BEFORE USING PRODUCT, READ THE FOLLOWING
|
|
|
- Dale O’Connor’
- 9 years ago
- Views:
Transcription
1 Caution: Federal (USA) law restricts this device to sale by or on the order of a licensed physician or properly licensed practitioner. BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY. 1. DEVICE DESCRIPTION JUVÉDERM VOLUMA XC is a sterile, biodegradable, non-pyrogenic, viscoelastic, clear, colorless, homogenized gel implant. It consists of crosslinked hyaluronic acid (HA) produced by Streptococcus equi bacteria, formulated to a concentration of 20 mg/ml and 0.3% w/w lidocaine in a physiologic buffer. 2. INTENDED USE/INDICATIONS JUVÉDERM VOLUMA XC is indicated for deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation to correct age-related volume deficit in the mid-face in adults over the age of CONTRAINDICATIONS JUVÉDERM VOLUMA XC is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies JUVÉDERM VOLUMA XC contains trace amounts of gram-positive bacterial proteins and is contraindicated for patients with a history of allergies to such material JUVÉDERM VOLUMA XC contains lidocaine and is contraindicated for patients with a history of allergies to such material 4. WARNINGS The product must not be injected into blood vessels. Introduction of JUVÉDERM VOLUMA XC into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Symptoms of vascular occlusion and embolization include pain that is disproportionate to the procedure or remote to the injection site, immediate blanching that extends beyond the injected area and that may represent vascular tributary distribution, and color changes that reflect ischemic tissue such as a dusky or reticular appearance. The treating healthcare professional should be knowledgeable regarding appropriate interventions in the event of intravascular disseminated injection. Intervention should be taken should these signs occur (see Healthcare Professional Instructions #13) As with all dermal filler procedures, JUVÉDERM VOLUMA XC should not be used in vascular rich areas. Use in these areas, such as glabella and nose, has resulted in cases of vascular embolization and symptoms consistent with ocular vessel occlusion, such as blindness Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled Treatment site reactions consist mainly of short-term inflammatory symptoms and generally resolve within 2 to 4 weeks. Refer to the ADVERSE EVENTS section for details 5. PRECAUTIONS JUVÉDERM VOLUMA XC is packaged for single-patient use. Do not resterilize. Do not use if package is open or damaged Based on preclinical studies and a toxicological risk assessment, patients should be limited to 20 ml of JUVÉDERM VOLUMA XC per 60 kg (130 lbs) body mass per year. The safety of injecting greater amounts has not been established The safety and effectiveness for the treatment of anatomic regions other than the mid-face have not been established in controlled clinical studies As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed JUVÉDERM VOLUMA XC is to be used as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, homogeneity, and performance of the product The safety for use during pregnancy, in breastfeeding females, and in patients with very thin skin in the mid-face region has not been established The safety for use in patients under 35 years or over 65 years has not been established The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied JUVÉDERM VOLUMA XC should be used with caution in patients on immunosuppressive therapy Patients who are using substances that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at treatment sites Patients who experience skin injury near the site of JUVÉDERM VOLUMA XC implantation may be at a higher risk for adverse events Patients may experience late onset nodules with use of dermal fillers, including JUVÉDERM VOLUMA XC. Refer to ADVERSE EVENTS section for details After use, treatment syringes and needles may be potential biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements JUVÉDERM VOLUMA XC injectable gel is a clear, colorless gel without particulates. In the event that the content of a syringe shows signs of separation and/or appears cloudy, do not use the syringe; notify Allergan Product Surveillance at (877) Adverse event data after repeat treatment with JUVÉDERM VOLUMA XC is being collected as part of a post-approval study The long-term safety of repeat treatments has not been established JUVÉDERM VOLUMA XC should only be used by healthcare professionals who have appropriate experience and who are knowledgeable about the anatomy and the product for use in deep (subcutaneous and/or supraperiosteal) injection for cheek augmentation Failure to comply with the needle attachment instructions could result in needle disengagement and/or product leakage at the LUER-LOK and needle hub connection 6. ADVERSE EVENTS A. Clinical Evaluation of JUVÉDERM VOLUMA XC In the randomized, controlled clinical trial to evaluate the safety and effectiveness of JUVÉDERM VOLUMA XC, there were 238 subjects treated with JUVÉDERM VOLUMA XC in the mid-face (zygomaticomalar region, anteromedial cheek, and/or submalar region, see Figure 1) during the primary phase of the study. Touch-up treatments occurred approximately 30 days after initial injection. After the 6-month blinded no treatment control period, control subjects were allowed to receive treatment; 32
2 control subjects were treated in the study. Preprinted diary forms were used by subjects after treatment to record specific signs and symptoms experienced during each of the first 30 days after initial, touch-up, and repeat treatments in each region of the mid-face. Of the 270 subjects who underwent treatment (from both the treatment and control groups), 265 completed the diary forms. A subset of subjects has also undergone repeat treatment following completion of the extended follow-up phase of the study, with 120 subjects completing diary forms after repeat treatment. Subjects were instructed to rate each treatment site response listed on the diary as Mild (barely noticeable), Moderate (uncomfortable), Severe (severe discomfort), or None. After initial treatment with JUVÉDERM VOLUMA XC, 98% of subjects reported experiencing a local treatment site response. Subjects rated treatment site responses as predominantly mild (21.5%) or moderate (59.2%) in severity with a duration of 2 to 4 weeks. For those treatment site responses evaluated as moderate or severe, the median duration as moderate or severe was 2 days, and the median time to complete resolution was 6 days. Based on available data from 120 subjects, the severity of CTRs following repeat treatment is similar, with a reduced incidence and duration compared to initial treatment. Treatment site responses reported by > 5% of subjects after initial treatments are summarized by severity in Table 1 and by duration in Table 2. Treatment Site Response Any Treatment Site Response Tenderness Swelling Firmness Lumps/Bumps Bruising Pain Redness Discoloration Itching Table 1: Treatment Site Responses by Maximum Severity Occurring in > 5% of Subjects After Initial Treatment (N = 265) Severity a Total Mild Moderate Severe % (n/n b ) % (n/n) % (n/n) % (n/n) 98.1% (260/265) 92.1% (244/265) 85.7% (227/265) 82.3% (218/265) 81.1% (215/265) 77.7% (206/265) 66.4% (176/265) 66.0% (175/265) 41.1% (109/265) 38.5% (102/265) 21.5% (56/260) 46.3% (113/244) 46.7% (106/227) 37.6% (82/218) 41.4% (89/215) 37.4% (77/206) 59.1% (104/176) 60.0% (105/175) 62.4% (68/109) 70.6% (72/102) 59.2% (154/260) 50.0% (122/244) 43.6% (99/227) 54.6% (119/218) 48.8% (105/215) 51.5% (106/206) 38.6% (68/176) 36.0% (63/175) 27.5% (30/109) 18.6% (19/102) 19.2% (50/260) 3.7% (9/244) 9.7% (22/227) 7.8% (17/218) 9.8% (21/215) 11.2% (23/206) 2.3% (4/176) 4.0% (7/175) 10.1% (11/109) 10.8% (11/102) a Maximum severity reported in the diary. The denominator for percentages by severity is the number of subjects with the corresponding treatment site response. b N denotes number of subjects who recorded responses in the diaries after the initial treatment. Treatment site responses reported by 5% of subjects included ache, acne, bulge, bumps, cheek larger upon waking up, dry patch, fine wrinkles, injection/needle marks, numbness, pigmentation from treatment, puffiness, rash, scratch near injection point, soreness, tightness, and yellowness. Treatment Site Response Any Treatment Site Response Tenderness Swelling Firmness Lumps/Bumps Bruising Pain Redness Discoloration Itching Table 2: Duration of Treatment Site Responses After Initial Treatment (N = 265) Duration a Total 1-3 Days 4-7 Days 8-14 Days Days > 30 Days % (n/n b ) % (n/n) % (n/n) % (n/n) % (n/n) % (n/n) 98.1% (260/265) 92.1% (244/265) 85.7% (227/265) 82.3% (218/265) 81.1% (215/265) 