Monitoring and Audit of Clinical Research Studies
|
|
|
- Bernard Hart
- 7 years ago
- Views:
Transcription
1 Mnitring and Audit f Clinical Research Studies Categry: Summary: Equality Analysis undertaken: Plicy Implementatin f this plicy will ensure that the Trust fulfils its statutry bligatins which in turn will maintain public cnfidence in the safety, rights and well-being f participants f research studies undertaken at the OUH Trust and that the reprted trial data are accurate, cmplete, and verifiable frm the surce dcuments. Sep 2015 Valid Frm: Jan 2016 Date f Next Review: Jan 2019 Apprval Date/ Via: Distributin: Related Dcuments: Authr(s): Further Infrmatin: This Dcument replaces: Medical Directr Trust Management Executive Via Research and Develpment t: Researchers within ORH Trust Research and Develpment Web Site Spnsrship f Clinical Research Studies Plicy Trust Management Apprval f Clinical Research Plicy Integrity in Research Plicy Research Prtcl Amendments Plicy Safety Reprting in Clinical Research Plicy Incident Reprting Plicy Fina Parker, Research and Develpment Manager Fina Parker, Fina.Parker@uh.nhs.uk Mnitring & Audit f Research Studies Plicy Versin 2.0 Lead Directr: Dr Tny Berendt Issue Date: 14 th January 2016 Page 1 f 9
2 Cntents Intrductin 3 Plicy Statement.. 3 Scpe Aim. 3 Definitins.. 3 Respnsibilities. 5 Chief Executive. The Medical Directr / Directr f R&D / Research & Develpment Lead / Research and Develpment Manager.... Chief Investigatr (CI) / Principal Investigatr (PI)... Research and Develpment Staff.. Cntent f the Plicy 5 Training. 6 Mnitring cmpliance 6 Review f this plicy 7 References... 7 Equality Analysis.. 7 Dcument Histry 8 Appendices: 1 Equality Analysis... 8 Page Page 2 f 9
3 Intrductin 1. The Research Gvernance Framewrk fr Health and Scial Care (RGF) requires that, where an rganisatin is prviding care t research participants within studies with external Spnsrs, the rganisatin must ensure that legislatin related t research is fllwed. 2. The RGF requires that an rganisatin taking n the rle f Spnsr must cnfirm that there are prper arrangements in place t initiate, manage, mnitr and finance a study. 3. The Medicines fr Human Use (Clinical Trials) Regulatins 2004 require that rganisatins which take n the rle f Spnsr f clinical trials must have systems in place fr the management f that trial. 4. The bjective f mnitring, audit and cmpliance checks is t verify that: 4.1. The rights and well-being f the human subjects are prtected The reprted trial data are accurate, cmplete, and verifiable frm the surce dcuments The cnduct f the study is in cmpliance with the currently apprved prtcl; with Gd Clinical Practice (GCP); and in accrdance with the applicable lcal regulatry requirement(s). Plicy Statement 5. It is the plicy f the Oxfrd University Hspitals NHS Fundatin Trust ( the Trust ) t: Scpe 5.1. Prtect the safety, dignity, rights and well-being f all patients invlved in clinical research Ensure that arrangements are in place fr the management and mnitring f research studies, where the Trust has taken n the rle f Spnsr r Hst Institutin, including cmpliance with the relevant regulatins. 6. This Plicy applies t anyne cnducting research within the Trust, whether such research is spnsred r hsted by the Trust, including Clinical Trials f Investigatinal Medicinal Prducts (CTIMPs). 7. This dcument applies t all areas f the Trust, and all emplyees f the Trust, including individuals emplyed by a third party, by external cntractrs, as vluntary wrkers, as students, as lcums r as agency staff. Aim 8. This plicy sets ut a cnsistent prcedure fr versight f the prgress f clinical research studies and prmting quality and cmpliance with the relevant legislatin. Definitins 9. The terms in use in this dcument are defined as fllws: 9.1. Spnsr - The rganisatin taking respnsibility fr initiatin, management and financing (r arranging the financing) f a clinical trial r research study Hst Institutin The rganisatin where the clinical research will take place. Page 3 f 9
4 9.3. Mnitring - The act f verseeing the prgress f a clinical trial, and f ensuring that it is cnducted, recrded, and reprted in accrdance with the prtcl, Standard Operating Prcedures (SOPs), Gd Clinical Practice (GCP), and the applicable regulatry requirement(s). Mnitring is an nging activity, thrughut the cnduct f the trial Audit - A systematic and independent examinatin f trial related activities and dcuments t determine whether the trial related activities were cnducted, recrded and reprted accrding t the prtcl, the spnsr s SOPs, GCP and the applicable regulatry requirements. Audit is an assessment f cmpliance with these standards at a given mment in time Cmpliance Check A review f a specific aspect f a research study r a grup f studies, fr example: Cnsent r safety reprting Clinical Trial f Investigatinal Medicinal Prduct (CTIMP) - Any investigatin in human subjects, ther than a nn-interventinal trial*, intended: T discver r verify the clinical, pharmaclgical r ther pharmacdynamic effects f ne r mre medicinal prducts, T identify any adverse reactins t ne r mre medicinal prducts r T study the absrptin, distributin, metablism and excretin f ne r mre such prducts With the bject f ascertaining the safety r efficacy f thse prducts Such trials will be gverned by the Medicines fr Human Use (Clinical Trials) Regulatins 2004 and updates. * The Medicines and Healthcare prducts Regulatry Agency (MHRA) view a trial as interventinal where a drug is being given as an interventin and assessments are being undertaken t assess the effects. This is nt dependant n the prescriptin f that drug being undertaken as part f the prtcl Device Trial - A clinical investigatin designed t establish the perfrmance f a medical device which is intended t reveal adverse events under nrmal cnditins f use, and permit assessment f the acceptable risks having regard t the intended perfrmance f the medical device. Such trials are regulated by the RGF and wuld require the apprval f an ethics cmmittee. Trials using nn-ce marked devices are als regulated by the Medical Devices Regulatins (2002) 9.8. Interventinal Trial / Study - Any investigatin in human subjects which invlves sme frm f medical interventin: surgical, medical, r psychlgical, but which is nt classified as a CTIMP as defined in 9.6. Such studies are regulated by the RGF and wuld require the apprval f an ethics cmmittee Nn-interventinal Study - Any investigatin in human subjects, wh are patients, which is bservatinal and des nt invlve any interventin in additin t their nrmal clinical care. Such studies are regulated by the RGF and wuld require the apprval f an ethics cmmittee Investigatr Site File a file cntaining all the infrmatin that a site will need t cnduct a trial at that site Chief Investigatr -The individual, as identified in the ethics applicatin, wh takes verall respnsibility fr the cnduct f a clinical study Principal Investigatr - The individual wh takes n respnsibility fr cnduct f the study at a particular site. Page 4 f 9
5 9.13. Standard Operating Prcedure - SOPs are dcuments that describe the prcedures that shuld be fllwed t ensure cnsistency in the perfrmance f specific prcesses r activities. Respnsibilities 10. The Chief Executive has verall respnsibility fr this plicy. 11. The Medical Directr / Directr f R&D / R&D Lead / R&D Manager have delegated authrity n behalf f the Trust t review and apprve mnitring/audit plans and reprts. 12. Chief Investigatr (CI) / Principal Investigatr (PI): Overall respnsibility fr the cnduct f the trial; Facilitate access t trial dcuments and participant medical recrds, fr the purpses f mnitring, auditing and inspectins; Ensure nging cmmunicatin, thrugh cpying f relevant crrespndence with Research Ethics Cmmittees (REC) and MHRA. 13. Research and Develpment Staff has the respnsibility t prvide advice and infrmatin t investigatrs n issues f cmpliance; t prepare fr and cnduct the mnitring visit/audit/cmpliance check; t write the resultant reprt; and t cmplete fllw-up visits as required; t facilitate cmmunicatin between the Trust and the investigatr / study team; t update electrnic study files and database with new infrmatin derived frm ethics prgress reprts, annual safety reprts, end f study ntificatins fllwing the Trust R&D Standard Operating Prcedures. Cntent f the Plicy Trust Spnsred Clinical Trials f Investigatinal Medicinal Prducts (CTIMPs) 14. Prir t trial cmmencement, a mnitring plan will be written by R&D staff, in cnjunctin with the trials staff. The mnitring plan will define the apprach t be taken in line with the Risk-adapted Apprach t the Management f CTIMPs. 15. An initiatin visit will be cnducted prir t trial start t ensure that everything is in place and that staff are fully aware f their respnsibilities fr cmpliance with GCP. 16. During the trial, all trials spnsred by the Trust will be mnitred at least twice yearly with fllw-up visits if required. These mnitring visits will cmprise sme r all f the fllwing: examinatin f the cntents f the Trial Master File; examinatin f a selectin f Case Reprt Frms and the crrespnding medical ntes, fr the purpse f Surce Data Verificatin; and discussin f the trial prgress with the investigatr team. R&D staff will generate a mnitring reprt, utlining actins required, and will fllw up n these actins. 17. Peridic visits will be made t pharmacy fr the purpses f mnitring f IMP handling and examinatin f the relevant pharmacy files. Trust Hsted CTIMPs 18. A selectin f hsted trials will be audited. The selectin prcedure will, in the main, be randm, but will include the facility t select specific trials that have previusly been identified as high risk. 19. Audits will cmprise examinatin f the cntents f the Investigatr Site File; examinatin f a selectin f Case Reprt Frms and the crrespnding medical ntes; and interviews with the investigatr team. R&D staff will generate an audit reprt, which will be prvided t the investigatr, alng with any recmmended Page 5 f 9
