GUIDE FOR FEED MANUFACTURERS TO COMPLY WITH THE FDA FINAL RULE PROHIBITING MAMMALIAN PROTEIN IN RUMINANT FEED
|
|
|
- Arlene Marshall
- 7 years ago
- Views:
Transcription
1 GUIDE FOR FEED MANUFACTURERS TO COMPLY WITH THE FDA FINAL RULE PROHIBITING MAMMALIAN PROTEIN IN RUMINANT FEED Introduction On June 5, 1997, FDA published a final rule prohibiting the use of mammalian protein (i.e. animal protein products, such as meat and bone meal) in ruminant feeds. Mammalian protein is defined as protein from mammals, but excludes porcine (pork) and equine (horse) protein from single-species slaughter plants. There are exemptions from this prohibition for proteins derived from blood, milk, gelatin and processed meat products which have been cooked and offered for human consumption and further heat processed for feed (such as plate waste and cellulosic food casings). Fat, tallow, amino acids, and dicalcium phosphate produced as a by-product of gelatin manufacturing, are not covered by this rule and may be used in ruminant feeds. Similarly, poultry and fish meals are not included in this rule and may continue to be used. The rule took effect August 4, This means the required warning statements and written recordkeeping and cleanout procedures should have been in place on this date. FDA is allowed an additional 60 days to exhaust labeling and products from the marketplace for products and labels produced before June 5, Therefore, all products and labels were to be in compliance with this rule by October 3, This guide is designed to assist you in complying with this new rule in a timely manner. However, it is difficult to design a broad-based guide of this type for all situations and scenarios in the feed industry. Much of the guide will be devoted to discussing the end-point for complying, so a firm can design compliance procedures to reach that endpoint in a manner appropriate for its particular operations. Areas of Concern Three principal areas are detailed in this guide. These are labeling, recordkeeping, and the main point of this guide, equipment cleanout. Each of these areas has specific requirements as part of the final rule. For the purpose of this guide, it is assumed your firm manufactures feed for more than one species, and likely uses meat and bone meal or other animal protein products from mammalian sources. If your firm utilizes only animal protein products from exempt sources, such as pork or horse, no equipment cleanout is required, nor is special labeling required. However, you may wish to acquire a letter of certification, such as the one appearing in Appendix A. Source AFIA %20Biosecurity%20Awareness/BSE%20Guidelines.pdf
2 Labeling The rule requires ail feed labeling, including brochures and other leaflets distributed with feed products containing (or likely to contain) prohibited mammalian protein, to have the statement "Do not feed to cattle or other ruminants" in a prominent place on the label or other printed material. FDA suggests the statement be distinguished by different type size or color or other means of highlighting the statement. Pet foods for retail sale and feed for non-ruminant laboratory animals are exempt from the labeling requirement. For existing Labeling, some firms may find it helpful to use a rubber stamp with the required statement, thus saving reprinting costs. Use of such a stamp should not obliterate or make unreadable other required labeling information. Similarly, printed bags may be labeled with a rubber stamp of the statement, stick-on labels, or other means of printing the statement prominently on the bag. For bulk shipments, the required statement may be stamped or otherwise printed on invoices or other shipping documents. PLEASE NOTE: The rule does not require the use of the words "Caution" or "Warning". Only the statement highlighted above is required. According to FDA, feed not bearing the required label statement are assumed to be in compliance with the rule, and the feed does not contain prohibited substances. FDA and state investigators will likely review animal protein product records of firms listing no cautionary statements on product labels. In other words, failing to use the cautionary statement implies compliance with the rule and means a firm is not using prohibited mammalian proteins. Feed produced by integrated operators for distribution to on-farm producers is not exempt from the labeling requirement unless the firm is using only exempt protein substances. This should be documented by adequate records. The Association of American Feed Control Officials (AAFCO) model feed regulations allow use of collective terms, such as "animal protein products." Nearly all states (except California and Hawaii) allow such terms in lieu of the individual ingredient name, such as, "meat and bone meal." This allowance makes it easier to utilize least-cost or best-cost formulation programs without reprinting labels each time an ingredient is changed. The final rule does not change the ability to use collective terms. Firms may continue to use "animal protein products" on feed labels, where one or more animal protein ingredients from the AAFCO list are utilized. Firms may voluntarily choose to utilize more specific ingredient names than "meat and bone meal" in ingredient listings by utilizing such specifically named ingredients like pork and bone meal. (Please note the use of a specific species name in the meat and bone name. The usual rule is to utilize the meat name, i.e. pork, beef, lamb, etc. followed by "... and bone meal.") It is unclear if feed control agencies will allow use of terms, such as "non-ruminant meat and bone meal." However, AFIA can petition such if needed. 2
3 Protein From Single Species Slaughter Facilities The rule allows certain exemptions from labeling and recordkeeping for firms purchasing and utilizing exempt products. For example, if your firm chooses to utilize only pork and bone meal, this ingredient must he obtained from a single-species slaughter facility. If such single species ingredients are utilized, neither recordkeeping nor the cautionary label statement is required. While not required to do so by the FDA regulations, ASIA strongly recommends that such a facility certify to the feed manufacturer that only porcine (or equine) species are slaughtered in its facility. Appendix A is an example of a certification letter (or such a statement may be included on invoices or other shipping document). Recordkeeping The rule requires records sufficient to track ingredients and finished products throughout receipt, processing and distribution for firms utilizing mammalian ingredients prohibited in ruminant feeds. These records must be available for inspection and copying by state and federal investigators, and must be maintained for one year after distribution. Normal business records may be used to document compliance with the rule during inspections by state and/or federal investigators. Most firms should have existing records sufficient to be in compliance with this rule. Any voluntary certification statements, detailed in the "Protein From Single Species Slaughter Facilities" section above and the "Ingredient Receiving" section below, should be maintained with ingredient invoices or other shipping documents. Labels of such products should be maintained as well. FDA advises that the recordkeeping requirement can be satisfied by an invoice or similar document reflecting receipt or purchase, and sale or delivery of the product. The information normally included in these documents includes: 1) Date of receipt or purchase, or sale or delivery; 2) seller's name and address; 3) consignee's name and address; 4) identification of the product, and 5) quantity. FDA notes invoices or similar sales documents may serve as labels for bulk products. Customer Recordkeeping Your customers may request information about recordkeeping required to comply with the rule, or you may wish to advise them about their recordkeeping requirements, especially dairy and beef cattle customers. AFIA offers the following suggestions for your customers: Records to document compliance with the rule for customers feeding ruminants must be available for FDA or state inspection and maintained for one year. Such records are similar to those described above for feed manufacturers and include the labeling for all feed products containing animal protein products. If a customer further mixes feeds for use at other facilities, these feeds must be labeled with the required cautionary statement if the feeds contain prohibited animal protein products. 3
4 Facilities and Equipment Cleanout For facilities which choose to separate mammalian and non-mammalian materials, FDA suggests that all equipment which comes in contact with feeds containing mammalian and nonmammalian product follow all reasonable and effective procedures to prevent contamination of manufactured feed. The agency notes that only equipment and storage facilities that are used for proteins derived from both mammalian and nonmammalian tissues are subject to the clean-out requirement. FDA will consider the use of clean-out procedures to be adequate for the purposes of this final rule if the procedures are based on the equipment clean-out procedures in 21 CFR (CGMPs for medicated feed manufacturers). Federally licensed, medicated feed mills are required to comply with these CGMP requirements, including having predetermined, written compliance procedures for each facility. Many feed manufacturers that do not hold a feed mill license (formerly MFAs or 1900s) may not have written procedures. These firms also must have written procedures to prevent unsafe carryover of prohibited mammalian protein into either ruminant feeds and/or ingredients used in ruminant feeds. These procedures may include physical cleanout, flushing, or sequencing. Ingredient Receiving In most instances, sequencing of received ingredients will enhance- operating efficiencies. For example, after unloading rendered mammalian protein, the area around the receiving pit should be swept, and the conveying system operated until it is completely cleaned out. Next, depending upon the operating capacity of the receiving system, an adequate amount of a high volume, routinely received ingredient, such as soybean meal, should be received to clean the receiving pit and conveying equipment of rendered mammalian protein residue. Ideally, this material should be directed to a second soybean meal bin, for example, and not used in ruminant feeds. In cases where the preferred sequence of ingredients cannot occur, then flushing is an option. In such a case, an adequate amount of soybean meal should be transferred to a bulk load out bin, loaded onto a truck, received to clean the receiving pit and conveying system, and directed to the soybean meal bin designated for non-ruminant use. The amount of sequenced or flushed material deemed adequate to clean the receiving system of rendered protein residue should he decided by someone knowledgeable in plant operations and/or FDA compliance. If inspected, FDA investigators will review your written procedures for receiving rendered protein, verify your plant personnel are following the procedures, and make an assessment as to whether the amount of material used for sequencing and/or flushing is adequate to prevent carryover of rendered protein. In order to document cleanout and minimize carryover from ingredient suppliers and carriers, each firm may request from ingredient haulers certification (Appendix B) that transport vehicles have been flushed with non-prohibited ingredients and/or sequenced in a manner adequate to prevent carryover or prohibited substances. Although certificates are not required by FDA, AFIA strongly recommends this approach. 4
