Cardiovascular System
|
|
|
- Godfrey Hunter
- 7 years ago
- Views:
Transcription
1 Heart and Circulation by Dr Mary Sheppard Cardiovascular System Galen Theory The ancient model of the blood system was accepted until Two types of blood, the venous and the arterial with distinct pathways and functions, relating to the three chief body centres: the liver (responsible for nutrition and growth), the heart (vitality), and the brain (sensation and reason). The heart did not drive blood through the arteries. Blood s movement through the arteries was explained by an innate pulsative faculty in the arteries themselves. This was until William Harvey s demonstration of the circulation of the blood, building on Vesalian anatomy. So much blood left the heart in a minute that it could not conceivably be absorbed by the body and be continually replaced by blood made in the liver. Harvey noted that the amount of blood forced out of the heart in an hour far exceeded its volume in the whole animal. This quantitative evidence established that the blood must constantly move in a circuit, otherwise the arteries and body would explode under the pressure. The Circulation The circulation comprises the heart, arteries, veins and capillaries. These components function together with the purpose of supplying nutrients such as oxygen and glucose to organs and removing waste products such as carbon dioxide and urea. The heart is located in the middle of the chest behind the sternum, between the lungs. It lies between the 1 st and the 4 th intercostal space. The heart rests in a moistened chamber called the pericardial cavity which is surrounded by the ribcage. The diaphragm, a tough layer of muscle, lies below. The heart is very well protected. A human being s heart is about the size of a clenched fist. The average adult heart weighs about grams. The heart consists of four chambers, atria and ventricles in one way communication. The body s circulation has two parts, with the heart acting as a double pump. The body has 5 litres of blood continually travelling through it in the circulatory system. The heart, the lungs, and the blood vessels work together to form the circulatory system. There are two circulations. One circulates blood from the body to the lungs (the pulmonary circulation), which is small with little peripheral resistance, so low pressures are needed and the walls of the right ventricle are thin. The other circulation circulates blood from the lungs around the body (the systemic circulation). The left side pumps the same volume against greater peripheral resistance, so the cardiac left ventricular walls are more muscular and thicker. The pulmonary artery connects the right ventricle of the heart with the lungs as part of the pulmonary circulation system. The two veins that carry blood into the heart (into the right atrium) are the superior and the inferior vena cavae. The superior vena cava brings blood from the head, neck and upper limbs. The inferior vena brings blood from the abdomen and lower limbs. 1
2 Blood Flow through the Heart The right atrium receives deoxygenated blood from the superior and inferior vena cavae. During contraction of the atrium, blood moves into the right ventricle through the tricuspid valve. Once the right ventricle is filled with blood, this chamber contracts, pumping blood via the pulmonary arteries to the lungs. Blood from the right side pump is dark red (bluish) and low in oxygen. The main pulmonary artery divides into right and left arteries to enter the lungs at the hilum. Blood travels in the pulmonary arteries and branches within the lungs where it receives fresh supplies of oxygen and becomes bright red in the capillary bed. Blood leaves the lungs in the pulmonary veins (two from each lung) back to the heart s left side pump. The oxygenated blood enters the left atrium through the pulmonary veins. Once this chamber is filled, the left atrial wall contracts, pushing blood through the mitral valve. After the left ventricle is filled, this chamber contracts, forcing blood out of the ventricle and into the aorta. The left ventricle is stronger in order to pump oxygenated blood through the entire body (left ventricle = 15mm; right = 3mm thick). In a cardiac cycle there are about beats per minute, with each cycle lasting 0.8 seconds. In an average lifetime, the heart beats more than two and a half billion times, without ever pausing to rest. Heart Rate The heart s rate of pumping oxygen-rich blood is fastest in infancy (about 120 beats per minute). As the child grows, the heart rate slows. A seven year old child s heart beats about 90 times per minute. By the age of 18, the heart rate has stabilised to about 70 beats per minute. Heart Chambers The human heart is primarily a muscle shell. There are four cavities inside the heart that fill with blood. Upper two of these cavities are called atria. The lower two are called ventricles. The two atria form the top of the heart, and the ventricles meet at the bottom of the heart to form a pointed base which points towards the left of your chest. The left ventricle contracts most forcefully, so you can best feel your heart pumping on the left side of your chest. A wall called the septum separates the right and left sides of the heart. An atrioventricular valve connects each atrium to the ventricle below it. The mitral valve connects the left atrium with the left ventricle. The tricuspid valve connects the right atrium with the right ventricle. Heart Muscle Tissue Epicardium is the fibrous and adipose connective tissue forming the adventitia of the heart, with autonomic nerves and vessels present. The myocardium is the muscle layer. Individual myocytes are seen with a pink stain with pale connective tissue in between containing capillaries. The Systemic Circulation The human heart beats 100,000 times a day, propelling 5.5 litres of blood through 60,000 miles of vessels. The blood is pumped from a 300 gram heart so forcefully that large arteries, when severed, can send a jet of blood several feet into the air. The forceful contraction of the heart s left ventricle forces the oxygenated blood into the aorta which then branches into many smaller arteries which run throughout the body. The inside layer of an artery is very smooth, allowing the blood to flow quickly. The outside layer of an artery is very strong muscle, allowing the blood to flow forcefully. The oxygen-rich blood eventually enters capillaries within organs where the oxygen and nutrients are released. In the systemic circulation, the blood leaves the left side of the heart and travels through arteries which gradually divide into capillaries. In the capillaries, food and oxygen are released to the body cells, and carbon dioxide and other waste products are returned to the bloodstream. The blood then travels in veins back to the 2
3 right side of the heart. Blood passes through the kidneys. This phase of systemic circulation is known as renal circulation. Kidneys filter much of the waste from blood and excrete it as urine. Classification of Blood Vessels Elastic arteries e.g. the aorta have a wide lumen and elastic wall. They have damp pressure variations. Muscular arteries have wide lumen, strong non-elastic walls and have low resistance conduit. Arterioles are resistance vessels with narrow lumen and thick contractile walls. They control resistance and flow, allowing regional redirection of blood Capillaries are exchange vessels with narrow lumen and thin walls. Venules and veins are capacitance vessels with wide lumen and distendable walls. They have low resistance conduit and reservoir. They allow frictional distribution of blood. The Coronary Circulation The heart needs large amounts of oxygen. This is delivered by the coronary circulation. Heart damage occurs when it does not receive a normal supply of food and oxygen. Damage to the coronary arteries and the heart is the commonest cause of death in the UK - atheroma and atherosclerosis or myocardial infarction. Blood Flow to the Limbs 3
4 Exchange vessels Capillaries are composed of a single endothelial cell. Across their walls occurs exchange between blood and tissue fluids, oxygen, CO 2, nutrients, water, inorganic ions, vitamins, hormones, metabolic products, immune substances, even immune competent cells. Capillaries may be plain, fenestrated or sinusoidal - to slow blood flow. Capillaries are concentrated into capillary beds. Some capillaries have small pores between the cells of the capillary wall, allowing materials to flow in and out of capillaries as well as the passage of white blood cells. Capillaries are microscopic in size. Blushing is one manifestation of blood flow into capillaries. Control of blood flow into capillary beds is done by nerve-controlled sphincters. Veins After capillary beds blood is collected in venules which are tributaries of veins. These vessels provide a low pressure blood reservoir through which blood returns to the heart. Veins have the same basic histological structure as arteries, but are greater in cross-sectional area, because of slower flow rate. Arteries are often accompanied by veins. Because only a little external pressure can stop flow, veins are confined to the dorsum of the foot and back of the hand, and often run on the flexor aspects of joints. The waste products are collected and the waste-rich blood flows into the veins and return in the venous circulation back to the right side of the heart via inferior and superior caval veins. Blood Vessel Structure Adventitia - outermost layer which is composed of connective tissue and nerve fibres with a small network of vessels called the vasa vasorum. Elastic lamina - layers of elastic fibres which provide elasticity to the vessel wall. Two layers, an internal elastic lamina just below the intima, and an external elastic lamina, sandwiched between the adventitia and media. Media - the layer of smooth muscle and elastic fibres which provide much of the strength and elasticity of the vessel. Intima - inner lining of the blood vessel formed by the endothelial cells and a small amount of connective tissue. Endothelial cells - form the lining of the blood vessels. They maintain the permeability, helping to regulate inflammation, and playing a critical role in blood coagulation and clotting. Pulse and Blood Pressure Besides circulating blood, the blood vessels provide two important means of measuring vital health statistics: pulse and blood pressure. We measure heart rate, or pulse, by touching an artery. The rhythmic contraction of the artery keeps pace with the beat of the heart. Since the radial artery is near the surface of the skin at the wrist, we can easily touch the artery here and get an accurate measure of the heart s pulse. 4
5 The blood flowing through the arteries has a higher pressure than the blood in the veins. Your blood pressure is measured using two numbers. The first number, which is higher, is taken when the heart beats during the systole phase. The second number is taken when the heart relaxes during the diastole phase. Those two numbers stand for millimetres. Normal blood pressure ranges from 110 to 150 millimetres (as the heart beats) over 60 to 80 millimetres (as the heart relaxes). It is normal for your blood pressure to increase when you are exercising and to decrease when you are sleeping. Increased blood pressure is called hypertension which is very common in older people. 5
6 Mechanical Properties of the Heart by Dr Ken MacLeod The contraction of the whole heart can be viewed by nuclear resonance spectroscopy - in a normal heart powerful left and right sided contractions are visible. It can also be seen that each contraction in a cell is accompanied by a flash increase and decrease in calcium ions (calcium ion transient). In a contraction, an electrical event (cardiac action potential) causes this Ca 2+ transient which gives rise to the contractile event. Single Cell Structure Ventricular cells are 100μm long and 15 μm wide. The T-tubules are closely opposed to the junctional sarcoplasmic reticulum (muscle cell equivalent of endoplasmic reticulum in other cells). The sarcoplasmic reticulum is the store of calcium in the cells. Morphologically, the SR is a lace-like structure that overruns and covers up the myofibrils. The store of calcium therefore lies very close to where it is to be used. Myofibrils make up about 46% of the cell, and mitochondria make up about 36% of the cardiac cell, and the SR is important but only makes up about 4% of the cardiac cell. T-tubules (transverse tubules) are finger-like invaginations from the cell surface. T-tubule openings are up to 200nm in diameter. They carry the surface depolarisation deep into the cell. They are spaced approximately 2μm apart so that a T-tubule lies alongside each Z line of every myofibril. Excitation-Contraction Coupling in the Heart When an action potential is produced at the extracellular surface, the wave of depolarisation is pushed down the T-tubules into the middle of the cell. This is sensed by the L-type calcium channels present in the T- tubules, and they open up. Calcium from outside the cell is then allowed to influx into the cell. Some of this influx activates the myofibrils directly, but most of it binds to the SR-Calcium release channel in the SR membrane. The release channel is activated, and allows stored calcium to be released from the SR into the cytoplasm and activate contraction. This is a calcium-induced calcium release process that happens in the heart. Skeletal muscle does not need this influx of calcium to release the calcium store. In heart muscle, it must have this external calcium trigger. Calcium is pumped back into the store by Calcium-ATPase (uses ATP to take calcium up against the concentration gradient, keeping it in storage, ready to be released in the next beat). The calcium that comes into the cell is effluxed by the sodium-calcium exchanger during relaxation. It uses a downhill energy gradient, as there is a low concentration of sodium inside the cell. 6
7 The Relationship between Force Production and Intracellular Ca 2+ concentration Cardiac muscle has a length-tension relationship, i.e. if it is stretched it will change its force. Cardiac muscle is very resistant to stretch, as it is less compliant than skeletal muscle. This is due to the properties of the extracellular matrix and the cytoskeleton. Only ascending limb is important for cardiac muscle. Preload and afterload Preload is the weight that stretches muscle before it is stimulated to contract. Afterload is the weight not apparent to muscle in the resting state - it is only encountered when muscle has started to contract. Isometric contraction - muscle doesn t shorten when the force is produced. Isotonic contraction - muscle shortens when the force is produced. As blood fills the ventricles during the relaxation phase (or diastole) of the cardiac cycle it stretches the resting ventricular walls. The stretch or filling determines the preload on the ventricles before ejection. Preload is dependent upon venous return to the heart. Measures of preload include end-diastolic volume, end diastolic pressure and right atrial pressure. Afterload is the load against which the left ventricle ejects blood after opening of the aortic valve. A simple measure of afterload is the diastolic arterial blood pressure. Any increase in afterload decreases the amount of isotonic shortening that occurs and decreases the velocity of shortening. 7
8 Frank-Starling relationship From observations by Otto Frank (1895) and EH Starling (1914) that as the filling of the heart increased there was a more forceful contraction, i.e. increased diastolic fibre length increases ventricular contraction. As a consequence, the ventricles pump greater stroke volume so that, at equilibrium, cardiac output exactly balances the augmented venous return. This is due to two factors. Firstly, there are changes in the number of myofilament cross bridges that interact. Secondly, there are changes in the calcium sensitivity of the myofilaments. At shorter lengths than optimal the actin filaments overlap on themselves so reducing the number of myosin cross bridges that can be made. Calcium is required for myofilament activation. Troponin C is a thin filament protein that binds calcium. TnC regulates the formation of cross-bridges between actin and myosin. At longer sarcomere lengths the affinity of troponin C for calcium is increased. Less calcium is required for the same amount of force. The amount of work done by the heart to eject blood under pressure into the aorta and pulmonary artery is called the stroke work. Stroke work is the volume of blood ejected during each stoke (SV) times the pressure at which the blood is ejected (P). Stroke Work = SV x P Law of Laplace The law of Laplace states that when the pressure within a cylinder is held constant, the tension on its walls increases with increasing radius. In order to increase P whilst keeping wall stress (T) constant, either decrease radius (R) or increase wall thickness (h). The radius of curvature of the walls of the left ventricle are less than that of the right ventricle allowing the left ventricle to generate higher pressures with similar wall stress. This facilitates late ejection. Wall stress is kept low in e.g. a giraffe by a long, narrow, thick-walled ventricle. In a frog, where pressures are low, the ventricle is almost spherical. Failing hearts often become dilated which decreases pressure generation and ejection of blood and increases wall stress. 8
9 Electrical Activity of the Heart by Dr Frank Harrison Human cardiac myocytes are small cells and are attached to adjacent ones. They join at intercalated discs (end-to-end junctions) and have gap junctions with very low electrical resistance. Because of this, they have action potentials that easily spread between cells, which means that the cells act together (syncytium). Cardiac muscle contains actin and myosin as contractile proteins. Sino-Atrial Node Left Atrium Atrio-Ventricular Node Right Atrium Left Ventricle Bundle of His Right Ventricle Purkinje Fibres Bundle Branches There are five important components. Firstly the Sino-Atrial Node is a strip of modified muscle tissue located on the posterial wall of the right atrium, near the vena cava. The second component is the Atrio-Ventricular Node. The two atria and the two ventricles are separated from one another by a non-conductive fibrous ring of tissue. The AVN provides an electrical bridge between the atria and the ventricles. The third component is the Bundle of His, which is a group of rapidly conducting muscle fibres. The Bundle of His conveys the electrical depolarisation from the AVN towards the septum (wall that divides ventricles). The Bundle of His divides into two bundle branches running down the right and left sides of the septum. These too are conducting muscle fibres. The final structure is that the bundle branches give rise to the Purkinje fibres, located on the inner surface of the ventricular muscle (the endocardium). The purpose of this specialised conducting fibre system ensures that the muscle mass contracts as near simultaneously as possible. Because this contraction is quite short, it enables the ventricles to develop substantial force in a short time which generates systolic blood pressure. Cardiac muscle is perfectly capable of beating without any outside influence at all. If the heart is taken from an animal and put in a suitable warm salty solution with appropriate solutes at the right ph, oxygenated, with glucose etc, the heart will keep beating more many hours! This is in contrast to skeletal muscle, which needs a stimulus to contract. A Heart Beat The wave of depolarisation is initiated at the SAN. It spreads from the SAN across the right and left atria, causing them to contract. The wave of depolarisation reaches the AVN, and it does so after a brief delay. The AVN depolarises, and this spreads down into the Bundle of His, down the branches and into the Purkinje fibres, initiating ventricular contraction. 9
10 The Electrical Activity Action potentials can be recorded from the SAN. The resting membrane potential is about -65mV, but it is not stable. It doesn t stay level, it shows some spontaneous depolarisation, known as the pre-potential. When the prepotential takes the cell membrane to a particular value (e.g. about -50mV), it reaches the threshold, at which point there is an influx of sodium ions and the cell depolarises. The cause of that slowly decaying pre-potential has two main factors contributing to it. Firstly, there is a special current that is innate to pacemaker cells in so far as there is a slow inward sodium current. The positive sodium ions make the inside of the cells less negative (depolarise). Secondly, the membrane permeability of potassium ions falls, which inhibits the outward movement of positively charged potassium ions. The combined effect is therefore that the cell slowly depolarises. The slope of the pre-potential determines how quickly the cell returns to the threshold and therefore how quickly the next beat is produced, i.e. the heart rate. Under the influence of the sympathetic nervous system, the slope of the pre-potential increases, which means the threshold is reached more quickly and the heart rate increases. On the other hand, under the influence of the parasympathetic nervous system (supply down the Vegas nerve), the slope of the pre-potential is reduced, and consequently the heart rate goes down. The reason for these two parts of the autonomous nervous system controlling heart rate is because of their transmitters - noradrenaline in the sympathetic pathway increases the inward sodium current, and acetylcholine in the parasympathetic (Vegas) pathway decreases the sodium current. The action potential of an atrial cell lacks the pre-potential, i.e. the resting membrane potential is stable and is also a bit more negative than the resting membrane potential of the pacemaker cells at around -90mV. The duration of the action potential is about 90ms. The shape is vaguely triangular, except where it starts to repolarise. In a ventricular cell, the resting membrane potential is again around -90mV. However, the duration is much longer, at around 250 to 300ms. The obvious feature that can be seen is known as the plateau phase, which is caused by an inward calcium ion current. As the membrane depolarises, passing around - 35mV, there are voltage sensitive calcium ion channels that open and allow calcium to move into the cell down its electrochemical gradient. Calcium ions carry two positive charges and hence delay the process of repolarisation. The calcium ion current is very important. The action potential of AVN cells resembles that of ventricular cells as it has a plateau phase, however is also has a pre-potential. One problem of the structures of the conducting system described is that it relies upon the current spread from the atria to the ventricles across that fibrous ring at the AVN. Potentially if the AVN goes wrong, then there is in principle a loss in mechanism for the atrial depolarisation to spread to the ventricles. This is called heart block. If there was no back-up system for ventricular depolarisation, the consequences would be dire. There is a back-up mechanism, in which the AVN cells take over the function of pacemaker activity for the ventricles. There is a difference, however, as the resting heart rate is generated by the depolarisation rate in the SAN. The equivalent rhythm of the AVN is a bit slower. 10
