The format of this leaflet was determined by the Ministry of Health and its content was checked and approved by it in April 2010.
|
|
|
- Sandra Thompson
- 7 years ago
- Views:
Transcription
1 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved by it in April Ultralan Cream Prescribing Information 1. NAME OF THE MEDICINAL PRODUCT U L T R A L A N 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Cream: 1 g contains 2.5 mg (0.25%) fluocortolone pivalate and 2.5 mg (0.25%) fluocortolone caproate. 3. PHARMACEUTICAL FORM Cream 4. CLINICAL PARTICULARS 4.1 Therapeutic indications: All skin diseases which respond to topical corticoid therapy, e.g.: - contact dermatitis, contact eczema - occupational eczema - vulgar, nummular, degenerative and seborrheic eczema - dishidrotic eczema - eczema in varicose syndrome (but not directly onto lower limb ulcers) - anal eczema - eczema in children - neurodermatitis (endogenous eczema, atopic dermatitis) - psoriasis - lichen ruber planus et verrucosus - lupus erythematosus discoides - first degree burns, sunburn, insect bites. 4.2 Dosage and method of administration At the commencement of treatment, Ultralan Cream is applied thinly twice or, in more severly affectd skin areas, three times per day. Once the clinical picture has improvedone application per day usually suffices. Babies and children up to the age of 4 years should not be treated with Ultralan Cream for longer than three weeks, particularly on skin areas covered by nappies. Ultralan Cream has a high water and low fat content. In weeping skin diseases it allows
2 -2- secretions to drain away, thus providing for rapid subsidence and drying up of the skin. Ultralan Cream is also suitable for application to moist, exposed and hairy areas of the body. Occlusive dressings In refractory cases an occlusive dressing may improve the efficacy of Ultralan Cream and should be managed as follows: After application of Ultralan Cream, the area under treatment should be covered with a plastic foil which should then be fixed firmly all round to healthy skin by means of adhesive plaster. Plastic gloves can be used to occlude the hands. The dressing should be kept in place for as long as can be expected of the patient, but generally not for longer than 24 hours. If the occlusive treatment is expected to be prolonged, it is advisable to change the dressing every 12 hours. If an infection develops under the dressing, occlusive treatment must be interrupted and a specific therapy instituted. (See also SPECIAL WARNINGS below). 4.3 Contra-indications Not suitable for the treatment of ophthalmic conditions. Tuberculous or syphilitic processes in the area to be treated; virus diseases (e.g. vaccinia, chickenpox, shingles). Acne vulgaris, undiagnosed perianal and genital pruritus, napkin eruptions, primary bacterial or fungal infections of the skin. Secondary infections in the absence of appropriate anti-infective therapy. 4.4 Special warnings and special precautions for use As with all topical steroids, there is a risk of skin atrophy following extensive therapy. Long-term continuous therapy should be avoided irrespective of age. Occlusion should be restricted to dermatoses involving limited areas. Adrenal suppresion can occur even without occlusion. If the skin dries out too much under protracted use of Ultralan Cream, the patient should be switched to a form which contains more fat. Ultralan Cream should not be allowed to come into contact with the eyes being applied to the face. If rosacea or perioral dermatitis is present, Ultralan Cream must not be applied to the face. Topical corticosteroids may be hazardous in psoriasis for a number of reasons including rebound relapses following development of tolerance, risk of generalised pustular psoriasis, and local and systemic toxicity due to impaired barrier function of the skin. Steroids may have a place in psoriasis of the scalp and chronic plaque psoriasis of the hands and feet. Careful patient supervision is important in psoriasis. 4.5 Interaction with other medicaments and other forms of interaction