77.7% (206/265) 66.4% (176/265) 66.0% (175/265) 41.1% (109/265) 38.5% (102/265) 8.1% (21/260) 29.9% (73/244) 41.0% (93/227) 26.6% (58/218) 21.4% (46/215) 24.8% (51/206) 56.3% (99/176) 59.4% (104/175) 64.2% (70/109) 81.4% (83/102) 22.7% (59/260) 30.7% (75/244) 33.0% (75/227) 29.8% (65/218) 22.3% (48/215) 30.6% (63/206) 31.3% (55/176) 28.0% (49/175) 19.3% (21/109) 16.7% (17/102) 24.6% (64/260) 27.9% (68/244) 17.6% (40/227) 20.2% (44/218) 22.3% (48/215) 29.6% (61/206) 9.7% (17/176) 8.6% (15/175) 6.4% (7/109) 2.0% (2/102) 24.6% (64/260) 8.6% (21/244) 5.3% (12/227) 11.0% (24/218) 18.1% (39/215) 14.6% (30/206) 2.8% (5/176) 2.3% (4/175) 5.5% (6/109) 0% (0/102) 20.0% (52/260) 2.9% (7/244) 3.1% (7/227) 12.4% (27/218) 15.8% (34/215) 0.5% (1/206) 0% (0/176) 1.7% (3/175) 4.6% (5/109) 0% (0/102) a Maximum duration reported in the diary. The denominator for percentages by duration is the number of subjects with the corresponding treatment site response. b N denotes number of subjects who recorded responses in the diaries after the initial treatment. Treatment site responses reported in subject diaries that lasted longer than 30 days were considered adverse events (AEs). AEs were also reported by the Treating Investigator at all follow-up visits where applicable. Table 3 summarizes device- and injection-related AEs that occurred with a frequency of > 1%. These adverse events were seen more frequently in subjects that received injection volumes greater than 9 ml, and in older subjects (> 60 years). Rarely, adverse events occurred weeks to months after the injection procedure. Among the 270 treated subjects, 32.6% (88/270) experienced device- and injection-related AEs following initial and touch-up treatment, 99% (624/627) of which were reported at a treatment site. The treatment site AEs were evenly divided across the 3 mid-facial regions. Information on AEs following repeat treatment is being collected as part of the post-approval study. Table 3: Device- and Injection-Related Adverse Events Reported by Treating Investigator and Subjects Occurring in > 1% of Treated Subjects (N = 270) Adverse Event Treated Subjects % (n/n) Treatment site mass 18.9% (51/270) Treatment site induration 14.1% (38/270) Treatment site swelling 7.0% (19/270) Treatment site pain 5.9% (16/270) Treatment site hematoma 3.7% (10/270) Treatment site discoloration 2.2% (6/270) Treatment site erythema 1.9% (5/270) Treatment site reaction 1.5% (4/270) Device- and injection related adverse events occurring in 1% of subjects included injection site hypertrophy (0.7%), nodule (0.7%), inflammation (0.4%), injection site anesthesia (0.4%), injection site dryness (0.4%), injection site erosion (0.4%), mass (0.4%), contusion (0.4%) and syncope (0.4%).
3 Two subjects (0.7%; 2/270) reported 3 serious adverse events (SAEs) that were considered to be related to the device. Approximately 6 months after treatment, after being scratched near the treated area by a tree branch, one subject experienced inflammation under the left eye. The subject also experienced nodularity in the right cheek approximately 7 months after treatment. The second subject experienced lumps in the cheeks approximately 7 months after treatment. A couple of days before the onset, the subject experienced myofascial pain and body aches. Treatment of the SAEs included topical steroids, oral antibiotics, intralesional steroids, anti-inflammatory medication, and hyaluronidase. All events resolved. Figure 1. Mid-Face Regions Treated B. Other Safety Data Post-Market Surveillance JUVÉDERM VOLUMA without lidocaine has been marketed outside the US since 2005, and JUVÉDERM VOLUMA with lidocaine has been marketed outside the US since As of December 31, 2012, the following AEs were received from post-market surveillance for JUVÉDERM VOLUMA with and without lidocaine with a frequency 5 and were not observed in the clinical study; this includes reports received globally from all sources including scientific journals and voluntary reports. All AEs obtained through post-market surveillance are listed in order of number of reports received: inflammatory reaction, lack of correction, infection, migration, granuloma, allergic reaction, abscess, necrosis, numbness, and vision abnormalities. Reported treatments include: antibiotics, steroids, hyaluronidase, anti-inflammatories, anti-histamines, aspiration, radio frequency therapy, laser treatment, ice, massage, warm compress, analgesics, anti-viral, ultrasound, excision, drainage, and surgery. Vision abnormalities have been reported following injection of JUVÉDERM VOLUMA, with and without lidocaine, into the nose, glabella, periorbital area, and/or cheek, with a time to onset ranging from immediate to 1 week following injection. Reported treatments include anticoagulant, steroid treatment, and surgery. Outcomes ranged from resolved to ongoing at the time of last contact. Events requiring medical intervention, and events where resolution information is not available, were reported after injection of JUVÉDERM VOLUMA with and without lidocaine in the highly vascularized areas of the glabella, nose, and periorbital area, which are outside the device indications for use (see WARNINGS section). 7. CLINICAL STUDIES A. Pivotal Study for JUVÉDERM VOLUMA XC Pivotal Study Design A multi-center, single-blind, randomized, no-treatment controlled pivotal clinical study was conducted to evaluate the safety and effectiveness of JUVÉDERM VOLUMA XC for cheek augmentation to correct age-related volume deficit in the midface. Subjects were randomized to treatment or no-treatment control in a 5.3:1 ratio. Treatment group subjects underwent treatment with JUVÉDERM VOLUMA XC at the outset of the study. Up to 2 treatments approximately 1 month apart (initial treatment and up to 1 touch-up treatment) were allowed. The Treating Investigator determined the appropriate volume of JUVÉDERM VOLUMA XC to be injected in the 3 sub-regions of the mid-face: zygomaticomalar region, anteromedial cheek region, and submalar region, which are depicted in Figure 1. Treatment of the nasolabial folds and periorbital region was prohibited. The no-treatment control subjects had treatment delayed for 6 months. Treated subjects returned for routine safety visits with the Treating Investigator at 1, 3, and 6 months after the last treatment during the primary safety and effectiveness phase. All subjects returned for effectiveness follow-up visits with 2 independent Evaluating Investigators (EI) at 1, 3, and 6 months after the last treatment. EIs assessed subjects overall mid-face volume deficit on the validated 6-point photometric Mid-Face Volume Deficit Scale (MFVDS) as well as volume deficit for each of the 3 facial sub-regions. EIs also assessed subjects improvement on the 5-point Global Aesthetic Improvement Scale (GAIS), the 5-point photometric Nasolabial Fold Photo Severity Scale (NLFSS), and the 11-point Other Aesthetic Features of the Mid-Face questionnaire. Subjects performed self-assessments on MFVDS, GAIS, NLFSS, treatment goal achievement, satisfaction with mid-facial regions, selfperception of age, look and feel of the face, and satisfaction with facial appearance. Further, 3D facial photography was performed, and volume changes were calculated. During the extended follow-up period, subjects returned for safety and effectiveness evaluations at quarterly intervals up to 24 months or until any visit at or after Month 12 when the average of the EIs live assessments of the MFVDS returned to, or was worse than, the pre-treatment level. Control subjects followed a similar effectiveness evaluation schedule through Month 6 but were not treated and not required to undergo safety evaluations or self-assessments of effectiveness. After Month 6, control subjects received treatment and followed the same treatment and follow-up schedule as the treatment group. An optional repeat treatment was offered to all subjects after completion of the extended follow-up period, with continued follow-up through 12 months after repeat treatment. Study Endpoints The primary effectiveness measure was the average of the 2 blinded EIs live assessments of the subject s overall mid-face volume deficit on the validated 6-point photometric MFVDS. A responder was defined as a subject with 1 grade improvement in the average MFVDS score since baseline. Effectiveness of JUVÉDERM VOLUMA XC was demonstrated if at least 70% of subjects treated with JUVÉDERM VOLUMA XC were responders at Month 6, and if the responder rate for the treatment group was statistically superior to that of the no-treatment control group at Month 6. Secondary measures included the level of improvement on the GAIS and MFVDS assessments for each region of the mid-face as assessed by the blinded EIs.