11 Timing of Venctricular Action Potential and Isometric Force The diagram illustrates that the electrical activity of the ventricle occurs before the mechanical events. What is obvious from this is that the duration of the two events are not that much different. This is in great contrast to skeletal muscle in which the action potential has a much shorter duration than the mechanical response. The refractory period of ventricular tissue is shown in the diagram, showing that the muscle is still refractory while it has started to relax, so the force is declining during the membrane potential s refractory period. Consequently, a fused tetanus cannot be produced in cardiac muscle, again in contrast to skeletal muscle. Cardiac muscle functions rhythmically. Clinical Cardiac Calcium It is the calcium ion inward current that extends the refractory period, and influences the force of the contractions of the heart. This has significant clinical implications, as some patients have conditions in which the heart is failing (not beating forcefully enough). This can be treated with a drug that increases the intracellular calcium ion concentration. Patients with angina can be treated by reducing the amount of work that the heart is doing (whilst simultaneously treating the hypoxia) with a calcium ion hooking drug (e.g. Verapamil) to reduce the amount of calcium ions that enter cardiac muscle. Introduction to ECG The effects of a wave of depolarisation are detected as the potential difference between two electrodes. When a wave of depolarisation is moving towards the positive electrode, it causes an upward deflection. When it is moving away from the positive electrode it produces a downward deflection. In practice, the equipment used to record ECG has to have high amplification as the signal being looked for is small. The characteristic shape of the ECG is caused by the wave of depolarisation that occurs in the heart. The wave of activity travels in several directions as it spreads across, but overall it has a net direction towards the left. This cumulative sum effect of depolarisation is recorded as a little upward hump at the beginning called the P wave (depolarisation of atria). The wave of depolarisation reaches the AVN, which has small junctional fibres of low conduction. They delay the spread into the ventricular system. During this delay, the atria contract, pumping the blood into the ventricles. Most of the filling of the ventricles happens during diastole, as the valves are open and filling occurs passively, but the last bit of blood is propelled by atrial contraction. The wave passes through the Bundle of His, with a net effect being to the left side. The wave then passes through the Purkinje cells, causing the ventricles to contract. The sequence of depolarisation of the ventricles is that the endocardium depolarises before the epicardium, and the apex depolarises before the base. The effect of this is that there is a powerful, short-lasting contraction of the ventricles, which gives rise to the QRS complex on the ECG. It represents ventricular depolarisation, followed by repolarisation of the ventricles seen on the ECG as the T wave. The net direction of the wave is to the left for good reason. The wall of the left ventricle is thicker than the wall of the right ventricle. Broadly speaking, both sides of the heart pump out the same volume of blood. The pressure in the systemic circulation (left) is much higher than that in the pulmonary circulation (right). This mean direction to the left is known as the Mean Frontal Plane Axis of the ventricle. 11
12 The Microcirculation by Dr Chris John The Branching Structure of the Microvasculature: 1 st Order Arterioles Terminal Arterioles Capillary Pericytic (post-capillary) Venule Venule The overall aim of the cardiovascular system is to provide adequate blood flow through the capillaries. This is the only way the tissues can function - if blood is supplied to them by the capillaries. A measure of this is known as blood flow rate, which is the volume of blood passing through a vessel per unit time. Where: F = ΔP R F is the blood flow rate P is the pressure, so ΔP is the pressure gradient R is the vascular resistance. The pressure gradient is the pressure at the beginning of the arteriole (the pressure as the blood exits the major artery) compared to the pressure as the blood leaves the arteriole. This is determined by the heart contraction force vs. the frictional loss. Resistance is the hindrance to blood flow due to friction between moving fluid and stationary vascular walls. There are a number of factors that influence this, such as the blood viscosity, the vessel length and the vessel radius. Blood viscosity as a general rule remains fairly constant, and the vessel length doesn t change. So the vessel radius is the main factor that influences resistance. Resistance has a proportional relationship with vessel radius described by: R 1 r 4 According to this equation, if the radium is halved, then the resistance in the vessel will increase 16 fold. The arterioles are the major resistance vessels in the cardiovascular system. They have the greatest influence on blood pressure. The pressure as blood enters the arterioles is on average 93mmHg (mean arterial pressure), and the pressure as it leaves the arterioles is on average 37mmHg. The blood is slowed sufficiently so that as it passes through the capillaries it is passing at a rate suitable for exchange of materials. The principle of blood flow rate can be applied to whole tissues or organs. F organ = ΔP / R organ, where the pressure gradient is pretty much the same as the mean arterial pressure. Without this pressure difference blood would not reach tissue capillary beds. 12
13 Blood flow across any given tissue is driven by the extent to which arterioles are either constricted or dilated. With powerful vasoconstriction the muscle in the arterioles contracts, the radius decreases massively, the resistance increases massively and the blood flow decreases. This is how blood flow can be restricted to particular tissues. With vasodilation, the arteriole muscles relax and the radius increases, the resistance decreases as the flow rate increases as the arterioles open up. Arteriolar smooth muscle normally displays a state of partial constriction - this is known as vascular tone. The radii of arterioles are adjusted independently to accomplish two functions: 1) Match blood flow to the metabolic needs of specific tissues (depending on the body s momentary needs). This is regulated by local (intrinsic) controls - independent of nerves or hormones. 2) Help regulate arterial blood pressure - regulated by extrinsic controls. Chemical alterations mean that if a tissue becomes more metabolically active, the blood flow through that tissue increases. For example in skeletal muscle, at rest about 10% of the capillaries are open. During exercise there is a massive increase in blood flow to the skeletal muscles, as more oxygen and more glucose is used. This is detected locally within the tissue (oxygen concentration changes) and so vasodilation would occur. This process is termed active hyperaemia. Physical manipulations are mainly due to heat loss - the need to prevent it or cause it. If the blood temperature starts to rise, there is a massive dilation of the peripheral arteriolar beds to try to dissipate that temperature. If the blood temperature starts to fall, then blood is diverted away from the periphery to try to maintain the blood pressure. For example applying cold compress like frozen peas diverts blood away from that area of the body which reduces swelling. Other physical alterations include myogenic vasoconstriction, which is a response to increased stretch of the arterioles. E.g. during exercise, the tissues that are massively metabolically active have increased blood flow, but this needs to be compensated for by constriction in other tissues. Commonly, in tissues such as the gut tissue, there is a rebound vasoconstriction to conserve the blood pressure. If the blood pressure is increased in one part of the body, it has to be decreased in another part of the body - autoregulation. In terms of extrinsic controls, arterial blood pressure control can be described by the basic equation F = ΔP / R as applied to the entire circulation. In this case: blood flow is equivalent to cardiac output (flow per unit time), pressure gradient is the mean arterial pressure, and the resistance is the total peripheral resistance. Therefore the resultant equation is: CO = MAP / TPR, and so MAP = CO x TPR Therefore, the body can preserve blood pressure when it has to by massive vasoconstriction of arterioles. Particular tissues are targeted more than others - no vasoconstriction in important tissues. E.g. at times of haemorrhage the cardiovascular control centre in the brain stem (medulla) sends neural signals to large numbers of tissue beds to cause profound vasoconstriction. This preserves blood pressure, but can also have disastrous circulatory failure effects. The receptors mediating the sympathetic nervous system effects are α receptors (α1) which cause vasoconstriction. β receptors are found on the heart. There are a number of hormonal mediators that can help to preserve blood pressure by their effects on the microcirculation. For example: vasopressin (vasoconstrictor), angiotensin II (vasoconstrictor), adrenaline / noradrenaline (effects on vasculature and heart - sympathetic output). Capillaries are arguably the most important vessels in the microcirculation. Capillary exchange is the ultimate function of the cardiovascular system where there is movement of metabolic substrates and removal of waste products - it allows tissues to function normally and in a coordinated fashion. Capillaries are well designed to facilitate this process. They are incredibly narrow 13
14 (7μm in diameter), have very thin walls (1μm) and form extensive and dense networks. This means that diffusion distances are very small, and the surface area for it is very large - the tissue is never far away from the capillary. Highly metabolically active tissues require a denser capillary network. Different tissues therefore have different densities of capillary network. Skeletal muscle has dense capillary network of 100cm²/g, the myocardium and also the brain have denser capillary networks of 500cm²/g. The lungs have the densest capillary network, but that is because of their role in gas exchange cm²/g. Just because there is a dense capillary network doesn t mean all the capillaries are open at all times. The precapillary sphincters control the opening and closing of the capillaries. For example in the skeletal muscle at rest, only about 10% of the capillaries are open and 90% of the blood bypasses the other capillaries in the system. They open up (active hyperaemia) when exercise begins to allow blood to flow into the tissues. There are three major forms of capillary structure. The large majority of capillaries are made up of continuous endothelium. The capillaries are endothelial cells lined up in a row, with water-filled gap junctions which allows movement of certain electrolytes and water, but protein cannot get out of the capillaries into the tissues. In certain tissues, there are incredibly tight gap-junctions, for example in the brain (the blood-brain barrier). In some tissues there are discontinuous capillaries where there are large gaps in the capillary structure. For example in the bone marrow, where white blood cells that have been generated pass out of the tissue and into the blood stream. Capillary structure depends on what the needs and function of the tissue is. Fluid movement across capillary - a volume of protein free plasma filters out of the capillary, mixes with the surrounding interstitial fluid and is reabsorbed. This is process known as bulk flow. Bulk flow is driven by two factors. The first that drives fluid out of the capillaries and into the tissues is hydrostatic pressure - the pressure generated by the heart, by the cardiac output. The next structure is the fenestrated capillary. There are small windows within the capillary structure. A lot of the endocrine glands have this capillary structure to allow small protein hormones to diffuse into or out of the endocrine tissue. The kidney is also a good example - Glomerulus filtration is through a capillary structure such as this. The second pressure that draws fluid back in again is the oncotic pressure. A strong osmotic pull is generated pulling fluid back into the capillaries from the tissues as proteins don t leave the blood stream. These forces are termed Starling s Forces. Starling s hypothesis of 1896 was that there must be a balance between the hydrostatic pressure of the blood in the capillaries and the osmotic attraction of the blood for the surrounding fluids and whereas capillary pressure determines transudation, the osmotic pressure of the proteins of the serum determines absorption. 14
15 If the pressure inside the capillary is greater than in the interstitial fluid, the result is ultrafiltration. This occurs early in the capillary. If the inward driving pressure is greater than the outward pressures across the capillary, the result is reabsorption. This occurs later in the capillary. There is always a net movement of fluid that takes the fluid overall in and out of the capillary system, but there is also always a small net loss. The role of the lymphatic system is to make sure that the net loss of fluid that occurs over the course of the day is eventually returned back into the bloodstream. Rather like the microcirculation, there is a lymphatic circulation that runs in parallel to the microcirculation. The lymphatics feed into all the tissues much like the microcirculation. As the lymphatics enter the tissues, they become blind-ended - it is not a circular system, they end in the tissues. Lymphatic capillaries are designed in such a way that fluid can flow into the lymph vessels, but cannot flow back out. So the interstitial fluid is taken away into the rest of the lymphatic system. The lymphatic capillaries drain into larger and larger lymphatic vessels, and eventually have entry points (e.g. in the thoracic area / lower neck) back into the venous system to ensure that blood pressure is preserved. Lymph nodes found in the lymphatic system are part of the immune system. They are filled with lymphocytes, and any sort of infection that passes through the lymphatic system will be detected in the lymph nodes and the various lymphocytes will attack that infection, whether it be through the generation of antibodies or otherwise. During an infection the lymph nodes tend to get larger, and this can be heard as a common complaint of swollen glands. There is no heart to drive lymphatic flow, and so lymphatic fluid gets from the tissues to the thoracic duct by skeletal muscle contractions and also by negative pressures exerted within the thoracic cavity - e.g. the contracting diaphragm. Around 3 litres of lymph are returned to the blood stream every day. Oedema refers to tissue swelling due to fluid accumulation, which occurs when the rate of production of the fluid is greater than the capacity of the lymphatic system to remove it (lymphatic failure). An unpleasant disease is the parasitic blockage of the lymph nodes - Elephantiasis. There is massive oedema throughout the body, primarily because the lymphatic system is blocked and cannot drain properly. 15
16 Understanding the ECG by Dr Frank Harrison Electrocardiography is a transthoracic interpretation of the electrical activity of the heart over time captured and externally recorded by skin electrodes. The ECG is based around the concept of an equilateral triangle (Einthoven s triangle) with the heart at the centre. The points of the triangle are approximated by the limb leads, connected to the right arm, left arm and left foot. The potential difference between two leads will depend upon the amplitude of the current, related to the muscle mass and direction of current flow. Attachment of Electrodes: the right foot is always used as a zero volt reference point. This leaves two arms and the left foot for recording signals. Lead I is LA RA where LA is + Lead II is LF RA where LF is + Lead III is LF LA where LF is + These standard limb leads are bipolar electrodes formed by connecting together two limb leads. In each case it is necessary to designate one electrode as positive and one negative. By convention, the apparatus is connected so that as a wave of depolarisation moves towards the positive electrode, an upward deflection is recorded. Another 3 limb leads can be connected up which use one single limb lead as one electrode (+) and another two leads connected together to make the indifferent negative electrode. avr RA LA+LF where RA is + avl LA RA+LF where LA is + avf LF RA+LA where LF is + These are the augmented limb leads. The negative electrode may be assumed to be halfway between the two points of the triangle that are connected together. The advantage of this arrangement is that it provides three more angles from which the electrical activity of the heart may be recorded. The three angles are still in the same frontal plane as the standard limb leads. The augmented limb leads and the standard limb leads, together give rise to the hexagonal reference system. The 6 limb leads therefore give a view of the electrical activity of the heart every 30 degrees. The magnitude of the ECG results may vary due to the fact that the leads assess the heart from different angles, therefore some leads will be more parallel to the direction of the electrical impulse and therefore give large ECG readings (normally lead II), whereas others may be more perpendicular to the direction of depolarisation/repolarisation and therefore five a smaller ECG reading. The Mean Frontal Plane Axis is the mean direction of the wave of depolarisation, which is usually towards the left ventricle as it is respective of tissue mass. Generally the mean frontal plane axis is between -30 and +90 because of the large muscle mass of the left ventricle. It does however depend on the way in which the heart lies as well as the amount of muscle in the left ventricle. 16
17 If the mean frontal plane axis lies beyond -30 then it is called left axis deviation. This occurs in left ventricular hypertrophy. If the mean frontal plane axis lies beyond +90 then it is right axis deviation. This occurs in right ventricle hypertrophy, associated with pulmonary conditions. Lead I Lead II Lead III Lead avl Lead avr Lead avf MFPA 0 o MFPA 90 o The value of cos90 is zero, hence the value of MFPAcos90 is also zero. This explains why a lead with its axis at right angles to the MFPA shows no signal (or a small equipotential). Cosines of angles between 90 and 270 are negative, thus when a lead is more than 90 to the MFPA the ECG will show downward rather than upward deflections. Whereas the limb leads measure depolarisation in the frontal plane, the 6 chest leads measure depolarisation in different horizontal planes. They are labelled V1 to V6 respectively. They are all positive electrodes. V1 = right 4 th intercostal space - parasternal V2 = left 4 th intercostal space - parasternal V3 = left midway between V2 and V4 V4 = left 5 th intercostal space - mid-clavicular line V5 = left anterior axillary line - in line with V4 V6 = left mid axillary line (right ventricle) (right ventricle) (septum and anterior wall of left ventricle) (septum and anterior left ventricle) (anterior and lateral left ventricle) (anterior and lateral left ventricle) The first part of the heart to depolarise is the septum, then the general direction of depolarisation is towards the left ventricle. Therefore, V1 will have a small upward deflection followed by a large downward one as the small septal depolarisation is toward the electrode and the large left ventricle depolarisation is away from the electrode. 17
18 The ECG - Identifying Some Basic Disturbances of Rhythm by Dr Sanjay Prasad The baseline (isoelectric line) is represented as a straight line on the ECG paper where there are no positive or negative charges of electricity to create deflections. The above grid shows the small and large squares that an ECG is commonly recorded on. A small square is 1mm by 1mm and a large square is 5mm by 5mm. Above is a normal 12 lead ECG with three additional rhythm strips at the bottom. Waveforms are representations of electrical activity created by depolarisation and repolarisation of the atria and ventricles. If the electrical current is flowing towards the lead then a positive deflection will be seen. If the electrical current is flowing away from the lead then a negative deflection will be seen. Waveforms that are above and below the isoelectric line are called biphasic. Electrical impulses originating in the SA node trigger atrial depolarisation. The normal P wave is no more than 0.1 seconds in duration and 2.5mm high. The direction of electrical activity is from the SA to the AV node. The P wave is a representation of the time it takes for atrial depolarisation. It is viewed normally as small and curved with a positive deflection, seen at it s tallest on lead II. The QRS complex represents ventricular depolarisation. It consists of three waveforms. The normal complex begins with a downward deflection known as the Q wave, followed by an upward deflection called the R wave. The next downward deflection will be the S wave. All ventricular complexes are known as QRS complexes even if every wave is not present in all complexes. The normal QRS complex is 0.04 to 0.12 seconds. Ventricular repolarisation (which follows ventricular depolarisation) is represented by the T wave. Its shape is rounded and taller and wider than the P wave. It is also more sensitive to physiological and hormonal changes in shape but usually presents as a positive deflection: 5-10mm in height. After T wave an ECG can sometimes show a U wave. It is of the same deflection as the T wave and similar to the shape of P wave. The U wave is thought to represent late repolarisation of the Purkinje fibres in the ventricles and is more often not shown on a rhythm strip. 18
19 Interval refers to the length of a wave plus the isoelectric line that follows it. The length of an interval ends when another wave begins. They are named by using the letters of both waves on either side. Intervals contain waves. Segments refers to the baseline between the end of one wave and the beginning of the next wave. Segments are the lines between waves. The PR interval is the length along the baseline from the beginning of the P wave to the beginning of the QRS complex. This is normally 0.12 to 0.20 seconds in duration (3 to 5 small squares). The QT interval is the beginning of the QRS complex to the end of the T wave. In the presence of a U wave the measure should be from the beginning of the QRS complex to the end of the U wave. The ST segment is the length between the end of the S wave of the QRS complex and the beginning of the T wave. It is electrically neutral. The PR segment represents the delay in conduction from atrial depolarisation to the beginning of ventricular depolarisation. It is also electrically neutral. Estimating rates and rhythm Graph ECG paper is divided into vertical and horizontal lines, whereby small squares are 1mm sq. and the larger squares are 5mm sq. The time or rate is estimated by measuring the number of square blocks along the horizontal line. The distance across one small square is 0.04 seconds. The distance across one large square is 0.2 seconds. Vertical lines measure amplitude or voltage and is measured in millivolts. Each small square along the vertical line equates to 0.1mV. One large square equals 0.5mV. Horizontal lines measure time. Vertical lines measure voltage. A one second strip consists of 5 large blocks, three seconds equates to 15, six seconds equates to 30 and ten seconds equates to 50. You begin by counting the R waves in a ten second strip. Multiply that number by 6 to determine the heart rate in one minute. For example, if there were 16 R waves in a ten second strip this would equate to 16 x 6 = 96 beats/min. A normal 12 lead ECG page (A4 landscape) is just over 10 seconds (25cm). Count the large blocks that fall between two R waves. Start by finding an R wave that falls on or close to a dark line. To determine a rhythm or pattern, you must measure the distances between complexes and compare this against the next grouping of complexes. This is done by measuring the distance between one P wave and the next P wave or from one R wave to the next. Consistent intervals = normal rhythm. 19
20 Common cardiac arrhythmias: - Bradycardia - resting heart rate of under 60 beats per minute. - Tachycardia - heart rate that exceeds the normal range for a resting heart rate (varies with age). Over 100 beats per minute for adults. - Cardiac conduction abnormalities - Supraventricular arrhythmias o Atrial fibrillation, atrial flutter, AVNRT - Ventricular arrhythmias o Ventricular tachycardia, fibrillation Normal ECG values: P wave Duration < 0.11s; Amplitude < 2.5mm in lead II PR interval s QRS complex Duration < 0.12s; Amplitude: R wave in V6 < 25mm; Axis -30 to +90 degrees Q wave Duration < 0.04s; Amplitude < 25% of total QRS complex amplitude QT interval s ST segment Should be isoelectric T wave May be inverted in lead III, avr, V1 and V2 without being abnormal. Checking an ECG: 1. Is it the correct recording? 2. Identify the leads 3. Check the calibration and speed of the paper 4. Identify the rhythm 5. Look at the QRS axis 6. Look at the P wave 7. Look at the PR interval 8. Look at the QRS complex 9. Determine the position of the ST segment 10. Calculate the QT interval 11. Look at the T wave 12. Check again Sinus Tachycardia P waves have normal morphology, but there is an atrial rate of beats per minute. There is a regular ventricular rhythm, but a ventricular rate of beats per minute. One P wave precedes every QRS complex. 20
21 Atrial Fibrillation P waves absent; oscillating baseline f (fibrillation) waves. Atrial rate beats per minute, and irregular ventricular rhythm. Ventricular rate beats per minute. Atrial Flutter Undulating saw-toothed baseline F (flutter) waves. Atrial rate beats per minute. Regular ventricular rhythm. Ventricular rate typically 150 beats per minute (with 2:1 atrioventricular block). 4:1 is also common (3:1 and 1:1 block uncommon). Preexcitation Syndrome 21
22 Heart Block This is also known as AV nodal block. 1 st Degree AV nodal block shows a prolonged PR interval. 2 nd Degree AV nodal block is Mobitz Type I (Wenckebach) or Mobitz Type II. 3 rd Degree AV nodal block is complete heart block. 22
