Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood
|
|
|
- Camron Morrison
- 7 years ago
- Views:
Transcription
1 PREPARATION OF HUMAN MONONUCLEAR CELL POPULATIONS AND SUBPOPULATIONS SECTION I This section describes procedures for the preparation of human mononuclear cell populations from peripheral blood (UNIT 7.1) and tissues (UNIT 7.8). Additional protocols detail the isolation of specific subpopulations of T cells (UNITS ), B cells (UNIT 7.5), monocytes/macrophages (UNIT 7.6), and lymphokine-activated killer/natural killer cells (LAK/NK; UNIT 7.7). Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood UNIT 7.1 Peripheral blood is the primary source of lymphoid cells for investigations of the human immune system. Its use is facilitated by Ficoll-Hypaque density gradient centrifugation a simple and rapid method of purifying peripheral blood mononuclear cells (PBMC) that takes advantage of the density differences between mononuclear cells and other elements found in the blood sample. Mononuclear cells and platelets collect on top of the Ficoll-Hypaque layer because they have a lower density; in contrast, red blood cells (RBC) and granulocytes have a higher density than Ficoll-Hypaque and collect at the bottom of the Ficoll-Hypaque layer (Fig ). Platelets are separated from the mononuclear cells by subsequent washing or by centrifugation through a fetal-bovine-serum (FBS) cushion gradient that allows penetration of mononuclear cells but not platelets. The mononuclear cell sample can be purified from monocytes by adherence or by exposure to L-leucine methyl ester (see Support Protocol 1 and Support Protocol 2). Cord blood and peripheral blood from infants contain immature cells, including nucleated red cells, that can result in significant contamination of the mononuclear cell layer. Removal of these cells requires additional steps (see Support Protocol 3). The isolation procedures described here can also be applied to cell populations derived from tissues (UNIT 7.8). CAUTION: When working with human blood, cells, or infectious agents, biosafety practices must be followed (see Chapter 7 introduction). NOTE: All solutions and equipment coming into contact with cells must be sterile, and proper sterile technique should be used accordingly. Figure Separation of blood components on a Ficoll-Hypaque gradient. plasma platelets lymphocytes Ficoll-Hypaque granulocytes erythrocytes Copyright 1996 by John Wiley & Sons, Inc. Supplement 19 CPI
2 BASIC PROTOCOL ISOLATION OF MONONUCLEAR CELLS BY FICOLL-HYPAQUE GRADIENT CENTRIFUGATION Materials Heparinized blood or heparinized cord blood (APPENDIX 3F) PBS (APPENDIX 2) Ficoll-Hypaque solution (density 1.77 g/liter; see recipe) Hanks balanced salt solution (HBSS; APPENDIX 2) FBS (e.g., HyClone), with or without heat inactivation (1 hr, 56 C; APPENDIX 2) Complete RPMI-1 medium (APPENDIX 2) 15- or 5-ml conical centrifuge tubes Beckman GPR centrifuge with GH-3.7 horizontal rotor (or equivalent temperature-controlled centrifuge) Additional reagents and equipment for counting cells and trypan blue exclusion for determining viability (APPENDIX 3A & 3B), flow cytometry (UNIT 5.4; optional), and depletion of contaminating cells from mononuclear fractions (see Support Protocol 3; optional) 1. Place fresh heparinized blood into 15- or 5-ml conical centrifuge tubes. Using a sterile pipet, add an equal volume of room-temperature PBS. Mix well. When isolating cells from a leukapheresis donor, dilute blood with PBS (1:4 blood/ PBS). Cord blood is readily available in 1-ml volumes; larger volumes can be obtained if required. Cord blood is prone to clotting and it is therefore helpful to add to each 1-ml aliquot 1 ml PBS containing 25 U heparin to supplement the heparin already contained in heparin-coated tubes. The blood should immediately be gently mixed, and should be examined when it reaches the laboratory for small blood clots. Because the clotting reaction releases proteins that can affect lymphocyte phenotype and function, samples containing clots should be discarded. 2. Slowly layer the Ficoll-Hypaque solution underneath the blood/pbs mixture by placing the tip of the pipet containing the Ficoll-Hypaque at the bottom of the sample tube. Use 3 ml Ficoll-Hypaque per 1 ml blood/pbs mixture. To maintain the Ficoll-Hypaque/blood interface, it is helpful to hold the centrifuge tube at a 45 angle. Alternatively, the blood/pbs mixture may be slowly layered over the Ficoll-Hypaque solution. Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood 3. Centrifuge 3 min in a GH-3.7 rotor at 2 rpm (9 g), 18 to 2 C, with no brake. 4. Using a sterile pipet, remove the upper layer that contains the plasma and most of the platelets (Fig ). Using another pipet, transfer the mononuclear cell layer to another centrifuge tube. Wash cells by adding excess HBSS ( 3 times the volume of the mononuclear cell layer) and centrifuging 1 min at 13 rpm (4 g), 18 to 2 C. Remove supernatant, resuspend cells in HBSS, and repeat the wash once to remove most of the platelets. The washing steps described above usually remove most of the platelets from mononuclear cell suspension. There are certain disease states associated with increased platelet concentrations (mononuclear cell to platelet cell ratio >1:1) in the peripheral blood, and additional steps are needed to remove the extra platelets in these cases. Add 3 ml FBS to a centrifuge tube for each milliliter of mononuclear cells. Layer the cell suspension ( cells/ml) over the FBS (alternatively, carefully layer the FBS under the cell suspension, which will rise as FBS is added). Centrifuge 15 min at 8 rpm (2 g), 18 to 2 C. Discard the supernatant containing the platelets. Resuspend cell pellet in complete RPMI-1 and proceed as in step Supplement 19 Current Protocols in Immunology
3 5. Resuspend mononuclear cells in complete RPMI-1. Count cells (APPENDIX 3A) and determine viability by trypan blue exclusion (APPENDIX 3B). Cord blood, and to a lesser extent peripheral blood from infants, gives a population of cells that is contaminated with erythrocytes and their precursors. Pure mononuclear cell populations may be obtained either by subjecting the cells to a second cycle of Ficoll-Hypaque gradient separation as described here, or by lysing the erythrocytes (see Support Protocol 3). 6. If desired, determine the purity of the PBMC population by flow cytometry (UNIT 5.4). DEPLETION OF MONOCYTES/MACROPHAGES FROM MONONUCLEAR CELLS USING ADHERENCE METHOD Approximately 4% of the isolated mononuclear cells obtained in the Basic Protocol are monocytes and macrophages. Monocytes can be depleted from the isolated mononuclear cell suspension by taking advantage of the fact that monocytes adhere to plastic whereas lymphocytes do not. SUPPORT PROTOCOL 1 Additional Materials (also see Basic Protocol) Mononuclear cell suspension (see Basic Protocol) Complete RPMI-2 medium (APPENDIX 2), room temperature 15-cm 2 tissue culture flasks, slanted-neck 1. Centrifuge mononuclear cells for 1 min at 14 rpm (3 g), 18 to 2 C. Remove supernatant and resuspend cell pellet in complete RPMI-2 to a final concentration of cells/ml. Transfer 5 ml cell suspension to a 15-cm 2 tissue culture flask. A biological-grade petri dish can be used, but adherence is increased when a tissue culture flask is used. 2. Incubate horizontally for 1 hr in a 37 C, 5% CO 2 humidified incubator. 