5 Processing Similar to CGMPs for medicated feed, the manufacture of feed formulated with mammalian protein can be properly sequenced. For example, prior to the production of ruminant feed, a non-ruminant feed, not formulated with mammalian protein, can be produced after feed containing mammalian protein. In most cases, this sequence should continue through each process in the plant, up to and including bulk load-out: and packaging, to prevent unsafe carryover of feed containing mammalian protein into any ruminant feeds. In cases where feed sequencing is not practical, then flushing is an option. In such a case, prior to ruminant feed production, an adequate amount of flush material, such as a high-volume ingredient like soybean meal, should be transferred through the plant processes to remove residues of feed containing mammalian protein. Typically, this amount will range between 200 pounds and 1,000 pounds, depending -upon the size of the operating systems. This flush material can be sacked or held in a bulk tote, must be properly identified as "mammalian protein flush", and can be reused into feed production containing mammalian protein by replacing the same ingredient pound for pound. The amount of flush material deemed adequate to clean the mixing and other plant systems of residue from feed containing rendered protein should be determined by someone knowledgeable in plant operations and/or FDA compliance. If inspected, FDA investigators will review your written procedures for production of rendered protein feeds, verify your plant personnel are following the procedures, and make an assessment as to whether the amount of material used for flushing is adequate to prevent unsafe carryover of feed containing rendered protein. Remember, written procedures must be developed for each site. Moreover, any procedures developed must be used consistently in the facility for which the procedures were developed. Summary In order to comply with this rule, feed manufacturers choosing to utilize nonmammalian and mammalian protein ingredients in she same facility must do the following: Label all products containing or which nay contain prohibited mammalian protein with the required warning statement. Maintain adequate records for one year to document compliance with the FDA rule on mammalian protein. Develop, maintain and utilize written procedures adequate to prevent the carryover of prohibited mammalian ingredients into ruminant feed. Allow state and/or federal investigators access to records and procedures concerning the rule. Feed manufacturers choosing to utilize non-prohibited mammalian proteins are not required to label products with the cautionary statement. However, they should maintain records sufficient to document compliance with the rule, including any certification statement from suppliers. Questions on compliance with this rule may be addressed to either Brian Bursiek or Richard Sellers at AFIA. 5
6 Appendix A Letter Certifying Pure Porcine/Equine Protein Product Compliance I/We certify that our product sold to at the following location contains only porcine/equine protein product and is therefore an exempt mammalian protein under FDA regulation 21 CFR I/We certify this product was produced at a single-species slaughter facility. I/we further certify that this letter will cover all future shipments until I/We provide written notification that this letter is no longer in effect. Name Signature Title Address Firm City/State/ZIP Phone Number _ Date 6
7 Appendix B Letter Requesting Adequate Cleanout of Conveyance Vehicles In Compliance with FDA Regulation 21 CFR This is to notify you that (Firm Name) requires your firm (shipper's name) to be in compliance with FDA regulation 2] CFR cleanout procedures. Your cleanout procedures should be adequate to prevent any carryover of mammalian protein products from one load to the next. Such procedures may be by flushing, physical cleanout or by sequencing your loads. You must provide this firm with written concurrence of this request. If there are any questions about this request, please contact the person in the office listed below. Name Signature Title Address Firm City/State/ZIP Phone Number _ 7
8 I/We concur with this request and certify our/my firm is in compliance with the stated rule and adequately clean out my/our vehicles after transportation of mammalian protein products. I/We further certify that this letter will cover all future shipments until I/we provide written notification that this letter is no longer in effect. Name Signature Title Address Firm City/State/ZIP Phone Number _ Date 8
PRIOR NOTICE OF IMPORTED FOOD SHIPMENTS A
 U.S. Food and Drug Administration Protecting the U.S. Food Supply U.S. Department of Health and Human Services What You Need to Know About PRIOR NOTICE OF IMPORTED FOOD SHIPMENTS A Small Entity Compliance
U.S. Food and Drug Administration Protecting the U.S. Food Supply U.S. Department of Health and Human Services What You Need to Know About PRIOR NOTICE OF IMPORTED FOOD SHIPMENTS A Small Entity Compliance
Safe%Feed/Safe%Food% Overview of Certification Programs: Why Should a Feed Mill Get Certified?
 Safe%Feed/Safe%Food% Overview of Certification Programs: Why Should a Feed Mill Get Certified? Henry Turlington, Ph.D. Director, Quality & Manufacturing Regulatory Affairs American Feed Industry Association
Safe%Feed/Safe%Food% Overview of Certification Programs: Why Should a Feed Mill Get Certified? Henry Turlington, Ph.D. Director, Quality & Manufacturing Regulatory Affairs American Feed Industry Association
SQFI Guidance RE: 2.8.3 Allergen Cleaning and Sanitation Practices
 1. INTRODUCTION SQFI Guidance The SQF Institute provides updates to expand on the meaning of the SQF Code, and provide further guidance on the important issues addressed in the SQF Code. The management
1. INTRODUCTION SQFI Guidance The SQF Institute provides updates to expand on the meaning of the SQF Code, and provide further guidance on the important issues addressed in the SQF Code. The management
Requirements for transport sequence, cleaning and disinfection
 Requirements for transport sequence, cleaning and disinfection 1.1 General Prior to the acceptance of a transport commission, the participant should determine the cleaning regime of the new cargo. Before
Requirements for transport sequence, cleaning and disinfection 1.1 General Prior to the acceptance of a transport commission, the participant should determine the cleaning regime of the new cargo. Before
Medicine Record Book
 Medicine Record Book Medicine Administration and Purchase Record Book (and Beef/Lamb Stock Health Plan) Name:.. Address:........ Assurance Number:. CONTENTS Foreword Animal Health Plan (Beef& Lamb only)
Medicine Record Book Medicine Administration and Purchase Record Book (and Beef/Lamb Stock Health Plan) Name:.. Address:........ Assurance Number:. CONTENTS Foreword Animal Health Plan (Beef& Lamb only)
Department of Health and Human Services. No. 66 April 6, 2016. Part III
 Vol. 81 Wednesday, No. 66 April 6, 2016 Part III Department of Health and Human Services Food and Drug Administration 21 CFR Parts 1 and 11 Sanitary Transportation of Human and Animal Food; Final Rule
Vol. 81 Wednesday, No. 66 April 6, 2016 Part III Department of Health and Human Services Food and Drug Administration 21 CFR Parts 1 and 11 Sanitary Transportation of Human and Animal Food; Final Rule
Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products
 Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products Chapter 1 General Principle Article 1 In order to enhance inspection,
Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products Chapter 1 General Principle Article 1 In order to enhance inspection,
It s all about managing food. Food Recall Plan Template For Food Manufacturers
 It s all about managing food. Food Recall Plan Template For Food Manufacturers What to Do In The Event Of a Product Recall Every Food Distributor and Food Manufacturer must track the products they manufacture
It s all about managing food. Food Recall Plan Template For Food Manufacturers What to Do In The Event Of a Product Recall Every Food Distributor and Food Manufacturer must track the products they manufacture
ADVICE FOR OWNERS OF PET PIGS AND MICRO PIGS
 ADVICE FOR OWNERS OF PET PIGS AND MICRO PIGS General guidance for keeping your pig ADVICE AND GUIDANCE Keeping pigs or micro pigs animals specially bred to be smaller in adulthood than most other pig species
ADVICE FOR OWNERS OF PET PIGS AND MICRO PIGS General guidance for keeping your pig ADVICE AND GUIDANCE Keeping pigs or micro pigs animals specially bred to be smaller in adulthood than most other pig species
United States Department of Agriculture Office of Inspector General
 United States Department of Agriculture Office of Inspector General United States Department of Agriculture Office of Inspector General Washington, D.C. 20250 DATE: February 27, 2012 AUDIT NUMBER: 01601-0001-Te
United States Department of Agriculture Office of Inspector General United States Department of Agriculture Office of Inspector General Washington, D.C. 20250 DATE: February 27, 2012 AUDIT NUMBER: 01601-0001-Te
TAMPA BAY WATER Supplying Water To The Region
 TAMPA BAY WATER Supplying Water To The Region REQUEST FOR QUOTATION TAMPA BAY WATER, A Regional Water Supply Authority (TAMPA BAY WATER), is requesting written quotations from vendors who are able to provide
TAMPA BAY WATER Supplying Water To The Region REQUEST FOR QUOTATION TAMPA BAY WATER, A Regional Water Supply Authority (TAMPA BAY WATER), is requesting written quotations from vendors who are able to provide
Helical Products Co., Inc.
