SAFETY OF Bacillus thuringiensis Proteins Used to Control Insect Pests in Agricultural Crops
|
|
|
- Egbert O’Connor’
- 7 years ago
- Views:
Transcription
1 SAFETY OF Bacillus thuringiensis Proteins Used to Control Insect Pests in Agricultural Crops I. INTRODUCTION This document reviews the safety assessment for Bacillus thuringiensis (Bt) delta-endotoxin proteins that have been introduced into agricultural crops to provide protection against insect pests. Information is provided on the scientific basis for U.S. EPA approvals for the introduction of Bt proteins into agricultural crops. Relevant to these approvals is the extensive data base on Bt protein safety. These proteins are the insect control components of Bt microbial formulations that have a history of safety when used on agricultural crops for over 30 years. A summary of the extensive data base on Bt protein safety is provided in tables at the end of this document. II. BACKGROUND The Environmental Protection Agency (EPA) has approved the commercial use of the Bacillus thuringiensis (Bt) delta-endotoxin proteins,, and Cry3A as expressed in genetically engineered corn, cotton, and potato, respectively. A a plant pesticide is currently under review at EPA. Cry5 and Cry9 proteins have been reported to exhibit useful insecticidal properties and may also be developed for expression in plants (Tailor et al. 1992, and Lambert et al. 1996, respectively). To assess the implications of human dietary exposure to these plant pesticides, EPA has asked registrants to submit results of acute oral mammalian toxicology studies (oral LD 50 ) and in vitro digestibility studies. These tests have been conducted using microbially produced Bt proteins that have been shown to be equivalent to the plant-expressed protein. In all cases, the test results have shown that Cry proteins are not toxic to mammals, even at the highest dose tested, and that they are rapidly degraded in simulated gastric fluid. Thus, the Agency has been able to conclude that Cry protein plant pesticides pose no foreseeable risks to human health and has granted exemptions from the requirement of a tolerance for all of the plant pesticides registered thus far (60 FR 21725, May 3, 1995; 60 FR 42443, August 16, 1995; 60 FR 47871, September 15, 1995; 61 FR 40340, August 2, 1996; 62 FR 17720, April 11, 1997). The first exemptions from tolerance were limited to a specific Cry protein as expressed in a single crop, such as Cry3A in potato and in cotton. More recently, in approving Monsanto s expressed in corn (61 FR 40340, August 2, 1996) and Dekalb s expressed in corn (62 FR 17720, April 11, 1997), EPA established a broad tolerance exemption for and proteins in all plant raw agricultural commodities. No additional exemptions from tolerance for the or proteins will be necessary to support other crops engineered to express either of these proteins. However, under current Agency policy, the acute oral toxicity and in vitro digestibility studies must be conducted on
2 each new Cry protein to be registered as a plant pesticide (e.g.,, Cry1Ba). An exemption from the requirement of a tolerance must also be established for each new Cry protein. Cry proteins are named according to their amino acid similarity to established holotype proteins (Crickmore et al. 1995). Cry proteins with similar amino acid sequences are grouped together. This nomenclature scheme replaces that of Hofte and Whitely (1989), in which Bt insecticidal crystal proteins are classified as CryI, CryII, or CryIII proteins, based on their insecticidal activities. As defined by the current nomenclature (Crickmore et al. 1995), 1 Cry proteins with the same Arabic numeral (e.g., Cry1) share at least a 45 percent amino acid sequence identity. Those with the same Arabic numeral and upper case letter (e.g., Cry1A) share at least a 75 percent sequence identity. The same Arabic numeral and upper and lower case letter (e.g., ) designates a greater than 95 percent sequence identity. Therefore, one can apply safety conclusions from testing one or a few representative Cry proteins to a broader, but closely related, group of proteins proteins that by definition share significant amino acid sequence identity. There currently exists an extensive body of scientific data demonstrating the safety of Cry proteins. A review of the literature establishes that many different Cry proteins have been evaluated in a variety of mammalian toxicology tests over the past 35 years. No adverse effects have been observed in mammals upon oral exposure to any of these Cry