of, or risk factors for, thromboembolic disorders (see below) Hypertension Liver disorders (e.g. liver adenoma) Diabetes mellitus with or without vascular involvement Cholelithiasis Migraine or (severe) headache Systemic lupus erythematosus A history of endometrial hyperplasia (see below) Epilepsy Asthma Otosclerosis. Due to the local administration of low dose estradi- NOVO-Vagifem - p.1/5
2 NOVO-Vagifem - p.2/5 Venous thromboembolism Systemic HRT is associated with a higher relative risk of developing venous thromboembolism (VTE), i.e. deep vein thrombosis or pulmonary embolism. One randomised controlled trial and epidemiological studies found a two- to three-fold higher risk for users compared with non-users. For non-users it is estimated that the number of cases of VTE that will occur over a 5 year period is about 3 per 1000 women aged years and 8 per 1000 women aged between years. It is estimated that in healthy women who use HRT for 5 years, the number of additional cases of VTE over a 5 year period will be between 2 and 6 (best estimate = 4) per 1000 women aged and between 5 and 15 (best estimate = 9) per 1000 women aged years. The occurrence of such an event is more likely in the first year of HRT than later. Generally recognised risk factors for VTE include a personal history or family history, severe obesity (BMI >30 kg/m²) and systemic lupus erythematosus (SLE). There is no consensus about the possible role of varicose veins in VTE. Patients with a history of VTE or known thrombophilic states have an increased risk of VTE. HRT may add to this risk. Personal or strong family history of thromboembolism, or recurrent spontaneous abortion, should be investigated in order to exclude a thrombophilic predisposition. Until a thorough evaluation of thrombophilic factors has been made or anticoagulant treatment initiated, use of HRT in such patients should be viewed as contraindicated. Those women already on anticoagulant treatment require careful consideration of the benefit-risk of use of HRT. The risk of VTE may be temporarily increased with prolonged immobilisation, major trauma or major surgery. As in all postoperative patients, scrupulous attention should be given to prophylactic measures to prevent VTE following surgery. Where prolonged immobilisation is liable to follow elective surgery, particularly abdominal or orthopaedic surgery to the lower limbs, consideration should be given to temporarily stopping HRT 4 to 6 weeks earlier, if posol in Vagifem, the recurrence or aggravation of the above mentioned conditions is less likely than with systemic oestrogen treatment. Reasons for immediate withdrawal of therapy: Therapy should be discontinued in case a contraindication is discovered and in the following situations: Jaundice or deterioration in liver function Significant increase in blood pressure New onset of migraine-type headache Pregnancy. Endometrial hyperplasia Women with an intact uterus with abnormal bleeding of unknown aetiology or women with an intact uterus, who have previously been treated with unopposed oestrogens, should be examined with special care in order to disclose a possible hyperstimulation/ malignancy of the endometrium before initiation of treatment with Vagifem. The risk of endometrial cancer after treatment with oral unopposed oestrogens is dependent on both duration of treatment and on oestrogen dose. The dose of estradiol in Vagifem is low and treatment is local. A minor degree of systemic absorption may occur in some patients. However, Vagifem has not been associated with an increased risk of endometrial hyperplasia or uterine cancer. Because there is no systemic effect under the local oestrogen treatment with Vagifem, the addition of a progestagen is not recommended. As a general rule, oestrogen replacement therapy should not be prescribed for longer than one year without another physical examination including gynaecological examination being performed. Vagifem is a local low dose estradiol preparation and therefore the occurrence of the below mentioned conditions is less likely than with systemic oestrogen treatment. Breast cancer Systemic oestrogen or oestrogenprogestagen treatment may increase the risk of breast cancer. Relative risk of breast cancer with conjugated equine oestrogens (CEE) or estradiol (E2) was greater when a progestagen was added, either sequentially or continuously, and regardless of type of progestagen.
