Cisplatin and Etoposide Concurrent with Radiotherapy in Non-Small Cell Lung Cancer: Single Institution Experience
|
|
|
- Elijah Perry
- 7 years ago
- Views:
Transcription
1 Med. J. Cairo Univ., Vol. 81, No. 1, June: , Cisplatin and Etoposide Concurrent with Radiotherapy in Non-Small Cell Lung Cancer: Single Institution Experience HANY H. ELDEEB, M.D.* and SEHAM E. ABD-ELKHALK, M.D.** The Departments of Clinical Oncology, Northampton Center for Oncology, UK* and Clinical Oncology, Mansoura University Hospital, Egypt** Abstract Introduction: Lung cancer is the most common cancer in the world and the leading cause of cancer-related deaths in Western countries. Phase III trials have demonstrated an improvement in survival with the combination of chemotherapy and radiotherapy over radiation alone for patients with a good performance status with stage III NSCLC. Patients and Methods: This is a retrospective study of patients with unresectable NSCLC treated with radical chemoradiotherapy at Northampton Oncology Centre in the period of May 2005-December Results: There is a statistically significant advantage in overall survival for adenocarcinoma patients over those with squamous cell carcinoma p= Median overall survival (OS) for patients with adenocarcinoma was 37 months (std. error , 95% CI ) while squamous cell carcinoma OS was 13 months (std error 7.115, 95% CI ). Conclusion: Our survival data for concurrent chemoradiotherapy matches with the international published data. Future research is needed to define the best concurrent chemotherapy regimen as well as the role of consolidation and maintenance chemotherapy. Key Words: Non-Small Cell Lung Cancer (NSCLC) Cisplatin Etoposide Radiotherapy. Introduction LUNG cancer is the most common cancer in the world and the leading cause of cancer-related deaths in Western countries. Non-small cell lung cancer (NSCLC) represents approximately 80-85% of all lung cancerl and approximately one third of patients will have stage III disease at the time of diagnosis. For patients with early stage NSCLC, surgical resection remains the standard of care. Five year survival for NSCLC remains low, ranging from 24% for patients with stage IIIA to 73% for patients with stage I disease [2]. Correspondence to: Dr. Hany Hashim Eldeeb hheldeeb@yahoo.com Phase III trials have demonstrated an improvement in survival with the combination of chemotherapy and radiotherapy over radiation alone for patients with a good performance status with stage III NSCLC. Combined modality therapy is the standard of care in United States [3]. However, there has been significant variability in the overall survival observed across combined modality therapy trials [4]. It is important to identify optimal regimens of combined chemotherapy and radiotherapy and to evaluate the feasibility and efficacy of such combinations 151. The goals of these approaches are to gain control of localized disease and to eradicate distant micrometastatic disease [6]. A meta analysis of 11 randomized trials has shown that the addition of cisplatin- based chemotherapy to radiotherapy is associated with a 10% reduction in the risk of death, relative to radiotherapy alone (p=0.006). Sequential platinum-based chemotherapy followed by radiotherapy improved survival in unresectable stage III NSCLC when compared with radiotherapy alone [7]. The most notable results in this respect were reported by the Cancer and Leukaemia Group B (CALGB) [8] and the Radiation Therapy Oncology Group (RTOG) [9]. More recently, concurrent administration of chemotherapy and radiotherapy has been reported to be superior to sequential therapy by the RTOG [10] and by Japanese investigators [11,12]. However, chemoradiotherapy is also associated with significantly higher rates of grade 3 or more toxic effects [13]. As a result studies such as the phase III trial conducted by the West Japan Thoracic Oncology Group, which aimed to identify more tolerable chemotherapy regimens to be used con- 325
2 326 Cisplatin & Etoposide Concurrent with Radiotherapy currently with thoracic radiation are of particular clinical relevance [14,15]. In this retrospective study we present Northampton Oncology Centre experience using concomitant chemoradiotherapy. Material and Methods This is a retrospective study of patients with unresectable NSCLC stages (I-III) treated at Northampton Oncology Centre in the period of May 2005-December Eligibility criteria for this study were: Cytologic or histologic diagnosis of NSCLC, stages (I-III), with the presence of measurable or assessable disease, patient age 18 years, Eastern Cooperative Oncology Group (EC- OG) performance status of 0 to 2, absolute neutrophil count 2,000/mL, platelet count 100,000, hemoglobin 9.5gm/d1, serum creatinin <1.2mg/d1, forced expiratory volume in 1 second (FEV1) >800mL, and adequate liver values: Total bilirubin <1.5 times the institutional upper limits of normal (ULN), AST and ALT 2.5 times the institutional (ULN). Patients were excluded from study participation if they had any of the following conditions: Malignant pleural effusion, distant metastases, serious medical illness or active concurrent malignancy. Baseline assessment included a complete medical history, evaluation of PS, physical examination and assessment of vital signs, blood tests (complete blood cell count with differential and platelet counts and blood chemistry evaluation); chest radiographs and computed tomography (CT) scans of chest and upper abdomen and PET scan. Baseline evaluation had to be performed within 2 weeks before therapy initiation. All measurable lesions were identified at baseline and monitored throughout. Treatment plan: Patients started concurrent chemo-radiotherapy. The regimen used in these patients was cisplatin 50mg/m 2 days 1, 8, 29 and 36 and intravenous etoposide 50mg/m2 days 1 and 29 followed by oral etoposide once daily 100mg/m 2 on days 2-5 and during the course of radiotherapy. Radiotherapy was