proteins. Briefly, the following sets of data and lines of scientific reasoning support EPA making generic human health safety determinations for these classes of Bt Cry proteins: Studies on representative proteins from three classes of Cry proteins (Cry1, Cry2, and Cry3) indicate that these materials are not toxic to mammals when administered orally at high doses, and that they degrade rapidly in simulated gastric fluid. Modified Cry proteins, a priori, pose no unique human health concerns. Natural and modified Cry proteins warrant the same regulatory considerations. Cry proteins have a complex, highly specific mode of action and have specific, effective binding sites in invertebrates. Immunocytochemical analyses of Cry1A have revealed no comparable binding sites in mammals. Results of extensive acute oral or dietary studies representing numerous commercial Bt microbial pesticide products containing different combinations of Cry proteins establish no mammalian toxicity. 1 Current information concerning the Bt holotype protein nomenclature and a continually updated database of Bt holotype proteins can be found on the world wide web at: 2
3 Bt microbial products have a long history (> 30 years) of safe use. Reports of adverse effects in humans from the use of microbial Bt products are rare. None of the incidents are attributable to exposure to Cry proteins (EPA, 1988). A. Cry Proteins Are Nontoxic to Mammals and Are Rapidly Digested Dietary exposure is the major route by which humans can be exposed to Cry proteins expressed in plants. Dermal and inhalation exposures are anticipated to be negligible because Cry proteins are expressed and contained within the cells of the plants and are not volatile. This is consistent with EPA s testing scheme for Cry proteins (McClintock et al. 1992), which reflects a focus on dietary exposure. Acute oral mammalian toxicity and protein digestibility are the end points for EPA s human health risk assessment. All of the mammalian toxicity testing of Bt proteins that are expressed in plants has, to the best of our knowledge, yielded negative results. Thus, no further mammalian toxicology testing (beyond acute and digestibility studies) has been required to support registration and exemptions from tolerance. No treatment-related adverse effects have been observed in any of the acute oral mammalian toxicity studies conducted with microbially produced,,, and Cry3A proteins (Table 1). Six oral gavage studies in mice established the LD 50 to be >3,280 mg/kg to >5,200 mg/kg for these proteins. Based on these results there is a safety factor of greater than 50,000 for human dietary exposure to and proteins in corn or cottonseed, greater than one million for Cry3A protein in potato, and greater than two million for protein in tomato. The no-observed-effect-level (NOEL) for was > 0.45 mg/kg/day in a 28-day repeated dose oral toxicity study in mice and > 0.06 mg/kg/day in a 31-day repeated dose study in rabbits. Treatment doses in the 28-day and 31-day studies were estimated to be 1,000 to 4,000 times the maximum anticipated human exposure from consuming tomatoes genetically engineered to produce (Noteborn et al. 1993). Based on the lack of toxic effects and the large margins of safety for both acute and 30-day exposures, these Cry proteins pose no foreseeable risks to human health. Moreover, these proteins are unlikely to cause endocrine effects because they exhibit no structural or functional similarity to estrogen or estrogen-mimicking compounds. EPA has stated that when proteins are toxic, they are known to act via acute mechanisms and at very low dose levels (Sjoblad et al. 1992). The Cry proteins tested so far are judged to be nontoxic to mammals. In addition, we believe that the acute toxicity data on these representative Cry proteins and the extensive data base on microbial Bt products support a broader conclusion: All Cry proteins classified by their amino acid sequence to be Cry1, Cry2, or Cry3 are highly unlikely to be toxic to humans. Further scientific evidence of the safety of Cry proteins is that they have been shown to be rapidly degraded in vitro using simulated gastric fluids (Table 2). Results of seven in vitro 3