3. Decant nonadherent lymphocytes into a centrifuge tube. Rinse tissue culture flask gently with 37 C complete RPMI-1; add this wash to the centrifuge tube. Centrifuge 1 min at 14 rpm (3 g), 18 to 2 C. Repeat steps 1 to 3 one time. 4. Discard supernatant. Resuspend cells in 5 to 1 ml complete RPMI-1. Count cells (APPENDIX 3A) and determine viability by trypan blue exclusion (APPENDIX 3B). Cells should be >9% viable. The adherent cells can be recovered using the procedures outlined in UNIT If desired, determine the purity of the PBMC population by flow cytometry (UNIT 5.4). DEPLETION OF MONOCYTES/MACROPHAGES FROM MONONUCLEAR CELLS USING L-LEUCINE METHYL ESTER Monocytes/macrophages can also be depleted from PBMC suspensions by a procedure that takes advantage of their rich lysosomal enzyme content (Thiele et al., 1983). This procedure employs a lysosomotropic agent L-leucine methyl ester that is taken up by phagocytic and cytotoxic cells and is concentrated in lysosomes, where it is converted by lysosomal enzymes to L-leucyl-L-leucine methyl ester, which is toxic to the cells. B cells and most T cells (cells not rich in lysosomes) are unaffected by exposure to L-leucine methyl ester. However, this agent does eliminate natural killer (NK) cells and cytotoxic T cells (UNIT 7.7). Additional Materials (also see Basic Protocol) 1 mm L-leucine methyl ester (Sigma) in complete RPMI medium (serum-free and filter sterilized; APPENDIX 2) SUPPORT PROTOCOL Current Protocols in Immunology Supplement 19
4 Additional reagents and equipment for nonspecific esterase staining (APPENDIX 3C) or for flow cytometry (UNIT 7.9) and preparation of monocyte-specific fluorescence-labeled antibodies (UNIT 5.3) 1. Incubate mononuclear cells in 1 mm L-leucine methyl ester prepared in complete serum-free RPMI medium at cells/ml for 4 min at room temperature. 2. Wash cells twice in HBSS (see Basic Protocol, step 4) and resuspend in complete RPMI Confirm that remaining lymphocytes are not contaminated by monocytes (large cells with eccentric, crescented nuclei) by light microscopy or by nonspecific esterase staining (APPENDIX 3C), which results in staining of monocytes but not other cells. Alternatively, examine the remaining lymphocytes by flow cytometry (UNIT 7.9) for the presence of cells staining with monocyte-specific fluorescence-labeled antibodies (UNIT 5.3). SUPPORT PROTOCOL 3 DEPLETION OF CONTAMINATING CELLS FROM MONONUCLEAR CELL FRACTIONS FROM CORD OR INFANT PERIPHERAL BLOOD When blood from very young infants is fractionated under the same conditions as adult blood, the mononuclear cell layer is often visibly contaminated with red blood cells (Fig ). In addition to this visible contamination, the mononuclear cell layer contains nucleated red cell precursors. Flow cytometric analysis shows that the proportion of cells that fail to react with CD45 (a leucocyte-common marker) can exceed 5%, even after mature red cells have been excluded on the basis of light-scatter parameters (Fig ). The contaminating cells can be removed by lysis in hypotonic ammonium chloride. This treatment does not affect the viability or most functions of mononuclear cells, but may affect antigen processing function, because ammonium ions inhibit lysosomal function. For studies that may be adversely affected by hypotonic ammonium chloride, the contaminating cells may be removed almost as efficiently by using two rounds of Ficoll-Hypaque density gradient separation. The improvement in purity produced by this second round of Ficoll-Hypaque density centrifugation suggests that the conditions (e.g., cell concentration and viscosity) in the first round do not allow all the cells to reach their isopycnic position. Figure Separation of adult (A) and cord (B) blood under identical conditions. The cord blood sample shows significant contamination of the interface layer with red blood cells. A B Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood Supplement 19 Current Protocols in Immunology
5 Additional Materials (also see Basic Protocol) ACK lysing buffer (UNIT 3.1) 1. Centrifuge the cell suspension obtained from the Ficoll-Hypaque separation 1 min at 4 g, room temperature, and remove the supernatant fluid. 2. Resuspend the cell pellet in ACK lysing buffer, using 5 ml solution per 1 ml of original blood volume. Allow to stand 5 min at room temperature, add 25 ml PBS, mix, and centrifuge 15 min at 3 g, room temperature. Remove supernatant, resuspend cell in 3 ml RPMI-1 or PBS, and centrifuge once more at 3 g, room temperature. Alternatively, centrifuge the cell suspension obtained from the Ficoll-Hypaque separation 1 min at 4 g, room temperature, remove the supernatant fluid, and resuspend the cell pellet in a volume of PBS equal to the original blood volume. Separate this suspension on a Ficoll-Hypaque density gradient (see Basic Protocol). 3. Determine the purity of the mononuclear cell population by flow cytometry using a CD45 (leucocyte common antigen) monoclonal antibody (Chapter 5). REAGENTS AND SOLUTIONS Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2; for suppliers, see APPENDIX 5. Ficoll-Hypaque solution (density 1.77 g/liter) 64. g Ficoll (molecular weight 4,; e.g., Sigma) 99. g sodium diatrizoate (e.g., Sigma).7 g NaCl Dissolve Ficoll and NaCl in 6 ml water using a magnetic stirrer at low speed. Add sodium diatrizoate. When all components are in solution, add water to 1 liter. Filter sterilize with a.22-µm filter unit (Nalgene). Store at 4 to 25 C and protect from direct light. Alternatively, Ficoll-Hypaque may be purchased already in solution (Ficoll-Paque, Pharmacia Biotech). COMMENTARY Background Information Ficoll-Hypaque gradient centrifugation allows rapid and efficient isolation of mononuclear cells from human peripheral blood (Boyum, 1968). As such, this technique is the starting point of most studies of human lymphoid cells. In general, Ficoll-Hypaque centrifugation does not change either the phenotype or the function of the isolated mononuclear cell population. However, it may be best to verify this in studies of cells from patients with various diseases (Kaplan et al., 1982). Monocytes/macrophages are removed from the mononuclear cell population in order to study lymphocyte function in the absence of monocytes/macrophages, or monocyte function in the absence of lymphocytes. The use of a single Ficoll-Hypaque density separation gives variable results with cord blood and peripheral blood from infants. The mononuclear cell layer is frequently contaminated with erythrocytes, usually visible as cell clumps sticking to the underside of the mononuclear layer (Fig ). Flow cytometric analysis (Fig ) shows that even when mature erythrocytes are excluded on the basis of light scatter, a significant number of CD45-negative cells remain. These cells include glycophorin-positive red cell precursors, although the number of glycophorin-positive cells is less than the number of CD45-negative cells (Ridings et al., 1996). The number of CD45- negative cells varies between cord preparations, from 1% to 5%. Cord blood also contains a small number of CD34-positive stem cells. These cells are too few to affect phenotype significantly but may nonetheless affect functional cells. It should also be borne in mind that the number of CD45-negative cells is not fully accounted for by red cell precursors; this implies Current Protocols in Immunology Supplement 19
