DCVax Novel Personalized Immune Therapies For Solid Tumor Cancers. SMi 4 th Annual Cancer Vaccines Conference September 16, 2015
|
|
|
- Evelyn Fletcher
- 8 years ago
- Views:
Transcription
1 DCVax Novel Personalized Immune Therapies For Solid Tumor Cancers SMi 4 th Annual Cancer Vaccines Conference September 16, 2015
2 Disclaimer Certain statements made in this presentation are forward-looking statements of NW Bio as defined by the Securities and Exchange Commission ( SEC ). All statements, other than statements of historical fact, included in this presentation that address activities, events or developments that NW Bio believes or anticipates will or may occur in the future are forward-looking statements. These statements are based on certain assumptions made based on experience, expected future developments and other factors NW Bio believes are appropriate in the circumstances. Such statements are subject to a number of assumptions, risks and uncertainties, many of which are beyond the control of NW Bio. Investors and others are cautioned that any such statements are not guarantees of future performance. These forward-looking statements could cause actual results and developments to differ materially from those expressed or implied in such statements, including our ability to raise funds for general corporate purposes and operations, including our clinical trials, the commercial feasibility and success of our technology, our ability to recruit qualified management and technical personnel, our ability to scale up the manufacturing of our product candidates for commercialization, the success of our clinical trials and our ability to obtain and maintain required regulatory approvals for our products. Furthermore, NW Bio does not intend (and is not obligated) to update publicly any forward-looking statements. The contents of this presentation should be considered in conjunction with the risk factors contained in NW Bio s recent filings with the SEC, including its most recent Form 10K. This communication is neither an offer to sell nor a solicitation of an offer to buy any securities mentioned herein. This publication is confidential for the information of the addressee only and may not be reproduced in whole or in part; copies circulated, or disclosed to another party, without the prior written consent of Northwest Biotherapeutics (NW Bio) are strictly prohibited. 2
3 DCVax : A Fully Personalized Immune Therapy Personalized Product: autologous (personalized) dendritic cells Allows repeat doses, ongoing treatment Personalized Targets: tumor antigens from patient s own tumor Avoids Russian Roulette Broad Scope of Targets: full set of tumor antigens Not just 1 or a few pre-selected, standardized antigens Maximize obstacles to tumor escape Excellent safety profile Some flu-like symptoms; no additional drugs, no hospital stays 3
4 Cancer Is A Personalized Disease Extensive variation among cancers Extensive variation among patients, even with same cancer Significant variations within a single patient as disease progresses Extensive heterogeneity even within a single tumor 4
5 Cancer Is A Personalized Disease LB Alexandrov et al. Nature 000, 1-7 (2013) doi: /nature
6 Two Key Issues With Existing Cancer Treatments Current treatments are largely standardized, not personalized. This raises 2 key issues Russian Roulette The patient s tumor may or may not express the targets. (e.g., EGFRvIII mutation in GBM only expressed on ~30% of GBMs) Tumor escape Tumors can and do stop expressing 1 or a few targets. But tumor cells cannot stop expressing all targets. 6
7 Personalized Treatments Vary Across A Spectrum Standardized Product Standardized Targets Personalized Profiling Personalized Product Standardized Targets Personalized Product Personalized Targets Peptide vaccines DNA vaccines Checkpoint inhibitors CAR-Ts DC vaccines with pre-selected peptide antigens DCVax 7
8 Dendritic Cells Mobilize Overall Immune System DENDRITIC CELLS the master immune cells Signals activate & biomarkers educate Dendritic Cells INNATE IMMUNE SYSTEM First-responders (within hours/days) Response is automatic Response is non-specific (not tailored to each particular threat) Natural Killer Cells, Neutrophils, Granulocytes, Macrophages ADAPTIVE IMMUNE SYSTEM Follow-on defense (within week or weeks) Response is triggered by exposure to particular threat (activation + education ) Response is specific & creates memory (tailored to each particular threat) HUMORAL IMMUNITY B Cells Antibodies CELLULAR IMMUNITY Helper T Cells Killer T Cells Tumor Cell Death 8
9 Large Multiplier: Each Dendritic Cell Activates Hundreds of Anti-Cancer T Cells Dendritic Cell activated anti-cancer T cells travel to tumor site tumor target proteins anti-cancer T cell activated resting anti-cancer T cell attaches to DC activated anti-cancer T cells divide rapidly 9
10 DCVax Potentially Applicable to All Types of Solid Tumors (Both Operable & Inoperable) Market Product Composition Lead Program All Operable Solid Tumors DCVax -L Dendritic cells + biomarkers from tumor tissue sample surgically removed Brain cancer 348-patient Phase III trial underway Small ovarian cancer Phase I/II trial completed All Inoperable Solid Tumors DCVax - Direct Dendritic cells injected directly into tumor(s) + biomarkers picked up onsite in tumor All solid tumor cancers 60-patient Phase I/II trial underway Other Hormone independent prostate cancer DCVax - Prostate* Dendritic cells + recombinant prostate cancer biomarker (PSMA) Prostate cancer 600-patient Phase III trial previously cleared by FDA * The Company will seek to out-license this program 10
11 DCVax Is Expanding the Reach of Immunotherapy Front line treatment for newly diagnosed disease DCVax-L for newly diagnosed GBM, with SOC (Phase III) Front line treatment for lower grade cancers DCVax-L for all grades of gliomas, with SOC (Hosp. Exemp., Germany) Treatment for patients with very heavy tumor burdens DCVax-L for late stage, metastatic ovarian cancer DCVax-L for rapid progressor GBM DCVax-Direct for 4-5+ inoperable metastatic tumors DCVax-Direct for very large tumors (10 cm. lung; 15x19x17 cm. sarcoma) Long tail of Overall Survival 11
12 Potentially Complementary With Checkpoint Inhibitors Large number of checkpoints built into the immune system Response rates (%s) to single checkpoint inhibitors limited to date T cells must be activated, to express checkpoints DCVax activates T cells DCVax mobilizes diverse T cell populations against diverse tumor antigens (targets) Source: Allison, J. (2014). Targeting Immune Checkpoints in Cancer Therapy: New Insights and Opportunities, meetings/sitc2014/primer 12