4 Subject Demographics A total of 345 subjects were enrolled in the study: 16 were screen failures primarily due to ineligibility, 30 were runin subjects, and 299 were randomized per protocol, 17 of whom discontinued prior to treatment. Of the remaining 282 subjects, 235 were randomized to the treatment group, and 47 were randomized to the control group. Three-fourths (74.0%, 174/235) of the treatment group completed the extended followup period. Sixty-one subjects (26.0%, 61/235) discontinued the study primarily due to loss to follow-up (34.4%, 21/61) or withdrawal of consent (36.1%, 22/61). At baseline, the majority of subjects in the treatment group (93.6%, 220/235) and all subjects in the control group (100%, 46/46) had moderate, significant, or severe volume deficit (encompassing scores of 2.5 through 5 on the MFVDS scale) in their mid-face according to the average of EI assessments. Subject demographics and pre-treatment characteristics are presented in Table 4. Table 4: Demographics and Pre-treatment Characteristics (N = 282) Treatment Group Control Group (N = 235) (N = 47) Characteristic % (n) % (n) Gender Female 80% (189) 79% (37) Male 20% (46) 21% (10) Age (years) Median Range (min, max) (35-65) (36-65) Race Caucasian 58% (137) 60% (28) Hispanic 15% (35) 9% (4) African-American 19% (44) 26% (12) Asian 4% (9) 6% (3) Other 4% (10) 0% (0) Fitzpatrick Skin Type I 3% (6) 4% (2) Treatment Characteristics II 26% (62) 21% (10) III 29% (67) 23% (11) IV 18% (43) 30% (14) V 19% (44) 19% (9) VI 6% (13) 2% (1) Multiple injection techniques were used for 95% of subjects, with the most common being tunneling, fanning, and serial puncture. Subjects were injected equally in the 3 facial sub-regions for a total median volume of 2.0 ml for the zygomaticomalar region, 2.0 ml for the anteromedial cheek, and 2.1 ml for the submalar region. The overall total volume used to achieve optimal correction for all 3 sub-regions ranged from 1.2 ml to 13.9 ml, with a median of 6.6 ml. The median volume at initial treatment was 4.8 ml. A touch-up treatment was performed for 82% (195/238) of subjects. The median total volume used for touch-up treatment was 1.9 ml. The volume of JUVÉDERM VOLUMA XC varied depending on the subject s volume deficit and treatment goal. rate for the treatment group was significantly greater (p < ) than the responder rate for the control group (a difference of 46.7%) at Month 6 (Table 5). JUVÉDERM VOLUMA XC was found to be effective in all Fitzpatrick skin phototypes, for males and females, and across the studied age range. Table 5: Effectiveness Summary Responder Rate at 6 Months Based on Evaluating Investigators Assessments Responder Rate at Month 6 p-value Treatment Group 85.6% (178/208) < Control Group a 38.9% (14/36) Difference in Responder Rates (Treatment rate - Control rate) 46.7% < a Includes 2 subjects who were treated in error. Secondary Effectiveness Results The GAIS responder rate for the treatment group was 82.2% (171/208) at Month 6, where the responder rate was the percent of subjects with a score of 1 (improved or much improved) on the GAIS for overall mid-face volume based on EIs assessments. At Month 6 the MFVDS responder rate for each of the facial sub-regions was above 75%. Extended Follow-Up Table 6 shows the mean MFVDS scores during the extended follow-up period (Months 9 to 24). The mean improvement was clinically significant ( 1 point), with the majority of subjects demonstrating improvement. 86.6% (181/209) at Month % (172/203) at Month % (128/179) at Month % (112/167) at Month 24 Table 6: Mean MFVDS Scores Over 24 Months Visit N Mean MFVDS Score Mean Change Since Baseline Baseline N/A Month Month Month Month Subject Self-Assessments Subjects performed numerous self-assessments, including satisfaction with facial appearance, self-perception of age, and NLF severity. At each time point more than three-fourths of the treatment group subjects demonstrated an improvement in the overall satisfaction with facial appearance since baseline. In addition, the majority of treatment group subjects perceived themselves as looking younger than perceived at baseline, from 76.4% at Month 1 to 55.4% at Month 24. Subjects, on average, reported themselves as looking approximately 5 years younger at Month 6 and 3 years younger at Month 24. Lastly, more than half (57%, 236/414) of the treatment group subjects at Month 6 observed a 1-point improvement in their NLFs. Primary Effectiveness Results JUVÉDERM VOLUMA XC provided a clinically and statistically significant improvement in mid-face volume deficit compared to the no-treatment control group. Primary effectiveness was met in that significantly greater than 70% of subjects in the treatment group were responders (85.6% improved by 1 grade compared with their pre-treatment assessment, p < against the 70% responder rate threshold), and the responder
5 8. INSTRUCTIONS FOR USE A. To Attach Needle to Syringe STEP 1: Remove tip cap Hold syringe and pull tip cap off the syringe, as shown in Figure A. STEP 2: Insert needle Figure A Hold the syringe body and firmly insert the hub of the needle (provided in the JUVÉDERM VOLUMA XC package) into the LUER-LOK end of the syringe. STEP 3: Tighten the needle Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position, as shown in Figure C. NOTE: If the position of the needle cap is as shown in Figure D, it is not attached correctly. Continue to tighten until the needle is seated in the proper position. STEP 4: Remove the needle cap Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap, as shown in Figure E. Figure B Figure C Figure D Figure E B. Healthcare Professional Instructions 1. JUVÉDERM VOLUMA XC injectable gel is a crosslinked, robust, injectable gel formulation, injected using a 27G ½" or 25G 1" needle to volumize and contour the cheek for correction of mid-face volume deficit. 2. Prior to treatment, the patient s medical history should be obtained, and the patient should be fully apprised of the indications, contraindications, warnings, precautions, treatment responses, adverse reactions, and method of administration. Patients also should be advised that supplemental touch-up implantations may be required to achieve and maintain maximum correction. 3. The patient s soft-tissue deficiencies should be characterized with regard to etiology, distensibility, stress at the site, and depth of lesion. Pre-treatment photographs are recommended. 4. After ensuring that the patient has thoroughly washed the treatment area with soap and water, the area should be prepped with alcohol or other antiseptic. Prior to injecting, depress the plunger rod until the product flows out of the needle. 5. If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the injection and replace the needle. 6. After insertion of the needle, and just before injection, the plunger rod should be withdrawn slightly to aspirate and verify the needle is not intravascular. 7. After the first small amount of material has been injected into the patient, wait a full 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection. 8. The injection technique for JUVÉDERM VOLUMA XC with regard to the angle and orientation of the bevel, the depth (subcutaneous and/or submuscular/supraperiosteal) of injection, and the quantity administered may vary depending on the area being treated. Injection of JUVÉDERM VOLUMA XC too superficially (intradermally), or in large volumes over a small area, may result in visible and persistent lumps and/or discoloration. 9. Tunneling, fanning, serial puncture, crosshatching, and ferning techniques may be used with JUVÉDERM VOLUMA XC to achieve optimal results. Injection may be administered in an antegrade or retrograde fashion. Inject JUVÉDERM VOLUMA XC while applying even pressure on the plunger rod and slowly moving the needle in the subcutaneous or submuscular/supraperiosteal plane. 10. JUVÉDERM VOLUMA XC should be distributed in small aliquots (small boluses of 0.1 ml to 0.2 ml) over a large area to reduce the risk of persistent lumpiness. 11. With submuscular/supraperiosteal injection, the number of times the needle passes through the muscle should be minimized to reduce the risk of bruising. It is important to stop injecting before the needle tip reaches the level of the deep dermis to prevent material from being placed too superficially in the skin. 12. Correct to 100% of the desired volume effect. Do not overcorrect. The degree and duration of the correction depend on the character of the defect treated, the tissue stress at the implant site, the depth of the implant in the tissue, and the injection technique. Markedly indurated defects may be difficult to correct.