3 NOVO-Vagifem - p.3/5 sible. Treatment should not be restarted until the woman is completely mobilised. If VTE develops after initiating therapy, the drug should be discontinued. Patients should be told to contact their doctors immediately when they are aware of a potential thromboembolic symptom (e.g., painful swelling of a leg, sudden pain in the chest, dyspnea). Stroke One large randomised clinical trial (WHI-trial) found, as a secondary outcome, an increased risk of ischaemic stroke in healthy women during treatment with continuous combined conjugated oestrogens and MPA. For women who do not use HRT, it is estimated that the number of cases of stroke that will occur over a 5 year period is about 3 per 1000 women aged years and 11 per 1000 women aged years. It is estimated that for women who use conjugated oestrogens and MPA for 5 years, the number of additional cases will be between 0 and 3 (best estimate = 1) per 1000 users aged years and between 1 and 9 (best estimate = 4) per 1000 users aged years. It is unknown whether the increased risk also extends to other HRT products. Ovarian cancer Long-term (at least 5-10 years) use of oestrogenonly HRT products in hysterectomised women has been associated with an increased risk of ovarian cancer in some epidemiological studies. It is unlikely that long-term use of combined HRT leads to any risk increase for ovarian cancer. Other conditions Oestrogens may cause fluid retention, and therefore patients with cardiac or renal dysfunction should be carefully observed during the first weeks of treatment. Patients with terminal renal insufficiency should also be closely observed, since it is expected that the level of circulating active ingredients in Vagifem is increased. There is no conclusive evidence for improvement of cognitive function. There is some evidence from the WHI trial of increased risk of probable dementia in women who start using continuous combined CEE and MPA after the age of 65. It is unknown whether the findings apply to younger post-menopausal women or other HRT products. Interactions Due to a topic administration of the low dose of estradiol in Vagifem, interactions of clinical relevance are not expected. Pregnancy and lactation Vagifem is not indicated during pregnancy. If pregnancy occurs during medication with Vagifem, treatment should be withdrawn immediately. The results of most epidemiological studies to date relevant to inadvertent fetal exposure to oestrogens indicate no teratogenic or foetotoxic effects. Vagifem is not indicated during lactation. Effects on ability to drive and use machines No effects known. Dosage and administration Vagifem is administered intravaginally using the Initial dose: One vaginal tablet daily for two weeks. Maintenance dose: One vaginal tablet twice a week. Treatment may be started on any convenient day. If a dose is forgotten, it should be taken as soon as the patient remembers. A double dose should be avoided. For initiation and continuation of treatment of menopausal symptoms, the lowest effective dose for the shortest duration (see Special warnings and precautions for use ) should be used. Vagifem may be used in women with or without an intact uterus. During treatment especially during the first 2 weeks minimal absorption may be seen but as plasma estradiol levels after the first 2 weeks usually do not exceed postmenopausal levels the addition of a progestagen is not recommended. Overdose No cases of overdose have been reported. Vagifem is intended for intravaginal use. The dose of estradiol is so low that a considerable number of
4 NOVO-Vagifem - p.4/5 tablets would have to be ingested to approach the dose normally used for oral systemic treatment. Treatment should be symptomatic. Side effects More than 640 patients have been treated with Vagifem in clinical trials, including over 200 patients treated from 28 weeks and up to 64 weeks. Well known oestrogen related adverse events which occurred with a higher frequency in the treated group as compared with the placebo group, are presented as Common (>1/100, <1/10). The spontaneous reporting rate on Vagifem corresponds to approximately 1 case pr. 10,000 patient years. Adverse events for which an increased frequency has not been observed in clinical trials, but which have been spontaneously reported and which on an overall judgement are considered possibly related to Vagifem treatment are therefore presented as Very rare (<1/10,000). Post-marketing experience is subject to underreporting especially with regard to trivial and well known adverse drug reactions. The presented frequencies should be interpreted in that light. The most commonly reported adverse drug reactions are: Vaginal discharge and vaginal discomfort. Oestrogen related adverse events such as breast pain, peripheral oedema and postmenopausal bleedings are most likely present at the beginning of Vagifem treatment. Common (>1/100, <1/10): Infections and infestations: Genital candidiasis or vaginitis Nervous system disorders: Headache Gastrointestinal disorders: Nausea, abdominal pain, abdominal distension or abdominal discomfort, dyspepsia, vomiting, flatulence Reproductive system and breast disorders: Vaginal haemorrhage, vaginal discharge or vaginal discomfort, breast oedema, breast enlargement, breast pain or breast tenderness General disorders and administration site conditions: Oedema peripheral. Very rare (<1/10,000): Neoplasms benign and malignant (incl. cysts and polyps): Breast cancer, endometrial cancer Immune system disorders: Hypersensitivity, NOS Metabolism and nutrition disorders: Fluid retention Psychiatric disorders: Insomnia, depression Nervous system disorders: Migraine aggravated Vascular disorders: Deep venous thrombosis Gastrointestinal disorders: Diarrhoea Skin and subcutaneous tissue disorders: Urticaria, rash erythematous, rash NOS, rash pruritic, genital pruritus Reproductive system and breast disorders: Hyperplasia endometrial, vaginal irritation, vaginal pain, vaginismus, vaginal ulceration General disorders and administration site conditions: Drug ineffective Investigations: Weight increased, blood oestrogen increased. The following adverse reactions have been reported in association with other oestrogen treatment: Myocardial infarction, congestive heart disease Gall bladder disease Skin and subcutaneous disorders: Chloasma, erythema multiforme, erythema nodosum, vascular purpura, pruritus Vaginal candidiasis Risk of developing endometrial cancer, endometrial hyperplasia or increase in size of uterine fibroids* Insomnia Epilepsy Libido disorder NOS (not otherwise specified) Deterioration of asthma Probable dementia. *In non-hysterectomised women Storage conditions Do not store above 25ºC. Do not refrigerate. Store in the original package. Instructions for use 1. Tear off one single blisterpack and open the end as shown.
5 NOVO-Vagifem - p.5/5 2. Insert the applicator carefully into the vagina until you can feel some resistance. 3. To release the tablet, gently press the push button until you feel a click. The tablet becomes secure in the wall of the vagina immediately. It will not fall out if you stand up/or walk. 4. Withdraw and throw away the Please go to for more information.
