URGENT FIELD CORRECTION. REVISED ED 530XT OPERATION MANUALS: Cleaning, Disinfection and Storage, CODE 202B D Operation
|
|
|
- Rafe Caldwell
- 7 years ago
- Views:
Transcription
1 URGENT FIELD CORRECTION December 23, 2015 REVISED ED 530XT OPERATION MANUALS: Cleaning, Disinfection and Storage, CODE 202B D Operation and Preparation, CODE 202B G Dear Valued Customer: This letter is to advise you of the revised FUJIFILM Operation Manuals for the FUJIFILM ED 530XT Duodenoscope ( ED 530XT ). The ED 530XT is a medical endoscope for the duodenum and upper G.I. tract. It is intended for observation, diagnosis, and endoscopic treatment of the esophagus, stomach and duodenum. FUJIFILM Medical Systems, U.S.A., Inc., Endoscopy Division is providing all users of the ED 530XT with revised Operation Manuals and a box of new disposable distal end cleaning brushes at no charge. The Operation Manuals (identified above) have been revised to reflect newly validated manual cleaning and high level disinfection procedures. This action is being taken as a result of publicized reports of multi drug resistant bacteria on endoscopes used for Endoscopic Retrograde Cholangiopancreatogram (ERCP) procedures. Given these reports and in an abundance of caution, FUJIFILM Medical Systems, U.S.A., Inc. has been working with the U.S. Food and Drug Administration ( FDA ) to validate the reprocessing procedures that are provided in the revised Operation Manuals. Per the company s discussions with FDA, comprehensive revisions, as described below, have been made to the ED 530XT Operation Manuals, Preparation and Operation and Cleaning, Disinfection and Storage. These revisions modify the cleaning and disinfection processes and require the use of a new disposable distal end cleaning brush [Model WB1318DE] to be used for the cleaning of the duodenoscope s distal tip, forceps elevator and elevator recess, in addition to use of the existing Fujifilm valve cylinder cleaning brush [Model WB11002FW2]. A box containing twenty WB1318DE disposable distal end brushes is being provided per scope model along with this Field Correction letter and a copy of the revised ED 530XT Operation Manuals containing FUJIFILM reprocessing recommendations. These new distal end brushes [Model WB1318DE] will be provided at no charge to accommodate up to six months of scope usage after the release date of this notification letter.
2 Below are the main differences in the revised ED 530XT manual reprocessing (cleaning and high level disinfection) procedures: Pre Cleaning During immersion of the scope tip in detergent solution, move the forceps elevator back and forth and aspirate detergent solution while the forceps elevator is raised and while lowered. Manual Cleaning Additional brushing of the distal tip, forceps elevator and elevator recess first using the existing Fujifilm valve cylinder cleaning brush [Model WB11002FW2] and then using the new [Model WB1318DE] disposable cleaning brush. Additional flushing of detergent and rinse water onto the forceps elevator/recess while the elevator is both raised and lowered. Additional flushing steps and increased channel flushing volumes of detergent and rinse water. Manual High Level Disinfection Additional flushing of disinfectant and rinse water onto the forceps elevator/recess while the elevator is both raised and lowered. Additional raising and lowering of the elevator while immersed in disinfectant solution and rinse water. Additional flushing steps and increased flushing volumes of disinfectant and rinse water through the scope s internal channels. The revised reprocessing techniques were developed a n d v a l i d a t e d in accordance with FDA s recommendations set forth in its March 17, 2015, guidance document entitled, Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. While the revised reprocessing instructions have not been validated for the other FUJIFILM duodenoscope models, the revised operation manuals may be used to reprocess the following duodenoscope models while they undergo validation testing: ED 250XT5, ED 450XT5, ED 250XL5, and ED 450XL5 Customers using the above listed duodenoscopes will be provided a revised Cleaning, Disinfection and Storage operation manual under a separate notice.
3 One copy of each of the revised Operation Manuals and one box of the disposable distal end cleaning brushes [Model WB1318DE], with 20 brushes per box are included with this notice. Additional copies of the manuals and boxes of brushes can be requested through your local sales representative. PLEASE NOTE: NO OTHER FUJIFILM MEDICAL SYSTEMS, U.S.A., INC., ENDOSCOPY DIVISION PRODUCTS ARE INVOLVED IN THIS FIELD ACTION. All prior versions of the Operation Manuals for the ED 530XT are superseded by these revised Operation Manuals and should be destroyed. Please check with your facility s applicable supply departments to determine if you have any copies of the superseded operation manuals. Please complete the enclosed tracking/verification form and return it to FUJIFILM Medical Systems USA, Inc., Endoscopy Division via e mail to fnonmedicalqa@fujifilm.com or via fax to (973) Please note that you must return the completed form even if you do not have any Operation Manuals to destroy. Your local sales representative can assist you in completing this form. This information is essential in order to maintain recall effectiveness information required by FDA. FUJIFILM Medical Systems, U.S.A., Inc., Endoscopy Division has a dedicated team of Clinical Specialists (CS) who visit customer facilities and perform reprocessing in servicing and training. If you would like to set up a site visit with our CS team to have an in service regarding these updated FUJIFILM duodenoscope reprocessing instructions, please contact Stephen Budda, Senior Manager, Clinical Education and Product Coordination at (316) or via at fmsuesdin service@fujifilm.com. Thank you for your support. Please contact your FUJIFILM Medical Systems U.S.A., Inc., Endoscopy Division sales representative if you have any questions regarding this field correction, any of our products, or would like assistance. We regret any inconvenience that this action may cause, but we appreciate your understanding as we take action to ensure patient safety and customer satisfaction. Sincerely, Faye Dunn Director Regulatory Affairs Enclosures Page 3 of 6 TRC
4 STEPS FOR URGENT FIELD CORRECTION The Tracking/Verification Form attached to this letter must be completed and returned even if you do not have any superseded operation manuals to destroy. 1. Segregate the Product. Please immediately remove all superseded operation manuals from your inventory (regardless of their location) and destroy this product. 2. Complete the Tracking/Verification Form. Complete and return the enclosed tracking/verification form (even if you do not currently have any of the superseded operation manuals to destroy). Your FUJIFILM Medical Systems, U.S.A., Inc., Endoscopy Division sales representative can assist you in completing the form. 3. Return the Complete Tracking/Verification Form via e mail to fnonmedicalqa@fujifilm.com or via fax to (973) Page 4 of 6 TRC
5 VOLUNTARY FIELD CORRECTION Tracking/Verification Form for Effectiveness Check PLEASE FILL OUT AND RETURN (via e mail to fnonmedicalqa@fujifilm.com or fax to ) We have received the current version of the OPERATION MANUALS: Cleaning, Disinfection and Storage, CODE 202B D and Operation and Preparation, CODE 202B G (Please check one of the following options) 1. We do not have superseded versions of the aforementioned operation manuals in stock or on hand. 2. We have destroyed all superseded versions of the aforementioned operation manuals we had in stock or on hand. (Please also let us know if you no longer own the ED 530XT endoscope by checking below) 1. We no longer own ED 530XT endoscope(s). ACCOUNT NAME: ADDRESS: CITY: STATE: ZIP CODE: PRINTED NAME: TITLE: SIGNATURE/ DATE: CURRENT E MAIL / TEL. NO.: Page 5 of 6 TRC