6 6 A 28 B Relative cell frequency C D Mean fluorescence intensity Figure Contamination of cord blood mononuclear cell population by CD45-negative cells. CD45 distribution (after gating on lymphocyte-rich region of dual-scatter plot), as indicated by staining with anti-cd45 antibody, is shown for adult cells (A) and cord blood cells (B) fractionated under the same conditions. The cord population contains a significant peak of CD45-negative cells. These cells may be removed either by hypotonic lysis (C) or by a second round of Ficoll-Hypaque fractionation (D). Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood Supplement 19 that there are uncharacterized additional cells in cord blood which may affect functional cells. Critical Parameters and Troubleshooting The yield of mononuclear cells from peripheral blood depends on the percentage of contaminating granulocytes and platelets and the efficiency of erythrocyte removal. For maximum yield and purity, it is essential to remove all the material at the Ficoll-Hypaque interface and to ensure that no Ficoll-Hypaque solution or supernatant is removed with the sample. Including Ficoll-Hypaque will increase the granulocyte contamination; including supernatant will increase the platelet contamination. Erythrocytes can aggregate and trap lymphocytes in the clumps. These clumps will sediment into the pellet and reduce the yield of lymphocytes. Diluting the blood with PBS before Ficoll-Hypaque centrifugation will reduce the clumping. In addition, the temperature of the centrifuge can affect the yield of lymphocytes. At low temperatures, lymphocyte yields are reduced because a longer centrifugation time is required. At high temperatures, lymphocyte viability is decreased and erythrocyte aggregation is increased. During the gradient centrifugation, the temperature should be maintained at 18 to 2 C. Occasionally, it is necessary to isolate mononuclear cells from clotted blood. This has been successfully accomplished by using streptokinase to dissolve the blood clots. Lymphocytes isolated from clotted blood function in some respects similarly to those isolated from heparinized blood, although the lymphocyte yield is only 6% of the yield from heparinized blood (Niku et al., 1987). Anticipated Results When blood is obtained from a normal donor, the yield should be mononuclear cells/ml of blood. Approximately 6% to 7% of the mononuclear cells are lymphocytes with a viability of >95%. The platelet count is <.5% of the total platelet content of the original blood sample. After depletion of monocytes/macrophages by plastic adherence, 95% of the mononuclear cells are lympho- Current Protocols in Immunology
7 cytes with a viability of >95%. After depletion using L-leucine methyl ester, >99% of the mononuclear cells are lymphocytes with a viability of >95%. Yields of cells from cord blood tend to be somewhat higher than those from adult peripheral blood, ranging from.7 to cells per ml. Viabilities of cord blood cells range from 86% to 1%, with most samples giving 1%. Time Considerations Isolating mononuclear cells from peripheral blood takes 1 hr. This is dependent on the initial volume of blood obtained; the larger the blood volume, the longer the preparative time before the samples are ready for centrifuging. Lysis of erythrocytes followed by washing adds approximately 2 min to the procedures, while the alternative procedure of introducing a second round of Ficoll-Hypaque separation adds 4 min. Decreasing the concentration of platelets from the isolated mononuclear cells takes 3 min. If the isolated mononuclear cells are to be stored, platelets should be removed before freezing the cells. Removing monocytes/macrophages from the mononuclear cell population takes 2 to 3 hr using the adherence method, and 1 to 2 hr using L-leucine methyl ester. If the isolated mononuclear cells are frozen, further purification can be done later. Literature Cited Boyum, A Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. Suppl. 21: Kaplan, J., Nolan, D., and Ree, A Altered lymphocyte markers and blastogenic responses associated with 24 hour delay in processing of blood samples. J. Immunol. Methods 5: Niku, S.D., Hoon, D.S.B., Cochran, A.J., and Morton, D.L Isolation of lymphocytes from clotted blood. J. Immunol. Methods 15:9-14. Ridings, J., Weedon, H., Ioannu, C., Flego, L., Macardle, P.J., and Zola, H Purification of cord blood lymphocytes. J. Immunol. Methods. In press. Thiele, D.L., Kurosaka, M., and Lipsky, P.E Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: Delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J. Immunol. 131: Key References Ficoll-Paque: For in vitro isolation of lymphocytes (booklet) Pharmacia Biotech, Uppsala, Sweden. Excellent publication that describes the theory and application of Ficoll-Paque for isolation of lymphocytes. Hughes, W.T. and Buescher, E.T Collection of Blood Specimens. In Pediatric Procedures, pp W.B. Saunders, New York. Excellent resource with clear and simple description (with pictures) about how to collect peripheral blood. Contributed by Marjorie E. Kanof Massachusetts General Hospital and Harvard Medical School Boston, Massachusetts Phillip D. Smith National Institute of Dental Research/NIH Bethesda, Maryland Heddy Zola (cord blood) Child Health Research Institue Inc. North Adelaide, Australia Current Protocols in Immunology Supplement 19
Human Peripheral Blood Mononuclear Cell (PBMC) Manual
 Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Human Peripheral Blood Mononuclear Cell (PBMC) Manual INSTRUCTION MANUAL ZBM0063.04 SHIPPING CONDITIONS Human Peripheral Blood Mononuclear Cells, cryopreserved Cryopreserved human peripheral blood mononuclear
Ficoll-Paque PREMIUM Ficoll-Paque PREMIUM 1.084 Ficoll-Paque PREMIUM 1.073
 Instructions 28-4039-56 AE Cell Preparation Media Ficoll-Paque PREMIUM Ficoll-Paque PREMIUM 1.084 Ficoll-Paque PREMIUM 1.073 Intended use For in vitro isolation of mononuclear cells and/or granulocytes
Instructions 28-4039-56 AE Cell Preparation Media Ficoll-Paque PREMIUM Ficoll-Paque PREMIUM 1.084 Ficoll-Paque PREMIUM 1.073 Intended use For in vitro isolation of mononuclear cells and/or granulocytes
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.02 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
For in vitro preparation of human mononuclear cells from peripheral blood, bone marrow, and umbilical cord blood. Not for in vitro diagnostic use.