4 assays conducted with representative Cry1, Cry2, and Cry3 proteins establish that the proteins are rapidly degraded, usually within 30 seconds. These results support the broader conclusion that members of these groups of Cry proteins (that share significant amino acid sequence identity) are likely to be rapidly degraded following ingestion by humans. Another area of consideration is whether Cry proteins may induce an allergenic reaction. The demonstrated rapid in vitro degradation of Cry proteins should minimize the potential for such an occurrence. By comparison, food allergens generally persisted in the in vitro gastrointestinal model, whereas common food proteins with no allergenic history degraded rapidly in simulated gastric fluid (Metcalfe et al. 1996). To further investigate the potential for allergenicity, searches of allergen sequence databases have been conducted. These searches have shown no significant matches with the Cry proteins (Metcalfe et al. 1996). Moreover, Cry proteins do not share characteristics often exhibited by known food allergens. Unlike many known food allergens, the Cry proteins as expressed in plants are present in relatively low concentrations, and are heat labile. In addition, in the more than 30-year history of their commercial use, there have been no reported cases of allergenic reactions to the microbial Bt products (61 FR 40430, August 2, 1996). B. Modified Cry Proteins Pose No Unique Human Health Concerns There is no reason to expect that genetically modified proteins designated as Cry1, Cry2, or Cry3, according to the Crickmore nomenclature, pose any unique human health concerns compared to their naturally occurring counterparts. There is evidence that modified Cry proteins have already been generated in nature. The delta endotoxin appears to have arisen from a recombination event between ancestral cry1aa and cry1ac-like toxin genes (Geiser et al. 1986). Similarly, amino acid sequence alignments of Cry1Ca, Cry1Cb, Cry1Ea, and Cry1Eb provide evidence that cry1ea and cry1cb could have arisen from a recombination event between ancestral cry1ca and cry1eb toxin genes (Thompson et al. 1995). Multiple alignments of the Cry1Ca, Cry1Cb, Cry1Ea, and Cry1Eb amino acid sequences highlight the probable recombination site near amino acid 450. Analyses such as this suggest that recombination between related cry genes is a normal process in cry gene evolution. Regardless of its origin (i.e., naturally occurring or genetically engineered), a protein with sufficient amino acid identity with a holotype Bt protein to be classified as a Cry1, Cry2, or Cry3 protein warrants the same level of regulatory treatment. EPA has registered microbial Bt products containing Cry proteins modified through genetic engineering. Two examples are Ecogen s Lepinox and Mycogen s Mattch bioinsecticides. The Agency s regulatory decisions related to these products are consistent with, and in support of, the rationale presented herein for applying the same standards to products containing either natural or modified Cry proteins. Lepinox is exempt from the requirement of a tolerance under the existing broad Bt tolerance exemption (40 CFR ). The Agency concluded that although Lepinox was developed using recombinant DNA 4
5 technology, its properties are no different from the range of properties of strains of Bt that might be found in nature. EPA required an acute oral toxicity/pathogenicity study and an in vitro digestibility study to support the tolerance exemption for the and Cry1C derived delta endotoxins in Mattch (40 FR 47487, September 13, 1995). C. No Cry Protein Binding Sites Have Been Identified in Mammals Cry proteins are surface-acting insecticidal proteins that exhibit a complex, multicomponent mode of action (English and Slatin 1992). Ultimately, the proteins bind to specific sites in the midgut epithelium cells of susceptible insects, opening cation-selective channels in the cell membrane. The cells swell due to an influx of ions and water, leading to cell lysis and ultimately the death of the insect (Hofte and Whitely 1989). Mammalian species are not susceptible to Cry proteins. This may be explained, in part, by the fact that conditions required for the complex steps in the mode of action described by English and Slatin (1992) do not exist in mammals or most invertebrates. Delta-endotoxins must first be solubilized. Some of the Cry proteins must then be proteolytically digested to the insecticidally active form. They must remain active rather than being further degraded. Receptor-mediated binding to the brush border membrane in midgut epithelium cells leads to membrane-bound forms of the endotoxin. This is believed to take place in three steps: binding to midgut receptor proteins, partitioning into the brush-border membrane, and finally, formation of channels and pores. The number of Cry proteins required to form a channel is uncertain. Only a relatively