Transplantation Reviews
|
|
|
- Jonah Horton
- 7 years ago
- Views:
Transcription
1 Transplantation Reviews 27 (2013) Contents lists available at SciVerse ScienceDirect Transplantation Reviews journal homepage: Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States Susan Leppke a,, Tabitha Leighton a, David Zaun a, Shu-Cheng Chen a, Melissa Skeans a, Ajay K. Israni a,b,c, Jon J. Snyder a,c, Bertram L. Kasiske a,b a Scientific Registry of Transplant Recipients, Minneapolis Medical Research Foundation, Minneapolis, Minnesota b Department of Medicine, Hennepin County Medical Center, University of Minnesota, Minneapolis, Minnesota c Department of Epidemiology and Community Health, University of Minnesota, Minneapolis, Minnesota abstract Founded in 1987, the Scientific Registry of Transplant Recipients (SRTR) operates under a contract from the US government administered by the Health Resources and Services Administration (HRSA). SRTR maintains a database of comprehensive information on all solid organ transplantation in the US. The registry supports the ongoing evaluation of the clinical status of solid organ transplantation, including kidney, heart, liver, lung, intestine, pancreas, and multi-organ transplants. Data in the registry are from multiple sources, but most are collected by the Organ Procurement and Transplantation Network (OPTN) from hospitals, organ procurement organizations, and immunology laboratories. The data include information on current and past organ donors, transplant candidates, transplant recipients, transplant outcomes, and outcomes of living donors. SRTR uses these data to create reports and analyses for HRSA, OPTN committees that make organ allocation policy, and the Centers for Medicare & Medicaid Services to carry out quality assurance surveillance activities; SRTR also creates standard analysis files for scientific investigators. In addition, SRTR and OPTN produce an Annual Data Report and provide information upon request for the general public. Thus, SRTR supports the transplant community with information services and statistical analyses to improve patient access to and outcomes of organ transplant Elsevier Inc. All rights reserved. 1. Historical background The first successful kidney transplant in the US was performed in However, large numbers of transplants were not performed routinely until the late 1960s (Table 1). In 1969, the Southeastern Regional Organ Procurement Program (SEROPP) was formed to help transplant centers procure organs. One of the seven original organ procurement programs funded by the US government, SEROPP subsequently became the Southeastern Organ Procurement Foundation (SEOPF), the precursor to United Network for Organ Sharing (UNOS). In 1984, the US Congress passed the National Organ Transplantation Act (NOTA) to address a critical organ donation shortage and improve the organ matching and placement process [1]. NOTA established the Organ Procurement and Transplantation Network (OPTN) to maintain a national registry for organ matching. In September 1986, the US Department of Health and Human Services Corresponding author. Scientific Registry of Transplant Recipients, Minneapolis Medical Research Foundation, 914 South 8th Street, Suite S4.100, Minneapolis, MN Tel.: ; fax: address: SLeppke@cdrg.org (S. Leppke). (HHS) awarded the initial OPTN contract to UNOS, a private, nonprofit organization. In addition, NOTA stipulates, The Secretary [of HHS] shall, by grant or contract, develop and maintain a scientific registry of the recipients of organ transplants. The registry shall include such information respecting patients and transplant procedures as the Secretary deems necessary to an ongoing evaluation of the scientific and clinical status of organ transplantation. The Health Resources and Services Administration (HRSA), an agency within HHS (Fig. 1), administers contracts for both OPTN and the Scientific Registry of Transplant Recipients (SRTR). From 1987 to 2000, HRSA contracted with UNOS to develop and operate SRTR. In September 2000, HRSA awarded the SRTR contract to the University Renal Research and Education Association, which became Arbor Research Collaborative for Health in July In September 2010, HRSA awarded the SRTR contract to the current contractor, the Minneapolis Medical Research Foundation (MMRF), whose Chronic Disease Research Group (CDRG) executes the contract. The SRTR contract, NOTA, and the Transplantation Amendment Act of 1990 include requirements for the reporting of outcomes to the public. Under NOTA, SRTR and OPTN publish an Annual Data Report, which reports patient outcomes associated with organ procurement and transplantation at transplant centers across the US. The Transplantation X/$ see front matter 2013 Elsevier Inc. All rights reserved.
2 S. Leppke et al. / Transplantation Reviews 27 (2013) Table 1 Historical Highlights of Organ Transplantation in the US. Year Event 1954 First living donor kidney transplant 1962 First deceased donor kidney transplant 1966 First simultaneous kidney/pancreas transplant 1967 First liver transplant 1968 First heart transplant 1969 SEROPP formed in Richmond, Virginia 1977 First match run system under SEOPF, called UNOS 1984 National Organ Transplant Act P.L First federal OPTN contract awarded to UNOS 1987 SRTR founded and managed by UNOS 1992 First-ever comprehensive report on transplant survival rates for all active US transplant centers published by UNOS as OPTN contractor 2000 HHS Final Rule regulatory framework established 2000 SRTR contract awarded by HRSA to URREA (URREA became Arbor Research Collaborative for Health in 2006) 2010 SRTR contract awarded to MMRF HHS, US Department of Health and Human Services; HRSA, Health Resources and Services Administration; MMRF, Minneapolis Medical Research Foundation; SEOPF, The South-Eastern Organ Procurement Foundation; SEROPP, Southeastern Regional Organ Procurement Program; SRTR, Scientific Registry of Transplant Recipients; UNOS, United Network for Organ Sharing; OPTN, Organ Procurement and Transplantation Network; URREA, University Renal Research and Education Association. Amendment Act of 1990 requires SRTR to report program-specific transplant survival rates for all solid organ transplants in the US. The first such report was published in 1992 and included 28,858 transplants performed between October 1, 1987, and December 31, 1989 [2]. In 1999, the program-specific reports (PSRs) were posted on the Internet for the first time. Currently, SRTR publishes PSRs on its Web site every 6 months, and these reports include statistics reported by the nation's organ procurement organizations (OPOs). On March 16, 2000, HHS implemented a Final Rule establishing a regulatory framework for the structure and operations of OPTN and SRTR [3]. The Final Rule stipulates that SRTR provide statistics and analyses to the public regarding the performance of transplant programs at least twice per year. The Final Rule also stipulates that OPTN and SRTR respond to reasonable requests from the public for data needed for research. Finally, OPTN and SRTR carry out analyses requested by OPTN committees in support of efforts to improve organ allocation policies in the US (Table 2). Table 2 Contrasting Analytical Responsibilities of SRTR and OPTN. Parameter SRTR OPTN Purpose Research and policy evaluation Organ allocation policy development Data and analyses Inferential analyses and simulated allocation modeling Data collection and descriptive statistics OPTN, Organ Procurement and Transplantation Network; SRTR, Scientific Registry of Transplant Recipients. 2. SRTR operational structure The Division of Transplantation in the Healthcare Systems Bureau at HRSA provides oversight of the SRTR contract. The SRTR Project Director, Deputy Project Director, and Director of Operations oversee a team of SRTR Senior Staff. The 18 Senior Staff are transplant clinicians and experts in operations research, histocompatibility, health care economics, biostatistics, and epidemiology. The Senior Staff work closely with SRTR support staff to provide: 1. Data and analytical support. 2. Publications and other communications. 3. Information technology and data management. 4. Project management Data and analytic support The data and analytic support function is SRTR's largest operational component. SRTR provides research support to HRSA and OPTN. OPTN operates 20 committees charged with policy development and enforcement regarding solid organ transplantation in the US. When the committees need research support to inform policy development, SRTR provides that support, often working closely with the OPTN contractor. To fulfill this role, SRTR employs biostatisticians, epidemiologists, research and policy liaisons, and clinicians. SRTR assigns a team consisting of a research and policy liaison, a biostatistician/ epidemiologist, and a clinician to support each of the major OPTN committees. SRTR also supports the broader research community by providing analysis files and responding to requests for data and data analyses, and develops and maintains a suite of simulated allocation modeling (SAM) software. The SAMs are used internally to model potential effects of changes to organ allocation policy, and are made available to external researchers Publications and other communications The SRTR publications group consists of medical editors and production editors. SRTR publishes the Annual Data Report and scientific articles in peer-reviewed journals, and presents data and analyses at major scientific meetings Information technology and data management Fig. 1. Relationship between the US Department of Health and Human Services, its Divisions, and the two contractors. MMRF, Minneapolis Medical research Foundation; OPTN, Organ Procurement and Transplantation Network; SRTR, Scientific Registry of Transplant Recipients; UNOS, United Network of Organ Sharing. SRTR receives monthly data updates from the OPTN contractor. These updates include current information on all wait-list candidates, transplant recipients, deceased donors, and living donors. The SRTR information technology (IT)/data management team processes these data monthly and generates standard analysis files (SAFs) for use by SRTR biostatisticians and epidemiologists. In addition, the IT/data management team constructs ad hoc analysis files in support of SRTR research and responds to numerous simple data requests from external researchers. SRTR currently maintains two public Web sites ( transplant.hrsa.gov and along with a private or secure Web site, all managed by the IT/data management team.