 GE Healthcare Instructions 28-4039-56 AA Cell Preparation Media Ficoll-Paque PREMIUM Intended use For in vitro preparation of human mononuclear cells from peripheral blood, bone marrow, and umbilical cord
GE Healthcare Instructions 28-4039-56 AA Cell Preparation Media Ficoll-Paque PREMIUM Intended use For in vitro preparation of human mononuclear cells from peripheral blood, bone marrow, and umbilical cord
NCL Method ITA-6. Leukocyte Proliferation Assay
 NCL Method ITA-6 Leukocyte Proliferation Assay Nanotechnology Characterization Laboratory National Cancer Institute-Frederick SAIC-Frederick Frederick, MD 21702 (301) 846-6939 ncl@mail.nih.gov December
NCL Method ITA-6 Leukocyte Proliferation Assay Nanotechnology Characterization Laboratory National Cancer Institute-Frederick SAIC-Frederick Frederick, MD 21702 (301) 846-6939 ncl@mail.nih.gov December
QIAGEN Supplementary Protocol
 QIAGEN Supplementary Protocol Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Purification of Total RNA from PBMC Using the RNeasy Micro or Mini Kit This protocol describes how to isolate PBMC
QIAGEN Supplementary Protocol Isolation of Peripheral Blood Mononuclear Cells (PBMC) and Purification of Total RNA from PBMC Using the RNeasy Micro or Mini Kit This protocol describes how to isolate PBMC
Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer
 Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer Purpose This procedure gives instruction on minimal residual disease (MRD) detection in patients with
Minimal residual disease detection in Acute Myeloid Leukaemia on a Becton Dickinson flow cytometer Purpose This procedure gives instruction on minimal residual disease (MRD) detection in patients with
RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method)
 Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
www.gbo.com/bioscience Tissue Culture 1 Cell/ Microplates 2 HTS- 3 Immunology/ HLA 4 Microbiology/ Bacteriology Purpose Beakers 5 Tubes/Multi-
 11 Cryo www.gbo.com/bioscience Technical Information 9 I 2 Leucosep TM 9 I 3 12 ml Leucosep TM Tubes 9 I 3 50 ml Leucosep TM Tubes 9 I 3 Instruction Manual Leucosep TM 9 I 4 OncoQuick 9 I 5 11 Cryo www.gbo.com/bioscience
11 Cryo www.gbo.com/bioscience Technical Information 9 I 2 Leucosep TM 9 I 3 12 ml Leucosep TM Tubes 9 I 3 50 ml Leucosep TM Tubes 9 I 3 Instruction Manual Leucosep TM 9 I 4 OncoQuick 9 I 5 11 Cryo www.gbo.com/bioscience
WHOLE BLOOD LYSING SOLUTION FOR FLOW CYTOMETRIC APPLICATIONS
 FOR IN-VITRO DIAGNOSTIC USE INVITROGEN CAL-LYSE TM Lysing Solution WHOLE BLOOD LYSING SOLUTION FOR FLOW CYTOMETRIC APPLICATIONS CAL-LYSE TM CATALOG No. GAS-010 250 tests 25 ml CATALOG No. GAS-010S-100
FOR IN-VITRO DIAGNOSTIC USE INVITROGEN CAL-LYSE TM Lysing Solution WHOLE BLOOD LYSING SOLUTION FOR FLOW CYTOMETRIC APPLICATIONS CAL-LYSE TM CATALOG No. GAS-010 250 tests 25 ml CATALOG No. GAS-010S-100
No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer
 APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
APPLICATION NOTE Attune NxT Flow Cytometer No-wash, no-lyse detection of leukocytes in human whole blood on the Attune NxT Flow Cytometer Introduction Standard methods for isolating and detecting leukocytes
CFSE Cell Division Assay Kit
 CFSE Cell Division Assay Kit Item No. 10009853 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 4 Precautions
CFSE Cell Division Assay Kit Item No. 10009853 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 4 Precautions
Measuring Cell Viability/Cytotoxicity: Cell Counting Kit-F
 Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Introduction The Cell Counting Kit-F is a fluorometic assay for the determination of viable cell numbers. Calcein-AM in this kit passes through the cell membrane and is hydrolized by the esterase in the
Lab 2. Isolation of mononuclear cells from peripheral blood and separation into subpopulations
 Lab 2 Isolation of mononuclear cells from peripheral blood and separation into subpopulations Supervisors: Sissela Broos sissela.broos@immun.lth.se tel: 222 96 78 Niclas Olsson niclas.olsson@immun.lth.se
Lab 2 Isolation of mononuclear cells from peripheral blood and separation into subpopulations Supervisors: Sissela Broos sissela.broos@immun.lth.se tel: 222 96 78 Niclas Olsson niclas.olsson@immun.lth.se
Instructions. Torpedo sirna. Material. Important Guidelines. Specifications. Quality Control
 is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
is a is a state of the art transfection reagent, specifically designed for the transfer of sirna and mirna into a variety of eukaryotic cell types. is a state of the art transfection reagent, specifically
Amaxa Mouse T Cell Nucleofector Kit
 Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Amaxa Mouse T Cell Nucleofector Kit For T cells isolated from C57BL/6 & BALB/c mice Evaluated for murine T cells isolated from C57BL/6 & BALB/c mice This protocol is designed for murine lymphocytes or
Islet Viability Assessment by Single Cell Flow Cytometry
 Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Islet Viability Assessment by Single Cell Flow Cytometry Page 1 of 8 Purpose: To comprehensively assess the viability of the islet cell preparation prior to transplantation. Tissue Samples: A sample containing
Ficoll-Paque PLUS. Ficoll-Paque. Intended Use For in vitro isolation of lymphocytes.