13 Potentially Complementary With T Cell & Targeted Therapies T cell therapies (CAR-Ts, TCR, etc.) Focus on a particular tumor antigen (CD-19, EGFR, etc.) Designed to boost T cell numbers, affinity, etc. for that antigen Durability not yet clear Addressing diverse antigens may be more important in solid tumors Targeted therapies Focus on a particular cellular pathway Alternate/redundant pathways, tumor escape are an issue DCVax offers broad spectrum anti-tumor action which can complement key rifle shots 13
14 Robust Pipeline of DCVax Programs DCVax -L: Brain cancer Phase III under way (348 pts) Brain cancer Info Arm outside trial (51 pts) Brain cancer Hospital Ex./Other Early Access Metastatic ovarian cancer Phase I completed Compassionate use diverse cancers DCVax - Direct: All solid tumor cancers Phase I/II underway Additional Phase II trial(s) -- pending Pre-clinical Ph I Ph II Ph III DCVax - Prostate Hormone indep. -- Phase I/II completed (36 pts) 14
15 DCVax Direct Program
16 DCVax-Direct: Underlying Biology Immature DCs will effectively take up antigens (biomarkers) in situ in tumors, but will not activate T cells. Also, immature DCs are highly susceptible to suppression by the tumor. Mature DCs will not effectively take up antigens in situ in tumors, but will present antigens to T cells and activate them. ****************************** Partially mature, activated DCs. at a certain stage. will effectively take up antigens (biomarkers) in situ in tumors, and will effectively present antigens to T cells and activate them. 16
17 DCVax-Direct: Activated DCs CD1a: DC-specific marker involved in antigen presentation (not on all DCs, and large patient-to-patient variability) CD209: DC-specific marker involved in binding pathogens (also found on macrophages) pstat-1: phosphorylated STAT-1 signal transduction pathway molecule required for IL-12 production MHC-I, II: MHC molecules involved in antigen presentation CD83: DC activation marker CD40, CD80, CD86: surface molecules involved in T cell activation
18 DCVax Direct: Potential Clinical Effects Possible Clinical Effects Causes Seen in Pre Clinical Studies? Local effects in tumors injected with DCVax-Direct Tumor necrosis (tumor cell death) Secretion of cytokines (e.g., TNFα, IL-6, IL-8) by activated DCs Systemic effects in tumors not injected Tumor shrinkage or elimination Immune system activation Immune memory Lack of recurrence even when re-challenged Immune system activation 18
19 DCVax-Direct Phase I Trial: Overview 40 patients enrolled; 39 evaluable Patients had multiple metastases; had failed other treatments 13 different cancers treated 3 dose levels tested: 2M, 6M and 15M cells Only 1 tumor injected; treatments widely spaced Feasibility of image-guided injections tested (multiple methods) Both imaging and biopsies used to monitor responses, correlate with clinical outcomes and evaluate treatment schedule Both local and systemic responses evaluated Safety and potential endpoints evaluated 19
20 DCVax-Direct Phase I Trial -- Highlights Half of patients still alive, at up to ~22 mos. after first injection Treatment effects observed in diverse cancers Survival correlates with dose levels Survival correlates with number of injections Survival correlates with absence of progression Stable disease (SD) at Wk 8 (4 th injection visit) strongly correlates with survival 21 of 35 patients achieved SD at Wk 8 (progression data n/a on 4 pts) Encouraging immunological responses observed post treatment Induction of immune checkpoint expression Both local effects (in injected tumor) and systemic effects (in non-injected tumors) observed 20
21 DCVax-Direct Phase I Trial Update: Ongoing OS As of Sept 2015 Pancr mcrc Sarcoma mcrc Pancr NET Melan Other Lung Melan Breast mcrc Lung Lung NET Ovarian Bladder NET Pancreas Sarcoma Sarcoma Sarcoma Sarcoma Sarcoma Sarcoma mcrc Melan Melan Sarcoma Pancr Desmo Melan mcrc Pancr NET Melan Lung mcrc Pancreas mcrc Pancr Breast Alive Dead Months
22 Dose Level Correlates With Survival As of Feb Alive Dead million 6 million 15 million 22
23 Number of Injections Correlates With Survival As of Feb Alive Dead injections 3 injections 4 injections 5 injections 6 injections 23
24 Tumor Control (Stable Disease At Week 8) Correlates With Survival As of Feb 2015 p= alive dead SD week 8 PD week 8 24
25 Stable Disease at Week 8 Correlates With Survival As of Sept p=0.031 Proportion Alive Months
26 TNFα Correlates With SD vs. PD At Week 8 As of Feb 2015 p<0.01 In a multivariate analysis, TNFα production is significantly associated with survival (p=0.018) 26
27 IL-8 Production Correlates with Survival As of Feb IL-8 >1,000 Proportion alive IL-8 <1, Months
28 Immunological Responses: Tumor Infiltrating T Cells TILs, including both CD4 + helper T cells and CD8 + killer T cells increased from baseline in 15 of 27 assessed patients Example: clear cell sarcoma CD3 CD4 CD8 Day 0 Day 7 TILs sharing sequences with peripheral T cells also increased, indicating a systemic response 28
29 Immunological Responses: Intra-tumoral Responses Day 0 CD3 + T cells Interferon gamma TNF α Week 8 Expression of T cell cytokines demonstrate anti-tumor activity of infiltrating T cells following DCVax-Direct administration 29
30 Induction of Immune Checkpoint Expression Expression of immune checkpoint molecules in tumor tissue modulates antitumor immune responses Checkpoint inhibitors (CIs) can unblock an existing anti-tumor immune response, but may be ineffective in absence of such pre-existing response 14 of 22 evaluable patients (64%) in DCVax-Direct Phase I trial, showed either de novo or significantly increased expression of the PDL-1 checkpoint molecule after DCVax-Direct treatment; potential candidates for CI treatment Example: De novo PDL-1 staining on sarcoma tissue, 8 weeks after initiation of DCVax-Direct treatment 30
31 PD-L1 On Macrophages In Tumor Correlates With Survival As of Sept Emerging or significantly increased PD-L1 expression 0.8 Proportion Alive No emerging or significantly increased PDL-1 expression Months
32 Phase II Trial Plans Injection of multiple tumors, as well as multiple injections into larger tumors, at each visit More frequent injection schedule Indications with promising activity, as well as diverse indications Soft tissue sarcoma Include Quality of Life measurements Trials both in US and outside US 32
