ISO 14971: Overview of the standard
|
|
|
- Paul Simpson
- 7 years ago
- Views:
Transcription
1 FDA Medical Device Industry Coalition ISO 14971: Overview of the standard Risk Management Through Product Life Cycle: An Educational Forum William A. Hyman Department of Biomedical Engineering Texas A&M University Medical devices Application of risk management to medical devices - 14 pages in body - 63 pages in 10 Annexes April William Hyman, Sc.D. 1
2 Contents Scope Terms and definitions General requirements Risk analysis Risk evaluation Risk control Residual risk acceptability Report/documentation Post-production April Contents Scope Terms and definitions General requirements Risk analysis Risk evaluation Risk control Residual risk acceptability Report/documentation Post-production April William Hyman, Sc.D. 2
3 Annexes Rationale Overview Identifying device characteristics that have risk Risk concepts Examples of hazards Risk management plan Techniques In vitro Biological hazards Residual risk April Observations on It is voluntary It is cited and recognized by FDA A search within FDA for ISO produces 564 items! It s useful application requires knowledge and diligence April 2010 William Hyman, Sc.D. 3
4 Observations on The reasons to undertake risk management (guided by 14971) are that: Reducing risk is a good thing Compliance is also a good thing Don t let the compliance imperative overwhelm the risk control imperative April 2010 Direct citations of in submitted documents From an MDR: risk analysis (performed according to ISO From a 510(k) Summary: risk analysis preformed identified by ISO 14971and QSR and internal procedures for risk analysis. April William Hyman, Sc.D. 4
5 General principles Risk is commonly described as having two principle components: > Severity if the harm occurs > Probability of the harm occurring April A few definitions Harm is the adverse event Hazard is the potential cause of the harm Note: You can detect a hazard before it causes harm, but detecting harm means the harm already occurred Safety is the absence of unacceptable risks But unacceptable to whom? April William Hyman, Sc.D. 5
6 General principles Risk management is something that you have to actively do It is not simply a byproduct of general good intentions and good engineering April General principles Risk can often not be reduced to zero but this is not an excuse for all hazards and harms There may be residual risks after appropriate risk evaluation and control Residual risks must undergo acceptance and communication activities 12 William Hyman, Sc.D. 6
7 The process part 1 Risk Analysis Risk Evaluation Risk Assessment Risk Control April The process part 1 Cycle! Risk Analysis Risk Evaluation Risk Control Risk Assessment April William Hyman, Sc.D. 7
8 The process part 2 Risk Analysis Risk Evaluation Cycle! Risk Control Acceptability? 15 The process part 3 Risk Analysis Risk Evaluation Cycle! Risk Control Acceptability? Repor t Post Production Risk Management 16 William Hyman, Sc.D. 8
9 A note on post production Manufacturing deviations Complaints and complaint handling CAPA Capture Recalls Evaluate Act Evaluate April General requirements in (Section 3) A formal (documented) process and plan in place Management commitment Resources Personnel Qualified personnel Documented results April William Hyman, Sc.D. 9
10 Risk analysis (Section 4) Intended use and misuse Risk related characteristics Hazard identification > known and foreseeable Risk estimation > systematic > based on available and general information April Risk evaluation (Section 5) Manufacturer determined criteria for Risk acceptability decision making There is not a predetermined, all purpose acceptable level of risk > no equation > no regulation April William Hyman, Sc.D. 10
11 Risk control (Section 6) - Engineering Elimination by (re)design Protective measures to protect against the risk both physical and alarms Information (IFUs, training) Preferably in this order! You shouldn t try to fix a dangerous design with a warning (if you could have reasonably designed it out)! April Risk control (Section 6) -Management Residual risk evaluation after controls are applied Another round of acceptance decision making including Risk/benefit analysis (Section 6.5) > an effort to make otherwise unacceptable risks acceptable which is potentially confusing If accepted --- disclosure decision making April 2010 William Hyman, Sc.D. 11
12 Overall risk (Section 7) - Management After all individual risk control activity has been done then decision making applied to complete design Note: assessing the collective risk that results from the individual risks is a challenging and imprecise procedure April Report (Section 8) Review pre product release Appropriately implemented Overall residual risk is acceptable Post production processes are in place Documented April William Hyman, Sc.D. 12
13 Post production(section 9) Collect and review Attention to previously unrecognized risks Attention to severity or rate of occurrence above originally estimated Include feedback into risk management process itself April Annexes Rationale Overview Identifying device characteristics associated with risk Risk concepts Examples of hazards Risk management plan Techniques In vitro Biological hazards Residual risk 26 William Hyman, Sc.D. 13
14 Annex C Questions -examples Intended and means of use (& user) Materials and components Sterile, or user sterilization Measurements and data interpretation Use in conjunction with interfacing Unwanted outputs (e.g. Noise, heat, EMI) Susceptible to environment, forces, etc. 27 Annex C Questions (cont) Software (& menus) Reuse, intended and single use Installation & use training New manufacturing processes Transmittal of user information User interface issues human factors distractions alarms predictable misuse 28 William Hyman, Sc.D. 14
15 Note Lists of questions, lists of hazards, check lists, pull down menus, etc are not a substitute for thoughtful analysis by a knowledgeable person The more unique/different your device is, the less likely it is that pre-prepared lists will be comprehensive April Annexes Rationale Overview Identifying device characteristics associated with risk Risk concepts Examples of hazards Risk management plan Techniques In vitro Biological hazards Residual risk 30 William Hyman, Sc.D. 15