23 Bundle Branch Blocks In a normal impulse conduction, the impulse moves from the sinoatrial node, to the AV node, through the bundle of His, through the bundle branches, on to the Purkinje fibres. So depolarisation of the bundle branches and Purkinje fibres are seen on an ECG as the QRS complex. Therefore, a conduction block of the bundle branches would be reflected as a change in the QRS complex. With bundle branch blocks there are usually two changes: 1) the QRS complex widens (>0.12 seconds) 2) the QRS morphology changes (varies depending on the ECG lead, and if it is a right or left bundle branch block) The QRS complex widens because when the conduction pathway is blocked, it will take longer for the electrical signal to pass throughout the ventricles. For right bundle branch block, the wide QRS assumes a unique, virtually diagnostic shape in those leads overlying the right ventricle (V1 and V2): rabbit ears. For left bundle branch block, the wide QRS complex assumes a characteristic change in shape in those leads opposite the left ventricle (right ventricular leads - V1 and V2). They show broad, deep S waves. Ventricular Fibrillation 23
24 Blood Vessel Order, Function and Specialisation by Dr Adrian H Chester The cardiovascular system comprises the heart, blood vessels and the blood. The functions of the cardiovascular system are mainly associated with the rapid convective transport of nutrients (such as oxygen, glucose, amino acids, fatty acids, vitamins and water) as well as wastes (such as carbon dioxide, urea, creatine). The cardiovascular system is also responsible homeostatic components such as hormone transport, temperature regulation, etc. Endothelial cells generate and release a whole range of mediators. For example: - Nitric oxide: a smooth muscle relaxant. It increases the size of blood vessels. It can also influence the growth of the smooth muscle cells with an inhibitory effect. It can increase the blood flow to myocytes, and can inhibit platelet aggregation. - Prostacyclin: can also cause smooth muscle cell relaxation, inhibition of growth and also platelet aggregation. Acts synergistically with nitric oxide. - Thromboxane: contracts smooth muscle cells, reduces blood flow and stimulates platelet aggregation. Directly opposes nitric oxide and prostacyclin. - Endothelin-1: very powerful contractile agent to vascular smooth muscle cells and so reduces blood flow. Also has a weak ability to stimulate growth of the cells. - Angiotensin II: similar to endothelin, increases contraction and stimulates growth (production of extracellular matrix) and therefore reduces blood flow. The matrix production can cause remodelling and fibrosis in the heart. All of these are produced at the same time, and so there is a balancing mechanism so that vasodilation and vasoconstriction can be controlled by tipping the scale to one side or another as appropriate. Nitric oxide The endothelium has a key role in vascular tone. Nitric oxide, known as the endothelium-derived relaxing factor, is one of the few gaseous signalling molecules known, playing a role in a variety of biological processes. The endothelium of blood vessels uses nitric oxide to signal the surrounding smooth muscle to relax, thus resulting in vasodilation and increasing blood flow. It is highly reactive but diffuses freely across membranes. A signalling molecule that causes the release of NO is acetylcholine in endothelium-dependent vasodilation. It stimulates L-arginine to be cleaved by endothelial nitric oxide synthase to release nitric oxide, which then stimulates guanate cyclase, which then stimulates the formation of cgmp, which causes a decrease in Ca 2+ concentration = relaxation. In blood vessels, there is flow induced vasodilation, as NO is released in response to increased sheer stress. Vasodilation in the skin is usually due to thermoregulation (temperature change). Penile erection involved flow mediated dilation of the corpus cavernosus. Prostacyclin Both prostacyclin and thromboxane are made by the family of enzymes called cyclo-oxygenase (COX). There are two cyclo-oxygenase isoforms. COX-1 is associated with healthy maintenance of the cardiovascular system. COX-2 mediates inflammation and pain. These enzymes act on Arachidonic Acid to synthesise membrane phospholipids, and via a cascade of intermediates produce prostaglandins and thromboxane. Although produced by the same enzymes, the molecular structures and the receptor binding of prostacyclin and thromboxane differ. Prostacyclin receptors are IP receptors, whereas thromboxane receptors are TP receptors. Prostacyclin activates the camp signalling pathway, and thromboxane activates the IP3 signalling 24
25 pathway. Prostacyclin is a vasodilator, anti-atherogenic and anti-platelet molecule. Thromboxane is a vasoconstrictor, pro-atherogenic and pro-platelet molecule. Endothelin Endothelin is a 21 amino acid polypeptide. It can be stimulated by a whole range of different inflammatory mediators or compounds that control vascular tone (adrenaline, angiotensin II, vasopressin, steroids, IL-1, etc) and can be counter-acted by a range of molecules (prostacyclin, nitric oxide, heparin, etc). When stimulated it relies on synthesis within the endothelial cell by stimulation of the prepro-endothelin-1 mrna gene. Prepro-endothelin-1 goes through a series of steps to form endothelin-1, which is released by the cell and acts on the underlying smooth muscle cells. There are ET A and ET B receptors. The ET A receptor mediates smooth muscle cell contraction, and the ET B receptor is present on endothelial cells, and promotes the release of nitric oxide in a self-limiting mechanism (negative feedback) and also mediates smooth muscle cell contraction. It is a potent vasoconstrictor, and so inhibiting the ET-1 pathway should produce vasodilation. ACE and Angiotensin II Angiotensin II is made from angiotensin I by the action of angiotensin converting enzyme (ACE), which is found predominantly in the lung capillaries. Angiotensin I is formed by the action of renin from a longer polypeptide angiotensinogen. Bradykinin is also broken down by ACE, which can mediate the release of nitric oxide. Angiotensin II has a whole range of effects as an endocrine, autocrine and paracrine hormone throughout the body: - Cardiovascular effects o vasoconstrictor - Neural effects o thirst sensation o decrease baroreflex o secretion of vasopressin o secretion of ACTH - Adrenal effects o aldosterone release - Renal effects o increase Na + reabsorption o increase glomerular capillary hydrostatic pressure Adrenergic Hormones Noradrenaline and adrenaline can come from sympathetic nerve endings or the adrenal glands, contributing to circulating and locally released levels of catecholamines. Adrenaline stimulates a whole range of adrenergic receptors - α1 and 2, and also β1 and 2 in vascular smooth muscle cells. These receptors have differing effects on different tissues. Adrenaline can cause an increase in the systolic blood pressure, contractility of the heart, and heart rate. It also causes vasoconstriction in the arterioles of the skin, mucosa and intestinal blood vessels (splanchnic areas). In the skeletal muscles, it causes dilatation as a first-line response to stress. It does this because it acts on different receptors in these different locations of the body. There are well characterised chronotropic and inotropic effects on the heart. 25
26 Noradrenaline is an agonist of α and β1 adrenergic receptors. It principally causes vasoconstriction. Circulating levels come from spill-over from sympathetic nerve terminals and from adrenal medullary cells. Noradrenaline exerts direct positive inotropic and chronotropic effects in the heart. Hypertension is caused by increasing peripheral vascular resistance. Aspirin Inhibits the COX system, and therefore blocks the synthesis of prostacyclin and thromboxane. With low dose aspirin daily over a period of time, prostacyclin levels drop but then remain constant. Thromboxane levels drop until 70% inhibited, and so the balance is tipped in favour of prostacyclin. The endothelial cell that releases prostacyclin is nucleated and so can make new proteins as and when needed. If a little bit of COX is inhibited each day, the cell can make a bit more and maintain the level of prostacyclin release. Platelets (principle source of thromboxane) don t have a nucleus, so once the enzyme is inhibited, that s it! Nitric oxide drugs Drugs that are classified as NO donors (such as nitroglycerine and nitroprusside) supply nitric oxide. enos activators (such as endothelium-dependent vasodilators) stimulate nitric oxide. Phosphodiesterase inhibitors (such as Viagra and Zaprinast) prevent nitric oxide breakdown by preventing the breakdown of cgmp. β-blockers Beta-blockers are able to inhibit the binding of adrenaline and noradrenaline to β-receptors. They prevent the normal ligand from binding to the beta-adrenoreceptor by competing for the binding site. The heart has both β1 (predominant) and β2 receptors, and so cardiac effects are blocked. Vascular smooth muscle has β2 receptors (normally activated by noradrenaline released by sympathetic adrenergic nerves or by circulating noradrenaline), and so vascular effects are blocked. β1-blockers are cardioselective. They can decrease contractility (negative inotropy), decrease relaxation rate, decrease heart rate (negative chronotropy), and decrease conduction velocity. Therapeutic use of β- blockers can therefore be for hypertension, angina, myocardial infarction, arrhythmias and heart failure, as these drugs lack the vascular effects of β2 receptor blockade. All of the mentioned compounds ultimately regulate the release of calcium from a cell to induce relaxation or contraction. Calcium entry into cells is regulated by calcium channels in the plasma membrane. Therefore, compounds that block the calcium channels (such as dihydropyridines like Nifedipine or phenylalkylamines like Verapamil) can also be of use in the regulation of vascular tone (non-specific). The vasodilation reduces afterload (cardiac output increases). Negative inotropic effects occur (decreased work done by the heart), and oxygen demand is also reduced. They prevent coronary artery vasospasm, which makes them very useful in the treatment of variant angina. Drugs that have ability to block β2 receptors as well may cause vasoconstriction and/or bronchoconstriction. Voltage-gated calcium channels mediate calcium influx in response to membrane depolarisation. They regulate intracellular processes such as contraction, secretion, neurotransmission and gene expression. Activity is essential to couple electrical signals in the cell surface to physiological events in cells. Their affinity for the channel is directly related to the membrane potential of the target cells. By blocking calcium entry into a smooth muscle cell, vasodilatation can be caused and this reduces the afterload on the heart and cardiac output will increase (higher negative potentials). There can also be a negative inotropic effect on myocytes to decrease the work done by the heart and decrease the oxygen demand (lower negative potentials). Dihydropyridine calcium channel blockers are often used to reduce systemic vascular resistance and arterial pressure, but are not used to treat angina because the vasodilation and hypotension can lead to reflex tachycardia. Why do Drugs have Side Effects? Our body often uses the same chemical to regulate more than one process, and there is always interaction between different systems in the body. Unfortunately, drugs are not always as selective. There is just tissue specific distribution of receptors. It is also a fact that two people taking the same medicine can have very different experiences. 26
27 Viagra was originally developed as an anti-hypertensive, but there were some interesting side effects. Viagra was much more selective for inducing penile erection rather than lowering blood pressure. This is because Phosphodiesterase enzymes aren t all the same - there are 5 types of the enzyme and expression varies between tissues. In the corpus cavernosum, there is phosphodiesterase 5, and Viagra is selective for that particular enzyme. Patients prone to asthma may get attacks when they take aspirin. This is because if COX-1 and COX-2 are blocked, then the metabolism of arachidonic acid is shunted towards an alternative pathway to produce leukotrienes which causes asthma in 3 to 5% of patients. 27
28 Mechanical Properties of the Heart 2 by Dr Ken MacLeod In the cardiac cycle, the heart beat is divided into two main phases: diastole and systole. Diastole is ventricular relaxation during which the ventricles fill with blood. This is split into four sub-phases. Systole is ventricular contraction when blood is pumped into the arteries. This is split into two sub-phases. The cardiac cycle is a description of mechanical and electrical events, volume changes and sounds associated with the heart beat. Atrial systole Just prior to atrial systole blood flows passively through the open atrioventricular valves. The atria contract, topping off the volume of blood in the ventricles. Atrial contraction is complete before the ventricle starts. As the atria contract, there is increased pressure in the atria and blood is pushed back up the jugular vein causing the first discernable wave in a jugular venous pulse. It is the sino-atrial node activation which depolarises the atria. On an ECG, the P wave is atrial depolarisation. Sometimes a 4 th heart sound can be heard during this time. It is an abnormal sound, and occurs with congestive heart failure, pulmonary embolism or tricuspid valve incompetence. Isovolumic contraction This is the interval between the atrioventricular valves closing and the semi-lunar valves opening. During this time, the ventricles are isolated from the rest of the circulation. There is then contraction of the ventricles with no change in volume. The atrioventricular valves close as ventricular pressure exceeds the atrial pressure. Pressure in the ventricles increases without a volume change and approaches aortic pressure. On an ECG, the QRS complex marks ventricular depolarisation. The 1 st heart sound (lub) can be heard due to closure of the atrioventricular valves and associated vibrations. Rapid ejection Aortic and pulmonary valves open and mark the start of this phase. As the ventricles contract, pressure within them exceeds the pressure in the aorta and pulmonary arteries. The semi-lunar valves open and blood is pumped out and volumes in the ventricles decrease. Right ventricular contraction pushes the tricuspid valve into the atrium and creates a small pressure into the jugular vein. Reduced ejection This phase marks the end of systole. Aortic and pulmonary valves begin to close. Blood flow from the ventricles decreases and the ventricular volume decreases more slowly. As pressures in the ventricles fall below that in the arteries, blood begins to flow back causing the semi-lunar valves to close. 28
29 On an ECG, the T wave is due to ventricular repolarisation marking the end of ventricular systole. Isovolumic relaxation This is the beginning of diastole. The aortic and pulmonary valves have now just shut. The atrioventricular valves remain closed until the end of this phase. The atria have now filled with blood, but the atrioventricular valves have shut, so the atrial pressure rises. Blood pushing the tricuspid valve gives a second jugular pulse. There is also a rebound pressure against the aortic valve as the distended aortic wall relaxes. The 2 nd heart sound is heard (dubb) when the aortic and pulmonary valves close. Rapid ventricular filling Once the atrioventricular valves open, blood in the atria flows rapidly into the ventricles. The ventricular volume increases and atrial pressures fall. The presence of a 3 rd heart sound is usually abnormal and can signify turbulent ventricular filling. This gallop can be due to severe hypertension or mitral valve incompetence. Reduced ventricular filling This phase can be called diastasis. Ventricular volume increases more slowly. The diagram on the right is the Wiggers Diagram. Pulmonary Circulation Pressures The patterns of pressure changes in the right heart are essentially identical to those of the left. Quantitatively, the pressures in the right heart and pulmonary circulation are much lower (peak of systole - 25mmHg in pulmonary artery). Despite the lower pressures, the right ventricle ejects the same amount of blood as the left. Systemic and pulmonary circulations have different pressures - 120/80 vs 25/5 mmhg. 29
30 The Pressure-Volume Loop in the Heart Left ventricular pressure and left ventricular volume can be represented graphically. Starting at point 1 (end diastolic volume), there is a low ventricular pressure. At point 2, there is the period of isovolumic contraction where the pressure rises, but there is no change in volume. As the aortic pressure is reached and exceeded, blood is ejected and point 3 is reached (end systolic volume). Pressure in the ventricle drops again with no volume change, and this takes the line to point 4. The heart is then filled with blood again with little change in pressure, back to the end diastolic volume. Blood filling the ventricle during the diastolic period determines the preload (the stretch on the resting ventricle). The blood pressures seen at point 2 determine the afterload, as these are the pressures in the great vessels. Increasing preload increase the stroke volume, and increasing afterload decreases stroke volume. This is because as afterload increases, the amount of shortening that occurs decreases. Cardiac output = heart rate x stroke volume preload, afterload, contractility Contractility is the contractile capacity (strength of contraction) of the heart. A simple measure of cardiac contractility is ejection fraction. Contractility is increased by sympathetic stimulation. There is a family of different Frank- Starling relations as cardiac contractility changes. During exercise, contractility is increased due to increased sympathetic activity. During exercise end diastolic volume is increased due to changes in peripheral circulation (venoconstriction and muscle pump). 30
31 Blood Vessels and Flow by Professor Alun Hughes The primary role of the circulation is to deliver oxygen and nutrients and to remove metabolites and carbon dioxide. This achieved by the simple device of connecting a pump to a system of branching pipes which converge at the pump to complete the circuit. The pump (heart) generates a pressure gradient that drives bulk flow of blood through the network of blood vessels. At the capillary level, gas and nutrient exchange is accomplished by diffusion. This means that for exchange to work effectively no cell should be further than 10 m from a capillary. These considerations impose a number of limitations on circulatory design and from a fluid dynamics perspective the circulation is extremely complex. In reality the circulatory system consists of two such circuits both originating and terminating in the heart. Blood is pumped by the right heart through the low resistance pulmonary circulation to the left heart. En route the blood is oxygenated. The left heart then pumps blood through the systemic circulation which supplies the tissues and returns blood to the right heart. The structure of vessels is highly appropriate for their function e.g. large elastic arteries act as conduits and dampening vessels, while muscular arteries and arterioles have extensive smooth muscle in their walls so they can regulate their diameter and the resistance to blood flow. While capillaries are very numerous and have very thin walls to facilitate transport and diffusion and veins are highly compliant and act as a reservoir for blood volume. The circulation has exchange function as well as reservoir function. The diameter of the blood vessels changes dramatically from the aorta (25mm in man) to the capillaries (5 m = 0.005mm). As a result of the change in diameter and the expansion of components of the vascular system due to branching there are large changes in the crosssectional area of the vasculature at different levels. There are billions of capillaries and this resents by far the largest cross-sectional area of the circulation. Capillary beds present a huge surface area for exchange to take place. Although the volume in a single capillary is tiny, the equivalent of the whole cardiac output passes through the capillary bed every minute. The majority of blood volume is contained within the venous part of the circulation. Regulation of the capacitance of the veins and venules regulates how much blood is stored and influences venous return to the heart and ventricular work via the Frank-Starling effect in the heart. Why does blood flow? The diagram on the right is a very simple model of the circulation but it is useful in understanding how the system works. It assumes that the action of the heart (pump) has established a pressure in the tank (the aorta) equivalent to 8 ft of water (as measured by Hales in 1733). This drives a steady flow (Q) through the circulation. The branching vessels of the circulation are simplified into a single long rigid pipe for the purposes of this model. Pressure drops along this pipe due to viscous losses of energy (friction), so that the pressure measured at the end (P 2 ) is lower than at P 1 this pressure difference drives the flow (Q). At the end of the circulation the system empties into the right atrium of the heart which is almost at atmospheric pressure. In its simplest form the circulation can be equated to an electrical circuit. The pressure difference (P) is equivalent to the potential difference (V); the fluid flow (Q) is equivalent to current flow (I) and the fluid resistance (R) equates to the electrical resistance (R). Ohm s law can be used to describe the relationship between V, I and R or P, Q and R. 31
32 The haemodynamic determinants of mean blood pressure In the circulation (systemic & pulmonary) Ohm s relationship between pressure, flow and resistance can be restated in physiological terms as: Mean Blood Pressure = Cardiac Output x Peripheral Vascular Resistance This relationship is an approximation since flow in the circulation is not steady (due to the intermittent pumping of the heart) and blood vessels are not rigid. Nevertheless it is a simple and useful relationship which is applicable in many situations. The relationship between pressure and flow can be used to estimate the resistance of the circulation using estimates of cardiac output as bulk flow/unit time and the difference between mean arterial and venous pressure as the pressure drop across the circulation. It is pressure drop not absolute pressure itself that drives flow. If this is done for the systemic and pulmonary circulation then it is clear that the resistance of the pulmonary circulation is substantially less than the systemic. Physiologically, regulation of flow is achieved by variation in resistance while blood pressure remains relatively constant. Pressure is not constant in the circulation. It falls due to the resistance to blood flow provided by the blood vessels. The magnitude of oscillation in pressure (pulse pressure) is damped in the smaller arteries and arterioles. The major site of resistance (i.e. major region of pressure drop) is in small muscular arteries (<0.5mm internal diameter) and arterioles. The pulmonary circulation operates at lower pressures but shows a broadly similar distribution of pressure across the difference components of the circulation. Why is there resistance to blood flow? In the normal circulation flow is laminar, i.e. the fluid behaves as if it flows in layers or streamlines. Laminar flow can be demonstrated by injecting a dye into fluid, showing the existence of a clearly defined streamline. Dynamic viscosity (μ) is a measure of the resistance of a fluid to deform under shear stress. Resistance arises as a result of the resistance due to friction between fluid laminae moving at different velocities. A force per unit area (the pressure difference) is needed to move the fluid in opposition to viscosity. The flow velocity on the surface of the vessel wall is zero (so called no slip condition) but in a flowing fluid, the velocity of each lamina increases progressively as you move further way from the wall. The spatial velocity gradient is called the shear rate and the shear rate multiplied by the dynamic viscosity is the shear stress. The shear stress near the wall is believed to be an important influence on endothelial function in health and disease (e.g. the development of atherosclerosis). What accounts for resistance is Poiseuille s Law and vessel calibre. Experiments performed by Jean Poiseuille ( ) in long glass tubes began to show the relationship between pressure and laminar flow (i.e. resistance) in long straight tubes. Subsequently the theoretical basis of this relationship was derived by Wiedman, and Neumann and Hagenbach. The resistance to flow in a long straight rigid tube depends on the viscosity of the fluid (μ), the length of the tube (L) and the radius of the tube (r) and is described by Poiseuille s equation: Resistance = 8 L/ r 4 This equation emphasises the importance of arterial diameter as a determinant of resistance. Consequently relatively small changes in vascular tone (vasoconstriction/vasodilatation) can achieve marked changes in flow. A striking example of this is during exercise where dilation of the arteries and arterioles feeding skeletal muscle results in a 30fold increase in muscle blood flow. 32