33 DCVax L Program
34 DCVax-L for Operable Tumors: Newly Diagnosed GBM PHASE I/II TRIALS 20 newly diagnosed GBM; 14 recurrent GBM; 5 lower grade gliomas Standard of care (surgery & 6 weeks radiation & chemo) + DCVax-L Primary endpoint: safety; Secondary endpoint: progression free survival Standard of Care* Matched Concurrent DCVax-L Controls** Progression 6.9 mos 8.1 mos 2 years (Tumor Recurrence) Overall Survival 14.6 mos 17 mos 3 years Long Tail of Survival 2 3% alive at 5 years To date: 33% alive >4 yrs 27% alive >6 yrs 2 pts alive >12 yrs * N Engl J Med 352: , 2005 **matched for age, gender, Karnofsky score, extent of surgical resection, and same std of care treatment, at same hospital, in same time period 34
35 International Phase III Trial With DCVax-L for GBM Newly diagnosed GBM; trial under way in both US & Europe 348 patient, randomized (2:1), double blind, placebo controlled Phase III trial: the gold standard in clinical trial design o o Primary endpoint: PFS (progression free survival) Secondary endpoints include OS (overall survival) o 3 DCVax-L treatments upfront (Day 0, 10, 20), then 3 boosters (months 2, 4, 8) then 4 treatments twice/year for maintenance phase (months 12, 18, 24, 30) Multiple protections built into trial design 4-month extension of PFS required to meet primary endpoint (less than 1/3 as long as extension of PFS seen in Phase I/II trials) Trial designed to reach p value = 0.02 if 4-month difference in PFS shown Trial also powered for secondary endpoint of Overall Survival Multiple sub-group analyses were prospectively included 35
36 Information Arm open label, for patients with apparent PD post chemo-radiation 51 patients had evidence (25% increase or new lesion) of apparent disease progression in imaging at Baseline Visit following 6 weeks chemo radiation Patients re imaged at Month 2 after Baseline Visit to confirm actual disease progression (another 25% increase or new lesion) (patients categorized by independent medical imaging company) 20 patients had further progression (another 25% increase or new lesion) at Month 2: Rapid Progressors 25 patients had stable disease, or modest (<25%) progression or some (<25%) regression at Month 2 Indeterminate 1 patient had all original 25% increase gone at Month 2: Confirmed Pseudoprogressor 5 patients Unclassified due to lack of images 36
37 20 Rapid-Progressor Patients: OS in Sept. vs Jan Patient alive in Jan remains alive; OS now > 3-1/2 years Overall, median OS 15.3 months (with Std of Care, months) Alive Dead Recurrent GBM median OS months in literature Months
38 25 Indeterminate Patients: OS in Sept. vs Jan Only 1 patient died during Jan. to Sept, % of patients (10 of 25) now have OS approx. 3 years 20% of patients now have OS 3-1/2 years Median OS is 21.5 months (vs months with Std of Care) Alive Sept 2015 ** Alive Jan ** ** (extension periods vary based on timing of data collection) Dead Sept 2015 Dead Jan Months Median OS of regular GBM patients with Std of Care: ~14.6 mos
39 DCVax -L: Cost-Effective, Rapid Batch Manufacturing DAY 1: Tumor tissue & blood at manufacturing facility. DAY 2: Precursors of dendritic cells isolated. DAY 2-7: Precursors differentiated into dendritic cells. DAY 7: DAY 8: Dendritic cells educated by exposure to biomarkers from tumor tissue. Educated dendritic cells harvested & frozen. Manufacturing finished. (Release tests follow) Single manufacturing run yields 3 5 years of doses of DCVax L product. Only 2 grams of tumor tissue needed for full batch. With <2 grams of tumor tissue, a partial batch can be produced. 39
40 Manufacturing Established At Logistics Hubs In US & Europe Memphis, TN: worldwide hub for both FedEx & UPS 80,000 sq ft facility; capacity for up to 5,000 DCVax patients/year Leipzig, Germany: Europe-wide hub for DHL, Lufthansa Cargo, others 40
41 Large Intellectual Property Portfolio DCVax -L for brain cancer: Orphan status granted in US and EU DCVax -Prostate and DCVax -Direct: Composition and methods Other platform coverage: Manufacturing for more potent dendritic cells Isolation, maturation and education of dendritic cells TFF system - automation of manufacturing Methods of delivery to patients Over 180 patents issued and pending, worldwide 41
42 Experienced Management Team Linda F. Powers Chairman & Chief Executive Officer 12+ years experience building biotechs, developing cell therapy products 15+ years experience in corporate finance, M&A, joint ventures, and IP Largest shareholder of NW: >$20 million invested Dr. Marnix Bosch Chief Technical Officer Former Head of Molecular Biology at Dutch National Institutes of Health Former Associate Professor at Univ. Washington Author of >50 research publications in immunology, virology & cancer Dr. Alton Boynton Chief Scientific Officer & Co-founder Formerly Director of the Department of Molecular Medicine of Northwest Hospital, Director of Foundation from which Fred Hutchinson Cancer Center was spun off Associate Director of Cancer Research Center of Hawaii. Dr. Anthony Maida Senior VP, Clinical Research Over 20 years experience building oncology companies; expertise in the business, financial, clinical, regulatory and science. Former controller of multi-billion dollar division of public companies Les Goldman SVP, Business Development Former Partner at Skadden, Arps; over 30 years corporate finance, legal and media experience 42
Oncos Therapeutics: ONCOS THERAPEUTICS Personalized Cancer Immunotherapy. March 2015. Antti Vuolanto, COO and co-founder
 Oncos Therapeutics: Personalized Cancer Immunotherapy ONCOS THERAPEUTICS Personalized Cancer Immunotherapy March 2015 Antti Vuolanto, COO and co-founder 1 History of Oncos Therapeutics 2002 2007 2009 Research
Oncos Therapeutics: Personalized Cancer Immunotherapy ONCOS THERAPEUTICS Personalized Cancer Immunotherapy March 2015 Antti Vuolanto, COO and co-founder 1 History of Oncos Therapeutics 2002 2007 2009 Research
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY. Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds
 NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
NEW CLINICAL RESEARCH OPTIONS IN PANCREATIC CANCER IMMUNOTHERAPY Alan Melcher Professor of Clinical Oncology and Biotherapy Leeds CANCER IMMUNOTHERAPY - Breakthrough of the Year in Science magazine 2013.