16 Annex D Risk concepts Probability and severity scales Qualitative 3 and 5 level scales are shown Severity Catastrophic Critical Serious Minor Negligible Death Permanent impairment or life threatening Medical intervention No medical intervention Inconvenience or temporary 31 Annex D Risk concepts Probability and severity scales Probability with verbal or numerical descriptors other Frequent >10-3 highly likely Probable < 10-3 and >= 10-4 will occur Occasional < 10-4 and >=10-5 may occur Remote < 10-5 and >=10-6 possible but unlikely Improbable < 10-6 very unlikely 32 William Hyman, Sc.D. 16
17 Annex D Risk concepts Scale issues How many levels? 3? 5? 10? Levels are often ill defined especially probability Often imprecise, yet precision is pretended Tendency to be optimistic (if not cheat) 33 Annex D Risk concepts Matrix Probability Severity William Hyman, Sc.D. 17
18 Annex D Risk concepts Matrix Probability Good (more or less) Severity Annex D Risk concepts Matrix Bad (more or less) Probability Good (more or less) Severity William Hyman, Sc.D. 18
19 Annex D Risk concepts Matrix Bad (more or less) Probability Good (more or less) Severity Challenging! Annex D Risk concepts Matrix Bad (more or less) And the rest? Probability Good (more or less) Severity Challenging! William Hyman, Sc.D. 19
20 Annex D Risk concepts There is no zone that is automatically acceptable or unacceptable The manufacturer must have their own decision and sign off process April Annex D Risk concepts Analysis of Risk: Are Current Methods Theoretically Sound? Applying risk assessment may not give manufacturers the answers they think they are getting Nataly F. Youssef and William A. Hyman April William Hyman, Sc.D. 20
21 Annexes Rationale Overview Identifying device characteristics associated with risk Risk concepts Examples of hazards Risk management plan Techniques In vitro Biological hazards Residual risk 41 Annex G- Risk management techniques Preliminary hazard analysis (PHA) early review of potential risks and their possible causation early guidance on what will need to be controlled April William Hyman, Sc.D. 21
22 Annex G- Risk management techniques Fault tree analysis (FTA) > define a bad event > identify what can lead to that event > identify what can lead to the things that lead to the event > etc April FTA Bad event #1 Cause #1 Cause #2 Cause #3 Cause (a) of cause #1 Cause (b) of cause #1 April William Hyman, Sc.D. 22
23 Annex G- Risk management techniques Failure Modes and Effects Analysis(FMEA) > identify a failure mode of compoent, device, or user > identify the effects of that failure mode > perform risk assessment to determine if the effect requires action April Annex G- Risk management techniques Hazard and Operability Study(HAZOP) > similar to FMEA > emphasis on use of system Hazard Analysis and Critical Control Points > similar to or uses many of the same methods > emphasis on processes (e.g. manufacturing) April William Hyman, Sc.D. 23
24 Annexes Rationale Overview Identifying device characteristics associated with risk Risk concepts Examples of hazards Risk management plan Techniques In vitro Biological hazards Residual risk 47 Annex J- Residual risk Communication Audience Method Effectiveness April William Hyman, Sc.D. 24
25 SUMMARY Risk management is a good thing even if there wasn t an FDA is a well recognized guide to risk management methodology But it is not a cook book, or a check list, or an alternative to conscientious effort The objective is to use it thoughtfully as opposed to going through the motions to meet regulatory requirements April Questions? April William Hyman, Sc.D. 25
Risk Assessment for Medical Devices. Linda Braddon, Ph.D. Bring your medical device to market faster 1
 Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Risk Assessment for Medical Devices Linda Braddon, Ph.D. Bring your medical device to market faster 1 My Perspective Work with start up medical device companies Goal: Making great ideas into profitable
Risk Management and the Impact of EN ISO 14971:2012 Annex Z
 Risk Management and the Impact of EN ISO 14971:2012 Annex Z BSI 2014 Medical Device Mini-Roadshow Ibim Tariah Ph.D Technical Director, Healthcare Solutions Copyright 2014 BSI. All rights reserved. 1 Risk
Risk Management and the Impact of EN ISO 14971:2012 Annex Z BSI 2014 Medical Device Mini-Roadshow Ibim Tariah Ph.D Technical Director, Healthcare Solutions Copyright 2014 BSI. All rights reserved. 1 Risk
Quality Risk Management - The Medical Device Experience. Niamh Nolan Principal Design Assurance Engineer Boston Scientific
 Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
Quality Risk Management - The Medical Device Experience Niamh Nolan Principal Design Assurance Engineer Boston Scientific Agenda Intent of Risk Management (RM) and Associated Regulations Overview of RM
Medical Software Development. International standards requirements and practice
 Medical Software Development International standards requirements and practice Food and Drug Administration What? A public health agency Why? Protect American consumers How? By enforcing the Federal Food,
Medical Software Development International standards requirements and practice Food and Drug Administration What? A public health agency Why? Protect American consumers How? By enforcing the Federal Food,
Title: Basic Principles of Risk Management for Medical Device Design
 Title: Basic Principles of Risk Management for Medical Device Design WHITE PAPER Author: Ganeshkumar Palanichamy Abstract Medical devices developed for human application are used for diagnostic or treatment
Title: Basic Principles of Risk Management for Medical Device Design WHITE PAPER Author: Ganeshkumar Palanichamy Abstract Medical devices developed for human application are used for diagnostic or treatment
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix
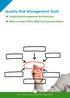 Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
FINAL DOCUMENT. Implementation of risk management principles and activities within a Quality Management System. The Global Harmonization Task Force