6 13. If immediate blanching occurs, the injection should be stopped and the area massaged until it returns to a normal color. Blanching may represent a vessel occlusion. If normal skin coloring does not return, do not continue with the injection. Treat in accordance with American Society for Dermatologic Surgery guidelines, which include hyaluronidase injection The area of lost facial volume should be lifted by the end of the injection. When injection is completed, the treated site may be gently massaged to mold the product to the contour of the surrounding tissue and assure that it is evenly distributed and conforms to the contour of the surrounding tissues. If overcorrection occurs, massage the area between your fingers or against an underlying superficial bone to obtain optimal results. 15. With patients who have localized swelling, the degree of correction is sometimes difficult to judge at the time of treatment. In these cases, it is better to invite the patient back to the office for a touch-up treatment. 16. After the initial treatment, an additional treatment may be necessary to achieve the desired level of correction. The same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as mid-face volume deficit severity, skin elasticity, and dermal thickness at the treatment site. 17. Patients may experience treatment site responses, which typically resolve within 2 to 4 weeks. Ice may be applied for a brief period following treatment to minimize swelling and reduce pain. 18. The healthcare professional should instruct the patient to promptly report any evidence of problems possibly associated with the use of JUVÉDERM VOLUMA XC. C. Patient Instructions It is recommended that the following information be shared with patients: Within the first 24 hours, patients should avoid strenuous exercise and extensive sun or heat exposure. Exposure to any of the above may cause temporary redness, swelling, and/or itching at the treatment sites If the treated area is swollen, an ice pack may be applied to the site for a short period To report an adverse reaction, phone the Allergan Product Surveillance Department at (877) HOW SUPPLIED JUVÉDERM VOLUMA XC injectable gel is supplied in individual treatment syringes with needles as indicated on the carton. JUVÉDERM VOLUMA XC can be injected with either a 27G ½" or a 25G 1" needle. The volume in each syringe is as stated on the syringe label and on the carton. The contents of the syringe are sterile and non-pyrogenic. Do not resterilize. Do not use if package is open or damaged. 10. STORAGE Store at room temperature (up to 25ºC/77ºF). DO NOT FREEZE. JUVÉDERM VOLUMA XC injectable gel has a clear appearance. In the event that a syringe contains material that is not clear, do not use the syringe; notify Allergan Product Surveillance immediately at (877) To place an order, contact Allergan at (800) Irvine, CA USA Made in France 2014 Allergan, Inc. marks owned by Allergan, Inc. Patented. See: BD LUER-LOK is a registered trademark of Becton, Dickinson and Company. 1 Alam M, Gladstone H, Kramer EM, et al. ASDS guidelines of care: injectable fillers. Dermatol Surg. 2008;34(suppl 1):S115-S L3781 Rev02
NEHSNORTH EASTERN HEALTH SPECIALISTS
 COSMETIC DERMATOLOGY NEHSNORTH EASTERN HEALTH SPECIALISTS nehs.com.au CONSENT FORM ACNE Treatment I, DOB:, of authorize of North Eastern Health Specialist to perform hair removal with the BBL / Nd-Yag
COSMETIC DERMATOLOGY NEHSNORTH EASTERN HEALTH SPECIALISTS nehs.com.au CONSENT FORM ACNE Treatment I, DOB:, of authorize of North Eastern Health Specialist to perform hair removal with the BBL / Nd-Yag
There are different types of lasers that according the wavelength of their beams have different indications; the most common ones used on skin are:
 What is Laser Surgery? Laser (Light Amplification by Stimulated Emission of Radiation) surgery uses a very hot, focused beam of light to remove or cauterise a skin lesion and control bleeding without affecting
What is Laser Surgery? Laser (Light Amplification by Stimulated Emission of Radiation) surgery uses a very hot, focused beam of light to remove or cauterise a skin lesion and control bleeding without affecting
Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â)
 Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â) Please read this leaflet carefully before you start to use Rebif and each time your prescription is refilled since there may
Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â) Please read this leaflet carefully before you start to use Rebif and each time your prescription is refilled since there may
CONSENT FOR STEROID INJECTION
 CONSENT FOR STEROID INJECTION What is Cortisone? Cortisone is the name used to describe a group of drugs correctly known as corticosteroids. Cortisone is used to treat pain in various parts of the body
CONSENT FOR STEROID INJECTION What is Cortisone? Cortisone is the name used to describe a group of drugs correctly known as corticosteroids. Cortisone is used to treat pain in various parts of the body
Informed Consent for Cosmetic Laser Skin Resurfacing with the DOT laser
 Informed Consent for Cosmetic Laser Skin Resurfacing with the DOT laser INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedure, its risks, as well as alternative
Informed Consent for Cosmetic Laser Skin Resurfacing with the DOT laser INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedure, its risks, as well as alternative
New 7/1/2015 MCFRS 1
 New 7/1/2015 MCFRS 1 The providers will summarize the need for this change from an epinephrine auto injector The provider will define the proper dosage of epinephrine for the adult and pediatric patient
New 7/1/2015 MCFRS 1 The providers will summarize the need for this change from an epinephrine auto injector The provider will define the proper dosage of epinephrine for the adult and pediatric patient
Blepharoplasty - Eyelid Surgery
 Blepharoplasty - Eyelid Surgery Introduction Eyelid surgery repairs sagging or drooping eyelids. The surgery is also known as blepharoplasty, or an eyelid lift. Sagging or drooping eyelids happen naturally
Blepharoplasty - Eyelid Surgery Introduction Eyelid surgery repairs sagging or drooping eyelids. The surgery is also known as blepharoplasty, or an eyelid lift. Sagging or drooping eyelids happen naturally
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
Chapter 11. Everting skin edges
 Chapter 11 PRIMARY WOUND CLOSURE KEY FIGURE: Everting skin edges In primary wound closure, the skin edges of the wound are sutured together to close the defect. Whenever possible and practical, primary
Chapter 11 PRIMARY WOUND CLOSURE KEY FIGURE: Everting skin edges In primary wound closure, the skin edges of the wound are sutured together to close the defect. Whenever possible and practical, primary
STANDARDIZED PROCEDURE BONE MARROW ASPIRATION
 I. Definition: This protocol covers the task of bone marrow aspiration by an Allied Health Professional. The purpose of this standardized procedure is to allow the Allied Health Professional to safely
I. Definition: This protocol covers the task of bone marrow aspiration by an Allied Health Professional. The purpose of this standardized procedure is to allow the Allied Health Professional to safely
TREATMENT CONSULTATION FORM
 TREATMENT CONSULTATION FORM Consultation led by: Gender: M / F Weight Has your patient had other aesthetic procedures for the body? How did your patient hear about the CoolSculpting procedure? TREATMENT
TREATMENT CONSULTATION FORM Consultation led by: Gender: M / F Weight Has your patient had other aesthetic procedures for the body? How did your patient hear about the CoolSculpting procedure? TREATMENT
INFORMED CONSENT DERMABRASION
 INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
INFORMED CONSENT DERMABRASION INSTRUCTION This is an informed-consent document which has been prepared to help your plastic surgeon inform you about dermabrasion, its risks, and alternative treatments.