specific B cells Humoral immunity lymphocytes antibodies B cells bone marrow Cell-mediated immunity: T cells antibodies proteins
 Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Adaptive Immunity Chapter 17: Adaptive (specific) Immunity Bio 139 Dr. Amy Rogers Host defenses that are specific to a particular infectious agent Can be innate or genetic for humans as a group: most microbes
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO
 Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
Transgene Presents Additional Positive Clinical Data from Phase 2b Part of TIME Trial with TG4010 at ESMO Statistically significant difference in progression-free survival continues to be seen in non-squamous
18.5 Percent Overall Response Rate Observed in Pembrolizumab-Treated Patients with this Aggressive Form of Breast Cancer
 News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
News Release Media Contacts: Annick Robinson Investor Contacts: Joseph Romanelli (514) 837-2550 (908) 740-1986 Stephanie Lyttle NATIONAL Public Relations (514) 843-2365 Justin Holko (908) 740-1879 Merck
New Advances in Cancer Treatments. March 2015
 New Advances in Cancer Treatments March 2015 Safe Harbour Statement This presentation document contains certain forward-looking statements and information (collectively, forward-looking statements ) within
New Advances in Cancer Treatments March 2015 Safe Harbour Statement This presentation document contains certain forward-looking statements and information (collectively, forward-looking statements ) within
A Letter from MabVax Therapeutics President and Chief Executive Officer
 A Letter from MabVax Therapeutics President and Chief Executive Officer Dear Fellow Stockholder: You have invested in MabVax Therapeutics because you share our passion for finding new therapies for the
A Letter from MabVax Therapeutics President and Chief Executive Officer Dear Fellow Stockholder: You have invested in MabVax Therapeutics because you share our passion for finding new therapies for the
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Pfizer Forms Global Alliance with Merck KGaA, Darmstadt, Germany to Accelerate Presence in Immuno-Oncology. November 17, 2014
 Pfizer Forms Global Alliance with Merck KGaA, Darmstadt, Germany to Accelerate Presence in Immuno-Oncology November 17, 2014 Forward-looking statements Our discussions during this conference call will
Pfizer Forms Global Alliance with Merck KGaA, Darmstadt, Germany to Accelerate Presence in Immuno-Oncology November 17, 2014 Forward-looking statements Our discussions during this conference call will
Heat Biologics, Inc. (Exact name of registrant as specified in charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (date of earliest event
BNC105 PHASE II RENAL CANCER TRIAL RESULTS
 ABN 53 075 582 740 ASX ANNOUNCEMENT 19 March 2014 BNC105 PHASE II RENAL CANCER TRIAL RESULTS Results show BNC105 utility in patients with advanced disease Identified biomarkers which correlate with patient
ABN 53 075 582 740 ASX ANNOUNCEMENT 19 March 2014 BNC105 PHASE II RENAL CANCER TRIAL RESULTS Results show BNC105 utility in patients with advanced disease Identified biomarkers which correlate with patient
Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF)
 June 2015 Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF) Strong, Fast and Long Lasting Vaccines For Cancer and Infectious Diseases FORWARD-LOOKING STATEMENTS Except for historical information, this presentation
June 2015 Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF) Strong, Fast and Long Lasting Vaccines For Cancer and Infectious Diseases FORWARD-LOOKING STATEMENTS Except for historical information, this presentation
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Your Immune System & Lung Cancer Treatment
 Your Immune System & Lung Cancer Treatment Immunotherapy and Lung Cancer Immunotherapy is quickly developing as an important approach to treating many forms of cancer, including lung cancer. Immunotherapy
Your Immune System & Lung Cancer Treatment Immunotherapy and Lung Cancer Immunotherapy is quickly developing as an important approach to treating many forms of cancer, including lung cancer. Immunotherapy
High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem
 High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem rmerrell@northshore.org Objectives Provide updates on
High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem rmerrell@northshore.org Objectives Provide updates on
Metastatic Breast Cancer 201. Carolyn B. Hendricks, MD October 29, 2011
 Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
Metastatic Breast Cancer 201 Carolyn B. Hendricks, MD October 29, 2011 Overview Is rebiopsy necessary at the time of recurrence or progression of disease? How dose a very aggressive treatment upfront compare
IMMUNOMEDICS, INC. February 2016. Advanced Antibody-Based Therapeutics. Oncology Autoimmune Diseases
 IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
IMMUNOMEDICS, INC. Advanced Antibody-Based Therapeutics Oncology Autoimmune Diseases February 2016 Forward-Looking Statements This presentation, in addition to historical information, contains certain
Immunovaccine Inc. (TSX-V: IMV)
 May 2014 Immunovaccine Inc. (TSX-V: IMV) Targeted T cell Activation Immunotherapies FORWARD-LOOKING STATEMENTS This document contains forward-looking information pursuant to applicable securities law.