33 What is the effect of blood pressure on the vessel wall? Pressure difference between two locations in the circulation is important for flow, but the pressure inside the vessel (transmural pressure) determines the distension of the vessel wall. The relationship between transmural pressure and wall tension is determined by Laplace s law. This and the wall thickness determine the circumferential stress. In extreme cases over a prolonged period in a weakened vessel high circumferential stress can cause a balloon like distension (aneurysm) or even rupture. Compliance properties of arteries and veins The elastic properties of blood vessels depend mainly on structural proteins, elastin and collagen. Elastin is much more distensible than collagen. The combination of elastin and collagen in vessels results in a non-linear relationship between vessel pressure and volume (i.e. non-linear compliance). The elastic properties of arteries and veins differ and this is important for their function. Veins are highly compliant at low pressures while arteries are compliant over a wider pressure range. This means that relatively small changes in venous pressure distend veins and increase the volume of blood stored in them. This is important when the pressure in veins changes for example on standing. In man the venous reservoir is not always at the same level as the heart. On standing gravity increases pressure in the lower limbs (80mmHg). Since veins are compliant this increases the volume of blood in these vessels and (transiently) reduces the venous volume returning to the heart. This would reduce cardiac output and blood pressure if there were no compensatory response. The effect of gravity and posture affects the transmural pressure in all vessels, but at any particular location the gradient of pressure from large artery to capillary to vein is maintained so flow still occurs in the same way. The major effect of gravity is on the distensible veins in the leg and the volume of blood contained in them. A number of mechanisms act to limit the effect of blood pooling in the lower limb veins on the circulation. Veins act as an important reservoir for blood. This is because despite the low pressures, vein walls are relatively thin and compliant therefore they accommodate large volumes of blood (2/3 total blood volume) at low pressures. This reservoir/compliance function is physiologically regulated. Vein walls, although thin, do contain smooth muscle. The role of this muscle is not to narrow venous diameter and affect resistance (as it does in small arteries), but to stiffen the wall i.e. reduce compliance. Stimulation of the sympathetic nervous system which innervates this smooth muscle (noradrenaline acting via α adrenoceptors) therefore reduces venous compliance and hence increases venous return to the heart. This is an important site of action of the sympathetic nervous system and contributes to reflex responses to standing and haemorrhage. Return of blood to the heart during upright posture is assisted by the contraction of skeletal muscle in the lower limb which compresses veins within the muscle and forces blood back to the heart. This is called the muscle pump. Another mechanism called the respiratory pump also assists venous return. During respiration expansion of the chest and diaphragm causes a negative pressure within the thorax which effectively sucks blood into the central veins by reducing the extra-vascular pressure in the thorax and increasing it in the abdominal cavity. Both the skeletal and respiratory pumps depend on the presence of valves in the veins outside the chest to prevent retrograde flow. Incompetent calves cause dilated superficial veins in the leg (varicose veins). Prolonged elevation of venous pressure causes oedema in the feet. 33
34 Pulsatility and arterial compliance Conventionally blood pressure measurements are made in the arm. Blood pressure varies over the cardiac cycle with a peak in systole and a minimum in diastole. The systolic (SBP) and diastolic pressure (DBP) are usually recorded in clinic as SBP/DBP (e.g. 110/70). Values of blood pressure vary widely in a community. High levels of blood pressure are termed hypertension. The ability of the aorta and the elastic arteries to buffer or damp the oscillation in blood pressure is often termed a Windkessel (German for air chamber) after the device used in early fire-engines. In systole more blood is ejected into the aorta and large elastic arteries than leaves them. This distends these vessels. In effect some of the pressure energy generated in systole is converted to elastic energy in the artery wall which is stored during systole. Once the heart ceases ejection and the aortic valve closes, pressure starts to fall. Consequently the walls of the aorta and elastic arteries recoil and the elastic energy is reconverted into pressure and the stored volume is discharged. This process damps the magnitude of pressure change and accounts (to a large extent) for the diastolic component of arterial pressure. It also accounts for the maintenance of flow in the microcirculation during diastole. If arterial compliance is reduced (i.e. arteries get stiffer e.g. with age) then this mechanism is less able to damp the fluctuation in pressure and pulse pressure increases. 34
35 Sympathetic Nervous System and Renin-Angiotensin System by Dr Mike Schachter Most of the sympathetic outflow to the rest of the body comes from the spinal cord - particularly from the thoracic spinal cord and some from the upper lumbar part. This is all ultimately controlled and influenced by factors much higher up in the central nervous system, e.g. in the medulla of the brain and to some extent beyond that (conscious response to stress). One important aspect of autonomic cardiovascular control is focussed on baroreceptors. Arterial baroreceptors respond to changes in pressure, and changes in distension of the blood vessels (particularly the carotid sinus and aortic arch). Increasing stretch of the vessels corresponds to increasing blood pressure. The purpose of the receptors is to respond to changes in blood pressure so that a decrease in blood pressure causes an increase in sympathetic nervous activity. On the other hand if the blood pressure goes too high, there is activation of the parasympathetic system to lower blood pressure. The overall purpose is to maintain blood pressure and cardiac output. Autonomic effector nerves control and regulate effector organs such as vascular smooth muscle. Adrenergic receptors are present on the effector organs, where the sympathetic nerve endings release noradrenaline. Adrenaline is a hormone that is not produced by nerves, but the adrenal medulla is technically a sort of modified sympathetic ganglion (producing adrenaline in preference to noradrenaline). Noradrenaline is generated from dopamine, and there is a system for inactivating the transmitter by uptake into the neurone and the effector organ. Any transmitter that is released is indeed limited by inactivation both pre-synaptically and postsynaptically - a very carefully regulated process. Synthesis of adrenaline and noradrenaline occurs in the terminal varicosity of a sympathetic nerve. A vesicle which contains the transmitter fuses with the plasma membrane, and there is a channel opening from the vesicle into the outside world so the neurotransmitter is released. It is actively expelled, and it requires ATP to release the transmitter. The process of reuptake ensures the neurotransmitter that is not destroyed is recycled. There is a constant process of synthesis, recycling and reuptake, which maintains (under normal circumstances) the quantity of noradrenaline in the sympathetic nerves. There are two main ways to remove the noradrenaline from the synapse. Neuronal uptake is called uptake 1. Extraneuronal uptake is called uptake 2, usually into the effector organ. Uptake 1 tends to be a recycling process, whereas uptake 2 is an inactivation process where the transmitter is broken down. Two enzymes are responsible for this: catetechol-o-methyltransferase (COMT) and monoamine-oxidase (MAO). Adrenoceptors is the name given to all receptors which interact with adrenergic transmitters such as adrenaline or noradrenaline. There are two groups of effects. Excitatory effects on smooth muscle (αadrenoreceptor mediated), and relaxant effects on smooth muscle but stimulatory effects on the heart (βadrenoreceptor mediated). β1-adrenoceptors are located on cardiac muscle and the smooth muscle of the gastrointestinal tract. β2- adrenoceptors are located on bronchial, vascular and uterine smooth muscle. β3-adrenoceptors are found on fat cells and possibly on smooth muscle of gastrointestinal tract. 35
36 α1-adrenoceptors are located post-synaptically i.e. predominantly on effector cells. This is important in mediating constriction of resistance vessels in response to sympathetic vasoconstriction. α2-adrenoceptors are located on pre-synaptic nerve terminal membranes. Their activation by released transmitter causes negative feedback inhibition of further transmitter release. Some are post-synaptic on vascular smooth muscle, and also mediate vasoconstriction. The α1 receptor is a classical calcium-dependent receptor. It is linked to G proteins, which are in turn linked to phospho-lipase C, which in turn release phosphate, which release calcium. Calcium is the lead mediator for muscle constriction. β receptors and α2 receptors are different, as they are linked to cyclic AMP. β receptors increase levels of camp, and in smooth muscle this leads to relaxation, as camp is an antagonist of calcium. In the heart, camp actually increases calcium and therefore increases heart rate and contractility. In most cases however, camp is considered a relaxant, as an inhibitor transmitter. α2 receptor activation inhibits camp synthesis, and this directly increases calcium levels. Cardiovascular effects of catecholamines in man: Isoprenaline is a synthetic compound - a pure β agonist. Noradrenaline is predominantly an α agonist and adrenaline is a bit of both. Noradrenaline decrease heart rate due to a reflex effect from vasoconstriction, which activates baroreceptors to slow the heart. Adrenaline lowers diastolic pressure because it causes peripheral dilatation in some muscle beds. There is a rise in heart rate due to the direct effect of adrenaline via β receptors. So noradrenaline and adrenaline have opposing effects on heart rate but similar effects on mean blood pressure. Isoprenaline causes no vasoconstriction; it causes vasodilatation which leads to decreased diastolic blood pressure. It causes some increase in systolic pressure because of its direct effect on the heart, activating contractility. This is why the heart rate goes up quite strongly. The effects of all three of these can be measured with blood pressure. - Noradrenaline: α1, α2, β1 - Adrenaline: α1, α2, β1, β2 - Dopamine: α1, β1 (weak effects, has its own receptors) - Isoprenaline: β1, β2 - Phenylephrine: α1 36
37 Regulation of the Cardiovascular System by Dr Ken MacLeod Stroke volume = end diastolic volume - end systolic volume Cardiac output = heart rate x stroke volume Mean systemic arterial pressure = cardiac output x total peripheral resistance The design of the cardiovascular system is such that there are systemic and pulmonary circulations. Blood is pumped from the right heart to the lungs back to the left heart. Veins are vessels that have capacitance. Venous volume distribution is affected by peripheral venous tone, gravity, skeletal muscle pump and respiratory pump (breathing). Central venous pressure (mean pressure in the right atrium) determines the amount of blood flowing back to the heart. The amount of blood flowing back to the heart determines stroke volume (using Starling s Law of the heart). Flow Control In veins, constriction determines compliance and venous return. In arterioles, constriction determines blood flow to organs they serve, mean arterial blood pressure and the pattern of distribution of blood to organs. Flow is changed primarily by altering vessel radius. F = ΔP R R = 1 r 4 There are various ways in which blood flow is regulated. There are local mechanisms, intrinsic to the smooth muscle itself or closely associated. There is systemic regulation in the form of hormones, and there is also the influence of the autonomic nervous system. Local Mechanisms Regulating Blood Flow Autoregulation is the intrinsic capacity to compensate for changes in perfusion pressure by changing vascular resistance. Myogenic theory is that smooth muscle fibres respond to tension in the vessel wall - as pressure rises muscle fibres contract. Stretch sensitive channels are involved. Metabolic theory is that as blood flow decreases, metabolites accumulate and vessels dilate. When flow increases, metabolites are washed away, e.g. CO 2, H +, adenosine, K +. Substances released from the endothelium: - Nitric oxide (endothelium derived relaxing factor) synthesised from arginine and plays a key role in vasodilation. - Prostacyclin and thromboxane (vasodilator and vasoconstrictor) relative amounts are important for clotting. - Endothelins (potent vasoconstrictors). 37
38 Systemic Regulation of Blood Flow by Hormones Circulating hormones affecting the vascular system: - Kinins: e.g. bradykinin, have complex interactions with renin-angiotensin system to relax vascular smooth muscle. - ANP: atrial natriuretic peptide, secreted from the cardiac atria is a vasodilator. - Circulating vasoconstrictors: ADH (vasopressin) secreted from posterior pituitary, noradrenaline released from adrenal medulla, angiotensin II formed by increased renin secretion from kidney. The Autonomic Nervous System Sympathetic nerve fibres innervate all vessels except capillaries and precapillary sphincters and some metarterioles. Large veins and the heart are also sympathetically innervated. Distribution of sympathetic fibres is variable. There are more innervating vessels supplying the kidneys, gut, spleen and skin and fewer innervating the skeletal muscle and brain. The vasomotor centre (VMC) in the brain is located bilaterally in the reticular substance of the medulla and the lower third of the pons. The VMC is composed of a vasoconstrictor area, a vasodilator area and a cardioregulatory inhibitory area. The VMC transmits impulses distally through the spinal cord to almost all blood vessels. Many higher centres of the brain such as the hypothalamus can exert powerful excitatory or inhibitory effects on the VMC. Lateral portions of the VMC controls heart activity by influencing heart rate and contractility. The medial portion of the VMC transmits signals via the vagus nerve to the heart that tend to decrease heart rate. All blood vessels receive sympathetic post-ganglionic innervation. The transmitter is noradrenaline. There is always some level of tonic activity. Control of nerve activity can accomplish dilation or constriction. Generally, there is no parasympathetic innervation to the vascular system. 38
39 Cardiac innervation has a number of different mechanisms. For example, increased activity of sympathetic nerves to the heart, increased plasma adrenaline, or decreased parasympathetic activity to the heart will all cause a rise in heart rate. The force of contraction is ultimately controlled by calcium ions. In both cardiac and skeletal muscle, the force-generating molecular motors (crossbridges) are turned on by increasing the intracellular free calcium level that regulates the troponin-tropomyosin system. The force of contraction can be controlled by increasing Ca 2+ influx or Ca 2+ uptake into intracellular stores increased. Stroke volume can be increased in a number of ways, for example, by increasing sympathetic innervation of the heart and by increasing plasma adrenaline. These are extrinsic controls. Intrinsic controls involve increasing end diastolic ventricular volume. This can be done by increasing atrial pressure, increasing venous return or increasing respiratory movements to create a low intrathoracic pressure. Feedback Baroreceptors detect changes in stretch in arteries such as the aortic arch and the carotid sinus. The baroreceptors fire signals to the vasomotor centre in the medulla oblongata via the vagus nerve and the glossopharyngeal nerve. Carotid sinus baroreceptors respond to pressures between 60 and 180mmHg. Baroreceptors respond to changes in arterial pressure. Baroreceptor reflex is most sensitive at pressures around 90 to 100mmHg. Reciprocal innervation involves an afferent input which stimulates parasympathetic nerves to the heart and simultaneously inhibits sympathetic innervation to the heart, arterioles and veins. Increased parasympathetic stimulation of the heart decreases the heart rate. Decreased sympathetic stimulation of the heart decreases the heart rate and stroke volume. Venous Return The regulation of venous return can be achieved by controlling venous pressure. Venous pressure can be increased in a number of ways, e.g. increasing blood volume, respiratory movements, sympathetic activity and by using the skeletal muscle pump. Increasing venous pressure increases venous return and this in turn has an effect on atrial pressure and end-diastolic volume. 39
40 40
41 Cardiovascular Stress by Dr Chris John Cardiovascular stress is applicable in change of posture, haemorrhage and exercise. Change of posture The problem for the cardiovascular system going from a sitting or lying (supine) to standing position is a severe challenge to human circulation. The problem is the addition of gravity to the situation. Going from a supine position to a vertical position, the standard mean arterial pressure (95-100mmHg) in the body is altered by gravity as you go away from the heart. Gravity pushes down on the column of blood, and therefore the pressure in the head is reduced, and the pressure in the feet is increased. The blood pressure in the arteries supplying the feet is considerably greater than the pressure generated by the heart itself. This is then a problem when this blood is to return to the heart. The pressure in the feet is less when it enters the veins. The venous pressure in the head is negative, as gravity simply forces the blood back down to the heart. Venous pressure in the lower limbs is going to take a lot of effort to get the blood back to the heart. For example, in a foot capillary, the usual pressure resulting from cardiac contraction is 25mmHg, but the added effect of gravity on the column of blood is 80mmHg. The result is an increased hydrostatic pressure in the blood vessels of the legs. As the blood enters the venous system at high pressure, there is venous distension. This means blood effectively pools in the legs as the veins stretch to maintain the blood at high pressure, but this makes it more difficult to get it back up to the heart. Another problem of increased hydrostatic pressure (forcing fluid out of the capillaries into the interstitium) with added gravity is that there is a lot more fluid leaving the capillaries further away from the heart downwards. In the feet, a fair amount of fluid is lost from the capillaries into the interstitium. The end result is a reduction in the effective circulating blood volume as a lot of the volume is lost into the interstitium, as the oncotic pressure normally drawing the fluid back into the blood is not sufficient. Starling s Law states that ventricular filling during diastole (end diastolic volume) determines the volume of blood ejected during systolic contraction (stroke volume). If a large amount of blood is being maintained within the distended veins in the legs and if fluid is also lost via the capillaries in those regions, then the amount of blood that can be returned to the heart is reduced. If this is the case, then there is a lower end diastolic volume and hence a reduced stroke volume. This leads to a drop in blood pressure - transient hypotension. Feelings of dizziness during transient hypotension quickly pass as there are compensatory mechanisms that deal with the problems. The first mechanism relates to arterial baroreceptors (mainly in the carotid sinus and within the aortic arch). These are nerve endings that protrude out into the blood flow. They are predominantly stretch receptors, responding to the stretch of the arteries. The higher the pressure, the more the baroreceptors fire; and the lower the pressure, the less they fire. The baroreceptors are exceptionally sensitive around the mean arterial pressure, so any increase or decrease here will be detected and responded to accordingly with an increase in firing rate (to raise BP) or fall in firing rate (to lower BP). Arterial baroreceptors send afferent nerves to the medulla in the brain, innervating the cardiovascular control centre. The cardiovascular control centre takes that information and passes it on to the autonomic nervous system. Baroreceptor firing mechanisms preserve blood pressure by influencing sympathetic or parasympathetic discharge. The secondary compensatory mechanism to transient hypotension is an increased sympathetic discharge. The firing rate of the baroreceptors decreases, which causes the loss of the parasympathetic drive. An increased sympathetic discharge increases the heart rate, increased contractility, increased splanchnic/renal vasoconstriction, and veno constriction in the legs to try to return the blood to the heart. 41
42 Haemorrhage The problem here is a reduction in the actual circulating blood volume. Again, the body tries to compensate for the loss of blood to preserve blood pressure, particularly in the brain and heart. The mechanisms are very similar for change of posture. Baroreceptor rate falls massively, which leads to increased heart rate and increased contractility. There is massive organ specific vasoconstriction, e.g. in the skin, to centralise the blood to preserve the pressure. There are extra compensatory mechanisms with regards to haemorrhage. If there is significant blood loss, then the hydrostatic pressure in the capillaries falls, so the amount of fluid going into the interstitium is reduced. On average the pressure difference at the arteriolar end is about 34mmHg and about 17mmHg at the venular end. After haemorrhage, these values fall markedly and the hydrostatic pressure falls massively. But there is still the colloid osmotic pressure that draws fluid out of the interstitium back into the blood. In a normal individual the balance between ultrafiltration and reabsorption is tipped more towards ultrafiltration. In someone who has undergone haemorrhage, reabsorption is the predominant force in the capillaries, which is known as autotransfusion. The blood bulks up using the fluid from the interstitium. Although the erythrocytes are not replaced, increasing blood volume will help to preserve pressure. Other compensatory mechanisms include hormone-driven decrease in urinary output (activation of the reninangiotensin system). As blood pressure falls, the reduced renal blood flow stimulates the production of renin, and so eventually the production of angiotensin II, which is a powerful vasoconstrictor, particularly in the kidney. Increased aldosterone production will promote sodium and water retention, and also the stimulation of ADH secretion will lead to water retention in the kidneys. All these factors act to prevent fluid loss and preserve blood pressure. The body can cope with less than 10% of blood loss (about 500ml), and there will be no noticeable change in blood pressure. If the body loses up to 30% of blood, there will be a fall in blood pressure but survival is highly likely. Above 30% blood loss, blood has to be redirected away from certain organs which results in shock, where certain tissues aren t receiving adequate blood supply. Fluid resuscitation is the best course of action when dealing with haemorrhage. Exercise The problem here is that it is necessary to increase blood flow to the heart and skeletal muscles. By increasing blood flow, there is going to be a massive fall in total peripheral resistance. In theory, exercise should be associated with a fall in blood pressure, but clearly it is not. This is the challenge for the cardiovascular system - increasing blood flow to tissues whilst preserving and maintaining blood pressure. As the skeletal muscle and the heart start to work harder, they use up more oxygen and generate more waste products, which is detected locally and causes profound vasodilation within the arterioles of that tissue. This is the process of active hyperaemia. There are control mechanisms by which the cardiovascular system maintains the blood pressure. The preprogrammed pattern is a system whereby the medullary cardiovascular centre starts to adapt to the exercise even before exercise has started - an anticipatory pre-programmed effect. This signal increases sympathetic outflow. On top of this, once the exercise starts, the muscle chemoreceptors pick up increasing metabolites being produced and this sends afferent signals to the medullary cardiovascular centre, which again causes it to act to preserve blood pressure. Local effects are in the heart and lungs and in the skeletal muscle to increase blood flow. The pre-programmed pattern decreases sympathetic drive to the skin and increased sympathetic drive to the GI tract and the kidneys. The net result is that there is profound vasoconstriction in the gut and kidneys, and this increases total peripheral resistance. With regards to the skin (an exception to the pre-programmed pattern), there is increased blood flow (reduced sympathetic activity) to dissipate the heat that is generated. There is a 10 fold increase in blood flow to skeletal muscle during exercise, and a 4 fold increase in blood flow to the heart. This decreases total peripheral resistance. But it is the vasoconstriction in other organs that increases total peripheral resistance. The overall result, however, is a decrease in total peripheral resistance, so it is clear that the skeletal muscle predominates. 42