3 52 S. Leppke et al. / Transplantation Reviews 27 (2013) The.gov public Web site hosts SRTR Annual Data Reports. It complies with requirements of Section 508 of the Rehabilitation Act to assure that people with disabilities can access Web content. The second public Web site hosts all PSRs and OPO-specific reports (OSRs). In addition, the.org public Web site provides supporting documentation, such as analytical methods, maps of transplant program locations, detailed flowcharts describing the organ allocation system for each organ, and links to publications and presentations. The secure Web site is available to all transplant programs and OPOs in the US; it posts preview versions of the PSRs and OSRs for programs to review prior to public release. Finally, the IT/data management team manages software and application development in support of SRTR activities. For example, SRTR developed the donor yield calculator to help OPOs compare their actual rates of organs recovered for transplant (donor yield) to rates that would be expected based on the models maintained by SRTR Project management The SRTR project management team maintains all contracts and subcontracts, produces mandated progress reports, manages invoicing, and monitors budgets. In addition, the project management team assists with budget development for SRTR special projects requested by HRSA. 3. Principal SRTR tasks The SRTR mission is to support the transplant community with statistical analyses to improve patient outcomes. To fulfill this mission, SRTR is required to: 1. Maintain a Steering Committee. This committee consists of SRTR leadership, HRSA leadership, and OPTN leadership. It provides oversight of SRTR activities, prioritizes tasks, and addresses issues relevant to the operation of the OPTN and SRTR contracts. 2. Maintain an SRTR Technical Advisory Committee (STAC). This committee is made up of nine members and ex-officio members. It provides scientific oversight of SRTR activities and methodologies, and oversees two subcommittees. The STAC-SAM subcommittee provides expertise regarding operations research in support of the development of SAM software. The STAC-PSR subcommittee advises SRTR on the development and application of advanced statistical methodologies for assessing transplant program performance. 3. Provide research and analytic support to OPTN committees. SRTR currently supports 20 OPTN committees and several subcommittees. 4. Provide research and analytic support to HRSA. SRTR provides research support to HRSA leadership when special analyses are required to support HRSA initiatives or inquiries. 5. Analyze OPO and transplant program performance. The PSRs and OSRs are released to the public every 6 months. 6. Publish and present results from research activities. SRTR regularly publishes scientific articles in peer-reviewed journals and presents study results at scientific meetings. 7. Develop and enhance SAM software. This ongoing activity enhances SRTR's ability to model the potential impact of changes to national allocation policies. 8. Make data available to the transplant community and the public. 9. Develop and publish an Annual Data Report. This report is an annual summary of statistics pertinent to organ transplantation in the US. 10. Produce a biennial report to Congress. This report, mandated by Congress, describes all federal activities related to organ transplantation. 11. Conduct special studies. Occasionally, HRSA identifies topics requiring special study. For example, SRTR is currently engaged in a special study to assess optimal systems for allocating deceased donor livers, with the goal of reducing disparities in access to deceased donor livers based on where the candidate is listed. 4. The SRTR database 4.1. Overview SRTR receives data collected by other organizations, manages and analyzes these data, and supplies data, summary reports, and analyses to the transplant community (Fig. 2). Data in the SRTR database are largely from OPTN (from transplant centers, OPOs, and histocompatibility laboratories), and also from the Centers for Medicare & Medicaid Services (CMS) and the Social Security Administration Death Master File (SSADMF). SRTR processes all of the data it receives and provides information upon request to HRSA, CMS, private insurance providers, OPTN committees, external investigators, and the public. The publicly released data include SAFs, PSRs, OSRs, Annual Data Reports, articles in the scientific literature, and presentations at scientific meetings. The HHS Final Rule requires that SRTR make all data available to the public while honoring patient privacy and applicable statutes. The Final Rule also requires SRTR to respond to requests from the public for data needed for bona fide research or analytical purposes, data needed to assess the performance of HRSA contractors and transplant programs, and data needed for other purposes Data sources SRTR's primary source of data is OPTN (Fig. 2). OPTN collects data from transplant centers, OPOs, and histocompatibility laboratories. UNOS first developed the OPTN data collection system in 1986 under contract to HHS. This system has undergone numerous changes; it is currently an Internet-based system called UNet TM [4], which enables organ transplant programs to: Register candidates for transplant. Match donated organs to waiting candidates. Submit data on donors, candidates, and recipients before and after transplant. The UNet TM database system includes data from every transplant and organ donation that has occurred in the US since October 1, Fig. 2. Sources and uses of SRTR data. CMS, Centers for Medicare & Medicaid Services; ESRD, end-stage renal disease; HRSA, Health Resources and Services Administration; MPSC, Membership and Professional Standards Committee; OPO, organ procurement organization; OPTN, Organ Procurement and Transplantation Network; SSADMF, Social Security Administration Death Master File; SRTR, Scientific Registry of Transplant Recipients.
4 S. Leppke et al. / Transplantation Reviews 27 (2013) Once a month, SRTR receives a snapshot of the OPTN database, which provides current and complete data including all new and existing information as well as any historical revisions. SRTR electronically evaluates and compiles these and other data into the SRTR database. SRTR and OPTN also receive monthly updates from the SSADMF. SRTR uses an internally developed person-matching algorithm against the SSADMF to determine when transplant candidates, living donors, and transplant recipients have died. These death dates are extremely important, since OPTN does not require transplant centers to follow transplant candidates who have been removed from the waiting list, or living donors for more than 2 years after donation. Centers may voluntarily submit death dates after these defined follow-up intervals, but they are not required to do so. SRTR also accesses CMS data for kidney transplant candidates and recipients collected by its Consolidated Renal Operations in a Web- Enabled Network (CROWN). CROWN is a CMS Web-based datacompilation system that allows dialysis facilities to comply with mandatory Medicare data submission policies. CROWN data include: Patient admission and discharge history. Form CMS-2728, End-Stage Renal Disease (ESRD) Medical Evidence Report: Medicare Entitlement and/or Patient Registration. Form CMS-2746, Death Notification. Form CMS-2744, ESRD Facility Survey. Patient Attributes and Related Treatment (monthly Patient Activity Reports). Hemodialysis, peritoneal dialysis, and vascular access information. Through the person-matching process against this CMS data source, SRTR obtains information on ESRD death date, first dialysis date, cause of death, and primary cause of kidney disease. The final SRTR database consists of hundreds of tables and many components, including the national transplant waiting lists, the donor recipient matching process, and data on donors and recipients Data processing The SRTR monthly update process starts with the arrival of the OPTN data snapshot. SRTR first evaluates, cleans, and reorganizes the OPTN data, then combines these data with data from other sources. Through the person-matching process, SRTR identifies and combines any duplicate patient or donor entries that relate to the same person. Then, three processes are performed to generate usable data: SAF generation. Data files are created in the Statistical Analysis System (SAS ; SAS Institute, Inc., Cary, North Carolina, US) format used by researchers and biostatisticians. Data dictionary generation. Documentation is created for the SAFs. Data quality validation. Numerous tests are performed to identify potential errors in the data. A report is generated and sent to OPTN so these issues can be addressed and resolved, enhancing the overall quality of the data in future releases Standard analysis file structure The SAFs include files in SAS format organized by candidate, donor, transplant, and transplant follow-up information (Table 3). Candidate files include status history and status justification; donor files include donor disposition and living donor follow-up information; transplant files include summarized candidate, donor, and follow-up information and transplant immunosuppression, recipient histocompatibility, and cross-match information; and transplant follow-up files include follow-up immunosuppression, malignancy, and malignancy treatment information. All files can be linked to each other at the person level and are accompanied by an electronic data dictionary. Table 3 Data Elements Included in the Public SAF. Files All organs Deceased donor information Donor disposition Living donor information Living donor follow-up information Immunosuppression medications given during follow-up Immunosuppression medications given at transplant Malignancies diagnosed Recipient histocompatibility Recipient histocompatibility cross-match Treatment data Candidate information Transplant follow-up information Transplant table Status history table Specific organs Kidney/pancreas PRA history Liver MELD exception MELD exception tumors Liver status justification Thoracic status justification 5. SRTR data uses Data 5.1. Health Resources and Services Administration All deceased donor information Organ dispositions of each organ for each recovered deceased donor All living donor information Information on living donors who are followed at 6 months and 1 year posttransplant Data on immunosuppression medications given to a transplant recipient during the follow-up period Data on immunosuppression medications given to a transplant recipient at the time of transplant Data on any malignancies diagnosed during the follow-up period One record for the recipient histocompatibility lab results for each transplant performed Cross-match results Treatment data for each de nova solid tumor reported Includes candidates registered on the OPTN waiting list and candidates who received a living donor organ, even if they were never wait-listed; gives candidate information during the waiting time For each transplant, a record at 1 year posttransplant and annually until retransplant, death, or loss to follow-up; information from the TRF form, including patient characteristics at the time of follow-up and posttransplant (1 year) or last follow-up (all others) One record per transplant; TRR form information, including patient characteristics at the time of transplant and transplant characteristics; donor characteristics, donor recipient interactions (e.g., calculated HLA mismatch scores, blood compatibilities, and organ sharing, based on the relationship between the OPO and the transplant center. At least one record for each wait-list registration; characteristics that may change during the wait History of kidney, kidney-pancreas, and pancreas wait-list records based on changes in PRA and copra MELD exception information Original and current MELD exception HCC tumors Liver status justification for 1, 2A, and 2B Status justification for 1A and 1B HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease; OPO, organ procurement organization; OPTN, organ procurement and Transplantation Network; PRA, panel-reactive antibody; TRF, transplant recipient follow-up; TRR, transplant recipient registration. Ultimately, HHS is responsible for administering deceased donor organ allocation in the US (Fig. 1). SRTR provides data and data analyses to HRSA as necessary and as defined in its contract with MMRF/CDRG. In addition to data reporting outlined in the contract and described below, Congress has mandated that HHS report every 2 years on the involvement of HHS in organ transplantation. SRTR provides much of the data and helps HHS produce this Biennial Report to Congress. The Secretary of HHS convenes an Advisory Committee