May 2014 Immunovaccine Inc. (TSX-V: IMV) Targeted T cell Activation Immunotherapies FORWARD-LOOKING STATEMENTS This document contains forward-looking information pursuant to applicable securities law.
Recruiting now. Could you help by joining this study?
 Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Equity markets Major advances in cancer therapeutics 18 August 2015
 Major advances in cancer therapeutics 18 August 2015 CIO WM Research Jerome Brimeyer, Equity Sector Strategist, jerome.brimeyer@ubs.com Our cancer therapeutics investment theme recommends companies that
Major advances in cancer therapeutics 18 August 2015 CIO WM Research Jerome Brimeyer, Equity Sector Strategist, jerome.brimeyer@ubs.com Our cancer therapeutics investment theme recommends companies that
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development
 Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Predictive Biomarkers for PD1 Pathway Inhibitor Immunotherapy
 Predictive Biomarkers for PD1 Pathway Inhibitor Immunotherapy Michael B. Atkins, M.D. Deputy Director Georgetown-Lombardi Comprehensive Cancer Center Michael Atkins, MD Consulting Fees (e.g., advisory
Predictive Biomarkers for PD1 Pathway Inhibitor Immunotherapy Michael B. Atkins, M.D. Deputy Director Georgetown-Lombardi Comprehensive Cancer Center Michael Atkins, MD Consulting Fees (e.g., advisory
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO
 Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking
 Q1/2016 This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking statements involve risks and uncertainties
Q1/2016 This presentation may contain forward-looking statements, which reflect Trillium's current expectation regarding future events. These forward-looking statements involve risks and uncertainties
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
Nuevas tecnologías basadas en biomarcadores para oncología
 Nuevas tecnologías basadas en biomarcadores para oncología Simposio ASEBIO 14 de marzo 2013, PCB Jose Jimeno, MD, PhD Co-Founder / Vice Chairman Pangaea Biotech SL Barcelona, Spain PANGAEA BIOTECH BUSINESS
Nuevas tecnologías basadas en biomarcadores para oncología Simposio ASEBIO 14 de marzo 2013, PCB Jose Jimeno, MD, PhD Co-Founder / Vice Chairman Pangaea Biotech SL Barcelona, Spain PANGAEA BIOTECH BUSINESS
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
SORRENTO THERAPEUTICS, INC. (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Localised Cancer Treatment. PCI Biotech. Amphinex a new product for localised cancer treatment
 Localised Cancer Treatment PCI Biotech Amphinex a new product for localised cancer treatment Disclaimer This document (the Presentation ) has been produced by PCI Biotech Holding ASA (the Company ). The
Localised Cancer Treatment PCI Biotech Amphinex a new product for localised cancer treatment Disclaimer This document (the Presentation ) has been produced by PCI Biotech Holding ASA (the Company ). The
Enhancing Anti-Tumor Activity of Checkpoint Inhibition
 Enhancing Anti-Tumor Activity of Checkpoint Inhibition Jeffrey Schlom, Ph.D. Laboratory of Tumor Immunology and Biology (LTIB) Center for Cancer Research National Cancer Institute, NIH Laboratory of Tumor
Enhancing Anti-Tumor Activity of Checkpoint Inhibition Jeffrey Schlom, Ph.D. Laboratory of Tumor Immunology and Biology (LTIB) Center for Cancer Research National Cancer Institute, NIH Laboratory of Tumor
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
Foundational Issues Related to Immunotherapy and Melanoma
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Immunovaccine Inc. (TSX-V: IMV) July 2011
 Immunovaccine Inc. (TSX-V: IMV) July 2011 2 Forward Looking Statements This document contains forward-looking information pursuant to applicable securities law. All information that addresses activities
Immunovaccine Inc. (TSX-V: IMV) July 2011 2 Forward Looking Statements This document contains forward-looking information pursuant to applicable securities law. All information that addresses activities
An Introduction To Immunotherapy And The Promise Of Tissue Phenomics
 An Introduction To Immunotherapy And The Promise Of Tissue Phenomics INSIDE: n The Potential of Immunotherapy n Towards an Understanding of Immunotherapy n Current Approaches to Immunotherapy n The Immunotherapy
An Introduction To Immunotherapy And The Promise Of Tissue Phenomics INSIDE: n The Potential of Immunotherapy n Towards an Understanding of Immunotherapy n Current Approaches to Immunotherapy n The Immunotherapy
Cytotoxic and Biotherapies Credentialing Programme Module 2
 Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Cytotoxic and Biotherapies Credentialing Programme Module 2 1. The Cell Cycle 2. Cancer Therapies 3. Adjunctive Therapies On completion of this module the RN will State the difference between a normal
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Corporate Presentation June 2, 2015
 Corporate Presentation June 2, 2015 SAFE HARBOR STATEMENT This presentation contains forward-looking statements. Although the Company believes its expectations are based on reasonable assumptions, these
Corporate Presentation June 2, 2015 SAFE HARBOR STATEMENT This presentation contains forward-looking statements. Although the Company believes its expectations are based on reasonable assumptions, these
Immunotherapy Concept Turned Reality
 Authored by: Jennifer Dolan Fox, PhD VirtualScopics Inc. jennifer_fox@virtualscopics.com +1 585 249 6231 Immunotherapy Concept Turned Reality Introduction While using the body s own immune system as a