6 actins. The trials team will respnd t the findings within the reprt, utlining any crrective actins t be cmpleted. Other Research Studies (Nn-CTIMPs) 20. In rder t avid duplicatin f effrt, the R&D team will endeavur t mnitr the prgress f research studies thrugh cpies f crrespndence with the relevant ethics cmmittee: prgress reprts; substantial amendments; end f study ntificatins. 21. Investigatr teams are required t assist with this prcess thrugh cpying f all such crrespndence t R&D. 22. The R&D staff will mnitr the submissin f such updates and update the R&D database accrdingly, thus ensuring that indemnity fr each study is nging. 23. These studies may be audited fr cmpliance with RGF and GCP, where it is deemed t be apprpriate. Cmpliance Checks All study types 24. The R&D team may als perfrm a cmpliance check n any study r grups f studies. This may be at the request f a study team; a result f an issue cming t light and being reprted t the R&D team; r as a randm selectin. Particular aspects f study management r participant safety are fcused n fr example the participant cnsent prcess r safety reprting. Training 25. All staff invlved in the cnduct f clinical trials will undertake training in Gd Clinical Practice (GCP) prir t beginning their invlvement in a trial. The prcess f mnitring and audit is cvered within this training. 26. All staff invlved in the cnduct f mnitring, audit and cmpliance checks receive training in the use f the trust R&D SOPs. Each individual s training needs will be identified thrugh annual appraisal and supervisin. Mnitring Cmpliance 27. Cmpliance with the dcument will be mnitred in the fllwing ways. Aspect f cmpliance r effectiveness being mnitred Staff f the Trust R&D Department will mnitr the implementatin f the plicy. Mnitring methd Review f database Respnsibility fr mnitring (jb title) The R&D Manager/Lead Frequency f mnitring At least annually Grup r Cmmittee that will review the findings and mnitr cmpletin f any resulting actin plan R&D Team and if required Directr f R&D. The Trust R&D Department review this plicy t reflect changes in legislatin and examples f best practice. Review f plicy R&D Lead and delegated staff At least Annually R&D Team and if required Directr f R&D. Page 6 f 9
7 Review 28. This plicy will be reviewed in 3 years, as set ut in the Plicy fr the Develpment and Implementatin f Prcedural Dcuments. 29. The Trust Management Executive has delegated authrity t the Research & Develpment Lead fr the apprval f any further supprting r assciated dcuments. References 30. The Medicines fr Human Use (Clinical Trials) Regulatins 2004 and Amendments 31. The Department f Health Research Gvernance Framewrk fr Health and Scial Care ICH Harmnised Tripartite Guideline fr Gd Clinical Practice. 33. MRC/DH/MHRA Jint Prject n the Risk-adapted Appraches t the Management f Clinical Trials f Investigatinal Medicinal Prducts Versin 28th March 2011 Equality Analysis 34. As part f its develpment, this plicy and its impact n equality, diversity and human rights has been reviewed, an equality analysis undertaken (see appendix attached) and in rder t minimize the ptential t discriminate, n adjustments have been identified: Hw des this plicy affect each characteristic? Prtected Characteristic: Disability (all disability including dementia and learning disability) Sex Age Race Sexual Orientatin Pregnancy and maternity Religin r belief Gender re-assignment Marriage r civil partnerships Carers Safeguarding peple wh are vulnerable Reasnable adjustments required Page 7 f 9
8 Dcument Histry Date f revisin Versin number Reasn fr review r update March Reviewed and Updated May Updated t reflect change in prcesses July Reviewed and Update September Reviewed and update transferred t new template Appendix 1: Equality Analysis Equality Analysis Plicy / Plan / prpsal name: Mnitring, Audit and Cmpliance Checks f Research Studies Date f Plicy: September 2015 Date due fr review September 2018 Lead persn fr plicy and equality analysis: R&D Lead Des the plicy /prpsal relate t peple? If yes please cmplete the whle frm. YES 1. Identify the main aim and bjectives and intended utcmes f the plicy. T ensure that the Trust has a rbust prcess in place t prvide cntinued assurance that the rights and wellbeing f trial participants are safeguarded, the participants will benefit frm the assurance that the trials undertaken at the OUH Trust are apprpriately managed and mnitred. 2. Invlvement f stakehlders. Based n the previus versin, this update has drawn n feedback frm researchers and ther staff including members f the R&D team. Evidence. Ppulatin infrmatin n search fr Oxfrdshire. Disability Nt applicable Disability: learning disability Nt applicable Sex Nt applicable Age: Nt applicable Race: Nt applicable Sexual rientatin: Nt applicable Pregnancy and maternity: Nt applicable Religin r belief. Nt applicable Gender re-assignment. Nt applicable Marriage r civil partnerships: Nt applicable Carers Nt applicable Page 8 f 9
9 Safeguarding peple wh are vulnerable: Nt applicable Other ptential impacts e.g. culture, human rights, sci ecnmic e.g. hmeless peple Nt applicable Sectin 4 Summary f Analysis This plicy des nt shw any ptential t discriminate. The mnitring, audit r cmpliance checks (as well as the array f legislatin) gverning each trial ensures all patients are adequately prtected. Hw des the plicy advance equality f pprtunity? Nt applicable Hw des the plicy prmte gd relatins between grups? Nt applicable. Page 9 f 9
CASSOWARY COAST REGIONAL COUNCIL POLICY ENTERPRISE RISK MANAGEMENT
 CASSOWARY COAST REGIONAL COUNCIL POLICY ENTERPRISE RISK MANAGEMENT Plicy Number: 2.20 1. Authrity Lcal Gvernment Act 2009 Lcal Gvernment Regulatin 2012 AS/NZS ISO 31000-2009 Risk Management Principles
CASSOWARY COAST REGIONAL COUNCIL POLICY ENTERPRISE RISK MANAGEMENT Plicy Number: 2.20 1. Authrity Lcal Gvernment Act 2009 Lcal Gvernment Regulatin 2012 AS/NZS ISO 31000-2009 Risk Management Principles
Audit Committee Charter. St Andrew s Insurance (Australia) Pty Ltd St Andrew s Life Insurance Pty Ltd St Andrew s Australia Services Pty Ltd
 Audit Cmmittee Charter St Andrew s Insurance (Australia) Pty Ltd St Andrew s Life Insurance Pty Ltd St Andrew s Australia Services Pty Ltd Versin 2.0, 22 February 2016 Apprver Bard f Directrs St Andrew
Audit Cmmittee Charter St Andrew s Insurance (Australia) Pty Ltd St Andrew s Life Insurance Pty Ltd St Andrew s Australia Services Pty Ltd Versin 2.0, 22 February 2016 Apprver Bard f Directrs St Andrew
SECTION J QUALITY ASSURANCE AND IMPROVEMENT PROGRAM
 Audit Manual Sectin J SECTION J QUALITY ASSURANCE AND IMPROVEMENT PROGRAM Ref. Plicy and Practice Requirements IIA Standards and Other references J 1 Plicy: The Head f Internal Audit shall develp and maintain
Audit Manual Sectin J SECTION J QUALITY ASSURANCE AND IMPROVEMENT PROGRAM Ref. Plicy and Practice Requirements IIA Standards and Other references J 1 Plicy: The Head f Internal Audit shall develp and maintain
Risk Management Policy AGL Energy Limited
 Risk Management Plicy AGL Energy Limited AUGUST 2014 Table f Cntents 1. Abut this Dcument... 2 2. Plicy Statement... 2 3. Purpse... 2 4. AGL Risk Cntext... 3 5. Scpe... 3 6. Objectives... 3 7. Accuntabilities...
Risk Management Plicy AGL Energy Limited AUGUST 2014 Table f Cntents 1. Abut this Dcument... 2 2. Plicy Statement... 2 3. Purpse... 2 4. AGL Risk Cntext... 3 5. Scpe... 3 6. Objectives... 3 7. Accuntabilities...