6 ATTACHMENT 1 REVISED OPERATION MANUALS: Cleaning, Disinfection and Storage (CODE 202B D) Operation and Preparation (CODE 202B G) Page 6 of 6 TRC
ON TRACK Reprocessing Inservice / Competency for GI Endoscopes (EVIS, EXERA, EXERA II)
 THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
THIS CHECKLIST IS DESIGNED FOR USE SOLELY AS A CUSTOMER EDUCATIONAL TOOL, AND IS NOT INTENDED TO REPLACE OR ANY WAY MODIFY THE OLYMPUS INSTRUCTION MANUAL/REPROCESSING MANUAL. BE SURE TO FOLLOW THE DETAILED
URGENT FIELD SAFETY NOTICE
 29 December 2014 Product: Type of Action: Product Codes: Range of Serial #s: Recall of Dear HeartWare Clinician, In 2013, HeartWare advised you on measures to take in relation to patient safety risks associated
29 December 2014 Product: Type of Action: Product Codes: Range of Serial #s: Recall of Dear HeartWare Clinician, In 2013, HeartWare advised you on measures to take in relation to patient safety risks associated
URGENT: VOLUNTARY PRODUCT RECALL / MARKET WITHDRAWAL ***LIMITED ONLY TO SPECIFIC LOTS OF DAYTRANA***
 July 18, 2014 Dear Valued Customer: You should have already received a notification from Stericycle Inc. about a Voluntary Limited Recall/Market Withdrawal of Specific Lots of DAYTRANA (methylphenidate
July 18, 2014 Dear Valued Customer: You should have already received a notification from Stericycle Inc. about a Voluntary Limited Recall/Market Withdrawal of Specific Lots of DAYTRANA (methylphenidate
Christina Bradley Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK
 Christina Bradley Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK Inadequate decontamination procedures and equipment malfunction were two leading causes of post endoscopic
Christina Bradley Hospital Infection Research Laboratory Queen Elizabeth Hospital Birmingham, UK Inadequate decontamination procedures and equipment malfunction were two leading causes of post endoscopic
DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION DATE(S) OF INSPECTION. 10 Highpoint Drive
 (b) (b) (4) (4) (b) (4) DISTRICT ADDRESS AND PHONE NUMBER FEINUMBER NAME AND TITlE OF INDIVIDUAL TO WHOM REPORT ISSUED This document lists observations made by the FDA representative(s) during the inspection
(b) (b) (4) (4) (b) (4) DISTRICT ADDRESS AND PHONE NUMBER FEINUMBER NAME AND TITlE OF INDIVIDUAL TO WHOM REPORT ISSUED This document lists observations made by the FDA representative(s) during the inspection
URGENT NOTICE: MEDICAL DEVICE RECALL Synthes Trochanteric Fixation Nailing (TFN) System
 URGENT NOTICE: MEDICAL DEVICE RECALL Synthes Trochanteric Fixation Nailing (TFN) System Dear Sir/ Madam: Synthes is initiating a voluntary recall of all lots of the Trochanteric Fixation Nailing (TFN)
URGENT NOTICE: MEDICAL DEVICE RECALL Synthes Trochanteric Fixation Nailing (TFN) System Dear Sir/ Madam: Synthes is initiating a voluntary recall of all lots of the Trochanteric Fixation Nailing (TFN)
National Endoscopy Programme. Decontamination Standards for Flexible Endoscopes
 National Endoscopy Programme Decontamination Standards for Flexible Endoscopes Updated March 2009 Introduction Providing an effective endoscope decontamination service within a safe environment is an essential
National Endoscopy Programme Decontamination Standards for Flexible Endoscopes Updated March 2009 Introduction Providing an effective endoscope decontamination service within a safe environment is an essential
Back to Basics: Flexible Endoscope Processing 1.6 www.aornjournal.org/content/cme LISA SPRUCE, DNP, RN, CNS-CP, CNOR, ACNS, ACNP, FAAN
 CONTINUING EDUCATION Back to Basics: Flexible Endoscope Processing 1.6 www.aornjournal.org/content/cme LISA SPRUCE, DNP, RN, CNS-CP, CNOR, ACNS, ACNP, FAAN Continuing Education Contact Hours indicates
CONTINUING EDUCATION Back to Basics: Flexible Endoscope Processing 1.6 www.aornjournal.org/content/cme LISA SPRUCE, DNP, RN, CNS-CP, CNOR, ACNS, ACNP, FAAN Continuing Education Contact Hours indicates
A Guide for Patients Living with a Biliary Metal Stent
 A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
A Guide for Patients Living with a Biliary Metal Stent What is a biliary metal stent? A biliary metal stent (also known as a bile duct stent ) is a flexible metallic tube specially designed to hold your
Antibiotic Resistant Bacteria: Economic & Legal Threats
 : Economic & Legal Threats : Economic & Legal Threats 05 November, 2015 Thomas Blanz-Gilbert, Global Casualty Manager, Zurich Insurance Group Annual Liability Regimes Conference 2015 Economic Burden Case
: Economic & Legal Threats : Economic & Legal Threats 05 November, 2015 Thomas Blanz-Gilbert, Global Casualty Manager, Zurich Insurance Group Annual Liability Regimes Conference 2015 Economic Burden Case
In use test of a Washer-disinfector reprocessing of flexible endoscopes
 of a Washer-disinfector reprocessing of flexible endoscopes Olympus ETD 3 with PAA Clean Endoscope Copenhagen Hospitals Denmark In-use test of Olympus ETD 3 Washer-disinfector with PAA Cleaner and disinfectant
of a Washer-disinfector reprocessing of flexible endoscopes Olympus ETD 3 with PAA Clean Endoscope Copenhagen Hospitals Denmark In-use test of Olympus ETD 3 Washer-disinfector with PAA Cleaner and disinfectant
Termination of National Bank Status
 Termination of National Bank Status Other Changes and Activities Comptroller s Corporate Manual Washington, DC April 1998 Termination of National Bank Status Table of Contents Introduction Background
Termination of National Bank Status Other Changes and Activities Comptroller s Corporate Manual Washington, DC April 1998 Termination of National Bank Status Table of Contents Introduction Background
Page 1 of 2. Dear Occupant:
 Page 1 of 2 Dear Occupant: You contacted the City of Lincoln Park to claim that you discovered that you had suffered property damage or personal injury as a result of a sewage disposal system event. Enclosed,
Page 1 of 2 Dear Occupant: You contacted the City of Lincoln Park to claim that you discovered that you had suffered property damage or personal injury as a result of a sewage disposal system event. Enclosed,
The NJSSA Pulse FROM THE PRESIDENT PETER GOLDZWEIG, DO NEW JERSEY STATE SOCIETY OF ANESTHESIOLOGISTS. March 2015
 NEW JERSEY STATE SOCIETY OF ANESTHESIOLOGISTS The NJSSA Pulse March 2015 FROM THE PRESIDENT PETER GOLDZWEIG, DO I do want to update everyone on the Novitas MAC LCD (local coverage determinant). The draft
NEW JERSEY STATE SOCIETY OF ANESTHESIOLOGISTS The NJSSA Pulse March 2015 FROM THE PRESIDENT PETER GOLDZWEIG, DO I do want to update everyone on the Novitas MAC LCD (local coverage determinant). The draft
Switch Kit (Complete these forms to make your moving process easier) Account Closing Report
 Switch Kit (Complete these forms to make your moving process easier) Account Closing Report Date: To: (Current bank, credit union, etc.) From: (Primary Account Holder) (Secondary Account Holder ) Address:
Switch Kit (Complete these forms to make your moving process easier) Account Closing Report Date: To: (Current bank, credit union, etc.) From: (Primary Account Holder) (Secondary Account Holder ) Address:
Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, NE Washington, DC 20426. Compliance Filing Docket No.