43 By the pre-programmed pattern as well as input from muscle chemoreceptors, there is increased sympathetic innervation to the heart. This causes increased heart rate, increased force of contraction, and also increased venoconstriction to increase stroke volume. All these factors will increase cardiac output. Contracting skeletal muscles will also cause an increase in venous return, which again will increase stroke volume. Cardiac Output = Stroke Volume x Heart Rate There are negative effects of this. A slight decrease in plasma volume opposes increasing venous return. Increased capillary pressure across the muscle walls - by dilating the capillaries of the skeletal muscle, there is more blood flowing through this muscle and so more fluid is lost into the interstitium. This reduces the effective blood volume to a certain degree, and is added to by the loss of salt and water due to sweat. The net result, however, is an increase in cardiac output due to the sympathetic drive predominating. Cardiac output increases massively due to sympathetic effects, and peripheral resistance reduces due to vasodilation in the skeletal muscle and the heart (although it is mitigated by vasoconstriction elsewhere). Overall the increased cardiac output is more important than the reduction in total peripheral resistance, which is why blood pressure increases during exercise. 43
44 Chambers, Valves, Conduction System & Coronary Circulation by Professor Yen Ho The heart is a hollow muscular organ that comprises of two pumps in parallel. One pump, the right heart, receives systemic venous return and pumps it to the lungs for oxygenation. The second pump, the left heart, receives oxygenated blood from the lungs and sends it around the body. The two pumps are separated by a partition the cardiac septum. Each pump is made up of an atrium and a ventricle. Each ventricle has an inlet valve that stops back flow to the atrium and an outlet valve that stops reflux from the conduits (the great arteries) leaving the heart. Thus, the heart has four chambers and four main valves. [Deoxygenated blood from body and heart] Right atrium (tricuspid valve) Right ventricle (pulmonary valve) Pulmonary trunk [Oxygenated blood from lungs] Left atrium (mitral valve) Left ventricle (aortic valve) Aorta Lungs Body Arrangement of chambers understanding the cardiac silhouette Although the two pumps are designated right heart and left heart, the chambers are not strictly to the right nor to the left. Indeed, the arrangement of the cardiac chambers to one another is complex. When viewed from the front, the right heart chambers are anterior and to the right of the left heart chambers. Very little of the left heart is visible. This is the strip of left ventricle forming the left heart border (Figure 1). The atrial chambers are to the right of their respective ventricular chambers. The left atrium is the most posterior cardiac chamber. The cardiac valves are arranged at an angle to one another with the pulmonary valve being sited most superiorly. Figure 1. Arrangement of the cardiac chambers and valves. Left heart chambers are pale (stippled), right heart chambers are dark grey. P= pulmonary, A= aortic, M= mitral, T= tricuspid valve. 44
45 The Cardiac Chambers Right atrium This chamber comprises of an appendage and a venous component. 1. The appendage is large and lined by parallel ridges, the pectinate muscles. 2. The venous component receives the openings of the systemic veins: the superior caval vein, the inferior caval vein and the coronary sinus. The right atrium shares the septum with the left atrium. The oval fossa is an oval depression where the septum is thin. The muscular rim around the oval fossa is distinctive of the right atrium. The smooth vestibule of the right atrium leads to the opening of the tricuspid valve. Left atrium This chamber comprises of an appendage and a venous component. 1. The appendage is small and finger-like. 2. The venous component receives the pulmonary veins from the lungs. The septal surface is not marked by an oval depression. The left atrium leads to the mitral valve. Right ventricle The right ventricle is elliptical in shape. There are three components to a normal ventricle. 1. Inlet: This contains the tricuspid valve and its tensor apparatus. 2. Outlet: This is the infundibulum that supports the pulmonary valve. The tricuspid and pulmonary valves are widely separated from one another by an expanse of muscle, the supraventricular crest. 3. The trabecular portion is at the apex comprises of irregular bundles of muscle that project into the cavity. The trabeculations in the right ventricle are coarse relative to that in the left ventricle. A prominent bundle crossing the ventricular cavity near the apex is termed the moderator band. The ventricular septum is curved and runs obliquely. The major part of the septum is muscular. A tiny part near the atrium is membranous. Left ventricle This chamber is more conical in shape and has a rounded cross-section. It is also thicker walled than the right ventricle in the normal heart. 1. Inlet: This contains the mitral valve. Unlike the right heart, the aortic and mitral valves are adjacent to each other. There is an extensive area of fibrous continuity between these two valves. The thicker areas at each end of the area of fibrous continuity are termed the right and left fibrous trigones. Together with the membranous septum, the right fibrous trigone forms the central fibrous body. 2. Outlet: Owing to the central position of the aortic valve in the heart, the aortic outlet lies between the ventricular septum and the mitral valve. 3. The trabecular portion comprises of thin criss-crossing muscle bundles that mainly line the apical third of the ventricular cavity. The upper part of the ventricular septum is smooth. There is no equivalent of the moderator band although occasionally thin tendons stretch across the cavity. The valves of the heart Atrioventricular (or inlet) valves These valves guard the junctions between atrial and ventricular chambers and prevent backflow to the atria when the ventricles contract in systole. They are characterised by having a hinge-like attachment of leaflets to the atrioventricular junction and tendinous cords that attach the leaflets to papillary muscles. The papillary muscles attach to the septum or ventricular wall. The attachments of the cords prevent the leaflets from billowing into the atrial chambers when the valves are closed during systole. Tricuspid valve As its name suggests, this valve comprises of three leaflets. Named according to their locations, these are the antero-superior, mural(or inferior) and septal leaflets. Tendinous cords attach to the underside (ventricular aspect) toward the free margins of the leaflets. The septal leaflet of the tricuspid valve is distinctive in having direct cordal attachments to the ventricular septum. Mitral valve This valve has two leaflets the anterior (or aortic) and mural (or posterior). The aortic leaflet interposes between left ventricular inlet and outlet. The mural leaflet is often scalloped in appearance. Two 45
46 groups of papillary muscles in antero-lateral and postero-medial positions support the mitral valve. Tendinous cords from both leaflets are attached to each group of muscles. The attachment of the mitral valve is strengthened by strut cords and basal cords. Arterial valves (or outlet valves) The arterial valve guards the junction between the ventricular chamber and a great artery, preventing reflux. They open when the ventricles contract in systole and close when the ventricles are filled in diastole. Unlike the atrioventricular valves, arterial valves are not attached to cords or papillary muscles. Each valve has three semilunar shaped leaflets. Their hingelines mark crescents that cross the ventriculo-arterial junctions. At the valvar insertions, the arterial walls are dilated to form the arterial sinuses corresponding to the leaflets. In the middle of each free margin is a thickened area termed the nodule. Each leaflet forms a pocket that fills with blood and closes the valve in diastole but are pushed apart in systole allowing flow to escape from the ventricle. Pulmonary valve Guarding the end of the right ventricular infundibulum, the pulmonary valve is hinged in part to the musculature of the infundibulum and in part to the wall of the pulmonary trunk. Aortic valve Similar in structure to the pulmonary valve, the nodules are slightly thicker. The coronary orifices are located in two of the aortic sinuses. Half of the circumference of the aortic valve is in fibrous continuity with the mitral valve. Coronary Circulation The muscle of the heart is nourished by the coronary arteries. The deoxygenated blood from the myocardium drains back to the heart mainly by the coronary veins, with a small portion draining directly to the cardiac chambers via thebesian orifices. Coronary arteries The coronary arteries arise from the sinuses of the aorta. In the normal heart there are usually two coronary orifices, the left and right coronary orifices (ostia). The major coronary arteries are the right coronary, the anterior descending, the circumflex and the posterior descending coronary arteries (Figure 2). Smaller arteries arise from the major arteries to supply the atrial and ventricular musculature including the septum. In 90% of individuals, the right coronary artery continues into the posterior descending (interventricular) coronary artery that supplies the ventricular septum, and, in many of these cases, the right coronary artery continues to run into the inferior sector of the left atrioventricular groove to supply also the postero-medial wall and the papillary muscle of the mitral valve. The arrangement whereby the right coronary artery supplies the posterior descending coronary artery is termed right coronary dominance (Figure 3). Similarly, left coronary dominance is the term used in cases where the left system continues into the posterior descending coronary artery. Figure 2. The major coronary arteries. The posterior descending (interventricular artery is represented by the broken line) 46
47 Figure 3. The concept of coronary dominance Coronary veins Coronary veins convey the major portion of coronary venous return back to the heart. The main cardiac veins are the known as the great, middle, and small cardiac veins. These veins drain into the coronary sinus that, in turn, drains into the right atrium. Smaller veins, known as thebesian veins, drain directly into the cardiac chambers. The cardiac conduction system In addition to the contractile or working myocardium, the heart possesses histologically specialised myocardium that forms the cardiac conduction system (Figure 4). The initiation and conduction of impulses for the contraction of the heart occurs with the specialized myocytes of this system. The cardiac conduction system consists of: 1) The sinus node (the pacemaker of the heart) 2) The atrioventricular conduction system which comprises of The atrioventricular node The penetrating atrioventricular bundle of His The atrioventricular bundle which branches into the right bundle branch and the left bundle branch The peripheral ramifications of the bundle branches (so-called Purkinje network) Figure 4. The components of the cardiac conduction system 47
48 Haemostasis and Thrombosis by Professor David Lane and Professor Mike Laffan Knowledge of haemostatic mechanisms is important for diagnosis and treatment of bleeding disorders, as well as identification of risks and treatment for thrombotic disease. It is also important for monitoring anticoagulant drugs. The functions of haemostasis: - Prevention of blood loss from intact vessels - Arrest of bleeding from injured vessels The haemostatic plug formation is a sequential response to injury. The first step is vessel constriction, which happens primarily in the small blood vessels to stop local blood loss. The second step involves the blood platelets - the formation of an unstable platelet plug. There are two mechanisms to this, which are platelet adhesion and platelet aggregation. Next is the stabilisation of the plug with fibrin, which involves blood coagulation. The last step is the break down (dissolution) of the clot and the initiation of vessel repair, which involves fibrinolysis (an enzyme cascade system). There is a very rapid response to vessel injury, initiated by platelets recognising a site of injury Platelet adhesion: Disruption in endothelial cells exposes subendothelial structures such as collagen, which is particularly important in the haemostatic response. One mechanism by which platelets adhere to the site of injury is through a protein called Von Willebrand factor. Von Willebrand factor recognises the exposed collagen, and forms a bridge that links the platelet to the site of vessel injury. The platelet has a glycoprotein 1b receptor which binds to the Von Willebrand factor. Platelets can also directly bind to the site of injury via a second receptor - glycoprotein 1a, which directly binds to collagen at the site of injury. Platelet aggregation: The glycoprotein bound platelets become activated and release ADP (stored within the platelet) and generate prostaglandins, which cause the platelets to aggregate. Platelet aggregation is the clumping of the platelets, mediated by glycoprotein 2b / 3a and fibrinogen forming bridges. Blood Coagulation: The main sites of synthesis of clotting factors, fibrinolytic factors and inhibitors are the liver, endothelial cells and megakaryocytes. Most synthesis is in the liver but some proteins are produced in high local concentration in the endothelium (e.g. Von Willebrand factor) and in megakaryocytes (e.g. factor V). Factor XII is an inactive protein which can be activated to factor XIIa, which activates XI to XIa, etc as a cascade amplification mechanism. This is called the intrinsic pathway. There are co-factors that help these reactions work effectively, efficiently and help to localise them. The first of these co-factors is factor VIII. Activated factor VIII interacts with platelet membrane phospholipid (Pl) and calcium ions to accelerate and localise the reaction on the surface of the platelet. The activation of coagulation occurs on the activated platelets of the haemostatic plug. The second mechanism by which coagulation is activated is where tissue factor (protein exposed on damaged vessel) becomes activated and interacts with factor VII or factor VIIa to activate the cascade for X to Xa. This 48
49 leads to the common pathway, whereby factor Xa activates thrombin from prothrombin on the surface of the platelet. Throughout the cascade, zymogens (inactive) are activated to protease enzymes. Prothrombin is activated to thrombin, which activates platelets and also activates factor VIII to VIIIa and factor V to Va. Fibrinogen is a normal plasma protein circulating in high concentrations, which is converted by thrombin into fibrin to stabilise the platelet plug. Thrombin also stabilises the fibrin by activating factor XIII to XIIIa to form cross-linked fibrin. Simply viewed, the coagulation system is a cascade or amplification system. There are zymogens which are converted to proteases, as well as co-factors which need to be activated on surfaces. The surface might be platelets, which localise and accelerate the reactions. The trigger to initiate coagulation in vivo is tissue factor. Although factor XII can be activated to factor XIIa, this is mainly an in vitro reaction, useful for some diagnostic tests. Fibrinolysis This involves plasminogen (a zymogen). Tissue plasminogen activator (tpa) activates plasminogen into plasmin. It is activated when a fibrin clot forms. The formation of the fibrin clot is the trigger for the activation of plasminogen into plasmin. Plasmin is a proteolytic enzyme which breaks down the clot, producing fibrin degradation products, which can be used in diagnostic tests. tpa and a bacterial activator (streptokinase) are used in therapeutical thrombolysis for myocardial infarction (clot busters). The clotting cascade is an amplification system where a small amount of factor VIIa produces a large amount of thrombin. However, blood does not clot completely whenever clotting is initiated by vessel injury because there are inhibitory mechanisms to prevent this. There are two general mechanisms: 1) Direct inhibition: e.g. antithrombin, this is an inhibitor of thrombin and other clotting proteinases. 2) Indirect inhibition: e.g. inhibition of thrombin generation by the protein C anticoagulant pathway. 1) Antithrombin can inhibit most of the coagulation proteinases highlighted in red. The enzymes are released, but are inhibited and controlled by a natural inhibitor. Heparin is used for the immediate anticoagulation in venous thrombosis and in pulmonary embolism. Heparin accelerates the action of antithrombin. 2) Involves co-factors VIIIa and Va, which are activated by trace amounts of thrombin and become co-factors. Indirect inhibition inactivates the generation of thrombin. Thrombin binds to a receptor on the endothelium called thrombomodulin, which activates protein C. Activated protein C is an anticoagulant, which inactivates the two co-factors VIIIa and Va. This is the mechanism of control. 49
50 These mechanisms are important in thrombosis, as 5% of the population have a variant of factor V, which is the polymorphism called Factor V Leiden, which is not easily inactivated by activated protein C, and so is a risk factor for thrombosis. Coagulation inhibitory mechanisms can fail if there is antithrombin deficiency, protein C deficiency, protein S deficiency, or due to Factor V Leiden - all these are risk factors for thrombosis. Normal haemostasis is a state of equilibrium. It is a balance between fibrinolytic factors and anticoagulant proteins against coagulation factors and platelets. Bleeding is when the balance tips towards fibrinolytic factors and anticoagulant proteins. Abnormal bleeding doesn t stop. For example with disorders such as haemophilia, the bleeding is not necessarily severe, but it will not stop. Abnormal bleeding is usually spontaneous, out of proportion to the trauma, unduly prolonged and restarts after appearing to stop. For example, many people get nose bleeds. Significant Bleeding History: Examples - Epistaxis not stopped by 10 minutes compression or requiring medical attention/transfusion. - Cutaneous haemorrhage or bruising without apparent trauma (especially multiple/large). - Prolonged (>15 minutes) bleeding from trivial wounds, or in oral cavity or recurring spontaneously in 7 days after wound. - Spontaneous GI bleeding leading to anaemia. - Menorrhagia requiring treatment or leading to anaemia, not due to structural lesions of the uterus. - Heavy, prolonged or recurrent bleeding after surgery or dental extractions. Defects in Primary Haemostasis Abnormal bleeding is mainly as a result of problems with primary haemostasis - forming the platelet plug. This requires collagen, Von Willebrand factor and platelets. Patients can have problems with all of these, most commonly Von Willebrand factor deficiency or low platelet numbers. Defective collagen in the vessel wall is a rare disorder, but could be due to steroid therapy or age. Deficient Von Willebrand factor can be as a result of Von Willebrand disease (genetic), and platelets may be compromised by use of aspirin and other drugs, or by thrombocytopenia. There is usually a pattern of bleeding in defects of primary haemostasis. Typically: easy bruising, nosebleeds, gum bleeding, Menorrhagia, bleeding after trauma/surgery, petechaie (specific for thrombocytopenia). Defects in Secondary Haemostasis Defects of secondary haemostasis are to do with stabilisation of the plug with fibrin (blood coagulation). Fibrin mesh formation will be affected if there is deficiency or defect of coagulation factors (I-XIII). Common examples are haemophilia (factor VIII or IX; genetic), drugs (warfarin), liver disease (acquired), consumption (e.g. Disseminated Intravascular Coagulation; acquired). Disseminated intravascular coagulation is where there is generalised activation of coagulation (tissue factor). It is associated with sepsis, major tissue damage and inflammation. Coagulation factors and platelets are consumed and depleted, and the activation of fibrinolysis depletes fibrinogen. The consequences are widespread bleeding and bruising, and deposition of fibrin in vessels causing organ failure. The patterns of bleeding in secondary haemostatic defects are typically: delayed bleeding, deeper bleeding from joints and muscles, not from small cuts, nosebleeds are rare, bleeding after trauma/surgery, bleeding after intramuscular injections. 50
51 Defects in Fibrinolysis Defects of clot stability usually involve fibrinolysis. Clot stability is affected if there is excess fibrinolytic factors (e.g. plasmin, tpa), with common examples being therapeutic administration and some tumours. Alternatively there may be deficient antifibrinolytic factors (e.g. antiplasmin), as in antiplasmin deficiency (genetic). Unbalanced haemostasis may be due to anticoagulant excess, which is only due to therapeutic administration e.g. heparin or hirudin. If the balance is tipped in favour of coagulation factors and platelets, the result may be thrombosis. Thrombosis is inappropriate intravascular coagulation, not preceded by bleeding. Thrombi may be venous or arterial. Effects of thrombosis vary. Obstructed flow of blood in an artery could lead to myocardial infarction, stroke of limb ischaemia. In a vein, obstruction will lead to pain and swelling. An embolus from veins may go to the lungs and cause pulmonary embolus, and arterial emboli (usually from the heart) may cause stroke or limb ischaemia. Deep vein thrombosis is where venous return is obstructed, and the patient will typically present with a painful and swollen leg. Pulmonary embolism will cause shortness of breath, chest pain and potentially sudden death. The prevalence of venous thromboembolism is overall 1 in 1,000 to 10,000 per year. The incidence doubles with each decade. Pulmonary embolism is present in 13% of hospital deaths, and is the cause of 5 to 10% of hospital deaths. Thromboembolism has a mortality rate of about 5%, with 20% recurrence in the first 2 years and 4% per year thereafter. Severe thrombophlebitic syndrome is seen in 23% of patients at 2 years (11% with stockings). Pulmonary hypertension is seen in 4% at 2 years. People may get thrombosis due to genetic constitution and risks, the effects of age and previous events, illnesses or due to acute stimulus. Genetic and acquired factors cause an increased risk of thrombosis. Virchow s triad shows there are three main contributory factors to thrombosis: 1) Endothelial injury (vessel wall is dominant cause in arterial thrombosis). 2) Abnormal blood flow (complex, contributes to both arterial and venous). 3) Hypercoagulability (blood is the dominant cause in venous thrombosis). Venous thrombosis is usually due to the balance being tipped in favour of too much coagulative activity and too little fibrinolytic activity. There is increased risk of thrombosis if there is a deficiency of anticoagulant proteins (e.g. antithrombin, protein C, protein S). On the other hand the risk also increases if there are increased coagulant proteins and activity (e.g. factor VIII, factor II, factor V Leiden, thrombocytosis). Increased risk of thrombosis due to the vessel wall involves endothelial cell proteins. Many proteins active in coagulation are expressed on the surface of endothelial cells and their expression, which is altered in inflammation (e.g. thrombomodulin, tissue factor, tissue factor pathway inhibitor). Changes in the endothelium make it more pro-coagulate. Increased risk of thrombosis due to blood flow can be due to reduced flow (stasis). This can occur after surgery, fracture, during a long haul flight, or in prolonged bed rest. Thrombophilia is a term used for anyone with an increased risk of thrombosis. Thrombosis can occur at a young age, or spontaneously. There can be multiple thromboses, even whilst anti-coagulated. The laboratory tests will measure anti-thrombin, Factor V Leiden, protein C, etc. 51