Business Continuity Management Policy
 Business Cntinuity Management Plicy Versin: 1.0 Last Amendment: Apprved by: Library Cuncil f New Suth Wales Plicy wner/spnsr: Directr, Operatins and Chief Financial Officer Plicy Cntact Officer: Senir
Business Cntinuity Management Plicy Versin: 1.0 Last Amendment: Apprved by: Library Cuncil f New Suth Wales Plicy wner/spnsr: Directr, Operatins and Chief Financial Officer Plicy Cntact Officer: Senir
17 Construction environmental management plan (CEMP)
 17 Cnstructin envirnmental management plan (CEMP) Bur Happld Cntents 17 Cnstructin Envirnmental Management Plan (CEMP) 17-1 17.1 Intrductin 17-1 17.2 Intrductin t EMS 17-1 17.2.1 Plicy 17-2 17.2.2 Planning
17 Cnstructin envirnmental management plan (CEMP) Bur Happld Cntents 17 Cnstructin Envirnmental Management Plan (CEMP) 17-1 17.1 Intrductin 17-1 17.2 Intrductin t EMS 17-1 17.2.1 Plicy 17-2 17.2.2 Planning
Business Continuity Management Policy
 The Public Trustee Business Cntinuity Management Plicy Octber 2015 Business Cntinuity Management Plicy Octber 2015 Page 1 f 6 Dcument Infrmatin Apprved Name Psitin Signature Date Mark Crftn A/Public Trustee
The Public Trustee Business Cntinuity Management Plicy Octber 2015 Business Cntinuity Management Plicy Octber 2015 Page 1 f 6 Dcument Infrmatin Apprved Name Psitin Signature Date Mark Crftn A/Public Trustee
Finance, Performance and Risk Committee 2014/2015
 Finance, Perfrmance and Risk Cmmittee 2014/2015 Date f Meeting: 17 December 2014 Agenda Item: Click here t enter text. Subject: Infrmatin Gvernance Plicy Reprting Officer: Paul Byrne Lead IG Manager Aim
Finance, Perfrmance and Risk Cmmittee 2014/2015 Date f Meeting: 17 December 2014 Agenda Item: Click here t enter text. Subject: Infrmatin Gvernance Plicy Reprting Officer: Paul Byrne Lead IG Manager Aim
Change Management Process
 Change Management Prcess B1.10 Change Management Prcess 1. Intrductin This plicy utlines [Yur Cmpany] s apprach t managing change within the rganisatin. All changes in strategy, activities and prcesses
Change Management Prcess B1.10 Change Management Prcess 1. Intrductin This plicy utlines [Yur Cmpany] s apprach t managing change within the rganisatin. All changes in strategy, activities and prcesses
Audit Committee Charter
 Audit Cmmittee Charter Membership The Audit Cmmittee (the "Cmmittee") f the Bard f Directrs (the "Bard") f Philip Mrris Internatinal Inc. (the "Cmpany") shall cnsist f at least three directrs all f whm
Audit Cmmittee Charter Membership The Audit Cmmittee (the "Cmmittee") f the Bard f Directrs (the "Bard") f Philip Mrris Internatinal Inc. (the "Cmpany") shall cnsist f at least three directrs all f whm
MSB FINANCIAL CORP. MILLINGTON BANK AUDIT COMMITTEE CHARTER
 MSB FINANCIAL CORP. MILLINGTON BANK AUDIT COMMITTEE CHARTER This Audit Cmmittee Charter has been amended as f July 17, 2015. The Audit Cmmittee shall review and reassess this Charter annually and recmmend
MSB FINANCIAL CORP. MILLINGTON BANK AUDIT COMMITTEE CHARTER This Audit Cmmittee Charter has been amended as f July 17, 2015. The Audit Cmmittee shall review and reassess this Charter annually and recmmend
ENTERPRISE RISK MANAGEMENT ENTERPRISE RISK MANAGEMENT POLICY
 ENTERPRISE RISK MANAGEMENT POLICY Plicy N. 10014 Review Date Octber 1, 2014 Effective Date March 1, 2014 Crss- Respnsibility Vice President, Reference Administratin Apprver Executive Cuncil 1. 1. Plicy
ENTERPRISE RISK MANAGEMENT POLICY Plicy N. 10014 Review Date Octber 1, 2014 Effective Date March 1, 2014 Crss- Respnsibility Vice President, Reference Administratin Apprver Executive Cuncil 1. 1. Plicy
RATIONALE TERMS OF REFERENCE FOR THE QUALITY COMMITTEE UNDER THE EXCELLENT CARE FOR ALL ACT. Authority
 RATIONALE With the intrductin f the Excellent Care fr All Act, hspital bards must nw have a quality cmmittee that reprts t the bard. The template prvides sample terms f references fr rganizatins t adapt
RATIONALE With the intrductin f the Excellent Care fr All Act, hspital bards must nw have a quality cmmittee that reprts t the bard. The template prvides sample terms f references fr rganizatins t adapt
AUDIT AND RISK COMMITTEE TERMS OF REFERENCE
 AUDIT AND RISK COMMITTEE TERMS OF REFERENCE 1. TITLE OF COMMITTEE Audit and Risk Cmmittee 2. ESTABLISHMENT The Audit and Risk Cmmittee is established under Part 3 Sectin 19(1) f the Charles Darwin University
AUDIT AND RISK COMMITTEE TERMS OF REFERENCE 1. TITLE OF COMMITTEE Audit and Risk Cmmittee 2. ESTABLISHMENT The Audit and Risk Cmmittee is established under Part 3 Sectin 19(1) f the Charles Darwin University
BIBH Duty Statements and Governance chart reviewed and approved April 2014. BIBH Executive Governance & Management Arrangements
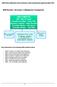 BIBH Duty Statements and Gvernance chart reviewed and apprved April 2014 BIBH Executive Gvernance & Management Arrangements BIBH COMMITTEE CEO - Paul O Cnnell Executive Secretary - Brian Firth Executive
BIBH Duty Statements and Gvernance chart reviewed and apprved April 2014 BIBH Executive Gvernance & Management Arrangements BIBH COMMITTEE CEO - Paul O Cnnell Executive Secretary - Brian Firth Executive
Waitemata District Health Board, 15 Shea Terrace, Takapuna
 Date: Octber 2015 Jb Title: Quality and Audit Manager Department: Planning, Funding and Outcmes Unit Lcatin: Waitemata District Health Bard, 15 Shea Terrace, Takapuna Reprting t: Directr Funding Direct
Date: Octber 2015 Jb Title: Quality and Audit Manager Department: Planning, Funding and Outcmes Unit Lcatin: Waitemata District Health Bard, 15 Shea Terrace, Takapuna Reprting t: Directr Funding Direct
How To Write An Ehsms Training, Awareness And Competency Procedure
 Envirnmental, Health & Safety Management System (EHSMS) Dcument Number: 00122 Issue Date: 05/07/2014 Training, Awareness and Cmpetency Prcedure Revisin Number: 7 Prepared By: Stalcup, Bryce Apprved By:
Envirnmental, Health & Safety Management System (EHSMS) Dcument Number: 00122 Issue Date: 05/07/2014 Training, Awareness and Cmpetency Prcedure Revisin Number: 7 Prepared By: Stalcup, Bryce Apprved By:
Gravesham Borough Council
 Classificatin: Part 1 Public Key Decisin: Please specify - N Gravesham Brugh Cuncil Reprt t: Perfrmance and Administratin Cmmittee Date: 12 Nvember 2015 Reprting fficer: Subject: Crprate Perfrmance Manager
Classificatin: Part 1 Public Key Decisin: Please specify - N Gravesham Brugh Cuncil Reprt t: Perfrmance and Administratin Cmmittee Date: 12 Nvember 2015 Reprting fficer: Subject: Crprate Perfrmance Manager
CMS Eligibility Requirements Checklist for MSSP ACO Participation
 ATTACHMENT 1 CMS Eligibility Requirements Checklist fr MSSP ACO Participatin 1. General Eligibility Requirements ACO participants wrk tgether t manage and crdinate care fr Medicare fee-fr-service beneficiaries.
ATTACHMENT 1 CMS Eligibility Requirements Checklist fr MSSP ACO Participatin 1. General Eligibility Requirements ACO participants wrk tgether t manage and crdinate care fr Medicare fee-fr-service beneficiaries.
