NSAID PREPARATIONS. COMPOSITION : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50. Clofenac SR. Sodium BP 50 mg.
|
|
|
- Stephen Scot Shields
- 7 years ago
- Views:
Transcription
1 Diclofenac Analgesic & Anti-inflammatory COMPOSITION Clofenac 25 : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50 : Each enteric coated tablet contains Diclofenac Sodium BP 50 mg. Clofenac SR : Each enteric coated tablet contains Diclofenac Sodium BP 100 mg in a sustained release formulation. Clofenac injection : Each 3 ml ampoule contains Diclofenac Sodium BP 75 mg. Clofenac Plus injection : Each 2 ml ampoule contains Diclofenac Sodium BP 75 mg and Lidocaine Hydrochloride USP 20 mg. Clofenac Gel : Each 100 gm gel contains 1.16 gm Diclofenac Diethyl ammonium salt which corresponds to 1 gm Diclofenac Sodium BP. Clofenac 12.5 Suppository : Each suppository contains Diclofenac Sodium BP 12.5 mg. Clofenac 50 Suppository : Each suppository contains Diclofenac Sodium BP 50 mg. PHARMACOLOGY Clofenac contains Diclofenac, which is a potent non-steroidal antiinflammatory drug (NSAID) with marked analgesic and antipyretic properties. It also has some uricosuric effect. The actions of diclofenac appear to be associated principally with the inhibition of prostaglandin synthesis - by inhibiting cyclooxygenase - the enzyme that catalyzes the formation of prostaglandin precursors (endoperoxides) from arachidonic acid. Following oral administration, diclofenac is rapidly absorbed from the gastro-intestinal tract. Peak plasma concentration following ingestion of an enteric coated tablet occurs at about 2 to 3 hours and that following injection occurs within half an hour. Diclofenac is 99.7% bound to plasma proteins and plasma half-life for the terminal elimination phase is 1-2 hours. Diclofenac enters the synovial fluid, where maximum concentrations are measured 2-4 hours after the peak plasma values have been obtained. The apparent half-life for elimination from the synovial fluid is 3-6 hours. Diclofenac is extensively metabolized to
2 a range of phenolic compounds. About 60% of the administered dose is excreted via the kidneys in the form of metabolites and less than 1% in unchanged form. The remainder is excreted via the bile in metabolised form. Clofenac Plus Injection contains Diclofenac Sodium BP and Lidocaine Hydrochloride USP. Lidocaine is the most widely used local anaesthetic drug. It acts more rapidly and is more stable than most other local anaesthetics. It is a very useful surface anaesthetic. Like other local anaesthetic, lidocaine impairs the generation and conduction of the nerve impulses by slowing depolarization. The onset of anaesthesia of lidocaine HCl is more rapid and the duration of action is longer. A 1%-2% solution has a duration of action of about 1-2 hours. Binding of lidocaine to plasma proteins is variable and concentration dependent. Approximately 90% of a parenteral dose of lidocaine is rapidly metabolized in the liver. Less than 10% of a dose is excreted unchanged in the urine. Clofenac Gel contains diclofenac sodium BP 1% as diclofenac diethyl ammonium salt. It has been designed for external use. The white creamy non-greasy preparation can easily be rubbed into the skin and its aqueous alcoholic base exerts a soothing and cooling effect on the skin. After local application, the active substance penetrates the skin, accumulates in the underlying tissue and combats both acute and chronic inflammatory reaction. The anti-inflammatory and analgesic properties of Clofenac Gel elicits a clinical response characterized by a marked decrease in inflammatory swelling of traumatic or rheumatic origin. It affords effective relief from tenderness and pain on movement. INDICATION Clofenac tablets, Clofenac injection, Clofenac Plus injection and Clofenac suppositories contain diclofenac sodium, which is used to relief all grades of pain and inflammation in a wide range of conditions including: a) Arthritic conditions: Rheumatoid Arthritis, Osteoarthritis, Ankylosing spondylitis, Acute gout. b) acute musculoskeletal disorders such as periarthritis (e.g., frozen shoulder), tendinitis, tenosynovitis, bursitis. c) other painful conditions resulting from trauma, including fracture, low back pain, sprains, strains, dislocations, orthopaedic, dental and other minor surgery. Clofenac Plus injection also contains lidocaine, which acts as a local