 GHTF/SG3/N15R8 FINAL DOCUMENT Title: Implementation of risk management principles and activities within a Quality Management System Authoring Group: GHTF Study Group 3 Endorsed by: The Global Harmonization
GHTF/SG3/N15R8 FINAL DOCUMENT Title: Implementation of risk management principles and activities within a Quality Management System Authoring Group: GHTF Study Group 3 Endorsed by: The Global Harmonization
Designing an Effective Risk Matrix
 Designing an Effective Risk Matrix HENRY OZOG INTRODUCTION Risk assessment is an effective means of identifying process safety risks and determining the most cost-effective means to reduce risk. Many organizations
Designing an Effective Risk Matrix HENRY OZOG INTRODUCTION Risk assessment is an effective means of identifying process safety risks and determining the most cost-effective means to reduce risk. Many organizations
Corrective and Preventive Action Background & Examples Presented by:
 Corrective and Preventive Action Background & Examples Presented by: Kimberly Lewandowski-Walker Food and Drug Administration Division of Domestic Field Investigations Office of Regulatory Affairs Overview
Corrective and Preventive Action Background & Examples Presented by: Kimberly Lewandowski-Walker Food and Drug Administration Division of Domestic Field Investigations Office of Regulatory Affairs Overview
Guidance for Industry: Quality Risk Management
 Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
Guidance for Industry: Quality Risk Management Version 1.0 Drug Office Department of Health Contents 1. Introduction... 3 2. Purpose of this document... 3 3. Scope... 3 4. What is risk?... 4 5. Integrating
RISK MANAGEMENT FOR INFRASTRUCTURE
 RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
RISK MANAGEMENT FOR INFRASTRUCTURE CONTENTS 1.0 PURPOSE & SCOPE 2.0 DEFINITIONS 3.0 FLOWCHART 4.0 PROCEDURAL TEXT 5.0 REFERENCES 6.0 ATTACHMENTS This document is the property of Thiess Infraco and all
Software-based medical devices from defibrillators
 C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
C O V E R F E A T U R E Coping with Defective Software in Medical Devices Steven R. Rakitin Software Quality Consulting Inc. Embedding defective software in medical devices increases safety risks. Given
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays
 Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
Risk Assessment Tools for Identifying Hazards and Evaluating Risks Associated with IVD Assays Robert C. Menson, PhD AACC Annual Meeting Philadelphia, PA 22 July 2003 What Risks Must Be Managed? Risk to
QUALITY RISK MANAGEMENT (QRM): A REVIEW
 Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
Lotlikar et al Journal of Drug Delivery & Therapeutics; 2013, 3(2), 149-154 149 Available online at http://jddtonline.info REVIEW ARTICLE QUALITY RISK MANAGEMENT (QRM): A REVIEW Lotlikar MV Head Corporate
Quality Risk Management ICH Q9 & ISO 14971. Presented by Michael Kerr 11 th November 2011
 Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Quality Risk Management ICH Q9 & ISO 14971 Presented by Michael Kerr 11 th November 2011 Agenda Risk Concept QRM Fundamentals Regulatory Expectations Warning Letters / Observations Application of QRM Introduction:
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS. April 2008 1
 Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
Safety Regulation Group SAFETY MANAGEMENT SYSTEMS GUIDANCE TO ORGANISATIONS April 2008 1 Contents 1 Introduction 3 2 Management Systems 2.1 Management Systems Introduction 3 2.2 Quality Management System
CAPA - the importance of data analysis
 CAPA - the importance of data analysis Presented by: Sue Jacobs QMS Consulting, Inc. 1 847 359 4456 sue@qmsconsultant.com QMS Consulting, Inc. 2007 1 Topics Regulatory Requirements Design Controls and
CAPA - the importance of data analysis Presented by: Sue Jacobs QMS Consulting, Inc. 1 847 359 4456 sue@qmsconsultant.com QMS Consulting, Inc. 2007 1 Topics Regulatory Requirements Design Controls and
Media fills Periodic performance qualification (Re-Validation)
 Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
Media fills Periodic performance qualification (Re-Validation) Minimum number of Simulations Number of units Contaminated Units Action a Two per Year (Retrospective & Prospective Validation) < 5000 5000
140.01.3 REQUIREMENTS OF SAFETY MANAGEMENT SYSTEM
 SA-CATS 140 Safety management system List of technical standards 140.01.3 REQUIREMENTS OF SAFETY MANAGEMENT SYSTEM 1. Minimum standards for the safety management system 140.01.3 REQUIREMENTS OF A SAFETY
SA-CATS 140 Safety management system List of technical standards 140.01.3 REQUIREMENTS OF SAFETY MANAGEMENT SYSTEM 1. Minimum standards for the safety management system 140.01.3 REQUIREMENTS OF A SAFETY
Hazard Identification, Risk Assessment and Management Procedure. Documentation Control
 Hazard Identification, Risk Assessment and Management Procedure Reference: Date approved: Approving Body: Implementation Date: Version: 3 Documentation Control GG/CM/007 Trust Board Supersedes: Version
Hazard Identification, Risk Assessment and Management Procedure Reference: Date approved: Approving Body: Implementation Date: Version: 3 Documentation Control GG/CM/007 Trust Board Supersedes: Version
Introduction into IEC 62304 Software life cycle for medical devices
 Introduction into IEC 62304 Software life cycle for medical devices Christoph Gerber 4. September 2008 SPIQ 9/5/2008 1 Agenda Current Picture Regulatory requirements for medical device software IEC 62304
Introduction into IEC 62304 Software life cycle for medical devices Christoph Gerber 4. September 2008 SPIQ 9/5/2008 1 Agenda Current Picture Regulatory requirements for medical device software IEC 62304
Safety Management Systems (SMS) guidance for organisations
 Safety and Airspace Regulation Group Safety Management Systems (SMS) guidance for organisations CAP 795 Published by the Civil Aviation Authority, 2014 Civil Aviation Authority, CAA House, 45-59 Kingsway,
Safety and Airspace Regulation Group Safety Management Systems (SMS) guidance for organisations CAP 795 Published by the Civil Aviation Authority, 2014 Civil Aviation Authority, CAA House, 45-59 Kingsway,
Vigilance Reporting. Vicky Medley - Head of QMS, Medical Devices. September 2015. Copyright 2015 BSI. All rights reserved.