 Q01 GENERAL REQUIREMENTS 1. Supplier Responsibilities 1.1. Delivery Certification By delivering products or services on the Contract, the Supplier certifies that such products or services are in compliance
Q01 GENERAL REQUIREMENTS 1. Supplier Responsibilities 1.1. Delivery Certification By delivering products or services on the Contract, the Supplier certifies that such products or services are in compliance
Pelleting Process. Pelleting Process
 Evolution of fish feeds: Pelleting Process Moist feeds; beef liver, spleen 1960 Powder mixture Semi moist feeds; trash fish and powder 1970 Dry pelleted feeds with lots of fines 1980 Extruded feeds 1990
Evolution of fish feeds: Pelleting Process Moist feeds; beef liver, spleen 1960 Powder mixture Semi moist feeds; trash fish and powder 1970 Dry pelleted feeds with lots of fines 1980 Extruded feeds 1990
USE BLUE OR BLACK INK ONLY. 1c. ARE YOU THE NEW OWNER OF A PREVIOUSLY REGISTERED FACILITY? Yes O No O
 FDA USE ONLY USE BLUE OR BLACK INK ONLY Date: (MM/DD/YYYY) Section 1 - TYPE OF REGISTRATION 1a. O DOMESTIC REGISTRATION O FOREIGN REGISTRATION 1b. O INITIAL REGISTRATION O UPDATE OF REGISTRATION INFORMATION
FDA USE ONLY USE BLUE OR BLACK INK ONLY Date: (MM/DD/YYYY) Section 1 - TYPE OF REGISTRATION 1a. O DOMESTIC REGISTRATION O FOREIGN REGISTRATION 1b. O INITIAL REGISTRATION O UPDATE OF REGISTRATION INFORMATION
Components of an Effective Allergen Control Plan A FRAMEWORK FOR FOOD PROCESSORS
 Components of an Effective Allergen Control Plan A FRAMEWORK FOR FOOD PROCESSORS An Allergen Control Plan protects consumers and your company. When a food safety issue due to mishandling of allergenic
Components of an Effective Allergen Control Plan A FRAMEWORK FOR FOOD PROCESSORS An Allergen Control Plan protects consumers and your company. When a food safety issue due to mishandling of allergenic
FOOD FACILITIES REGISTRATION OF. Protecting the U.S. Food Supply. What You Need to Know About
 U.S. Food and Drug Administration Protecting the U.S. Food Supply U.S. Department of Health and Human Services What You Need to Know About REGISTRATION OF FOOD FACILITIES FDA Food Security Information
U.S. Food and Drug Administration Protecting the U.S. Food Supply U.S. Department of Health and Human Services What You Need to Know About REGISTRATION OF FOOD FACILITIES FDA Food Security Information
FDA Inspection Observations The FDA 483 and Beyond. Objectives
 FDA Inspection Observations The FDA 483 and Beyond Presenter: David L. Chesney Vice President and Practice Lead PAREXEL Consulting, Waltham, MA Dave.chesney@parexel.com Objectives Describe history and
FDA Inspection Observations The FDA 483 and Beyond Presenter: David L. Chesney Vice President and Practice Lead PAREXEL Consulting, Waltham, MA Dave.chesney@parexel.com Objectives Describe history and
Complying with the Philadelphia Trans Fat Ban
 Complying with the Philadelphia Trans Fat Ban A Guide for Restaurants, Caterers, Mobile Food-Vending Units and Other Food Service Establishments `` The Philadelphia City Council passed legislation in February
Complying with the Philadelphia Trans Fat Ban A Guide for Restaurants, Caterers, Mobile Food-Vending Units and Other Food Service Establishments `` The Philadelphia City Council passed legislation in February
Food Safety Modernization Act Sanitary Food Transportation Act Proposed Rule. Erik R. Lieberman February 18, 2014
 Food Safety Modernization Act Sanitary Food Transportation Act Proposed Rule Erik R. Lieberman February 18, 2014 Background During the late 1980s, there were a number of press reports that trucks that
Food Safety Modernization Act Sanitary Food Transportation Act Proposed Rule Erik R. Lieberman February 18, 2014 Background During the late 1980s, there were a number of press reports that trucks that
Agribusiness Presentation Scott McCain, President & COO, Agribusiness Group
 Agribusiness Presentation Scott McCain, President & COO, Agribusiness Group 1 Scott McCain President & COO, Agribusiness Group President and COO, Maple Leaf Agribusiness Group since 1995 Over 25 years
Agribusiness Presentation Scott McCain, President & COO, Agribusiness Group 1 Scott McCain President & COO, Agribusiness Group President and COO, Maple Leaf Agribusiness Group since 1995 Over 25 years
Fish Of The Order Siluriformes And Their Importance To FishCommercial Investigation Program
 UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS NOTICE 09-16 2/3/16 OFFICE OF INVESTIGATION, ENFORCEMENT AND AUDIT (OIEA) RESPONSIBILITIES RELATED TO SILURIFORMES
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS NOTICE 09-16 2/3/16 OFFICE OF INVESTIGATION, ENFORCEMENT AND AUDIT (OIEA) RESPONSIBILITIES RELATED TO SILURIFORMES
Road Haulage. Code of Practice. Contents
 Road Haulage Code of Practice Contents 1. General Overview 3 2. Commitment 4 3. Traceability 5 4. Safety 5 5. Hygiene 5 6. Collection and Delivery 7 7. Sub-Contractors 7 8. Training 8 9. Conditions of
Road Haulage Code of Practice Contents 1. General Overview 3 2. Commitment 4 3. Traceability 5 4. Safety 5 5. Hygiene 5 6. Collection and Delivery 7 7. Sub-Contractors 7 8. Training 8 9. Conditions of
Veterinary Compounding
 Veterinary Compounding Veterinarians occasionally use compounded preparations to meet a specific patient s medical need. The purpose of this brochure, created jointly by the Animal Health Institute (AHI),
Veterinary Compounding Veterinarians occasionally use compounded preparations to meet a specific patient s medical need. The purpose of this brochure, created jointly by the Animal Health Institute (AHI),
Keeping It Legal: Regulations and Licenses for Growing and Selling Food in Oregon
 Keeping It Legal: Regulations and Licenses for Growing and Selling Food in Oregon Adapted from Growing Farms Online, a comprehensive training program for beginning farmers, from the OSU Small Farms Program.