Australian Institute of Psychology. Human Research Ethics Committee. Terms of Reference
 Australian Institute f Psychlgy Human Research Ethics Cmmittee Terms f Reference What is research? Accrding t the Natinal Statement research... is widely understd t include at least investigatin undertaken
Australian Institute f Psychlgy Human Research Ethics Cmmittee Terms f Reference What is research? Accrding t the Natinal Statement research... is widely understd t include at least investigatin undertaken
General Records Authority 33. Accredited Training
 General Recrds Authrity 33 2012/00579704 Accredited Training February 2013 This is an accurate reprductin f the authrised recrds authrity cntent, created fr accessibility purpses CONTENTS INTRODUCTION
General Recrds Authrity 33 2012/00579704 Accredited Training February 2013 This is an accurate reprductin f the authrised recrds authrity cntent, created fr accessibility purpses CONTENTS INTRODUCTION
THIRD PARTY PROCUREMENT PROCEDURES
 ADDENDUM #1 THIRD PARTY PROCUREMENT PROCEDURES NORTH CENTRAL TEXAS COUNCIL OF GOVERNMENTS TRANSPORTATION DEPARTMENT JUNE 2011 OVERVIEW These prcedures establish standards and guidelines fr the Nrth Central
ADDENDUM #1 THIRD PARTY PROCUREMENT PROCEDURES NORTH CENTRAL TEXAS COUNCIL OF GOVERNMENTS TRANSPORTATION DEPARTMENT JUNE 2011 OVERVIEW These prcedures establish standards and guidelines fr the Nrth Central
PENETRATION TEST OF THE INDIAN HEALTH SERVICE S COMPUTER NETWORK
 Department f Health and Human Services OFFICE OF INSPECTOR GENERAL PENETRATION TEST OF THE INDIAN HEALTH SERVICE S COMPUTER NETWORK Inquiries abut this reprt may be addressed t the Office f Public Affairs
Department f Health and Human Services OFFICE OF INSPECTOR GENERAL PENETRATION TEST OF THE INDIAN HEALTH SERVICE S COMPUTER NETWORK Inquiries abut this reprt may be addressed t the Office f Public Affairs
Malpractice and Maladministration Policy
 TR340 Malpractice and Maladministratin Plicy This plicy aims t: Define malpractice and maladministratin in the cntext f CIM/CAM studying members, Accredited study centres (ASCs), examinatin centres, invigilatrs
TR340 Malpractice and Maladministratin Plicy This plicy aims t: Define malpractice and maladministratin in the cntext f CIM/CAM studying members, Accredited study centres (ASCs), examinatin centres, invigilatrs
Quality Assurance/Control Procedures
 2015 Prgrammatic Categrical Exclusin {PCE} Agreement Oregn Department f Transprtatin Federal Highway Administratin, Oregn Divisin Quality Assurance/Cntrl Prcedures Intrductin The Prgrammatic Agreement
2015 Prgrammatic Categrical Exclusin {PCE} Agreement Oregn Department f Transprtatin Federal Highway Administratin, Oregn Divisin Quality Assurance/Cntrl Prcedures Intrductin The Prgrammatic Agreement
Privacy and Security Training Policy (PS.Pol.051)
 Privacy and Security Training Plicy (PS.Pl.051) Purpse T define the plicies and prcedures fr prviding privacy and security training in respect f the CnnectingGTA Slutin. Definitins Electrnic Service Prvider
Privacy and Security Training Plicy (PS.Pl.051) Purpse T define the plicies and prcedures fr prviding privacy and security training in respect f the CnnectingGTA Slutin. Definitins Electrnic Service Prvider
Internal Audit Charter and operating standards
 Internal Audit Charter and perating standards 2 1 verview This dcument sets ut the basis fr internal audit: (i) the Internal Audit charter, which establishes the framewrk fr Internal Audit; and (ii) hw
Internal Audit Charter and perating standards 2 1 verview This dcument sets ut the basis fr internal audit: (i) the Internal Audit charter, which establishes the framewrk fr Internal Audit; and (ii) hw
UNIVERSITY OF CALIFORNIA MERCED PERFORMANCE MANAGEMENT GUIDELINES
 UNIVERSITY OF CALIFORNIA MERCED PERFORMANCE MANAGEMENT GUIDELINES REFERENCES AND RELATED POLICIES A. UC PPSM 2 -Definitin f Terms B. UC PPSM 12 -Nndiscriminatin in Emplyment C. UC PPSM 14 -Affirmative
UNIVERSITY OF CALIFORNIA MERCED PERFORMANCE MANAGEMENT GUIDELINES REFERENCES AND RELATED POLICIES A. UC PPSM 2 -Definitin f Terms B. UC PPSM 12 -Nndiscriminatin in Emplyment C. UC PPSM 14 -Affirmative
GUIDELINE INFORMATION MANAGEMENT (IM) PROGRAM PLAN
 Gvernment f Newfundland and Labradr Office f the Chief Infrmatin Officer Infrmatin Management Branch GUIDELINE INFORMATION MANAGEMENT (IM) PROGRAM PLAN Guideline (Definitin): OCIO Guidelines derive frm
Gvernment f Newfundland and Labradr Office f the Chief Infrmatin Officer Infrmatin Management Branch GUIDELINE INFORMATION MANAGEMENT (IM) PROGRAM PLAN Guideline (Definitin): OCIO Guidelines derive frm
Change Management Process For [Project Name]
![Change Management Process For [Project Name] Change Management Process For [Project Name]](/thumbs/27/10006925.jpg) Management Prcess Fr [Prject Name] i 1 Intrductin The is fllwed during the Executin phase f the Prject Management Life Cycle, nce the prject has been frmally defined and planned. 1.1 What is a Management
Management Prcess Fr [Prject Name] i 1 Intrductin The is fllwed during the Executin phase f the Prject Management Life Cycle, nce the prject has been frmally defined and planned. 1.1 What is a Management
Helicopter Landing Sites Planning, Implementation and Management
 Directive # QH-HSD-039:2013 Effective Date: 01 July 2013 Review Date: 01 July 2016 Supersedes: Nil Landing Sites Planning, Implementatin and Management Purpse The purpse f this Health Service Directive
Directive # QH-HSD-039:2013 Effective Date: 01 July 2013 Review Date: 01 July 2016 Supersedes: Nil Landing Sites Planning, Implementatin and Management Purpse The purpse f this Health Service Directive
Template on written coordination and cooperation arrangements of the supervisory college established for the <XY> Group/<A> Institution
 COORDINATION AND COOPERATION ARRANGEMENTS EBA/RTS/2014/16 EBA/ITS/2014/07 Annex II Template n written crdinatin and cperatin arrangements f the supervisry cllege established fr the Grup/ Institutin
COORDINATION AND COOPERATION ARRANGEMENTS EBA/RTS/2014/16 EBA/ITS/2014/07 Annex II Template n written crdinatin and cperatin arrangements f the supervisry cllege established fr the Grup/ Institutin
Environment Protection Authority
 Envirnment Prtectin Authrity EPA Cmplaints Management Plicy Intrductin This plicy sets ut the purpse, principles and prcess fr hw custmer feedback, including cmplaints, will be managed in the EPA t imprve
Envirnment Prtectin Authrity EPA Cmplaints Management Plicy Intrductin This plicy sets ut the purpse, principles and prcess fr hw custmer feedback, including cmplaints, will be managed in the EPA t imprve
Human Resources Policy pol-020
 Human Resurces Plicy pl-020 Versin: 2.00 Last amendment: Jul 2014 Next Review: Jul 2017 Apprved By: Cuncil Date: 04 May 2005 Cntact Officer: Directr, Office f Human Resurce Services INTRODUCTION The University
Human Resurces Plicy pl-020 Versin: 2.00 Last amendment: Jul 2014 Next Review: Jul 2017 Apprved By: Cuncil Date: 04 May 2005 Cntact Officer: Directr, Office f Human Resurce Services INTRODUCTION The University
Multi-Year Accessibility Policy and Plan for NSF Canada and NSF International Strategic Registrations Canada Company, 2014-2021
 Multi-Year Accessibility Plicy and Plan fr NSF Canada and NSF Internatinal Strategic Registratins Canada Cmpany, 2014-2021 This 2014-21 accessibility plan utlines the plicies and actins that NSF Canada
Multi-Year Accessibility Plicy and Plan fr NSF Canada and NSF Internatinal Strategic Registratins Canada Cmpany, 2014-2021 This 2014-21 accessibility plan utlines the plicies and actins that NSF Canada
Projects Director Report Guidelines. IPMA Level A