5 54 S. Leppke et al. / Transplantation Reviews 27 (2013) on Organ Transplantation (ACOT) that occasionally may request data and analyses from OPTN or SRTR. HHS also participates with the transplant community in task forces designed to improve the supply of organs for transplant and transplant outcomes; SRTR may provide data or analyses to assist these endeavors Centers for Medicare & Medicaid Services CMS pays for many organ transplants in the US and maintains its own quality assurance process for transplant programs [5]. Much of the data used in the CMS surveillance of transplant programs are supplied by SRTR to CMS through its contractor, the University of Michigan Kidney Epidemiology and Cost Center (UM-KECC). Data include the PSRs and OSRs, and other data as requested. To date, CMS has chosen to use criteria for identifying transplant programs needing scrutiny and audit similar to the criteria used by the OPTN's Membership and Professional Standards Committee (MPSC) Private payers Private payers use the PSRs and other information to select transplant programs to provide services for their insured patients. Payers often request additional information on transplant program outcomes that is not provided in the PSRs. Transplant programs may voluntarily request that UNOS release information to insurance providers under a uniform request for information (RFI) program developed for transplant programs in conjunction with UNOS. SRTR provides some of the RFI data to UNOS for this purpose Organ Procurement and Transplantation Network Committees OPTN committees often request information that requires data analyses. OPTN provides descriptive statistics, and SRTR provides inferential analyses of data. Typically, SRTR carries out analyses regarding the potential effects of newly proposed allocation policies at the request of organ-specific OPTN committees. To support these analyses, SRTR maintains and updates the SAMs. There are currently three SAMs, the kidney-pancreas simulation allocation model (KPSAM), the liver simulation allocation model (LSAM), and the thoracic simulation allocation model (TSAM). The SAMs use actual data previously collected for transplant donors, candidates, and recipients, but change organ allocation rules to simulate reallocation of organs using committee-requested what if? scenarios. The SAM programs have the flexibility to specify new candidate and donor characteristics along with new organ allocation policies. The resulting output can provide information about allograft survival, relisting rate, number of transplants performed, and wait-list mortality under a proposed allocation policy. Comparisons can be made with simulations using current allocation policy Researchers One of SRTR's primary missions is to provide data to individuals or groups performing research. Researchers and clinicians can request data by completing a data use agreement (DUA) specific to each project. Each DUA includes a research plan and a security plan. All DUAs are approved by SRTR prior to the release of data. The STAC advises SRTR regarding data release practices, and reviews all requests for data linkages and patient-identified data. These policies and procedures help protect patient privacy. SRTR fulfills four types of data requests: 1. Simple data requests. These are requests for existing data and do not require additional programming or analyses; they can generally be fulfilled in less than 4 h and do not require a DUA or formal approval. 2. Data requests for standard analysis files or simulated allocation models. These requests can be fulfilled with a SAF and/or a SAM; they require a DUA. 3. Data requests requiring linkages. Data requests that require a linkage to an external data source or use of personal identifiers to link with other public or private data sources can usually be accommodated. All such requests must be authorized by the STAC and the HRSA SRTR Project Officer. Once the request is approved, performing or arranging a linkage with SRTR data may take a few weeks to several months. 4. Data request for additional SRTR programming. Data requests requiring additional SRTR analyses are considered depending on resources available, and are reviewed on a case-by-case basis by SRTR and the HRSA SRTR Project Officer. The SRTR SAF provides data elements from the SRTR database, including heart, lung, kidney, pancreas, liver, and intestine transplants and combinations thereof. The SAF includes encrypted patient identifiers, encrypted transplant center codes, and encrypted OPO codes. Currently, the SAF does not include geographic data or text fields. Some data elements that are not part of the standard files, such as OPTN region, may be included upon request. The SAF is released quarterly (March, June, September, and December of each year) and is available only in SAS formatted files. SRTR provides the SAMs to the public for use in research. The three types of SAMs available allow for analysis of changes to organ allocation policies. The SAMs have limitations and require programming skills to use OPTN Membership and Professional Standards Committee Like CMS, the OPTN MPSC quality assurance process monitors outcomes of transplant programs and OPOs. The MPSC applies performance criteria to data reported in the PSRs and OSRs to determine transplant programs and OPOs that may need further scrutiny. SRTR produces PSRs and OSRs every 6 months and publishes them on its Web site. SRTR provides other data to the MPSC as requested General public SRTR publishes PSRs and OSRs on its Web site every 6 months. In addition, in conjunction with OPTN, SRTR publishes an Annual Data Report providing the latest transplant statistics. These reports provide the information needed to answer most inquiries. However, SRTR also responds to more specific requests for information, as described above. 6. Reports 6.1. Program-specific reports The PSRs contain information on wait-list candidates, deceased donors, living donors, transplant recipients, and outcomes for all OPTN-approved transplant programs in the US. These include heart, intestine, kidney, liver, lung, and pancreas programs. In addition, PSRs are produced for two types of multi-organ transplant programs, heart lung and kidney pancreas, since OPTN separately certifies these types of programs. If an institution maintains multiple transplant programs, e.g., a kidney program and a liver program, separate reports are produced for each organ type. This results in a large number of PSRs (Table 4). The PSRs follow the same basic structure, with slight modifications for each organ type. For example, the liver and kidney reports contain data on living donors but the other PSRs do not, because living donor transplants are not performed or are uncommon. The structure of the
6 S. Leppke et al. / Transplantation Reviews 27 (2013) Table 4 Numbers of PSRs Produced by SRTR, July Program Type Number of Programs, July 2012 PSR Heart 146 Kidney 264 Intestine 35 Liver 137 Lung 70 Pancreas 143 Heart-lung 41 Kidney-pancreas 148 Total PSRs 984 PSR, program-specific report; SRTR, Scientific Registry of Transplant Recipients. PSRs follows the transplant process from registration on the waiting list, through transplant, through death: 6.2. Section A: Program Summary Wait-list activity (total candidates, active candidates, and new candidates). Census of transplant recipients the program is following. Observed and expected transplant rates. Observed and expected mortality rates following registration on the waiting list. First-year, adult and pediatric, observed and expected patient and graft survival Section B: Wait-List Information Wait-list activity, with comparisons to the OPTN region and to the nation as a whole. Demographic characteristics of wait-list candidates. Medical characteristics of wait-list candidates. Transplant rates for all wait-list candidates, adult and pediatric. Mortality rates following registration on the waiting list, adult and pediatric. Wait-list candidate status 6, 12, and 18 months after listing. Percent of candidates who undergo deceased donor transplants by demographic and medical characteristics. Estimates of time to transplant for candidates in each program, donation service area (DSA), OPTN region, and the nation as a whole Section C: Transplant Information Transplant recipient demographic characteristics, including age, sex, and race. Transplant recipient medical characteristics. Characteristics of deceased donors compared with the OPTN region and the nation. Characteristics of living donors compared with the OPTN region and the nation. Graft and patient survival compared with the nation. For each outcome, an expected adjusted survival is provided. Each program is then determined to be performing as expected, better than expected, or worse than expected based on a statistical comparison of its observed and expected graft failure and death counts. These statistics are provided for the most recent 30-month cohort of transplant recipients in the program. The statistical models that determine the expected event counts for each transplant program are publicly available on the SRTR Web site Organ procurement organization-specific reports There are currently 58 OPOs in the US (Fig. 3). Each OPO serves a DSA defined by a grouping of counties. The OPO services each donor hospital in its DSA to manage the donation process and facilitate placement of the donated organs. The OSRs contain information on deceased donation in each of the 58 DSAs, including information on eligible deaths at donor hospitals in the DSA, conversion rates of eligible deaths to actual organ donors, characteristics of deceased donors procured by the OPO, and organ utilization statistics per deceased donor. The OSRs include information on: Organs recovered and transplanted per donor in the DSA. Number and location of organs transplanted in the US. Donation rates for all donors and for donors meeting eligibility criteria. Observed and expected organ-specific yields. Donor characteristics including ethnicity/race, age, sex, blood type, cause of death, and donation after circulatory death or donation after brain death status. Percent of candidates who undergo transplant by 30 days, 1, 2, and 3 years. Recipient characteristics by organ type. Fig. 3. The 58 donation service areas in the US identified by organ procurement organization.