Keeping It Legal: Regulations and Licenses for Growing and Selling Food in Oregon Adapted from Growing Farms Online, a comprehensive training program for beginning farmers, from the OSU Small Farms Program.
Land O Lakes Feed DDGS. Nutrients Concentrate: United States Ethanol Outlook. A Growing Opportunity
 DDGS A Growing Opportunity Dr. Harold Tilstra Region Manager Land O Lakes Feed hdtilstra@landolakes.com 4/9/2004 Land O' Lakes Feed; Tilstra 2 Land O Lakes Feed Vision: To To be the leading animal nutrition
DDGS A Growing Opportunity Dr. Harold Tilstra Region Manager Land O Lakes Feed hdtilstra@landolakes.com 4/9/2004 Land O' Lakes Feed; Tilstra 2 Land O Lakes Feed Vision: To To be the leading animal nutrition
FSIS Security Guidelines for Food Processors
 United States Department of Agriculture Food Safety and Inspection Service FSIS Security Guidelines for Food Processors Food Security Plan Management Dear Establishment Owner/Operator: The Food Safety
United States Department of Agriculture Food Safety and Inspection Service FSIS Security Guidelines for Food Processors Food Security Plan Management Dear Establishment Owner/Operator: The Food Safety
It s all about managing food. Food Recall Plan Template For Food Distributors
 It s all about managing food. Food Recall Plan Template For Food Distributors What to Do In The Event Of a Product Recall Every Food Distributor and Food Manufacturer must track the products they manufacture
It s all about managing food. Food Recall Plan Template For Food Distributors What to Do In The Event Of a Product Recall Every Food Distributor and Food Manufacturer must track the products they manufacture
Horse Meat Production in Canada
 Horse Meat Production in Canada How can Canadian consumers be sure that the meat they are buying is exactly what is stated on the package (i.e. if it is labelled beef, then it only contains beef, not horse
Horse Meat Production in Canada How can Canadian consumers be sure that the meat they are buying is exactly what is stated on the package (i.e. if it is labelled beef, then it only contains beef, not horse
Food Production (Dairy Food Safety Scheme) Amendment (Goat and Sheep Dairy Products) Regulation 2003
 New South Wales Food Production (Dairy Food Safety Scheme) Amendment (Goat and Sheep Dairy Products) Regulation 2003 under the Food Production (Safety) Act 1998 Her Excellency the Governor, with the advice
New South Wales Food Production (Dairy Food Safety Scheme) Amendment (Goat and Sheep Dairy Products) Regulation 2003 under the Food Production (Safety) Act 1998 Her Excellency the Governor, with the advice
Industry Best Practices for Holding Tested Products
 Industry Best Practices for Holding Tested Products Coordinated By: American Association of Meat Processors American Meat Institute Food Products Association National Chicken Council National Meat Association
Industry Best Practices for Holding Tested Products Coordinated By: American Association of Meat Processors American Meat Institute Food Products Association National Chicken Council National Meat Association
SALES AND USE TAX TECHNICAL BULLETINS SECTION 7 PRINTERS, NEWSPAPER OR MAGAZINE PUBLISHERS AND BOOKBINDERS
 SECTION 7 - PRINTERS, NEWSPAPER OR MAGAZINE PUBLISHERS AND BOOKBINDERS 7-1 COMMERCIAL PRINTERS AND PUBLISHERS A. All retail sales of tangible personal property by commercial printers or publishers are
SECTION 7 - PRINTERS, NEWSPAPER OR MAGAZINE PUBLISHERS AND BOOKBINDERS 7-1 COMMERCIAL PRINTERS AND PUBLISHERS A. All retail sales of tangible personal property by commercial printers or publishers are
The latest updates are in blue. 11/3/00;Revised 11/8/00 1 C:\WINDOWS\Desktop\StarLink\ActionC1 00-11-08.doc
 The latest updates are in blue. 11/3/00;Revised 11/8/00 1 C:\WINDOWS\Desktop\StarLink\ActionC1 00-11-08.doc ACTION CHECKLIST FOR STARLINK CORN PRODUCERS AND ELEVATORS DR. CHARLES R. HURBURGH, JR. Background
The latest updates are in blue. 11/3/00;Revised 11/8/00 1 C:\WINDOWS\Desktop\StarLink\ActionC1 00-11-08.doc ACTION CHECKLIST FOR STARLINK CORN PRODUCERS AND ELEVATORS DR. CHARLES R. HURBURGH, JR. Background
Food Defense Self-Assessment Checklist for. Slaughter and Processing Plants
 Food Defense Self-Assessment Checklist for Slaughter and Processing Plants Outside Security 1. What food defense measures does your plant have in place for the exterior of the building? Are the plant s
Food Defense Self-Assessment Checklist for Slaughter and Processing Plants Outside Security 1. What food defense measures does your plant have in place for the exterior of the building? Are the plant s
The Sheep Safety and Quality Assurance Program. Melissa Garrod-VanLaningham, DVM
 The Sheep Safety and Quality Assurance Program Melissa Garrod-VanLaningham, DVM THE MISSION OF SSQA Maximize consumer confidence in, and acceptance of, sheep products by using research and education to
The Sheep Safety and Quality Assurance Program Melissa Garrod-VanLaningham, DVM THE MISSION OF SSQA Maximize consumer confidence in, and acceptance of, sheep products by using research and education to
Feeding Flood-Damaged or Sprouted Crops to Livestock
 Feeding Flood-Damaged or Sprouted Crops to Livestock High moisture conditions often lead to crop damage and losses. Some losses are from grain sprouting in the head before harvest. These sprouted grains
Feeding Flood-Damaged or Sprouted Crops to Livestock High moisture conditions often lead to crop damage and losses. Some losses are from grain sprouting in the head before harvest. These sprouted grains
10 GUIDANCE NOTE. Product Recall and Traceability (Revision 3) Guidance Note No. 10: Product Recall and Traceability (Revision 3)
 10 GUIDANCE NOTE Product Recall and Traceability (Revision 3) Guidance Note No. 10: Product Recall and Traceability (Revision 3) B Guidance Note No. 10 Product Recall and Traceability (Revision 3) Published
10 GUIDANCE NOTE Product Recall and Traceability (Revision 3) Guidance Note No. 10: Product Recall and Traceability (Revision 3) B Guidance Note No. 10 Product Recall and Traceability (Revision 3) Published
Check that you have the correct paper. Please complete the information above. You do not need to use complete sentences for the reading assessment.
 Learner name Learner registration number Learner signature Centre Assessment date Functional Skills English Assessment Reading Level 1 NOCN USE ONLY Question Mark 1 2 3 4 5 6 7 Total Instructions to candidates
Learner name Learner registration number Learner signature Centre Assessment date Functional Skills English Assessment Reading Level 1 NOCN USE ONLY Question Mark 1 2 3 4 5 6 7 Total Instructions to candidates
Acid, Acidified and Low-acid Foods
 EC 705 April 2000 Acid, Acidified and Low-acid Foods Canning Guidelines for Food Processors Department of Food Science and Human Nutrition College of Agriculture, Forestry & Life Sciences Clemson University
EC 705 April 2000 Acid, Acidified and Low-acid Foods Canning Guidelines for Food Processors Department of Food Science and Human Nutrition College of Agriculture, Forestry & Life Sciences Clemson University
Sales tax must be computed on the total receipt of $549.95.