3 anaesthetic. Therefore, the possibility of pain at the injection site, which is most likely to occur after intramuscular injection of normal diclofenac, is minimized if Clofenac Plus Injection is used. Clofenac Gel is used for the local symptomatic relief of pain and inflammation in: a) trauma of the tendons, ligaments, muscles and joints, e.g, due to sprains, strains and bruises, b) localised forms of soft tissue rheumatism. DOSAGE AND ADMINISTRATION Clofenac 25 mg and 50 mg enteric coated tablets : Adults: mg daily in 2 to 3 divided doses, preferably after food. Dose should be reduced in long term use. Clofenac SR : 1 tablet daily, taken whole with liquid, preferably at meal times. If necessary, the daily dose can be increased to 150 mg by supplementation with conventional tablets. Children: 1-3 mg of diclofenac/kg body wt. daily in divided doses. Elderly patients: In elderly or debilitated patients, the lowest effective dosage is recommended, although the pharmacokinetics of diclofenac sodium are not impaired to any clinically relevant extent in elderly patients. Clofenac injection/clofenac Plus injection : Adults: One ampoule once (or in severe cases, twice) daily by intramuscular injection. Renal colic: One ampoule once daily intramuscularly. A further ampoule may be administered after 30 minutes, if necessary. The recommended maximum daily dose of diclofenac is 150 mg, by any route. The recommended maximum daily dose of lidocaine is 200 mg. Children: In juvenile chronic arthritis, 1-3 mg of diclofenac/kg body wt. daily in divided doses. Elderly patients: In elderly or debilitated patients, the lowest effective dosage is recommended, commensurate with age and physical status. Clofenac Gel : Clofenac Gel should be rubbed gently into the skin. Depending on the size of the affected site to be treated 2-4 gm Clofenac Gel should be applied 3-4 times daily and rubbed in lightly. After application, the hands should be washed unless they are the site being treated.
4 Use in the elderly: The usual adult dosage may be used. Clofenac Gel may also be given to further treatment with other dosage forms of Clofenac. or as prescribed by the physician. Clofenac Suppository : Suppository should be administered rectally. Adults: 50 mg suppository 2-3 times daily. Maximum daily dose is 150 mg. Children: 12.5 mg suppository 2-3 times daily, or 1-3 mg/kg per day in divided doses. CONTRAINDICATION AND PRECAUTION Diclofenac is contra-indicated for those patients who are hypersensitive to diclofenac. In patients with active or suspected peptic ulcer or gastrointestinal bleeding, or for those patients in whom attacks of asthma, urticaria or acute rhinitis are precipitated by aspirin or other NSAIDs possessing prostaglandin synthetase inhibiting activity, diclofenac is also contra-indicated. Because of the presence of lidocaine, Clofenac -Plus injection is also contra-indicated for those patients who are hypersensitive to local anaesthetics of the amide type, although the incidence is very rare. In patients with Adams-Stokes syndrome or with severe degrees of SA, AV, or intraventricular heart block in the absence of an artificial pacemaker, and for those patients who are hypersensitive to any of the excipients used in the formulation (sodium metabisulphite, mannitol, benzyl alcohol, propylene glycol), this injection is also contra-indicated. Clofenac Gel is also contra-indicated to those who are hypersensitive to the gel base. Renal: Patients with severe hepatic, cardiac or renal insufficiency or the elderly should be kept under close surveillance, since the use of NSAIDs may result in deterioration of renal function. The lowest effective dose should be used and renal function monitored. Hepatic: If abnormal liver function tests persist or worsen, clinical signs or symptoms consistent with liver disease develop or if other manifestations occur (eosinophilia, rash), diclofenac should be discontinued. All patients who are receiving long term treatment with NSAIDs should be monitored as a precautionary measure (e.g., renal, hepatic function and blood counts).