PROVERA (medroxyprogesterone acetate) Product Monograph Page 34 of 38
 PART III: CONSUMER INFORMATION PR PROVERA* (medroxyprogesterone acetate) This leaflet is part III of a three-part "Product Monograph" published when PROVERA was approved for sale in Canada and is designed
PART III: CONSUMER INFORMATION PR PROVERA* (medroxyprogesterone acetate) This leaflet is part III of a three-part "Product Monograph" published when PROVERA was approved for sale in Canada and is designed
Public Assessment Report. Decentralised Procedure
 PAR Vagifem 10 microgram Vaginal Tablets Public Assessment Report Decentralised Procedure VAGIFEM 10 MICROGRAMS VAGINAL TABLETS UK licence no: PL 04668/0237 Novo Nordisk AS 1 PAR Vagifem 10 microgram Vaginal
PAR Vagifem 10 microgram Vaginal Tablets Public Assessment Report Decentralised Procedure VAGIFEM 10 MICROGRAMS VAGINAL TABLETS UK licence no: PL 04668/0237 Novo Nordisk AS 1 PAR Vagifem 10 microgram Vaginal
Now that your Doctor has prescribed Livial for you
 Now that your Doctor has prescribed Livial for you This educational brochure is only for use by patients prescribed LIVIAL The Menopause The term menopause refers to the very last menstrual period a woman
Now that your Doctor has prescribed Livial for you This educational brochure is only for use by patients prescribed LIVIAL The Menopause The term menopause refers to the very last menstrual period a woman
Why is Cerazette used? To prevent pregnancy.
 CERAZETTE Tablets for oral use Consumer Medicine Information What is in this leaflet Keep this leaflet. You may need to read it again. This leaflet will provide information about the benefits and risks
CERAZETTE Tablets for oral use Consumer Medicine Information What is in this leaflet Keep this leaflet. You may need to read it again. This leaflet will provide information about the benefits and risks
PART III: CONSUMER INFORMATION. 0.5 mg Estradiol and 0.1 mg Norethindrone acetate Film-coated tablets
 PART III: CONSUMER INFORMATION Pr Activelle LD 0.5 mg Estradiol and 0.1 mg Norethindrone acetate Film-coated tablets This leaflet is Part III of a three-part "Product Monograph" published when Activelle
PART III: CONSUMER INFORMATION Pr Activelle LD 0.5 mg Estradiol and 0.1 mg Norethindrone acetate Film-coated tablets This leaflet is Part III of a three-part "Product Monograph" published when Activelle
1. What Ovestin is and what it is used for
 PACKAGE LEAFLET: INFORMATION FOR THE USER OVESTIN 1 mg CREAM ESTRIOL 1mg in 1g of cream Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER OVESTIN 1 mg CREAM ESTRIOL 1mg in 1g of cream Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read
USER PACKAGE LEAFLET: INFORMATION FOR THE USER Exluton, tablets 0.5 mg Lynestrenol
 Lynestrenol 0.5mg tablets, (N.V.Organon), RH021 WHOPAR part 3 supplier s translation of original SRA approved text May 2012 USER PACKAGE LEAFLET: INFORMATION FOR THE USER Exluton, Lynestrenol Read this
Lynestrenol 0.5mg tablets, (N.V.Organon), RH021 WHOPAR part 3 supplier s translation of original SRA approved text May 2012 USER PACKAGE LEAFLET: INFORMATION FOR THE USER Exluton, Lynestrenol Read this
Information for you Treatment of venous thrombosis in pregnancy and after birth. What are the symptoms of a DVT during pregnancy?
 Information for you Treatment of venous thrombosis in pregnancy and after birth Published in September 2011 What is venous thrombosis? Thrombosis is a blood clot in a blood vessel (a vein or an artery).
Information for you Treatment of venous thrombosis in pregnancy and after birth Published in September 2011 What is venous thrombosis? Thrombosis is a blood clot in a blood vessel (a vein or an artery).
Dr. Friedman s Guide to Estrogen Replacement
 Dr. Friedman s Guide to Estrogen Replacement There are risks and benefits with all medicines and estrogen replacement is no exception. In fact, estrogen replacement is one of the most controversial topics
Dr. Friedman s Guide to Estrogen Replacement There are risks and benefits with all medicines and estrogen replacement is no exception. In fact, estrogen replacement is one of the most controversial topics
Hormone. Replacement. Therapy. Information leaflet. This information is also available on request in other formats by phoning 01387 241053.
 Hormone Replacement Therapy This information is also available on request in other formats by phoning 01387 241053. Information leaflet Produced by Dr H Currie & Sr. K Martin May 2005 Updated Dec. 2013
Hormone Replacement Therapy This information is also available on request in other formats by phoning 01387 241053. Information leaflet Produced by Dr H Currie & Sr. K Martin May 2005 Updated Dec. 2013
INFORMATION FOR THE PATIENT PATIENT PACKAGE INSERT CLÉO -35 Acne Treatment
 INFORMATION FOR THE PATIENT PATIENT PACKAGE INSERT CLÉO -35 Acne Treatment Composition CLÉO -35 is a preparation which contains 2 sex hormones, cyproterone acetate and ethinyl estradiol in a specific ratio.