7 56 S. Leppke et al. / Transplantation Reviews 27 (2013) Annual data report Each year, with OPTN support and HRSA review and approval, SRTR produces a report that provides data on organ donation and transplantation in the US, showing recent activity and trends over time. The report includes chapters on kidney, pancreas, liver, intestine, heart, and lung transplantation, with sections describing the waiting list, deceased donor organ donation, living donor organ donation, transplant, donor recipient matching, outcomes, immunosuppression, and pediatric transplant. The report also includes chapters on deceased donor organ donation and international transplant rate comparisons and an appendix. The 2011 report contains approximately 500 figures and tables. The full report is published on the Internet at Online viewing options include e-reading, printer-friendly PDFs, and Power- Point slides. A print version of the report appears in the American Journal of Transplantation [6]. Conclusion SRTR is a registry that contains current and past information on the full continuum of transplant activity in the US. Data in the registry come largely from OPTN, and from CMS and the SSADMF. SRTR employs an extensive system involving several technologies to combine, reorganize, and clean data to create the SRTR database. Data in the database are then evaluated, reorganized, and eventually extracted into more useful forms, such as the SAFs. Researchers and clinicians request the SAFs and other data elements for research at their institutions. SRTR provides data for research; analyses for US government agencies, including HRSA and CMS; and information for transplant centers, OPOs, OPTN committees, and the general public. SRTR thereby supports the development of policy and research to shape the future of solid organ transplantation in the US. Acknowledgments This work was conducted under the auspices of the Minneapolis Medical Research Foundation, contractor for the Scientific Registry of Transplant Recipients, as a deliverable under contract no. HHSH C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Governmentsponsored work, there are no restrictions on its use. The views expressed herein are those of the authors and not necessarily those of the US Government. The authors have no conflicts of interest. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing. References [1] National Organ Transplantation Act of Public law , title 42-section Stat [2] Lin HM, Kauffman HM, McBride MA, et al. Center-specific graft and patient survival rates: 1997 United Network for Organ Sharing (UNOS) report. JAMA 1998;280: [3] Public Health Service, Department of Health and Human Services. Final rule Available at: Title42/42cfr121_main_02.tpl. Accessed December 21, [4] United Network for Organ Sharing. Data collection. Available at: org/donation/index.php?topic=data_collection. Accessed December 21, [5] Hamilton TE. Improving organ transplantation in the United States a regulatory perspective. Am J Transplant 2008;8: [6] Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2011 data report. Am J Transplant 2013;13(Suppl s1):1 234.
Center Activity (07/01/2009-06/30/2010) Center Region United States Tables for More Information
 Program Summary Center Activity (07/01/2009-06/30/2010) Center Region United States Tables for More Information Deceased donor transplants (n=number) 0 169 0 07C,08C,09C On waitlist at start (n) 0 0 0
Program Summary Center Activity (07/01/2009-06/30/2010) Center Region United States Tables for More Information Deceased donor transplants (n=number) 0 169 0 07C,08C,09C On waitlist at start (n) 0 0 0
Demystifying Transplant Performance Reviews. Robyn Zernhelt Performance Analyst
 Demystifying Transplant Performance Reviews Robyn Zernhelt Performance Analyst Pre-Assessment Transplant Performance Reviews The PAIS reviews which transplant programs? 1. Kidney, Pancreas, Intestine 2.
Demystifying Transplant Performance Reviews Robyn Zernhelt Performance Analyst Pre-Assessment Transplant Performance Reviews The PAIS reviews which transplant programs? 1. Kidney, Pancreas, Intestine 2.
THE BENEFITS OF LIVING DONOR KIDNEY TRANSPLANTATION. feel better knowing
 THE BENEFITS OF LIVING DONOR KIDNEY TRANSPLANTATION feel better knowing your choice will help create more memories. Methods of Kidney Donation Kidneys for transplantation are made available through deceased
THE BENEFITS OF LIVING DONOR KIDNEY TRANSPLANTATION feel better knowing your choice will help create more memories. Methods of Kidney Donation Kidneys for transplantation are made available through deceased
*6816* 6816 CONSENT FOR DECEASED KIDNEY DONOR ORGAN OPTIONS
 The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
User Guide. A. Program Summary B. Waiting List Information C. Transplant Information
 User Guide This report contains a wide range of useful information about the pancreas transplant program at Saint Louis University Hospital (MOSL). The report has three main sections: A. Program Summary
User Guide This report contains a wide range of useful information about the pancreas transplant program at Saint Louis University Hospital (MOSL). The report has three main sections: A. Program Summary
The New Kidney Allocation System: Resources for Protocols and Processes webinar.
 The New Kidney Allocation System: Resources for Protocols and Processes webinar. 1 Speakers for the webinar are: Dr. Richard Formica Associate Professor of Medicine and Surgery, Yale University School
The New Kidney Allocation System: Resources for Protocols and Processes webinar. 1 Speakers for the webinar are: Dr. Richard Formica Associate Professor of Medicine and Surgery, Yale University School
David Axelrod, MD, MBA. Associate Professor of Surgery Section Chief- Solid Organ Transplantation Dartmouth- Hitchcock Medical Center
 David Axelrod, MD, MBA Associate Professor of Surgery Section Chief- Solid Organ Transplantation Dartmouth- Hitchcock Medical Center Owner of XynManagement which produces software to track and improve
David Axelrod, MD, MBA Associate Professor of Surgery Section Chief- Solid Organ Transplantation Dartmouth- Hitchcock Medical Center Owner of XynManagement which produces software to track and improve
Candidates about Multiple Listing and Waiting Time Transfer
 TA L K I N G A B O U T T R A N S P L A N TAT I O N Questions & A n s we r s for Transplant Candidates about Multiple Listing and Waiting Time Transfer U N I T E D N E T W O R K F O R O R G A N S H A R
TA L K I N G A B O U T T R A N S P L A N TAT I O N Questions & A n s we r s for Transplant Candidates about Multiple Listing and Waiting Time Transfer U N I T E D N E T W O R K F O R O R G A N S H A R
4/25/2016. Transplant Journey. Objectives. Reason for Transplantation at Mayo Clinic. Mayo Clinic Model of Care
 Transplant Journey Lynette Fix, RN, BAN, CCTC Objectives Identify key components of transplant evaluation process Identify the patient follow-up process Describe diseases indicated for transplantation
Transplant Journey Lynette Fix, RN, BAN, CCTC Objectives Identify key components of transplant evaluation process Identify the patient follow-up process Describe diseases indicated for transplantation
How To Fix An Electronic Medical Record
 PROBLEMS FOR ORGAN PROCUREMENT ORGANIZATIONS CAUSED BY THE IMPLEMENTATION OF ELECTRONIC MEDICAL RECORDS The nationwide adoption of Electronic Medical Records (EMRs) should provide Organ Procurement Organizations
PROBLEMS FOR ORGAN PROCUREMENT ORGANIZATIONS CAUSED BY THE IMPLEMENTATION OF ELECTRONIC MEDICAL RECORDS The nationwide adoption of Electronic Medical Records (EMRs) should provide Organ Procurement Organizations
Preparing for ABO Determination, Reporting and Verification Policy Changes Webinar Script
 Our speaker for today s webinar is Theresa Daly. Theresa is the Director of Clinical Operations for the Transplant Hospital at the New York Presbyterian Hospital Columbia University. In this role she oversees
Our speaker for today s webinar is Theresa Daly. Theresa is the Director of Clinical Operations for the Transplant Hospital at the New York Presbyterian Hospital Columbia University. In this role she oversees
New Kidney Allocation and What it Means to Your Transplant Center and Your Patients
 New Kidney Allocation and What it Means to Your Transplant Center and Your Patients Alexander Wiseman, M.D. Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas
New Kidney Allocation and What it Means to Your Transplant Center and Your Patients Alexander Wiseman, M.D. Professor, Division of Renal Diseases and Hypertension Medical Director, Kidney and Pancreas
Questions and Answers for Transplant Candidates about the New Kidney Allocation System
 TA L K I N G A B O U T T R A N S P L A N TAT I O N U N I T E D N E T W O R K F O R O R G A N S H A R I N G Questions and Answers for Transplant Candidates about the New Kidney Allocation System United
TA L K I N G A B O U T T R A N S P L A N TAT I O N U N I T E D N E T W O R K F O R O R G A N S H A R I N G Questions and Answers for Transplant Candidates about the New Kidney Allocation System United
OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Report to the Board of Directors June 25-26, 2012 Richmond, VA.
 OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Report to the Board of Directors June 25-26, 2012 Richmond, VA Summary I. Action Items for Board Consideration The Board is asked to approve
OPTN/UNOS Liver and Intestinal Organ Transplantation Committee Report to the Board of Directors June 25-26, 2012 Richmond, VA Summary I. Action Items for Board Consideration The Board is asked to approve
Department of Health and Human Services
 Friday, March 30, 2007 Part II Department of Health and Human Services Centers for Medicare & Medicaid Services 42 CFR Parts 405, 482, 488, and 498 Medicare Program; Hospital Conditions of Participation:
Friday, March 30, 2007 Part II Department of Health and Human Services Centers for Medicare & Medicaid Services 42 CFR Parts 405, 482, 488, and 498 Medicare Program; Hospital Conditions of Participation:
Partnering With Your Transplant Team
 Partnering With Your Transplant Team The Patient s Guide to Transplantation U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Health Resources and Services Administration This booklet was prepared for the Health
Partnering With Your Transplant Team The Patient s Guide to Transplantation U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Health Resources and Services Administration This booklet was prepared for the Health
Is a kidney transplant right for me?
 Is a kidney transplant right for me? Your guide to the transplant process www.esrdncc.org Contents Introduction 3 What is a Transplant Center? 4 What is the Waiting List? 5 What Happens After the Surgery?
Is a kidney transplant right for me? Your guide to the transplant process www.esrdncc.org Contents Introduction 3 What is a Transplant Center? 4 What is the Waiting List? 5 What Happens After the Surgery?