52 There are of course acquired risks for thrombosis. Numerous conditions will alter blood coagulation, the vessel wall and blood flow to precipitate thrombosis or make it more likely. For example: oral contraceptive pill, pregnancy, malignancy, surgery, inflammatory response. The prevention of thrombosis is based on the assessment of individual risk and circumstantial risk. The potential risk factors should be avoided or prophylactic anticoagulant therapy should be given. Treatment is to limit recurrence, for example by increasing anticoagulant activity (heparin) or by lowering pro-coagulant factors (warfarin). Clots can be lysed by using tpa. 52
53 Hypertension by Professor Alun Hughes Hypertension is a major cause of mortality and morbidity, as currently it affects nearly a billion individuals worldwide. It accounts for approximately one in eight of all deaths globally. But blood pressure shows a continuous relationship with cardiovascular risk. Most disease that is attributable to increased blood pressure occurs in individuals that are not labelled as hypertensive. Blood pressure distribution is unimodal and any distinction between normal and abnormal is arbitrary. Hypertension is the level of blood pressure above which investigation and treatment do more good than harm. An important factor to bear in mind is that an individual s blood pressure does not remain constant with age. Mean pressure difference between systolic and diastolic pressures rises with age, but particularly after the age of about 50 there is a significant change. The majority of people over the age of 60 are hypertensive by current definitions. The pulse pressure also rises with age. Blood pressure is continuously distributed in a population and there is no threshold for blood pressure risk. The major risks attributable to elevated blood pressure are the risks of coronary heart disease, stroke, heart failure and peripheral vascular disease / atheromatous disease. The risks are increased between 3 to 6 fold in hypertensives. 95% of cases of hypertension have an unidentifiable cause, and this is called primary or essential hypertension. 5% of cases have an identifiable cause, and this is called secondary hypertension. Secondary causes of hypertension may include: - Renal disease, including renal artery stenosis - Tumours secreting aldosterone (Conn s syndrome) - Tumours secreting catecholamines (pheochromocytoma) - Oral contraceptive pill - Pre-eclampsia / pregnancy associated hypertension - Rare genetic causes (e.g. Liddle s syndrome) Pathophysiology of primary hypertension The haemodynamics of hypertension can be expressed by the equation: Blood pressure = Cardiac Output x Peripheral Vascular Resistance Typically, established hypertension is associated with several factors. For example: uniformly increased peripheral resistance, reduced arterial compliance, normal cardiac output, normal blood volume / extracellular volume, and central shift in blood volume. Established primary hypertension is characterised by elevated total peripheral resistance and normal or reduced carbon dioxide. Elevated peripheral vascular resistance is caused by the active narrowing of arteries (vasoconstriction) and the structural narrowing of arteries by growth and remodelling (possibly adaptive?). 53
54 In a genetic sense, hypertension is unlikely to have a monogenic cause. Commonly hypertension is a complex polygenic. Environmental factors include dietary salt, obesity, alcohol, pre-natal environment and others. Twins and other studies suggest 30 to 50% of variation in blood pressure is attributable to genetic variation. Monogenic causes (under 1% of hypertension) include Liddle s syndrome, which is a mutation in amiloridesensitive tubular epithelial Na + channel. Complex polygenic causes are due to multiple genes with small effects, interactions with sex, other genes and the environment. There is strong evidence that the kidneys (in relation to salt intake) affect hypertension. Impaired renal function or blood flow is the commonest secondary cause of hypertension (e.g. renal parenchymal disease, renal artery stenosis). Most monogenic causes of hypertension affect renal Na + excretion. Salt intake is strongly linked with blood pressures of human populations. Populations with low salt have low population blood pressures and no rise in blood pressure with age. Animals with reduced renal sodium handling develop hypertension. Excess salt intake in many animals results in elevated blood pressure. In rats, hypertension can be transplanted with the kidney, and there is similar (though incomplete) data in man. Also high sympathetic activity in children may contribute to hypertension. There is inconsistent evidence for endocrine / paracrine factors. Effects of Hypertension High blood pressure has adverse effects on numerous organs, such as the brain (strokes and dementia), eyes (microvascular disease and retinopathy), the entire circulation (peripheral vascular disease), the heart (myocardial infarction, heart failure, atrial fibrillation) and kidneys (renal disease and failure). The increased peripheral resistance places an excessive load on the heart, and one of the immediate consequences of this is that the heart has to work harder. It adapts structurally to this - there is often marked thickening of the heart wall. Hypertension is commonly associated with an increase in left ventricular wall mass and changes in chamber size. Thickening of the wall is also a feature of large arteries in hypertension. This is attributable both to a structural remodelling of the wall (hypertrophy to help artery to resist higher pressures) and also to an increased acceleration of atherosclerosis. Hypertension may cause arterial rupture or dilations (aneurysms). This can lead to thrombosis or haemorrhage (e.g. strokes). In smaller arteries, hypertension is associated with hypertrophy and/or narrowing of the lumen of small arteries. This normalises wall stress but increases resistance. In the microvasculature, high blood pressure is associated with vessel loss. There is a reduction in capillary density, which leads to impaired perfusion and increased peripheral vascular resistance. There is also elevated capillary pressure, which leads to damage and leakage. The retina illustrates microvascular damage in hypertension. There is thickening of the wall of small arteries, vasospasm, rarefaction, impaired perfusion and increased leakage into the surrounding tissue. In a Grade III retinopathy, silver wiring can be seen (wall thickening), haemorrhages (wall rupture), AV nipping (wall thickening) and hard exudates (plasma leaks). Renal dysfunction is common in hypertension. Extreme (accelerated/malignant hypertension) is now rare but leads to progressive renal failure. In primary hypertension the kidney degenerates to a granular capsular surface, with cortical thinning and renal atrophy. With accelerated hypertension there are subcapsular haemorrhages. Increased blood pressure is associated with increased albumin loss in the urine, which is indicative of renal microvascular damage. 54
55 Introduction to Atherosclerosis by Professor Dorian O Haskard Ischaemic heart disease and cerebrovascular disease have become very significant world disease burdens in the last few decades, and it is estimated that by 2020 ischaemic heart disease will be the top burden. Atherosclerosis is an unavoidable disease in medicine. It affects general practice, acute medicine, metabolic medicine, endocrinology, vascular surgery, cardiac surgery, cardiology and neurology. Atherosclerosis was noted by Virchow is the deposition of lipids in arteries, although he considered the condition an arteritis. The condition doesn t necessarily involve the full circumference of the artery, it usually only occurs on one side. There is accumulation of lipid deposited in the arterial wall in relation to macrophages. These depositions become more numerous, and there ends up being lipid, dead cell and necrotic material covered by a hard fibrous cap. This will lead to the gradual narrowing of the lumen which will lead to ischaemic manifestations. Or it could fracture or rupture, and this will lead to the exposure of the clotting cascade to tissue factor and other thrombogenic stimuli in the necrotic core. This will lead to thrombotic occlusion of the artery and infarction. The risk factors for atherosclerosis are modifiable and non-modifiable. The potentially modifiable risks include smoking, lipids, blood pressure, diabetes, obesity and lack or exercise. The non-modifiable risk factors include age, sex, and genetic background. Paradigms of atherosclerosis pathogenesis 1. Inevitable consequence of aging, which is true to some extent. 2. The cholesterol hypothesis - this is probably the main driver to atherosclerosis. 3. Inflammation and immunity - is linked to the cholesterol. The cholesterol hypothesis was devised by N. N. Anitschkow, who fed cholesterol to rabbits and showed that they did indeed develop very human looking atherosclerosis, with necrotic cores, the fibrous plaque and foam cells. When he took the rabbits off the cholesterol diet, the problems largely went away. Today there is overwhelming evidence that cholesterol is the major aetiological factor in atherosclerosis. There is experimental evidence, clinical genetic evidence (familial hypercholesterolaemia), epidemiological evidence (Framlingham), and interventional evidence (randomised controlled trials of statins). Cholesterol is deposited in the form of LDLs that penetrate the endothelial barrier into the subintimal space of the arterial wall. The LDL gets physically trapped in to the matrix proteoglycans in the intima. The LDL particles become susceptible to various forms of modification which denature them and render them recognisable by macrophages. An LDL particle has a core of cholesterol and tricglycerides, encased by a layer of phospholipids and held together by a large protein. When this particle is trapped in the wall, free radicals and enzymes such as phospholipases modify the particle. Normally the LDL particle is taken up into cells such as macrophages by a 55
56 molecule called the LDL receptor. This takes the LDL particle into the cell and provides the cell with as much cholesterol as it needs for the membrane etc. If the cell has enough cholesterol, it shuts down expression of the LDL receptor. When the LDL particle is modified by free radicals or enzymatic action, the recognition element of the LDL receptor is damaged, and so the LDL receptor is no longer recognised. Instead, the macrophage recognises the modified particle as denatured material by its scavenger receptors. Unlike the homeostatic process of taking in the LDL through the LDL receptor, the scavenger receptor mediated mechanism is not regulated and the cell can t have enough - it goes on consuming until it becomes a foam cell with lots of lipid within the cell. This is a mechanism that essentially is protective, as it is for removing debris. Monocytes are recruited to take up LDL and dispose of it. When there are risk factors such as high cholesterol, this homeostatic process becomes pro-inflammatory leading to lesions. The macrophages die when they have too much cholesterol, which contributes to the junk in the necrotic core. They may also make or recruit angiogenic factors, VSMC growth factors, proteases and free radicals to cause further damage. Manifestations of Atherosclerosis Atherosclerosis doesn t affect the entire circumference of arteries and it doesn t affect all arteries. Generally, atherosclerosis occurs at branch points of arteries and at curvatures. For example, at the carotid bifurcation where the laminar arterial flow becomes a complex flow pattern with reversals and oscillations - the endothelium is exposed to different mechanical forces. Atherosclerosis leads to gradual narrowing of arteries. Stenosis refers to gradual loss of luminal diameter leading to critical reduction in blood flow. This can be visualised by angiography where there is little blood flowing through the artery. The effect of stenosis is ischaemia. Ischaemia is insufficient blood supply to meet metabolic demands of the tissues, which leads to hypoxic cellular dysfunction. It is typically experienced as pain on exertion, for example in the heart as angina pectoris or in the legs as intermittent claudication. Ischaemia causes discomfort, though it is not necessarily life threatening. A more serious manifestation is when the plaque ruptures or erodes. The localised area of fat deposition and tissue breakdown (necrosis) within the arterial wall is referred to as atherosclerotic plaque. Plaque erosion is the breakdown of the endothelial lining of the lesion without the full rupture of the fibrous cap. Plaque rupture is the breakdown of the fibrous cap of tissue separating the plaque from the blood. The effects of plaque erosion or rupture vary. Platelet recruitment and blood coagulation at the site of erosion or minor rupture may lead to silent nonocclusive thrombus and plaque growth. However, blood coagulation at the site of rupture may lead to an occlusive thrombus and cessation of blood flow - infarction. Embolism is the dislodgement of solid material (e.g. platelet plug, thrombus, cholesterol-rich plaque contents) into the arterial circulation leading to occlusion at distant sites. The consequences depend on the size of the embolus and the target organ (e.g. brain, eye, bowel, limbs). 56
57 The effects of arterial occlusion from thrombosis or embolism also vary: - Transient occlusion: short ischaemia from an occlusion that spontaneously resolves, e.g. in the brain Transient Ischaemic Attack or in the eye amaurosis fugax. - Infarction: death of tissue due to unresolved ischaemia, e.g. in the heart (myocardial infarction) or in the brain (cerebrovascular accident). Timeline of Atherosclerosis Evidence has shown that lifestyles associated with a western culture such as a diet rich in saturated fats and high in calories, smoking and physical inactivity, are some of the modifiable risk factors leading to an increase in the prevalence of CV events. Of these, three are considered to be of prime importance: Smoking is responsible for 50% of all avoidable deaths, of which half are due to CVD. Raised blood pressure has been found to be an important risk factor for the development of CHD, cardiac failure and cerebrovascular disease. The greater the increase in blood pressure the higher the risk. Greatest benefit of blood pressure lowering is seen in those at higher risk. Even modest reductions produce substantial benefits in those with multiple risk factors. Dyslipidaemia, in particular, raised low-density lipoprotein (LDL) cholesterol and triglyceride levels, and low high-density lipoprotein (HDL) cholesterol are associated with increased risk of CVD. However, raised LDL cholesterol has been shown to be most strongly associated with the development of atherosclerosis and the risk of CV events. Between the ages of 40 and 50 is a period of time known as the window of opportunity for primary prevention. This depends on lifestyle and risk factor management. Over 60 years of age is a time called the window of clinical prevention, which refers to secondary prevention. For example catheter based interventions, revascularisation surgery or treatment of heart failure. Pathogenesis of Atherosclerosis Atherosclerosis is a chronic inflammatory response in the walls of arteries, in large part in reaction to the deposition of lipoproteins (plasma proteins that carry cholesterol and triglycerides). The main cell types involved in atherosclerosis are: - vascular endothelial cells - white blood cells (leukocytes), particularly monocytes recruited to macrophages - platelets - vascular smooth muscle cells 57
58 Vascular Endothelium in Atherosclerosis by Dr Anna M. Randi To understand the effects of atherosclerosis on the vascular endothelium, it is important to have knowledge of the structure of arteries and veins. There are generally three layers (except for capillaries and venules): - Tunica intima o Endothelium - Tunica media o Smooth muscle cells - Tunica adventitia o Vasa vasorum, nerves The endothelium is the surface separating blood from other tissues. This is very extensive, with a surface area of over 1000m² and a weight of over 100g. The vascular endothelium acts as a vital barrier separating blood from tissues, formed by a monolayer of endothelial cells (contact inhibition). Endothelial cells are very flat, about 1 to 2μm thick and 10 to 20μm in diameter. Endothelial cells regulate a variety of factors, such as: - Leukocyte transmigration - Permeability - Angiogenesis - Mechano transduction - Inflammation - Thrombosis and haemostasis - Vascular tone However, not all endothelial cells in the body are the same. There are structural, functional and genetic differences according to the position in the cardiovascular tree. There is no universal endothelial cell marker, as different vascular beds express different proteins. The intact endothelium displays ultrastructural diversity and molecular heterogeneity. 58
59 Regulation of endothelial homeostasis is a balance. There are anti and pro factors for inflammation, thrombosis, and proliferation and angiogenesis. Usually, the balance is tipped to the anti side. Endothelial activation can lead to a chronic increase in thrombosis, angiogenesis, leukocyte recruitment, permeability and ultimately the risk factors contributing to atherosclerosis. Permeability The endothelium regulates the flux of fluids and molecules from blood to tissues and vice versa. Increased vascular permeability results in leakage of plasma proteins into the subendothelial space (below the endothelium). The subendothelial space is composed of extracellular matrix and separates the endothelium from the internal elastic lamina and the media (vascular smooth muscle cells). Activation of the endothelium causes the endothelial junctions to come apart, and lipoproteins can get modified (oxidative) and trapped in proteoglycans in the subendothelial space. Macrophages then become foam cells when they phagocytose too much lipid, which causes chronic inflammation. Leukocyte Recruitment Leukocyte recruitment involves the endothelial junctions. Between the endothelial junctions, there are many proteins present that bind to form a zipper. If a leukocyte wants to go through, it squeezes past the proteins. Leukocytes will normally flow in the blood circulation. If, however, there is inflammation and activation of the endothelium, the endothelial cells express proteins that can bind to proteins on the leukocytes. The leukocytes roll, change shape, stick and go through the endothelial junction. The problem with atherosclerosis is that the leukocyte goes through in the wrong type of vessel. It goes through a blood vessel that has a thick vessel wall, and so when it goes through the wall and chews up the basement membrane, it finds a thick vessel wall where it gets stuck. It finds lipids there, it starts gobbling them up and this begins the atherosclerotic problem. In capillaries, the endothelial cells are surrounded by basement membrane and pericapillary cells (pericytes). The post-capillary venule is a structure similar to capillaries but has more pericytes. In an artery there are three thick layers, rich in cells and extracellular matrix. Recruitment of blood leukocytes into tissues takes place normally during inflammation. Leukocytes adhere to the endothelium of post-capillary venules and transmigrate into tissues. In atherosclerosis, leukocytes adhere to the activated endothelium of large arteries and get stuck in the subendothelial space. Newly formed postcapillary venules at the base of developing lesions provide a further portal for leukocyte entry. 59
60 Plaque macrophages express inflammatory factors involved in monocytes recruitment. Cytokines capable of activating endothelial cell adhesion molecules (e.g. E-selectin, ICAM-1, VCAM-1) include interleukin-1 and tumour necrosis factor α. Proteins with direct chemoattractant (chemokine) properties on monocytes include interleukin-8 and monocytes chemotactic protein-1 (MCP-1). Blood Flow Laminar flow is streamlined flow, with the outermost layer moving slowest and the centre moving fastest. Laminar blood flow promotes nitric oxide production, and factors that inhibit coagulation, leukocyte adhesion, and smooth muscle cell proliferation. Laminar blood flow promotes endothelial survival. Disturbed flow is interrupted, and the rate of flow exceeds the critical velocity. This can occur when the fluid passes a constriction, a sharp turn, or a rough surface. Disturbed flow promotes coagulation, leukocyte adhesion, smooth muscle cell proliferation and endothelial apoptosis. Nitric oxide is a gas identified in 1980 as the endothelium derived relaxing factor (EDRF). NO is lipophilic, and passes easily through cell membranes. Nitric oxide is synthesised by the reaction of oxygen with L-arginine to produce NO and L-citrulline under the catalytic activity of NO synthase. There are 3 isoforms of NO synthase. Endothelial NO synthase = enos. The main function of nitric oxide in endothelial cells is the regulation of vascular tone (blood supply to organs). Nitric oxide is reduced by risk factors. Loss of nitric oxide bioactivity is mainly due to quenching by superoxide, released in response to risk factors such as diabetes and smoking. The superoxide reacts with nitric oxide to form peroxynitrite, which causes tissue injury. Angiogenesis Angiogenesis is the formation of new blood vessels by sprouting from pre-existing vessels. Atherosclerosis lesions can be vascularised by a network of capillaries that arise from the adventitial vasa vasorum. These capillaries may be important regulators of plaque growth and instability. Macrophages within the plaque release growth factors that stimulate angiogenesis: - Platelet derived growth factor - Fibroblast growth factor - Transforming growth factor beta - Vascular endothelial growth factor The new capillaries are immature, fragile and prone to rupture. Consequences of neovascularisation can therefore include haemorrhage leading to debris addition in the necrotic core, increase in plaque size and plaque rupture, as well as being a further portal of entry for leukocyte recruitment. 60
61 Lipids, Macrophages and Vascular Smooth Muscle Cells in Atherosclerosis by Dr Joseph J Boyle Atherosclerosis is a stereotypic arterial response to injury, the exact pathology of which is determined by the nature and duration of the stimulus. Risk factors have been identified to predispose an individual to the development or progression of coronary heart disease and the occurrence of cardiovascular events. Evidence has shown that lifestyles associated with a western culture such as a diet rich in saturated fats and high in calories, smoking and physical inactivity, are some of the modifiable risk factors leading to an increase in the prevalence of cardiovascular events. Of these, three are considered to be of prime importance: - Smoking is responsible for 50% of all avoidable deaths, of which half are cardiovascular problems. - Raised blood pressure has been found to be an important risk factor for the development of CHD, cardiac failure and cerebrovascular disease. The greater the increase in blood pressure the higher the risk. Greatest benefit of blood pressure lowering is seen in those at higher risk. Even modest reductions produce substantial benefits in those with multiple risk factors. - Dyslipidaemia, in particular, raised low-density lipoprotein (LDL) cholesterol and triglyceride levels, and low high-density lipoprotein (HDL) cholesterol are associated with increased risk of CVD. However, raised LDL cholesterol has been shown to be most strongly associated with the development of atherosclerosis and the risk of CV events. Monocytes Monocytes are a type of white blood cell. White blood cells are defence cells, which normally kill microorganisms (germs) by a number of mechanisms. White blood cells can sometimes injure host tissue if they are activated excessively or inappropriately. Low density lipoproteins (LDLs) leak through the endothelial barrier and become trapped in the sub-endothelium. Trapped LDLs become oxidatively modified by free radicals, and then they are phagocytosed by macrophages. This stimulates chronic inflammation. Lipoproteins Low density lipoproteins are the bad cholesterol, synthesised in the liver. LDLs carry cholesterol from the liver to the rest of the body, including the arteries themselves. High density lipoproteins are the good cholesterol. They carry cholesterol from peripheral tissues including arteries back to the liver. This is known as reverse cholesterol transport. Oxidised LDLs or modified LDLs are created by the action of free radicals. These are not one single substance. There are whole families of highly inflammatory and toxic forms of LDL found in vessel walls. Familial hyperlipidaemia is due to a failure to clear LDL particles from the blood. This results in xanthomas, which are small collections of fat visible on the skin as lumps, and early atherosclerosis. Brown and Goldstein received the Nobel Prize for medicine in 1985, when they hunted for the gene responsible for familial hypercholesterolaemia (high LDL and severe atherosclerosis). They discovered the LDL receptor (expression of which is negatively regulated by intracellular cholesterol). This led to the discovery of HMG-CoA Reductase inhibitors (statins) for lowering plasma cholesterol. They also proposed a second LDL receptor (this time not under feedback control) involved in atherosclerotic lesions. These were called the scavenger receptors since they hoovered up chemically modified (oxidised) LDLs. Today there are known to be a family of scavenger receptors expressed by macrophages. 61