3 -3- None so far known. 4.6 Pregnancy and lactation Topical administration of corticosteriods to pregnant animals can cause abnormalities of foetal development, including cleft palate and intra-uterine growth retardation. As a general rule, topical preparations containing corticoids should not be applied during the first trimester of pregnancy. In particular, application to large areas of the body or for prolonged periods must be avoided. 4.7 Effects on ability to drive and use machines ` Not applicable. 4.8 Undesirable effects The following reactions may occur when Ultralan Cream is applied to large areas of the body (about 10% and more) and\or for long periods of time (more than 4 weeks), occlusive dressings are used: local concomitant symptoms such as atrophy of the skin, telangiectasia, striae, acneform changes of the skin, perioral dermatitis, increased growth of body hair (hypertrichosis) and systematic effects of the corticoid due to absorption. In rare cases allergic skin reactions may occur. Side effects cannot be excluded in neonates whose mothers have been treated extensively or for a prolonged period of time during pregnancy or while lactating (for example, reduced adrenocortical function, when applied during the last weeks of pregnancy). 4.9 Overdose None stated. 5. PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties Ultralan Cream suppresses inflammation in inflammatory and allergic skin conditions and alleviates subjective complains such as itching, burning and pain. Owing to the different speeds with which the two forms of its corticoid component take effect, Ultralan Cream is both fast-acting and long-lasting in its effectiveness. Capillary dilatation, intracellular oedema and tissue infiltration regress, capillary proliferation is suppressed. This leads to fading of inflamed skin surfaces. 5.2 Pharmacokinetic properties
4 -4- A combination of two corticosteroids (steroid alcohol with ester or two different esters of the same steroid) in a dermatological preparation is pharmacokinetically meaningful, since a higher concentration of corticosteroids can be achieved and maintained over a longer period of time in the skin after application of a combination as compared with a single compound. Due to a different lipophilicity two compounds distribute in a different way into the horny layer and diffuse at different rates through the skin, leading to a rapid onset of action but also to a protracted activity. Investigation on the time course of the vasoconstriction in volunteers as well as investigations on the percutaneous absorption indicate that fluocortolone and fluocortolone pivalate penetrate more rapidly into and through human skin than fluocortolone caproate, giving evidence that the above mentioned principles have been realized with Ultralan Cream. Measurement of the drug recovery on the skin surface at the end of the exposure time in healthy skin volunteers as well as in eczema and psoriasis patients overestimate the extent of the percutaneous absorption and are therefore only of limited value for an assessment of the systemic drug load and for risk assessment. Pharmacodynamic investigations of the effect on the pituitary adrenal axis show only a small risk of systematic effects if not large areas of the body are treated over a long time. Fluocortolone-21-monoesters are hydrolized like a series of other 21-esters of corticosteroids most probably already in the skin, at the latest immediately after percutaneous absorption, into fluocortolone and the corresponding fatty acid. Fluocortolone itself possesses the shortest plasma half-life of all synthetic corticosteroids (approx minutes determined after i.v. administration) comparable to that of the endogenous cortisol. Fluocortolone is inactivated in the human organism via a series of reduction, oxidation and conjugation reactions with glucoronic and sulfuric acid and is excreted as metabolites mainly with the urine. 5.3 Preclinical safety data In systemic tolerance studies following repeated oral and parenteral administration the effect of fluocortolone, fluocortolone caproate and fluocortolone pivalate was that of a typical glucocorticoid. It can be derived from these results that no systematic side effects further to those which are typical of glucocorticoids are to be expected following therapeutic use of the Ultralan Cream even under extreme conditions such as application over large areas and\or occlusion. Specific embryotoxicity studies with the active substances contained in Ultralan Cream led to results typical of glucocorticoids, i.e. following sufficiently high exposure embryolethal and\or teratogenic effects could be induced given the appropriate test systems. Since epidemiological studies have as yet given no indications of embryotoxic effects due to systemic glucocorticoid therapy, no embryotoxic effects are to be expected following the therapeutic use of Ultralan Cream. However, taking animal-experimental results into consideration particular care should be taken when deciding to use Ultralan Cream. Investigation of floucortolone in a bacterial test system for the detection of pointmutagenic effects gave no indications of a genotoxic potential. Since no relevant indications of genotoxic effects have been found for any of the glucocorticoids, such effects are not to be