INFORMATION FOR THE PATIENT PATIENT PACKAGE INSERT CLÉO -35 Acne Treatment Composition CLÉO -35 is a preparation which contains 2 sex hormones, cyproterone acetate and ethinyl estradiol in a specific ratio.
Abnormal Uterine Bleeding FAQ Sheet
 Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
Abnormal Uterine Bleeding FAQ Sheet What is abnormal uterine bleeding? Under normal circumstances, a woman's uterus sheds a limited amount of blood during each menstrual period. Bleeding that occurs between
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 13 October 2005 Doc. Ref. EMEA/CHMP/021/97 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 13 October 2005 Doc. Ref. EMEA/CHMP/021/97 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
(212) 733-2324 mackay.jimeson@pfizer.com
 For immediate release: July 9, 2012 Media Contacts: MacKay Jimeson (212) 733-2324 mackay.jimeson@pfizer.com Pfizer Recognizes 10 th Anniversary Of The Women s Health Initiative: A Modern Day Perspective
For immediate release: July 9, 2012 Media Contacts: MacKay Jimeson (212) 733-2324 mackay.jimeson@pfizer.com Pfizer Recognizes 10 th Anniversary Of The Women s Health Initiative: A Modern Day Perspective
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust.
 Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Risk Management Plan
 Risk Management Plan Active substance: Drospirenone/ethinylestradiol Version number: 4.0 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Oral contraception Effective control
Risk Management Plan Active substance: Drospirenone/ethinylestradiol Version number: 4.0 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Oral contraception Effective control
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Birth Control Pills
 Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
Birth Control Pills WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Birth control pills (also called oral contraceptives or "the pill") are used by millions of women in the United States to
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500. Hormone Therapy
 Hormone Therapy WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 At menopause, a woman's body makes less estrogen and she stops having menstrual periods. This is a natural stage in a woman's
Hormone Therapy WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 At menopause, a woman's body makes less estrogen and she stops having menstrual periods. This is a natural stage in a woman's
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cerazette, 75 microgram film-coated tablets desogestrel
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cerazette, 75 microgram film-coated tablets desogestrel Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Cerazette, 75 microgram film-coated tablets desogestrel Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
Understanding Endometriosis - Information Pack
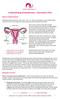 What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
Hull & East Riding Prescribing Committee
 Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
Hull & East Riding Prescribing Committee Guideline on Prescribing of Gonadorelin (GnRH) Analogues and Progesterone Receptor Modulators in treatment of Endometriosis or prior to Endometrial Ablation, or
1. WHAT ESTRADOT IS AND WHAT IT IS USED FOR
 PACKAGE LEAFLET: INFORMATION FOR THE USER Estradot 25 micrograms/24 hours transdermal patch Estradot 37.5 micrograms/24 hours transdermal patch Estradot 50 micrograms/24 hours transdermal patch Estradot
PACKAGE LEAFLET: INFORMATION FOR THE USER Estradot 25 micrograms/24 hours transdermal patch Estradot 37.5 micrograms/24 hours transdermal patch Estradot 50 micrograms/24 hours transdermal patch Estradot
Preventing Blood Clots in Adult Patients. Information For Patients
 Preventing Blood Clots in Adult Patients Information For Patients 1 This leaflet will give you information on how to reduce the risk of developing blood clots during and after your stay in hospital. If
Preventing Blood Clots in Adult Patients Information For Patients 1 This leaflet will give you information on how to reduce the risk of developing blood clots during and after your stay in hospital. If
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
CONSUMER INFORMATION IMPORTANT: PLEASE READ
 CONSUMER INFORMATION Pr MICRONOR norethindrone tablets, USP This leaflet is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about MICRONOR. Contact your
CONSUMER INFORMATION Pr MICRONOR norethindrone tablets, USP This leaflet is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about MICRONOR. Contact your
Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride
 Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride This medicine is for use in men only Read all of this leaflet carefully before you start taking this medicine because
Package Leaflet: Information for the user Propecia 1 mg film-coated Tablets Finasteride This medicine is for use in men only Read all of this leaflet carefully before you start taking this medicine because
Read all of this leaflet carefully before you start taking this medicine.
 YAZ PIL Page: 1 of 15 PACKAGE LEAFLET: INFORMATION FOR THE USER Yaz 0.02 mg / 3 mg film-coated tablets Ethinylestradiol / Drospirenone Read all of this leaflet carefully before you start taking this medicine.
YAZ PIL Page: 1 of 15 PACKAGE LEAFLET: INFORMATION FOR THE USER Yaz 0.02 mg / 3 mg film-coated tablets Ethinylestradiol / Drospirenone Read all of this leaflet carefully before you start taking this medicine.