Authored by: Jennifer Dolan Fox, PhD VirtualScopics Inc. jennifer_fox@virtualscopics.com +1 585 249 6231 Immunotherapy Concept Turned Reality Introduction While using the body s own immune system as a
Endpoint Selection in Phase II Oncology trials
 Endpoint Selection in Phase II Oncology trials Anastasia Ivanova Department of Biostatistics UNC at Chapel Hill aivanova@bios.unc.edu Primary endpoints in Phase II trials Recently looked at journal articles
Endpoint Selection in Phase II Oncology trials Anastasia Ivanova Department of Biostatistics UNC at Chapel Hill aivanova@bios.unc.edu Primary endpoints in Phase II trials Recently looked at journal articles
Understanding CA 125 Levels A GUIDE FOR OVARIAN CANCER PATIENTS. foundationforwomenscancer.org
 Understanding CA 125 Levels A GUIDE FOR OVARIAN CANCER PATIENTS foundationforwomenscancer.org Contents Introduction...1 CA 125................................... 1 The CA 125 Test...2 The Use of the CA
Understanding CA 125 Levels A GUIDE FOR OVARIAN CANCER PATIENTS foundationforwomenscancer.org Contents Introduction...1 CA 125................................... 1 The CA 125 Test...2 The Use of the CA
Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF)
 November 2015 Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF) Strong, Fast and Long Lasting Vaccines For Cancer and Infectious Diseases FORWARD-LOOKING STATEMENTS Except for historical information, this presentation
November 2015 Immunovaccine Inc. (TSX: IMV) (OTCQX: IMMVF) Strong, Fast and Long Lasting Vaccines For Cancer and Infectious Diseases FORWARD-LOOKING STATEMENTS Except for historical information, this presentation
Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation
 Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation Hans S. Keirstead President, NeoStem Oncology NeoStem Presented by GTC, a conference production company 626-256-6405
Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation Hans S. Keirstead President, NeoStem Oncology NeoStem Presented by GTC, a conference production company 626-256-6405
Chapter 43: The Immune System
 Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Name Period Our students consider this chapter to be a particularly challenging and important one. Expect to work your way slowly through the first three concepts. Take particular care with Concepts 43.2
Breakthrough oral drug delivery technology for diabetes, drugs and vaccines November 5, 2014
 Oramed Pharmaceuticals, Inc. Initiating Report Breakthrough oral drug delivery technology for diabetes, drugs and vaccines November 5, 2014 Key data Sector Rating Biotechnology BUY Target Price $45.00
Oramed Pharmaceuticals, Inc. Initiating Report Breakthrough oral drug delivery technology for diabetes, drugs and vaccines November 5, 2014 Key data Sector Rating Biotechnology BUY Target Price $45.00
The role of IBV proteins in protection: cellular immune responses. COST meeting WG2 + WG3 Budapest, Hungary, 2015
 The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
Understanding series. new. directions. 1-800-298-2436 LungCancerAlliance.org. A guide for the patient
 Understanding series LUNG CANCER: new treatment directions 1-800-298-2436 LungCancerAlliance.org A guide for the patient TABLE OF CONTENTS What s New in lung cancer? Advancements...4 Changes in genes that
Understanding series LUNG CANCER: new treatment directions 1-800-298-2436 LungCancerAlliance.org A guide for the patient TABLE OF CONTENTS What s New in lung cancer? Advancements...4 Changes in genes that
Overview of Phase 1 Oncology Trials of Biologic Therapeutics
 Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Advances in Treatment of Malignant Pleural Mesothelioma: A Reason for Hope
 Advances in Treatment of Malignant Pleural Mesothelioma: A Reason for Hope Daniel H. Sterman, M.D. Associate Professor of Medicine and Surgery Co-Director, PENN Mesothelioma and Pleural Program University
Advances in Treatment of Malignant Pleural Mesothelioma: A Reason for Hope Daniel H. Sterman, M.D. Associate Professor of Medicine and Surgery Co-Director, PENN Mesothelioma and Pleural Program University
The immune system. Bone marrow. Thymus. Spleen. Bone marrow. NK cell. B-cell. T-cell. Basophil Neutrophil. Eosinophil. Myeloid progenitor
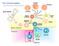 The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
The immune system Basophil Neutrophil Bone marrow Eosinophil Myeloid progenitor Dendritic cell Pluripotent Stem cell Lymphoid progenitor Platelets Bone marrow Thymus NK cell T-cell B-cell Spleen Cancer
Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells. Corporate Presentation May 2015
 Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells Corporate Presentation May 2015 Forward-looking statements / safe harbor This presentation and the accompanying oral
Breakthrough Cancer Therapies: Directing the Immune System to Eliminate Tumor Cells Corporate Presentation May 2015 Forward-looking statements / safe harbor This presentation and the accompanying oral
Robert Bristow MD PhD FRCPC
 Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
MOLOGEN AG German Equity Forum 2015
 German Equity Forum 2015 Dr. Mariola Söhngen Chief Executive Officer Frankfurt am Main 24 November 2015 We are Pioneers in the Field of Immunotherapies Biotechnology company with focus on immunotherapies
German Equity Forum 2015 Dr. Mariola Söhngen Chief Executive Officer Frankfurt am Main 24 November 2015 We are Pioneers in the Field of Immunotherapies Biotechnology company with focus on immunotherapies
Moving forward, where are we with Clinical Trials?
 Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED
 ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
 September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
September 27, 2013 ONO PHARMACEUTICAL CO., LTD. Corporate Communications Phone: +81-6-6263-5670 Decision to Continue the Development of Tecemotide (L-BLP25) in Non-Small Cell Lung Cancer to be Announced
Future Oncology: Technology, Products, Market and Service Opportunities
 Brochure More information from http://www.researchandmarkets.com/reports/296370/ Future Oncology: Technology, Products, Market and Service Opportunities Description: Future Oncology is an analytical newsletter
Brochure More information from http://www.researchandmarkets.com/reports/296370/ Future Oncology: Technology, Products, Market and Service Opportunities Description: Future Oncology is an analytical newsletter
CLINICAL POLICY Department: Medical Management Document Name: Opdivo Reference Number: CP.PHAR.121 Effective Date: 07/15
 Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Your Immune System & Melanoma Treatment
 Your Immune System & Melanoma Treatment Immunotherapy and Melanoma Immunotherapy is rapidly emerging as an important approach to treating many forms of cancer. For people with melanoma, the news is particularly
Your Immune System & Melanoma Treatment Immunotherapy and Melanoma Immunotherapy is rapidly emerging as an important approach to treating many forms of cancer. For people with melanoma, the news is particularly
B Cells and Antibodies
 B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
B Cells and Antibodies Andrew Lichtman, MD PhD Brigham and Women's Hospital Harvard Medical School Lecture outline Functions of antibodies B cell activation; the role of helper T cells in antibody production
Future strategies for myeloma: An overview of novel treatments In development
 Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
Future strategies for myeloma: An overview of novel treatments In development Dr. Matthew Streetly Guys and St. Thomas NHS Trust How far have we come? Melphalan and prednisolone VAD Autologous SCT Thalidomide
MOLOGEN AG. Dr. Matthias Schroff Chief Executive Officer Dr. Alfredo Zurlo Chief Medical Officer. Roadshow Abu Dhabi, Dubai April 2015
 Dr. Matthias Schroff Chief Executive Officer Dr. Alfredo Zurlo Chief Medical Officer Roadshow Abu Dhabi, Dubai April 2015 Disclaimer Certain statements in this presentation contain formulations or terms
Dr. Matthias Schroff Chief Executive Officer Dr. Alfredo Zurlo Chief Medical Officer Roadshow Abu Dhabi, Dubai April 2015 Disclaimer Certain statements in this presentation contain formulations or terms
Genomic Medicine The Future of Cancer Care. Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America
 Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Genomic Medicine The Future of Cancer Care Shayma Master Kazmi, M.D. Medical Oncology/Hematology Cancer Treatment Centers of America Personalized Medicine Personalized health care is a broad term for interventions
Principal Investigator: Valerie W. Rusch, MD, FACS, Chief, Thoracic Surgery Memorial Sloan-Kettering Cancer Center
 Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
CAN-FITE BIOPHARMA LTD.
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 Under the Securities Exchange Act of 1934 For the Month
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 Under the Securities Exchange Act of 1934 For the Month
2) Macrophages function to engulf and present antigen to other immune cells.
 Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immune Therapy for Pancreatic Cancer
 Immune Therapy for Pancreatic Cancer December 16, 2014 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-229-3239 Toll Only:
Immune Therapy for Pancreatic Cancer December 16, 2014 If you experience technical difficulty during the presentation: Contact WebEx Technical Support directly at: US Toll Free: 1-866-229-3239 Toll Only:
AFFITECH and XOMA Sign Antibody Collaboration and Cross-License Agreement
 FOR IMMEDIATE RELEASE Ref 05AFF05 Contacts for Affitech: Contacts for XOMA Affitech (Norway): Investor Inquiries Dr. Martin Welschof Ellen M Martin Chief Executive Officer Kureczka/Martin Associates Phone:
FOR IMMEDIATE RELEASE Ref 05AFF05 Contacts for Affitech: Contacts for XOMA Affitech (Norway): Investor Inquiries Dr. Martin Welschof Ellen M Martin Chief Executive Officer Kureczka/Martin Associates Phone:
Immuno-Oncology Therapies to Treat Lung Cancer
 Immuno-Oncology Therapies to Treat Lung Cancer What you need to know ONCHQ14NP07519 Introduction: Immuno-oncology represents an innovative approach to cancer research that seeks to harness the body s own
Immuno-Oncology Therapies to Treat Lung Cancer What you need to know ONCHQ14NP07519 Introduction: Immuno-oncology represents an innovative approach to cancer research that seeks to harness the body s own
The Immune System: A Tutorial
 The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
The Immune System: A Tutorial Modeling and Simulation of Biological Systems 21-366B Shlomo Ta asan Images taken from http://rex.nci.nih.gov/behindthenews/uis/uisframe.htm http://copewithcytokines.de/ The
Navigating GIST. The Life Raft Group June 12, 2008
 Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Company Presentation June 2011 Biotest AG 0
 Company Presentation June 2011 0 Disclaimer This document contains forward-looking statements on overall economic development as well as on the business, earnings, financial and asset situation of and
Company Presentation June 2011 0 Disclaimer This document contains forward-looking statements on overall economic development as well as on the business, earnings, financial and asset situation of and
Cancer Immunotherapy: immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapies - Overview
 Brochure More information from http://www.researchandmarkets.com/reports/3066973/ Cancer Immunotherapy: immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapies - Overview Description:
Brochure More information from http://www.researchandmarkets.com/reports/3066973/ Cancer Immunotherapy: immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapies - Overview Description:
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center
 What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
LOOKING FORWARD PUMA BIOTECHNOLOGY, INC. 2014 ANNUAL REPORT
 LOOKING FORWARD PUMA BIOTECHNOLOGY, INC. 2014 ANNUAL REPORT Puma Biotechnology, Inc. is a development stage biopharmaceutical company that acquires and develops innovative products for the treatment of
LOOKING FORWARD PUMA BIOTECHNOLOGY, INC. 2014 ANNUAL REPORT Puma Biotechnology, Inc. is a development stage biopharmaceutical company that acquires and develops innovative products for the treatment of
Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL)
![Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL)](/thumbs/26/8616095.jpg) PRESS RELEASE Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Meta-analysis of three late-stage trials in people with type 2 diabetes shows decreases