62 Macrophages within Plaques Monocyte-macrophage differentiation occurs after transendothelial migration. Macrophage scavenger receptors mediate the clearance of microorganism, apoptotic cells and oxidised lipoproteins. Macrophages within atherosclerotic plaques generate free radicals that modify (oxidise) lipoproteins 1. The macrophages phagocytose modified lipoproteins and become foam cells. On activation, they express and secrete: - Cytokine mediators that recruit more monocytes (e.g. TNFα, IL-1, MCP-1) 2 - Chemoattractants and growth factors for vascular smooth muscle cells 3 - Proteinases that degrade tissue (e.g. the fibrous cap) 4 - Tissue factor that stimulates coagulation upon contact with blood 5 Macrophages die by apoptosis, which further contributes to the lipid-rich core of the plaque. 1. Macrophages have oxidative enzymes that can modify native LDLs. - NADPH oxidase is found in the membrane of the macrophages and generates superoxide by transferring electrons from NAPDH inside the cell across the membrane and coupling these to molecular oxygen to produce the free radical. In the macrophage, superoxide can spontaneously form hydrogen peroxide that will undergo further reactions to generate reactive oxygen species (ROS). Another enzyme called superoxide dismutase converts superoxide into hydrogen peroxide. - Myeloperoxidase produces hypochlorous acid (HOCl) from reactive oxygen species and chloride. It can also produce peroxynitrite, which is an oxidant and nitrating agent. 2. Plaque macrophages express inflammatory factors involved in monocyte recruitment. Cytokines are capable of activating endothelial cell adhesion molecules (e.g. E-selectin, ICAM-1, VCAM-1). For example, interleukin- 1 and tumour necrosis factor α. Proteins with direct chemoattractant (chemokine) properties on monocytes include interleukin-8 and monocyte chemotactic protein-1 (MCL-1). 3. This is to do with the wound healing role of macrophages in atherosclerosis. Macrophages release protein growth factors that recruit vascular smooth muscle cells and stimulate them to proliferate and deposit extracellular matrix. Platelet derived growth factor causes vascular smooth muscle cell chemotaxis and cell division. Fibroblast growth factor promotes smooth muscle cell survival and cell division. Transforming growth factor beta is key in increasing collagen synthesis. Vascular smooth muscle cells are non-striated, elongated cells. They are normally specialised for contraction, which constricts the vessel lumen and regulates blood flow. Under injury or free radical stress, vascular smooth muscle cells may switch to a synthetic phenotype, specialised for synthesis of extracellular matrix. 4. Macrophages proteolyse extracellular matrix. Metalloproteinases are a family of about 28 homologous enzymes. They activate each other by proteolysis and degrade collagen by a catalytic mechanism based on zinc. Vulnerable and stable plaques are large soft eccentric lipid-rich necrotic cores with a thin fibrous cap. They have reduced vascular smooth muscle cells and collagen content, and there is increased vascular smooth muscle cell apoptosis. There is an infiltrate of activated macrophages expressing metalloproteinases. 62
Exchange solutes and water with cells of the body
 Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Chapter 8 Heart and Blood Vessels Three Types of Blood Vessels Transport Blood Arteries Carry blood away from the heart Transport blood under high pressure Capillaries Exchange solutes and water with cells
Functions of Blood System. Blood Cells
 Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Functions of Blood System Transport: to and from tissue cells Nutrients to cells: amino acids, glucose, vitamins, minerals, lipids (as lipoproteins). Oxygen: by red blood corpuscles (oxyhaemoglobin - 4
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back
 Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
Vascular System The heart can be thought of 2 separate pumps from the right ventricle, blood is pumped at a low pressure to the lungs and then back to the left atria from the left ventricle, blood is pumped
Heart and Vascular System Practice Questions
 Heart and Vascular System Practice Questions Student: 1. The pulmonary veins are unusual as veins because they are transporting. A. oxygenated blood B. de-oxygenated blood C. high fat blood D. nutrient-rich
Heart and Vascular System Practice Questions Student: 1. The pulmonary veins are unusual as veins because they are transporting. A. oxygenated blood B. de-oxygenated blood C. high fat blood D. nutrient-rich
Chapter 20: The Cardiovascular System: The Heart
 Chapter 20: The Cardiovascular System: The Heart Chapter Objectives ANATOMY OF THE HEART 1. Describe the location and orientation of the heart within the thorax and mediastinal cavity. 2. Describe the
Chapter 20: The Cardiovascular System: The Heart Chapter Objectives ANATOMY OF THE HEART 1. Describe the location and orientation of the heart within the thorax and mediastinal cavity. 2. Describe the
Cardiovascular Physiology
 Cardiovascular Physiology Heart Physiology for the heart to work properly contraction and relaxation of chambers must be coordinated cardiac muscle tissue differs from smooth and skeletal muscle tissues
Cardiovascular Physiology Heart Physiology for the heart to work properly contraction and relaxation of chambers must be coordinated cardiac muscle tissue differs from smooth and skeletal muscle tissues
Anatomi & Fysiologi 060301. The cardiovascular system (chapter 20) The circulation system transports; What the heart can do;
 The cardiovascular system consists of; The cardiovascular system (chapter 20) Principles of Anatomy & Physiology 2009 Blood 2 separate pumps (heart) Many blood vessels with varying diameter and elasticity
The cardiovascular system consists of; The cardiovascular system (chapter 20) Principles of Anatomy & Physiology 2009 Blood 2 separate pumps (heart) Many blood vessels with varying diameter and elasticity
Electrodes placed on the body s surface can detect electrical activity, APPLIED ANATOMY AND PHYSIOLOGY. Circulatory system
 4 READING AND INTERPRETING THE ELECTROCARDIOGRAM Electrodes placed on the body s surface can detect electrical activity, which occurs in the heart. The recording of these electrical events comprises an
4 READING AND INTERPRETING THE ELECTROCARDIOGRAM Electrodes placed on the body s surface can detect electrical activity, which occurs in the heart. The recording of these electrical events comprises an
Introduction to Electrocardiography. The Genesis and Conduction of Cardiac Rhythm
 Introduction to Electrocardiography Munther K. Homoud, M.D. Tufts-New England Medical Center Spring 2008 The Genesis and Conduction of Cardiac Rhythm Automaticity is the cardiac cell s ability to spontaneously
Introduction to Electrocardiography Munther K. Homoud, M.D. Tufts-New England Medical Center Spring 2008 The Genesis and Conduction of Cardiac Rhythm Automaticity is the cardiac cell s ability to spontaneously
The Circulatory System. Chapter 17 Lesson 1
 The Circulatory System Chapter 17 Lesson 1 Functions of the Circulatory System Your circulatory system maintains an internal environment in which all the cells in your body are nourished. As your heart
The Circulatory System Chapter 17 Lesson 1 Functions of the Circulatory System Your circulatory system maintains an internal environment in which all the cells in your body are nourished. As your heart
Lecture Outline. Cardiovascular Physiology. Cardiovascular System Function. Functional Anatomy of the Heart
 Lecture Outline Cardiovascular Physiology Cardiac Output Controls & Blood Pressure Cardiovascular System Function Functional components of the cardiovascular system: Heart Blood Vessels Blood General functions
Lecture Outline Cardiovascular Physiology Cardiac Output Controls & Blood Pressure Cardiovascular System Function Functional components of the cardiovascular system: Heart Blood Vessels Blood General functions
Circulatory System Review
 Circulatory System Review 1. Draw a table to describe the similarities and differences between arteries and veins? Anatomy Direction of blood flow: Oxygen concentration: Arteries Thick, elastic smooth
Circulatory System Review 1. Draw a table to describe the similarities and differences between arteries and veins? Anatomy Direction of blood flow: Oxygen concentration: Arteries Thick, elastic smooth
Electrophysiology Introduction, Basics. The Myocardial Cell. Chapter 1- Thaler
 Electrophysiology Introduction, Basics Chapter 1- Thaler The Myocardial Cell Syncytium Resting state Polarized negative Membrane pump Depolarization fundamental electrical event of the heart Repolarization
Electrophysiology Introduction, Basics Chapter 1- Thaler The Myocardial Cell Syncytium Resting state Polarized negative Membrane pump Depolarization fundamental electrical event of the heart Repolarization
12.1: The Function of Circulation page 478
 12.1: The Function of Circulation page 478 Key Terms: Circulatory system, heart, blood vessel, blood, open circulatory system, closed circulatory system, pulmonary artery, pulmonary vein, aorta, atrioventricular
12.1: The Function of Circulation page 478 Key Terms: Circulatory system, heart, blood vessel, blood, open circulatory system, closed circulatory system, pulmonary artery, pulmonary vein, aorta, atrioventricular
THE HEART Dr. Ali Ebneshahidi
 THE HEART Dr. Ali Ebneshahidi Functions is of the heart & blood vessels 1. The heart is an essential pumping organ in the cardiovascular system where the right heart pumps deoxygenated blood (returned
THE HEART Dr. Ali Ebneshahidi Functions is of the heart & blood vessels 1. The heart is an essential pumping organ in the cardiovascular system where the right heart pumps deoxygenated blood (returned
Electrocardiography I Laboratory
 Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Cardiovascular System
 Topics to Review Diffusion Skeletal muscle fiber (cell) anatomy Membrane potential and action potentials Action potential propagation Excitation-contraction coupling in skeletal muscle skeletal muscle
Topics to Review Diffusion Skeletal muscle fiber (cell) anatomy Membrane potential and action potentials Action potential propagation Excitation-contraction coupling in skeletal muscle skeletal muscle
3. Tunica adventitia is the outermost layer; it is composed of loosely woven connective tissue infiltrated by nerves, blood vessels and lymphatics
 Blood vessels and blood pressure I. Introduction - distribution of CO at rest II. General structure of blood vessel walls - walls are composed of three distinct layers: 1. Tunica intima is the innermost
Blood vessels and blood pressure I. Introduction - distribution of CO at rest II. General structure of blood vessel walls - walls are composed of three distinct layers: 1. Tunica intima is the innermost
Practical class 3 THE HEART
 Practical class 3 THE HEART OBJECTIVES By the time you have completed this assignment and any necessary further reading or study you should be able to:- 1. Describe the fibrous pericardium and serous pericardium,
Practical class 3 THE HEART OBJECTIVES By the time you have completed this assignment and any necessary further reading or study you should be able to:- 1. Describe the fibrous pericardium and serous pericardium,
Chapter 19 Ci C r i cula l t a i t o i n
 Chapter 19 Circulation A closed system Circulatory System Consisting of Heart, Arteries, Veins, Capillaries, Blood & the Lymphatic system Blood Make up The blood is made up of Plasma and three main types
Chapter 19 Circulation A closed system Circulatory System Consisting of Heart, Arteries, Veins, Capillaries, Blood & the Lymphatic system Blood Make up The blood is made up of Plasma and three main types
Page 1. Introduction The blood vessels of the body form a closed delivery system that begins and ends at the heart.
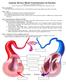 Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction The blood vessels
Blood Vessels and Circulation
 13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
13 Blood Vessels and Circulation FOCUS: Blood flows from the heart through the arterial blood vessels to capillaries, and from capillaries back to the heart through veins. The pulmonary circulation transports
Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School
 Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School This guide is for middle and high school students participating in AIMS Anatomy of the Human Heart and
Distance Learning Program Anatomy of the Human Heart/Pig Heart Dissection Middle School/ High School This guide is for middle and high school students participating in AIMS Anatomy of the Human Heart and
Human Anatomy & Physiology II with Dr. Hubley
 Human Anatomy & Physiology II with Dr. Hubley Exam #1 Name: Instructions This exam consists of 40 multiple-choice questions. Each multiple-choice question answered correctly is worth one point, and the
Human Anatomy & Physiology II with Dr. Hubley Exam #1 Name: Instructions This exam consists of 40 multiple-choice questions. Each multiple-choice question answered correctly is worth one point, and the
Circulatory System and Blood
 Circulatory System and Blood 1. Identify the arteries in the diagram and give one function for each. Y: Common carotid artery: sends oxygenated blood to the brain, provide nutrients. X: Subclavian artery:
Circulatory System and Blood 1. Identify the arteries in the diagram and give one function for each. Y: Common carotid artery: sends oxygenated blood to the brain, provide nutrients. X: Subclavian artery:
Overview of the Cardiovascular System
 Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
Overview of the Cardiovascular System 2 vascular (blood vessel) loops: Pulmonary circulation: from heart to lungs and back) Systemic circulation: from heart to other organs and back Flow through systemic
Understanding the Electrocardiogram. David C. Kasarda M.D. FAAEM St. Luke s Hospital, Bethlehem
 Understanding the Electrocardiogram David C. Kasarda M.D. FAAEM St. Luke s Hospital, Bethlehem Overview 1. History 2. Review of the conduction system 3. EKG: Electrodes and Leads 4. EKG: Waves and Intervals
Understanding the Electrocardiogram David C. Kasarda M.D. FAAEM St. Luke s Hospital, Bethlehem Overview 1. History 2. Review of the conduction system 3. EKG: Electrodes and Leads 4. EKG: Waves and Intervals
Blood vessels. transport blood throughout the body
 Circulatory System Parts and Organs Blood vessels transport blood throughout the body Arteries blood vessels that carry blood AWAY from the heart Pulmonary arteries carry the deoxygenated blood from heart
Circulatory System Parts and Organs Blood vessels transport blood throughout the body Arteries blood vessels that carry blood AWAY from the heart Pulmonary arteries carry the deoxygenated blood from heart
Muscles How muscles contract - The Sliding Filament Theory
 Muscles How muscles contract - The Sliding Filament Theory A muscle contains many muscle fibers A muscle fiber is a series of fused cells Each fiber contains a bundle of 4-20 myofibrils Myofibrils are
Muscles How muscles contract - The Sliding Filament Theory A muscle contains many muscle fibers A muscle fiber is a series of fused cells Each fiber contains a bundle of 4-20 myofibrils Myofibrils are
2161-1 - Page 1. Name: 1) Choose the disease that is most closely related to the given phrase. Questions 10 and 11 refer to the following:
 Name: 2161-1 - Page 1 1) Choose the disease that is most closely related to the given phrase. a disease of the bone marrow characterized by uncontrolled production of white blood cells A) meningitis B)
Name: 2161-1 - Page 1 1) Choose the disease that is most closely related to the given phrase. a disease of the bone marrow characterized by uncontrolled production of white blood cells A) meningitis B)
The heart then repolarises (or refills) in time for the next stimulus and contraction.
 Atrial Fibrillation BRIEFLY, HOW DOES THE HEART PUMP? The heart has four chambers. The upper chambers are called atria. One chamber is called an atrium, and the lower chambers are called ventricles. In
Atrial Fibrillation BRIEFLY, HOW DOES THE HEART PUMP? The heart has four chambers. The upper chambers are called atria. One chamber is called an atrium, and the lower chambers are called ventricles. In
BIOL 1108 Vertebrate Anatomy Lab
 BIOL 1108 Vertebrate Anatomy Lab This lab explores major organs associated with the circulatory, excretory, and nervous systems of mammals. Circulatory System Vertebrates are among the organisms that have
BIOL 1108 Vertebrate Anatomy Lab This lab explores major organs associated with the circulatory, excretory, and nervous systems of mammals. Circulatory System Vertebrates are among the organisms that have
ACLS Chapter 3 Rhythm Review Instructor Lesson Plan to Accompany ACLS Study Guide 3e
 ACLS Chapter 3 Rhythm Review Lesson Plan Required reading before this lesson: ACLS Study Guide 3e Textbook Chapter 3 Materials needed: Multimedia projector, computer, ACLS Chapter 3 Recommended minimum
ACLS Chapter 3 Rhythm Review Lesson Plan Required reading before this lesson: ACLS Study Guide 3e Textbook Chapter 3 Materials needed: Multimedia projector, computer, ACLS Chapter 3 Recommended minimum
Note: The left and right sides of the heart must pump exactly the same volume of blood when averaged over a period of time
 page 1 HEART AS A PUMP A. Functional Anatomy of the Heart 1. Two pumps, arranged in series a. right heart: receives blood from the systemic circulation (via the great veins and vena cava) and pumps blood
page 1 HEART AS A PUMP A. Functional Anatomy of the Heart 1. Two pumps, arranged in series a. right heart: receives blood from the systemic circulation (via the great veins and vena cava) and pumps blood
Renal Blood Flow GFR. Glomerulus Fluid Flow and Forces. Renal Blood Flow (cont d)
 GFR Glomerular filtration rate: about 120 ml /minute (180 L a day) Decreases with age (about 10 ml/min for each decade over 40) GFR = Sum of the filtration of two million glomeruli Each glomerulus probably
GFR Glomerular filtration rate: about 120 ml /minute (180 L a day) Decreases with age (about 10 ml/min for each decade over 40) GFR = Sum of the filtration of two million glomeruli Each glomerulus probably
Starling s Law Regulation of Myocardial Performance Intrinsic Regulation of Myocardial Performance
 Regulation of Myocardial Performance Intrinsic Regulation of Myocardial Performance Just as the heart can initiate its own beat in the absence of any nervous or hormonal control, so also can the myocardium
Regulation of Myocardial Performance Intrinsic Regulation of Myocardial Performance Just as the heart can initiate its own beat in the absence of any nervous or hormonal control, so also can the myocardium
12-Lead EKG Interpretation. Judith M. Haluka BS, RCIS, EMT-P
 12-Lead EKG Interpretation Judith M. Haluka BS, RCIS, EMT-P ECG Grid Left to Right = Time/duration Vertical measure of voltage (amplitude) Expressed in mm P-Wave Depolarization of atrial muscle Low voltage
12-Lead EKG Interpretation Judith M. Haluka BS, RCIS, EMT-P ECG Grid Left to Right = Time/duration Vertical measure of voltage (amplitude) Expressed in mm P-Wave Depolarization of atrial muscle Low voltage
Chapter 16: Circulation
 Section 1 (The Body s Transport System) Chapter 16: Circulation 7 th Grade Cardiovascular system (the circulatory system) includes the heart, blood vessels, and blood carries needed substances to the cells
Section 1 (The Body s Transport System) Chapter 16: Circulation 7 th Grade Cardiovascular system (the circulatory system) includes the heart, blood vessels, and blood carries needed substances to the cells
CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM
 CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM INTRODUCTION Lung cancer affects a life-sustaining system of the body, the respiratory system. The respiratory system is responsible for one of the essential
CHAPTER 1: THE LUNGS AND RESPIRATORY SYSTEM INTRODUCTION Lung cancer affects a life-sustaining system of the body, the respiratory system. The respiratory system is responsible for one of the essential
Sign up to receive ATOTW weekly email worldanaesthesia@mac.com
 INTRODUCTION TO CARDIOVASCULAR PHYSIOLOGY ANAESTHESIA TUTORIAL OF THE WEEK 125 16 TH MARCH 2009 Toby Elkington, Specialist Registrar Carl Gwinnutt, Consultant Department of Anaesthesia, Salford Royal NHS
INTRODUCTION TO CARDIOVASCULAR PHYSIOLOGY ANAESTHESIA TUTORIAL OF THE WEEK 125 16 TH MARCH 2009 Toby Elkington, Specialist Registrar Carl Gwinnutt, Consultant Department of Anaesthesia, Salford Royal NHS
Blood Pressure Regulation
 Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
Blood Pressure Regulation Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction There are two basic mechanisms for regulating
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What term is used to refer to the process of electrical discharge and the flow of electrical
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What term is used to refer to the process of electrical discharge and the flow of electrical
CHAPTER XV PDL 101 HUMAN ANATOMY & PHYSIOLOGY. Ms. K. GOWRI. M.Pharm., Lecturer.
 CHAPTER XV PDL 101 HUMAN ANATOMY & PHYSIOLOGY Ms. K. GOWRI. M.Pharm., Lecturer. Types of Muscle Tissue Classified by location, appearance, and by the type of nervous system control or innervation. Skeletal
CHAPTER XV PDL 101 HUMAN ANATOMY & PHYSIOLOGY Ms. K. GOWRI. M.Pharm., Lecturer. Types of Muscle Tissue Classified by location, appearance, and by the type of nervous system control or innervation. Skeletal
ECG made extra easy. medics.cc
 ElectroCardioGraphyraphy ECG made extra easy Overview Objectives for this tutorial What is an ECG? Overview of performing electrocardiography on a patient Simple physiology Interpreting the ECG Objectives
ElectroCardioGraphyraphy ECG made extra easy Overview Objectives for this tutorial What is an ECG? Overview of performing electrocardiography on a patient Simple physiology Interpreting the ECG Objectives
the basics Perfect Heart Institue, Piyavate Hospital
 ECG INTERPRETATION: the basics Damrong Sukitpunyaroj MD Damrong Sukitpunyaroj, MD Perfect Heart Institue, Piyavate Hospital Overview Conduction Pathways Systematic Interpretation Common abnormalities in
ECG INTERPRETATION: the basics Damrong Sukitpunyaroj MD Damrong Sukitpunyaroj, MD Perfect Heart Institue, Piyavate Hospital Overview Conduction Pathways Systematic Interpretation Common abnormalities in
HEART HEALTH WEEK 3 SUPPLEMENT. A Beginner s Guide to Cardiovascular Disease HEART FAILURE. Relatively mild, symptoms with intense exercise
 WEEK 3 SUPPLEMENT HEART HEALTH A Beginner s Guide to Cardiovascular Disease HEART FAILURE Heart failure can be defined as the failing (insufficiency) of the heart as a mechanical pump due to either acute
WEEK 3 SUPPLEMENT HEART HEALTH A Beginner s Guide to Cardiovascular Disease HEART FAILURE Heart failure can be defined as the failing (insufficiency) of the heart as a mechanical pump due to either acute
PSIO 603/BME 511 1 Dr. Janis Burt February 19, 2007 MRB 422; 626-6833 jburt@u.arizona.edu. MUSCLE EXCITABILITY - Ventricle
 SIO 63/BME 511 1 Dr. Janis Burt February 19, 27 MRB 422; 626-6833 MUSCLE EXCITABILITY - Ventricle READING: Boron & Boulpaep pages: 483-57 OBJECTIVES: 1. Draw a picture of the heart in vertical (frontal
SIO 63/BME 511 1 Dr. Janis Burt February 19, 27 MRB 422; 626-6833 MUSCLE EXCITABILITY - Ventricle READING: Boron & Boulpaep pages: 483-57 OBJECTIVES: 1. Draw a picture of the heart in vertical (frontal
April 18, 2008 Dr. Alan H. Stephenson Pharmacological and Physiological Science
 Renal Mechanisms for Regulating Urine Concentration April 18, 2008 Dr. Alan H. Stephenson Pharmacological and Physiological Science Amount Filtered Reabsorption is selective Examples of substances that
Renal Mechanisms for Regulating Urine Concentration April 18, 2008 Dr. Alan H. Stephenson Pharmacological and Physiological Science Amount Filtered Reabsorption is selective Examples of substances that
Smooth Muscle. Learning Objectives.