5 Clofenac Gel should not be taken by mouth and should not be used under occlusive, airtight dressings. The gel should not be allowed to come in contact with the eyes or mucous membranes. SIDE EFFECT Side effects to diclofenac are usually mild and transient. However, if serious side effects occur, diclofenac should be discontinued. Gastrointestinal: Occasional: epigastric pain, other gastro-intestinal disorders (e.g., nausea, vomiting, diarrhoea, abdominal cramps, dyspepsia, flatulence, anorexia). Rare: gastro-intestinal bleeding, peptic ulcer (with or without bleeding or perforation), bloody diarrhoea. In isolated cases: lower gut disorders (e.g., non-specific haemorrhagic colitis and exacerbations of ulcerative colitis or Crohn's proctocolitis), pancreatitis, glossitis,constipation, etc. Clofenac Plus injection: In very rare instances, injection site disorders may occur. In isolated cases, abscesses and local necrosis may occur. The adverse effects due to lidocaine mainly involve the CNS, are usually of short duration, and are dose related. The CNS reactions may be manifested by drowsiness, dizziness, disorientation, confusion, lightheadedness, etc. Clofenac Gel is usually well tolerated. Local irritation, erythema, pruritus or dermatitis may occasionally occur. Although the gel has low systemic absorption, yet the possibility of the systemic side-effects cannot be completely excluded because of use to a relatively large area of skin. DRUG INTERACTION Clofenac Gel: No drug interaction during treatment with diclofenac gel have been reported. All other dosage forms may have the following drug interactions : Lithium and digoxin: Diclofenac may increase plasma concentrations of lithium and digoxin. Anticoagulants: There are isolated reports of an increased risk of haemorrhage with the combined use of diclofenac and anticoagulant therapy, although clinical investigations do not appear to indicate any influence on anticoagulant effect. Antidiabetic agents: Clinical studies have shown that diclofenac can be given together with oral antidiabetic agents without influencing their clinical effect. Cyclosporin: Cases of nephrotoxicity have been reported in patients receiving cyclosporin and diclofenac concomitantly.
6 Methotrexate: Cases of serious toxicity have been reported when methotrexate and NSAIDs are given within 24 hours of each other. Quinolone antimicrobials: Convulsions may occur due to an interaction between quinolones and NSAIDs. Therefore, caution should be exercised when considering concomitant therapy of NSAID and quinolones. Other NSAIDs and steroids: Co-administration of diclofenac with other systemic NSAIDs and steroids may increase the frequency of unwanted effects. With aspirin, the plasma levels of each is lowered, although no clinical significance is known. Diuretics: Various NSAIDs areliable to inhibit the activity of diuretics. Concomitant treatment with potassium-sparing diuretics may be associated with increased serum potassium levels. So, serum potassium should be monitored. USE IN PREGNANCY AND LACTATION Diclofenac tablets and injection should not be prescribed during pregnancy, unless there are compelling reasons for doing so. The lowest effective dosage should be used. This type of drugs are not recommended during the last trimester of pregnancy. Very small quantities of diclofenac may be detected in breast milk, but no undesirable effects on the infant are to be expected. Since no experience has been acquired with diclofenac gel in pregnancy or lactation, it is not recommended for use in these circumstances. STORAGE CONDITION Protect from heat, light, and moisture. Clofenac Suppository: Store below 30 C. HOW SUPPLIED Clofenac 25 : Box containing 10 x 10 tablets in blister pack. Clofenac 50 : Box containing 20 x 10 tablets in blister pack. Clofenac SR : Box containing 10 x 10 tablets in blister pack. Clofenac injection : Box containing 5 x 2 ampoules of 3 ml in blister pack. Clofenac Plus injection : Box containing 2 x 5 ampoules of 2 ml in blister pack. Clofenac Gel 10 gm : Tube containing 10 gm gel. Clofenac 12.5 mg Suppository : Box containing 2 x 5 suppositories in blister pack. Clofenac 50 mg Suppository : Box containing 2 x 5 suppositories in blister pack.