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to:
 Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
Objectives At the completion of this module, unlicensed assistive personnel (UAP) should be able to: 1. administer medications by subcutaneous injections. 2. document medication administration in the client
YOUR GUIDE TO THE LANTUS SOLOSTAR INSULIN PEN
 Important Safety Information for Lantus You must test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare
Important Safety Information for Lantus You must test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare
MOHS MICROGRAPHIC SURGERY
 Mary E. Maloney, MD Director Professor of Medicine David E. Geist, MD Assistant Professor of Medicine Dori Goldberg, MD Assistant Professor of Medicine Mark J. Scharf, MD Professor of Medicine Jason D.
Mary E. Maloney, MD Director Professor of Medicine David E. Geist, MD Assistant Professor of Medicine Dori Goldberg, MD Assistant Professor of Medicine Mark J. Scharf, MD Professor of Medicine Jason D.
RADIESSE INJECTABLE IMPLANT INSTRUCTIONS FOR USE
 RADIESSE IJECTABLE IMPLAT ISTRUCTIOS FOR USE DEVICE DESCRIPTIO RADIESSE injectable implant is a sterile, non-pyrogenic, semi-solid, cohesive implant, whose principle component is synthetic calcium hydroxylapatite
RADIESSE IJECTABLE IMPLAT ISTRUCTIOS FOR USE DEVICE DESCRIPTIO RADIESSE injectable implant is a sterile, non-pyrogenic, semi-solid, cohesive implant, whose principle component is synthetic calcium hydroxylapatite
Acne (Acne Vulgaris) A common type of bacteria that lives on the skin, known as Propionibacterium acnes, sometimes
 Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
Acne (Acne Vulgaris) Acne, clinically known as acne vulgaris, is the most common skin disease. It affects 85% of teenagers, some as young as 12, and often continues into adulthood. It is also called pimples,
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML SINGLE-USE PEN
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
Chapter 4 Physiological Therapeutics. 1 Cryotherapy
 Chapter 4 Physiological Therapeutics 1 Cryotherapy CRYOTHERAPY PHYSIOLOGIC EFFECTS OF ICE APPLICATION 1. Decreased circulation 5. Increased tissue stiffness 2. Local vasoconstriction 6. Decreased muscle
Chapter 4 Physiological Therapeutics 1 Cryotherapy CRYOTHERAPY PHYSIOLOGIC EFFECTS OF ICE APPLICATION 1. Decreased circulation 5. Increased tissue stiffness 2. Local vasoconstriction 6. Decreased muscle
MEDICATION GUIDE REBIF interferon beta-1a Injection for subcutaneous use
 MEDICATION GUIDE REBIF interferon beta-1a Injection for subcutaneous use Read this Medication Guide before you start using REBIF and each time you get a refill. There may be new information. The information
MEDICATION GUIDE REBIF interferon beta-1a Injection for subcutaneous use Read this Medication Guide before you start using REBIF and each time you get a refill. There may be new information. The information
Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use.
 Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Questions for my Doctor Refer to Coaptite Injectable Implant Instructions for Use provided with product for complete instructions for use. INDICATIONS: Coaptite Injectable Implant is indicated for soft
Informed Consent For Laser Hair Removal
 Informed Consent For Laser Hair Removal INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedures, its risks, as well as alternative treatment(s). It is important
Informed Consent For Laser Hair Removal INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedures, its risks, as well as alternative treatment(s). It is important
STANDARD OPERATING PROCEDURE. Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol
 STANDARD OPERATING PROCEDURE Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 03
STANDARD OPERATING PROCEDURE Administration of High Dose Muscular Vitamin Supplements for Undergoing Alcohol DOCUMENT CONTROL: Version: 2 Ratified by: Clinical Effectiveness Committee Date ratified: 03
Instructions and User Guide. Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150. June 8, 2011 Rev 2.
 Instructions and User Guide Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150 June 8, 2011 Rev 2.1 Contents 3 Chapter 1: Overview 3 Giving you the confidence only
Instructions and User Guide Select Series: Vascular Access Ultrasound Training Models BPO100, BPBV110, BPP120, BPNB150 June 8, 2011 Rev 2.1 Contents 3 Chapter 1: Overview 3 Giving you the confidence only
Varicose veins and spider veins
 Varicose veins and spider veins Summary Varicose veins are knobbly, twisted and darkish-blue in appearance, and are most commonly found on people s legs. Varicose veins are caused by faulty valves within
Varicose veins and spider veins Summary Varicose veins are knobbly, twisted and darkish-blue in appearance, and are most commonly found on people s legs. Varicose veins are caused by faulty valves within
CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE
 Introduction CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE Original authors: Niren Angle, John J. Bergan, Joshua I. Greenberg, and J. Leonel Villavicencio Abstracted by Teresa L. Carman New technology has
Introduction CHAPTER 15 SCLEROTHERAPY FOR VENOUS DISEASE Original authors: Niren Angle, John J. Bergan, Joshua I. Greenberg, and J. Leonel Villavicencio Abstracted by Teresa L. Carman New technology has
Beaumont Hospital. Varicose Veins. and their TREATMENT. Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM
 Beaumont Hospital Varicose Veins and their TREATMENT Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM Department of Surgery Beaumont Hospital and Royal College of Surgeons in Ireland
Beaumont Hospital Varicose Veins and their TREATMENT Professor Austin Leahy, MCh, FRCS, FRCSI WWW.VEINCLINICSOFIRELAND.COM Department of Surgery Beaumont Hospital and Royal College of Surgeons in Ireland
INFORMED CONSENT - CARPAL TUNNEL RELEASE
 . Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein and reproduce the modified version for use in the Purchaser's own practice only.
Treating HIV-related lipoatrophy by injecting a non-absorbable gel polymer
 Issue date January 2013 Information for the public NICE interventional procedures guidance advises the NHS on when and how new procedures can be used in clinical practice. Treating HIV-related lipoatrophy
Issue date January 2013 Information for the public NICE interventional procedures guidance advises the NHS on when and how new procedures can be used in clinical practice. Treating HIV-related lipoatrophy
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary
 X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
X-Plain Subclavian Inserted Central Catheter (SICC Line) Reference Summary Introduction A Subclavian Inserted Central Catheter, or subclavian line, is a long thin hollow tube inserted in a vein under the
PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D.
 PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D. A. INDICATIONS: Periocular steroid injection involves placement of steroid around the eye to treat intraocular inflammation or swelling of
PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D. A. INDICATIONS: Periocular steroid injection involves placement of steroid around the eye to treat intraocular inflammation or swelling of
Breast Implants: Local Complications and Adverse Outcomes
 Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
Breast Implants: Local Complications and Adverse Outcomes This booklet highlights the most common problems associated with silicone gel-filled and saline-filled breast implants: those that occur in the
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter
 Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
Patient Information Guide Morpheus CT Peripherally Inserted Central Catheter IC 192 Rev C A measure of flexibility and strength. Table of Contents 1. Introduction 2. What is the Morpheus CT PICC? 3. What
Ankle Block. Indications The ankle block is suitable for the following: Orthopedic and podiatry surgical procedures of the distal foot.
 Ankle Block The ankle block is a common peripheral nerve block. It is useful for procedures of the foot and toes, as long as a tourniquet is not required above the ankle. It is a safe and effective technique.
Ankle Block The ankle block is a common peripheral nerve block. It is useful for procedures of the foot and toes, as long as a tourniquet is not required above the ankle. It is a safe and effective technique.