 New York State Department of Taxation and Finance Taxpayer Services Division Technical Services Bureau DELIVERY CHARGE ADDED TO TAXABLE RECEIPT EFFECTIVE SEPTEMBER 1, 1991 The sales tax law had provided,
New York State Department of Taxation and Finance Taxpayer Services Division Technical Services Bureau DELIVERY CHARGE ADDED TO TAXABLE RECEIPT EFFECTIVE SEPTEMBER 1, 1991 The sales tax law had provided,
How To Control Veterinary Drug Use
 CAC/GL 71-2009 page 1 of 38 GUIDELINES FOR THE DESIGN AND IMPLEMENTATION OF NATIONAL REGULATORY FOOD SAFETY ASSURANCE PROGRAMME ASSOCIATED WITH THE USE OF VETERINARY DRUGS IN FOOD PRODUCING ANIMALS CAC/GL
CAC/GL 71-2009 page 1 of 38 GUIDELINES FOR THE DESIGN AND IMPLEMENTATION OF NATIONAL REGULATORY FOOD SAFETY ASSURANCE PROGRAMME ASSOCIATED WITH THE USE OF VETERINARY DRUGS IN FOOD PRODUCING ANIMALS CAC/GL
Voice: 402.223.2411 Fax: 402.223.2401
 Hello!! Thank you for checking out our website. I founded Eclipse Transervices Corpora on in 1992 near Virginia, Nebraska. In August of 1997, we moved our opera on to Beatrice, Nebraska. We offer a variety
Hello!! Thank you for checking out our website. I founded Eclipse Transervices Corpora on in 1992 near Virginia, Nebraska. In August of 1997, we moved our opera on to Beatrice, Nebraska. We offer a variety
How To Control A Dog
 TITLE 8: AGRICULTURE AND ANIMALS CHAPTER I: DEPARTMENT OF AGRICULTURE : ANIMALS AND ANIMAL PRODUCTS (EXCEPT MEAT AND POULTRY INSPECTION ACT REGULATIONS) PART 30 ANIMAL CONTROL ACT Section 30.10 Definitions
TITLE 8: AGRICULTURE AND ANIMALS CHAPTER I: DEPARTMENT OF AGRICULTURE : ANIMALS AND ANIMAL PRODUCTS (EXCEPT MEAT AND POULTRY INSPECTION ACT REGULATIONS) PART 30 ANIMAL CONTROL ACT Section 30.10 Definitions
impact analysis (PRIA); the PRIA is available at http://www.regulations.gov Docket No.
 Preliminary Regulatory Impact Analysis for the proposed rule on Sanitary Transportation of Human and Animal Food (Docket No. FDA-2013-N-0013) under Executive Order 12866, Executive Order 13563, the Regulatory
Preliminary Regulatory Impact Analysis for the proposed rule on Sanitary Transportation of Human and Animal Food (Docket No. FDA-2013-N-0013) under Executive Order 12866, Executive Order 13563, the Regulatory
Manufacturing Cost Annual. California 2011 Data
 Manufacturing Cost Annual California 2011 Data C A L I F O R N I A Manufacturing Cost Annual 2011 Data Table of Contents Manufacturing Cost Overview Introduction... 3 Manufacturing Cost Overview... 4 Butter
Manufacturing Cost Annual California 2011 Data C A L I F O R N I A Manufacturing Cost Annual 2011 Data Table of Contents Manufacturing Cost Overview Introduction... 3 Manufacturing Cost Overview... 4 Butter
Feed Mill Operations & Key Performance Indicators. Charles Stark North Carolina State University
 Feed Mill Operations & Key Performance Indicators Charles Stark North Carolina State University Major Areas of Responsibilities Production Quality Costs Safety Housekeeping Human Resources Strategic Planning/Cost
Feed Mill Operations & Key Performance Indicators Charles Stark North Carolina State University Major Areas of Responsibilities Production Quality Costs Safety Housekeeping Human Resources Strategic Planning/Cost
- 1 - First Time Pharmacy Managers (Revised 02/02/2011)
 State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
State of Connecticut Department of Consumer Protection Commission of Pharmacy 165 Capitol Avenue, Room 147 Hartford, CT 06106 - Telephone: 860-713-6070 ALL FIRST-TIME PHARMACY MANAGERS ARE REQUIRED TO
HOW TO IMPORT FOREIGN SOIL and HOW TO MOVE SOIL within the UNITED STATES
 Circular Q-330.300-2 Soil (01/2001) U.S. Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine 4700 River Road, Unit 133 Riverdale, Maryland 20737-1228 HOW
Circular Q-330.300-2 Soil (01/2001) U.S. Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine 4700 River Road, Unit 133 Riverdale, Maryland 20737-1228 HOW
Food Safety Guidance for Farmers Markets
 Food Safety Guidance for Farmers Markets Anyone selling food or drink from a market stall must comply with food hygiene legislation. Foods which are categorised as high risk include cooked meats, fish
Food Safety Guidance for Farmers Markets Anyone selling food or drink from a market stall must comply with food hygiene legislation. Foods which are categorised as high risk include cooked meats, fish
Selenium and Selenium Yeast Use in Feed. Division of Regulatory Services University of Kentucky April 25, 2005
 Selenium and Selenium Yeast Use in Feed Division of Regulatory Services University of Kentucky April 25, 2005 REVISED JULY 19, 2007 Meagan Davis, Feed Registration Specialist Selenium, long known for its
Selenium and Selenium Yeast Use in Feed Division of Regulatory Services University of Kentucky April 25, 2005 REVISED JULY 19, 2007 Meagan Davis, Feed Registration Specialist Selenium, long known for its
WORLD BANK STUDY Livestock and Slaughter Waste Management. Presentation 2 Conditions at Slaughterhouses
 WORLD BANK STUDY Livestock and Slaughter Waste Management Workshop at World Bank H.Q. 27 February 2008 Presentation 2 Conditions at Slaughterhouses NIPPON KOEI Co. Ltd. in association with ProAnd Associates
WORLD BANK STUDY Livestock and Slaughter Waste Management Workshop at World Bank H.Q. 27 February 2008 Presentation 2 Conditions at Slaughterhouses NIPPON KOEI Co. Ltd. in association with ProAnd Associates
Subject: Lot, Batch & Serial # System - 1 - Created QC/ Edited by Paul DiMarco Approval/Effective Date: 01/01/2002
 - 1-1.) Scope: This procedure covers the issuance and use of Lot, batch and serial numbers for Certificate of Analysis, Batch Records and Product Labels. 2.) Normative References: SOP 4-2-2 Quality Manual
- 1-1.) Scope: This procedure covers the issuance and use of Lot, batch and serial numbers for Certificate of Analysis, Batch Records and Product Labels. 2.) Normative References: SOP 4-2-2 Quality Manual
Guidelines and Good Use Practices for the Use of Seed Applied Products and of Treated Seed (May 2014)
 Guidelines and Good Use Practices for the Use of Seed Applied Products and of Treated Seed (May 2014) Prepared by the Seed Applied Technologies Committee (SAT-Com) of the International Seed Federation
Guidelines and Good Use Practices for the Use of Seed Applied Products and of Treated Seed (May 2014) Prepared by the Seed Applied Technologies Committee (SAT-Com) of the International Seed Federation
Grayson Farmers Market 2015 June 3 - October 28, 2015 Connecting Friends, Food, and Farmers
 Grayson Farmers Market 2015 June 3 - October 28, 2015 Connecting Friends, Food, and Farmers Please find below the application for the 2015 Season of the Grayson Farmers Market located 475 Grayson Parkway,
Grayson Farmers Market 2015 June 3 - October 28, 2015 Connecting Friends, Food, and Farmers Please find below the application for the 2015 Season of the Grayson Farmers Market located 475 Grayson Parkway,
phasing out ARTIFICIAL TRANS FAT How to Comply: What Restaurants, Caterers, Mobile Food Vendors, and Others Need to Know
 phasing out ARTIFICIAL TRANS FAT in Cambridge Food Service Establishments How to Comply: What Restaurants, Caterers, Mobile Food Vendors, and Others Need to Know SUMMARY OF REGULATION In 2009, the City
phasing out ARTIFICIAL TRANS FAT in Cambridge Food Service Establishments How to Comply: What Restaurants, Caterers, Mobile Food Vendors, and Others Need to Know SUMMARY OF REGULATION In 2009, the City
ENVIRONMENTAL HEALTH AND SAFETY HAZARDOUS MATERIALS MANAGEMENT PLAN
 ENVIRONMENTAL HEALTH AND SAFETY HAZARDOUS MATERIALS MANAGEMENT PLAN November 2011 University of Northern Colorado Hazardous Materials Management Plan I. General II. III. IV. Responsibilities Definition
ENVIRONMENTAL HEALTH AND SAFETY HAZARDOUS MATERIALS MANAGEMENT PLAN November 2011 University of Northern Colorado Hazardous Materials Management Plan I. General II. III. IV. Responsibilities Definition
NORTH CAROLINA COOPERATIVE EXTENSION SERVICE. North Carolina State University. College of Agriculture & Life Sciences