 GE Healthcare Instructions 71-7167-00 AG Cell preparation Ficoll-Paque PLUS Intended Use For in vitro isolation of lymphocytes. Introduction and Summary of the Method There is a need for a rapid, simple
GE Healthcare Instructions 71-7167-00 AG Cell preparation Ficoll-Paque PLUS Intended Use For in vitro isolation of lymphocytes. Introduction and Summary of the Method There is a need for a rapid, simple
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
Immunophenotyping peripheral blood cells
 IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
IMMUNOPHENOTYPING Attune Accoustic Focusing Cytometer Immunophenotyping peripheral blood cells A no-lyse, no-wash, no cell loss method for immunophenotyping nucleated peripheral blood cells using the Attune
HighPure Maxi Plasmid Kit
 HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
Plant Genomic DNA Extraction using CTAB
 Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
Biology 29 Cell Structure and Function Spring, 2009 Springer LABORATORY 2:CHLOROPLASTS AND PHOTOREDUCTION
 Biology 29 Cell Structure and Function Spring, 2009 Springer LABORATORY 2:CHLOROPLASTS AND PHOTOREDUCTION In this laboratory we will purify chloroplasts from spinach by differential centrifugation, then
Biology 29 Cell Structure and Function Spring, 2009 Springer LABORATORY 2:CHLOROPLASTS AND PHOTOREDUCTION In this laboratory we will purify chloroplasts from spinach by differential centrifugation, then
Xpert TM Karyotyping Teaching Kit
 Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
Xpert TM Karyotyping Teaching Kit Product Code: CCK012 Contents 1. About the kit 2. Kit contents and storage instructions 3. Materials required but not provided in the kit 4. Aseptic techniques and good
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
STANDARD OPERATING PROCEDURE Title: Antibody Production at Strategic Diagnostics Inc. SOP#: M-119 Version #: 1 Date Approved: August 6, 2009 Author: Strategic Diagnostic Inc. Date Modified: 1. PURPOSE
NimbleGen DNA Methylation Microarrays and Services
 NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
UltraClean Soil DNA Isolation Kit
 PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
PAGE 1 UltraClean Soil DNA Isolation Kit Catalog # 12800-50 50 preps New improved PCR inhibitor removal solution (IRS) included Instruction Manual (New Alternative Protocol maximizes yields) Introduction
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity
 Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
Lab 10: Bacterial Transformation, part 2, DNA plasmid preps, Determining DNA Concentration and Purity Today you analyze the results of your bacterial transformation from last week and determine the efficiency
TransIT -2020 Transfection Reagent
 Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
Quick Reference Protocol, MSDS and Certificate of Analysis available at mirusbio.com/5400 INTRODUCTION TransIT -2020 Transfection Reagent is a high-performance, animal-origin free, broad spectrum reagent
MagExtractor -Genome-
 Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
HiPer Ion Exchange Chromatography Teaching Kit
 HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
HiPer Ion Exchange Chromatography Teaching Kit Product Code: HTC001 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 5-6 hours Storage Instructions: The kit is stable for
Below is a list of things you should be aware of before you schedule your sort.
 Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
Sorting at the Flow Cytometry Facility At the present time, the assistance of a trained cell sorter operator is needed for all sorting applications. As such, if you are planning a first time sort you will
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
123count ebeads Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use.
 Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
Page 1 of 1 Catalog Number: 01-1234 Also known as: Absolute cell count beads GPR: General Purpose Reagents. For Laboratory Use. Normal human peripheral blood was stained with Anti- Human CD45 PE (cat.
EdU Flow Cytometry Kit. User Manual
 User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
User Manual Ordering information: (for detailed kit content see Table 2) EdU Flow Cytometry Kits for 50 assays: Product number EdU Used fluorescent dye BCK-FC488-50 10 mg 6-FAM Azide BCK-FC555-50 10 mg
ArC Amine Reactive Compensation Bead Kit
 ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
Title: Mapping T cell epitopes in PCV2 capsid protein - NPB #08-159. Date Submitted: 12-11-09
 Title: Mapping T cell epitopes in PCV2 capsid protein - NPB #08-159 Investigator: Institution: Carol Wyatt Kansas State University Date Submitted: 12-11-09 Industry summary: Effective circovirus vaccines
Title: Mapping T cell epitopes in PCV2 capsid protein - NPB #08-159 Investigator: Institution: Carol Wyatt Kansas State University Date Submitted: 12-11-09 Industry summary: Effective circovirus vaccines
Enumeration and Viability of Nucleated Cells from Bone Marrow, Cord Blood, and Mobilized Peripheral Blood
 Application Note Primary Cell Analysis Enumeration and Viability of Nucleated Cells from Bone Marrow, Cord Blood, and Mobilized Peripheral Blood Cell Viability Introduction: Bone Marrow, Cord Blood, Stem
Application Note Primary Cell Analysis Enumeration and Viability of Nucleated Cells from Bone Marrow, Cord Blood, and Mobilized Peripheral Blood Cell Viability Introduction: Bone Marrow, Cord Blood, Stem
MANUAL PLATELET COUNT
 MANUAL PLATELET COUNT Principle Whole blood is diluted with a 1% ammonium oxalate solution. The isotonic balance of the diluent is such that all erythrocytes are lysed while the leukocytes, platelets,
MANUAL PLATELET COUNT Principle Whole blood is diluted with a 1% ammonium oxalate solution. The isotonic balance of the diluent is such that all erythrocytes are lysed while the leukocytes, platelets,
NCL Method ITA-14. Analysis of Nanoparticle Effects on Maturation of Monocyte Derived Dendritic Cells In Vitro
 NCL Method ITA-14 Analysis of Nanoparticle Effects on Maturation of Monocyte Derived Dendritic Cells In Vitro Nanotechnology Characterization Laboratory Frederick National Laboratory for Cancer Research
NCL Method ITA-14 Analysis of Nanoparticle Effects on Maturation of Monocyte Derived Dendritic Cells In Vitro Nanotechnology Characterization Laboratory Frederick National Laboratory for Cancer Research
6 Characterization of Casein and Bovine Serum Albumin
 6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus
 Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
Related topics: Application Note 27 Data Analysis of Tube Formation Assays.