small subset of insects has been identified that supports this complex series of events that leads to cell death. Noteborn et al. (1993) detected no specific binding of protein to mouse and rat gastrointestinal tract tissue in vivo. These researchers also adapted an in vitro immunocytochemical assay (for detecting Cry protein binding in insect cells) to evaluate binding of protein to mammalian gut tissue sections. Their analysis of mouse, rat, monkey, and human tissue sections did not reveal any binding sites in these tissues. These findings further support the safety of Cry proteins exposed to mammals through the diet. D. Bacillus thuringiensis Microbial Pesticides Are Nontoxic to Mammals Results of testing microbial Bt preparations for oral mammalian toxicity over the past 35 years provide an extensive body of scientific data that supports their safe use. Collectively, these studies demonstrate the total lack of acute, subchronic, and chronic oral toxicity associated with Bt microbial pesticides (Table 3). These findings are directly relevant to this petition because these microbial preparations contain genes encoded for the production of at least four different classes of Cry proteins, including seven Cry1 proteins and two each of the Cry2, Cry3, and Cry9 proteins (Table 4). Therefore, these studies reflect mammalian oral exposures to some or all of the 13 Cry proteins expressed by these genes. 5
6 Acute oral studies conducted in rats and rabbits revealed no mortalities at the highest doses tested, which ranged from 10 8 to colony forming units per animal or >2,670 to 5,050 mg Bt product/kg. There were no deleterious effects based on observations including body weight, food consumption, and gross necropsy. Four subchronic oral studies in rats demonstrated NOELs of > 4,000 to >8,400 mg Bt product/kg/day and > 10 9 Bt spores/kg/day. The rat two-year chronic NOEL was also >8,400 mg Bt product/kg/day, the highest dose tested. Humans fed one gram of a Bt preparation per day for three days exhibited no symptoms of toxicity or other ill effects. The Bt preparation used in the latter study was a Bt subspecies strain HD-1 containing genes encoded for the production of four Cry proteins:,,, and. The literature contains numerous additional references to mammalian toxicology studies in which animals have been administered Bt microbial preparations via one of several other routes of exposure, such as pulmonary, dermal, ocular, intraperitoneal, subcutaneous, intravenous, or intracerebral injection. Some of these studies report adverse effects in test animals, including mortalities, often related to the microbes themselves and not the Cry proteins in the microbial formulations. These studies are not summarized in this report because we do not believe they are relevant to assessing risks from oral exposure to Bt proteins in plants. EPA s plant pesticide testing scheme focuses on oral ingestion via the diet for plant pesticide risk assessments. Finally, EPA s interpretation of the microbial Bt mammalian toxicology database has led them to conclude that laboratory findings and favorable use history of Bt microbial pesticides clearly argue for the human safety of these active microbial pesticide ingredients (McClintock et al. 1995). E. Bacillus thuringiensis Microbial Products Have a Long History of Safe Use Bt microbial products were first registered in 1961 and have been applied continuously since then for an expanding number of uses in agriculture, disease vector control, and forestry. McClintock et al. (1995) reviewed the adverse effects that have been suggested might occur in humans from Bt microbial products. None of these reported incidents involved or implicated Cry proteins as the causative agent, nor did the authors (McClintock et al. [1995]) find them to be significant concerns in view of the currently registered products and the quality assurance safeguards that are in place for these products. They cited favorable use history in support of the overall human safety of the Bt microbial pesticides. In establishing the existing tolerance exemptions for Cry protein plant pesticides, EPA has stated that FIFRA section 6(a)2 reports claiming allergic reactions were not due to Bacillus thuringiensis itself or any of the Cry toxins. 6