delivered with photon beams generated by Linear accelerator. Administered in 2Gy/ fractions/day, 5 days/week. The Gross Target Volume (GTV) included the entire tumor and involved lymph nodes on the PET scan with no elective nodal irradiation. The clinical target volume (CTV) was obtained by adding 8mm to the GTV and the PTV was generated by adding a further 7mm to CTV. Conformal radiotherapy planning was utilized to target the PTV. Statistical analysis: The primary end point of this study was the overall survival. Survival was calculated from the date of diagnosis to death or last follow-up evaluation at January Survival curves were established with the Kaplan-meier method and were compared using the Log-rank test and Cox model. Usual statistical tests (x 2 test and Fisher's exact probability test) were used to compare variables in the same treatment group. Differences were considered significant at p<.05. These tests were run on an IBM compatible personal computer using the statistical Package for Social scientists (SPSS) for windows 10.0 (SPSS Inc., Chicago, IL,USA). Results A total of 32 patients were treated between May 2005 and December There were 23 male and 9 females. Eleven patients had PS 0 (35.5%), 16 patients (51.6%) had PS 1 while only 4 (12.9%) patients with PS 2. Most of patients (12) had adenocarcinoma (37.5%), ten patients (31.3%) had squamous cell carcinoma, three with adenosquamous and the remaining three patients had unspecified Non-Small Cell Carcinoma. (Tables 1,2) show patient's characteristics. Although the prescribed dose was Gy, three patients were treated with 56Gy due to limitation of lung V20 and V5 values. There is a statistically significant advantage in overall survival for adenocarcinoma patients over those with squamous cell carcinoma p= Median overall survival (OS) for patients with adenocarcinoma was 37 months (Std. Error , 95% CI ) while squamous cell carcinoma OS was 13 months (Std. Error 7.115, 95% CI ). Median overall survival for patients with stage I was 37 months (Std. Errorl4.5OO, 95% CI ), stage II was 40 months (Std 2.449, 95% CI ), stage Ma is 17 months (Std. Error ) and for stage Illb is 20 months (Std ). By pair wise comparison there is statistically significant difference in overall survival for stage I versus Illb (p=0.026), stage II vs Illb (p=0.009), also better overall survival, but not statistically significant for Ma vs Mb (p=0.08).
3 Hany H. Eldeeb & Seham E. Abd-Elkhalk 327 Median overall survival for patients that were treated with radiation dose 64 Gy/32 fractions was 24 months (Std. Error 4.829, 95% CI ), 60 Gy/30 fractions was 33 months (std , 95% CI ) and 56 Gy/28 fractions was 13 months (std 8.981, 95% CI ). Of 32 patients, 21 patients after progression received second line chemotherapy different regimens, while 11 patients had best supportive care (34.4%). Only 6 patients received third line chemotherapy. Toxicity: By reviewing patients files; regarding to nonhematological toxicities, most of the patients complained of anorexia and nausea, difficult swallowing due to grade 3 esophagitis (9.3%), dry cough due to radiation pneumonitis grade 3 and 4 (6.25%). Pneumonitis and esophagitis induced have been considered serious, but they were easily manageable in the present study. For hematological toxicities, group of patients developed myelosuppresion in the form of leucopenia and neutropenia in 8 patients (25%), thrombocytopenia (12.5%), anaemia (12.5%), only one patient developed febrile neutropenia (3.1%). There was no enough data on the database to comment on the quality of life or toxicity in this patients series. Table (1): Descriptive statistics (frequency) of different categorical risk factors. Count Column N % Sex: Male Female PS Histology: Adeno Scc Non specified Adenosquam Staging: II Ma Mb Co morbidities: Non significant Average/mild Moderate Severe RT dose: 64/ / / Table (2): Descriptive statistics. N Minimum Maximum Mean Std. Deviation Age FEV Survival Valid N 32 (listwise) Discussion Much progress has been made in recent decades in treating stage III NSCLC. Concomitant chemoradiotherapy clearly represents the current standard and the preferred approach over induction chemotherapy. There have been no phase III comparisons of different chemoradiotherapy regimens. Therefore, a specific standard regimen cannot be recommended at this time [16]. Meta-analyses of studies comparing concurrent with sequential therapy showed that there was a relative improvement of about 20% with concurrent therapy over sequential therapy in these patients and that concurrent chemoradiation is more toxic than the sequential approach, particularly with regard to oesophagitis. The incidence of pneumonitis is not significantly higher with concurrent therapy [17]. Interestingly, despite the growing popularity of second and third generation chemotherapy regimens to be used with thoracic irradiation, the National Comprehensive Cancer network (NCCN) continues to recommend cisplatin and etoposide as its preferred regimen [18]. In this retrospective study we present Northampton Oncology Centre experience using concomitant chemo radiotherapy; for 32 patients with non-small cell lung cancer, unresectable stages (I-III), received cisplatin/etoposide regimen concomitant with radiotherapy. We were one of few centers in UK who adopted the concurrent CRT in 2005 and as our center only serves less than a million, the number of eligible patients was small. Many trials have included inoperable stage I-II as our trail done. We assessed overall survival and evaluated different risk factors. The median overall survival was 24 months. There was a significant overall survival advantage for those with adenocarcinoma over those with squamous cell carcinoma and those with stage I and II compared to stage III although the number of stage I and II patients was small. There was better overall survival with no significant difference for those receiving more than 56Gy/28.