5 -5- expected from the active substances in Ultralan Cream. Specific tumorigenicity studies have not been carried out with the active substances contained in Ultralan Cream. On the basis of knowledge concerning the structures, the pharmacological action pattern and the results of systemic tolerance studies with chronic administration, there is no suspicion of a tumorigenic potential. Since systematically effective immunosuppressive dosages will not be reached after dermal application of Ultralan Cream if used as directed, no influence on the occurrence of tumors is to be expected. Following repeated dermal administration of fluocortolone and the two esters in different combinations and preparations, no substance-related dermal changes were observed. 6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients: White soft paraffin (petrolatum) Heavy liquid paraffin Stearyl alcohol Polyoxyl 40 stearate Polyacrylic acid (carbopol 980) sodium edetate Perfume oil citrus rose Sodium hydroxide Methyl and propyl parahydroxybenzoate Purified water. 6.2 Incompatibilities None so far known. 6.3 Shelf life 5 years 6.4 Special precautions for storage Store below 25 C 6.5 Nature and contents of container Standard tubes with 5, 10, 15, 20, 30, 50, 100 and 200 g with membrane closure and screw cap (tube material aluminium, internal coating done with epoxide, end seal band made of polyamide-based compound, external coating made of polyester, screw cap made of highdensity polyethylene). Thread jar with 300 g made of opal glass and a sealing disk of polyethylene, screw cap made of polystyrene. 6.6 Instructions for use \ handling
6 -6- Store all drugs properly and keep them out of reach of children. Manufacturer Intendis manufacturing SPA, Italy Via E. Schering Segrate, Milano Importer Bayer Israel Ltd., 36 Hacharash St., Hod Hasharon 45240
7 -7-
NERIDERM Cream, Ointment, Fatty Ointment
 PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
PRESCRIBING INFORMATION NERIDERM Cream, Ointment, Fatty Ointment Qualitative and Quantitive Composition 1 g Neriderm contains 1 mg (0.1%) diflucortolone valerate. Pharmaceutical Form Cream, Ointment, Fatty
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT
 NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
NEW ZEALAND DATA SHEET BETNOVATE -C CREAM and OINTMENT Name of each active ingredient Betamethasone valerate 0.1% w/w and clioquinol 3% w/w Presentations BETNOVATE-C Cream is a smooth, straw-coloured water-miscible
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol
 [GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
[GSK Logo] Package Leaflet: Information for the User Betnovate -C 0.1% / 3% w/w Cream Betamethasone (as valerate) and Clioquinol Read all of this leaflet carefully before you start using this medicine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis
 For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
For Mild to Moderate Plaque Psoriasis and Moderate to Severe Atopic Dermatitis OLUX-E (clobetasol propionate) Foam, 0.05% Please see Important Safety Information on back page and accompanying Full Prescribing
X-Plain Psoriasis Reference Summary
 X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
X-Plain Psoriasis Reference Summary Introduction Psoriasis is a long-lasting skin disease that causes the skin to become inflamed. Patches of thick, red skin are covered with silvery scales. It affects
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665
 Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON AVENA SATIVA L., FRUCTUS
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
PRODUCT INFORMATION OINTMENT, CREAM & LOTION
 PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
PRDUCT INFRMATIN ADVANTAN INTMENT, FATTY INTMENT, CREAM & LTIN NAME F THE DRUG The chemical name is 21-acetoxy-11β-hydroxy-6α-methyl-17-propionyloxy-1, 4- pregnadiene-3, 20-dione. The chemical structure
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Department of Dermatology, Churchill Hospital PUVA Treatment
 Oxford University Hospitals NHS Trust Department of Dermatology, Churchill Hospital PUVA Treatment information for patients CONTENTS What is PUVA 3 What conditions are treated with PUVA? 3 How is PUVA
Oxford University Hospitals NHS Trust Department of Dermatology, Churchill Hospital PUVA Treatment information for patients CONTENTS What is PUVA 3 What conditions are treated with PUVA? 3 How is PUVA
Professor Andrew Wright,
 Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
Professor Andrew Wright, Systemic treatments are drugs taken as tablets or injections that travel through the bloodstream, dampening down the immune system to reach and treat eczema all over the body.