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Spotlight Series: Interventional Radiology. Varicose Veins and Venous Insufficiency
 Spotlight Series: Interventional Radiology Varicose Veins and Venous Insufficiency What is venous insufficiency? Spectrum of Disease Spider veins and telangiectasias Small reddish and purple veins near
Spotlight Series: Interventional Radiology Varicose Veins and Venous Insufficiency What is venous insufficiency? Spectrum of Disease Spider veins and telangiectasias Small reddish and purple veins near
Biliary Drain. What is a biliary drain?
 Biliary Drain What is a biliary drain? A biliary drain is a tube to drain bile from your liver. It is put in by a doctor called an Interventional Radiologist. The tube or catheter is placed through your
Biliary Drain What is a biliary drain? A biliary drain is a tube to drain bile from your liver. It is put in by a doctor called an Interventional Radiologist. The tube or catheter is placed through your
Breast Augmentation. If you are dissatisfied with your breast size, augmentation surgery is a choice to consider. Breast augmentation can:
 Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
Breast Augmentation What is Breast Augmentation? Also known as augmentation mammaplasty, breast augmentation involves using implants to fulfill your desire for fuller breasts or to restore breast volume
A. ADMINISTERING SUBCUTANEOUS MEDICATIONS INTERMITTENTLY/CONTINUOUSLY B. (SUBCUTANEOUS INFUSION) HYDRODERMOCLYSIS
 SUBCUTANEOUS THERAPY A. ADMINISTERING SUBCUTANEOUS MEDICATIONS INTERMITTENTLY/CONTINUOUSLY B. (SUBCUTANEOUS INFUSION) HYDRODERMOCLYSIS PARTS I. Purposes II. General Information III. Responsibilities IV.
SUBCUTANEOUS THERAPY A. ADMINISTERING SUBCUTANEOUS MEDICATIONS INTERMITTENTLY/CONTINUOUSLY B. (SUBCUTANEOUS INFUSION) HYDRODERMOCLYSIS PARTS I. Purposes II. General Information III. Responsibilities IV.
PowerLight LED Light Therapy. The FUTURE of corrective skin
 PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
PowerLight LED Light Therapy The FUTURE of corrective skin care TODAY LED facial treatments Effective when used with correct protocols Non thermal stimulation of collagen Increases circulation and lymphatic
VIVITROL (EXTENDED-RELEASE INJECTABLE NALTREXONE) MEDICAL PROTOCOL AND PROCEDURES
 VIVITROL (EXTENDED-RELEASE INJECTABLE NALTREXONE) MEDICAL PROTOCOL AND PROCEDURES INTRODUCTION This is the protocol and procedures to administer VIVITROL (extended-release naltrexone injection, or XR-NTX)
VIVITROL (EXTENDED-RELEASE INJECTABLE NALTREXONE) MEDICAL PROTOCOL AND PROCEDURES INTRODUCTION This is the protocol and procedures to administer VIVITROL (extended-release naltrexone injection, or XR-NTX)
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use
 FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
FDA-Approved Patient Labeling IMPLANON (etonogestrel implant) Subdermal Use IMPLANON does not protect against HIV infection (the virus that causes AIDS) or other sexually transmitted diseases. Read this
8/6/2010. Name of medication Concentration (1:1,000 or 1mg/1ml) Expiration date
 Learning Objectives: Anaphylaxis & Epinephrine Administration by the EMT Adapted with permission from the Pilot Project for the Administration of Epinephrine by Washington EMTs With successful completion
Learning Objectives: Anaphylaxis & Epinephrine Administration by the EMT Adapted with permission from the Pilot Project for the Administration of Epinephrine by Washington EMTs With successful completion
An introduction to the principles and practice of safe and effective administration of injections
 An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
An introduction to the principles and practice of safe and effective administration of injections Introduction Giving an injection safely is considered to be a routine nursing activity. However it requires
Instructions for Use PROCRIT (PRO KRIT) (epoetin alfa)
 Instructions for Use PROCRIT (PROKRIT) (epoetin alfa) Use these Instructions for Use if you or your caregiver has been trained to give PROCRIT injections at home. Do not give yourself the injection unless
Instructions for Use PROCRIT (PROKRIT) (epoetin alfa) Use these Instructions for Use if you or your caregiver has been trained to give PROCRIT injections at home. Do not give yourself the injection unless
PICC- Peripherally Inserted Central Catheter PROCEDURAL CONSENT FORM. A. Interpreter / cultural needs. B. Procedure. C. Risks of the procedure
 DO NOT WRITE IN THIS BINDING MARGIN v3.00-03/2011 SW9266 Facility: PICC- Peripherally Inserted Central Catheter A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
DO NOT WRITE IN THIS BINDING MARGIN v3.00-03/2011 SW9266 Facility: PICC- Peripherally Inserted Central Catheter A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
Anaphylaxis and Epinephrine Auto-Injector
 Lesson Guide Anaphylaxis and Epinephrine Auto-Injector Lesson Length: 45 minutes Guidance for the Instructor To complete this lesson and meet the lesson objectives, you must: Welcome participants and explain
Lesson Guide Anaphylaxis and Epinephrine Auto-Injector Lesson Length: 45 minutes Guidance for the Instructor To complete this lesson and meet the lesson objectives, you must: Welcome participants and explain
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004
 Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
ICON PRE AND POST OP INSTRUCTIONS. MAXG or 1540 (XD/XF)
 ICON PRE AND POST OP INSTRUCTIONS MAXG or 1540 (XD/XF) Pre-Op Instructions 1. Please arrive to appointment 15 minutes before your scheduled time. Please be advised, running late may result in less than
ICON PRE AND POST OP INSTRUCTIONS MAXG or 1540 (XD/XF) Pre-Op Instructions 1. Please arrive to appointment 15 minutes before your scheduled time. Please be advised, running late may result in less than
Emory Eye Center New Patient Questionnaire
 Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
INJECTION TECHNIQUE. IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole
 IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
IVF NURSING OFFICE: (301) 400-2151 Darshana (301) 400-2146 Nicole PLEASE NOTE: If you do not have medications for the next day s dose, you MUST go to the clinic that morning at 6:30 AM for more medications.
Welcome to Bella! Give the Gift of Bella. A few tips to prepare you for your first visit: Gift Certificates are just $100 for a $150 value!