4 328 Cisplatin & Etoposide Concurrent with Radiotherapy A multicenter French study reported by Le Chevalier et al. [19] also confirmed improved survival for the chemotherapy plus radiotherapy arm compared to radiotherapy alone (3-year survival rates of 11% vs 5%, respectively) with an improved distant failure rate for chemotherapy plus radiotherapy (22% vs 46% at 1 year, respectively). Unfortunately, both treatment groups showed similarly high loco regional failure with 1-year local control rates of only 15% and 17%, illustrating the vexing problem of obtaining good loco regional control of disease in the locally advanced setting. A Phase II study used concurrent cisplatin 20mg /m 2 /day plus etoposide 50mg/m 2 /day, from day 1 through day 5, every 4 weeks for four cycles, and radiotherapy administered to a total dose of 60 Gy for unresectable stage III non-small cell lung cancer. Response rate was 84%, including 68% complete response. With a minimum follow-up of 23 months, overall survival was 70% at 1 year, 39.7% at 2 years, and 34.7% at 3 years [20]. The most common detected toxicities by this protocol are considered amenable, manageable. Grade 3 esophagitis (9.3%), radiation pneumonitis grade 3 and 4 (6.25%). Leucopenia and neutropenia in 8 patients (25%), thrombocytopenia (12.5%), anaemia (12.5%), only one patient developed febrile neutropenia (3.1%). Which is comparable to common toxicities by using Concurrent Chemoradiotherapy with Carboplatin and Docetaxel for Stage III Non-Small-Cell Lung Cancer were grade 3/4 neutropenia (95%), grade 3 esophagitis (5%), grade 3 anorexia (30%), grade 3 febrile neutropenia (35%) and grade 5 radiation pneumonitis (5%) [21]. The role of induction chemotherapy followed by concurrent chemotherapy or concurrent chemoradiotherapy followed by consolidative chemotherapy remains investigational. Phase III Study by the Hoosier Oncology Group and U.S. Oncology 22 evaluated the results of phase II study by the Southwest Oncology Group 23 using consolidation docetaxel after cisplatin (P), etoposide (E), and radiation (XRT) resulted in a median survival time (MST) of 26 months, whether consolidation docetaxel was responsible for this improved survival. This randomized phase III trial failed to achieve the primary objective of improved survival with the addition of consolidation docetaxel after PE/XRT for this population of patients with stage III NSCLC. Despite the use of older chemotherapeutic agents (PE) and only 59.4Gy of XRT, the PE/XRT regimen in this study resulted in a median survival time superior to historic controls and a 3- year survival rate comparable with other regimens using newer chemotherapeutic agents and higher doses of XRT. Finally, they concluded that consolidation docetaxel after PE/XRT results in increased toxicities but does not further improve survival compared with PE/XRT alone in patients with stage III inoperable NSCLC [22] Simiraly, randomized phase III trial evaluated whether consolidation The Cancer and Leukaemia Group B (CALGB) Trial was designed to investigate the impact of induction chemotherapy in addition to standard chemoradiotherapy on overall survival [24]. The difference in survival was not statistically significant (p=0.3) and the median survival observed on the induction chemotherapy followed by chemoradiotherapy and the chemoradiotherapy alone arms was 14 and 12 months respectively [4]. The use of weekly low dose carboplatin and paclitaxel with concurrent radiotherapy may have compromised the efficacy of both treatment paradigms investigated in this trial. The trials have demonstrated a benefit of concurrent chemoradiotherapy over sequential chemotherapy [10,12]. However, when newer agents (e.g. Taxanes and gemcitabine) are used the chemotherapy dose must be reduced to avoid excessive toxicity. A phase III trial that compared weekly paclitaxel with concurrent TRT versus TRT alone after induction chemotherapy with carboplatin and paclitaxel demonstrated a significant improvement in time to tumor progression (p<0.001), but not overall survival in comparison to TRT alone (p=0.091) [25]. A randomized Phase III trial of combined paclitaxel, carboplatin, and radiation therapy followed by weekly paclitaxel or observation for patients with locally advanced inoperable non-small cell lung cancer (radiotherapy dose=66.6gy in 37 fractions). Patints then randomized for either observation or maintenance (6 cycles of paclitaxel 70) a median of 5 of 6 planned cycles that was