PRODUCT MONOGRAPH. HydroVal. (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid
 PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate USP) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. September 02, 2003 130 East Drive
MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%
![MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%](/thumbs/27/10084771.jpg) MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
TOPICAL TREATMENTS FOR PSORIASIS
 TOPICAL TREATMENTS FOR PSORIASIS What are the aims of this leaflet? Patients with psoriasis are usually treated with preparations that are applied to the skin. This leaflet has been written to help you
TOPICAL TREATMENTS FOR PSORIASIS What are the aims of this leaflet? Patients with psoriasis are usually treated with preparations that are applied to the skin. This leaflet has been written to help you
Hydrozole Cream Hydrocortisone (microfine) 1% w/w and Clotrimazole 1% w/w
 CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
CONSUMER MEDICINE INFORMATION What is in this leaflet? This leaflet answers some common questions about Hydrozole It does not contain all the available information. It does not take the place of talking
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
MicroSilver BG TM. The innovative agent for beautiful, healthy skin.
 The innovative agent for beautiful, healthy skin. Inhalt Why MicroSilver BG TM? 3 What is MicroSilver BG TM? 3 How does MicroSilver BG TM work? 3 Products and usage 4 MicroSilver BG TM still used today
The innovative agent for beautiful, healthy skin. Inhalt Why MicroSilver BG TM? 3 What is MicroSilver BG TM? 3 How does MicroSilver BG TM work? 3 Products and usage 4 MicroSilver BG TM still used today
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
HYDROCORTISONE 10 MG TABLETS
 HYDROCORTISONE 10 MG TABLETS (Hydrocortisone) PL 20072/0238 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary
HYDROCORTISONE 10 MG TABLETS (Hydrocortisone) PL 20072/0238 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178
 Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
Beclometasone Dipropionate 50 micrograms per spray Aqueous Nasal Spray PL 16431/0178 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific Discussion Page 5 Steps Taken for Assessment Page 11 Steps Taken
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB)
 Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB) Your doctor has referred you to the Dowling Day Treatment Centre for a course of narrow band ultraviolet treatment for your
Treating your skin condition with narrowband ultraviolet B radiation (NB-UVB) Your doctor has referred you to the Dowling Day Treatment Centre for a course of narrow band ultraviolet treatment for your
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
4 Clinical Particulars
 SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
SUMMARYOF PRODUCT CHARACTERISTICS
 SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Community herbal monograph on Commiphora molmol Engler, gummi-resina
 12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
Summary of Product Characteristics Medical Helium
 Summary of Product Characteristics Medical Helium www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Helium 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Helium BP 1988 100%. 3. PHARMACEUTICAL
Summary of Product Characteristics Medical Helium www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Helium 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Helium BP 1988 100%. 3. PHARMACEUTICAL
Public Assessment Report Scientific discussion. Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC
 Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
VOLTAREN OPHTHA EYE DROPS
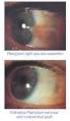 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
What Alcohol Does to the Body. Chapter 25 Lesson 2
 What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
What Alcohol Does to the Body Chapter 25 Lesson 2 Short-Term Effects of Drinking The short-term term effects of alcohol on the body depend on several factors including: amount of alcohol consumed, gender,
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
FLAMAZINE CREAM MSDS No. 185
 SECTION 1 IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Product Name FLAMAZINE CREAM Other Names - Manufacturer s Product Code 66800531, 66800532, 66150153, 66150155 Use Topical antibacterial cream,
SECTION 1 IDENTIFICATION OF THE MATERIAL AND SUPPLIER Product Product Name FLAMAZINE CREAM Other Names - Manufacturer s Product Code 66800531, 66800532, 66150153, 66150155 Use Topical antibacterial cream,
CETRABEN ORIGINAL EMOLLIENT CREAM (PL 06831/0262) UKPAR TABLE OF CONTENTS
 CETRABEN ORIGINAL EMOLLIENT CREAM (PL 06831/0262) UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary Page
CETRABEN ORIGINAL EMOLLIENT CREAM (PL 06831/0262) UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary Page
CONTRAINDICATIONS. None (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Taclonex Ointment safely and effectively. See Full Prescribing Information for Taclonex Ointment.