How To Take Xarelto
 A patient's guide Your clinic's contact details are: Name: Contact number: Contents 2 Why have I been prescribed Xarelto? 2 What is Xarelto? 3 How do I take Xarelto? 3 What should I do if I miss a dose
A patient's guide Your clinic's contact details are: Name: Contact number: Contents 2 Why have I been prescribed Xarelto? 2 What is Xarelto? 3 How do I take Xarelto? 3 What should I do if I miss a dose
Menopause Guidance on management and prescribing HRT for GPs based on NICE guidance 2015
 PRIMARY CARE WOMEN S HEALTH FORUM GUIDELINES Menopause Guidance on management and prescribing HRT for GPs based on NICE guidance 2015 Written by Dr Imogen Shaw This guidance is designed to support you
PRIMARY CARE WOMEN S HEALTH FORUM GUIDELINES Menopause Guidance on management and prescribing HRT for GPs based on NICE guidance 2015 Written by Dr Imogen Shaw This guidance is designed to support you
Uterine Fibroid Symptoms, Diagnosis and Treatment
 Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
Fibroids and IR Uterine Fibroid Symptoms, Diagnosis and Treatment Interventional radiologists use MRIs to determine if fibroids can be embolised, detect alternate causes for the symptoms and rule out misdiagnosis,
1/9 (Translation from Finnish / SK / March 16, 2012)
 1/9 (Translation from Finnish / SK / March 16, 2012) JADELLE sine inserter 2 x 75 mg implants Package leaflet Please read this leaflet carefully. It contains information on the preparation and its use.
1/9 (Translation from Finnish / SK / March 16, 2012) JADELLE sine inserter 2 x 75 mg implants Package leaflet Please read this leaflet carefully. It contains information on the preparation and its use.
Xarelto (rivaroxaban) Prescriber Guide
 Xarelto (rivaroxaban) Prescriber Guide Simple Protection For More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation 4 Patients
Xarelto (rivaroxaban) Prescriber Guide Simple Protection For More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation 4 Patients
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
What is the menopause and what are the symptoms?
 What is the menopause and what are the symptoms? Strictly speaking, the menopause is the last menstrual period. However, most women think of the menopause as the time of life leading up to, and after,
What is the menopause and what are the symptoms? Strictly speaking, the menopause is the last menstrual period. However, most women think of the menopause as the time of life leading up to, and after,
Gynecology Abnormal Pelvic Anatomy and Physiology: Cervix. Cervix. Nabothian cysts. cervical polyps. leiomyomas. Cervical stenosis
 Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
Gynecology Abnormal Pelvic Anatomy and Physiology: (Effective February 2007) pediatric, reproductive, and perimenopausal/postmenopausal (24-28 %) Cervix Nabothian cysts result from chronic cervicitis most
Informed Consent Form for Testosterone Therapy
 Student Health Services Oregon State University, 201 Plageman Building, Corvallis, Oregon 97331-8567 Tel 541-737-9355 General Fax 541-737-4530 Medical Fax 541-737-9665 http://studenthealth.oregonstate.edu/
Student Health Services Oregon State University, 201 Plageman Building, Corvallis, Oregon 97331-8567 Tel 541-737-9355 General Fax 541-737-4530 Medical Fax 541-737-9665 http://studenthealth.oregonstate.edu/
FDA-Approved Patient Labeling
 FDA-Approved Patient Labeling Guide for Using Lo Loestrin Fe WARNING TO WOMEN WHO SMOKE Do not use Lo Loestrin Fe if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious
FDA-Approved Patient Labeling Guide for Using Lo Loestrin Fe WARNING TO WOMEN WHO SMOKE Do not use Lo Loestrin Fe if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious
Treating heavy menstrual bleeding caused by fibroids or polyps
 Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Treating heavy menstrual bleeding caused by fibroids or polyps With today s medical advances the outlook for successful treatment of fibroids and polyps has never been better. You don t have to live with
Vagifem (estradiol vaginal inserts) Initial U.S. Approval: 1999
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VAGIFEM safely and effectively. See full prescribing information for VAGIFEM. Vagifem (estradiol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VAGIFEM safely and effectively. See full prescribing information for VAGIFEM. Vagifem (estradiol
PATIENT HISTORY FORM
 PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
Hormone Therapy with Tamoxifen
 What is hormone-receptor-positive breast cancer? Many breast cancers need estrogen and/or progesterone (female hormones), to grow and spread. When breast cancer is found, the cancer is tested for two proteins,
What is hormone-receptor-positive breast cancer? Many breast cancers need estrogen and/or progesterone (female hormones), to grow and spread. When breast cancer is found, the cancer is tested for two proteins,
Laila-35 ED cyproterone acetate and ethinyloestradiol
 Laila-35 ED cyproterone acetate and ethinyloestradiol Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about LAILA-35 ED. It does not contain all of the
Laila-35 ED cyproterone acetate and ethinyloestradiol Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about LAILA-35 ED. It does not contain all of the
What Does Pregnancy Have to Do With Blood Clots in a Woman s Legs?