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Standard Operating Procedure
 1.0 Purpose: 1.1 The characterisation of of main leukocyte subsets in peripheral blood cells from mice by flow cytometry. Reliable values of frequencies of leukocyte clusters are very much dependent on
1.0 Purpose: 1.1 The characterisation of of main leukocyte subsets in peripheral blood cells from mice by flow cytometry. Reliable values of frequencies of leukocyte clusters are very much dependent on
Monoclonal Antibody Supernatant and Ascites Fluid Production
 Monoclonal Antibody Supernatant and Ascites Fluid Production UNIT 2.6 A major advantage of using monoclonal antibodies over polyclonal antisera is the potential availability of large quantities of the
Monoclonal Antibody Supernatant and Ascites Fluid Production UNIT 2.6 A major advantage of using monoclonal antibodies over polyclonal antisera is the potential availability of large quantities of the
Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum
 SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
SUPPLEMENTAL PROTOCOL WHITE PAPER Agencourt RNAdvance Blood Kit for Free Circulating DNA and mirna/rna Isolation from 200-300μL of Plasma and Serum Bee Na Lee, Ph.D., Beckman Coulter Life Sciences Process
Frozen-EZ Yeast Transformation II Catalog No. T2001
 INSTRUCTIONS Frozen-EZ Yeast Transformation II Catalog No. T2001 Highlights High transformation efficiency that yields approximately 10 5-10 6 transformants per μg plasmid DNA (circular). Frozen storage
INSTRUCTIONS Frozen-EZ Yeast Transformation II Catalog No. T2001 Highlights High transformation efficiency that yields approximately 10 5-10 6 transformants per μg plasmid DNA (circular). Frozen storage
MACS cell separation - the faster way to ultrapure, Dr. Katharina Winnemoeller katharinaw@miltenyibiotec.com.au
 MACS cell separation - the faster way to ultrapure, viable cells + Dr. Katharina Winnemoeller katharinaw@miltenyibiotec.com.au About us Germany's largest independent, privately owned biotech company 1300
MACS cell separation - the faster way to ultrapure, viable cells + Dr. Katharina Winnemoeller katharinaw@miltenyibiotec.com.au About us Germany's largest independent, privately owned biotech company 1300
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION
 DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
DNA SPOOLING 1 ISOLATION OF DNA FROM ONION INTRODUCTION This laboratory protocol will demonstrate several basic steps required for isolation of chromosomal DNA from cells. To extract the chromosomal DNA,
Stepcount. Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom. Cat. Reference: STP-25T
 Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Product Description: Closed transparent tubes with a metal screen, including a white matrix at the bottom Cat. Reference: STP-25T Reagent provided:: 25 Stepcount tubes for 25 test INTENDED USE. Immunostep
Direct Antiglobulin Test (DAT)
 Exercise 8 Exercise 9 Direct Antiglobulin Test (DAT) Elution Study Task Aim Introduction To perform the DAT and elution procedure with correct interpretation of results. To perform with 100% accuracy the
Exercise 8 Exercise 9 Direct Antiglobulin Test (DAT) Elution Study Task Aim Introduction To perform the DAT and elution procedure with correct interpretation of results. To perform with 100% accuracy the
UltraClean Forensic DNA Isolation Kit (Single Prep Format)
 UltraClean Forensic DNA Isolation Kit (Single Prep Format) Catalog No. Quantity 14000-10 10 preps 14000-S 1 prep Instruction Manual Please recycle Version: 10302012 1 Table of Contents Introduction...
UltraClean Forensic DNA Isolation Kit (Single Prep Format) Catalog No. Quantity 14000-10 10 preps 14000-S 1 prep Instruction Manual Please recycle Version: 10302012 1 Table of Contents Introduction...
MTT Cell Proliferation Assay
 ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
ATCC 30-1010K Store at 4 C This product is intended for laboratory research purposes only. It is not intended for use in humans, animals or for diagnostics. Introduction Measurement of cell viability and
Marmara Üniversitesi Fen-Edebiyat Fakültesi Kimya Bölümü / Biyokimya Anabilim Dalı PURIFICATION AND CHARACTERIZATION OF PROTEINS
 EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
EXPERIMENT VI PURIFICATION AND CHARACTERIZATION OF PROTEINS I- Protein isolation and dialysis In order to investigate its structure and properties a protein must be obtained in pure form. Since proteins
LIVE/DEAD Fixable Dead Cell Stain Kits
 USER GUIDE LIVE/DEAD Fixable Dead Cell Stain Kits Pub. No. MAN0002416 (MP34955) Rev. A.0 Table 1. Contents and storage Material Amount Storage Stability Individual Kits: Blue, violet, aqua, yellow-, green,
USER GUIDE LIVE/DEAD Fixable Dead Cell Stain Kits Pub. No. MAN0002416 (MP34955) Rev. A.0 Table 1. Contents and storage Material Amount Storage Stability Individual Kits: Blue, violet, aqua, yellow-, green,
DNA Isolation Kit for Cells and Tissues
 DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
STEMCELL Quality Control Kits
 STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
STEMCELL Quality Control Kits i Table of Contents 1.0 Introduction...1 2.0 Thawing Cells, Plating and Colony Enumeration...2 2.1 Supplies and reagents included in the QC kit...2 2.2 Additional reagents
Optimized Protocol sirna Test Kit for Cell Lines and Adherent Primary Cells
 page 1 of 8 sirna Test Kit for Cell Lines and Adherent Primary Cells Kit principle Co-transfection of pmaxgfp, encoding the green fluorescent protein (GFP) from Pontellina p. with an sirna directed against
page 1 of 8 sirna Test Kit for Cell Lines and Adherent Primary Cells Kit principle Co-transfection of pmaxgfp, encoding the green fluorescent protein (GFP) from Pontellina p. with an sirna directed against
Cell Cycle Phase Determination Kit
 Cell Cycle Phase Determination Kit Item No. 10009349 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety
Cell Cycle Phase Determination Kit Item No. 10009349 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety
The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade plasmid DNA.