delivered in the maintenance arm. For the observation group vs. The maintenance group, the estimated 1 and 4-year survival rates were 77% vs. 66% and 38% vs. 17% (median, 26.9 months vs months, respectively [p=0.07]); the estimated 1 and 4-year performance-free survival (PFS) were 46% vs. 24% and 25% vs. 13% (median, 10.2 months vs. 8.2 months, respectively [p=0.04]) [26]. The novel concept of adding non cross resistant chemotherapy following the completion of concurrent chemoradiation was examined in a SWAG study. In this multi-institutional Single arm phase II study, 71 patients with unresectable stage IIIb NSCLC were treated with concurrent chemoradiation with cisplatin, etoposide and TRT followed by three cycles of docetaxel (75 to 100mg/m 2 given
5 Hany H. Eldeeb & Seham E. Abd-Elkhalk 329 every 3 weeks) [27]. The median survival was an impressive 27 months, and the projected 3-year survival was 47%, which encouraged SWAG investigators to conduct a phase III study comparing this regimen (cisplatin, etoposide, and radiation followed by three cycles of docetaxel) with an identical regimen followed by maintenance therapy with ZD 1839 (Iressa), a specific inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase. Yamamoto et al. [15] compared three different cytotoxic chemotherapy regimens administered during radiation treatment: Arm A (the control group) received four cycles of mitomycin (8mg/m 2 on day 1), vindesine (3mg/m 2 on days 1 and 8) and cisplatin (80mg/m 2 on day 1); arm B received weekly irinotecan (20mg/m 2 ) and carboplatin (area under the plasma concentration-time curve [AUC] 2) for 6 weeks, followed by two courses of irinotecan (50mg/m 2 on days 1 and 8) and carboplatin (AUC 5 on day 1); and arm C received weekly paclitaxel (40mg/m 2 ) and carboplatin (AUC 2) for 6 weeks followed by two courses of paclitaxel (200mg/m 2 on day 1) and carboplatin (AUC 5 on day 1). 5-year survival rates were essentially the same in all three chemotherapy arms, a difference favoring the cisplatin (control) arm was observed at 3 years (35%, 24% and 26% for arms A, B and C, respectively), but non-inferiority was not statistically achieved. It must also be recognized that the reported gains in the therapeutic ratio were quite modest. Conclusion: Our survival data for concurrent chemoradiotherapy matches with the international published data. Future research is needed to define the best concurrent chemotherapy regimen as well as the role of consolidation and maintenance chemotherapy. Conflict of interest: The authors have no financial or personal relationship that could inappropriately influence/bias this work. Limitations of the study: As it is a retrospective study, small sample number of patients and no control arm for comparison. References 1- ATHANAIOS KARAMPEASZIS, LAMBROS VAMVA- KAS, ATHINA AGELIDOU, et al.: Docetaxel Vs Vinorelbine in Elderly Patients With Advanced Non-Small Cell Lung Cancer: A Hellenic Oncology Research Group Randomized Phase III Study. Clinical Lung Cancer, Vol. 12, No. 3, 155: 60, DAVID P.: Carbone and Enriqueta Felip. Adjuvant Therapy in Non- Small Cell Lung Cancer: Future Treatment Prospects and Paradigms. Clinical Lung Cancer, Vol. 12, No. 5, , PFISTER D.G., JOHNSON D.H., AZZOLI C.G., et al.: American Society of Clinical Oncology Treatment of Unresectable Non-Small Cell Lung Cancer Guideline: Update J. Clin. Oncol., 22: , THOMAS E. STINCHOMBE, LYDDIA HODGSON, JAMES E. HERDON II, et al.: Treatment Outcomes of Different Prognostic Groups of Patients on Cancer and Leukemia Group B trial 39801: Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for unresectable stage III non-small cell lung cancer J. Thorac. Oncol., 4 (9): , KOJI TAKEDA, SHUNICHI NEGORO, MASAHIRO TANAKA, et al.: A Phase II Study of Cisplatin and Irinotecan As Induction Chemotherapy Followed by Concomitant Thoracic Radiotherapy with Weekly Lowdose Irinotecan in Unresectable, Stage III, Non-Small Cell Lung Cancer: JCOG Jpn J. Clin. Oncol., 41 (1) 25-31, GIORGIO V.: Scogliotti and Andrew T. Turrsii. Docetaxel- Based Combined Modality Chemoradiotherapy for locally advanced Non-small cell lung cancer. The Oncologist, 8: , Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in Non-Small Cell Lung Cancer: A metaanalysis using updated data on individual patients from 52 randomized clinical trials. BMJ, 311: , DILLMAN R.O., HERNDON J., SEAGREN S.L., et al.: Improved Survival in Stage III Non-Small Cell Lung Cancer; Seven year follow up of cancer and Leukaemia Group B (GALGB) 8433 Trial. J. Natl. Cancer Inst., 88: , SAUSE W., KOLESAR P., TAYLOR S.I.V., et al.: Final Results of Phase III Trial in Regionally Advanced Unresectable Non-Small Cell Lung Cancer. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group and Southwest Oncology Group. Chest, 117: , CURRAN W.J.J.R., SCOTT C., LANGER C., et al.: Phase III Comparison of Sequential vs Concurrent Chemoradiation for Patients with Unresectable stage III Non-Small Cell Lung Cancer: Initial report of Radiation Oncology Group (RTOG) Proc. Soc. Clin. Oncol., 19: 484a, FURUSE K., KUBOTA K., KAWAHARA M., et al.: Phase II Study of Concurrent Radiotherapy and Chemotherapy for Unresectable stage III Non-Small Cell Lung Cancer. Southern Osaka Lung Cancer Study Group. J. Clin. Oncol., 13: , FURUSE K., KUBOTA K., KAWAHARA M., et al.: Phase III Study of Concurrent versus Sequential Thoracic Radiotherapy in Combination with Mitomycin, Vindestin, Cisplatin in Unresectable stage III Non-Small Cell Lung Cancer. The West Japan Lung Cancer Group. J. Clin. Oncol., 17: , 1999.
6 330 Cisplatin & Etoposide Concurrent with Radiotherapy 13-AUPERIN A., et al.: Meta-Analysis of Concomitant versus Sequential Radiochemotherapy in Locally Advanced Nonsmall Cell Lung Cancer. J. Clin. Oncol., 28: , CURRAN W.J., et al.: Long-Term Benefit is Observed in a Phase III Comparison of Sequential vs. Concurrent Chemo-Radiation for Patients with Unresectable stage III NSCLC: RTOG 9410 [abstract]. Proc. Am. Soc. Clin. Oncol., 22: a2499, YAMAMOTO N., et al.: Phase III Study Comparing Second- and Third Generation Regimens with Concurrent Thoracic Radiotherapy in Patients with Unresectable stage III Non-Small Cell Lung Cancer: West Japan Thoracic Oncology Group WJTOG0105. J. Clin. Oncol., 28: , GERVAIS R., DUCOLONE A., LE CHEVALIER T., et al.: Conventional Radiation (RT) with Daily Carboplatin Compared to RT Alone After Induction Chemotherapy (ICT): Final results of a randomized phase III trial in stage III unresectable non-small cell lung cancer (NSCLC). Study CRG/BMS/NPC/96 of the French Lung Cancer Study Group (FNCLCC) and IFCT. Journal of Clinical Oncology, 23 abstract 7016, GADGEEL S.M.: The Optimal Chemotherapy for Stage III Non-Small Cell Lung Cancer Patients. Curr. Oncol. Rep., 13 (4): 272-9, National Comprehensive Cancer Network. Non-Small Cell Lung Cancer v. 2. NCCN Clinical Practice Guidelines in OncologyTM [online]. Available at: org/professionals/physician_gls/pdf/nscl.pdf, LE CHEVALIER T., ARRIAGADA R., QUOIX E., et al.: Radiotherapy Alone versus Combined Chemotherapy and Radiotherapy in nonresectable Non-Small Cell Lung Cancer: First analysis of a randomized trial of 353 patients. J. Natl. Cancer Inst., 83: , FRANCOIS REBOUL, YVELISE BREWER, PASCAL VINCENT, et al.: Concurrent Cisplatin, Etoposide, and Radiotherapy for Unresectable Stage III Non-Small Cell Lung Cancer: A Phase II study. International Journal of Radiation Oncology * Biology * Physics. Volume 35, Issue 2, Pages , TERASHIMA T., MATSUZAKI T., OGAWA R., et al.: Concurrent Chemoradiotherapy with Carboplatin and Docetaxel for Stage III Non-Small-Cell Lung Cancer. Gan To Kagaku Ryoho., 38 (13): [Abstract], NASSER HANNA, MARCUS NEUBAUER, CONSTAN- TIN YIANNOUTSOS, et al.: Phase III Study of Cisplatin, Etoposide, and Concurrent Chest Radiation With or Without Consolidation Docetaxel In Patients With Inoperable Stage III Non-Small-Cell Lung Cancer: The Hoosier Oncology Group and U.S. Oncology. J. Clin. Oncol. 26: , by American Society of Clinical Oncology, DAVID R. GANDARA, KARI CHANSKY, KATHY S. ALBAIN, et al.: Consolidation Docetaxel After Concurrent Chemoradiotherapy in Stage IIIB Non-Small-Cell Lung Cancer: Phase II Southwest Oncology Group Study J. Clin. Oncol., Vol. 21, No. 10: pp , VOKES E.E., HERNDON J.E. 2nd, KELLEY M.J., et al.: Induction Chemotherapy Followed by Chemoradiotherapy Compared with Chemoradiotherapy Alone for Regionally Advanced Unresectable stage III Non-small Cell Lung Cancer: Cancer and Leukemia Group B. J. Clin. Oncol., 25: [Pub Med: ], HUBER R.M., FLENTJE M., SCHMIDT M., et al.: Simultaneous Chemoradiotherapy Compared with Radiotherapy Alone after Induction Chemotherapy in Inoperable Stage IIIA or IIIB Non-Small Cell Lung Cancer: Study CTRT99/97 by the Bronchial Carcinoma Therapy Group. J. Clin. Oncol., 24: [PubMed: ], CARTER D.L., GARFIELD D., HATHORN J., et al.: A Randomized Phase III Trial of Combined Paclitaxel, Carboplatin, and Radiation Therapy Followed by Weekly Paclitaxel or Observation for Patients with Locally Advanced Inoperable Non-Small Cell Lung Cancer. Clin. Lung. Cancer, LAURIE G., CHANSKY J., ALBAIN K., et al.: Conslidation Docetaxel Following Concurrent Chemoradiotherapy in Pathologic Stage IIIB Non-Small Cell Lung Cancer (NSCLC) (SW0G9504): Patterns of failure and updated survival. proc Am. Soc. Clin. Oncol., 1225 (abst), 2001.