 Vigilance Reporting Vicky Medley - Head of QMS, Medical Devices September 2015 2 Why? 3 protecting and improving public health https://www.gov.uk/government/organisations/medicines-and-healthcareproducts-regulatory-agency/about
Vigilance Reporting Vicky Medley - Head of QMS, Medical Devices September 2015 2 Why? 3 protecting and improving public health https://www.gov.uk/government/organisations/medicines-and-healthcareproducts-regulatory-agency/about
Controlling Risks Risk Assessment
 Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Controlling Risks Risk Assessment Hazard/Risk Assessment Having identified the hazards, one must assess the risks by considering the severity and likelihood of bad outcomes. If the risks are not sufficiently
Safety Risk Management in RT: A Software Manufacturer Perspective
 Safety Risk Management in RT: A Software Manufacturer Perspective Jim Schewe, Ph.D. Philips Radiation Oncology Systems AAPM Spring Clinical Meeting March 16, 2014 1 Learning Objectives For the session
Safety Risk Management in RT: A Software Manufacturer Perspective Jim Schewe, Ph.D. Philips Radiation Oncology Systems AAPM Spring Clinical Meeting March 16, 2014 1 Learning Objectives For the session
Risk Management: Coordinated activities to direct and control an organisation with regard to risk.
 POLICY CG01 RISK MANAGEMENT Document Control Statement This Policy is maintained by the Governance and Organisational Strategy. Any printed copy may not be up to date and you are advised to check the electronic
POLICY CG01 RISK MANAGEMENT Document Control Statement This Policy is maintained by the Governance and Organisational Strategy. Any printed copy may not be up to date and you are advised to check the electronic
University of Paderborn Software Engineering Group II-25. Dr. Holger Giese. University of Paderborn Software Engineering Group. External facilities
 II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
II.2 Life Cycle and Safety Safety Life Cycle: The necessary activities involving safety-related systems, occurring during a period of time that starts at the concept phase of a project and finishes when
Bringing Legacy Medical Devices into Usability Compliance. Shannon Clark 4/29/2015
 Bringing Legacy Medical Devices into Usability Compliance Shannon Clark 4/29/2015 Agenda What is a legacy device? Background on Usability Engineering Standards What is the roadmap for bringing legacy devices
Bringing Legacy Medical Devices into Usability Compliance Shannon Clark 4/29/2015 Agenda What is a legacy device? Background on Usability Engineering Standards What is the roadmap for bringing legacy devices
ELECTRICAL SAFETY RISK ASSESSMENT
 ELECTRICAL SAFETY RISK ASSESSMENT The intent of this procedure is to perform a risk assessment, which includes a review of the electrical hazards, the associated foreseeable tasks, and the protective measures
ELECTRICAL SAFETY RISK ASSESSMENT The intent of this procedure is to perform a risk assessment, which includes a review of the electrical hazards, the associated foreseeable tasks, and the protective measures
3.0 Risk Assessment and Analysis Techniques and Tools
 3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
3.0 Risk Assessment and Analysis Techniques and Tools Risks are determined in terms of the likelihood that an uncontrolled event will occur and the consequences of that event occurring. Risk = Likelihood
a Medical Device Privacy Consortium White Paper
 a Medical Device Privacy Consortium White Paper Introduction The Medical Device Privacy Consortium (MDPC) is a group of leading companies addressing health privacy and security issues affecting the medical
a Medical Device Privacy Consortium White Paper Introduction The Medical Device Privacy Consortium (MDPC) is a group of leading companies addressing health privacy and security issues affecting the medical
Quality Risk Management
 PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
Risk Management in IEC 60601-1 3 rd Edition. Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service
 Risk Management in IEC 60601-1 3 rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service General Information Time of presentation: 50-60 min. Questions
Risk Management in IEC 60601-1 3 rd Edition Presented by Alberto Paduanelli Medical Devices Lead Auditor, MHS-UK, TÜV SÜD Product Service General Information Time of presentation: 50-60 min. Questions
Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.
 Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.. www.pharmout.net Page 1 of 15 Version-02 1. Scope 1.1. Purpose This paper reviews the implementation of the ANSI/AAMI/IEC
Implementation of ANSI/AAMI/IEC 62304 Medical Device Software Lifecycle Processes.. www.pharmout.net Page 1 of 15 Version-02 1. Scope 1.1. Purpose This paper reviews the implementation of the ANSI/AAMI/IEC
Best Practice In A Change Management System
 Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Quality & Compliance Associates, LLC Best Practice In A Change Management System President Quality & Compliance Associates, LLC Change Control and Its Role in a Continuous Improvement Environment 3 Benefits
Occupational safety risk management in Australian mining
 IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
IN-DEPTH REVIEW Occupational Medicine 2004;54:311 315 doi:10.1093/occmed/kqh074 Occupational safety risk management in Australian mining J. Joy Abstract Key words In the past 15 years, there has been a
Program Hazard Analysis
 For New, Modified, or Recognized Activities 10/20/2009 Revision and Update This evaluation process is used to systematically identify, assess, and resolve hazards associated with program activities that
For New, Modified, or Recognized Activities 10/20/2009 Revision and Update This evaluation process is used to systematically identify, assess, and resolve hazards associated with program activities that
FMEA Failure Risk Scoring Schemes
 FMEA Failure Risk Scoring Schemes 1-10 Scoring for Severity, Occurrence and Detection CATEGORY Severity 10 9 8 7 6 5 3 2 1 Hazardous, without warning Hazardous, with warning Very High High Moderate Low
FMEA Failure Risk Scoring Schemes 1-10 Scoring for Severity, Occurrence and Detection CATEGORY Severity 10 9 8 7 6 5 3 2 1 Hazardous, without warning Hazardous, with warning Very High High Moderate Low