APPENDIX E DATA REPORTING REGULATIONS
 APPENDIX E DATA REPORTING REGULATIONS DATA REPORTING REGULATION Section 4602(e) of the Balanced Budget Act of 1997 authorizes the Secretary of the Department of Health and Human Services (HHS) to require
APPENDIX E DATA REPORTING REGULATIONS DATA REPORTING REGULATION Section 4602(e) of the Balanced Budget Act of 1997 authorizes the Secretary of the Department of Health and Human Services (HHS) to require
Kidney Transplantation
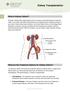 ilearning about your health Kidney Transplantation www.cpmc.org/learning What is Kidney Failure? Normally, kidneys filter waste products from your blood, control the balance of salt and water in your body,
ilearning about your health Kidney Transplantation www.cpmc.org/learning What is Kidney Failure? Normally, kidneys filter waste products from your blood, control the balance of salt and water in your body,
Kidney and Pancreas Transplant Evaluation Clinics and Committee: Inpatient Nephrology Transplant Consult Service
 Care of Renal Transplant Patients takes place in five distinct practice settings at the University of Michigan: a) Kidney and Pancreas Pre-transplant evaluation clinics (five half-day clinics per week)
Care of Renal Transplant Patients takes place in five distinct practice settings at the University of Michigan: a) Kidney and Pancreas Pre-transplant evaluation clinics (five half-day clinics per week)
ATTACHMENT I TO APPENDIX B OF UNOS BYLAWS
 ATTACHMENT I TO APPENDIX B OF UNOS BYLAWS Designated Transplant Program Criteria XIII. Transplant Programs. A. In order to qualify for membership, a transplant program must utilize, for its histocompatibility
ATTACHMENT I TO APPENDIX B OF UNOS BYLAWS Designated Transplant Program Criteria XIII. Transplant Programs. A. In order to qualify for membership, a transplant program must utilize, for its histocompatibility
Anthem Centers of Medical Excellence (CME) Transplant Network. CONTRACT OPERATIONS MANUAL for Transplant Program
 Anthem Centers of Medical Excellence (CME) Transplant Network CONTRACT OPERATIONS MANUAL for Transplant Program A supplemental document to the Anthem Blue Cross Facility Operations Manual TABLE OF CONTENTS
Anthem Centers of Medical Excellence (CME) Transplant Network CONTRACT OPERATIONS MANUAL for Transplant Program A supplemental document to the Anthem Blue Cross Facility Operations Manual TABLE OF CONTENTS
Organ, Tissue and Eye Donation: Glossary of Terms
 Organ, Tissue and Eye Donation: Glossary of Terms AATB: The American Association of Tissue Banks is a professional, non-profit, scientific and educational organization. It is the only national tissue banking
Organ, Tissue and Eye Donation: Glossary of Terms AATB: The American Association of Tissue Banks is a professional, non-profit, scientific and educational organization. It is the only national tissue banking
Modifications to How New Donor Information Received Post-Transplant is Reported to Recipient Centers
 Modifications to How New Donor Information Received Post-Transplant is Reported to Recipient Centers Sponsoring Committee Ad Hoc Disease Transmission Advisory Public Comment: 2016-January Board Date: 2016-June
Modifications to How New Donor Information Received Post-Transplant is Reported to Recipient Centers Sponsoring Committee Ad Hoc Disease Transmission Advisory Public Comment: 2016-January Board Date: 2016-June
OFFICE OF INSPECTOR GENERAL
 Department of Health and Human Services OFFICE OF INSPECTOR GENERAL FOSTERING EQUITY IN PATIENT ACCESS TO TRANSPLANTATION: Differences in Waiting Times for Kidneys JUNE GIBBS BROWN Inspector General MAY
Department of Health and Human Services OFFICE OF INSPECTOR GENERAL FOSTERING EQUITY IN PATIENT ACCESS TO TRANSPLANTATION: Differences in Waiting Times for Kidneys JUNE GIBBS BROWN Inspector General MAY
HEALTH POLICY STATEMENT
 Page 1 HEALTH POLICY STATEMENT PREAMBLE The American Nephrology Nurses Association (ANNA) is a national organization of registered nurses practicing in nephrology, which includes but is not limited to
Page 1 HEALTH POLICY STATEMENT PREAMBLE The American Nephrology Nurses Association (ANNA) is a national organization of registered nurses practicing in nephrology, which includes but is not limited to
DAIDS Bethesda, MD USA POLICY
 Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Overview NIH policy requiring independent data and safety monitoring boards (DSMB) for all multicenter Phase III trials has existed since 1979; the most recent restatement was issued in 1998 (NIH Policy
Development of the National Living Donor Assistance Center: reducing financial disincentives to living organ donation
 Development of the National Living Donor Assistance Center: reducing financial disincentives to living organ donation Over the years, the transplant community has worked to advance the care of living organ
Development of the National Living Donor Assistance Center: reducing financial disincentives to living organ donation Over the years, the transplant community has worked to advance the care of living organ
How does a kidney transplant differ from dialysis?
 TA L K I N G A B O U T T R A N S P L A N TAT I O N Frequently Asked Questions about Kidney Transplant Evaluation and Listing If your kidneys have stopped working properly, or may stop working soon, you
TA L K I N G A B O U T T R A N S P L A N TAT I O N Frequently Asked Questions about Kidney Transplant Evaluation and Listing If your kidneys have stopped working properly, or may stop working soon, you
OPTN/UNOS TRANSPLANT ADMINISTRATORS COMMITTEE REPORT June 19-20, 2008 SUMMARY
 OPTN/UNOS TRANSPLANT ADMINISTRATORS COMMITTEE REPORT June 19-20, 2008 SUMMARY I. Action Items for Board Consideration: None II. Other Significant Items: Improve OPTN Data Systems - The Committee continues
OPTN/UNOS TRANSPLANT ADMINISTRATORS COMMITTEE REPORT June 19-20, 2008 SUMMARY I. Action Items for Board Consideration: None II. Other Significant Items: Improve OPTN Data Systems - The Committee continues
ANNUAL REPORT ON KIDNEY TRANSPLANTATION
 ANNUAL REPORT ON KIDNEY TRANSPLANTATION REPORT FOR 2013/2014 (1 APRIL 2004 31 MARCH 2014) PUBLISHED SEPTEMBER 2014 PRODUCED IN COLLABORATION WITH NHS ENGLAND Contents 1 Executive Summary... 1 2 Introduction...
ANNUAL REPORT ON KIDNEY TRANSPLANTATION REPORT FOR 2013/2014 (1 APRIL 2004 31 MARCH 2014) PUBLISHED SEPTEMBER 2014 PRODUCED IN COLLABORATION WITH NHS ENGLAND Contents 1 Executive Summary... 1 2 Introduction...
Expanded criteria donors for kidney transplantation
 American Journal of Transplantation 2003; 3 (Suppl. 4): 114 125 Blackwell Munksgaard 2003 Blackwell Munksgaard ISSN 1601-2577 Expanded criteria donors for kidney transplantation Robert A. Metzger a, Francis
American Journal of Transplantation 2003; 3 (Suppl. 4): 114 125 Blackwell Munksgaard 2003 Blackwell Munksgaard ISSN 1601-2577 Expanded criteria donors for kidney transplantation Robert A. Metzger a, Francis
Public Law 98-507 98th Congress
 PUBLIC LAW 98-507-OCT. 19, 1984 98 STAT. 2339 Public Law 98-507 98th Congress An Act To provide for the establishment of the Task Force on Organ Transplantation and the act, 19, 1984 Organ Procurement
PUBLIC LAW 98-507-OCT. 19, 1984 98 STAT. 2339 Public Law 98-507 98th Congress An Act To provide for the establishment of the Task Force on Organ Transplantation and the act, 19, 1984 Organ Procurement
Preparing for Transplantation
 Preparing for Transplantation How Do I Get on a Transplant List? David A. Dreyfus John B. Valencia I have been told I need a kidney transplant? What s my first step DO Your Homework!! Without a living
Preparing for Transplantation How Do I Get on a Transplant List? David A. Dreyfus John B. Valencia I have been told I need a kidney transplant? What s my first step DO Your Homework!! Without a living
David Axelrod, MD, MBA Associate Professor of Surgery Section Chief Solid Organ Transplantation Dartmouth Hitchcock Medical Center
 David Axelrod, MD, MBA Associate Professor of Surgery Section Chief Solid Organ Transplantation Dartmouth Hitchcock Medical Center Economic analysis team Mark Schnitzler, Ph.D. Krista Lentine MD, Ph.D.
David Axelrod, MD, MBA Associate Professor of Surgery Section Chief Solid Organ Transplantation Dartmouth Hitchcock Medical Center Economic analysis team Mark Schnitzler, Ph.D. Krista Lentine MD, Ph.D.
TA L K I N G A B O U T T R A N S P L A N TAT I O N
 TA L K I N G A B O U T T R A N S P L A N TAT I O N UNITED NETWORK FOR ORGAN SHARING United Network for Organ Sharing (UNOS) is a private non-profit 501(c)(3) organization that operates the Organ Procurement
TA L K I N G A B O U T T R A N S P L A N TAT I O N UNITED NETWORK FOR ORGAN SHARING United Network for Organ Sharing (UNOS) is a private non-profit 501(c)(3) organization that operates the Organ Procurement
Kidney Transplant Program. Guide to Understanding Transplant
 Kidney Transplant Program Guide to Understanding Transplant The wonderful physicians and staff of the Stanford Kidney Transplant Program are always there to contact if I have a question or concern. They
Kidney Transplant Program Guide to Understanding Transplant The wonderful physicians and staff of the Stanford Kidney Transplant Program are always there to contact if I have a question or concern. They
Where Will my New Kidney Come From?