 Prjects Directr Reprt Guidelines IPMA Level A Cntents 1. GENERAL PROVISIONS.. 2 2. PROJECT PORTFOLIO / PROGRAMME DESCRIPTION...2 3. PROJECTS DIRECTOR REPORT 5 4. ANNEXES..7 Authr Classificatin Status Electrnic
Prjects Directr Reprt Guidelines IPMA Level A Cntents 1. GENERAL PROVISIONS.. 2 2. PROJECT PORTFOLIO / PROGRAMME DESCRIPTION...2 3. PROJECTS DIRECTOR REPORT 5 4. ANNEXES..7 Authr Classificatin Status Electrnic
E-Business Strategies For a Cmpany s Bard
 DATATEC LIMITED BOARD CHARTER / TERMS OF REFERENCE 1. CONSTITUTION The primary bjective f the Cmpany s Bard Charter is t set ut the rle and respnsibilities f the Bard f Directrs ( the Bard ) as well as
DATATEC LIMITED BOARD CHARTER / TERMS OF REFERENCE 1. CONSTITUTION The primary bjective f the Cmpany s Bard Charter is t set ut the rle and respnsibilities f the Bard f Directrs ( the Bard ) as well as
CHANGE MANAGEMENT STANDARD
 The electrnic versin is current, r when printed and stamped with the green cntrlled dcument stamp. All ther cpies are uncntrlled. DOCUMENT INFORMATION Descriptin Dcument Owner This standard utlines the
The electrnic versin is current, r when printed and stamped with the green cntrlled dcument stamp. All ther cpies are uncntrlled. DOCUMENT INFORMATION Descriptin Dcument Owner This standard utlines the
TITLE: Supplier Contracting Guidelines Process: FIN_PS_PSG_050 Replaces: Manual Sections 6.4, 7.1, 7.5, 7.6, 7.11 Effective Date: 10/1/2014 Contents
 TITLE: Supplier Cntracting Guidelines Prcess: FIN_PS_PSG_050 Replaces: Manual Sectins 6.4, 7.1, 7.5, 7.6, 7.11 Cntents 1 Abut university supplier cntracting... 2 2 When is a cntract required?... 2 3 Wh
TITLE: Supplier Cntracting Guidelines Prcess: FIN_PS_PSG_050 Replaces: Manual Sectins 6.4, 7.1, 7.5, 7.6, 7.11 Cntents 1 Abut university supplier cntracting... 2 2 When is a cntract required?... 2 3 Wh
Chapter 7 Business Continuity and Risk Management
 Chapter 7 Business Cntinuity and Risk Management Sectin 01 Business Cntinuity Management 070101 Initiating the Business Cntinuity Plan (BCP) Purpse: T establish the apprpriate level f business cntinuity
Chapter 7 Business Cntinuity and Risk Management Sectin 01 Business Cntinuity Management 070101 Initiating the Business Cntinuity Plan (BCP) Purpse: T establish the apprpriate level f business cntinuity
Sources of Federal Government and Employee Information
 Inf Surce Surces f Federal Gvernment and Emplyee Infrmatin Ridley Terminals Inc. TABLE OF CONTENTS General Infrmatin Intrductin t Inf Surce Backgrund Respnsibilities Institutinal Functins, Prgram and Activities
Inf Surce Surces f Federal Gvernment and Emplyee Infrmatin Ridley Terminals Inc. TABLE OF CONTENTS General Infrmatin Intrductin t Inf Surce Backgrund Respnsibilities Institutinal Functins, Prgram and Activities
FINANCIAL SERVICES FLASH REPORT
 FINANCIAL SERVICES FLASH REPORT Draft Regulatry Cmpliance Management Guideline Released by the Office f the Superintendent f Financial Institutins May 5, 2014 On April 30, 2014, the Office f the Superintendent
FINANCIAL SERVICES FLASH REPORT Draft Regulatry Cmpliance Management Guideline Released by the Office f the Superintendent f Financial Institutins May 5, 2014 On April 30, 2014, the Office f the Superintendent
Communal Property Institution Capacity Assessment Tool
 Cmmunal Prperty Institutin Capacity Assessment Tl Intrductin t cmmunal prperty institutins Cmmunal prperty institutins (CPIs) Participants in the land refrm prgramme can hld prperty thrugh different frms
Cmmunal Prperty Institutin Capacity Assessment Tl Intrductin t cmmunal prperty institutins Cmmunal prperty institutins (CPIs) Participants in the land refrm prgramme can hld prperty thrugh different frms
Public consultation paper
 Public cnsultatin paper Nvember 2012 Public cnsultatin n guidelines fr prfessinal indemnity insurance arrangements fr nurses and nurse practitiners. Please prvide feedback by email t: nmbafeedback@ahpra.gv.au
Public cnsultatin paper Nvember 2012 Public cnsultatin n guidelines fr prfessinal indemnity insurance arrangements fr nurses and nurse practitiners. Please prvide feedback by email t: nmbafeedback@ahpra.gv.au
Health and Safety Training and Supervision
 Intrductin: Health and Safety Training and Supervisin University f Nttingham is cmmitted t maintaining and develping standards f excellence in all aspects f its business. T that end, the University aspires
Intrductin: Health and Safety Training and Supervisin University f Nttingham is cmmitted t maintaining and develping standards f excellence in all aspects f its business. T that end, the University aspires
Key Steps for Organizations in Responding to Privacy Breaches
 Key Steps fr Organizatins in Respnding t Privacy Breaches Purpse The purpse f this dcument is t prvide guidance t private sectr rganizatins, bth small and large, when a privacy breach ccurs. Organizatins
Key Steps fr Organizatins in Respnding t Privacy Breaches Purpse The purpse f this dcument is t prvide guidance t private sectr rganizatins, bth small and large, when a privacy breach ccurs. Organizatins
Professional indemnity insurance arrangements for enrolled nurses, registered nurses and nurse practitioners
 Guideline August 2013 Prfessinal indemnity insurance arrangements fr enrlled nurses, registered nurses and nurse practitiners Intrductin This guideline has been develped by the Nursing and Midwifery Bard
Guideline August 2013 Prfessinal indemnity insurance arrangements fr enrlled nurses, registered nurses and nurse practitiners Intrductin This guideline has been develped by the Nursing and Midwifery Bard
Application for Inclusion of a Developed Practice Area in Professional Psychology for Purposes of Doctoral and Internship Program Accreditation
 Applicatin fr Inclusin f a Develped Practice Area in Prfessinal Psychlgy fr Purpses f Dctral and Internship Prgram Accreditatin Cmmittee n Accreditatin c/ Office f Prgram Cnsultatin and Accreditatin Educatin
Applicatin fr Inclusin f a Develped Practice Area in Prfessinal Psychlgy fr Purpses f Dctral and Internship Prgram Accreditatin Cmmittee n Accreditatin c/ Office f Prgram Cnsultatin and Accreditatin Educatin
Information Technology Services. University of Maine System. Version 0.07. December 20, 2012
 IT PROJECT MANAGEMENT OFFICE (PMO) CHARTER Infrmatin Technlgy Services University f Maine System Versin 0.07 December 20, 2012 Prepared by: Rbin Sherman Authrized by: [1] Table f Cntents EXECUTIVE SUMMARY...
IT PROJECT MANAGEMENT OFFICE (PMO) CHARTER Infrmatin Technlgy Services University f Maine System Versin 0.07 December 20, 2012 Prepared by: Rbin Sherman Authrized by: [1] Table f Cntents EXECUTIVE SUMMARY...
Loss Share Data Specifications Change Management Plan
 Lss Share Data Specificatins Change Management Plan Last Updated: 2/27/2013 Table f Cntents I. Purpse... 3 II. Change Management Apprach... 3 III. Categries f Revisins... 4 IV. Help and Supprt... 6 Lss
Lss Share Data Specificatins Change Management Plan Last Updated: 2/27/2013 Table f Cntents I. Purpse... 3 II. Change Management Apprach... 3 III. Categries f Revisins... 4 IV. Help and Supprt... 6 Lss
UNIVERSITY INCIDENT PLANNING COMMITTEE TERMS OF REFERENCE
 1. TITLE OF COMMITTEE UNIVERSITY INCIDENT PLANNING COMMITTEE University Incident Planning Cmmittee (IPC) 2. ESTABLISHMENT TERMS OF REFERENCE The University Incident Planning Cmmittee is established in
1. TITLE OF COMMITTEE UNIVERSITY INCIDENT PLANNING COMMITTEE University Incident Planning Cmmittee (IPC) 2. ESTABLISHMENT TERMS OF REFERENCE The University Incident Planning Cmmittee is established in
ACQUIRED RARE DISEASE DRUG THERAPY EXCEPTION PROCESS
 ADMINISTRATIVE POLICY ACQUIRED RARE DISEASE DRUG THERAPY EXCEPTION PROCESS Plicy Number: ADMINISTRATIVE 19.8 T Effective Date: Octber 1, 014 Table f Cntents CONDITIONS OF COVERAGE... BENEFIT CONSIDERATIONS...