7 III. Conclusion The Environmental Protection Agency has exempted from tolerance several Bt proteins for introduction into agricultural crops to control insect pests. These tolerance exemptions were based on the extensive safety data base and history of safe use that exist for microbial pesticide formulations that contain Bt proteins. Each of these proteins were also subjected to safety testing to confirm that they posed no meaningful risks to human health. Development of agricultural crop varieties that contain Bt proteins provides a safe alternative to the use of chemical insecticides to control insect pests. 7
8 Table 1. Mammalian Toxicity of Bacillus thuringiensis Cry Proteins (Oral Exposure in Mice) Findings Reference Cry Protein Doses Tested Results Dietary Exposure (Acute LD 50 ) Safety Factor 1 400, 1000, >4000 mg/kg >50,000 (corn) No deleterious effects based on body weight gain, food Monsanto and EPA (tryptic fragment) 4000 mg/kg consumption, and gross necropsy. Fact Sheet mg/kg >3280 mg/kg No mortalities and no treatment-related clinical signs of toxicity. EPA Memo 1995 One female failed to gain weight between day 7 and day 14. All animals gained weight by the end of the study (males gained more weight over the study than females). [Fact Sheet] (Ciba Seeds) 0.045, 0.45 mg/kg/day via drinking water 0.06 mg/kg/day via drinking water (full length) 500, 1000, 4200 mg/kg 5000 mg/kg (3325 mg of protein/kg) 399, 1001, 4011 mg/kg Cry3A 100; 500, 1000, 5220 mg/kg 1 Safety Factor Calculation: NOEL > 0.45 mg/kg/day NOEL > 0.06 mg/kg/day >1000 to >4000 (tomato) >1000 to >4000 (tomato) >4200 mg/kg >50,000 (cottonseed; cottonseed oil) >2,000,000 (tomato) >5000 mg/kg In the 28-day oral mouse study, no toxicity was observed. There was no production of immunological responses. Noteborn et al In the 31-day oral rabbit study, no toxicity was observed Noteborn et al No deleterious effects based on body weight gain, food consumption, and gross necropsy. No treatment-related toxicity or clinical abnormalities were observed. >4011 mg/kg >50,000 (cottonseed; cottonseed oil) No deleterious effects based on body weight gain, food consumption, and gross necropsy. >5220 mg/kg >1,000,000 (potato) No deleterious effects based on body weight gain, food consumption, and gross necropsy. Monsanto and EPA Fact Sheet,1995 (Monsanto) Spenser et al (Dekalb) Monsanto Monsanto 1995 Safety Factor = Mammalian LD 50 or NOEL (µg/kg BW/day) Human Cry Protein Consumption (µg/kg BW/day) Human Cry Protein consumption (µg/kg body weight/day) = Average Human Consumption of Food Item (grams/day) x Maximum Cry Protein Concentration (µg/g) Average Human Body Weight (60kg) 8
9 Table 2. In vitro Digestibility of Bacillus thuringiensis Cry Proteins in Simulated Gastric Fluid Cry Protein Results Findings Reference Degraded within No longer detectable after digestion for 30 seconds in gastric fluid; in intestinal fluid, Monsanto,1996 (tryptic fragment) 30 seconds converted to trypsin-resistant core, as expected. Degraded within 1 minute Rapidly degraded in the presence of pepsin. EPA Memo,1995 [Fact Sheet] (Ciba Seeds) Substantially degraded Study of the activity of under simulated G.I. tract conditions and applying multienzymatic methods the protein was substantially degraded via digestion. Noteborn et al (full length) Cry3A Degraded within 30 seconds Degraded within 30 seconds Degraded within 30 seconds Degraded within 30 seconds No longer detectable after digestion for 30 seconds in gastric fluid; in intestinal fluid, converted to trypsin-resistant core, as expected. The protein was found to rapidly degrade in full strength and diluted simulated gastric fluid; degraded to below detection limits after a few seconds in full strength simulated gastric fluid; in simulated gastric fluid in which the pepsin concentration had been reduced 110-fold, degraded to below detection in 5 minutes. No longer detectable after digestion for less than 30 seconds in gastric fluid; in intestinal fluid, converted to trypsin-resistant core, as expected. No longer detectable after digestion for less than 30 seconds in gastric fluid; in intestinal fluid, converted to trypsin-resistant core, as expected. Monsanto and (Monsanto) 1995 Spenser et al (Dekalb) Monsanto Monsanto,1995 9