 Where Will my New Kidney Come From? The Organ Shortage There is a severe shortage of organs for transplant. This means that the wait for a kidney transplant can be many years. The UW Transplant Program
Where Will my New Kidney Come From? The Organ Shortage There is a severe shortage of organs for transplant. This means that the wait for a kidney transplant can be many years. The UW Transplant Program
2014 U.S. organ and tissue transplant cost estimates and discussion
 Prepared by: T. Scott Bentley, FSA Principal and Consulting Actuary Peer Reviewed By: Steven G. Hanson, ASA Actuary 2014 U.S. organ and tissue transplant cost estimates and discussion Prepared by: T. Scott
Prepared by: T. Scott Bentley, FSA Principal and Consulting Actuary Peer Reviewed By: Steven G. Hanson, ASA Actuary 2014 U.S. organ and tissue transplant cost estimates and discussion Prepared by: T. Scott
OFFICE OF INSPECTOR GENERAL
 Department of Health and Human Services OFFICE OF INSPECTOR GENERAL Medicare Conditions of Participation for Organ Donation: An Early Assessment of the New Donation Rule JUNE GIBBS BROWN Inspector General
Department of Health and Human Services OFFICE OF INSPECTOR GENERAL Medicare Conditions of Participation for Organ Donation: An Early Assessment of the New Donation Rule JUNE GIBBS BROWN Inspector General
New Jersey Organ & Tissue Donation
 New Jersey Organ & Tissue Donation Contact Hours: 1.0 First Published: May 7, 2013 Course Expires: May 7, 2016 Copyright 2013 by RN.com All Rights Reserved Reproduction and distribution of these materials
New Jersey Organ & Tissue Donation Contact Hours: 1.0 First Published: May 7, 2013 Course Expires: May 7, 2016 Copyright 2013 by RN.com All Rights Reserved Reproduction and distribution of these materials
Living Donor Paired Exchange Registry. What is Living Kidney Donor Paired Exchange?
 Living Paired Exchange Registry What is Living Kidney Paired Exchange? The Living Paired Exchange Registry. At Canadian Blood Services, we are committed to helping Canadians help each other. By building
Living Paired Exchange Registry What is Living Kidney Paired Exchange? The Living Paired Exchange Registry. At Canadian Blood Services, we are committed to helping Canadians help each other. By building
Summary of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Status
 of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Shelley Grant Branch Chief, Blood Stem Cell Transplantation Program September 11, 2015 Overview December 20, 2005 - HRSA began
of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Shelley Grant Branch Chief, Blood Stem Cell Transplantation Program September 11, 2015 Overview December 20, 2005 - HRSA began
Is a Kidney Transplant Right for Me?
 Is a Kidney Transplant Right for Me? Network 8 Introduction What do singer Natalie Cole, actor Ken Howard, funny men Tracy Morgan and George Lopez and fashion reporter Steven Cojocaru have in common? They
Is a Kidney Transplant Right for Me? Network 8 Introduction What do singer Natalie Cole, actor Ken Howard, funny men Tracy Morgan and George Lopez and fashion reporter Steven Cojocaru have in common? They
STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005
 STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005 VerDate 14-DEC-2004 06:53 Jan 05, 2006 Jkt 039139 PO 00000 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL129.109 APPS10 PsN: PUBL129 119 STAT. 2550 PUBLIC LAW
STEM CELL THERAPEUTIC AND RESEARCH ACT OF 2005 VerDate 14-DEC-2004 06:53 Jan 05, 2006 Jkt 039139 PO 00000 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL129.109 APPS10 PsN: PUBL129 119 STAT. 2550 PUBLIC LAW
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance
 University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
ESRD FACILITY SURVEY (CMS-2744) INSTRUCTIONS FOR COMPLETION
 ESRD FACILITY SURVEY (CMS-2744) INSTRUCTIONS FOR COMPLETION REPORTING RESPONSIBILITY The ESRD Facility Survey is designed to capture only a limited amount of information concerning each Federally approved
ESRD FACILITY SURVEY (CMS-2744) INSTRUCTIONS FOR COMPLETION REPORTING RESPONSIBILITY The ESRD Facility Survey is designed to capture only a limited amount of information concerning each Federally approved
NATCO Introductory Education Course Objectives
 NATCO Introductory Education Course for the NEW Transplant Professional & Procurement Professional June 12-15, 2015 Tempe Mission Palms Hotel Tempe, AZ Friday, June 12, 2015 SESSION Course Participants
NATCO Introductory Education Course for the NEW Transplant Professional & Procurement Professional June 12-15, 2015 Tempe Mission Palms Hotel Tempe, AZ Friday, June 12, 2015 SESSION Course Participants
Hospital Quality Initiative Overview CENTERS FOR MEDICARE & MEDICAID SERVICES December 2005
 Hospital Quality Initiative Overview CENTERS FOR MEDICARE & MEDICAID SERVICES December 2005 Background Quality health care is a high priority for the Bush administration, the Department of Health and Human
Hospital Quality Initiative Overview CENTERS FOR MEDICARE & MEDICAID SERVICES December 2005 Background Quality health care is a high priority for the Bush administration, the Department of Health and Human
Arecent Institute of Medicine report titled Best care
 Original Clinical ScienceçGeneral Variation in Cost and Quality in Kidney Transplantation Bishara A. Nassir, MD, 1 Carl E. Dean, MD, 2 Suying Li, PhD, 3 Nicholas Salkowski, PhD, 4 Craig A. Solid, PhD,
Original Clinical ScienceçGeneral Variation in Cost and Quality in Kidney Transplantation Bishara A. Nassir, MD, 1 Carl E. Dean, MD, 2 Suying Li, PhD, 3 Nicholas Salkowski, PhD, 4 Craig A. Solid, PhD,
Florida Medicaid. Transplant Services Coverage Policy
 Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
The Paired Donation Network. E. Steve Woodle, M.D. Division of Transplantation University of Cincinnati
 The Paired Donation Network E. Steve Woodle, M.D. Division of Transplantation University of Cincinnati Paired Donation: Definition In a paired donation, two living kidney donor/recipient pairs (both of
The Paired Donation Network E. Steve Woodle, M.D. Division of Transplantation University of Cincinnati Paired Donation: Definition In a paired donation, two living kidney donor/recipient pairs (both of
DEPARTMENT OF HEALTH AND HUMAN SERVICES. Health Resources and Services Administration. Advisory Council on Blood Stem Cell Transplantation
 This document is scheduled to be published in the Federal Register on 01/21/2016 and available online at http://federalregister.gov/a/2016-01127, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
This document is scheduled to be published in the Federal Register on 01/21/2016 and available online at http://federalregister.gov/a/2016-01127, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2003 to 2012
 Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2003 to 2012 Types of Care Our Vision Better data. Better decisions. Healthier Canadians. Our Mandate
Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2003 to 2012 Types of Care Our Vision Better data. Better decisions. Healthier Canadians. Our Mandate
Changes for Calendar Year 2015 Physician Quality Programs and Other Programs in the Medicare Physician Fee Schedule
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services Room 352-G 200 Independence Avenue, SW Washington, DC 20201 FACT SHEET FOR IMMEDIATE RELEASE October 31, 2014 Contact: CMS
Overview of Organ Donation and Transplantation
 2 Overview of Organ Donation and Transplantation Overview of Organ Donation and Transplantation A summary of organ donation and transplantation activity in the UK during the financial year from 1 April
2 Overview of Organ Donation and Transplantation Overview of Organ Donation and Transplantation A summary of organ donation and transplantation activity in the UK during the financial year from 1 April
Saint Francis Kidney Transplant Program Issue Date: 6/9/15
 Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
Pennsylvania. Department of Health. Internship Opportunities
 Pennsylvania Department of Health Internship Opportunities Summer 2016 Applications for these internship opportunities are due Friday January 22, 2016. Please refer to the application instructions on page
Pennsylvania Department of Health Internship Opportunities Summer 2016 Applications for these internship opportunities are due Friday January 22, 2016. Please refer to the application instructions on page
WASHTENAW COUNTY DEMOCRATIC PARTY BYLAWS Adopted September 13, 2009 ARTICLE I. NAME
 WASHTENAW COUNTY DEMOCRATIC PARTY BYLAWS Adopted September 13, 2009 ARTICLE I. NAME This organization shall be known as the Washtenaw County Democratic Party (WCDP). It shall consist of the Officers, Executive
WASHTENAW COUNTY DEMOCRATIC PARTY BYLAWS Adopted September 13, 2009 ARTICLE I. NAME This organization shall be known as the Washtenaw County Democratic Party (WCDP). It shall consist of the Officers, Executive
Los Angeles County Metropolitan Transportation Authority Office of the Inspector General Medicare Part B Reimbursements to Retirees
 Los Angeles County Metropolitan Transportation Authority Medicare Part B Reimbursements to Retirees Several procedural refinements are needed to ensure that reimbursements are discontinued for deceased
Los Angeles County Metropolitan Transportation Authority Medicare Part B Reimbursements to Retirees Several procedural refinements are needed to ensure that reimbursements are discontinued for deceased
Kidneys. Kidney Failure4. Transplantation
 Kidneys & Kidney Failure4 Transplantation This booklet helps you understand the process of transplantation. It defines the criteria for a donor and the recipient. It also clearly explains the concepts
Kidneys & Kidney Failure4 Transplantation This booklet helps you understand the process of transplantation. It defines the criteria for a donor and the recipient. It also clearly explains the concepts
PENNSYLVANIA PRIMARY CARE LOAN REPAYMENT PROGRAM
 PENNSYLVANIA PRIMARY CARE LOAN REPAYMENT PROGRAM Practice Site Application Reference Guide & Instructions PENNSYLVANIA DEPARTMENT OF HEALTH Bureau of Health Planning Division of Health Professions Development