 October 31, 2014 Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, NE Washington, DC 20426 North Baja Pipeline, LLC 717 Texas Street, Suite 2400 Houston, TX 77002 John
October 31, 2014 Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, NE Washington, DC 20426 North Baja Pipeline, LLC 717 Texas Street, Suite 2400 Houston, TX 77002 John
2470 RO120 QUICK CONNECT
 IMPORTANT SAFETY and QUALITY NOTICE PENTAIR WATER PURIFICATION +1.262.238.4400 main +1.262.238.4404 fax 5730 North Glen Park Road Milwaukee, WI 53209 United States www.waterpurification.pentair.com June
IMPORTANT SAFETY and QUALITY NOTICE PENTAIR WATER PURIFICATION +1.262.238.4400 main +1.262.238.4404 fax 5730 North Glen Park Road Milwaukee, WI 53209 United States www.waterpurification.pentair.com June
Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes
 Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2012, Society of Gastroenterology Nurses and Associates, Inc. (SGNA). First published in
Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2012, Society of Gastroenterology Nurses and Associates, Inc. (SGNA). First published in
Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes
 Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2012, Society of Gastroenterology Nurses and Associates, Inc. (SGNA). First published in
Standards of Infection Control in Reprocessing of Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2012, Society of Gastroenterology Nurses and Associates, Inc. (SGNA). First published in
MANY OF TODAY S SURGERIES USE MINIMALLY INVASIVE
 Lesson No. CRCST 137 (Technical Continuing Education - TCE) Sponsored by: by Patti Koncur, CRCST Educational Specialist, IAHCSMM and Scott Davis, CMRP, CRCST, CHMMC Materials Manager University Medical
Lesson No. CRCST 137 (Technical Continuing Education - TCE) Sponsored by: by Patti Koncur, CRCST Educational Specialist, IAHCSMM and Scott Davis, CMRP, CRCST, CHMMC Materials Manager University Medical
Gastroenterology Nursing
 VOLUME 33 NUMBER 4 July/August 2010 www.gastroenterologynursing.com Endoscope Reprocessing Methods A Prospective Study on the Impact of Human Factors and Automation Gastroenterology Nursing The Official
VOLUME 33 NUMBER 4 July/August 2010 www.gastroenterologynursing.com Endoscope Reprocessing Methods A Prospective Study on the Impact of Human Factors and Automation Gastroenterology Nursing The Official
In December 2012, we notified patients of an end of service life date issue that will affect the Animas 2020 and IR 1200 insulin pumps as follows:
 On Animas Letterhead URGENT FIELD SAFETY NOTICE April XX, 2015 Important Information For Patients With Animas 2020 and IR 1200 Insulin Pumps Who Also Have An Animas Vibe Insulin Pump Dear Animas Pump User:
On Animas Letterhead URGENT FIELD SAFETY NOTICE April XX, 2015 Important Information For Patients With Animas 2020 and IR 1200 Insulin Pumps Who Also Have An Animas Vibe Insulin Pump Dear Animas Pump User:
University of Kentucky / UK HealthCare Policy and Procedure. Policy # A03-030
 University of Kentucky / UK HealthCare Policy and Procedure Policy # A03-030 Title/Description: High-Level Disinfection with Cidex OPA Purpose: To provide instructions for high level disinfection with
University of Kentucky / UK HealthCare Policy and Procedure Policy # A03-030 Title/Description: High-Level Disinfection with Cidex OPA Purpose: To provide instructions for high level disinfection with
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy!
 Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclosure:
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclosure:
Transducer and System Care and Cleaning. 2013 Koninklijke Philips N.V. All rights reserved. Published in USA.
 l Transducer and System Care and Cleaning 4535 617 39631 Rev B December 2013 2013 Koninklijke Philips N.V. All rights reserved. Published in USA. Philips Ultrasound 22100 Bothell-Everett Highway Bothell,
l Transducer and System Care and Cleaning 4535 617 39631 Rev B December 2013 2013 Koninklijke Philips N.V. All rights reserved. Published in USA. Philips Ultrasound 22100 Bothell-Everett Highway Bothell,
Creating the Improved Standard in Healthcare Sterile Reprocessing
 Creating the Improved Standard in Healthcare Sterile Reprocessing Corporate Presentation May 2016 Important Cautions Regarding Forward Looking Statements and Other Disclosures The statements in this presentation
Creating the Improved Standard in Healthcare Sterile Reprocessing Corporate Presentation May 2016 Important Cautions Regarding Forward Looking Statements and Other Disclosures The statements in this presentation
Ultimate Concepts Inc.