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination
 For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination DESCRIPTION UPRIGHT is a fixed dose combination
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only UpRight Aceclofenac 100 mg and Paracetamol 500 mg fixed dose combination DESCRIPTION UPRIGHT is a fixed dose combination
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
PACKAGE INSERT TEMPLATE FOR ACETYLSALICYLIC ACID/ASPIRIN TABLET
 PACKAGE INSERT TEMPLATE FOR ACETYLSALICYLIC ACID/ASPIRIN TABLET Brand or Product Name [Product name] tablet 500mg Name and Strength of Active Substance(s) Acetylsalicylic acid.500mg Product Description
PACKAGE INSERT TEMPLATE FOR ACETYLSALICYLIC ACID/ASPIRIN TABLET Brand or Product Name [Product name] tablet 500mg Name and Strength of Active Substance(s) Acetylsalicylic acid.500mg Product Description
Doxylamine succinate belongs to the ethanolamine class of antihistamines with sedative properties.
 Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
Data Sheet MERSYNDOL Tablet Paracetamol 450mg per tablet Codeine Phosphate 9.75mg per tablet Doxylamine Succinate 5mg per tablet MERSYNDOL FORTE Tablet Paracetamol 450mg per tablet Codeine Phosphate 30mg
PARACETAMOL REXIDOL. 600 mg Tablet. Analgesic-Antipyretic. Paracetamol 600 mg
 (Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
(Insert Text) UL Consumer Health PARACETAMOL REXIDOL 600 mg Tablet Analgesic-Antipyretic FORMULATION Each tablet contains: Paracetamol 600 mg PRODUCT DESCRIPTION Rexidol is a round, yellow, flat, bevel-edged
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
VOLTAROL Suppositories 12.5, 25, 50 and 100 mg (diclofenac sodium)
 VOLTAROL Suppositories 12.5, 25, 50 and 100 mg (diclofenac sodium) Patient Information Leaflet What you need to know about Voltarol Suppositories Your doctor has decided that you need this medicine to
VOLTAROL Suppositories 12.5, 25, 50 and 100 mg (diclofenac sodium) Patient Information Leaflet What you need to know about Voltarol Suppositories Your doctor has decided that you need this medicine to
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Influence of ph Most local anesthetics are weak bases.
 Local anesthetics The agent must depress nerve conduction. The agent must have both lipophilic and hydrophilic properties to be effective by parenteral injection. Structure-activity relationships The typical
Local anesthetics The agent must depress nerve conduction. The agent must have both lipophilic and hydrophilic properties to be effective by parenteral injection. Structure-activity relationships The typical
Essential Shared Care Agreement Drugs for Dementia
 Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
PRODUCT INFORMATION PANAMAX
 NAME OF THE MEDICINE Non-proprietary Name Paracetamol PRODUCT INFORMATION PANAMAX DESCRIPTION Each tablet contains paracetamol 500 mg. The inactive ingredients are: maize starch, purified talc, pregelatinised
NAME OF THE MEDICINE Non-proprietary Name Paracetamol PRODUCT INFORMATION PANAMAX DESCRIPTION Each tablet contains paracetamol 500 mg. The inactive ingredients are: maize starch, purified talc, pregelatinised
Review of Pharmacological Pain Management
 Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Psoriasis and Psoriatic Arthritis Alliance
 Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
SR 5. W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1
 W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
W2364A_NT.XATRAL SR5 PAK/SA 5/12/05 9:47 Page 1 alfuzosin SR 5 mg (Alfuzosin HCI) COMPOSITION Each sustained-release, film-coated tablet contains: Alfuzosin hydrochloride......... 5 mg Excipients... q.s.
PATIENT INFORMATION LEAFLET Phor Migraine (Ibuprofen)
 PATIENT INFORMATION LEAFLET Phor Migraine (Ibuprofen) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have any further
PATIENT INFORMATION LEAFLET Phor Migraine (Ibuprofen) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read it again. If you have any further
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent to 150 mg clindamycin base.
 APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
APPROVED PACKAGE INSERT DALACIN C 150 mg CAPSULES SCHEDULING STATUS: S4 PROPRIETARY NAME (and dosage form): DALACIN C TM 150 mg (Capsules) COMPOSITION: Each capsule contains clindamycin hydrochloride equivalent
LEFLUNOMIDE (Adults)
 Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER. 500 mg, film-coated tablet Active substance: metformin hydrochloride
 P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
P AC K AG E L E AF L E T: INFORMAT I ON FO R THE USER Siofor 500 500 mg, film-coated tablet Active substance: metformin hydrochloride For use in children above 10 years and adults Read all of this leaflet
Elderly: in elderly patients there is no need to reduce the daily dosage (see section 5.2).
 Mesulid-DL-Feb 2012-02 Doctor Leaflet 1. NAME OF THE MEDICINAL PRODUCT Mesulid 100 mg caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each caplet contains 100mg nimesulide. For excipients, see section
Mesulid-DL-Feb 2012-02 Doctor Leaflet 1. NAME OF THE MEDICINAL PRODUCT Mesulid 100 mg caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each caplet contains 100mg nimesulide. For excipients, see section
Calcium Folinate Ebewe Data Sheet
 NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package leaflet: Information for the user. < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid
 PACKAGE LEAFLET 1 Package leaflet: Information for the user < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET 1 Package leaflet: Information for the user < ASPROFLASH and associated names > 500 mg coated tablet Acetylsalicylic acid Read all of this leaflet carefully before you start using this
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
Nonsteroidal. Drugs (NSAIDs) Anti-Inflammatory. North American Spine Society Public Education Series
 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Nurse Initiated Medications Procedure
 1. Purpose This Procedure is performed as a means of ensuring the safe administration of therapeutic medication to patients in accordance with all legislative and regulatory requirements. 2. Application
1. Purpose This Procedure is performed as a means of ensuring the safe administration of therapeutic medication to patients in accordance with all legislative and regulatory requirements. 2. Application
DICLOFENAC POTASSIUM 12.5 MG TABLETS PL 24668/0001 UKPAR TABLE OF CONTENTS
 DICLOFENAC POTASSIUM 12.5 MG TABLETS PL 24668/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary Page
DICLOFENAC POTASSIUM 12.5 MG TABLETS PL 24668/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 12 Steps taken after authorisation summary Page
Nōdia DESCRIPTION PHARMACOLOGY. Loperamide hydrochloride USP 2 mg Tablets. Pharmacological classification - antidiarrhoeal.
 Nōdia Loperamide hydrochloride USP 2 mg Tablets DESCRIPTION Nōdia 2 mg tablets are green, capsule-shaped tablets with a break-line on one side. Each tablet contains 2 mg loperamide hydrochloride and typically
Nōdia Loperamide hydrochloride USP 2 mg Tablets DESCRIPTION Nōdia 2 mg tablets are green, capsule-shaped tablets with a break-line on one side. Each tablet contains 2 mg loperamide hydrochloride and typically
4 Clinical Particulars
 SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
SUMMARY OF PRODUCT CHARACTERISTICS 1 Name of the Medicinal Product Procyclidine Syrup 5mg/5ml 2. Qualitative and Quantitative Composition Each 5ml dose contains 5mg Procyclidine Hydrochloride BP. 3. Pharmaceutical
New Zealand Data Sheet
 New Zealand NAME OF MEDICINE VOLTAREN RAPID 25 Diclofenac potassium tablets 25 mg Diclofenac potassium liquid capsules 25 mg PRESENTATION Each Voltaren Rapid 25 tablet contains 25 mg of diclofenac potassium.
New Zealand NAME OF MEDICINE VOLTAREN RAPID 25 Diclofenac potassium tablets 25 mg Diclofenac potassium liquid capsules 25 mg PRESENTATION Each Voltaren Rapid 25 tablet contains 25 mg of diclofenac potassium.