PENNSYLVANIA PRIMARY CARE LOAN REPAYMENT PROGRAM Practice Site Application Reference Guide & Instructions PENNSYLVANIA DEPARTMENT OF HEALTH Bureau of Health Planning Division of Health Professions Development
. 4 " ~ f.".2 DEPARTMENT OF HEALTH & HUMAN SERVICES OFFICE OF INSPECTOR GENERAL. December 19,2003. Our Reference: Report Number A-O2-03-01016
 . 4 " ~..+.-"..i"..,. f.".2 '" '" ~ DEPARTMENT OF HEALTH & HUMAN SERVICES OFFICE OF INSPECTOR GENERAL Office of Audit Services Region II Jacob K. Javits Federal Building New York, New York 10278 (212)
. 4 " ~..+.-"..i"..,. f.".2 '" '" ~ DEPARTMENT OF HEALTH & HUMAN SERVICES OFFICE OF INSPECTOR GENERAL Office of Audit Services Region II Jacob K. Javits Federal Building New York, New York 10278 (212)
Hepatitis C Virus Infection: Prevalence Report, 2003 Data Source: Minnesota Department of Health HCV Surveillance System
 Hepatitis C Virus Infection: Prevalence Report, 2003 Data Source: Minnesota Department of Health HCV Surveillance System P.O. Box 9441 Minneapolis, MN 55440-9441 612-676-5414, 1-877-676-5414 www.health.state.mn.us/immunize
Hepatitis C Virus Infection: Prevalence Report, 2003 Data Source: Minnesota Department of Health HCV Surveillance System P.O. Box 9441 Minneapolis, MN 55440-9441 612-676-5414, 1-877-676-5414 www.health.state.mn.us/immunize
How To Qualify For EHR Stimulus Funds Under
 BEST PRACTICES: How To Qualify For EHR Stimulus Funds Under Meaningful Use & Certified EHR Technology The American Recovery and Reinvestment Act (ARRA) set aside early $20 billion in incentive payments
BEST PRACTICES: How To Qualify For EHR Stimulus Funds Under Meaningful Use & Certified EHR Technology The American Recovery and Reinvestment Act (ARRA) set aside early $20 billion in incentive payments
JOINT NOTICE OF OUR HEALTH INFORMATION PRACTICES
 JOINT NOTICE OF OUR HEALTH INFORMATION PRACTICES THIS NOTICE DESCRIBES HOW INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. Jennings
JOINT NOTICE OF OUR HEALTH INFORMATION PRACTICES THIS NOTICE DESCRIBES HOW INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. Jennings
GAO PREVENTION AND PUBLIC HEALTH FUND. Activities Funded in Fiscal Years 2010 and 2011. Report to Congressional Requesters
 GAO United States Government Accountability Office Report to Congressional Requesters September 2012 PREVENTION AND PUBLIC HEALTH FUND Activities Funded in Fiscal Years 2010 and 2011 To access this report
GAO United States Government Accountability Office Report to Congressional Requesters September 2012 PREVENTION AND PUBLIC HEALTH FUND Activities Funded in Fiscal Years 2010 and 2011 To access this report
Immunization Information System (IIS) Manager Sample Role Description
 Immunization Information System (IIS) Manager Sample Role Description March 2016 0 Note: This role description is meant to offer sample language and a comprehensive list of potential desired responsibilities
Immunization Information System (IIS) Manager Sample Role Description March 2016 0 Note: This role description is meant to offer sample language and a comprehensive list of potential desired responsibilities
Improving Hospital Performance
 Improving Hospital Performance Background AHA View Putting patients first ensuring their care is centered on the individual, rooted in best practices and utilizes the latest evidence-based medicine is
Improving Hospital Performance Background AHA View Putting patients first ensuring their care is centered on the individual, rooted in best practices and utilizes the latest evidence-based medicine is
PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Transmission Through Organ Transplantation
 PHS Guideline PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Transmission Through Organ Transplantation Debbie L. Seem, RN, MPH a Ingi Lee, MD, MSCE b
PHS Guideline PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Transmission Through Organ Transplantation Debbie L. Seem, RN, MPH a Ingi Lee, MD, MSCE b
CROWNWeb Data Management Guidelines
 Connecting the Pieces CROWNWeb Data Management Guidelines Document Control Document Information Information Sequence Number 0001 Document Type Guidelines Release Number 1.0 Release Date April 15, 2015
Connecting the Pieces CROWNWeb Data Management Guidelines Document Control Document Information Information Sequence Number 0001 Document Type Guidelines Release Number 1.0 Release Date April 15, 2015
Individual HealthPartners Wisconsin Freedom Plan (Cost) Enrollment Form
 Individual HealthPartners Wisconsin Freedom Plan (Cost) Enrollment Form This is the enrollment application for your HealthPartners Wisconsin Freedom plan (Cost) medical and prescription drug options. Follow
Individual HealthPartners Wisconsin Freedom Plan (Cost) Enrollment Form This is the enrollment application for your HealthPartners Wisconsin Freedom plan (Cost) medical and prescription drug options. Follow
HD RP. Health Disparities in Kidney Transplantation: An Equity Analysis. Shirley A. Wells, University of Texas-Pan American ABSTRACT INTRODUCTION
 JOURNAL OF HD RP Journal of Health Disparities Research and Practice Volume 3, Number 2, Fall 2009, pp. 1 12 2009 Center for Health Disparities Research School of Community Health Sciences University of
JOURNAL OF HD RP Journal of Health Disparities Research and Practice Volume 3, Number 2, Fall 2009, pp. 1 12 2009 Center for Health Disparities Research School of Community Health Sciences University of
DEPARTMENT OF HEALTH DETERMINING MEDICAID ELIGIBILITY. Report 2005-S-42 OFFICE OF THE NEW YORK STATE COMPTROLLER DIVISION OF STATE SERVICES
 Alan G. Hevesi COMPTROLLER OFFICE OF THE NEW YORK STATE COMPTROLLER DIVISION OF STATE SERVICES Audit Objective...2 Audit Results - Summary...2 Background...3 Audit Findings and Recommendations...4 Deceased
Alan G. Hevesi COMPTROLLER OFFICE OF THE NEW YORK STATE COMPTROLLER DIVISION OF STATE SERVICES Audit Objective...2 Audit Results - Summary...2 Background...3 Audit Findings and Recommendations...4 Deceased
United Network For Organ Sharing. Inquiring Minds Want to Know
 United Network For Organ Sharing Inquiring Minds Want to Know Introduction Tommy Trexler Resource Manager, Service Desk Andrea Loyd Service Desk Specialist Lauri Walker Service Desk Specialist Tara Taylor
United Network For Organ Sharing Inquiring Minds Want to Know Introduction Tommy Trexler Resource Manager, Service Desk Andrea Loyd Service Desk Specialist Lauri Walker Service Desk Specialist Tara Taylor
Meaningful Use. Medicare and Medicaid EHR Incentive Programs
 Meaningful Use Medicare and Medicaid Table of Contents What is Meaningful Use?... 1 Table 1: Patient Benefits... 2 What is an EP?... 4 How are Registration and Attestation Being Handled?... 5 What are
Meaningful Use Medicare and Medicaid Table of Contents What is Meaningful Use?... 1 Table 1: Patient Benefits... 2 What is an EP?... 4 How are Registration and Attestation Being Handled?... 5 What are
CHAPTER 1 BACKGROUND AND CORD BLOOD BANK (CBB) ORGANIZATION
 CHAPTER 1 BACKGROUND AND CORD BLOOD BANK (CBB) ORGANIZATION Chapter 1 BACKGROUND AND CORD BLOOD BANK (CBB) ORGANIZATION 1.1 OVERVIEW OF THE CORD BLOOD TRANSPLANTATION STUDY Bone marrow transplantation
CHAPTER 1 BACKGROUND AND CORD BLOOD BANK (CBB) ORGANIZATION Chapter 1 BACKGROUND AND CORD BLOOD BANK (CBB) ORGANIZATION 1.1 OVERVIEW OF THE CORD BLOOD TRANSPLANTATION STUDY Bone marrow transplantation
Incentives to Accelerate EHR Adoption
 Incentives to Accelerate EHR Adoption The passage of the American Recovery and Reinvestment Act (ARRA) of 2009 provides incentives for eligible professionals (EPs) to adopt and use electronic health records
Incentives to Accelerate EHR Adoption The passage of the American Recovery and Reinvestment Act (ARRA) of 2009 provides incentives for eligible professionals (EPs) to adopt and use electronic health records
Professional Level Public Health Informatician
 Professional Level Public Health Informatician Sample Position Description and Sample Career Ladder April 2014 Acknowledgements Public Health Informatics Institute (PHII) wishes to thank the Association
Professional Level Public Health Informatician Sample Position Description and Sample Career Ladder April 2014 Acknowledgements Public Health Informatics Institute (PHII) wishes to thank the Association
Chart 11-1. Number of dialysis facilities is growing, and share of for-profit and freestanding dialysis providers is increasing
 11 0 Chart 11-1. Number of dialysis facilities is growing, and share of for-profit and freestanding dialysis providers is increasing Average annual percent change 2014 2009 2014 2013 2014 Total number
11 0 Chart 11-1. Number of dialysis facilities is growing, and share of for-profit and freestanding dialysis providers is increasing Average annual percent change 2014 2009 2014 2013 2014 Total number
LIVING KIDNEY DONATION
 LIVING KIDNEY DONATION 1 THE MICHIGAN DIFFERENCE Why Living Donation? As of May 2011, there were close to 2,500 individuals on the waiting list for a kidney transplant in the state of Michigan and more
LIVING KIDNEY DONATION 1 THE MICHIGAN DIFFERENCE Why Living Donation? As of May 2011, there were close to 2,500 individuals on the waiting list for a kidney transplant in the state of Michigan and more
HEAL NY Phase 5 Health IT RGA Section 7.1: HEAL NY Phase 5 Health IT Candidate Use Cases Interoperable EHR Use Case for Medicaid
 HEAL NY Phase 5 Health IT RGA Section 7.1: HEAL NY Phase 5 Health IT Candidate Use Cases Interoperable EHR Use Case for Medicaid Interoperable Electronic Health Records (EHRs) Use Case for Medicaid (Medication
HEAL NY Phase 5 Health IT RGA Section 7.1: HEAL NY Phase 5 Health IT Candidate Use Cases Interoperable EHR Use Case for Medicaid Interoperable Electronic Health Records (EHRs) Use Case for Medicaid (Medication
Affordable Care Act Reviews
 Appendix A Affordable Care Act Reviews New Programs and Initiatives... 107 Pre-Existing Condition Insurance Plans, 1101... 107 Controls Over Pre-Existing Condition Insurance Plans and Collaborative Administration...