 Smooth Muscle. Learning Objectives. At the end of this course, you should be able to : 1. describe the structure of smooth muscle 2. describe where smooth muscle occurs within the body 3. discuss the structural
Smooth Muscle. Learning Objectives. At the end of this course, you should be able to : 1. describe the structure of smooth muscle 2. describe where smooth muscle occurs within the body 3. discuss the structural
To provide the body (cells) with oxygen, and remove CO 2. To provide the body (cells) with nutrients and remove wastes.
 Circulatory system. Basic function: To provide the body (cells) with oxygen, and remove CO 2. To provide the body (cells) with nutrients and remove wastes. Not all organisms have a circulatory system -
Circulatory system. Basic function: To provide the body (cells) with oxygen, and remove CO 2. To provide the body (cells) with nutrients and remove wastes. Not all organisms have a circulatory system -
Water Homeostasis. Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Water Homeostasis Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 1. Water Homeostasis The body maintains a balance of water intake
Water Homeostasis Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) 1. Water Homeostasis The body maintains a balance of water intake
Questions on The Nervous System and Gas Exchange
 Name: Questions on The Nervous System and Gas Exchange Directions: The following questions are taken from previous IB Final Papers on Topics 6.4 (Gas Exchange) and 6.5 (Nerves, hormones and homeostasis).
Name: Questions on The Nervous System and Gas Exchange Directions: The following questions are taken from previous IB Final Papers on Topics 6.4 (Gas Exchange) and 6.5 (Nerves, hormones and homeostasis).
The digestive system eliminated waste from the digestive tract. But we also need a way to eliminate waste from the rest of the body.
 Outline Urinary System Urinary System and Excretion Bio105 Lecture 20 Chapter 16 I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system 1
Outline Urinary System Urinary System and Excretion Bio105 Lecture 20 Chapter 16 I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system 1
QRS Complexes. Fast & Easy ECGs A Self-Paced Learning Program
 6 QRS Complexes Fast & Easy ECGs A Self-Paced Learning Program Q I A ECG Waveforms Normally the heart beats in a regular, rhythmic fashion producing a P wave, QRS complex and T wave I Step 4 of ECG Analysis
6 QRS Complexes Fast & Easy ECGs A Self-Paced Learning Program Q I A ECG Waveforms Normally the heart beats in a regular, rhythmic fashion producing a P wave, QRS complex and T wave I Step 4 of ECG Analysis
Human Anatomy and Physiology II Laboratory
 Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
Human Anatomy and Physiology II Laboratory The Circulation (Two Weeks) 1 This lab involves two weeks work studying the vasculature of the human body. Both weeks involve the exercise in the lab manual entitled
INTRODUCTORY GUIDE TO IDENTIFYING ECG IRREGULARITIES
 INTRODUCTORY GUIDE TO IDENTIFYING ECG IRREGULARITIES NOTICE: This is an introductory guide for a user to understand basic ECG tracings and parameters. The guide will allow user to identify some of the
INTRODUCTORY GUIDE TO IDENTIFYING ECG IRREGULARITIES NOTICE: This is an introductory guide for a user to understand basic ECG tracings and parameters. The guide will allow user to identify some of the
Anatomy and Physiology: Understanding the Importance of CPR
 Anatomy and Physiology: Understanding the Importance of CPR Overview This document gives you more information about the body s structure (anatomy) and function (physiology). This information will help
Anatomy and Physiology: Understanding the Importance of CPR Overview This document gives you more information about the body s structure (anatomy) and function (physiology). This information will help
Milwaukee School of Engineering Gerrits@msoe.edu. Case Study: Factors that Affect Blood Pressure Instructor Version
 Case Study: Factors that Affect Blood Pressure Instructor Version Goal This activity (case study and its associated questions) is designed to be a student-centered learning activity relating to the factors
Case Study: Factors that Affect Blood Pressure Instructor Version Goal This activity (case study and its associated questions) is designed to be a student-centered learning activity relating to the factors
CHAPTER 2: BLOOD CIRCULATION AND TRANSPORT
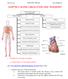 CHAPTER 2: BLOOD CIRCULATION AND TRANSPORT BLOOD CIRCULATION AND TRANSPORT HUMAN BEING PLANTS Function of heart Wilting Structure of heart Blood vessels: characteristics and functions Transpiration: function
CHAPTER 2: BLOOD CIRCULATION AND TRANSPORT BLOOD CIRCULATION AND TRANSPORT HUMAN BEING PLANTS Function of heart Wilting Structure of heart Blood vessels: characteristics and functions Transpiration: function
The Body s Transport System
 Circulation Name Date Class The Body s Transport System This section describes how the heart, blood vessels, and blood work together to carry materials throughout the body. Use Target Reading Skills As
Circulation Name Date Class The Body s Transport System This section describes how the heart, blood vessels, and blood work together to carry materials throughout the body. Use Target Reading Skills As
Biology 224 Human Anatomy and Physiology II Week 8; Lecture 1; Monday Dr. Stuart S. Sumida. Excretory Physiology
 Biology 224 Human Anatomy and Physiology II Week 8; Lecture 1; Monday Dr. Stuart S. Sumida Excretory Physiology The following ELEVEN slides are review. They will not be covered in lecture, but will be
Biology 224 Human Anatomy and Physiology II Week 8; Lecture 1; Monday Dr. Stuart S. Sumida Excretory Physiology The following ELEVEN slides are review. They will not be covered in lecture, but will be
ELECTROCARDIOGRAPHY (I) THE GENESIS OF THE ELECTROCARDIOGRAM
 ELECTROCARDIOGRAPHY (I) THE GENESIS OF THE ELECTROCARDIOGRAM Scridon Alina, Șerban Răzvan Constantin 1. Definition The electrocardiogram (abbreviated ECG or EKG) represents the graphic recording of electrical
ELECTROCARDIOGRAPHY (I) THE GENESIS OF THE ELECTROCARDIOGRAM Scridon Alina, Șerban Răzvan Constantin 1. Definition The electrocardiogram (abbreviated ECG or EKG) represents the graphic recording of electrical
UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM
 BIOLOGY - 2201 UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM What happens to your body as you run? Breathing, heart rate, temperature, muscle pain, thirsty... Homeotasis Homeostasis is the process of maintaining
BIOLOGY - 2201 UNIT 3 : MAINTAINING DYNAMIC EQUILIBRIUM What happens to your body as you run? Breathing, heart rate, temperature, muscle pain, thirsty... Homeotasis Homeostasis is the process of maintaining
Investigating the Human Body On-site student activities: Years 7-8 Investigating the Human Body On-site student activities Years 7 8
 Investigating the Human Body On-site student activities Years 7 8 Student activity (and record) sheets have been developed with alternative themes for students to use as guides and focus material during
Investigating the Human Body On-site student activities Years 7 8 Student activity (and record) sheets have been developed with alternative themes for students to use as guides and focus material during
Human Anatomy & Physiology I with Dr. Hubley. Practice Exam 1
 Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
Human Anatomy & Physiology I with Dr. Hubley Practice Exam 1 1. Which definition is the best definition of the term gross anatomy? a. The study of cells. b. The study of tissues. c. The study of structures
Cardiac Muscle. Learning Objectives.
 Cardiac Muscle. Learning Objectives. At the end of this course, you should be able to : 1. describe the structure of cardiac muscle 2. understand the concept of the functional syncytium 3. give a basic
Cardiac Muscle. Learning Objectives. At the end of this course, you should be able to : 1. describe the structure of cardiac muscle 2. understand the concept of the functional syncytium 3. give a basic
Animal Tissues. I. Epithelial Tissue
 Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Animal Tissues There are four types of tissues found in animals: epithelial tissue, connective tissue, muscle tissue, and nervous tissue. In this lab you will learn the major characteristics of each tissue
Diagram showing Systemic and Portal Circulation
 Diagram showing Systemic and Portal Circulation The Lymphatic System The Lymphatic System comprises of lymphatic capillaries, lymphatic vessels, nodes and ducts. Lymph fluid is not blood plasma, it contains
Diagram showing Systemic and Portal Circulation The Lymphatic System The Lymphatic System comprises of lymphatic capillaries, lymphatic vessels, nodes and ducts. Lymph fluid is not blood plasma, it contains
Cardiology. Anatomy and Physiology of the Heart.
 Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Heart. Module 1: Anatomy and Physiology of the Heart Page 1 CONTENT Introduction Page 3 How to use the ECG Self Learning package.page
Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Heart. Module 1: Anatomy and Physiology of the Heart Page 1 CONTENT Introduction Page 3 How to use the ECG Self Learning package.page
BIO 2401 MUSCLE TISSUE page 1 MUSCLES AND MUSCLE TISSUE. Striations Present or Absent?
 BIO 2401 MUSCLE TISSUE page 1 Types of Muscle MUSCLES AND MUSCLE TISSUE Type of Muscle Skeletal Location of Muscle attaches to and covers bony skeleton Striations Present or Absent? present Control of
BIO 2401 MUSCLE TISSUE page 1 Types of Muscle MUSCLES AND MUSCLE TISSUE Type of Muscle Skeletal Location of Muscle attaches to and covers bony skeleton Striations Present or Absent? present Control of
the Cardiovascular System
 5 Chapter Anatomy Jones and & Physiology Bartlett Learning, LLC of the Cardiovascular System OUTLINE Introduction The Heart Structures of the Heart Conduction System Functions of the Heart The Blood Vessels
5 Chapter Anatomy Jones and & Physiology Bartlett Learning, LLC of the Cardiovascular System OUTLINE Introduction The Heart Structures of the Heart Conduction System Functions of the Heart The Blood Vessels
How To Understand What You Know
 Heart Disorders Glossary ABG (Arterial Blood Gas) Test: A test that measures how much oxygen and carbon dioxide are in the blood. Anemia: A condition in which there are low levels of red blood cells in
Heart Disorders Glossary ABG (Arterial Blood Gas) Test: A test that measures how much oxygen and carbon dioxide are in the blood. Anemia: A condition in which there are low levels of red blood cells in
Provided by the American Venous Forum: veinforum.org
 CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
CHAPTER 1 NORMAL VENOUS CIRCULATION Original author: Frank Padberg Abstracted by Teresa L.Carman Introduction The circulatory system is responsible for circulating (moving) blood throughout the body. The
Section Four: Pulmonary Artery Waveform Interpretation
 Section Four: Pulmonary Artery Waveform Interpretation All hemodynamic pressures and waveforms are generated by pressure changes in the heart caused by myocardial contraction (systole) and relaxation/filling
Section Four: Pulmonary Artery Waveform Interpretation All hemodynamic pressures and waveforms are generated by pressure changes in the heart caused by myocardial contraction (systole) and relaxation/filling
Normal & Abnormal Intracardiac. Lancashire & South Cumbria Cardiac Network
 Normal & Abnormal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform
Normal & Abnormal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform
Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes.
 LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
LESSON 1. CELLS & TISSUES Lesson Aim To explain the human body at a microscopic level, including the structure and function of cells, tissues and membranes. THE CELL All living matter is composed of functional
Introduction to CV Pathophysiology. Introduction to Cardiovascular Pathophysiology
 Introduction to CV Pathophysiology Munther K. Homoud, MD Tufts-New England Medical Center Spring 2008 Introduction to Cardiovascular Pathophysiology 1. Basic Anatomy 2. Excitation Contraction Coupling
Introduction to CV Pathophysiology Munther K. Homoud, MD Tufts-New England Medical Center Spring 2008 Introduction to Cardiovascular Pathophysiology 1. Basic Anatomy 2. Excitation Contraction Coupling
For more information about the use of the Propaq monitor, refer to the Propaq Directions For Use.
 Clinical Support 8500 S.W. Creekside Pl. Beaverton, OR 97008-7107 U.S.A. Telephone: 503-526-4200 Toll Free: 800-289-2500 clinicalsupport@protocol.com ELECTROCARDIOGRAPHY Introduction This article provides
Clinical Support 8500 S.W. Creekside Pl. Beaverton, OR 97008-7107 U.S.A. Telephone: 503-526-4200 Toll Free: 800-289-2500 clinicalsupport@protocol.com ELECTROCARDIOGRAPHY Introduction This article provides
Tachyarrhythmias (fast heart rhythms)
 Patient information factsheet Tachyarrhythmias (fast heart rhythms) The normal electrical system of the heart The heart has its own electrical conduction system. The conduction system sends signals throughout
Patient information factsheet Tachyarrhythmias (fast heart rhythms) The normal electrical system of the heart The heart has its own electrical conduction system. The conduction system sends signals throughout
Biology 347 General Physiology Lab Advanced Cardiac Functions ECG Leads and Einthoven s Triangle
 Biology 347 General Physiology Lab Advanced Cardiac Functions ECG Leads and Einthoven s Triangle Objectives Students will record a six-lead ECG from a resting subject and determine the QRS axis of the
Biology 347 General Physiology Lab Advanced Cardiac Functions ECG Leads and Einthoven s Triangle Objectives Students will record a six-lead ECG from a resting subject and determine the QRS axis of the
33.1 The Circulatory System
 33.1 The Circulatory System Lesson Objectives Identify the functions of the human circulatory system. Describe the structure of the heart and explain how it pumps blood through the body. Name three types
33.1 The Circulatory System Lesson Objectives Identify the functions of the human circulatory system. Describe the structure of the heart and explain how it pumps blood through the body. Name three types
Factors Affecting Blood Pressure. Vessel Elasticity Blood Volume Cardiac Output
 Factors that Affect Pressure Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction pressure is affected by several factors:
Factors that Affect Pressure Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction pressure is affected by several factors:
Teppe Treppe: A staircase increase in tension production after repeated simulation, even though the muscle is allowed to relax between twitches.
 Part II, Muscle: Mechanisms of Contraction and Neural Control, Chapter 12 Outline of class notes Objectives: After studying part II of this chapter you should be able to: 1. Discuss how contractile force
Part II, Muscle: Mechanisms of Contraction and Neural Control, Chapter 12 Outline of class notes Objectives: After studying part II of this chapter you should be able to: 1. Discuss how contractile force
Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014
 Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014 Philip J. Bergmann Lab Objectives 1. To learn how blood flows through a dual circuit circulation with lungs. 2. To
Biol 111 Comparative & Human Anatomy Lab 9: Circulatory System of the Cat Spring 2014 Philip J. Bergmann Lab Objectives 1. To learn how blood flows through a dual circuit circulation with lungs. 2. To
Cardiovascular Biomechanics
 Cardiovascular Biomechanics Instructor Robin Shandas, Ph.D. Associate Professor of Pediatric Cardiology and Mechanical Engineering Robin.shandas@colorado.edu (303) 837-2586 (MWF) / (303) 492-0553 (T,Th)
Cardiovascular Biomechanics Instructor Robin Shandas, Ph.D. Associate Professor of Pediatric Cardiology and Mechanical Engineering Robin.shandas@colorado.edu (303) 837-2586 (MWF) / (303) 492-0553 (T,Th)
Our Human Body On-site student activities Years 5 6
 Our Human Body On-site student activities Years 5 6 Our Human Body On-site student activities: Years 5-6 Student activity (and record) sheets have been developed with alternative themes for students to
Our Human Body On-site student activities Years 5 6 Our Human Body On-site student activities: Years 5-6 Student activity (and record) sheets have been developed with alternative themes for students to
1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood?
 1 1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood? 2 The plan shows the blood system of a mammal. What does the part labelled X represent? A heart
1 1 The diagram shows blood as seen under a microscope. Which identifies parts P, Q, R and S of the blood? 2 The plan shows the blood system of a mammal. What does the part labelled X represent? A heart
Heart Rate and Physical Fitness
 Heart Rate and Physical Fitness The circulatory system is responsible for the internal transport of many vital substances in humans, including oxygen, carbon dioxide, and nutrients. The components of the
Heart Rate and Physical Fitness The circulatory system is responsible for the internal transport of many vital substances in humans, including oxygen, carbon dioxide, and nutrients. The components of the
Evaluation copy. Analyzing the Heart with EKG. Computer
 Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Analyzing the Heart with EKG Computer An electrocardiogram (ECG or EKG) is a graphical recording of the electrical events occurring within the heart. In a healthy heart there is a natural pacemaker in
Normal Intracardiac Pressures. Lancashire & South Cumbria Cardiac Network
 Normal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform display
Normal Intracardiac Pressures Lancashire & South Cumbria Cardiac Network Principle Pressures recorded from catheter tip Electrical transducer - wheatstone bridge mechanical to electrical waveform display
Exchange and transport
 Exchange and transport Examples of things which need to be interchanged between an organism and its environment include: Respiratory gases Nutrients Excretory products Heat This exchange can take place
Exchange and transport Examples of things which need to be interchanged between an organism and its environment include: Respiratory gases Nutrients Excretory products Heat This exchange can take place
Engage: Brainstorming Body Systems. Record the structures and function of each body system in the table below.
 Engage: Brainstorming Body s Record the structures and function of each body system in the table below. Body Nervous Circulatory Excretory Immune Digestive Respiratory Skeletal Muscular Endocrine Integumentary
Engage: Brainstorming Body s Record the structures and function of each body system in the table below. Body Nervous Circulatory Excretory Immune Digestive Respiratory Skeletal Muscular Endocrine Integumentary
THE CIRCULATORY SYSTEM and the LYMPHATIC SYSTEM
 CHAPTER 6: THE CIRCULATORY SYSTEM THE CIRCULATORY SYSTEM and the LYMPHATIC SYSTEM Most of the cells in the human body are not in direct contact with the external environment, so rely on the circulatory
CHAPTER 6: THE CIRCULATORY SYSTEM THE CIRCULATORY SYSTEM and the LYMPHATIC SYSTEM Most of the cells in the human body are not in direct contact with the external environment, so rely on the circulatory
Blood Pressure. Blood Pressure (mm Hg) pressure exerted by blood against arterial walls. Blood Pressure. Blood Pressure
 Blood Pressure Blood Pressure (mm Hg) pressure exerted by blood against arterial walls Systolic pressure exerted on arteries during systole Diastolic pressure in arteries during diastole 120/80 Borderline
Blood Pressure Blood Pressure (mm Hg) pressure exerted by blood against arterial walls Systolic pressure exerted on arteries during systole Diastolic pressure in arteries during diastole 120/80 Borderline
Muscle Tissue. Muscle Physiology. Skeletal Muscle. Types of Muscle. Skeletal Muscle Organization. Myofibril Structure
 Muscle Tissue Muscle Physiology Chapter 12 Specially designed to contract Generates mechanical force Functions locomotion and external movements internal movement (circulation, digestion) heat generation
Muscle Tissue Muscle Physiology Chapter 12 Specially designed to contract Generates mechanical force Functions locomotion and external movements internal movement (circulation, digestion) heat generation
2.2.1 Pressure and flow rate along a pipe: a few fundamental concepts
 1.1 INTRODUCTION Single-cell organisms live in direct contact with the environment from where they derive nutrients and into where they dispose of their waste. For living systems containing multiple cells,
1.1 INTRODUCTION Single-cell organisms live in direct contact with the environment from where they derive nutrients and into where they dispose of their waste. For living systems containing multiple cells,
Electrocardiogram and Heart Sounds
 Electrocardiogram and Heart Sounds An introduction to the recording and analysis of electrocardiograms, and the sounds of the heart. Written by Staff of ADInstruments Introduction The beating of the heart
Electrocardiogram and Heart Sounds An introduction to the recording and analysis of electrocardiograms, and the sounds of the heart. Written by Staff of ADInstruments Introduction The beating of the heart
Chapter 23. Urine Formation I Glomerular Filtration
 Chapter 23 Urine Formation I Glomerular Filtration Urine Formation I: Glomerular Filtration kidneys convert blood plasma to urine in three stages glomerular filtration tubular reabsorption and secretion
Chapter 23 Urine Formation I Glomerular Filtration Urine Formation I: Glomerular Filtration kidneys convert blood plasma to urine in three stages glomerular filtration tubular reabsorption and secretion
Cells, tissues and organs
 Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Chapter 8: Cells, tissues and organs Cells: building blocks of life Living things are made of cells. Many of the chemical reactions that keep organisms alive (metabolic functions) take place in cells.
Membrane Structure and Function
 Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