 Patient s Guide to Prevention of Blood Clots During Pregnancy: Use of Blood-Thinning A Patient s Guide to Prevention of Blood Clots During Pregnancy: Use of Blood-Thinning Drugs to Prevent Abnormal Blood
Patient s Guide to Prevention of Blood Clots During Pregnancy: Use of Blood-Thinning A Patient s Guide to Prevention of Blood Clots During Pregnancy: Use of Blood-Thinning Drugs to Prevent Abnormal Blood
Essential Shared Care Agreement Drugs for Dementia
 Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Ovarian Cyst. Homoeopathy Clinic. Introduction. Types of Ovarian Cysts. Contents. Case Reports. 21 August 2002
 Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Case Reports 21 August 2002 Ovarian Cyst Homoeopathy Clinic Check Yourself If you have any of the following symptoms call your doctor. Sense of fullness or pressure or a dull ache in the abdomen Pain during
Combination Birth Control Pills - FAQ
 Combination Birth Control Pills - FAQ How does the birth control pill work? prevents ovulation thickens cervical mucus, which makes it hard for sperm to enter the uterus thins the lining of the uterus,
Combination Birth Control Pills - FAQ How does the birth control pill work? prevents ovulation thickens cervical mucus, which makes it hard for sperm to enter the uterus thins the lining of the uterus,
PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride)
 PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride) This medicine is for use in men only Read all of this leaflet carefully before you start to take this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER PROPECIA 1 mg film-coated Tablets (finasteride) This medicine is for use in men only Read all of this leaflet carefully before you start to take this medicine.
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
11 Serious and life-threatening side effects can occur while taking EVISTA. These include 12 blood clots and dying from stroke:
 1 1 BNL 3123 AMP 2 Medication Guide 3 EVISTA (E-VISS-tah) 4 (raloxifene hydrochloride) 5 Tablets for Oral Use 6 Read the Medication Guide that comes with EVISTA before you start taking it and each time
1 1 BNL 3123 AMP 2 Medication Guide 3 EVISTA (E-VISS-tah) 4 (raloxifene hydrochloride) 5 Tablets for Oral Use 6 Read the Medication Guide that comes with EVISTA before you start taking it and each time
The Women s Health Initiative where are we a decade later?
 The Women s Health Initiative where are we a decade later? Henry G. Burger MIMR-PHI Institute & Jean Hailes for Women s Health, Melbourne, Australia Disclosures: Henry Burger Sources of Funding Pharmaceutical
The Women s Health Initiative where are we a decade later? Henry G. Burger MIMR-PHI Institute & Jean Hailes for Women s Health, Melbourne, Australia Disclosures: Henry Burger Sources of Funding Pharmaceutical
Abnormal Uterine Bleeding
 Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
Abnormal Uterine Bleeding WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 Abnormal uterine bleeding is one of the most common reasons women see their doctors. It can occur at any age and has
Shira Miller, M.D. Los Angeles, CA 310-734-8864 www.shiramillermd.com. The Compounding Pharmacy of Beverly Hills Beverly Hills Public Library
 Shira Miller, M.D. Los Angeles, CA 310-734-8864 The Compounding Pharmacy of Beverly Hills Beverly Hills Public Library 2 Outline What is hormone therapy? Why would healthy men and women need to think about
Shira Miller, M.D. Los Angeles, CA 310-734-8864 The Compounding Pharmacy of Beverly Hills Beverly Hills Public Library 2 Outline What is hormone therapy? Why would healthy men and women need to think about
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
The menopausal transition usually has three parts:
 The menopausal transition usually has three parts: Perimenopause begins several years before a woman s last menstrual period, when the ovaries gradually produce less estrogen. In the last 1-2 years of
The menopausal transition usually has three parts: Perimenopause begins several years before a woman s last menstrual period, when the ovaries gradually produce less estrogen. In the last 1-2 years of
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER. 500 mg, film-coated tablet Active substance: metformin hydrochloride
 P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING
 PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING What is combined hormonal contraception? Birth control which contains two hormones
PILLS & RING INFORMATION AND INSTRUCTIONS ON COMBINED HORMONAL CONTRACEPTION INCLUDING BIRTH CONTROL PILLS & NUVA RING What is combined hormonal contraception? Birth control which contains two hormones
Testosterone Therapy for Women
 Testosterone Therapy for Women The Facts You Need Contents 2 INTRODUCTION: The Facts You Need... 3-4 CHAPTER 1: Testosterone and Women... 5-9 CHAPTER 2: Testosterone Therapy for Women... 10-14 CONCLUSION:
Testosterone Therapy for Women The Facts You Need Contents 2 INTRODUCTION: The Facts You Need... 3-4 CHAPTER 1: Testosterone and Women... 5-9 CHAPTER 2: Testosterone Therapy for Women... 10-14 CONCLUSION:
A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT
 A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk
A PATIENT S GUIDE TO DEEP VEIN THROMBOSIS TREATMENT This medicine is subject to additional monitoring. This will allow quick identification of new safety information. If you get any side effects, talk
Package leaflet: Information for the user. Ondemet 4mg and 8mg Tablets (Ondansetron)