Appendix A Affordable Care Act Reviews New Programs and Initiatives... 107 Pre-Existing Condition Insurance Plans, 1101... 107 Controls Over Pre-Existing Condition Insurance Plans and Collaborative Administration...
Six Degrees of Separation No More: Using Data Linkages to Improve the Quality of Cancer Registry and Study Data
 Six Degrees of Separation No More: Using Data Linkages to Improve the Quality of Cancer Registry and Study Data Prepared for the 138th Annual Meeting of the American Public Health Association Denver, CO
Six Degrees of Separation No More: Using Data Linkages to Improve the Quality of Cancer Registry and Study Data Prepared for the 138th Annual Meeting of the American Public Health Association Denver, CO
Montgomery County, Unique Aspects of the Medicaid Control System
 MONTGOMERY COUNTY POLICY AND PROCEDURE Date Drafted: 12/07/09 Date Approved: 12/15/09 Date(s) Revised: I. POLICY: It is the policy of Montgomery County to promote compliance with all federal, state, and
MONTGOMERY COUNTY POLICY AND PROCEDURE Date Drafted: 12/07/09 Date Approved: 12/15/09 Date(s) Revised: I. POLICY: It is the policy of Montgomery County to promote compliance with all federal, state, and
The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future
 The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future November 2013 About Health Sciences South Carolina The HSSC Clinical Data Warehouse: Health Sciences South Carolina
The HSCC Clinical Data Warehouse: The South Carolina Biomedical Research Tool of the Future November 2013 About Health Sciences South Carolina The HSSC Clinical Data Warehouse: Health Sciences South Carolina
Transplant Report. UMassMemorial Medical Center A Member of UMass Memorial Health Care
 Transplant Report UMassMemorial Medical Center A Member of UMass Memorial Health Care A Message from the Chief Dear Colleague: In recent years, UMass Memorial Medical Center has made significant strides
Transplant Report UMassMemorial Medical Center A Member of UMass Memorial Health Care A Message from the Chief Dear Colleague: In recent years, UMass Memorial Medical Center has made significant strides
A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems
 A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems November 14, 2014 Submission to the Advisory Panel on Health Care Innovation
A Real Time Lab for Pan Canadian Innovation Leveraging Canadian Blood Services Model for Better Value to Health care Systems November 14, 2014 Submission to the Advisory Panel on Health Care Innovation
GAO MEDICARE ADVANTAGE. Relationship between Benefit Package Designs and Plans Average Beneficiary Health Status. Report to Congressional Requesters
 GAO United States Government Accountability Office Report to Congressional Requesters April 2010 MEDICARE ADVANTAGE Relationship between Benefit Package Designs and Plans Average Beneficiary Health Status
GAO United States Government Accountability Office Report to Congressional Requesters April 2010 MEDICARE ADVANTAGE Relationship between Benefit Package Designs and Plans Average Beneficiary Health Status
PROFESSIONAL SERVICES CONSULTING AGREEMENT
 PROFESSIONAL SERVICES CONSULTING AGREEMENT THIS AGREEMENT, effective as of the date of (the Effective Date ), is by and between New Jersey Institute of Technology ("NJIT"), a public research university,
PROFESSIONAL SERVICES CONSULTING AGREEMENT THIS AGREEMENT, effective as of the date of (the Effective Date ), is by and between New Jersey Institute of Technology ("NJIT"), a public research university,
Kidney Transplantation and Cancer - A Review
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2016 4/2017 4/2016 Description of Procedure or Service A kidney transplant
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2016 4/2017 4/2016 Description of Procedure or Service A kidney transplant
HEALTH CARE PROFESSIONAL MANUAL
 HEALTH CARE PROFESSIONAL MANUAL Cigna LifeSOURCE is committed to providing access to quality transplant care, improved health, and lower costs. Offered by: Cigna Health and Life Insurance Company, Connecticut
HEALTH CARE PROFESSIONAL MANUAL Cigna LifeSOURCE is committed to providing access to quality transplant care, improved health, and lower costs. Offered by: Cigna Health and Life Insurance Company, Connecticut
Chapter 4. Planning a cancer registry
 Chapter 4. Planning a cancer registry 0. M. Jensenl and S. Whelan2 Danish Cancer Registry, Danish Cancer Society, Rosenvaengets Hoveduej 35, PO Box 839, Copenhagen 21nternational Agency for Research on
Chapter 4. Planning a cancer registry 0. M. Jensenl and S. Whelan2 Danish Cancer Registry, Danish Cancer Society, Rosenvaengets Hoveduej 35, PO Box 839, Copenhagen 21nternational Agency for Research on
VETERANS TRUST FUND GRANT. COLORADO DEPARTMENT OF MILITARY AND VETERANS AFFAIRS Applications due: March 01, 2016
 VETERANS TRUST FUND GRANT COLORADO DEPARTMENT OF MILITARY AND VETERANS AFFAIRS Applications due: March 01, 2016 PROGRAM SUMMARY The Veteran s Trust Fund (VTF) grant was established in 2000 by the State
VETERANS TRUST FUND GRANT COLORADO DEPARTMENT OF MILITARY AND VETERANS AFFAIRS Applications due: March 01, 2016 PROGRAM SUMMARY The Veteran s Trust Fund (VTF) grant was established in 2000 by the State
How To Write A Traumatic Brain Injury Act Of 2008
 Traumatic Brain Injury Act of 2008: Overview Background The Reauthorization of the Traumatic Brain Injury Act (TBI) of 2008 reauthorized appropriations through Fiscal Year 2012 for TBI programs administered
Traumatic Brain Injury Act of 2008: Overview Background The Reauthorization of the Traumatic Brain Injury Act (TBI) of 2008 reauthorized appropriations through Fiscal Year 2012 for TBI programs administered
Introduction. The History of CMS/JCAHO Measure Alignment
 Release Notes: Introduction - Version 2.2 Introduction The History of CMS/JCAHO Measure Alignment In early 1999, the Joint Commission solicited input from a wide variety of stakeholders (e.g., clinical
Release Notes: Introduction - Version 2.2 Introduction The History of CMS/JCAHO Measure Alignment In early 1999, the Joint Commission solicited input from a wide variety of stakeholders (e.g., clinical
Community Living Exchange Collaborative: A National Technical Assistance Program. Funded by Centers for Medicare and Medicaid Services (CMS)
 Community Living Exchange Collaborative: A National Technical Assistance Program Funded by Centers for Medicare and Medicaid Services (CMS) Susan Reinhard, RN, PhD Robert Mollica, EdD Policy Brief Money
Community Living Exchange Collaborative: A National Technical Assistance Program Funded by Centers for Medicare and Medicaid Services (CMS) Susan Reinhard, RN, PhD Robert Mollica, EdD Policy Brief Money
Where World-Class Expertise and Genuine Compassion Come Together. AT THE FOREFRONT OF TRANSPLANT CARE Kidney Combined Kidney-Pancreas Pancreas Islets
 Where World-Class Expertise and Genuine Compassion Come Together 0011000110100111100110111100000101010011000101100010111000010010000100010000100001011110101010101111000 000010111000101110011000110100111100110111100000101010011000101100010111000010010000100010000100
Where World-Class Expertise and Genuine Compassion Come Together 0011000110100111100110111100000101010011000101100010111000010010000100010000100001011110101010101111000 000010111000101110011000110100111100110111100000101010011000101100010111000010010000100010000100
2014/15 National Tariff Payment System. Annex 7A: Specified services for acute services for local pricing
 2014/15 National Tariff Payment System Annex 7A: Specified services for acute services for local pricing 17 December 2013 Publications Gateway Reference 00883 Annex 7A: Specified services for acute services
2014/15 National Tariff Payment System Annex 7A: Specified services for acute services for local pricing 17 December 2013 Publications Gateway Reference 00883 Annex 7A: Specified services for acute services
Medicare and Medicaid Programs; EHR Incentive Programs
 Medicare and Medicaid Programs; EHR Incentive Programs Background The American Recovery and Reinvestment Act of 2009 establishes incentive payments under the Medicare and Medicaid programs for certain
Medicare and Medicaid Programs; EHR Incentive Programs Background The American Recovery and Reinvestment Act of 2009 establishes incentive payments under the Medicare and Medicaid programs for certain