10 10 Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial (Crymax) (Crymax) (Lepinox) (Raven) (Cutlass OF) (Foil OF) (Dipel) (Dipel) Cry Gene Content Cry1C Cry1C Cry3A Cry3Ba Cry3A Cry3Ba Cry2B Cry3A Test Substance or Formulation technical (Ecogen) WDG formulation (Ecogen) technical (Ecogen) technical (Ecogen) formulation-cutlass OF (Ecogen) formulation-foil OF (Ecogen) Type of Study Acute Oral Toxicity/ Pathogenicity (Rat) Acute Oral Toxicity Acute Oral Toxicity/ Pathogenicity (Rat) Acute Oral Toxicity/ Pathogenicity (Rat) Acute Oral Toxicity/ Pathogenicity (Rat) Acute Oral Toxicity/ Pathogenicity (Rat) Results Findings (LD 50 ) x 10 8 CFU Not pathogenic, toxic, nor infectious; EG7841 showed no evidence of toxicity or infectivity/pathogenicity to rats and rapid clearance was demonstrated. >5050 mg/kg > 1.19 x 10 8 CFU There was no evidence of toxicity or pathogenicity at any of the doses tested. >4 x 10 8 spores/animal There was no evidence of toxicity or infectivity/pathogenicity. >10 8 CFU/ml There was no evidence of toxicity or infectivity/pathogenicity. >10 8 CFU/ml There was no evidence of toxicity or infectivity/pathogenicity. Reference Carter and Liggett 1994 and EPA Fact Sheet 1996 (Ecogen) 1996 (Ecogen) Barbera 1995 Carter et al David 1989a David 1989b Acute Oral-Rat 2.67 g/kg No acute oral infectivity (Abbott) Acute Oral-Rat 4.7 x spores/kg No acute oral infectivity, pathogenicity, or toxicity (Abbott) 10
11 11 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial (Dipel) (Dipel) (Dipel) (Dipel) (Dipel) (Dipel) Cry Gene Content Test Substance or Formulation technical technical Type of Study Results (LD 50 ) Acute Oral-Rat >3.4 x spores/kg Acute Oral-Rat >4.6 x spores/kg Findings No acute oral infectivity. No acute oral infectivity. Reference 1986 (Abbott) 1986 (Abbott) Acute Oral-Rat >5 g/kg No toxic or virulent effects., 1986 (Abbott) 13-Week Oral- Rat 90-Day Oral- Rat 2-Year Chronic- Rat NOEL=1.3 x 10 9 spores/kg/day No toxicity or infectivity. NOEL>8400 mg/kg/day NOEL=8400 mg/kg/day Statistically significantly decreased body weight gain in females from week 10 to week 104; no infectivity/pathogenicity was found. McClintock et al McClintock et al McClintock et al
12 12 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial (Dipel) (Dipel) Cry Gene Content Crystal deltaendotoxin Cry 1Ac Cry 1Ac Test Substance or Formulation Type of Study Oral Administration to Mice Acute Oral- Rabbit Acute Oral- Rabbit Results (LD 50 ) µg of protein/g [0.1, 0.5, 1.0 mg] 2.0 x 10 9 spores/animal 6.9 x 10 7 spores/kg Findings Bt crystal delta-endotoxin produced no pathological effects by oral administration. When administered per os, the alkali-solubilized crystal deltaendotoxin was not toxic to adult (0.5 mg protein/animal) or suckling (200 µg protein/animal) mice. In addition, native subspecies crystal deltaendotoxin and soluble protein of crystal delta-endotoxin produced no effects in adult (1.0 mg/animal) or suckling (200 µg/animal) mice by oral administration. No acute oral infectivity. No acute oral infectivity. Reference Thomas and Ellar (Abbott) 1986 (Abbott) 12
13 13 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial (Dipel and Thuricide-HP) Cry Gene Content Cry 1Ac Cry 1Ac Test Substance or Formulation (Abbott and Sandoz) Type of Study 5-Month Dietary Toxicity/ Infectivity Study in Sheep Results (LD 50 ) NOEL = 500 mg/kg (~10 12 spores/day) Human-Oral NOEL = 1 g (1 x viable spores)/day for 3 days Findings The results of this study conclude that Bt was not a pathogen in sheep following oral administration. All sheep exposed to the test substances were blood and tissue culture positive for Bt in the absence of any pathological change, which suggests that Bt is an essentially avirulent bacterium in sheep. Considering the eating habits of sheep, the absence of lung lesions suggests that Bt is incapable of producing disease when introduced by inhalation. Treatment-related occasional loose stools were observed and may have been caused by either the carrier or the observed change in the bacterial content of the rumen. Mild to marked lymphoid hyperplasia in Peyer s patches of the colon and cecum was noted but could not be definitely linked to the test substances. The hyperplasia was not considered to be clinically significant. No toxicity/infectivity; all blood cultures were negative; 5 out of 10 patients showed viable Bt 30 days post-feeding. Reference Hadley et al (Abbott) and McClintock et al
14 14 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial aizawai (Strain GC-91) aizawai (Agree, Design) aizawai (Xentari) aizawai (Xentari) israelensis Cry Gene Content Cry 1Ac Cry1B-like Cry1C Cry1D Cry2B Cry9 Cry9 Cry1D Cry1C Cry2B Cry9 Cry1D Cry1C Cry2B Cry4A Cry4B Cry10A Cry11A Cyt1Aa Test Substance or Formulation technical (CGA ) Formulation technical formulation Type of Study Acute Oral Toxicity Acute Oral Toxicity Acute Oral Toxicity Acute Oral Toxicity Results (LD 50 ) 20 ml/kg or 10 8 CFU/animal 5050 mg/kg Not toxic Acute Oral-Rat 6.9 x 10 7 spores/kg Findings Not toxic, pathogenic, or infective mg/kg ( x CFU/rat) No acute oral infectivity. Reference 1993 (Ciba) 1993 (Ciba) Unknown Unknown 1986 (Abbott) 14