Quality Risk Management in Pharmaceutical Industry: A Review
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.6, No.3, pp 908-914, July-Aug 2014 Quality Risk Management in Pharmaceutical Industry: A Review V Vijayakumar Reddy*,
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.6, No.3, pp 908-914, July-Aug 2014 Quality Risk Management in Pharmaceutical Industry: A Review V Vijayakumar Reddy*,
Risk Assessment Tool and Guidance (Including guidance on application)
 Risk Assessment Tool and Guidance (Including guidance on application) Document reference number Revision number OQR012 Document developed by 5 Document approved by Revision date October 2011 Responsibility
Risk Assessment Tool and Guidance (Including guidance on application) Document reference number Revision number OQR012 Document developed by 5 Document approved by Revision date October 2011 Responsibility
Board of Directors 24 October 2014
 Board of Directors 24 October 2014 AGENDA ITEM: Item 16 PRESENTED BY: Richard Jones, Trust Secretary & Head of Governance PREPARED BY: DATE PREPARED: 19 September 2014 Richard Jones, Trust Secretary &
Board of Directors 24 October 2014 AGENDA ITEM: Item 16 PRESENTED BY: Richard Jones, Trust Secretary & Head of Governance PREPARED BY: DATE PREPARED: 19 September 2014 Richard Jones, Trust Secretary &
Risk Management. Policy
 Policy Risk Management Endorsed: 26 February 2014 Brief description The GPC Risk Management Policy and its supporting standards and procedures provide a framework to ensure that risks arising from our
Policy Risk Management Endorsed: 26 February 2014 Brief description The GPC Risk Management Policy and its supporting standards and procedures provide a framework to ensure that risks arising from our
Management and Reporting of Undergraduate Nurse Incidents During Clinical Placement at RCH
 Management and Reporting of Undergraduate Nurse Incidents During Clinical Placement at RCH Management and Reporting of Undergraduate Nurse Incidents During Clinical Placement at RCH Overview The purpose
Management and Reporting of Undergraduate Nurse Incidents During Clinical Placement at RCH Management and Reporting of Undergraduate Nurse Incidents During Clinical Placement at RCH Overview The purpose
Rulemaking Directorate. Preliminary Regulatory Impact Assessment Explanatory Note 2012/2013
 Rulemaking Directorate Preliminary Regulatory Impact Assessment Explanatory Note 2012/2013 1 What is the purpose of the Pre-RIA?... 2 2 What is new?... 2 3 Rulemaking procedure and workflow... 3 4 Description
Rulemaking Directorate Preliminary Regulatory Impact Assessment Explanatory Note 2012/2013 1 What is the purpose of the Pre-RIA?... 2 2 What is new?... 2 3 Rulemaking procedure and workflow... 3 4 Description
Standard Operating Procedure Title: Quality Risk Management Techniques
 Department Quality Management Document no QMS-135 Title Quality Risk Management Techniques Prepared by: Date: Supersedes: Checked by: Date: Date Issued: Approved by: Date: Review Date: 1.0 Purpose This
Department Quality Management Document no QMS-135 Title Quality Risk Management Techniques Prepared by: Date: Supersedes: Checked by: Date: Date Issued: Approved by: Date: Review Date: 1.0 Purpose This
A Risk Management Standard
 A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
A Risk Management Standard Introduction This Risk Management Standard is the result of work by a team drawn from the major risk management organisations in the UK, including the Institute of Risk management
Quality Risk Management Understanding and Control the Risk in Pharmaceutical Manufacturing Industry
 International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
International Journal of Pharmaceutical Science Invention ISSN (Online): 2319 6718, ISSN (Print): 2319 670X Volume 4 Issue 1 January 2015 PP.29-41 Quality Risk Management Understanding and Control the
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
LSST Hazard Analysis Plan
 LSST Hazard Analysis Plan Large Synoptic Survey Telescope 950 N. Cherry Avenue Tucson, AZ 85719 www.lsst.org 1. REVISION SUMMARY: Contents 1 Introduction... 5 2 Definition of Terms... 5 2.1 System... 5
LSST Hazard Analysis Plan Large Synoptic Survey Telescope 950 N. Cherry Avenue Tucson, AZ 85719 www.lsst.org 1. REVISION SUMMARY: Contents 1 Introduction... 5 2 Definition of Terms... 5 2.1 System... 5
Application of Quality Risk Management to Pharmaceutical Operations. Eldon Henson, Vice President, Quality Operations
 Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
Application of Quality Risk Management to Pharmaceutical Operations Eldon Henson, Vice President, Quality Operations Key Topics of Discussion Definition of Quality Risk Management (QRM) Overview of PDA
QUALITY RISK MANAGEMENT
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY RISK MANAGEMENT Q9 Current Step 4 version
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY RISK MANAGEMENT Q9 Current Step 4 version
How to conduct risk management & vulnerability assessments of medical devices using current ISO/IEC standards as a guidance
 How to conduct risk management & vulnerability assessments of medical devices using current ISO/IEC standards as a guidance Stephen L. Grimes, FACCE FHIMSS FAIMBE Chief Technology Officer ABM Healthcare
How to conduct risk management & vulnerability assessments of medical devices using current ISO/IEC standards as a guidance Stephen L. Grimes, FACCE FHIMSS FAIMBE Chief Technology Officer ABM Healthcare
Guidance for Industry
 Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q9 Quality Risk Management U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Hazard Identification, Risk Assessment and Control Management
 The Paraplegic and Quadriplegic Association of SA Inc Hazard Identification, Risk Assessment and Control Management STATEMENT The Paraplegic and Quadriplegic Association of South Australia Incorporated
The Paraplegic and Quadriplegic Association of SA Inc Hazard Identification, Risk Assessment and Control Management STATEMENT The Paraplegic and Quadriplegic Association of South Australia Incorporated
Risk Assessment and Management. Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc.
 Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
Risk Assessment and Management Allen L. Burgenson Manager, Regulatory Affairs Lonza Walkersville Inc. Standard Disclaimer Standard Disclaimer: This presentation is the opinion of the presenter, and does
Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle
 Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
Quality Risk Management 11 November 2011 Galway, Ireland Quality Risk Management The Pharmaceutical Experience Ann O Mahony Quality Assurance Specialist Pfizer Biotech Grange Castle Overview Regulatory
Overview of Medical Device Design Controls in the US. By Nandini Murthy, MS, RAC
 Overview of Medical Device Controls in the US By Nandini Murthy, MS, RAC 18 controls are a regulatory requirement for medical devices. In the US, compliance with the design controls section of 21 Code
Overview of Medical Device Controls in the US By Nandini Murthy, MS, RAC 18 controls are a regulatory requirement for medical devices. In the US, compliance with the design controls section of 21 Code
Risk Management and Cybersecurity for Devices that Contain Software. Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015
 Risk Management and Cybersecurity for Devices that Contain Software Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015 Main Points Establish a Cybersecurity Risk Management Program
Risk Management and Cybersecurity for Devices that Contain Software Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015 Main Points Establish a Cybersecurity Risk Management Program
Hazard/Risk Identification and Control Procedure
 Hazard/Risk Identification and Control Procedure Introduction Hazard identification and the steps taken to minimize the risks associated with identified hazards are a critical component of working safely.
Hazard/Risk Identification and Control Procedure Introduction Hazard identification and the steps taken to minimize the risks associated with identified hazards are a critical component of working safely.
Integrating System Safety and Software Assurance
 Integrating System Safety and Software Assurance Systems Certification and Integrity Directorate of Aviation Engineering Directorate General Technical Airworthiness 1 Overview Integration of software assurance
Integrating System Safety and Software Assurance Systems Certification and Integrity Directorate of Aviation Engineering Directorate General Technical Airworthiness 1 Overview Integration of software assurance
RISK MANAGEMENT POLICY. Version 3
 RISK MANAGEMENT POLICY Version 3 Version: Version 3 Version 3 Authors: Liz Hollman, Mary Klaus, Sarah Langan-Hart Approved by: Healthcare Governance Committee Trust Board Approved date: May 2009 Review
RISK MANAGEMENT POLICY Version 3 Version: Version 3 Version 3 Authors: Liz Hollman, Mary Klaus, Sarah Langan-Hart Approved by: Healthcare Governance Committee Trust Board Approved date: May 2009 Review
identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the hazard.
 What is a risk assessment? Risk assessment is the process where you: identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the
What is a risk assessment? Risk assessment is the process where you: identify hazards, analyze or evaluate the risk associated with that hazard, and determine appropriate ways to eliminate or control the
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach
 Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
Improved Utilization of Self-Inspection Programs within the GMP Environment A Quality Risk Management Approach Barbara Jeroncic Self-inspection is a well-established and vital part of the pharmaceutical
The Lowitja Institute Risk Management Plan
 The Lowitja Institute Risk Management Plan 1. PURPOSE This Plan provides instructions to management and staff for the implementation of consistent risk management practices throughout the Lowitja Institute
The Lowitja Institute Risk Management Plan 1. PURPOSE This Plan provides instructions to management and staff for the implementation of consistent risk management practices throughout the Lowitja Institute
Risk Management Policy
 Risk Management Policy Responsible Officer Author Ben Bennett, Business Planning & Resources Director Julian Lewis, Governance Manager Date effective from December 2008 Date last amended December 2012
Risk Management Policy Responsible Officer Author Ben Bennett, Business Planning & Resources Director Julian Lewis, Governance Manager Date effective from December 2008 Date last amended December 2012
Guidance Notes on Job Safety Analysis for the Marine and Offshore Industries
 Guidance Notes on Job Safety Analysis for the Marine and Offshore Industries Outline What is Job Safety Analysis (JSA)? Why is JSA important to the marine and offshore industries? What is in the ABS GN
Guidance Notes on Job Safety Analysis for the Marine and Offshore Industries Outline What is Job Safety Analysis (JSA)? Why is JSA important to the marine and offshore industries? What is in the ABS GN
Title: OHS Risk Management Procedure
 Issue Date: July 2011 Review Date: July 2013 Page Number: 1 of 9 1. Purpose: To outline the methodology by which Department of Education and Early Childhood Development (DEECD) identifies, assesses, controls
Issue Date: July 2011 Review Date: July 2013 Page Number: 1 of 9 1. Purpose: To outline the methodology by which Department of Education and Early Childhood Development (DEECD) identifies, assesses, controls
ICH Q9 Quality Risk Management - an industry view. Peter H. Gough, Eli Lilly and Company
 ICH Q9 Quality Risk Management - an industry view Peter H. Gough, Eli Lilly and Company Contents How did we get here? FDA 21 st Century GMP Initiative ICH activity Introduction to risk management Links
ICH Q9 Quality Risk Management - an industry view Peter H. Gough, Eli Lilly and Company Contents How did we get here? FDA 21 st Century GMP Initiative ICH activity Introduction to risk management Links
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device
 Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE
 WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 globalsales@maetrics.com
WHITEPAPER: SOFTWARE APPS AS MEDICAL DEVICES THE REGULATORY LANDSCAPE White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 globalsales@maetrics.com
When Your Life Depends on Software
 Published on Quality Digest (http://www.qualitydigest.com) Home > Content When Your Life Depends on Software IEC 62304 imposes requirements on software for medical devices. We re all aware of the importance
Published on Quality Digest (http://www.qualitydigest.com) Home > Content When Your Life Depends on Software IEC 62304 imposes requirements on software for medical devices. We re all aware of the importance
(1) Extremely high risk CASCOM Commander, Commandants of Quartermaster, Ordnance or Transportation Schools, and DeCA Commander.