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Diazepam Tablets BP 2mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Diazepam BP 2.00mg 3 PHARMACEUTICAL FORM Tablet 4. CLINICAL PARTICULARS
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Diazepam Tablets BP 2mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Diazepam BP 2.00mg 3 PHARMACEUTICAL FORM Tablet 4. CLINICAL PARTICULARS
Steroids. What are steroids?
 Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
Results of the first clinical study on a new treatment for Psori asis: the T.M.S.T. method
 MINERAL MOR LTD Results of the first clinical study on a new treatment for Psori asis: the TMST method Marco Harari MD 12122012 Conclusions: 1 No side effects were recorded during the 5week study period
MINERAL MOR LTD Results of the first clinical study on a new treatment for Psori asis: the TMST method Marco Harari MD 12122012 Conclusions: 1 No side effects were recorded during the 5week study period
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
Intravenous Methyl Prednisolone in Multiple Sclerosis
 Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Treatment of diseases affecting the kidney using steroids
 Treatment of diseases affecting the kidney using steroids Hope Building Renal 0161 206 5223 All Rights Reserved 2015. Document for issue as handout. This information has been written by the medical and
Treatment of diseases affecting the kidney using steroids Hope Building Renal 0161 206 5223 All Rights Reserved 2015. Document for issue as handout. This information has been written by the medical and
PRODUCT INFORMATION. Silver sulfadiazine is a sulfonamide and has broad antimicrobial activity against both Grampositive and Gram-negative organisms.
 PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Librium 10mg Hard capsule Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 10 mg of chlordiazepoxide hydrochloride. Excipients:
1 NAME OF THE MEDICINAL PRODUCT Librium 10mg Hard capsule Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 10 mg of chlordiazepoxide hydrochloride. Excipients:
UKPAR Goodnight THR 00904/0003. Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS. Lay Summary Page 2. Scientific discussion Page 3
 Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation Page 13 Summary of Product Characteristics
Goodnight THR 00904/0003 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation Page 13 Summary of Product Characteristics
Leukocytoclastic Vasculitis and Stasis Dermatitis With Id Reaction
 Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
Id Reaction December 01, 2007 By David L. Kaplan, MD [1] A Photo Quiz to Hone Dermatologic Skills Case 1: A slightly pruritic eruption developed on the lower legs of a 39-year-old woman after she had an
VOLTAREN OPHTHA EYE DROPS
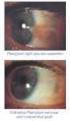 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
Facial Use of Low Potency Steroids With Anti-microbials and Retinoids
 Facial Use of Low Potency Steroids With Anti-microbials and Retinoids Steven L. Harlan, MD Medical Director, Dermatology Center, Des Moines, IA Introduction: There have been many reports of topical corticosteroids
Facial Use of Low Potency Steroids With Anti-microbials and Retinoids Steven L. Harlan, MD Medical Director, Dermatology Center, Des Moines, IA Introduction: There have been many reports of topical corticosteroids
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
FLAMAZINE CREAM 1.0% w/w
 PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Foam: A Unique Delivery Vehicle for Topically Applied Formulations
 : A Unique Delivery Vehicle for Topically Applied Formulations Dov Tamarkin, PhD ix Ltd. Key Words:, Emulsion, Lipophilic Emulsion, Nanoemulsion, Aqueous, Hydroethanolic, Potent-Solvent, Suspension, Ointment,
: A Unique Delivery Vehicle for Topically Applied Formulations Dov Tamarkin, PhD ix Ltd. Key Words:, Emulsion, Lipophilic Emulsion, Nanoemulsion, Aqueous, Hydroethanolic, Potent-Solvent, Suspension, Ointment,
Decentralised Procedure. Public Assessment Report
 Decentralised Procedure Public Assessment Report Articainhydrochlorid mit Epinephrin Pierrel 40 mg/ml + 0.01 mg/ml Injektionslösung Articainhydrochlorid mit Epinephrin Pierrel 40 mg/ml + 0.005 mg/ml Injektionslösung
Decentralised Procedure Public Assessment Report Articainhydrochlorid mit Epinephrin Pierrel 40 mg/ml + 0.01 mg/ml Injektionslösung Articainhydrochlorid mit Epinephrin Pierrel 40 mg/ml + 0.005 mg/ml Injektionslösung
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
Having regard to the Treaty establishing the European Economic Community, and in particular Article 100 thereof;
 DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved. Revised: 4/2015
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
CICLOSPORIN. What are the aims of this leaflet?