 Welcome to Bella! We are glad to have you as our guest. We encourage you to visit our website to see all of the exciting new laser and skincare treatments that we offer. Please be aware of our 24 hour
Welcome to Bella! We are glad to have you as our guest. We encourage you to visit our website to see all of the exciting new laser and skincare treatments that we offer. Please be aware of our 24 hour
X-Plain Varicose Veins Reference Summary
 X-Plain Varicose Veins Reference Summary Introduction Varicose veins are very common, in both women and men. Varicose veins can be painful and unattractive. Vein doctors use non-invasive ultrasound imaging
X-Plain Varicose Veins Reference Summary Introduction Varicose veins are very common, in both women and men. Varicose veins can be painful and unattractive. Vein doctors use non-invasive ultrasound imaging
Consent for Laser/Light Based Treatment
 Consent for Laser/Light Based Treatment I authorize Dr. Linda Cook to oversee laser/intense pulsed light cosmetic dermatology treatments on me, including but not limited to deep tissue heating, soft tissue
Consent for Laser/Light Based Treatment I authorize Dr. Linda Cook to oversee laser/intense pulsed light cosmetic dermatology treatments on me, including but not limited to deep tissue heating, soft tissue
X-Plain Trigeminal Neuralgia Reference Summary
 X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
X-Plain Trigeminal Neuralgia Reference Summary Introduction Trigeminal neuralgia is a condition that affects about 40,000 patients in the US every year. Its treatment mostly involves the usage of oral
I-140 Venipuncture for Blood Specimen Collection
 I-140 Venipuncture for Blood Specimen Collection Purpose Obtain a blood specimen by venipuncture for laboratory analysis using aseptic technique. Applies To Registered Nurses Licensed Practical/Vocational
I-140 Venipuncture for Blood Specimen Collection Purpose Obtain a blood specimen by venipuncture for laboratory analysis using aseptic technique. Applies To Registered Nurses Licensed Practical/Vocational
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery
 Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
Michael A. Boss, M.D. FMH Plastic, Reconstructive und Aesthetic Surgery Boss Aesthetic Center Schauplatzgasse 23 CH-3011 Bern Switzerland +41 31 311 7691 www.aesthetic-center.com B r e a s t A u g m e
TRANSFORM YOUR BODY WITHOUT SURGERY OR DOWNTIME. Freeze your fat away with CryoSculpting
 TRANSFORM YOUR BODY WITHOUT SURGERY OR DOWNTIME. Freeze your fat away with CryoSculpting BEFORE 12 WEEKS AFTER IT ALL BEGAN WITH NOT-SO-CHUBBY CHEEKS. 1 The revolutionary technology behind CoolSculpting
TRANSFORM YOUR BODY WITHOUT SURGERY OR DOWNTIME. Freeze your fat away with CryoSculpting BEFORE 12 WEEKS AFTER IT ALL BEGAN WITH NOT-SO-CHUBBY CHEEKS. 1 The revolutionary technology behind CoolSculpting
LASER TREATMENT FOR VARICOSE VEINS
 LASER TREATMENT FOR VARICOSE VEINS How can varicose veins be treated by laser? Laser treatment of varicose veins, Endovascular Laser Ablation (known as EVLA), is a minimally invasive procedure for treating
LASER TREATMENT FOR VARICOSE VEINS How can varicose veins be treated by laser? Laser treatment of varicose veins, Endovascular Laser Ablation (known as EVLA), is a minimally invasive procedure for treating
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167
 APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction
 Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Sientra Silicone Gel Breast Implants Quick Facts About Breast Augmentation And Reconstruction About This Brochure This brochure is intended to provide you with a high level overview of the facts about
Heart problems - What are the possible side effects of AVONEX? What is AVONEX? Who should not take AVONEX?
 MEDICATION GUIDE AVONEX Interferon beta-1a (Including appendix with instructions for using AVONEX Vials) Please read this guide carefully before you start to use AVONEX (a-vuh-necks) and each time your
MEDICATION GUIDE AVONEX Interferon beta-1a (Including appendix with instructions for using AVONEX Vials) Please read this guide carefully before you start to use AVONEX (a-vuh-necks) and each time your
What are spider veins? What is the best treatment for spider veins?
 Laser Spider/Leg Vein Q & A What are spider veins? Millions of women and men are bothered by unsightly spider veins on their faces and legs. Spider veins or telangiectasias are those small red, blue and
Laser Spider/Leg Vein Q & A What are spider veins? Millions of women and men are bothered by unsightly spider veins on their faces and legs. Spider veins or telangiectasias are those small red, blue and
MEDICATION GUIDE STELARA
 MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
MEDICATION GUIDE STELARA (stel ar a) (ustekinumab) Injection What is the most important information I should know about STELARA? STELARA is a medicine that affects your immune system. STELARA can increase
HUMULIN (HU-mu-lin) N
 Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
Instructions for Use HUMULIN (HU-mu-lin) N (human insulin [rdna origin] isophane suspension) vial (100 Units/mL, U-100) Read the Instructions for Use before you start taking HUMULIN N and each time you
The Enbrel SureClick autoinjector is a single-use prefilled autoinjector. It contains one 50-mg dose of Enbrel.
 Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-use prefilled autoinjector. It contains one 50-mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-use prefilled autoinjector. It contains one 50-mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
 SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
SOMAVERT pegvisomant for injection PATIENT INFORMATION SOMAVERT (SOM-ah-vert) (pegvisomant for injection) Read the patient information that comes with SOMAVERT before you start using it and each time you
PICC & Midline Catheters Patient Information Guide
 PICC & Midline Catheters Patient Information Guide medcompnet.com 1 table of contents Introduction 4 What is a PICC or Midline Catheter? 4 How is the PICC or Midline Catheter Inserted? 6 Catheter Care
PICC & Midline Catheters Patient Information Guide medcompnet.com 1 table of contents Introduction 4 What is a PICC or Midline Catheter? 4 How is the PICC or Midline Catheter Inserted? 6 Catheter Care
Use of Packing for Surgical Wounds. Maggie Benson Clinical Problem Solving II
 Use of Packing for Surgical Wounds Maggie Benson Clinical Problem Solving II Purpose Present patient management s/p Incision and Drainage in an outpatient setting Examine evidence for the use of wound
Use of Packing for Surgical Wounds Maggie Benson Clinical Problem Solving II Purpose Present patient management s/p Incision and Drainage in an outpatient setting Examine evidence for the use of wound
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
CERVICAL MEDIASTINOSCOPY WITH BIOPSY
 INFORMED CONSENT INFORMATION ADDRESSOGRAPH DATA CERVICAL MEDIASTINOSCOPY WITH BIOPSY You have decided to have an important procedure and we appreciate your selection of UCLA Healthcare to meet your needs.
INFORMED CONSENT INFORMATION ADDRESSOGRAPH DATA CERVICAL MEDIASTINOSCOPY WITH BIOPSY You have decided to have an important procedure and we appreciate your selection of UCLA Healthcare to meet your needs.
RENAL ANGIOMYOLIPOMA EMBOLIZATION
 RENAL ANGIOMYOLIPOMA EMBOLIZATION The information about renal angiomyolipomas on the next several pages includes questions commonly asked about the embolization procedure. Please take a few moments to
RENAL ANGIOMYOLIPOMA EMBOLIZATION The information about renal angiomyolipomas on the next several pages includes questions commonly asked about the embolization procedure. Please take a few moments to
Epinephrine Administration Training for Unlicensed School Personnel
 Epinephrine Administration Training for Unlicensed School Personnel Management of Life-Threatening Allergies in the School Setting Dover and Sherborn Schools EpiPen Administration This program is designed
Epinephrine Administration Training for Unlicensed School Personnel Management of Life-Threatening Allergies in the School Setting Dover and Sherborn Schools EpiPen Administration This program is designed
PICCs and Midline Catheters
 Patient Education PICCs and Midline Catheters Patient s guide to PICC (peripherally inserted central catheter) and midline catheters What are PICCs and midline catheters used for? Any medicine given over
Patient Education PICCs and Midline Catheters Patient s guide to PICC (peripherally inserted central catheter) and midline catheters What are PICCs and midline catheters used for? Any medicine given over
INFORMED CONSENT BLEPHAROPLASTY SURGERY
 INFORMED CONSENT BLEPHAROPLASTY SURGERY INSTRUCTIONS This is informed-consent document which had been prepared to help your plastic surgeon inform you about blepharoplasty surgery, its risks, and alternative
INFORMED CONSENT BLEPHAROPLASTY SURGERY INSTRUCTIONS This is informed-consent document which had been prepared to help your plastic surgeon inform you about blepharoplasty surgery, its risks, and alternative
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec)
 PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
PHYSICIAN OFFICE BILLING INFORMATION SHEET FOR IMLYGIC (talimogene laherparepvec) INDICATION IMLYGIC is a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable
Varicose Vein Treatment (Endovenous Ablation of Varicose Veins)
 Scan for mobile link. Varicose Vein Treatment (Endovenous Ablation of Varicose Veins) Varicose vein treatment, also known as endovenous ablation, uses radiofrequency or laser energy to cauterize and close
Scan for mobile link. Varicose Vein Treatment (Endovenous Ablation of Varicose Veins) Varicose vein treatment, also known as endovenous ablation, uses radiofrequency or laser energy to cauterize and close
Eye Injuries. The Eyes The eyes are sophisticated organs. They collect light and focus it on the back of the eye, allowing us to see.