PRESS RELEASE Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Meta-analysis of three late-stage trials in people with type 2 diabetes shows decreases
Coxsackievirus A21 (CAVATAK TM ) - mediated oncolytic immunotherapy in advanced melanoma patients
 Coxsackievirus A21 (CAVATAK TM ) - mediated oncolytic immunotherapy in advanced melanoma patients Robert H.I. Andtbacka 1, Brendan Curti 2, Mark Grose 3, Len Post 4, Jeffrey Ira Weisberg 4 and Darren Shafren
Coxsackievirus A21 (CAVATAK TM ) - mediated oncolytic immunotherapy in advanced melanoma patients Robert H.I. Andtbacka 1, Brendan Curti 2, Mark Grose 3, Len Post 4, Jeffrey Ira Weisberg 4 and Darren Shafren
Fulfilling the Promise
 Fulfilling the Promise Advancing the Fight Against Cancer: America s Medical Schools and Teaching Hospitals For more than a century, the nation s medical schools and teaching hospitals have worked to understand,
Fulfilling the Promise Advancing the Fight Against Cancer: America s Medical Schools and Teaching Hospitals For more than a century, the nation s medical schools and teaching hospitals have worked to understand,
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022
 Brochure More information from http://www.researchandmarkets.com/reports/2228475/ Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022 Description: Avastin (Renal Cell Carcinoma) Analysis and
Brochure More information from http://www.researchandmarkets.com/reports/2228475/ Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022 Description: Avastin (Renal Cell Carcinoma) Analysis and
CEL-SCI Corporation NYSE AMEX: CVM. Geert Kersten Chief Executive Officer 8229 Boone Boulevard, Suite 802 Vienna, VA 22182, USA Phone: (703) 506-9460
 CEL-SCI Corporation NYSE AMEX: CVM Geert Kersten Chief Executive Officer 8229 Boone Boulevard, Suite 802 Vienna, VA 22182, USA Phone: (703) 506-9460 November 2009 SEC Disclaimer Statements made during
CEL-SCI Corporation NYSE AMEX: CVM Geert Kersten Chief Executive Officer 8229 Boone Boulevard, Suite 802 Vienna, VA 22182, USA Phone: (703) 506-9460 November 2009 SEC Disclaimer Statements made during
STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016
 STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments, as well as negations of
STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments, as well as negations of
News Release. Merck KGaA, Darmstadt, Germany, and Pfizer Receive FDA Breakthrough Therapy Designation for Avelumab in Metastatic Merkel Cell Carcinoma
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer Drugs
 Provisional Translation (as of January 27, 2014)* November 15, 2013 Pharmaceuticals and Bio-products Subcommittees, Science Board Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer
Provisional Translation (as of January 27, 2014)* November 15, 2013 Pharmaceuticals and Bio-products Subcommittees, Science Board Summary of Discussion on Non-clinical Pharmacology Studies on Anticancer
Asthma (With a little SCID to start) Disclosures Outline Starting with the Immune System The Innate Immune System The Adaptive Immune System
 1 2 3 4 5 6 7 8 9 Asthma (With a little SCID to start) Lauren Smith, MD CHKD Pediatric Allergy/Immunology Disclosures None Will be discussing some medications that are not yet FDA approved Outline SCID
1 2 3 4 5 6 7 8 9 Asthma (With a little SCID to start) Lauren Smith, MD CHKD Pediatric Allergy/Immunology Disclosures None Will be discussing some medications that are not yet FDA approved Outline SCID
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ
 OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Strategic Consulting Services
 Services 1 Leadership Team Mark Levonyak President mlevonyak@davaonc.com Mobile: 214.460.5051 Martin W. Lee, MD EVP, Clinical Trial Services mlee@davaonc.com Mobile: 952.373.1405 John Eckardt, MD Chief
Services 1 Leadership Team Mark Levonyak President mlevonyak@davaonc.com Mobile: 214.460.5051 Martin W. Lee, MD EVP, Clinical Trial Services mlee@davaonc.com Mobile: 952.373.1405 John Eckardt, MD Chief
Immunotherapy of Uveal Melanoma
 Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Eye Am Not Alone (EANA) Patient Retreat Saturday Session - March 3, 2012 Immunotherapy of Uveal Melanoma Do not distribute or copy without the express permission of the Ocular Melanoma Foundation (OMF).
Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents
 Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents J. Jack Lee, Ph.D. Department of Biostatistics 3 Primary Goals for Clinical Trials Test safety and
Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents J. Jack Lee, Ph.D. Department of Biostatistics 3 Primary Goals for Clinical Trials Test safety and
HUMORAL IMMUNE RE- SPONSES: ACTIVATION OF B CELLS AND ANTIBODIES JASON CYSTER SECTION 13
 SECTION 13 HUMORAL IMMUNE RE- SPONSES: ACTIVATION OF B CELLS AND ANTIBODIES CONTACT INFORMATION Jason Cyster, PhD (Email) READING Basic Immunology: Functions and Disorders of the Immune System. Abbas,
SECTION 13 HUMORAL IMMUNE RE- SPONSES: ACTIVATION OF B CELLS AND ANTIBODIES CONTACT INFORMATION Jason Cyster, PhD (Email) READING Basic Immunology: Functions and Disorders of the Immune System. Abbas,
SUMMARY. Randolph Fillmore, Florida Science Communications
 SUMMARY Randolph Fillmore, Florida Science Communications The H. Lee Moffitt Cancer Center held its first Advanced Prostate Cancer Collaboration roundtable discussion November 24, 2008. The roundtable
SUMMARY Randolph Fillmore, Florida Science Communications The H. Lee Moffitt Cancer Center held its first Advanced Prostate Cancer Collaboration roundtable discussion November 24, 2008. The roundtable
Adaptimmune Reports Full Fiscal Year 2015 Financial Results. - Conference call to be held today at 8:00 AM ET (1:00 PM BST) -
 Adaptimmune Reports Full Fiscal Year 2015 Financial Results - Conference call to be held today at 8:00 AM ET (1:00 PM BST) - PHILADELPHIA, Pa. and OXFORD, United Kingdom., October 13, 2015 Adaptimmune
Adaptimmune Reports Full Fiscal Year 2015 Financial Results - Conference call to be held today at 8:00 AM ET (1:00 PM BST) - PHILADELPHIA, Pa. and OXFORD, United Kingdom., October 13, 2015 Adaptimmune
Newsletter. WntResearch AB, Medeon Science Park, Per Albin Hanssons väg 41, 205 12 Malmö, Sweden. Primary Objective:
 Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
Newsletter This resume of the results from the phase 1 study with Foxy-5 is based on clinical and laboratory data from the study, and these data have now been locked into the database. The full report
How To Collect A Leukapheresis Collection
 Leukapheresis Collection Insights in Unstimulated Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving a Combination of Autologous Immunotherapy (AGS-003) and Sunitinib: From the Ongoing
Leukapheresis Collection Insights in Unstimulated Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving a Combination of Autologous Immunotherapy (AGS-003) and Sunitinib: From the Ongoing
Ask Us About Clinical Trials
 Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