 CICLOSPORIN What are the aims of this leaflet? This leaflet has been written to help you understand more about ciclosporin. It tells you what it is, how it works, how it is used to treat skin conditions,
CICLOSPORIN What are the aims of this leaflet? This leaflet has been written to help you understand more about ciclosporin. It tells you what it is, how it works, how it is used to treat skin conditions,
SHINGLES (Herpes zoster infection)
 SHINGLES (Herpes zoster infection) What are the aims of this leaflet? This leaflet has been written to help you understand more about shingles. It will tell you what it is, what causes it, what can be
SHINGLES (Herpes zoster infection) What are the aims of this leaflet? This leaflet has been written to help you understand more about shingles. It will tell you what it is, what causes it, what can be
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Aciclovir 200mg Tablets Aciclovir 400mg Tablets Aciclovir 800mg Tablets PL 29831/0517 PL 29831/0518 PL 29831/0519 UKPAR
 Aciclovir 200mg Tablets Aciclovir 400mg Tablets Aciclovir 800mg Tablets PL 29831/0517 PL 29831/0518 PL 29831/0519 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for
Aciclovir 200mg Tablets Aciclovir 400mg Tablets Aciclovir 800mg Tablets PL 29831/0517 PL 29831/0518 PL 29831/0519 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for
Common Skin Conditions in Children. Liz Moore and Emma King Dermatology Nurse Consultants
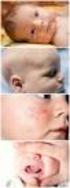 Common Skin Conditions in Children Liz Moore and Emma King Dermatology Nurse Consultants Diagnosis? Nummular Dermatitis Disc pattern rash (discoid eczema) Clearly demarcated edges Occurs at any age Can
Common Skin Conditions in Children Liz Moore and Emma King Dermatology Nurse Consultants Diagnosis? Nummular Dermatitis Disc pattern rash (discoid eczema) Clearly demarcated edges Occurs at any age Can
Treatment options a simple guide
 Guide Treatment options a simple guide To decide which treatment is right for you, a good starting point is to know what options you have and to understand the pros and cons of each one. People respond
Guide Treatment options a simple guide To decide which treatment is right for you, a good starting point is to know what options you have and to understand the pros and cons of each one. People respond
TOPICAL THERAPY. ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity
 TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
TOPICAL THERAPY ADVANTAGES - increased dose of medication to affected area. - reduced systemic side effects and toxicity DISADVANTAGES - takes time - may be greasy or messy - may have different preparations
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION
 NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (WELL-ESTABLISHED MEDICINAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
Summary of Product Characteristics Medical Air
 Summary of Product Characteristics Medical Air www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Air 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Air Ph.Eur. 100% 3. PHARMACEUTICAL FORM Inhalation
Summary of Product Characteristics Medical Air www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Air 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Air Ph.Eur. 100% 3. PHARMACEUTICAL FORM Inhalation
D.RIGOPOULOS, D.IOANNIDES,* D.KALOGEROMITROS, S.GREGORIOU AND A.KATSAMBAS
 British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
British Journal of Dermatology 24; 151: 171 175. DOI: 1.1111/j.1365-2133.24.628.x Therapeutics Pimecrolimus cream 1% vs. betamethasone 17-valerate Æ1% cream in the treatment of seborrhoeic dermatitis.
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
Public Assessment Report Decentralised Procedure Linezolid 2 mg/ml solution for infusion Linezolid 600mg film coated tablets Linezolid 100mg/5ml granules for oral suspension Procedure No: UK Licence No:
LEFLUNOMIDE (Adults)
 Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active

 More details >>> HERE
More details >>> HERE