This leaflet answers some common questions about ARROW - MELOXICAM.
 Arrow - Meloxicam Meloxicam Tablets 7.5mg What is in this leaflet This leaflet answers some common questions about ARROW - MELOXICAM. It does not contain all of the available information. It does not take
Arrow - Meloxicam Meloxicam Tablets 7.5mg What is in this leaflet This leaflet answers some common questions about ARROW - MELOXICAM. It does not contain all of the available information. It does not take
Elements for a public summary. VI.2.1 Overview of disease epidemiology. VI.2.2 Summary of treatment benefits
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
DATA SHEET. This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
 DATA SHEET METFORMIN GENERIC HEALTH Metformin hydrochloride 500mg and 850mg tablets This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
DATA SHEET METFORMIN GENERIC HEALTH Metformin hydrochloride 500mg and 850mg tablets This product is may not be interchangeable with other products containing this ingredient in the New Zealand Market.
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole
 PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
PACKAGE LEAFLET: INFORMATION FOR THE USER Omeprazol XXX 40 mg powder for solution for infusion omeprazole Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
Medications for chronic pain
 Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
ENZAR FORTE TABLETS. (derived from Pancreatin USP) Sodium tauroglycocholate BPC 65mg (with sugar coating containing essential carminative oils)
 ENZAR FORTE TABLETS COMPOSITION Each enteric-coated tablet contains: Amylase activity -15,000 USP units Lipase activity -4,000 USP units Protease activity -15,000 USP units of (derived from Pancreatin
ENZAR FORTE TABLETS COMPOSITION Each enteric-coated tablet contains: Amylase activity -15,000 USP units Lipase activity -4,000 USP units Protease activity -15,000 USP units of (derived from Pancreatin
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
SYNACTHEN i.m./i.v. tetracosactide hexaacetate
 New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
New Zealand Consumer Medicine Information SYNACTHEN i.m./i.v. tetracosactide hexaacetate 250 micrograms/ml solution for injection or infusion What is in this leaflet This leaflet answers some common questions
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only
 For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only Cholecalciferol Granules VITOMIN D3 COMPOSITION Each sachet of 1 g contains: Cholecalciferol
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only OR for Specialist Use only Cholecalciferol Granules VITOMIN D3 COMPOSITION Each sachet of 1 g contains: Cholecalciferol
PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol Read all of this leaflet carefully because it contains important information for you. This medicine is available
PACKAGE LEAFLET: INFORMATION FOR THE USER Paracetamol 500 mg Effervescent Tablets Paracetamol Read all of this leaflet carefully because it contains important information for you. This medicine is available
Public Assessment Report
 Public Assessment Report IBUPROFEN 200 MG TABLETS EXTRA STRENGTH IBUPROFEN 400 MG TABLETS (IBUPROFEN) PL 17907/0159-61 MHRA PAR Ibuprofen Tablets PL 17907/0159-61 - 1 - IBUPROFEN TABLETS (IBUPROFEN) PL
Public Assessment Report IBUPROFEN 200 MG TABLETS EXTRA STRENGTH IBUPROFEN 400 MG TABLETS (IBUPROFEN) PL 17907/0159-61 MHRA PAR Ibuprofen Tablets PL 17907/0159-61 - 1 - IBUPROFEN TABLETS (IBUPROFEN) PL
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection
 SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given SYNACTHEN
SYNACTHEN DEPOT i.m. (tetracosactide hexaacetate) 1 mg/ml Suspension for injection Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given SYNACTHEN
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
Data Sheet. Paraldehyde
 Data Sheet Paraldehyde Paraldehyde Injection Solution 100% Presentation Paraldehyde Injection BP is a sterile liquid containing paraldehyde BP with hydroquinone 100 micrograms/ml as an antioxidant. It
Data Sheet Paraldehyde Paraldehyde Injection Solution 100% Presentation Paraldehyde Injection BP is a sterile liquid containing paraldehyde BP with hydroquinone 100 micrograms/ml as an antioxidant. It
Arthritis and Rheumatology Clinics of Kansas Patient Education. Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis
 Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Arthritis and Rheumatology Clinics of Kansas Patient Education Reactive Arthritis (ReA) / Inflammatory Bowel Disease (IBD) Arthritis Introduction: For as long as scientists have studied rheumatic disease,