 Chapter 3 Composite Risk anagement (CR) 3-1. General a. CR is a leadership responsibility. Commanders/supervisors at every level will employ CR to effectively control safety and occupational health risks
Chapter 3 Composite Risk anagement (CR) 3-1. General a. CR is a leadership responsibility. Commanders/supervisors at every level will employ CR to effectively control safety and occupational health risks
PABIAC Safety-related Control Systems Workshop
 Health and and Safety Executive PABIAC Safety-related Control Systems Workshop KEY STANDARDS FOR ELECTRICAL & FUNCTIONAL SAFETY OF PAPERMAKING MACHINES: APPLICATION & USE Steve Frost HM Principal Electrical
Health and and Safety Executive PABIAC Safety-related Control Systems Workshop KEY STANDARDS FOR ELECTRICAL & FUNCTIONAL SAFETY OF PAPERMAKING MACHINES: APPLICATION & USE Steve Frost HM Principal Electrical
IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED
 IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Linda C. Bruno, M.A., MT(ASCP) Director, Microbiology and Molecular Labs ACL Laboratories, Rosemont, IL June 1, 2015 Disclosures - No disclosures 2 IQCP
IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Linda C. Bruno, M.A., MT(ASCP) Director, Microbiology and Molecular Labs ACL Laboratories, Rosemont, IL June 1, 2015 Disclosures - No disclosures 2 IQCP
Version: 3.0. Effective From: 19/06/2014
 Policy No: RM66 Version: 3.0 Name of Policy: Business Continuity Planning Policy Effective From: 19/06/2014 Date Ratified 05/06/2014 Ratified Business Service Development Committee Review Date 01/06/2016
Policy No: RM66 Version: 3.0 Name of Policy: Business Continuity Planning Policy Effective From: 19/06/2014 Date Ratified 05/06/2014 Ratified Business Service Development Committee Review Date 01/06/2016
BSI Road Show: September 8 th to 15 th, 2014
 BSI Road Show: September 8 th to 15 th, 2014 Post Market Surveillance (including PMCF): common non compliances Ibim Tariah Ph.D Technical Director, Healthcare Solutions Itoro Udofia Ph.D Global Head, Orthopaedics
BSI Road Show: September 8 th to 15 th, 2014 Post Market Surveillance (including PMCF): common non compliances Ibim Tariah Ph.D Technical Director, Healthcare Solutions Itoro Udofia Ph.D Global Head, Orthopaedics
Healthcare risk assessment made easy
 Healthcare risk assessment made easy March 2007 The purpose of this document is to provide: 1. an easy-to-use risk assessment tool that helps promote vigilance in identifying risk and the ways in which
Healthcare risk assessment made easy March 2007 The purpose of this document is to provide: 1. an easy-to-use risk assessment tool that helps promote vigilance in identifying risk and the ways in which
Analyzing Risks in Healthcare. February 12, 2014
 Analyzing s in Healthcare February 12, 2014 1 Content What is Enterprise Management (ERM) ERM Benefits ERM Standards / ISO 31000:2009 ERM Process Register ERM Governance Model s Q&A 2 What is Enterprise
Analyzing s in Healthcare February 12, 2014 1 Content What is Enterprise Management (ERM) ERM Benefits ERM Standards / ISO 31000:2009 ERM Process Register ERM Governance Model s Q&A 2 What is Enterprise
Design Verification The Case for Verification, Not Validation
 Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Overview: The FDA requires medical device companies to verify that all the design outputs meet the design inputs. The FDA also requires that the final medical device must be validated to the user needs.
Deviation Handling and Quality Risk Management
 Deviation Handling and Quality Risk Management A note for guidance for the manufacture of prequalified vaccines for supply to United Nations agencies July, 2013 Vaccine Quality and Regulations (VQR), Essential
Deviation Handling and Quality Risk Management A note for guidance for the manufacture of prequalified vaccines for supply to United Nations agencies July, 2013 Vaccine Quality and Regulations (VQR), Essential
Risk Management in the Medical Laboratory: Reducing Risk through Application of Standards
 Risk Management in the Medical Laboratory: Reducing Risk through Application of Standards Michael A Noble MD FRCPC Chair, Program Office for Laboratory Quality Management University of British Columbia
Risk Management in the Medical Laboratory: Reducing Risk through Application of Standards Michael A Noble MD FRCPC Chair, Program Office for Laboratory Quality Management University of British Columbia
Medical Device Reporting (MDR) 21 CFR Part 803
 Medical Device Reporting (MDR) 21 CFR Part 803 1 Objectives Review applicable sections of 21 CFR 803 and 21 CFR 820 Review and explain MDR reporting requirements Review FDA-483 observation examples 2 Medical
Medical Device Reporting (MDR) 21 CFR Part 803 1 Objectives Review applicable sections of 21 CFR 803 and 21 CFR 820 Review and explain MDR reporting requirements Review FDA-483 observation examples 2 Medical
White paper. Corrective action: The closed-loop system
 White paper Corrective action: The closed-loop system Contents Summary How corrective action works The steps 1 - Identify non-conformities - Opening a corrective action 6 - Responding to a corrective action
White paper Corrective action: The closed-loop system Contents Summary How corrective action works The steps 1 - Identify non-conformities - Opening a corrective action 6 - Responding to a corrective action
Document issued on: May 11, 2005
 Guidance for Industry and FDA Staff Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices Document issued on: May 11, 2005 This document supersedes Guidance for the
Guidance for Industry and FDA Staff Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices Document issued on: May 11, 2005 This document supersedes Guidance for the
FINAL DOCUMENT. Global Harmonization Task Force
 FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
FINAL DOCUMENT Global Harmonization Task Force Title: Quality management system Medical Devices Guidance on corrective action and preventive action and related QMS processes Authoring Group: Study Group
ORACLE CONSULTING GROUP
 ORACLE CONSULTING GROUP 9 Golder Ranch Rd., Ste. 1 Tucson, Arizona 9 Web Site: E-mail: 20-2-0 20-2-0 (FAX) CONSULTING MEMORANDUM QUALITY SYSTEM INSPECTION TECHNIQUE