 MAR 2 3 2004 Ultimate Concepts Inc. 7835 South 1300 East Sandy, Utah 84094 Phone 800-682-3241 or 801-566-3214 SUMMARY Submitter's name: Address: Ultimate Concepts, Inc. 7835 South 1300 East Sandy, Utah
MAR 2 3 2004 Ultimate Concepts Inc. 7835 South 1300 East Sandy, Utah 84094 Phone 800-682-3241 or 801-566-3214 SUMMARY Submitter's name: Address: Ultimate Concepts, Inc. 7835 South 1300 East Sandy, Utah
0 EC2 92011 V-,) 133 Lj9a
 0 EC2 92011 V-,) 133 Lj9a Section 5: 5 1 0(k) Summar 5 10(K) SUMMARY FOR SOMATOM DEFINITION Flash (with Stellar Detector) Submitted by: Siemens Medical Solutions USA, Inc. 51 Valley Stream Parkway Malvern,
0 EC2 92011 V-,) 133 Lj9a Section 5: 5 1 0(k) Summar 5 10(K) SUMMARY FOR SOMATOM DEFINITION Flash (with Stellar Detector) Submitted by: Siemens Medical Solutions USA, Inc. 51 Valley Stream Parkway Malvern,
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes
 Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2016 (SGNA). First published in 1996, revised 2000, 2005, 2007, 2008, 2011, 2012, 2015
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Acknowledgements Copyright 2016 (SGNA). First published in 1996, revised 2000, 2005, 2007, 2008, 2011, 2012, 2015
Strategies for Effective Management of the Sterile Processing Department
 Strategies for Effective Management of the Sterile Processing Department Objectives Upon completion, participants will be able to... 1) explain the importance of instrument reprocessing for infection prevention,
Strategies for Effective Management of the Sterile Processing Department Objectives Upon completion, participants will be able to... 1) explain the importance of instrument reprocessing for infection prevention,
Reprocessing. Critical & Semi-Critical Equipment
 Reprocessing (Cleaning, Disinfection & Sterilization) Critical & Semi-Critical Equipment - A Physician Toolkit - Reprocessing Critical & Semi-Critical Equipment - A Physician Tool Kit Table of Contents
Reprocessing (Cleaning, Disinfection & Sterilization) Critical & Semi-Critical Equipment - A Physician Toolkit - Reprocessing Critical & Semi-Critical Equipment - A Physician Tool Kit Table of Contents
DHCPL_RA2014-170-EXT Page 1 of 6
 September 11th 2015 URGENT: Field Safety Notice RA2014-170(EXT) FSCA identifier: Type of Action: Description: Product Field Action RA2014-170(EXT) Field Safety Corrective Action: Return to supplier. LFIT
September 11th 2015 URGENT: Field Safety Notice RA2014-170(EXT) FSCA identifier: Type of Action: Description: Product Field Action RA2014-170(EXT) Field Safety Corrective Action: Return to supplier. LFIT
JUN 2 6 butt. EkoSonicTM Endovascular System with Rapid Pulse Modulation
 KC6, IL(6/ SPECIAL 510(k) Notification EkoSonic TM Endovascular System JUN 2 6 butt Section 4. General Provisions 510(k) Summary Submitter's Name and Address: Contact Person: Classification Name: EKOS
KC6, IL(6/ SPECIAL 510(k) Notification EkoSonic TM Endovascular System JUN 2 6 butt Section 4. General Provisions 510(k) Summary Submitter's Name and Address: Contact Person: Classification Name: EKOS
kok1 UQ 510(k)J Device {Applicant 510(k) Summary (per 21 CFR 807.92(c)) OCT 2 7 2008 1. Applicant
 kok1 UQ 510(k) Summary (per 21 CFR 807.92(c)) OCT 2 7 2008 1. Applicant Tech Avenue Ventures d/b/a MPowRx Health and Wellness Products Inc. #510 3553-31 St. NW Calgary, Alberta T2L 2K7 Canada Contact Person:
kok1 UQ 510(k) Summary (per 21 CFR 807.92(c)) OCT 2 7 2008 1. Applicant Tech Avenue Ventures d/b/a MPowRx Health and Wellness Products Inc. #510 3553-31 St. NW Calgary, Alberta T2L 2K7 Canada Contact Person:
Service Manager Dealer Principal, North American Ducati Dealer Network. Electronic Control Unit Re-mapping
 . 10443 Bandley Drive Corporate Headquarters Cupertino, CA 95014 Tel: 408-253-0499 Fax: 408-253-4099 www.ducatiusa.com Recall Bulletin RCL-08-001 DNA Date: February 13, 2008 To: Subject: Service Manager
. 10443 Bandley Drive Corporate Headquarters Cupertino, CA 95014 Tel: 408-253-0499 Fax: 408-253-4099 www.ducatiusa.com Recall Bulletin RCL-08-001 DNA Date: February 13, 2008 To: Subject: Service Manager
March 25, 2015. Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, N.E. Washington, D.C. 20426
 March 25, 2015 Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, N.E. Washington, D.C. 20426 Tuscarora Gas Transmission Company 700 Louisiana Street, Suite 700 Houston,
March 25, 2015 Ms. Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street, N.E. Washington, D.C. 20426 Tuscarora Gas Transmission Company 700 Louisiana Street, Suite 700 Houston,
Michigan Department of Licensing and Regulatory Affairs Bureau of Professional Licensing Board of Pharmacy PO Box 30670 Lansing MI 48909 (517)
 Michigan Department of Licensing and Regulatory Affairs Bureau of Professional Licensing Board of Pharmacy PO Box 30670 Lansing MI 48909 (517) 373-8068 www.michigan.gov/bpl 1 MANUFACTURER/WHOLESALER LICENSE
Michigan Department of Licensing and Regulatory Affairs Bureau of Professional Licensing Board of Pharmacy PO Box 30670 Lansing MI 48909 (517) 373-8068 www.michigan.gov/bpl 1 MANUFACTURER/WHOLESALER LICENSE
March 20, 2006. 20100 First Name, Last Name Company Name Address 1 Address 2 City, State Zip. Dear Valued Broker:
 March 20, 2006 20100 First Name, Last Name Company Name Address 1 Address 2 City, State Zip Dear Valued Broker: On March 14, 2006, Vytra Health Plans formally merged with HIP Health Plan of New York. The
March 20, 2006 20100 First Name, Last Name Company Name Address 1 Address 2 City, State Zip Dear Valued Broker: On March 14, 2006, Vytra Health Plans formally merged with HIP Health Plan of New York. The
DEC 2 2 2005. The three components of the Axxent Electronic Brachytherapy System include the Controller, Balloon Applicators-BR and X-ray Source.