ADMINISTRATIVE POLICY ACQUIRED RARE DISEASE DRUG THERAPY EXCEPTION PROCESS Plicy Number: ADMINISTRATIVE 19.8 T Effective Date: Octber 1, 014 Table f Cntents CONDITIONS OF COVERAGE... BENEFIT CONSIDERATIONS...
Request for Resume (RFR) CATS II Master Contract. All Master Contract Provisions Apply
 Sectin 1 General Infrmatin RFR Number: (Reference BPO Number) Functinal Area (Enter One Only) F50B3400026 7 Infrmatin System Security Labr Categry A single supprt resurce may be engaged fr a perid nt t
Sectin 1 General Infrmatin RFR Number: (Reference BPO Number) Functinal Area (Enter One Only) F50B3400026 7 Infrmatin System Security Labr Categry A single supprt resurce may be engaged fr a perid nt t
Privacy Breach and Complaint Protocol
 Privacy Breach and Cmplaint Prtcl Effective: December 31, 2012 Apprved by: Le McKenna, CFO 1.0 General Privacy breaches and privacy cmplaints will be handled in accrdance with this prtcl. This prtcl is
Privacy Breach and Cmplaint Prtcl Effective: December 31, 2012 Apprved by: Le McKenna, CFO 1.0 General Privacy breaches and privacy cmplaints will be handled in accrdance with this prtcl. This prtcl is
Project Open Hand Atlanta. Health Insurance Portability and Accountability Act (HIPAA) NOTICE OF PRIVACY PRACTICES
 Prject Open Hand Atlanta Effective Date: April 14, 2003 Health Insurance Prtability and Accuntability Act (HIPAA) The Health Insurance Prtability and Accuntability Act f 1996 (HIPAA) directs health care
Prject Open Hand Atlanta Effective Date: April 14, 2003 Health Insurance Prtability and Accuntability Act (HIPAA) The Health Insurance Prtability and Accuntability Act f 1996 (HIPAA) directs health care
Texas Department of Insurance Division of Workers Compensation. Insurance Carrier/Utilization Review Agent Plan-Based Audit
 Texas Department f Insurance Divisin f Wrkers Cmpensatin Insurance Carrier/Utilizatin Review Agent Plan-Based Audit Octber 22, 2012 1 P age Sectin I: General Statement and Overview The Texas Department
Texas Department f Insurance Divisin f Wrkers Cmpensatin Insurance Carrier/Utilizatin Review Agent Plan-Based Audit Octber 22, 2012 1 P age Sectin I: General Statement and Overview The Texas Department
Personal Data Security Breach Management Policy
 Persnal Data Security Breach Management Plicy 1.0 Purpse The Data Prtectin Acts 1988 and 2003 impse bligatins n data cntrllers in Western Care Assciatin t prcess persnal data entrusted t them in a manner
Persnal Data Security Breach Management Plicy 1.0 Purpse The Data Prtectin Acts 1988 and 2003 impse bligatins n data cntrllers in Western Care Assciatin t prcess persnal data entrusted t them in a manner
Government of Malta. Reference: GMICT X 0004-1:2014 Version: 7.0. Effective: 07 January 2014
 Gvernment f Malta Reference: GMICT X 0004-1:2014 Versin: 7.0 Effective: 07 January 2014 This dcument is part f the http://ictplicies.gv.mt Underlined terms are defined in the Vcabulary. Purpse The purpse
Gvernment f Malta Reference: GMICT X 0004-1:2014 Versin: 7.0 Effective: 07 January 2014 This dcument is part f the http://ictplicies.gv.mt Underlined terms are defined in the Vcabulary. Purpse The purpse
MANITOBA SECURITIES COMMISSION STRATEGIC PLAN 2013-2016
 MANITOBA SECURITIES COMMISSION STRATEGIC PLAN 2013-2016 The Manitba Securities Cmmissin (the Cmmissin) is a divisin f the Manitba Financial Services Agency (MFSA). The ther divisin is the Financial Institutins
MANITOBA SECURITIES COMMISSION STRATEGIC PLAN 2013-2016 The Manitba Securities Cmmissin (the Cmmissin) is a divisin f the Manitba Financial Services Agency (MFSA). The ther divisin is the Financial Institutins
Handling professional conduct complaints against doctors
 Handling prfessinal cnduct cmplaints against dctrs Handling prfessinal cnduct cmplaints against dctrs Handling prfessinal cnduct cmplaints against dctrs Avant supprts: à a natinally cnsistent apprach t
Handling prfessinal cnduct cmplaints against dctrs Handling prfessinal cnduct cmplaints against dctrs Handling prfessinal cnduct cmplaints against dctrs Avant supprts: à a natinally cnsistent apprach t
CDC UNIFIED PROCESS PRACTICES GUIDE
 Dcument Purpse The purpse f this dcument is t prvide guidance n the practice f Risk Management and t describe the practice verview, requirements, best practices, activities, and key terms related t these
Dcument Purpse The purpse f this dcument is t prvide guidance n the practice f Risk Management and t describe the practice verview, requirements, best practices, activities, and key terms related t these
Duty Statement Manager The Early Years at Seymour (TEYS)
 Duty Statement Manager The Early Years at Seymur (TEYS) Psitin Title Respnsible T Time Fractin Status Salary and Cnditins Psitin Purpse: Manager The Early Years at Seymur (TEYS) Business Manager and Head
Duty Statement Manager The Early Years at Seymur (TEYS) Psitin Title Respnsible T Time Fractin Status Salary and Cnditins Psitin Purpse: Manager The Early Years at Seymur (TEYS) Business Manager and Head
NEW YORK STATE DEPARTMENT OF HEALTH BUREAU OF DENTAL HEALTH SCHOOL-BASED HEALTH CENTER DENTAL PROGRAM PERFORMANCE EFFECTIVENESS REVIEW TOOL (PERT)
 NEW YORK STATE DEPARTMENT OF HEALTH BUREAU OF DENTAL HEALTH SCHOOL-BASED HEALTH CENTER DENTAL PROGRAM PERFORMANCE EFFECTIVENESS REVIEW TOOL (PERT) March 1, 2007 TABLE OF CONTENTS SECTION I: INTRODUCTION
NEW YORK STATE DEPARTMENT OF HEALTH BUREAU OF DENTAL HEALTH SCHOOL-BASED HEALTH CENTER DENTAL PROGRAM PERFORMANCE EFFECTIVENESS REVIEW TOOL (PERT) March 1, 2007 TABLE OF CONTENTS SECTION I: INTRODUCTION
Care Plan Oversight. Home Health Certification. July 23, 2014. Agenda
 Care Plan Oversight Hme Health Certificatin July 23, 2014 Agenda Care Plan Oversight Why We Are Prviding the Educatin Prcedure cdes Descriptin f Services Wh Can Perfrm Frequency f Services Face-t-Face
Care Plan Oversight Hme Health Certificatin July 23, 2014 Agenda Care Plan Oversight Why We Are Prviding the Educatin Prcedure cdes Descriptin f Services Wh Can Perfrm Frequency f Services Face-t-Face
Corporate Standards for data quality and the collation of data for external presentation
 The University f Kent Crprate Standards fr data quality and the cllatin f data fr external presentatin This paper intrduces a set f standards with the aim f safeguarding the University s psitin in published
The University f Kent Crprate Standards fr data quality and the cllatin f data fr external presentatin This paper intrduces a set f standards with the aim f safeguarding the University s psitin in published
University of Texas at Dallas Policy for Accepting Credit Card and Electronic Payments
 University f Texas at Dallas Plicy fr Accepting Credit Card and Electrnic Payments Cntents: Purpse Applicability Plicy Statement Respnsibilities f a Merchant Department Prcess t Becme a Merchant Department