ORACLE CONSULTING GROUP 9 Golder Ranch Rd., Ste. 1 Tucson, Arizona 9 Web Site: E-mail: 20-2-0 20-2-0 (FAX) CONSULTING MEMORANDUM QUALITY SYSTEM INSPECTION TECHNIQUE
SAFETY and HEALTH MANAGEMENT STANDARDS
 SAFETY and HEALTH STANDARDS The Verve Energy Occupational Safety and Health Management Standards have been designed to: Meet the Recognised Industry Practices & Standards and AS/NZS 4801 Table of Contents
SAFETY and HEALTH STANDARDS The Verve Energy Occupational Safety and Health Management Standards have been designed to: Meet the Recognised Industry Practices & Standards and AS/NZS 4801 Table of Contents
Report EHR Safety Event
 Report EHR Safety Event Please create a separate report for each event or hazard identified. If this report relates to a previously reported incident, please provide Event ID: What is Being Reported? Required
Report EHR Safety Event Please create a separate report for each event or hazard identified. If this report relates to a previously reported incident, please provide Event ID: What is Being Reported? Required
POL ENTERPRISE RISK MANAGEMENT SC51. Executive Services Department BUSINESS UNIT: Executive Support Services SERVICE UNIT:
 POL ENTERPRISE RISK MANAGEMENT SC51 POLICY CODE: SC51 DIRECTORATE: Executive Services Department BUSINESS UNIT: Executive Support Services SERVICE UNIT: Executive Support Services RESPONSIBLE OFFICER:
POL ENTERPRISE RISK MANAGEMENT SC51 POLICY CODE: SC51 DIRECTORATE: Executive Services Department BUSINESS UNIT: Executive Support Services SERVICE UNIT: Executive Support Services RESPONSIBLE OFFICER:
FMEA and FTA Analysis
 FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
FMEA and FTA Analysis Why it is Coming to Your Hospital and Your Laboratory Tina A. Krenc Director, R&D Phase Systems Abbott Laboratories 1 Agenda Background on requirements for risk management Tools to
Waveney Lower Yare & Lothingland Internal Drainage Board Risk Management Strategy and Policy
 Waveney Lower Yare & Lothingland Internal Drainage Board Risk Management Strategy and Policy Page: 1 Contents 1. Purpose, Aims & Objectives 2. Accountabilities, Roles & Reporting Lines 3. Skills & Expertise
Waveney Lower Yare & Lothingland Internal Drainage Board Risk Management Strategy and Policy Page: 1 Contents 1. Purpose, Aims & Objectives 2. Accountabilities, Roles & Reporting Lines 3. Skills & Expertise
GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA : A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology MEDDEV. 2.7.1 April 2003 GUIDELINES
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology MEDDEV. 2.7.1 April 2003 GUIDELINES
Quality Assurance. Disclosure for Lilli Møller Andersen. No relevant financial relationships exist for any issue mentioned in this presentation
 Quality Assurance Disclosure for Lilli Møller Andersen No relevant financial relationships exist for any issue mentioned in this presentation Agenda Quality Assurance Quality Management System Quality
Quality Assurance Disclosure for Lilli Møller Andersen No relevant financial relationships exist for any issue mentioned in this presentation Agenda Quality Assurance Quality Management System Quality
Let s skip over the next ten
 FOCUS ON... COMPLIANCE Risk-Based Quality Management Systems What Are We Waiting For? by Carol DeSain Let s skip over the next ten years, the changes in FDA policy, politics and leadership; the age-old
FOCUS ON... COMPLIANCE Risk-Based Quality Management Systems What Are We Waiting For? by Carol DeSain Let s skip over the next ten years, the changes in FDA policy, politics and leadership; the age-old
How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters
 environmental failure analysis & prevention health technology development How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters Kevin L. Ong, Ph.D., P.E. Managing
environmental failure analysis & prevention health technology development How to Use the Design Process to Manage Risk: Elements of Design Controls and Why It Matters Kevin L. Ong, Ph.D., P.E. Managing
Quality Risk Management Principles and Industry Case Studies
 Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
Final Draft Rev. December 28, 2008 Quality Risk Management Principles and Industry Case Studies T. Frank 1, S. Brooks 2, R. Creekmore 3, B. Hasselbalch 4, K. Murray 5, K. Obeng 6, S. Reich 5, E. Sanchez
CORP 600 00 RISK MANAGEMENT POLICY & METHODOLOGY
 CORP 600 00 RISK MANAGEMENT POLICY & METHODOLOGY CORP 600 RISK MANAGEMENT POLICY Purpose In March 2003, the Australian Stock Exchange (ASX) Corporate Governance Council released the first version of its
CORP 600 00 RISK MANAGEMENT POLICY & METHODOLOGY CORP 600 RISK MANAGEMENT POLICY Purpose In March 2003, the Australian Stock Exchange (ASX) Corporate Governance Council released the first version of its
Title: Rio Tinto management system
 Standard Rio Tinto management system December 2014 Group Title: Rio Tinto management system Document No: HSEC-B-01 Standard Function: Health, Safety, Environment and Communities (HSEC) No. of pages: 23
Standard Rio Tinto management system December 2014 Group Title: Rio Tinto management system Document No: HSEC-B-01 Standard Function: Health, Safety, Environment and Communities (HSEC) No. of pages: 23
How To Write Software
 1 Medical Device Software - Software Life Cycle Processes IEC 62304 2 Credits John F. Murray Software Compliance Expert U.S. Food and Drug Administration Marcie R. Williams Medical Device Fellow Ph.D.
1 Medical Device Software - Software Life Cycle Processes IEC 62304 2 Credits John F. Murray Software Compliance Expert U.S. Food and Drug Administration Marcie R. Williams Medical Device Fellow Ph.D.
INCIDENT POLICY Page 1 of 13 November 2015
 Page 1 of 13 Policy Applies To All Mercy Hospital Staff Credentialed Medical Specialists and Allied Health Personnel are required to indicate understanding of the incident policy via the credentialing
Page 1 of 13 Policy Applies To All Mercy Hospital Staff Credentialed Medical Specialists and Allied Health Personnel are required to indicate understanding of the incident policy via the credentialing