15 15 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial israelensis israelensis israelensis israelensis israelensis Cry Gene Content Cry4A Cry4B Cry10A Cry11A Cyt1Aa Cry4A Cry4B Cry10A Cry11A Cyt1Aa Cry4A Cry4B Cry10A Cry11A Cyt1Aa Crystal deltaendotoxin Cry4A Cry4B Cry10A Cry11A Cyt1Aa Test Substance or Formulation Type of Study Results (LD 50 ) Acute Oral-Rat 2.67 g/kg Single Oral Administration to Weanling Rats 3-Month Feeding-Rat Oral Administration to Mice Acute Oral- Rabbit Findings > CFM No rats became ill or died; the authors concluded that Bt israelensis is not a virulent or invasive mammalian pathogen. Reference McClintock et al Siegel et al NOEL=4 g/kg/day No toxicity. McClintock et al > 0.5 mg/animal In a series of preliminary toxicity tests with adult and suckling mice, the native subspecies, israelensis crystal deltaendotoxin, produced no pathological effects by oral administration. When administered per os, the alkali-solubilized crystal delta-endotoxin was not toxic to adult (0.5 mg protein/animal) or suckling (200 µg protein/animal) mice 2 x 10 9 No acute oral infectivity. spores/animal Thomas and Ellar 1983 McClintock et al
16 16 (Continued) Table 3. Mammalian Toxicity of Bacillus thuringiensis Microbial Pesticides (Oral Exposure) Bt Microbial israelensis Berliner Cry Gene Content Cry4A Cry4B Cry10A Cry11A Cyt1Aa Cry1B Test Substance or Formulation technical Type of Study Acute Oral- Rabbit 5-Day Human Oral Exposure Results (LD 50 ) Findings Reference 6.28 g/kg No acute oral infectivity (Abbott) NOEL = 1 g of Bt (3 x 10 9 viable spore/gram) in capsules daily for 5 days All subjects remained well during the course of the experiment ( 5 weeks) and all laboratory findings were negative (subjects were evaluated before treatment, after the 5-day treatment period, and 4 to 5 weeks post treatment). Fisher and Rosner
17 17 Table 4. Gene Content of Bacillus thuringiensis Commercial Products Bt Product Organism Cry1Balike Cry1Ca Cry1Da Cry1F a 6 Cry3A Cry3B a Cry9B Cry9- like DiPel 2X X X X X DiPel 6AF X X X X DiPel ES X X X X DiPel ES-NT X X X X Raptor X X X X X Condor G X X X X Condor OF X X X X Foil BFC X X MVP X Biobit FC X X X X Biocot X X X X Foray 48B X X X X Delfin WG X X X X Javelin WG X X X X B1781 X X X X CryMax X X X Raven X X X Lepinox X X X Agree / X X X X X aizawai Design / X X X X X X aizawai Cutlass WP / X X X X X aizawai XenTari aizawai X X X X X X Florbac AF aizawai X X X X X X Novodor FC tenebrionis X X X X X M-1 tenebrionis X Novodor tenebrionis X 17
18 18 IV. REFERENCES Barbera, P.W. October Toxicity/pathogenicity testing of Bacillus thuringiensis strain EG 7826 following acute oral challenge in rats. IIT Research Institute, Chicago, Illinois, IITRI Project No. L Summary only. Carter, J.N., and M.P. Liggett. September 9, Acute oral toxicity and infectivity/pathogenicity to rats of EG7841. Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire, England. HRC Study Report No. ECO 6/ Summary only. Carter, J.N., et al. November 17, Acute oral toxicity and infectivity/pathogenicity to rats of EG7673. Huntingdon Research Centre Ltd., Huntingdon, Cambridgeshire, England. HRC Study Report No. ECO 1/ Summary only. Code of Federal Regulations, Viable spores of the microorganism Bacillus thuringiensis Berliner; exemption from the requirement of a tolerance; 40 CFR Crickmore, N., et al Revision of the nomenclature of Bacillus thuringiensis cry genes, p. 14. In Program and abstracts of the 28 th Annual Meeting of the Society for Invertebrate Pathology, Bethesda, Maryland. David, R.M. 1989a. Acute oral toxicity/pathogenicity study of Foil OF insecticide in rats. Microbiological Associates Inc., Bethesda, Maryland. Unpublished report for Ecogen Inc. MBA Study No. G Summary only. David, R.M. 1989b. Acute oral toxicity/pathogenicity study of Cutlass OF insecticide in rats. Microbiological Associates Inc., Bethesda, Maryland. Unpublished report for Ecogen Inc. MBA Study No. G Summary only. English, L. And Slatin, S.L Mode of action of delta-endotoxins from Bacillus thuringiensis: a comparison with other bacterial toxins. Insect Biochemical Molecular Biology, 22(1):1-7. EPA Guidance for the reregistration of pesticide products containing Bacillus thuringiensis as the active ingredient. Registration Standard 540/RS December, for Bacillus thuringiensis subsp., israelensis, and aizawai. September for Bacillus thuringiensis subsp. aizawai Strain GC November 2, 2001