 1 of 14 2/12/2013 2:13 PM NORTH CAROLINA COOPERATIVE EXTENSION SERVICE North Carolina State University College of Agriculture & Life Sciences In our health-conscious society, consumers are becoming increasingly
1 of 14 2/12/2013 2:13 PM NORTH CAROLINA COOPERATIVE EXTENSION SERVICE North Carolina State University College of Agriculture & Life Sciences In our health-conscious society, consumers are becoming increasingly
FLORIDA HAZARDOUS WASTE MANAGEMENT REGULATIONS THAT DIFFER FROM FEDERAL REQUIREMENTS
 FLORIDA HAZARDOUS WASTE MANAGEMENT REGULATIONS THAT DIFFER FROM FEDERAL REQUIREMENTS Chapter 1: Introduction General Notes Lead Agency: Florida Department of Environmental Protection (DEP) Division of
FLORIDA HAZARDOUS WASTE MANAGEMENT REGULATIONS THAT DIFFER FROM FEDERAL REQUIREMENTS Chapter 1: Introduction General Notes Lead Agency: Florida Department of Environmental Protection (DEP) Division of
GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM
 GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM FEBRUARY 2016 2 Contents Introduction... 4 Scope and objectives... 5 Scope... 5 Objectives... 5 Responsibilities... 5 The role of the licensee
GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM FEBRUARY 2016 2 Contents Introduction... 4 Scope and objectives... 5 Scope... 5 Objectives... 5 Responsibilities... 5 The role of the licensee
SECTION 6. The Codex code of practice on good animal feeding
 SECTION 6 The Codex code of practice on good animal feeding 60 The Codex code of practice on good animal feeding SECTION 6 61 CODE OF PRACTICE ON GOOD ANIMAL FEEDING CAC/RCP 54-2004 SECTION 1. INTRODUCTION
SECTION 6 The Codex code of practice on good animal feeding 60 The Codex code of practice on good animal feeding SECTION 6 61 CODE OF PRACTICE ON GOOD ANIMAL FEEDING CAC/RCP 54-2004 SECTION 1. INTRODUCTION
Triangle Compounding 11/2/15
 Triangle Compounding 11/2/15 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District 60 Eighth Street NE Atlanta, GA 30309 November 2, 2015 VIA UNITED
Triangle Compounding 11/2/15 Department of Health and Human Services Public Health Service Food and Drug Administration Atlanta District 60 Eighth Street NE Atlanta, GA 30309 November 2, 2015 VIA UNITED
FOOD SAFETY SYSTEM CERTIFICATION 22000 FSSC 22000
 FOOD SAFETY SYSTEM CERTIFICATION 22000 FSSC 22000 Certification scheme for food safety systems in compliance with ISO 22000: 2005 and technical specifications for sector PRPs Features Foundation for Food
FOOD SAFETY SYSTEM CERTIFICATION 22000 FSSC 22000 Certification scheme for food safety systems in compliance with ISO 22000: 2005 and technical specifications for sector PRPs Features Foundation for Food
Potentially Infectious Medical Waste
 Potentially Infectious Medical Waste A Summary of Regulatory Requirements General Requirements Title XV of the Illinois Environmental Protection Act (Act) establishes statutory requirements to ensure that
Potentially Infectious Medical Waste A Summary of Regulatory Requirements General Requirements Title XV of the Illinois Environmental Protection Act (Act) establishes statutory requirements to ensure that
CORN BY-PRODUCTS IN DAIRY COW RATIONS
 CORN BY-PRODUCTS IN DAIRY COW RATIONS Dennis Lunn, Ruminant Nutritionist Shur-Gain, Nutreco Canada Inc. CORN BY-PRODUCTS IN DAIRY COW RATIONS Dennis Lunn, Ruminant Nutritionist Shur-Gain, Nutreco Canada
CORN BY-PRODUCTS IN DAIRY COW RATIONS Dennis Lunn, Ruminant Nutritionist Shur-Gain, Nutreco Canada Inc. CORN BY-PRODUCTS IN DAIRY COW RATIONS Dennis Lunn, Ruminant Nutritionist Shur-Gain, Nutreco Canada
December 31, 2015. comments to the Office of Dietary Supplements (ODS) at the National Institutes of Health on the
 December 31, 2015 VIA ELECTRONIC SUBMISSION Richard Bailen, MBA, MHA Office of Dietary Supplements National Institutes of Health 6100 Executive Boulevard, Room 3B01 Bethesda, MD 20892-7517 Email: ODS@nih.gov
December 31, 2015 VIA ELECTRONIC SUBMISSION Richard Bailen, MBA, MHA Office of Dietary Supplements National Institutes of Health 6100 Executive Boulevard, Room 3B01 Bethesda, MD 20892-7517 Email: ODS@nih.gov
SILICON VALLEY CLEAN WATER. May 2015
 SILICON VALLEY CLEAN WATER May 2015 Slug Discharge Control and Spill Containment Guidelines This document was revised and used with the permission of the Los Angeles County Sanitation District, Industrial
SILICON VALLEY CLEAN WATER May 2015 Slug Discharge Control and Spill Containment Guidelines This document was revised and used with the permission of the Los Angeles County Sanitation District, Industrial
UDOT SPILL PREVENTION and RESPONSE PLAN for CONSTRUCTION SITES
 UDOT SPILL PREVENTION and RESPONSE PLAN for CONSTRUCTION SITES February 2014 The plan contained in the following pages was developed in part from UDOT Construction Division s Safety and Health Manual,
UDOT SPILL PREVENTION and RESPONSE PLAN for CONSTRUCTION SITES February 2014 The plan contained in the following pages was developed in part from UDOT Construction Division s Safety and Health Manual,
PROCESS FLOW DIAGRAM Figure 1
 PROCESS FLOW DIAGRAM Figure 1 PROCESS CATEGORY: SLAUGHTER PRODUCT: BEEF RECEIVING PACKAGING MATERIALS RECEIVING LIVE CATTLE STUNNING/BLEEDING HEAD/SHANK REMOVAL SKINNING EVISCERATION SPLITTING VISCERA
PROCESS FLOW DIAGRAM Figure 1 PROCESS CATEGORY: SLAUGHTER PRODUCT: BEEF RECEIVING PACKAGING MATERIALS RECEIVING LIVE CATTLE STUNNING/BLEEDING HEAD/SHANK REMOVAL SKINNING EVISCERATION SPLITTING VISCERA
The U.S. Trichinella Certification Program
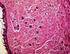 The U.S. Trichinella Certification Program H. Ray Gamble U.S. National Academies 500 Fifth Street NW Washington, DC 20001 rgamble@nas.edu 1-202-334-2787 Pre-Slaughter Control (Prevention) of Trichinella
The U.S. Trichinella Certification Program H. Ray Gamble U.S. National Academies 500 Fifth Street NW Washington, DC 20001 rgamble@nas.edu 1-202-334-2787 Pre-Slaughter Control (Prevention) of Trichinella
Texas Correlations CEV Pathway: Food Products/Processing Systems
 Pathways for Career Success Texas Correlations CEV Pathway: Food Products/Processing Systems Meets 100% of TEKS standards for: Correlations For: Food Products/Processing Systems Pathway New Course: Subject
Pathways for Career Success Texas Correlations CEV Pathway: Food Products/Processing Systems Meets 100% of TEKS standards for: Correlations For: Food Products/Processing Systems Pathway New Course: Subject
Apple Juice Concentrate (AJC) Manual
 Apple Juice Concentrate (AJC) Manual Minneapolis Grain Exchange, Inc. 400 South 4 th Street Minneapolis, MN 55415 www.mgex.com AJC Manual Table of Contents The below table of contents is being supplied
Apple Juice Concentrate (AJC) Manual Minneapolis Grain Exchange, Inc. 400 South 4 th Street Minneapolis, MN 55415 www.mgex.com AJC Manual Table of Contents The below table of contents is being supplied
Southern California Edison Company [SAMPLE AGREEMENT] PURCHASE ORDER EVAPORATIVE COOLER BULK PURCHASE GENERAL DESCRIPTION
![Southern California Edison Company [SAMPLE AGREEMENT] PURCHASE ORDER EVAPORATIVE COOLER BULK PURCHASE GENERAL DESCRIPTION Southern California Edison Company [SAMPLE AGREEMENT] PURCHASE ORDER EVAPORATIVE COOLER BULK PURCHASE GENERAL DESCRIPTION](/thumbs/40/21156222.jpg) Page 1 of 4 [SAMPLE AGREEMENT] DATE PURCHASE ORDER NO. PURCHASE ORDER EVAPORATIVE COOLER BULK PURCHASE SUPPLIER CODE COMM/SERV. CODE SHIP TO: REQUISITION NO. ACCOUNTING LOC.NO. MARK FOR: Mail the copy
Page 1 of 4 [SAMPLE AGREEMENT] DATE PURCHASE ORDER NO. PURCHASE ORDER EVAPORATIVE COOLER BULK PURCHASE SUPPLIER CODE COMM/SERV. CODE SHIP TO: REQUISITION NO. ACCOUNTING LOC.NO. MARK FOR: Mail the copy
11 III. Create additional market opportunities for New Hampshire producers who are not
 Rep. Moynihan, Coos 2 October, 13 13-23h 08/ Amendment to HB 660-FN 1 Amend the bill by replacing all after the enacting clause with the following: 3 1 New Subdivision; Genetically Engineered. Foods. Amend.