 INSTRUCTION MANUAL ZymoPURE Plasmid Gigaprep Kit Catalog Nos. D4204 (Patent Pending) Highlights The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade
INSTRUCTION MANUAL ZymoPURE Plasmid Gigaprep Kit Catalog Nos. D4204 (Patent Pending) Highlights The fastest spin-column based procedure for purifying up to 10 mg of ultra-pure endotoxin-free transfection-grade
Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-806)
 Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-86) The Hoechst purification was established for murine hematopoietic stem cells (HSC)
Hoechst 33342 HSC Staining and Stem Cell Purification Protocol (see Goodell, M., et al. (1996) J Exp Med 183, 1797-86) The Hoechst purification was established for murine hematopoietic stem cells (HSC)
RiboZol RNA Extraction Reagents
 RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
RiboZol RNA Extraction Reagents Code Description Size N580-30ML-SAMPLE Ribozol TM RNA Extraction Reagent 30 ml N580-30ML Ribozol TM RNA Extraction Reagent 30 ml N580-100ML Ribozol TM RNA Extraction Reagent
Chromatin Immunoprecipitation
 Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Generation of EBV-immortalized B cell lines
 UCANU 0014 Version November 01, 2011 Page 1 van 6 Generation of EBVimmortalized B cell lines CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written by Name Function
UCANU 0014 Version November 01, 2011 Page 1 van 6 Generation of EBVimmortalized B cell lines CMCI (Center for molecular and Cellular Intervention) University Medical Center Utrecht Written by Name Function
Mouse krebs von den lungen 6 (KL-6) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
Mouse IFN-gamma ELISpot Kit
 Page 1 of 8 Mouse IFN-gamma ELISpot Kit Without Plates With Plates With Sterile Plates Quantity Catalog Nos. 862.031.001 862.031.001P 862.031.001S 1 x 96 tests 862.031.005 862.031.005P 862.031.005S 5 x
Page 1 of 8 Mouse IFN-gamma ELISpot Kit Without Plates With Plates With Sterile Plates Quantity Catalog Nos. 862.031.001 862.031.001P 862.031.001S 1 x 96 tests 862.031.005 862.031.005P 862.031.005S 5 x
PRODUCT INFORMATION SHEET Monoclonal antibodies detecting human antigens
 www.iqproducts.nl PRODUCT INFORMATION SHEET Monoclonal antibodies detecting human antigens IFN- γ PURE [RUO] [REF] IQP-160P s 50 tests FITC [RUO] [REF] IQP-160F s 50 tests R-PE [RUO] [REF] IQP-160R s 50
www.iqproducts.nl PRODUCT INFORMATION SHEET Monoclonal antibodies detecting human antigens IFN- γ PURE [RUO] [REF] IQP-160P s 50 tests FITC [RUO] [REF] IQP-160F s 50 tests R-PE [RUO] [REF] IQP-160R s 50
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a
 Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA. kit
 BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis
 ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
ab139418 Propidium Iodide Flow Cytometry Kit for Cell Cycle Analysis Instructions for Use To determine cell cycle status in tissue culture cell lines by measuring DNA content using a flow cytometer. This
MLX BCG Buccal Cell Genomic DNA Extraction Kit. Performance Characteristics
 MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
MLX BCG Buccal Cell Genomic DNA Extraction Kit Performance Characteristics Monolythix, Inc. 4720 Calle Carga Camarillo, CA 93012 Tel: (805) 484-8478 monolythix.com Page 2 of 9 MLX BCG Buccal Cell Genomic
Procedure for RNA isolation from human muscle or fat
 Procedure for RNA isolation from human muscle or fat Reagents, all Rnase free: 20% SDS DEPC-H2O Rnase ZAP 75% EtOH Trizol Chloroform Isopropanol 0.8M NaCitrate/1.2M NaCl TE buffer, ph 7.0 1. Homogenizer-probe
Procedure for RNA isolation from human muscle or fat Reagents, all Rnase free: 20% SDS DEPC-H2O Rnase ZAP 75% EtOH Trizol Chloroform Isopropanol 0.8M NaCitrate/1.2M NaCl TE buffer, ph 7.0 1. Homogenizer-probe
Enhance Sensitivity of FISH Analysis with Highly Purified Multiple Myeloma Cells Using RoboSep, the Fully Automated Cell Separator
 Enhance Sensitivity of FISH Analysis with Highly Purified Multiple Myeloma Cells Using RoboSep, the Fully Automated Cell Separator Benoit Guilbault, PhD Field Applications Scientist t STEMCELL Technologies,
Enhance Sensitivity of FISH Analysis with Highly Purified Multiple Myeloma Cells Using RoboSep, the Fully Automated Cell Separator Benoit Guilbault, PhD Field Applications Scientist t STEMCELL Technologies,
ProteoMiner Protein Enrichment Kits
 ProteoMiner Protein Enrichment Kits Instruction Manual Catalog # 163-3003 163-3006 163-3007 163-3008 163-3009 163-3010 163-3011 163-3012 For Technical Support, contact your local Bio-Rad office, or in
ProteoMiner Protein Enrichment Kits Instruction Manual Catalog # 163-3003 163-3006 163-3007 163-3008 163-3009 163-3010 163-3011 163-3012 For Technical Support, contact your local Bio-Rad office, or in
Human Adult Mesothelial Cell Manual
 Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Human Adult Mesothelial Cell Manual INSTRUCTION MANUAL ZBM0025.01 SHIPPING CONDITIONS Human Adult Mesothelial Cells Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Chromatin Immunoprecipitation (ChIP)
 Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
INSTRUCTIONS 56-1190-98. Edition AC
 Sephacryl S-100 High Resolution Sephacryl S-200 High Resolution Sephacryl S-300 High Resolution Sephacryl S-400 High Resolution Sephacryl S-500 High Resolution INSTRUCTIONS Sephacryl High Resolution chromatography
Sephacryl S-100 High Resolution Sephacryl S-200 High Resolution Sephacryl S-300 High Resolution Sephacryl S-400 High Resolution Sephacryl S-500 High Resolution INSTRUCTIONS Sephacryl High Resolution chromatography
Titering Antibodies INTRODUCTION Materials Procedure for titering antibodies to extracellular antigens A. Directly conjugated antibodies
 C.C. Stewart, S.J. Stewart, Titering Antibodies. In: Current Protocols in Cytometry, (J.P. Robinson, Z. Darzynkiewicz, P. Dean. L. Dressler, P.Rabinovitch, C. Stewart, H. Tanke, L. Wheeless, eds.) J.Wiley