 DEC 2 2 2005 Xoft, Inc. Fremont, California Premarket Notification March 31, 2005 II. 510(k) Summary A. Name of Device Trade name: AxxentTM Electronic Brachytherapy System Common name: X-Ray Radiation
DEC 2 2 2005 Xoft, Inc. Fremont, California Premarket Notification March 31, 2005 II. 510(k) Summary A. Name of Device Trade name: AxxentTM Electronic Brachytherapy System Common name: X-Ray Radiation
NDA 202439/S-008 SUPPLEMENT APPROVAL
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202439/S-008 SUPPLEMENT APPROVAL Janssen Pharmaceuticals, Inc. ATTENTION: Alla Rhoge Pharm.D., Associate
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202439/S-008 SUPPLEMENT APPROVAL Janssen Pharmaceuticals, Inc. ATTENTION: Alla Rhoge Pharm.D., Associate
510(K) SUMMARY. 510(k) Number KOS'00-r
 " iirtual Ports Virtsua Ports Virtual Ports Ltd. 510(K) SUMMARY 510(k) Number KOS'00-r MAR 2 12008 Applicant's Name: Contact Person: Trade Name: Common name: Classification: Predicate Devices: Device Description:
" iirtual Ports Virtsua Ports Virtual Ports Ltd. 510(K) SUMMARY 510(k) Number KOS'00-r MAR 2 12008 Applicant's Name: Contact Person: Trade Name: Common name: Classification: Predicate Devices: Device Description:
How to Effectively Code for Endoscopic Procedures in Gastroenterology
 How to Effectively Code for Endoscopic Procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Interim Chief, Division of Gastroenterology Texas Tech University Health Science Center All rights
How to Effectively Code for Endoscopic Procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Interim Chief, Division of Gastroenterology Texas Tech University Health Science Center All rights
December 31, 2015. comments to the Office of Dietary Supplements (ODS) at the National Institutes of Health on the
 December 31, 2015 VIA ELECTRONIC SUBMISSION Richard Bailen, MBA, MHA Office of Dietary Supplements National Institutes of Health 6100 Executive Boulevard, Room 3B01 Bethesda, MD 20892-7517 Email: ODS@nih.gov
December 31, 2015 VIA ELECTRONIC SUBMISSION Richard Bailen, MBA, MHA Office of Dietary Supplements National Institutes of Health 6100 Executive Boulevard, Room 3B01 Bethesda, MD 20892-7517 Email: ODS@nih.gov
FSIS Security Guidelines for Food Processors
 United States Department of Agriculture Food Safety and Inspection Service FSIS Security Guidelines for Food Processors Food Security Plan Management Dear Establishment Owner/Operator: The Food Safety
United States Department of Agriculture Food Safety and Inspection Service FSIS Security Guidelines for Food Processors Food Security Plan Management Dear Establishment Owner/Operator: The Food Safety
FEB 2 2 2007. Mr. Gordon J. Peters Director Regulatory Compliance DocuSys, Incorporated 820 S. University Boulevard, Suite 3-H Mobile, Alabama 36609
 FEB 2 2 2007 510(k) Summary DocuSys Inc. Anesthesia Information and digital-drug Management System The following information is in accordance with 21 CFR 807.92 Submitter's Name, Address, Telephone Number,
FEB 2 2 2007 510(k) Summary DocuSys Inc. Anesthesia Information and digital-drug Management System The following information is in accordance with 21 CFR 807.92 Submitter's Name, Address, Telephone Number,
SOD (Sphincter of Oddi Dysfunction)
 SOD (Sphincter of Oddi Dysfunction) SOD refers to the mechanical malfunctioning of the Sphincter of Oddi, which is the valve muscle that regulates the flow of bile and pancreatic juice into the duodenum.
SOD (Sphincter of Oddi Dysfunction) SOD refers to the mechanical malfunctioning of the Sphincter of Oddi, which is the valve muscle that regulates the flow of bile and pancreatic juice into the duodenum.
50 Tannery Road, Unit 3 Branchburg, NJ 08822 1-800-407-3726 www.drain-net.com
 50 Tannery Road, Unit 3 Branchburg, NJ 08822 1-800-407-3726 www.drain-net.com Dear Sir/Madam: VAC S was founded in response to a real problem existing in healthcare facility management today. OSHA s standards
50 Tannery Road, Unit 3 Branchburg, NJ 08822 1-800-407-3726 www.drain-net.com Dear Sir/Madam: VAC S was founded in response to a real problem existing in healthcare facility management today. OSHA s standards
April 29, 2011 FIELD NOTIFICATION
 Haemonetics Corporation Braintree, MA 02184-9114 Tel: 781.848.7100 www.haemonetics.com April 29, 2011 FIELD NOTIFICATION URGENT - FIELD RECALL: OrthoPAT ORTHOPEDIC PERIOPERATIVE AUTOTRANSFUSION DEVICES
Haemonetics Corporation Braintree, MA 02184-9114 Tel: 781.848.7100 www.haemonetics.com April 29, 2011 FIELD NOTIFICATION URGENT - FIELD RECALL: OrthoPAT ORTHOPEDIC PERIOPERATIVE AUTOTRANSFUSION DEVICES
June 9, 2015. Stryker Corporation Garry T. Hayeck, Ph.D. Senior Regulatory Affairs Specialist 2 Pearl Court Allendale, New Jersey 07401
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Stryker Corporation Garry
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Stryker Corporation Garry
October 28, 2015. Cavex Holland Bv Mr. Richard Woortman Manager Technical Services Fustweg 5 Haarlem, 2031CJ The NETHERLANDS
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 October 28, 2015 Cavex
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 October 28, 2015 Cavex
CHARTER FIBERLINK SC-CCo, LLC
 CHARTER FIBERLINK SC-CCo, LLC Betty Sanders Director Regulatory Affairs Direct: 314-288 -3259 Email: bettv.sanders@chartercor(l.com April 12, 2010 VIA ELECTRONIC FILING Mr. Charles Terreni, Chief Clerk
CHARTER FIBERLINK SC-CCo, LLC Betty Sanders Director Regulatory Affairs Direct: 314-288 -3259 Email: bettv.sanders@chartercor(l.com April 12, 2010 VIA ELECTRONIC FILING Mr. Charles Terreni, Chief Clerk
FDIC Money Smart Financial Education Program
 Use this tip sheet to identify and address issues surrounding your credit and its history. This tip sheet covers the following topics: Ordering your credit report Reading a credit report Disputing information
Use this tip sheet to identify and address issues surrounding your credit and its history. This tip sheet covers the following topics: Ordering your credit report Reading a credit report Disputing information
510(k) SUMMARY. OPTION-vyM Urinary Catheter
 510(k) SUMMARY OPTICON MEDICAL, INC. OPTION-vyM Urinary Catheter Submitter s Name and Contact Information Opticon Medical, Inc. 7001 Post Road, Suite 100 Dublin, OH 430 16 Primary Contact: Glenn D. Brunner,
510(k) SUMMARY OPTICON MEDICAL, INC. OPTION-vyM Urinary Catheter Submitter s Name and Contact Information Opticon Medical, Inc. 7001 Post Road, Suite 100 Dublin, OH 430 16 Primary Contact: Glenn D. Brunner,
BMR ~131o-lvleccai Kesearcn Ltd.