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART
 Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board
 Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Table of Contents. Data Supplement 1: Summary of ASTRO Guideline Statements. Data Supplement 2: Definition of Terms
 Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer
 Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
Concurrent Chemotherapy and Radiotherapy for Head and Neck Cancer Ryan J. Burri; Nancy Y. Lee Published: 03/23/2009 Abstract and Introduction Abstract Head and neck cancer is best managed in a multidisciplinary
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Introduction. Acta Medica Mediterranea, 2015, 31: 883 BOZKURT DUMAN 5, SEMRA PAYDAŞ 4, VEHBI ERCOLAK 6 1
 Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology. Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Special Article. Received 5 December 2014; revised 18 February 2015; accepted 25 February 2015. Practical Radiation Oncology (2015) 5, 141-148
 Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
EVIDENCE TABLE. Study Objective. (Purpose of Study)
 . Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 997; (6):70-77.. Greene FL, Page DL, Fleming ID, et al, eds, for the American Joint Committee on Cancer. AJCC Cancer
. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 997; (6):70-77.. Greene FL, Page DL, Fleming ID, et al, eds, for the American Joint Committee on Cancer. AJCC Cancer
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
SECOND-LINE CHEMOTHERAPY in advanced non
 Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)?
 Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery
 Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
Outcomes of Patients With Stage III Nonsmall Cell Lung Cancer Treated With Chemotherapy and Radiation With and Without Surgery Hale B. Caglar, MD 1 ; Elizabeth H. Baldini, MD, MPH 1 ; Megan Othus, MS 2
Non-small Cell Lung Cancer: Locally Advanced
 Non-small Cell Lung Cancer: Locally Advanced Daniel W. Golden MD, PGY-5, Ryan Bair MD, PGY-3, and Matthew Koshy MD, Assistant Professor Pritzker School of Medicine, University of Chicago Chicago Clinical
Non-small Cell Lung Cancer: Locally Advanced Daniel W. Golden MD, PGY-5, Ryan Bair MD, PGY-3, and Matthew Koshy MD, Assistant Professor Pritzker School of Medicine, University of Chicago Chicago Clinical
Altered Fractionation of Radical Radiation Therapy in the Management of Unresectable Non-Small Cell Lung Cancer
 Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
Evidence-based Series #7-12 Version 2 A Quality Initiative of the Program in Evidence-based Care (PEBC), Cancer Care Ontario (CCO) Altered Fractionation of Radical Radiation Therapy in the Management of
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
Summary ID# 13095. Clinical Study Summary: Study H3E-EW-B012
 Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer
 Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
Protein kinase C alpha expression and resistance to neo-adjuvant gemcitabine-containing chemotherapy in non-small cell lung cancer Dan Vogl Lay Abstract Early stage non-small cell lung cancer can be cured
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer
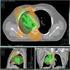 Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
Cancer Res Treat. 2005;37(5):273-278 T reatment O utcomes of T hree-dimensional Conformal Radiotherapy for Stage III Non-Small Cell Lung Cancer Seung-Gu Yeo, M.D. 1, Moon-June Cho, M.D. 1,4, Sun-Young
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC)
 Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Bahrain Medical Bulletin, Vol.23, No.4, December 2001 Recent Trends in Management of Unresectable Non-Small Cell Lung Cancer (NSCLC) Jalal Al-Maskati, MBChB, ABIM * Lung cancer is a major health problem
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
More than half of all lung cancer patients present with. New Combinations in the Treatment of Lung Cancer* A Time for Optimism
 New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