C.C. Stewart, S.J. Stewart, Titering Antibodies. In: Current Protocols in Cytometry, (J.P. Robinson, Z. Darzynkiewicz, P. Dean. L. Dressler, P.Rabinovitch, C. Stewart, H. Tanke, L. Wheeless, eds.) J.Wiley
Taqman TCID50 for AAV Vector Infectious Titer Determination
 Page 1 of 8 Purpose: To determine the concentration of infectious particles in an AAV vector sample. This process involves serial dilution of the vector in a TCID50 format and endpoint determination through
Page 1 of 8 Purpose: To determine the concentration of infectious particles in an AAV vector sample. This process involves serial dilution of the vector in a TCID50 format and endpoint determination through
Basic Science in Medicine
 Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
Medical Journal of th e Islamic Republic of Iran Volume 18 Number 3 Fall 1383 November 2004 Basic Science in Medicine ] EXPANSION OF HUMAN CORD BLOOD PRIMITIVE PROGENITORS IN SERUM-FREE MEDIA USING HUMAN
GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps)
 1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
Cell Culture Protocol for Biogelx Peptide Hydrogel 2D and 3D Cell Culture PRO/BGX/001
 Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Protocol (PRO) Cell Culture Protocol for Hydrogel 2D and 3D Cell Culture PRO/BGX/001 Materials Provided Gel solution (stiffness of Gel will vary as per type of product requested), packaged in a glass vial.
Production of Monoclonal Antibody Supernatant and Ascites Fluid
 Production of Monoclonal Antibody Supernatant and Ascites Fluid Wayne M. Yokoyama 1 UNIT 11.10 1 Howard Hughes Medical Institute and Washington University School of Medicine, St. Louis, Missouri ABSTRACT
Production of Monoclonal Antibody Supernatant and Ascites Fluid Wayne M. Yokoyama 1 UNIT 11.10 1 Howard Hughes Medical Institute and Washington University School of Medicine, St. Louis, Missouri ABSTRACT
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION
 Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
Day -1 RETRIEVAL OF OVARIES AND OOCYTE COLLECTION AND MATURATION OVARY COLLECTION Materials and Equipment Needed Thermos with 2 containers with 0.5 L of transport saline in each. Appropriate attire as
International Beryllium Conference, Montreal, Canada March 10, 2005
 Alternative Lymphocyte Proliferation Tests: BrdU and Flow Cytometry Based Tests International Beryllium Conference, Montreal, Canada March 10, 2005 Tim K. Takaro Department of Environmental and Occupational
Alternative Lymphocyte Proliferation Tests: BrdU and Flow Cytometry Based Tests International Beryllium Conference, Montreal, Canada March 10, 2005 Tim K. Takaro Department of Environmental and Occupational
ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
 #3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
Genomic DNA Extraction Kit INSTRUCTION MANUAL
 Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Malondialdehyde (MDA) ELISA
 Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Application Guide... 2
 Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit
 Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
Whole Blood Flow Cytometry
 Whole Blood Flow Cytometry y Nailin Li Department t of Medicine, i Clinical i l Pharmacology Unit Karolinska Institute/University Hospital, 171 76 Stockholm Department of Pathology & Pathophysiology Zhejiang
Whole Blood Flow Cytometry y Nailin Li Department t of Medicine, i Clinical i l Pharmacology Unit Karolinska Institute/University Hospital, 171 76 Stockholm Department of Pathology & Pathophysiology Zhejiang
Expression of CD163 on Bovine Alveolar Macrophages and Peripheral Blood Mononuclear Cells
 Expression of CD163 on Bovine Alveolar Macrophages and Peripheral Blood Mononuclear Cells Mary E. Kopechek Honor s Research Thesis May 17, 2007 Research Advisor: Dr. Jeff Lakritz, DVM, PhD The Ohio State
Expression of CD163 on Bovine Alveolar Macrophages and Peripheral Blood Mononuclear Cells Mary E. Kopechek Honor s Research Thesis May 17, 2007 Research Advisor: Dr. Jeff Lakritz, DVM, PhD The Ohio State
Bovine Vitamin B12 (VB12) ELISA Kit
 Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
Bovine Vitamin B12 (VB12) ELISA Kit Catalog Number. CSB-E14089B For the quantitative determination of endogenic bovine vitamin B12 (VB12) concentrations in serum, plasma, tissue homogenates. This package
Introduction to flow cytometry
 Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Introduction to flow cytometry Flow cytometry is a popular laser-based technology. Discover more with our introduction to flow cytometry. Flow cytometry is now a widely used method for analyzing the expression
Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement
 Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement Andreas Spittler, MD, Associate Professor for Pathophysiology, Medical University
Application Information Bulletin: Set-Up of the CytoFLEX Set-Up of the CytoFLEX* for Extracellular Vesicle Measurement Andreas Spittler, MD, Associate Professor for Pathophysiology, Medical University
Kevin Bogart and Justen Andrews. Extraction of Total RNA from Drosophila. CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610
 Kevin Bogart and Justen Andrews Extraction of Total RNA from Drosophila CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610 Bogart K and Andrews J. 2006. Extraction of Total RNA from Drosophila. CGB
Kevin Bogart and Justen Andrews Extraction of Total RNA from Drosophila CGB Technical Report 2006-10 doi:10.2506/cgbtr-200610 Bogart K and Andrews J. 2006. Extraction of Total RNA from Drosophila. CGB
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit
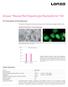 Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
Amaxa Mouse/Rat Hepatocyte Nucleofector Kit For Primary Mouse and Rat Hepatocytes This protocol is designed for freshly isolated mouse and rat hepatocytes; polygonal, adherent cells Example for Nucleofection