 BMR ~131o-lvleccai Kesearcn Ltd. Parkmnore Business Park West, Galway, Ireland Tel: +353 (0)91 774300 - Fax: +353 (0)91 774301 6 4& This summary is being submitted in accordance with the requirements of
BMR ~131o-lvleccai Kesearcn Ltd. Parkmnore Business Park West, Galway, Ireland Tel: +353 (0)91 774300 - Fax: +353 (0)91 774301 6 4& This summary is being submitted in accordance with the requirements of
Standard laryngoscope blade assemblies Directions for use
 1 2014 Welch Allyn, Inc. MM 721358 Ver. C Standard laryngoscope blade assemblies Directions for use Intended use About this document The rigid laryngoscope is a device used to examine and visualize a patient
1 2014 Welch Allyn, Inc. MM 721358 Ver. C Standard laryngoscope blade assemblies Directions for use Intended use About this document The rigid laryngoscope is a device used to examine and visualize a patient
2:4 Letter to client regarding choice between LLC and S corporation
 2:4 Letter to client regarding choice between LLC and S corporation Dear [Client]: I understand that you are interested in creating a new business entity for a [type of business] business. This letter
2:4 Letter to client regarding choice between LLC and S corporation Dear [Client]: I understand that you are interested in creating a new business entity for a [type of business] business. This letter
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS
 DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
Letter to United States Attorney Re: Filing of Abstract of Judgment, Abstract of Judgment Form, and Instructions
 Letter to United States Attorney Re: Filing of Abstract of Judgment, Abstract of Judgment Form, and Instructions - CMN United States Attorney Re: Dear : We enclose the original and two copies of an abstract
Letter to United States Attorney Re: Filing of Abstract of Judgment, Abstract of Judgment Form, and Instructions - CMN United States Attorney Re: Dear : We enclose the original and two copies of an abstract
sbchoi~kku.edu jeremy~sooil.com DANA Diabecare IlS Insulin Infusion pump Infusion pump Class II
 510(k) Summary for DANA Diabecare IS SPONSOR SOOIL Development Co, Ltd. 196-1, Dogok-dong, Kangnam-gu Seoul 135-270 Korea 2 2007 Contact Person: Soo Bong Choi Telephone: 82-2-3463-0041 Fax: 82-2-3463-7077
510(k) Summary for DANA Diabecare IS SPONSOR SOOIL Development Co, Ltd. 196-1, Dogok-dong, Kangnam-gu Seoul 135-270 Korea 2 2007 Contact Person: Soo Bong Choi Telephone: 82-2-3463-0041 Fax: 82-2-3463-7077
North Carolina State University Emergency Facilities Closure Checklist- Part I
 North Carolina State University Emergency Facilities Closure Checklist- Part I Unplanned or spontaneous events often disrupt daily operations on campus. In the event that an incident may interrupt your
North Carolina State University Emergency Facilities Closure Checklist- Part I Unplanned or spontaneous events often disrupt daily operations on campus. In the event that an incident may interrupt your
February 5, 2015. Dear Kristin Pabst,
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 February 5, 2015, Inc.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 February 5, 2015, Inc.
Make the Move to Metrum Community Credit Union
 Make the Move to Metrum Community Credit Union Making the move to a new financial doesn t have to be a hassle! We ve helped many members move their checking accounts to Metrum Community Credit Union (MCCU),
Make the Move to Metrum Community Credit Union Making the move to a new financial doesn t have to be a hassle! We ve helped many members move their checking accounts to Metrum Community Credit Union (MCCU),
September 17, 2014. Keeler Instruments Inc. Mr. Eugene R. Van Arsdale Marketing Manager 459 Parkway Broomall, PA 19008
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 September 17, 2014 Keeler
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 September 17, 2014 Keeler
AW Server 510 (k) Summary of Safety and Effectiveness
 SEP 2 2008 GE Medical Systems AW Server 510 (k) Summary of Safety and Effectiveness This 510(k) summary of safety and effectiveness information is submitted in accordance with the requirements of 21 CFR
SEP 2 2008 GE Medical Systems AW Server 510 (k) Summary of Safety and Effectiveness This 510(k) summary of safety and effectiveness information is submitted in accordance with the requirements of 21 CFR
t-doc Instrument Intelligence take command of your operations Always with you
 t-doc Instrument Intelligence take command of your operations Always with you 2 T-DOC TAKE COMMAND TAKE YOUR OPERATIONS TO NEW LEVELS Many hospitals lack an overview of their instrument inventory, not
t-doc Instrument Intelligence take command of your operations Always with you 2 T-DOC TAKE COMMAND TAKE YOUR OPERATIONS TO NEW LEVELS Many hospitals lack an overview of their instrument inventory, not
Confinement Waiver Instructions
 Confinement Waiver Instructions Mail or fax completed form to: P.O. Box 1555, Des Moines, IA 50306-1555 Fax: 800 531 0038 Contact us: Annuity Customer Contact Center Tel: 888 266 8489 Athene Annuity and
Confinement Waiver Instructions Mail or fax completed form to: P.O. Box 1555, Des Moines, IA 50306-1555 Fax: 800 531 0038 Contact us: Annuity Customer Contact Center Tel: 888 266 8489 Athene Annuity and
You recently called the Medicare Rights helpline for assistance with a denial from your Medicare private health plan.
 Date: Dear Helpline Caller: The Medicare Rights Center is a national, nonprofit organization. We help older adults and people with disabilities with their Medicare problems. We support caregivers and train
Date: Dear Helpline Caller: The Medicare Rights Center is a national, nonprofit organization. We help older adults and people with disabilities with their Medicare problems. We support caregivers and train
AORN Position Statement on Allied Health Care Providers and Support Personnel in the Perioperative Practice Setting
 AORN Position Statement on Allied Health Care Providers and Support Personnel in the Perioperative Practice Setting POSITION STATEMENT The perioperative RN is accountable for patient outcomes resulting
AORN Position Statement on Allied Health Care Providers and Support Personnel in the Perioperative Practice Setting POSITION STATEMENT The perioperative RN is accountable for patient outcomes resulting
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
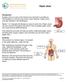 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS)
 Initial REMS approval: 02/2013 Most recent modification: 06/2015 Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS) This REMS applies
Initial REMS approval: 02/2013 Most recent modification: 06/2015 Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS) This REMS applies
Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry
 Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry The portion of this guidance document setting forth the submission procedures for risk evaluation and mitigation
Risk Evaluation and Mitigation Strategies: Modifications and Revisions Guidance for Industry The portion of this guidance document setting forth the submission procedures for risk evaluation and mitigation
August 21, 2012. Dear Mr. Marcinowski:
 August 21, 2012 Mr. Frank Marcinowski Deputy Assistant Secretary for Technical and Regulatory Support Office of Environmental Management U.S. Department of Energy 1000 Independence Avenue, S.W. Washington,
August 21, 2012 Mr. Frank Marcinowski Deputy Assistant Secretary for Technical and Regulatory Support Office of Environmental Management U.S. Department of Energy 1000 Independence Avenue, S.W. Washington,
CARE PATHS/DECISION POINT REVIEW
 Personal Service Insurance Company PO Box 3001 Plymouth Meeting, PA 19462 Ph: 610.832.4940 Fax: 610.832.2138 Toll Free: 800.954.2442 Date (##/##/####) Physician Name Street Address City, State, Zip Claimant:
Personal Service Insurance Company PO Box 3001 Plymouth Meeting, PA 19462 Ph: 610.832.4940 Fax: 610.832.2138 Toll Free: 800.954.2442 Date (##/##/####) Physician Name Street Address City, State, Zip Claimant:
Hot Topics In Infection Control!