INTRODUCTION. liver neoplasms, adrenal gland neoplasms, non small-cell lung cancer, radionuclide imaging, bisphosphonates,
 VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
VOLUME 22 NUMBER 2 JANUARY 15 2004 JOURNAL OF CLINICAL ONCOLOGY A S C O S P E C I A L A R T I C L E American Society of Clinical Oncology Treatment of Unresectable Non Small-Cell Lung Cancer Guideline:
Diagnosis and multimodality management of stage III non-small cell lung cancer Review Article
 Cancer Therapy Vol 6, page 81 Cancer Therapy Vol 6, 81-94, 2008 Diagnosis and multimodality management of stage III non-small cell lung cancer Review Article Kevin Sullivan, Zujun Li, John Rescigno, Michael
Cancer Therapy Vol 6, page 81 Cancer Therapy Vol 6, 81-94, 2008 Diagnosis and multimodality management of stage III non-small cell lung cancer Review Article Kevin Sullivan, Zujun Li, John Rescigno, Michael
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
NON-SMALL CELL LUNG CANCER STAGE III
 NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
National Clinical Trials Network Groups Update Fall 2014
 National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
Stage 3 Lung Cancer - Know YourSurviving Conditions
 Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
Survival of Stage IIIb and IV Non-Small Cell Lung Cancer Patients on Best Supportive Care in Manitoba, Canada Erich Kliewer Alain Demers Sri Navaratnam Coreen Hildebrand Grace Musto Report for AstraZeneca
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma
 Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma Marc de Perrot, Ronald Feld, Natasha B Leighl, Andrew Hope, Thomas K Waddell, Shaf Keshavjee,
Cetuximab (Erbitux) MM.04.005 05/10/2005. HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s) of Service: Office: Outpatient
 Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Pancreatic Cancer: FDA Approved Treatments and Clinical Trials
 Pancreatic Cancer: FDA Approved Treatments and Clinical Trials Vincent J Picozzi MD MMM Virginia Mason Medical Center Seattle WA 1 Pancreatic cancer is the hardest cancer of all to treat 2 Pancreatic cancer:
Pancreatic Cancer: FDA Approved Treatments and Clinical Trials Vincent J Picozzi MD MMM Virginia Mason Medical Center Seattle WA 1 Pancreatic cancer is the hardest cancer of all to treat 2 Pancreatic cancer:
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer
 REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
CLINICAL POLICY Department: Medical Management Document Name: Opdivo Reference Number: CP.PHAR.121 Effective Date: 07/15
 Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Page: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PII S0360-3016(99)00307-7 CLINICAL INVESTIGATION
 PII S0360-3016(99)00307-7 Int. J. Radiation Oncology Biol. Phys., Vol. 45, No. 5, pp. 1151 1156, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/99/$ see front
PII S0360-3016(99)00307-7 Int. J. Radiation Oncology Biol. Phys., Vol. 45, No. 5, pp. 1151 1156, 1999 Copyright 1999 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/99/$ see front
5. Non small Cell Lung Cancer
 5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
5. Non small Cell Lung Cancer Introduction The cancer registry in Sweden shows a continuous increase of lung cancer cases from 867 in 1958 when the registry started to 2 846 in 2000. The Swedish cancer
Cesare Gridelli 1, Francesca Casaluce 2, Assunta Sgambato 2, Fabio Monaco 3, Cesare Guida 4
 Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Editorial Treatment of limited-stage small cell lung cancer in the elderly, chemotherapy vs. sequential chemoradiotherapy vs. concurrent chemoradiotherapy: that s the question Cesare Gridelli 1, Francesca
Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery
 Special Report Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery Charu Aggarwal, MD, Linna Li, MD, Hossein Borghaei, DO, Ranee
Special Report Multidisciplinary Therapy of Stage IIIA Non Small-Cell Lung Cancer: Long-term Outcome of Chemoradiation With or Without Surgery Charu Aggarwal, MD, Linna Li, MD, Hossein Borghaei, DO, Ranee
New Trends & Current Research in the Treatment of Lung Cancer, Pt. II
 New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy on limited small cell lung cancer
 [Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
[Chinese Journal of Cancer 27:10, 353-357; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Efficacy of chemotherapy combined hyperfractionated accelerated radiotherapy
Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc.
 CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
CLINICAL FEATURE Helical TomoTherapy for Lung Cancer Radiotherapy: Good Science Pays Clinical Dividends Peter Hoban, Ph.D., TomoTherapy Inc. The Challenge Lung cancer kills more people each year in the
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer
 Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer ISBN/EAN 9789490122096 Cover photo: Monique Slagter-Verburg Layout: Karin van Rijnbach Print: Gildeprint Drukkerijen
Treatment of stage III non-small cell lung cancer and limited-disease small-cell lung cancer ISBN/EAN 9789490122096 Cover photo: Monique Slagter-Verburg Layout: Karin van Rijnbach Print: Gildeprint Drukkerijen
Radiotherapy in Lung Cancer. Dr. Vassilis Kouloulias. Radiotherapy in lung cancer. Dr. V. Kouloulias
 Radiotherapy in Lung Cancer Dr. Vassilis Kouloulias Assistant Professor of Radiation Oncologist, 2 nd Dpt. Radiology, University of Athens, Greece. Key Words Small cell lung cancer (SCLC), Non-small cell
Radiotherapy in Lung Cancer Dr. Vassilis Kouloulias Assistant Professor of Radiation Oncologist, 2 nd Dpt. Radiology, University of Athens, Greece. Key Words Small cell lung cancer (SCLC), Non-small cell
Future Directions in Clinical Research. Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center
 Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Lung Cancer. Public Outcomes Report. Submitted by Omar A. Majid, MD
 Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
Medication Policy Manual. Topic: Alimta, pemetrexed Date of Origin: May 12, 2010
 Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Moving forward, where are we with Clinical Trials?
 Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Treatment of Stage III Non-small Cell Lung Cancer
 CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Treatment of Stage III Non-small Cell Lung Cancer Diagnosis and Management of Lung Cancer, 3rd ed: American College of
CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Treatment of Stage III Non-small Cell Lung Cancer Diagnosis and Management of Lung Cancer, 3rd ed: American College of
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent
 ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent Richard M. Gewanter, MD, Kenneth E. Rosenzweig, MD, Joe Yujiao Chang, MD, PhD,
ACR Appropriateness Criteria Nonsurgical Treatment for Non-Small-Cell Lung Cancer: Good Performance Status/Definitive Intent Richard M. Gewanter, MD, Kenneth E. Rosenzweig, MD, Joe Yujiao Chang, MD, PhD,
Key words: chemotherapy; evidence-based medicine; guidelines; non-small cell lung cancer
 Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Chemotherapeutic Management of Stage IV Non-small Cell Lung Cancer* Mark A. Socinski, MD, FCCP; David E. Morris, MD; Gregory A. Masters, MD, FCCP; and Rogerio Lilenbaum, MD Stage IV non-small cell lung
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
The Need for Accurate Lung Cancer Staging
 The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
The Need for Accurate Lung Cancer Staging Peter Baik, DO Thoracic Surgery Cancer Treatment Centers of America Oklahoma Osteopathic Association 115th Annual Convention Financial Disclosures: None 2 Objectives
Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer. A Single Institution Experience
 ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
ORIGINAL ARTICLE Definitive Treatment of Poor-Risk Patients with Stage I Lung Cancer A Single Institution Experience Michael Hsie, MD,* Stefania Morbidini-Gaffney, MD,* Leslie J. Kohman, MD, Elisabeth
Malignant Pleural Mesothelioma in Singapore
 RESEARCH COMMUNICATION C SP Yip 1, HN Koong 2, CM Loo 3, KW Fong 1* Abstract Aim: To examine the clinical characteristics and outcomes of malignant pleural mesothelioma (MPM) in Singapore. Methods and
RESEARCH COMMUNICATION C SP Yip 1, HN Koong 2, CM Loo 3, KW Fong 1* Abstract Aim: To examine the clinical characteristics and outcomes of malignant pleural mesothelioma (MPM) in Singapore. Methods and
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka
 Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Adiuwantowe i neoadiuwantowe leczenie chorych na zaawansowanego raka żołądka Neoadiuvant and adiuvant therapy for advanced gastric cancer Franco Roviello, IT Neoadjuvant and adjuvant therapy for advanced
Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD
 Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Grading of Tumor Regression in Non-small Cell Lung Cancer* Morphology and Prognosis Klaus Junker, MD; Kathrin Langner, MD; Folker Klinke, MD; Ulrich Bosse, MD; and Michael Thomas, MD Objective: Different
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Radiotherapy for Non-Small Cell Lung Cancer. Standard Treatment Options Radiotherapy Planning
 Radiotherapy for Non-Small Cell Lung Cancer I II Standard Treatment Options Radiotherapy Planning TNM Staging System Disease Staging - Management is based on disease stage - Stage I-II: early stage - Stage
Radiotherapy for Non-Small Cell Lung Cancer I II Standard Treatment Options Radiotherapy Planning TNM Staging System Disease Staging - Management is based on disease stage - Stage I-II: early stage - Stage