University f Texas at Dallas Plicy fr Accepting Credit Card and Electrnic Payments Cntents: Purpse Applicability Plicy Statement Respnsibilities f a Merchant Department Prcess t Becme a Merchant Department
ITIL Release Control & Validation (RCV) Certification Program - 5 Days
 ITIL Release Cntrl & Validatin (RCV) Certificatin Prgram - 5 Days Prgram Overview ITIL is a set f best practices guidance that has becme a wrldwide-adpted framewrk fr Infrmatin Technlgy Services Management
ITIL Release Cntrl & Validatin (RCV) Certificatin Prgram - 5 Days Prgram Overview ITIL is a set f best practices guidance that has becme a wrldwide-adpted framewrk fr Infrmatin Technlgy Services Management
LINCOLNSHIRE POLICE Policy Document
 LINCOLNSHIRE POLICE Plicy Dcument 1. POLICY IDENTIFICATION PAGE POLICY TITLE: ICT CHANGE & RELEASE MANAGEMENT POLICY POLICY REFERENCE NO: PD 186 POLICY OWNERSHIP: ACPO Cmmissining Officer: Prtfli / Business-area
LINCOLNSHIRE POLICE Plicy Dcument 1. POLICY IDENTIFICATION PAGE POLICY TITLE: ICT CHANGE & RELEASE MANAGEMENT POLICY POLICY REFERENCE NO: PD 186 POLICY OWNERSHIP: ACPO Cmmissining Officer: Prtfli / Business-area
Drug Supply Management
 SOP Reference Number: Page 1 f 9 Versin n. and date: Standard perating prcedure title: Drug Supply Management SOP Reference number: Versin Number and Date: Supersedes versin: Issue date: Review date: Authr
SOP Reference Number: Page 1 f 9 Versin n. and date: Standard perating prcedure title: Drug Supply Management SOP Reference number: Versin Number and Date: Supersedes versin: Issue date: Review date: Authr
Purpose Statement. Objectives
 Apprved by Academic Affairs Cuncil, June 24, 2014 Faculty Handbk Part VI: Other Plicies and Prcedures Sectin R. Intellectual Prperty Classified Emplyee Handbk Part VI: Other Plicies and Prcedures Sectin
Apprved by Academic Affairs Cuncil, June 24, 2014 Faculty Handbk Part VI: Other Plicies and Prcedures Sectin R. Intellectual Prperty Classified Emplyee Handbk Part VI: Other Plicies and Prcedures Sectin
Code on Good Research Practice
 Cde n Gd Research Practice Revised June 2015 (appendix remved) Cntents PREAMBLE 1 PRINCIPLES 1 Statement f Principles 1 Observatin f the Cde 2 Breach f the Cde 2 Advice 2 GOOD RESEARCH PRACTICE 2 Characteristics
Cde n Gd Research Practice Revised June 2015 (appendix remved) Cntents PREAMBLE 1 PRINCIPLES 1 Statement f Principles 1 Observatin f the Cde 2 Breach f the Cde 2 Advice 2 GOOD RESEARCH PRACTICE 2 Characteristics
Version Date Comments / Changes 1.0 January 2015 Initial Policy Released
 Page 1 f 6 Vice President, Infrmatics and Transfrmatin Supprt APPROVED (S) REVISED / REVIEWED SUMMARY Versin Date Cmments / Changes 1.0 Initial Plicy Released INTENT / PURPOSE The Infrmatin and Data Gvernance
Page 1 f 6 Vice President, Infrmatics and Transfrmatin Supprt APPROVED (S) REVISED / REVIEWED SUMMARY Versin Date Cmments / Changes 1.0 Initial Plicy Released INTENT / PURPOSE The Infrmatin and Data Gvernance
Community Support Programs N9 Organizational Internship Program
 NAVY REGION SOUTHWEST Cmmunity Supprt Prgrams N9 Organizatinal Internship Prgram April 2011 Cntents Prgram... 3 Purpse... 3 Outcme... 3 Duratin... 3 Definitins... 3 Eligibility... 4 Prcess... 5 Participating
NAVY REGION SOUTHWEST Cmmunity Supprt Prgrams N9 Organizatinal Internship Prgram April 2011 Cntents Prgram... 3 Purpse... 3 Outcme... 3 Duratin... 3 Definitins... 3 Eligibility... 4 Prcess... 5 Participating
NYU Langone Medical Center NYU Hospitals Center NYU School of Medicine
 Title: Identity Theft Prgram Effective Date: July 2009 NYU Langne Medical Center NYU Hspitals Center NYU Schl f Medicine POLICY It is the plicy f the NYU Langne Medical Center t educate and train staff
Title: Identity Theft Prgram Effective Date: July 2009 NYU Langne Medical Center NYU Hspitals Center NYU Schl f Medicine POLICY It is the plicy f the NYU Langne Medical Center t educate and train staff
Heythrop College Disciplinary Procedure for Support Staff
 Heythrp Cllege Disciplinary Prcedure fr Supprt Staff Intrductin 1. This prcedural dcument des nt apply t thse academic-related staff wh are mentined in the Cllege s Ordinance, namely the Librarian and
Heythrp Cllege Disciplinary Prcedure fr Supprt Staff Intrductin 1. This prcedural dcument des nt apply t thse academic-related staff wh are mentined in the Cllege s Ordinance, namely the Librarian and
Research Governance Policy
 Research Gvernance Plicy 1. Scpe and Purpse 2. Need fr a research gvernance plicy T meet the requirements f funding bdies T manage risk T imprve the quality f research T prtect the quality f research T
Research Gvernance Plicy 1. Scpe and Purpse 2. Need fr a research gvernance plicy T meet the requirements f funding bdies T manage risk T imprve the quality f research T prtect the quality f research T
Risk Management Strategy 2014/2016
 Enclsure L Risk Management Strategy 2014/2016 Trust Bard Item: 8.4 29 th January 2014 Enclsure: L Purpse f the Reprt: T present the Trust Bard the updated Risk Management Strategy fr ratificatin. The Strategy
Enclsure L Risk Management Strategy 2014/2016 Trust Bard Item: 8.4 29 th January 2014 Enclsure: L Purpse f the Reprt: T present the Trust Bard the updated Risk Management Strategy fr ratificatin. The Strategy
Texas Woman's University University Policy Manual
 Texas Wman's University University Plicy Manual Plicy Name: Plicy Number: 6.06 Date Passed: July 2004 Health Insurance Prtability& Accuntability Act (HIPAA) Date Reviewed: September 2008 Next Review: September
Texas Wman's University University Plicy Manual Plicy Name: Plicy Number: 6.06 Date Passed: July 2004 Health Insurance Prtability& Accuntability Act (HIPAA) Date Reviewed: September 2008 Next Review: September
THE CITY UNIVERSITY OF NEW YORK IDENTITY THEFT PREVENTION PROGRAM
 THE CITY UNIVERSITY OF NEW YORK IDENTITY THEFT PREVENTION PROGRAM 1. Prgram Adptin The City University f New Yrk (the "University") develped this Identity Theft Preventin Prgram (the "Prgram") pursuant
THE CITY UNIVERSITY OF NEW YORK IDENTITY THEFT PREVENTION PROGRAM 1. Prgram Adptin The City University f New Yrk (the "University") develped this Identity Theft Preventin Prgram (the "Prgram") pursuant
Information Security Incident Response Plan
 Infrmatin Security Incident Respnse Plan Agency: Date: Cntact: 1 TABLE OF CONTENTS Intrductin... 3 Authrity... 4 Terms and Definitins... 4 Rles and Respnsibilities... 5 Prgram... 6 Educatin and Awareness...
Infrmatin Security Incident Respnse Plan Agency: Date: Cntact: 1 TABLE OF CONTENTS Intrductin... 3 Authrity... 4 Terms and Definitins... 4 Rles and Respnsibilities... 5 Prgram... 6 Educatin and Awareness...