 2015 SASKPIC Fall Conference Hot Topics In Infection Control! According to the Provincial ICCs that is Objectives Provide context for UTI prevention in Continuing Care settings Recommendations and tools
2015 SASKPIC Fall Conference Hot Topics In Infection Control! According to the Provincial ICCs that is Objectives Provide context for UTI prevention in Continuing Care settings Recommendations and tools
To provide direction for the safe handling, administration and disposal of hazardous drugs.
 Subsection: MEDICATION Related terms: Cytotoxic Drugs, Antineoplastic Drugs Authorized by: Clinical Directors CS-04-02-01 Page 1 of 9 Date Established: October 2006 Date For Review: September 2014 Dates
Subsection: MEDICATION Related terms: Cytotoxic Drugs, Antineoplastic Drugs Authorized by: Clinical Directors CS-04-02-01 Page 1 of 9 Date Established: October 2006 Date For Review: September 2014 Dates
L~l t l V~! MAY 23 200?
 L~l t l V~! MAY 23 200? 510 (k) Summay (As required by 21 CFR 807.92 and 21 CFR 807.93) NAME OF SPONSOR: 510(K) CONTACT: DePuy Orthopaedics, Inc. 700 Orthopaedic Drive Warsaw, Indiana 46582 Establishment
L~l t l V~! MAY 23 200? 510 (k) Summay (As required by 21 CFR 807.92 and 21 CFR 807.93) NAME OF SPONSOR: 510(K) CONTACT: DePuy Orthopaedics, Inc. 700 Orthopaedic Drive Warsaw, Indiana 46582 Establishment
Tax Basis Information Required by Internal Revenue Code Section 6045B as of October 4, 2011
 Tax Basis Information Required by Internal Revenue Code Section 6045B as of October 4, 2011 On October 3, 2011, Fortune Brands, Inc. ( Fortune Brands ) distributed shares of common stock of Fortune Brands
Tax Basis Information Required by Internal Revenue Code Section 6045B as of October 4, 2011 On October 3, 2011, Fortune Brands, Inc. ( Fortune Brands ) distributed shares of common stock of Fortune Brands
Title 16. Board of Pharmacy Proposed Text
 Title 16. Board of Pharmacy Proposed Text Proposal to add new Article 9.1 of Division 17 of Title 16 of the California Code of Regulations and a new Article title as follows: Article 9.1. Prescription
Title 16. Board of Pharmacy Proposed Text Proposal to add new Article 9.1 of Division 17 of Title 16 of the California Code of Regulations and a new Article title as follows: Article 9.1. Prescription
RFID Survives Sterilization to Deliver Medical Device Tracking Solution
 RFID Survives Sterilization to Deliver Medical Device Tracking Solution and UDI Compliance Introduction Healthcare providers are increasingly turning to technology solutions to help improve patient outcomes,
RFID Survives Sterilization to Deliver Medical Device Tracking Solution and UDI Compliance Introduction Healthcare providers are increasingly turning to technology solutions to help improve patient outcomes,
BIOHAZARDOUS/MEDICAL WASTE MANAGEMENT PLAN
 l BIOHAZARDOUS/MEDICAL WASTE MANAGEMENT PLAN MARCH 2014 1 Contents Purpose...3 Regulatory Reference...3 Definitions...3 Waste Generation Points...4 Student Health Center...4 Biology...4 Athletics...4 Trauma
l BIOHAZARDOUS/MEDICAL WASTE MANAGEMENT PLAN MARCH 2014 1 Contents Purpose...3 Regulatory Reference...3 Definitions...3 Waste Generation Points...4 Student Health Center...4 Biology...4 Athletics...4 Trauma
Bathroom Installation Guide
 Bathroom Installation Guide Step-by-step installation of your cabinets We do a lot of groundwork to make it as simple as possible for you to assemble and install your new bathroom furniture yourself. We
Bathroom Installation Guide Step-by-step installation of your cabinets We do a lot of groundwork to make it as simple as possible for you to assemble and install your new bathroom furniture yourself. We
By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA
 SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
SMALL BOWEL BLEEDING: CAUSES, DIAGNOSIS AND TREATMENT By Anne C. Travis, M.D., M.Sc. and John R. Saltzman, M.D., FACG Brigham and Women's Hospital Harvard Medical School Boston, MA 1. What is the small
Cleaning and High Level Disinfection of Semi-Critical Medical Devices
 I. Purpose: A. To provide guidance for cleaning, processing and high level disinfection (HLD) of semi-critical devices using cylinders, bins/pans, or automated endoscopic reprocessors. This process is
I. Purpose: A. To provide guidance for cleaning, processing and high level disinfection (HLD) of semi-critical devices using cylinders, bins/pans, or automated endoscopic reprocessors. This process is
SERVICEMEMBERS CIVIL RELIEF ACT LEASE TERMINATION
 ASA DIX LEGAL BRIEF A PREVENTIVE LAW SERVICE OF THE JOINT READINESS CENTER LEGAL SECTION UNITED STATES ARMY SUPPORT ACTIVITY DIX KEEPING YOU INFORMED ON YOUR PERSONAL LEGAL NEEDS SERVICEMEMBERS CIVIL RELIEF
ASA DIX LEGAL BRIEF A PREVENTIVE LAW SERVICE OF THE JOINT READINESS CENTER LEGAL SECTION UNITED STATES ARMY SUPPORT ACTIVITY DIX KEEPING YOU INFORMED ON YOUR PERSONAL LEGAL NEEDS SERVICEMEMBERS CIVIL RELIEF
PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric surgery: correlation with pulmonary function.