 Package leaflet: Information for the user Ondemet 4mg and 8mg Tablets (Ondansetron) Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Package leaflet: Information for the user Ondemet 4mg and 8mg Tablets (Ondansetron) Read all of this leaflet carefully before you start taking this medicine because it contains important information for
BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader. Two years ago, I was working as a bedside nurse. One of my colleagues felt
 Ali A. Kader, S. (2010). Breast cancer awareness for women and men. UCQ Nursing Journal of Academic Writing, Winter 2010, 70 76. BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader Two years
Ali A. Kader, S. (2010). Breast cancer awareness for women and men. UCQ Nursing Journal of Academic Writing, Winter 2010, 70 76. BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader Two years
Xarelto (rivaroxaban) Prescriber Guide November 2012
 Xarelto (rivaroxaban) Prescriber Guide November 2012 Simple Protection for More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation
Xarelto (rivaroxaban) Prescriber Guide November 2012 Simple Protection for More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
Uterine fibroids (Leiomyoma)
 Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
Uterine fibroids (Leiomyoma) What are uterine fibroids? Uterine fibroids are fairly common benign (not cancer) growths in the uterus. They occur in about 25 50% of all women. Many women who have fibroids
Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants
 Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants Traffic light classification- Amber 2 Information sheet for Primary Care Prescribers Relevant Licensed Indications
Enoxaparin for long term anticoagulation in patients unsuitable for oral anticoagulants Traffic light classification- Amber 2 Information sheet for Primary Care Prescribers Relevant Licensed Indications
vagina vaginal r vaginal ring vaginal vaginal ring aginal ring vaginal ring vaginal ring vaginal ring the contraceptive vaginal rin vaginal ring
 your guide to the contraceptive vaginal ring Helping you choose the method of contraception that is best for you vagina vaginal ring vaginal r vaginal ring vaginal ring vaginal rin vaginal aginal ring
your guide to the contraceptive vaginal ring Helping you choose the method of contraception that is best for you vagina vaginal ring vaginal r vaginal ring vaginal ring vaginal rin vaginal aginal ring
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
patient education Fact Sheet PFS003: Hormone Therapy APRIL 2015
 patient education Fact Sheet PFS003: Hormone Therapy APRIL 2015 Hormone Therapy Menopause is the time in a woman s life when she naturally stops having menstrual periods. Menopause marks the end of the
patient education Fact Sheet PFS003: Hormone Therapy APRIL 2015 Hormone Therapy Menopause is the time in a woman s life when she naturally stops having menstrual periods. Menopause marks the end of the
DVT/PE Management with Rivaroxaban (Xarelto)
 DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
DVT/PE Management with Rivaroxaban (Xarelto) Rivaroxaban is FDA approved for the acute treatment of DVT and PE and reduction in risk of recurrence of DVT and PE. FDA approved indications: Non valvular
Prescriber Guide. 20mg. 15mg. Simply Protecting More Patients. Simply Protecting More Patients
 Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
Prescriber Guide 20mg Simply Protecting More Patients 15mg Simply Protecting More Patients 1 Dear Doctor, This prescriber guide was produced by Bayer Israel in cooperation with the Ministry of Health as
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
European Medicines Agency recommends restricting use of trimetazidine-containing medicines
 22 June 2012 EMA/CHMP/417861/2012 Press Office Press release European Medicines Agency recommends restricting use of trimetazidine-containing medicines Restricted indication for patients with stable angina
22 June 2012 EMA/CHMP/417861/2012 Press Office Press release European Medicines Agency recommends restricting use of trimetazidine-containing medicines Restricted indication for patients with stable angina
Oxygen Therapy. Oxygen therapy quick guide V3 July 2012.
 PRESENTATION Oxygen (O 2 ) is a gas provided in a compressed form in a cylinder. It is also available in a liquid form. It is fed via a regulator and flow meter to the patient by means of plastic tubing
PRESENTATION Oxygen (O 2 ) is a gas provided in a compressed form in a cylinder. It is also available in a liquid form. It is fed via a regulator and flow meter to the patient by means of plastic tubing
Package leaflet: Information for the user Microgynon 30 Levonorgestrel Ethinylestradiol
 Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Frequently Asked Questions About Ovarian Cancer
 Media Contact: Gerri Gomez Howard Cell: 303-748-3933 gerri@gomezhowardgroup.com Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
Media Contact: Gerri Gomez Howard Cell: 303-748-3933 gerri@gomezhowardgroup.com Frequently Asked Questions About Ovarian Cancer What is ovarian cancer? Ovarian cancer is a cancer that forms in tissues
NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS
 NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS Date of First Issue 01/12/ 2012 Approved 15/11/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016
NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS Date of First Issue 01/12/ 2012 Approved 15/11/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM FEC-T. Patient s first names. Date of birth.
 Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM FEC-T Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas
Patient identifier/label: Page 1 of 6 PATIENT AGREEMENT TO SYSTEMIC THERAPY: CONSENT FORM FEC-T Patient s surname/family name Patient s first names Date of birth Hospital Name: Guy s Hospital St. Thomas
Xarelto (rivaroxaban) Prescriber Guide
 Xarelto (rivaroxaban) Prescriber Guide May 2013 Simple Protection for More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation
Xarelto (rivaroxaban) Prescriber Guide May 2013 Simple Protection for More Patients 2 Xarelto Prescriber Guide Patient Alert Card 4 Dosing Recommendations 4 Dosing in patients with atrial fibrillation
Acute pelvic inflammatory disease: tests and treatment
 Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
Acute pelvic inflammatory disease: tests and treatment Information for you Information for you Published August 2010 Published in August 2010 (next review date: 2014) Acute What is pelvic inflammatory
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
Confirmed Deep Vein Thrombosis (DVT)
 Confirmed Deep Vein Thrombosis (DVT) Information for patients What is deep vein thrombosis? Blood clotting provides us with essential protection against severe loss of blood from an injury to a vein or
Confirmed Deep Vein Thrombosis (DVT) Information for patients What is deep vein thrombosis? Blood clotting provides us with essential protection against severe loss of blood from an injury to a vein or
About the Uterus. Hysterectomy may be done to treat conditions that affect the uterus. Some reasons a hysterectomy may be needed include:
 Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Hysterectomy removal of the uterus is a way of treating problems that affect the uterus. Many conditions can be cured with hysterectomy. Because it is major surgery, your doctor may suggest trying other
Heavy periods (menstrual bleeding)
 Heavy periods (menstrual bleeding) This information sheet has been given to you to help answer some of the questions you may have about heavy periods and the treatments that are available. This leaflet
Heavy periods (menstrual bleeding) This information sheet has been given to you to help answer some of the questions you may have about heavy periods and the treatments that are available. This leaflet
MEDICAL HISTORY AND SCREENING FORM
 MEDICAL HISTORY AND SCREENING FORM The purpose of preventive exams is to screen for potential health problems and provide education to promote optimal health. It is best practice for chronic health problems
MEDICAL HISTORY AND SCREENING FORM The purpose of preventive exams is to screen for potential health problems and provide education to promote optimal health. It is best practice for chronic health problems
HARMONET 75 microgram/20 microgram Coated Tablets Gestodene and Ethinylestradiol. PATIENT INFORMATION LEAFLET (Republic of Ireland)
 HARMONET 75 microgram/20 microgram Coated Tablets Gestodene and Ethinylestradiol PATIENT INFORMATION LEAFLET (Republic of Ireland) Please read this carefully before you start to take your tablets. Keep
HARMONET 75 microgram/20 microgram Coated Tablets Gestodene and Ethinylestradiol PATIENT INFORMATION LEAFLET (Republic of Ireland) Please read this carefully before you start to take your tablets. Keep
Safety and Efficacy of Deferasirox (ICL670) in Patients With Iron Overload Resulting From Hereditary Hemochromatosis
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CICL670A2202 Previous Study Return to List Next Study Safety and Efficacy of Deferasirox (ICL670) in Patients With Iron Overload
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CICL670A2202 Previous Study Return to List Next Study Safety and Efficacy of Deferasirox (ICL670) in Patients With Iron Overload
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide)
 EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
EMA/467911/2014 Summary of the risk management plan (RMP) for Xultophy (insulin degludec / liraglutide) This is a summary of the risk management plan (RMP) for Xultophy, which details the measures to be
Medical criteria for IUCD s Based on the WHO MEC (2004- Annexure 3) system a woman s eligibility for IUCD insertion falls in 4 categories. These categ
 CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
CLIENT ASSESSMENT Ensure that the woman is not pregnant Determine the length and direction of uterus. Ensure that she does not have gonorrhea and chlamydia, and is not a high risk case of STI s Identify
Testosterone propionate, phenylpropionate, isocaproate and decanoate. Please read this leaflet carefully before you start using SUSTANON 250.
 SUSTANON 250 Testosterone propionate, phenylpropionate, isocaproate and decanoate What is in this leaflet Please read this leaflet carefully before you start using SUSTANON 250. This leaflet answers some
SUSTANON 250 Testosterone propionate, phenylpropionate, isocaproate and decanoate What is in this leaflet Please read this leaflet carefully before you start using SUSTANON 250. This leaflet answers some
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium)
 MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
MEDICATION GUIDE COUMADIN (COU-ma-din) (warfarin sodium) Read this Medication Guide before you start taking COUMADIN (warfarin sodium) and each time you get a refill. There may be new information. This
Suspected pulmonary embolism (PE) in pregnant women
 Suspected pulmonary embolism (PE) in pregnant women What is a pulmonary embolus? A deep vein thrombosis (DVT) is a blood clot that forms in one of the deep veins of the leg. If the clot moves to the lung,
Suspected pulmonary embolism (PE) in pregnant women What is a pulmonary embolus? A deep vein thrombosis (DVT) is a blood clot that forms in one of the deep veins of the leg. If the clot moves to the lung,
Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved by it.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved by it. Prescribing Information PROGYLUTON Tablets ESTROGENS INCREASE THE RISK OF ENDOMETRIAL
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved by it. Prescribing Information PROGYLUTON Tablets ESTROGENS INCREASE THE RISK OF ENDOMETRIAL
Doxylamine succinate belongs to the ethanolamine class of antihistamines with sedative properties.
 Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
Uterus myomatosus. 10-May-15. Clinical presentation. Incidence. Causes? 3 out of 4 women. Growth rate vary. Most common solid pelvic tumor in women
 Uterus myomatosus A.J. Henriquez March 14, 2015 Uterus myomatosus Definition, incidence, clinical presentation and diagnosis. New FIGO classification for uterine leiomyomas Brief description on treatment
Uterus myomatosus A.J. Henriquez March 14, 2015 Uterus myomatosus Definition, incidence, clinical presentation and diagnosis. New FIGO classification for uterine leiomyomas Brief description on treatment