 Eye Injuries Introduction The design of your face helps protect your eyes from injury. But injuries can still damage your eyes. Sometimes injuries are severe enough that you could lose your vision. Most
Eye Injuries Introduction The design of your face helps protect your eyes from injury. But injuries can still damage your eyes. Sometimes injuries are severe enough that you could lose your vision. Most
HUMULIN 70/30 KwikPen
 1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) Read the Instructions for Use before you start taking HUMULIN 70/30 and each
1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) Read the Instructions for Use before you start taking HUMULIN 70/30 and each
MEDICATION GUIDE (ex tā vee uh) What is the most important information I should know about EXTAVIA?
 MEDICATION GUIDE EXTAVIA (ex tā vee uh) Interferon beta-1b Read the Medication Guide that comes with EXTAVIA before you start taking it and each time you get a refill. There may be new information. This
MEDICATION GUIDE EXTAVIA (ex tā vee uh) Interferon beta-1b Read the Medication Guide that comes with EXTAVIA before you start taking it and each time you get a refill. There may be new information. This
FRAGMIN Please bring this booklet the day of your surgery.
 FRAGMIN Please bring this booklet the day of your surgery. QHC#72 Fragmin (dalteparin sodium injection); an anticoagulant (blood thinner) is used to help prevent blood clots after surgery. While on Fragmin,
FRAGMIN Please bring this booklet the day of your surgery. QHC#72 Fragmin (dalteparin sodium injection); an anticoagulant (blood thinner) is used to help prevent blood clots after surgery. While on Fragmin,
UW MEDICINE PATIENT EDUCATION. Using Insulin. Basic facts about insulin and self-injection. What is insulin? How does diabetes affect the body?
 UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
UW MEDICINE PATIENT EDUCATION Using Insulin Basic facts about insulin and self-injection This handout explains what insulin is, the different types of insulin, how to store it, how to give an injection
NeuroStar TMS Therapy Patient Guide for Treating Depression
 NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter
 Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Central venous catheter: Peripherally inserted central catheter This information leaflet aims to help answer some of the questions
17. Undiagnosed lumps and bumps and unexplained areas of pain. 2. Varicose veins (do not treat anything below the vein site).
 15. Acute rheumatism. 16. Asthma. 17. Undiagnosed lumps and bumps and unexplained areas of pain. 18. Whiplash. 19. Slipped Disc. LOCAL CONTRA-INDICATIONS 1. Skin diseases (non contagious). 2. Varicose
15. Acute rheumatism. 16. Asthma. 17. Undiagnosed lumps and bumps and unexplained areas of pain. 18. Whiplash. 19. Slipped Disc. LOCAL CONTRA-INDICATIONS 1. Skin diseases (non contagious). 2. Varicose
Basal Cell Carcinoma Affecting the Eye Your Treatment Explained
 Basal Cell Carcinoma Affecting the Eye Your Treatment Explained Patient Information Introduction This booklet is designed to give you information about having a Basal Cell Carcinoma near your eye and the
Basal Cell Carcinoma Affecting the Eye Your Treatment Explained Patient Information Introduction This booklet is designed to give you information about having a Basal Cell Carcinoma near your eye and the
2 What you need to know before you have Ampiclox
 Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
MARYLAND STATE SCHOOL HEALTH SERVICES GUIDELINES
 Department of Health and Mental Hygiene Maryland State Department of Education Maryland State School Health Council MARYLAND STATE SCHOOL HEALTH SERVICES GUIDELINES Emergency Management Guidelines for
Department of Health and Mental Hygiene Maryland State Department of Education Maryland State School Health Council MARYLAND STATE SCHOOL HEALTH SERVICES GUIDELINES Emergency Management Guidelines for
Allergies: ENT and Allergy Center of Missouri YOUR GUIDE TO TESTING AND TREATMENT. University of Missouri Health Care
 Allergies: YOUR GUIDE TO TESTING AND TREATMENT ENT and Allergy Center of Missouri University of Missouri Health Care 812 N. Keene St., Columbia, MO 65201 (573) 817-3000 www.muhealth.org WHAT CAUSES ALLERGIES
Allergies: YOUR GUIDE TO TESTING AND TREATMENT ENT and Allergy Center of Missouri University of Missouri Health Care 812 N. Keene St., Columbia, MO 65201 (573) 817-3000 www.muhealth.org WHAT CAUSES ALLERGIES
IVF CLASS. IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, nicole.l.sobers.ctr@health.
 IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, [email protected] PLEASE NOTE: If you do not have medications for the next
IVF CLASS IVF NURSE CONTACT INFORMATION: Darshana 301-400-2151, [email protected] Nicole 301-400-2146, [email protected] PLEASE NOTE: If you do not have medications for the next
ILIOTIBIAL BAND SYNDROME
 ILIOTIBIAL BAND SYNDROME Description The iliotibial band is the tendon attachment of hip muscles into the upper leg (tibia) just below the knee to the outer side of the front of the leg. Where the tendon
ILIOTIBIAL BAND SYNDROME Description The iliotibial band is the tendon attachment of hip muscles into the upper leg (tibia) just below the knee to the outer side of the front of the leg. Where the tendon
Executive Summary. Dermal Filler Devices
 Executive Summary Dermal Filler Devices Food and Drug Administration Center for Devices and Radiological Health Office of Device Evaluation General and Plastic Surgery Devices Panel Public Advisory Committee
Executive Summary Dermal Filler Devices Food and Drug Administration Center for Devices and Radiological Health Office of Device Evaluation General and Plastic Surgery Devices Panel Public Advisory Committee
Myelogram PROCEDURAL CONSENT FORM. A. Interpreter / cultural needs. B. Procedure. C. Risks of the procedure
 DO NOT WRITE IN THIS BINDING MARGIN v5.00-03/2011 SW9263 Facility: A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified Interpreter present? Yes No A Cultural
DO NOT WRITE IN THIS BINDING MARGIN v5.00-03/2011 SW9263 Facility: A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified Interpreter present? Yes No A Cultural
The Total Ankle Replacement
 The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
Hand Injuries and Disorders
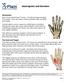 Hand Injuries and Disorders Introduction Each of your hands has 27 bones, 15 joints and approximately 20 muscles. There are many common problems that can affect your hands. Hand problems can be caused
Hand Injuries and Disorders Introduction Each of your hands has 27 bones, 15 joints and approximately 20 muscles. There are many common problems that can affect your hands. Hand problems can be caused
User Manual Important: First read the Medication Guide that comes inside your FORTEO carton.
 1 RA082FSAM02 User Manual Important: First read the Medication Guide that comes inside your FORTEO carton. Before you use your new FORTEO delivery device, please read the entire front and back of this
1 RA082FSAM02 User Manual Important: First read the Medication Guide that comes inside your FORTEO carton. Before you use your new FORTEO delivery device, please read the entire front and back of this
How To Prepare and Give a Prefilled Syringe Injection
 Diablo Valley Onc&Hem Med Grp, Inc Phone Number: (925)6775041 How To Prepare and Give a Prefilled Syringe Injection Patient Education Quick Reference Guide The following are instructions for use of prefilled
Diablo Valley Onc&Hem Med Grp, Inc Phone Number: (925)6775041 How To Prepare and Give a Prefilled Syringe Injection Patient Education Quick Reference Guide The following are instructions for use of prefilled
Patient Information Leaflet for Flixonase Aqueous Nasal Spray (fluticasone propionate)
 Patient Information Leaflet for Flixonase Aqueous Nasal Spray (fluticasone propionate) Your doctor has decided to prescribe Flixonase Aqueous Nasal Spray as part of your treatment. This leaflet tells you
Patient Information Leaflet for Flixonase Aqueous Nasal Spray (fluticasone propionate) Your doctor has decided to prescribe Flixonase Aqueous Nasal Spray as part of your treatment. This leaflet tells you