 Version 2. 8.09.2011 Hammersmith Hospital Du Cane Rd London W12 0HS Tel: 020 8383 1000 www.imperial.nhs.uk PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric
Version 2. 8.09.2011 Hammersmith Hospital Du Cane Rd London W12 0HS Tel: 020 8383 1000 www.imperial.nhs.uk PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric
Application Submitted on Received on Supplement type NDA 021875/S-005 July 24, 2008 July 25, 2008 Changes Being Effected
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 021875/S-005, S-008, S-015 SUPPLEMENT APPROVAL Cephalon Inc. Attention: Paul Kirsch Senior Director and Group
November 20, 2014. Vascular Flow Technologies Ltd. Edwin Lindsay VP of QA/RA Prospect Business Centre, Gemini Cresent Dundee DD2 1TY United Kingdom
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 November 20, 2014 Vascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 November 20, 2014 Vascular
REPROCESSING OF FLEXIBLE CYSTOSCOPES
 ALERT Joint AUA/SUNA White Paper on the REPROCESSING OF FLEXIBLE CYSTOSCOPES (2/8/14): The STERIS System 1E (SS1E) liquid chemical sterilant processing system has become available for reuseable processing
ALERT Joint AUA/SUNA White Paper on the REPROCESSING OF FLEXIBLE CYSTOSCOPES (2/8/14): The STERIS System 1E (SS1E) liquid chemical sterilant processing system has become available for reuseable processing
4.4 Attendance Management Policy
 Policy Statement The is committed to providing excellence in service to the general public. It is important for all employees of the Government of Nova Scotia to work as a team in the attainment of this
Policy Statement The is committed to providing excellence in service to the general public. It is important for all employees of the Government of Nova Scotia to work as a team in the attainment of this
Adoption of Business Net Income
 Adoption of Business Net Income 1 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking
Adoption of Business Net Income 1 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking
Document Control. White Paper By Galaxy Consulting. At Your Service Today Tomorrow We Appreciate The Privilege Of Serving You! Abstract.
 Document Control April White Paper By Galaxy Consulting A At Your Service Today Tomorrow We Appreciate The Privilege Of Serving You! Abstract December 2015 The primary purpose of document control is to
Document Control April White Paper By Galaxy Consulting A At Your Service Today Tomorrow We Appreciate The Privilege Of Serving You! Abstract December 2015 The primary purpose of document control is to
Frequently Asked Questions (FAQs) Troubled Asset Relief Program (TARP) Standards for Compensation and Corporate Governance
 Frequently Asked Questions (FAQs) Troubled Asset Relief Program (TARP) Standards for Compensation and Corporate Governance Section 111 of the Emergency Economic Stabilization Act of 2008 (EESA), as amended
Frequently Asked Questions (FAQs) Troubled Asset Relief Program (TARP) Standards for Compensation and Corporate Governance Section 111 of the Emergency Economic Stabilization Act of 2008 (EESA), as amended
CPT COD1NG UPDATES Gastroenterology CPT Advisors
 2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
Guidelines 85. Contents. 1. Scope of this guideline. 1. Scope of this guideline
 Guidelines 85 ESGE/ESGENA guideline for process validation and routine testing for reprocessing endoscopes in washer-disinfectors, according to the European Standard pren ISO 15883 parts 1, 4 and 5 Authors
Guidelines 85 ESGE/ESGENA guideline for process validation and routine testing for reprocessing endoscopes in washer-disinfectors, according to the European Standard pren ISO 15883 parts 1, 4 and 5 Authors
Reprocessing Flexible Endoscopes Avoiding Reprocessing Errors Critical for Infection Prevention and Control
 Reprocessing Flexible Endoscopes Avoiding Reprocessing Errors Critical for Infection Prevention and Control By Bradley Catalone, Ph.D., and George Koos Flexible endoscopy procedures are now a routine part
Reprocessing Flexible Endoscopes Avoiding Reprocessing Errors Critical for Infection Prevention and Control By Bradley Catalone, Ph.D., and George Koos Flexible endoscopy procedures are now a routine part
Disposal of chemical waste must be regarded as an integral part of all research projects and teaching programs involving chemical use.
 1. Purpose and Objectives This procedure provides information on the generation, collection, storage and disposal of chemical waste to ensure: that the collection, storage and disposal of chemical waste
1. Purpose and Objectives This procedure provides information on the generation, collection, storage and disposal of chemical waste to ensure: that the collection, storage and disposal of chemical waste
URGENT: FIELD SAFETY NOTICE MSS-15-637 FA. Medical Device Safety Advisory Notice BD Plastipak Syringes / BD Oral Syringes
 Becton Dickinson GmbH Tullastr. 8-12 69126 Heidelberg Postfach 10 16 29 69006 Heidelberg Tel.: 06221 3050 Fax: 06221 305216 www.bd.com/de Becton Dickinson GmbH Postfach 10 16 29 69006 Heidelberg URGENT:
Becton Dickinson GmbH Tullastr. 8-12 69126 Heidelberg Postfach 10 16 29 69006 Heidelberg Tel.: 06221 3050 Fax: 06221 305216 www.bd.com/de Becton Dickinson GmbH Postfach 10 16 29 69006 Heidelberg URGENT:
internet internet website: website: www.clalglobal.co.il. Email: clalglobalservice@clal-ins.co.il Fax: +972-77-6383448 Fax: +972-77-6383448
 Dear Customer, Dear Customer, Further Further to your to request your request to exercise to exercise your rights your in rights accordance in accordance with the with Insurance the Insurance Policy Policy
Dear Customer, Dear Customer, Further Further to your to request your request to exercise to exercise your rights your in rights accordance in accordance with the with Insurance the Insurance Policy Policy
LEVEL 2 AWARD IN THE SAFE USE OF PESTICIDES (QCF) (0216) FOUNDATION UNIT PA1
 NPTC Registered Charity No. 09649 ABBEY PARK, STARETON, WARWICKSHIRE, CV8 LY Tel: 04 7685 7300 Fax: 04 7669 68 Email: information@nptc.org.uk LEVEL AWARD IN THE SAFE USE OF PESTICIDES (QCF) (06) FOUNDATION
NPTC Registered Charity No. 09649 ABBEY PARK, STARETON, WARWICKSHIRE, CV8 LY Tel: 04 7685 7300 Fax: 04 7669 68 Email: information@nptc.org.uk LEVEL AWARD IN THE SAFE USE OF PESTICIDES (QCF) (06) FOUNDATION
BUSINESS CREDIT APPLICATION. Enclosed please find forms necessary for establishing an account with us.
 BUSINESS CREDIT APPLICATION Dear Prospective Customer, Enclosed please find forms necessary for establishing an account with us. Fill out BOTH pages of the credit application. An authorized company officer
BUSINESS CREDIT APPLICATION Dear Prospective Customer, Enclosed please find forms necessary for establishing an account with us. Fill out BOTH pages of the credit application. An authorized company officer