1.2 Supporting References For information relating to the Company Hardware Request project, see the SharePoint web site.
 Hardware Request System Visin 1 Intrductin 1.1 Dcument Purpse and Scpe This dcument utlines the visin fr the Hardware Request system. The purpses f this dcument are t: Identify and agree n the prblems
Hardware Request System Visin 1 Intrductin 1.1 Dcument Purpse and Scpe This dcument utlines the visin fr the Hardware Request system. The purpses f this dcument are t: Identify and agree n the prblems
Richmond Clinical Commissioning Group Report Summary
 Richmnd Clinical Cmmissining Grup Reprt Summary Meeting Title: Gverning Bdy Date: 16 September 2014 Reprt Title: Better Care Fund Plan Agenda Item: 8 Attachment: D Purpse: (please delete /N as apprpriate)
Richmnd Clinical Cmmissining Grup Reprt Summary Meeting Title: Gverning Bdy Date: 16 September 2014 Reprt Title: Better Care Fund Plan Agenda Item: 8 Attachment: D Purpse: (please delete /N as apprpriate)
Business Continuity Management Systems Foundation Training Course
 Certificatin criteria fr Business Cntinuity Management Systems Fundatin Training Curse CONTENTS 1. INTRODUCTION 2. LEARNING OBJECTIVES 3. ENABLING OBJECTIVES KNOWLEDGE & SKILLS 4. TRAINING METHODS 5. COURSE
Certificatin criteria fr Business Cntinuity Management Systems Fundatin Training Curse CONTENTS 1. INTRODUCTION 2. LEARNING OBJECTIVES 3. ENABLING OBJECTIVES KNOWLEDGE & SKILLS 4. TRAINING METHODS 5. COURSE
REQUEST FOR PROPOSAL SECURITY SERVICES
 REQUEST FOR PROPOSAL SECURITY SERVICES Sectin I INTRODUCTION [Cmpany] is seeking prpsals frm qualified Cntractrs t prvide unifrmed security service fr [Cmpany] facilities at [Lcatin(s)]. This dcument is
REQUEST FOR PROPOSAL SECURITY SERVICES Sectin I INTRODUCTION [Cmpany] is seeking prpsals frm qualified Cntractrs t prvide unifrmed security service fr [Cmpany] facilities at [Lcatin(s)]. This dcument is
CDC UNIFIED PROCESS PRACTICES GUIDE
 Dcument Purpse The purpse f this dcument is t prvide guidance n the practice f Business Case and t describe the practice verview, requirements, best practices, activities, and key terms related t these
Dcument Purpse The purpse f this dcument is t prvide guidance n the practice f Business Case and t describe the practice verview, requirements, best practices, activities, and key terms related t these
WHAT YOU NEED TO KNOW ABOUT. Protecting your Privacy
 WHAT YOU NEED TO KNOW ABOUT Prtecting yur Privacy YOUR PRIVACY IS OUR PRIORITY Credit unins have a histry f respecting the privacy f ur members and custmers. Yur Bard f Directrs has adpted the Credit Unin
WHAT YOU NEED TO KNOW ABOUT Prtecting yur Privacy YOUR PRIVACY IS OUR PRIORITY Credit unins have a histry f respecting the privacy f ur members and custmers. Yur Bard f Directrs has adpted the Credit Unin
JOINT BOARD OF MODERATORS GUIDELINES FOR CHECKING OUTPUT STANDARDS OF DEGREE PROGRAMMES
 JOINT BOARD OF MODERATORS GUIDELINES FOR CHECKING OUTPUT STANDARDS OF DEGREE PROGRAMMES 1. Intrductin 1.1 These Guidelines are fr higher educatin institutins (r ther educatinal establishments) prviding
JOINT BOARD OF MODERATORS GUIDELINES FOR CHECKING OUTPUT STANDARDS OF DEGREE PROGRAMMES 1. Intrductin 1.1 These Guidelines are fr higher educatin institutins (r ther educatinal establishments) prviding
Revised October 27, 2011 Page 1 of 6
 Keystne STARS Accreditatin Applicatin Philsphy The Keystne STARS prgram is Pennsylvania s QRIS which began in 2002. There are fur quality levels frm STAR 1 t STAR 4, each level building n the prir levels;
Keystne STARS Accreditatin Applicatin Philsphy The Keystne STARS prgram is Pennsylvania s QRIS which began in 2002. There are fur quality levels frm STAR 1 t STAR 4, each level building n the prir levels;
Doctoral Framework Guidelines
 Dctral Framewrk Guidelines UTS Framewrk fr Dctral Educatin UTS Business Schl Higher Degree Research 1. Intrductin The UTS Framewrk fr Dctral Educatin is a UTS-wide initiative directed twards imprving the
Dctral Framewrk Guidelines UTS Framewrk fr Dctral Educatin UTS Business Schl Higher Degree Research 1. Intrductin The UTS Framewrk fr Dctral Educatin is a UTS-wide initiative directed twards imprving the
How To Write A Scial Media Plicy
 Scial Media Plicy Scial Media Plicy Recrd Number D14/78 Respnsible Manager Directr Business Supprt and Strategy Manager Custmer and Cmmunicatins Last reviewed 11 February 2014 Adptin reference Cuncil Reslutin
Scial Media Plicy Scial Media Plicy Recrd Number D14/78 Respnsible Manager Directr Business Supprt and Strategy Manager Custmer and Cmmunicatins Last reviewed 11 February 2014 Adptin reference Cuncil Reslutin
POLICY 1390 Information Technology Continuity of Business Planning Issued: June 4, 2009 Revised: June 12, 2014
 State f Michigan POLICY 1390 Infrmatin Technlgy Cntinuity f Business Planning Issued: June 4, 2009 Revised: June 12, 2014 SUBJECT: APPLICATION: PURPOSE: CONTACT AGENCY: Plicy fr Infrmatin Technlgy (IT)
State f Michigan POLICY 1390 Infrmatin Technlgy Cntinuity f Business Planning Issued: June 4, 2009 Revised: June 12, 2014 SUBJECT: APPLICATION: PURPOSE: CONTACT AGENCY: Plicy fr Infrmatin Technlgy (IT)
IT CHANGE MANAGEMENT POLICY
 IT CHANGE MANAGEMENT POLICY Effective Date May 19, 2016 Crss-Reference 1. IT Operatins and Maintenance Plicy 2. IT Security Incident Management Plicy Respnsibility Apprver Review Schedule 1. Plicy Statement
IT CHANGE MANAGEMENT POLICY Effective Date May 19, 2016 Crss-Reference 1. IT Operatins and Maintenance Plicy 2. IT Security Incident Management Plicy Respnsibility Apprver Review Schedule 1. Plicy Statement
National Australia Bank Limited Group Disclosure & External Communications Policy
 Natinal Australia Bank Limited Grup Disclsure & External Cmmunicatins Plicy Grup Disclsure & External Cmmunicatins Plicy Page 2 f 7 Grup Disclsure & External Cmmunicatins Plicy ( the Plicy ) 1. Overview
Natinal Australia Bank Limited Grup Disclsure & External Cmmunicatins Plicy Grup Disclsure & External Cmmunicatins Plicy Page 2 f 7 Grup Disclsure & External Cmmunicatins Plicy ( the Plicy ) 1. Overview
Systems Support - Extended
 1 General Overview This is a Service Level Agreement ( SLA ) between and the Enterprise Windws Services t dcument: The technlgy services the Enterprise Windws Services prvides t the custmer. The targets
1 General Overview This is a Service Level Agreement ( SLA ) between and the Enterprise Windws Services t dcument: The technlgy services the Enterprise Windws Services prvides t the custmer. The targets
FINANCE SCRUTINY SUB-COMMITTEE
 REPORT FOR: PERFORMANCE AND FINANCE SCRUTINY SUB-COMMITTEE Date f Meeting: 6 January 2015 Subject: Staff Survey and Sickness Absence Mnitring Results and Actin plans Respnsible Officer: Scrutiny Lead Member
REPORT FOR: PERFORMANCE AND FINANCE SCRUTINY SUB-COMMITTEE Date f Meeting: 6 January 2015 Subject: Staff Survey and Sickness Absence Mnitring Results and Actin plans Respnsible Officer: Scrutiny Lead Member
Customer Care Policy
 Custmer Care Plicy Page 1 f 12 CUSTOMER CARE POLICY Keighley & District Vlunteer Centre and Bradfrd Vlunteer Centre are independent charities that wrk in partnership t prmte vlunteering and t supprt lcal
Custmer Care Plicy Page 1 f 12 CUSTOMER CARE POLICY Keighley & District Vlunteer Centre and Bradfrd Vlunteer Centre are independent charities that wrk in partnership t prmte vlunteering and t supprt lcal
0820.02 Workers Disability Compensation Claims Procedures Issued: January 1, 1994 Revised: March 29, 2012
 State f Michigan Administrative Guide t State Gvernment 0820.02 Wrkers Disability Cmpensatin Claims Prcedures Issued: January 1, 1994 Revised: March 29, 2012 SUBJECT: APPLICATION: PURPOSE: CONTACT AGENCY:
State f Michigan Administrative Guide t State Gvernment 0820.02 Wrkers Disability Cmpensatin Claims Prcedures Issued: January 1, 1994 Revised: March 29, 2012 SUBJECT: APPLICATION: PURPOSE: CONTACT AGENCY:
Requirements For Change Control in a Hospital Blood Bank
 NHS SCOTLAND DOCUMENT Mdel SOP t Meet Requirements f OIG Quality Management System Dcument Reference N: NHSSIG D78_05_01 Requirements Fr Change Cntrl in a Hspital Bld Bank Dcument Prepared Nvember 2005
NHS SCOTLAND DOCUMENT Mdel SOP t Meet Requirements f OIG Quality Management System Dcument Reference N: NHSSIG D78_05_01 Requirements Fr Change Cntrl in a Hspital Bld Bank Dcument Prepared Nvember 2005
TITLE: RECORDS AND INFORMATION MANAGEMENT POLICY
 TITLE: RECORDS AND INFORMATION MANAGEMENT POLICY REFERENCE NUMBER: 14/103368 RESPONSIBLE DEPARTMENT: Crprate Services APPLICABLE LEGISLATION: State Recrds Act 1997 Lcal Gvernment Act 1999 Crpratins Act
TITLE: RECORDS AND INFORMATION MANAGEMENT POLICY REFERENCE NUMBER: 14/103368 RESPONSIBLE DEPARTMENT: Crprate Services APPLICABLE LEGISLATION: State Recrds Act 1997 Lcal Gvernment Act 1999 Crpratins Act