Rep. Moynihan, Coos 2 October, 13 13-23h 08/ Amendment to HB 660-FN 1 Amend the bill by replacing all after the enacting clause with the following: 3 1 New Subdivision; Genetically Engineered. Foods. Amend.
MEAT CONTENT CALCULATION
 MEAT CONTENT CALCULATION Introduction Amendments to the European Labelling Directive have resulted in the need to harmonise the definition of meat across Europe. This new definition attempts to ensure
MEAT CONTENT CALCULATION Introduction Amendments to the European Labelling Directive have resulted in the need to harmonise the definition of meat across Europe. This new definition attempts to ensure
Trade Rules of the National Hay Association U.S. FORAGE EXPORT COUNCIL
 Trade Rules of the National Hay Association U.S. FORAGE EXPORT COUNCIL Adopted May 6, 2014 All Active members and other parties using these rules are free to agree upon any contractual provisions that
Trade Rules of the National Hay Association U.S. FORAGE EXPORT COUNCIL Adopted May 6, 2014 All Active members and other parties using these rules are free to agree upon any contractual provisions that
I-Track Software. A state of the art production and warehouse management system designed for Food and Beverage Manufacturers. Overview 2.
 Overview 2 Features 3 Benefits 4 I-Track Software A state of the art production and warehouse management system designed for Food and Beverage Manufacturers Site Assessment 5 Integrated Plant Floor Execution
Overview 2 Features 3 Benefits 4 I-Track Software A state of the art production and warehouse management system designed for Food and Beverage Manufacturers Site Assessment 5 Integrated Plant Floor Execution
The Colorado State Patrol Commercial Vehicle Safety Units --------------------------------------------------- January 2011
 The Colorado State Patrol Commercial Vehicle Safety Units --------------------------------------------------- January 2011 Intrastate Commercial Vehicle Operations For Farmers and Ranchers Colorado State
The Colorado State Patrol Commercial Vehicle Safety Units --------------------------------------------------- January 2011 Intrastate Commercial Vehicle Operations For Farmers and Ranchers Colorado State
Quality Agreement. by and between. Supplier Name. Address: and. Client Name: Address:
 NOTE TO USERS This Quality Agreement template was developed by the Bulk Pharmaceutical Task Force (BPTF), an affiliate organization of the Society of Chemical Manufacturers and Affiliates (SOCMA), as a
NOTE TO USERS This Quality Agreement template was developed by the Bulk Pharmaceutical Task Force (BPTF), an affiliate organization of the Society of Chemical Manufacturers and Affiliates (SOCMA), as a
ALTERNATIVE ABP PROCESSES AND
 ALTERNATIVE ABP PROCESSES AND TSE/BSE E. VANOPDENBOSCH, F. BARIZZONE BIOLOGICAL HAZARDS (BIOHAZ) - EFSA Outline What EFSA does How EFSA Scientific Opinions are issued Legislation in force Responsibility
ALTERNATIVE ABP PROCESSES AND TSE/BSE E. VANOPDENBOSCH, F. BARIZZONE BIOLOGICAL HAZARDS (BIOHAZ) - EFSA Outline What EFSA does How EFSA Scientific Opinions are issued Legislation in force Responsibility
General Legal Requirements A guide for pharmacists in Northern Ireland 2010 Edition. Medicines for Veterinary Use
 General Legal Requirements A guide for pharmacists in Northern Ireland 2010 Edition 5 Medicines for Veterinary Use 5 MEDICINES FOR VETERINARY USE The Veterinary Medicines Regulations SI No. 2297 came into
General Legal Requirements A guide for pharmacists in Northern Ireland 2010 Edition 5 Medicines for Veterinary Use 5 MEDICINES FOR VETERINARY USE The Veterinary Medicines Regulations SI No. 2297 came into
Texas Regulations on Medical Waste
 TCEQ REGULATORY GUIDANCE Waste Permits Division RG-001 Revised September 2012 Texas Regulations on Medical Waste This is a regulatory guide to assist generators of medical waste, transporters of untreated
TCEQ REGULATORY GUIDANCE Waste Permits Division RG-001 Revised September 2012 Texas Regulations on Medical Waste This is a regulatory guide to assist generators of medical waste, transporters of untreated
Kroger Manufacturing Trailer Seal Policy Revised 09/03/10
 Kroger Manufacturing Trailer Seal Policy Revised 09/03/10 1 Inbound Supplier After the product is loaded and the trailer doors are closed, the supplier (or shipping warehouse) is responsible for affixing
Kroger Manufacturing Trailer Seal Policy Revised 09/03/10 1 Inbound Supplier After the product is loaded and the trailer doors are closed, the supplier (or shipping warehouse) is responsible for affixing
Austin Used Cooking Oil Initiative Voluntary Compliance Standards
 Austin Used Cooking Oil Initiative Voluntary Compliance Standards The City of Austin invites Used Cooking Oil Generators - including brick-and-mortar restaurants, mobile food vendors and other food service
Austin Used Cooking Oil Initiative Voluntary Compliance Standards The City of Austin invites Used Cooking Oil Generators - including brick-and-mortar restaurants, mobile food vendors and other food service
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC
 UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS DIRECTIVE 5420.4, Revision 3 9/15/06 HOMELAND SECURITY THREAT CONDITION RESPONSE - EMERGENCY PROCEDURES FOR
UNITED STATES DEPARTMENT OF AGRICULTURE FOOD SAFETY AND INSPECTION SERVICE WASHINGTON, DC FSIS DIRECTIVE 5420.4, Revision 3 9/15/06 HOMELAND SECURITY THREAT CONDITION RESPONSE - EMERGENCY PROCEDURES FOR
Independent Contractor Policy
 Independent Contractor Policy This Policy has been implemented to prevent accidents and personal injuries. It is not intended to be entirely inclusive. It is the responsibility of the Independent Contractor
Independent Contractor Policy This Policy has been implemented to prevent accidents and personal injuries. It is not intended to be entirely inclusive. It is the responsibility of the Independent Contractor
HACCP: Hazard Analysis Critical Control Points. Dr. Angela Shaw Department of Food Science and Human Nutrition Extension and Outreach
 HACCP: Hazard Analysis Critical Control Points Dr. Angela Shaw Department of Food Science and Human Nutrition Extension and Outreach Information Adapted from: Hazard Analysis and Critical Control Point
HACCP: Hazard Analysis Critical Control Points Dr. Angela Shaw Department of Food Science and Human Nutrition Extension and Outreach Information Adapted from: Hazard Analysis and Critical Control Point
Methods of Making Sanitation Ratings Of Milk Shippers
 Methods of Making Sanitation Ratings Of Milk Shippers 2011 Revision U.S. Department of Health and Human Services Public Health Service Food and Drug Administration PREFACE The objective of a rating is