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
SUMMARY OF PRODUCT CHARACTERISTICS BREXIN. 3. Pharmaceutical Form Tablet Pale yellow, hexagonal tablet with a median score line on one side
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. Name of the Medicinal Product BREXIN 20 mg TABLETS BREXIN 2. Qualitative and Quantitative Composition Each tablet
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר SUMMARY OF PRODUCT CHARACTERISTICS 1. Name of the Medicinal Product BREXIN 20 mg TABLETS BREXIN 2. Qualitative and Quantitative Composition Each tablet
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES
 DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
Rheumatoid Arthritis monitoring of DMARDs
 www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
www.bpac.org.nz keyword: DMARDS Rheumatoid Arthritis monitoring of DMARDs Key reviewers: Professor John Highton, Head of Section, Department of Medical and Surgical Sciences, Dunedin School of Medicine,
Safety Information Card for Xarelto Patients
 Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
DULCOLAX Tablets and Suppositories Bisacodyl
 New Zealand Consumer Medicine Information DULCOLAX Tablets and Suppositories Bisacodyl What is in this leaflet This leaflet answers some common questions about DULCOLAX. It does not contain all available
New Zealand Consumer Medicine Information DULCOLAX Tablets and Suppositories Bisacodyl What is in this leaflet This leaflet answers some common questions about DULCOLAX. It does not contain all available
Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O.
 Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O. Kurt N. Woeller, DO, is an osteopathic physician specializing in natural medicine. Dr. Woeller began his study of natural medicine
Intestinal Permeability Leaky Gut Syndrome Protocol Dr. Kurt Woeller, D.O. Kurt N. Woeller, DO, is an osteopathic physician specializing in natural medicine. Dr. Woeller began his study of natural medicine
Arthritis of the Shoulder
 Arthritis of the Shoulder In 2011, more than 50 million people in the United States reported that they had been diagnosed with some form of arthritis, according to the National Health Interview Survey.
Arthritis of the Shoulder In 2011, more than 50 million people in the United States reported that they had been diagnosed with some form of arthritis, according to the National Health Interview Survey.
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection
 NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
CONSENT FOR STEROID INJECTION
 CONSENT FOR STEROID INJECTION What is Cortisone? Cortisone is the name used to describe a group of drugs correctly known as corticosteroids. Cortisone is used to treat pain in various parts of the body
CONSENT FOR STEROID INJECTION What is Cortisone? Cortisone is the name used to describe a group of drugs correctly known as corticosteroids. Cortisone is used to treat pain in various parts of the body
**Form 1: - Consultant Copy** Telephone Number: Fax Number: Email: Author: Dr Bernard Udeze Pharmacist: Claire Ault Date of issue July 2011
 Effective Shared Care Agreement for the treatment of Dementia in Alzheimer s Disease Donepezil tablets / orodispersible tablets (Aricept / Aricept Evess ) These forms (1 and 2) are to be completed by both
Effective Shared Care Agreement for the treatment of Dementia in Alzheimer s Disease Donepezil tablets / orodispersible tablets (Aricept / Aricept Evess ) These forms (1 and 2) are to be completed by both
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
Shared Care Guideline for the use of Leflunomide for Rheumatoid Arthritis
 Shared Care Guideline for the use of Leflunomide for Rheumatoid Section 1: Agreement for transfer of prescribing to GP Please sign this form and return it to the named consultant if you are willing to
Shared Care Guideline for the use of Leflunomide for Rheumatoid Section 1: Agreement for transfer of prescribing to GP Please sign this form and return it to the named consultant if you are willing to
2 What you need to know before you have Ampiclox
 Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Reason for update: GDS 14 & QRD Updates Response to questions for variation update section 4.1 of SPC MHRA Submission Date: 6 November 2014 MHRA Approval Date: Text Date: October 2014 Text Issue and Draft
Perfalgan 10 mg/ml, solution for infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Yes This controlled document shall not be copied in part or whole without the express permission of the author or the author s representative.
 Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
PHENYLEPHRINE HYDROCHLORIDE INJECTION USP
 PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Package leaflet: Information for the patient. Laxido Orange, powder for oral solution
 Package leaflet: Information for the patient Laxido Orange, powder for oral solution Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Package leaflet: Information for the patient Laxido Orange, powder for oral solution Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Amylase and Lipase Tests
 Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
IBUPROFEN 400MG TABLETS PL 20395/0080 UKPAR TABLE OF CONTENTS
 IBUPROFEN 400MG TABLETS PL 20395/0080 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Steps taken after authorisation summary Summary of Product
IBUPROFEN 400MG TABLETS PL 20395/0080 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Steps taken after authorisation summary Summary of Product
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
SUMMARY OF PRODUCT CHARACTERISTICS. Paracetamol...10.00 mg for 1 ml of solution for infusion
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol...10.00 mg for 1 ml of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol...10.00 mg for 1 ml of
Single Blind, Comparative Study of Ketoprofen Cream Vs Diclofenac and Piroxicam Cream in Management of Rheumatoid Arthritis Patients
 International Journal of Pharma Sciences Vol. 4, No. 5 (2014): 697-701 Research Article Open Access ISSN: 2320-6810 Single Blind, Comparative Study of Ketoprofen Vs Diclofenac and Piroxicam in Management
International Journal of Pharma Sciences Vol. 4, No. 5 (2014): 697-701 Research Article Open Access ISSN: 2320-6810 Single Blind, Comparative Study of Ketoprofen Vs Diclofenac and Piroxicam in Management
VOLTAREN OPHTHA EYE DROPS
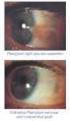 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Public Assessment Report. Table of Contents
 Public Assessment Report Lidocaine Injection BP with preservative 1% Lidocaine Injection BP with preservative 2% PL 01502/0070 PL 01502/0071 Hameln Pharmaceuticals Limited Table of Contents Page Lay Summary
Public Assessment Report Lidocaine Injection BP with preservative 1% Lidocaine Injection BP with preservative 2% PL 01502/0070 PL 01502/0071 Hameln Pharmaceuticals Limited Table of Contents Page Lay Summary
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
Before you use Voltaren. When you must not use it
 Voltaren(R) diclofenac sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Voltaren. It does not contain all the available information. It does
Voltaren(R) diclofenac sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Voltaren. It does not contain all the available information. It does
GLUCOSAMINE, CHONDROITIN, AND MSM
 GLUCOSAMINE, CHONDROITIN, AND MSM Compiled by Campbell M Gold (2009) (This material was compiled from various unverified sources) CMG Archives http://campbellmgold.com IMPORTANT The health information
GLUCOSAMINE, CHONDROITIN, AND MSM Compiled by Campbell M Gold (2009) (This material was compiled from various unverified sources) CMG Archives http://campbellmgold.com IMPORTANT The health information
Allopurinol Allopurinol
 Drug information Allopurinol Allopurinol This leaflet provides information on allopurinol and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Drug information Allopurinol Allopurinol This leaflet provides information on allopurinol and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (HCl) STEROP 0,8mg/1ml. Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust.
 Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Patient Group Direction Hospital: Bristol Royal Infirmary Department: UHBristol Thrombosis Service University Hospitals Bristol NHS Foundation Trust. This Patient Group Direction (PGD) has been written
Hand Injuries and Disorders
 Hand Injuries and Disorders Introduction Each of your hands has 27 bones, 15 joints and approximately 20 muscles. There are many common problems that can affect your hands. Hand problems can be caused
Hand Injuries and Disorders Introduction Each of your hands has 27 bones, 15 joints and approximately 20 muscles. There are many common problems that can affect your hands. Hand problems can be caused