Methods of Making Sanitation Ratings Of Milk Shippers 2011 Revision U.S. Department of Health and Human Services Public Health Service Food and Drug Administration PREFACE The objective of a rating is
MARSHALL UNIVERSITY HAZARDOUS WASTE DISPOSAL SECTION
 MARSHALL UNIVERSITY HAZARDOUS WASTE DISPOSAL SECTION TABLE OF CONTENTS POLICY STATEMENT.2 REFERENCE PROCEDURE 3 l.0 Purpose.3 2.0 Scope 3 3.0 Definitions 4 4.0 Responsibilities 5 5.0 Procedure.7 11/16/2005
MARSHALL UNIVERSITY HAZARDOUS WASTE DISPOSAL SECTION TABLE OF CONTENTS POLICY STATEMENT.2 REFERENCE PROCEDURE 3 l.0 Purpose.3 2.0 Scope 3 3.0 Definitions 4 4.0 Responsibilities 5 5.0 Procedure.7 11/16/2005
Scaling Up For Regional Markets Grading Standards and Wholesale Glossary of Terms
 Scaling Up For Regional Markets Grading Standards and Wholesale Glossary of Terms Wholesale food markets often use terms and phrases unique to the industry. To understand a market report, communicate with
Scaling Up For Regional Markets Grading Standards and Wholesale Glossary of Terms Wholesale food markets often use terms and phrases unique to the industry. To understand a market report, communicate with
9.1 OBJECTIVE... 9-1 9.2 SCOPE... 9-1 9.3 REQUIRED FORMS AND REFERENCES... 9-1. 9.3.1 Forms... 9-1. 9.3.2 References... 9-1
 9.1 OBJECTIVE.................................................................. 9-1 9.2 SCOPE..................................................................... 9-1 9.3 REQUIRED FORMS AND REFERENCES..........................................
9.1 OBJECTIVE.................................................................. 9-1 9.2 SCOPE..................................................................... 9-1 9.3 REQUIRED FORMS AND REFERENCES..........................................
IACUC POLICIES, PROCEDURES, and GUIDELINES ADOPTION OF ANIMALS DESIGNATED FOR RESEARCH
 Page 1 of 7 IACUC POLICIES, PROCEDURES, and GUIDELINES ADOPTION OF ANIMALS DESIGNATED FOR RESEARCH 131.1 PURPOSE This document establishes policies and provides guidelines for adoption of research animals
Page 1 of 7 IACUC POLICIES, PROCEDURES, and GUIDELINES ADOPTION OF ANIMALS DESIGNATED FOR RESEARCH 131.1 PURPOSE This document establishes policies and provides guidelines for adoption of research animals
TRACEABILITY IN THE FOOD SUPPLY CHAIN
 Department of Health and Human Services OFFICE OF INSPECTOR GENERAL TRACEABILITY IN THE FOOD SUPPLY CHAIN Daniel R. Levinson Inspector General March 2009 Office of Evaluation and Inspections The Office
Department of Health and Human Services OFFICE OF INSPECTOR GENERAL TRACEABILITY IN THE FOOD SUPPLY CHAIN Daniel R. Levinson Inspector General March 2009 Office of Evaluation and Inspections The Office
TITLE II FOOD ALLERGEN LABELING AND CONSUMER PROTECTION
 118 STAT. 905 (T) Section 108(b)(3) of Public Law 90 399 is amended by striking section 201(w) as added by this Act and inserting section 201(v). (6) REGULATIONS. On the date of enactment of this Act,
118 STAT. 905 (T) Section 108(b)(3) of Public Law 90 399 is amended by striking section 201(w) as added by this Act and inserting section 201(v). (6) REGULATIONS. On the date of enactment of this Act,
Guide for Preparing SHIPPING PAPERS
 Guide for Preparing SHIPPING PAPERS One of the most frequently cited safety violations of the Hazardous Materials Regulations (HMR), Title 49 CFR Parts 100-185, is the failure of the shipper to properly
Guide for Preparing SHIPPING PAPERS One of the most frequently cited safety violations of the Hazardous Materials Regulations (HMR), Title 49 CFR Parts 100-185, is the failure of the shipper to properly
Investigational Drugs: Investigational Drugs and Biologics
 : I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
: I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
EOC 0002. Quality Through Compliance. Policies and Procedures. HAWAII HEALTH SYSTEMS C O R P O R A T I O N Touching Lives Everyday" N/A
 HAWAII HEALTH SYSTEMS C O R P O R A T I O N Touching Lives Everyday" Policies and Procedures Subject: Corporate Policy on Medical Waste Quality Through Compliance Issued by: Corporate Compliance Committee
HAWAII HEALTH SYSTEMS C O R P O R A T I O N Touching Lives Everyday" Policies and Procedures Subject: Corporate Policy on Medical Waste Quality Through Compliance Issued by: Corporate Compliance Committee
CHAIN OF CUSTODY GLOSSARY OF TERMS AND DEFINITIONS
 CHAIN OF CUSTODY GLOSSARY OF TERMS AND DEFINITIONS March 2014 Sustainable Agriculture Network and Rainforest Alliance, 2012-2014. This document is available at the following websites: www.sanstandards.org
CHAIN OF CUSTODY GLOSSARY OF TERMS AND DEFINITIONS March 2014 Sustainable Agriculture Network and Rainforest Alliance, 2012-2014. This document is available at the following websites: www.sanstandards.org
Pre-Approval Process INAD vs. NADA
 Pre-Approval Process INAD vs. NADA Barbara M. Leotta, DVM CVM/ONADE/DTDNFA (240)276-8262 barbara.leotta@fda.hhs.gov Pre-Approval Process General Overview Pathways To Legal Marketing Office of New Animal
Pre-Approval Process INAD vs. NADA Barbara M. Leotta, DVM CVM/ONADE/DTDNFA (240)276-8262 barbara.leotta@fda.hhs.gov Pre-Approval Process General Overview Pathways To Legal Marketing Office of New Animal
Self-Audit Checklist
 Page 1 Company Name: Date of audit: Date of last audit performed: Name of person performing self-audit: Signature: Name of person responsible for quality system: Signature: Number of non-compliances: Page
Page 1 Company Name: Date of audit: Date of last audit performed: Name of person performing self-audit: Signature: Name of person responsible for quality system: Signature: Number of non-compliances: Page
The certification process
 TS004(COS)v01 The certification process for COSMOS standard Standard in force available on www.cosmos-standard.org or sent on request. 1 Contents I. When to apply... 3 II. The different steps in the certification
TS004(COS)v01 The certification process for COSMOS standard Standard in force available on www.cosmos-standard.org or sent on request. 1 Contents I. When to apply... 3 II. The different steps in the certification
CODEX STANDARD FOR FOLLOW-UP FORMULA CODEX STAN 156-1987. This standard applies to the composition and labelling of follow-up formula.
 CODEX STAN 156-1987 Page 1 of 9 CODEX STANDARD FOR FOLLOW-UP FORMULA CODEX STAN 156-1987 1. SCOPE This standard applies to the composition and labelling of follow-up formula. It does not apply to foods
CODEX STAN 156-1987 Page 1 of 9 CODEX STANDARD FOR FOLLOW-UP FORMULA CODEX STAN 156-1987 1. SCOPE This standard applies to the composition and labelling of follow-up formula. It does not apply to foods