19 19 EPA memorandum regarding registration of Bacillus thuringiensis delta-endotoxin as produced in corn by a gene (from Janet Andersen to Daniel Barolo). March 20, for Bacillus thuringiensis subsp. Cry1A(c) Delta-Endotoxin and Its Controlling Sequences as Expressed in Cotton. November 22, for Bacillus thuringiensis subsp. Strain EG September for Bacillus thuringiensis subsp. Cry1A(b) Delta-Endotoxin and Its Controlling Sequences as Expressed in Cotton. December 20, Federal Register, Plant Pesticide Bacillus thuringiensis CryIIIA Delta-Endotoxin and the Genetic Material Necessary for Its Production; Tolerance Exemption; Final Rule; 60 FR May 3, Federal Register, Plant Pesticide Bacillus thuringiensis CryIA(b) Delta-Endotoxin and the Genetic Material Necessary for Its Production (Plasmid Vector pcib4431) in Corn; Final Rule; 60 FR August 16, Federal Register, Lepidopteran Pheromones: Tolerance Exemption; Final Rule; 60 FR August 30, Federal Register, CryIA(c) and CryIc Derived Delta Endotoxins of Bacillus thuringiensis Encapsulated in Killed Pseudomonas fluorescens; Exemption from the Requirement of a Tolerance; 60 FR September 13, Federal Register, Plant Pesticide Bacillus thuringiensis CryIA(c) Delta-Endotoxin and the Genetic Material Necessary for Its Production in Cotton; Tolerance Exemption; Final Rule; 60 FR September 15, Federal Register, Bacillus thuringiensis CryIA(b) Delta-Endotoxin and the Genetic Material Necessary for Its Production in All Plants; Exemption from Requirement of a Tolerance; Final Rule; 61 FR August 2, Federal Register, Bacillus thuringiensis subspecies CryIA(c) and the Genetic Material Necessary for Its Production in All Plants; Exemption From the Requirement of a Tolerance on All Raw Agricultural Commodities; Final Rule; 62 FR April 11, Fisher, R. and L. Rosner Toxicology of the microbial insecticide, Thuricide. Agricultural and Food Chemistry, 7 (10):
20 20 Geiser, M., et al The hypervariable region in the genes coding for entomopathogenic crystal proteins of Bacillus thuringiensis: nucleotide sequence of the kurhd1 gene of subsp. HD1. Gene, 48: Hadley, W.M., et al Five-month oral (diet) toxicity/infectivity study of Bacillus thuringiensis insecticides in sheep. Fundamental and Applied Toxicology, 8: Hofte, H. and Whitely, H.R Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev., 53: Lambert, B., et al A Bacillus thuringiensis insecticidal crystal protein with a high activity against members of the family noctuidae. Appl. Environ. Micobiol. 62(1): McClintock, J.T., et al Toxicological evaluation of genetically engineered plant pesticides. In Food Safety Assessment, ACS Symposium Series 484: McClintock, J.T., et al A comparative review of the mammalian toxicity of Bacillus thuringiensis-based pesticides. Pestic. Sci., 45: Metcalfe, D.D., et al Assessment of the Allergenic Potential of Foods Derived from Genetically Engineered Crop Plants. Critical Rev. in Food Science and Nutrition. 36(s):S165-S186. Noteborn, H.P.J.M., et al Food safety of transgenic tomatoes expressing the insecticidal crystal protein from Bacillus thuringiensis and the marker enzyme APH(3 )II. Med. Fac. Landbouww. Univ. Gent, 58/4b. Noteborn, H.P.J.M., et al Consuming transgenic food crops: the toxicological and safety aspects of tomato expressing Cry1b and NPTII. ECB6: Proceedings of the 6 th European Congress on biotechnology. Elsevier Science. Siegel, J.P., et al Safety of the entomopathogen Bacillus thuringiensis var. israelensis for mammals. Journal of Economic Entomology, 80(4): Sjoblad, R.D., et al Toxicological considerations for protein components of biological pesticide products. Regulatory Toxicology and Pharmacology, 15:3-9. Spenser, T.M., et al. October 14, Petition for determination of nonregulated status: insect protected corn (Zea mays L.) with the cry1a(c) gene from Bacillus thuringiensis subsp.. Dekalb Genetics Corporation. 20
21 21 Tailor, R., et al Identification and characterization of a novel Bacillus thuringiensis delta-endotoxin entomocidal to coleopteran and lepidopteran larvae. Mol. Micro. 6(9):1,211-1,217. Thomas, W.E., and D.J. Ellar Bacillus thuringiensis var. israelensis crystal deltaendotoxin: effects on insect and mammalian cells in vitro and in vivo. J. Cell Science, 60: Thompson, M.A., et al Structure, function and engineering of Bacillus thuringiensis toxins. Genetic Engineering, 17:
Safety and Advantages of Bacillus thuringiensis-protected Plants to Control Insect Pests
 Regulatory Toxicology and Pharmacology 32, 156 173 (2000) doi:10.1006/rtph.2000.1426, available online at http://www.idealibrary.com on Safety and Advantages of Bacillus thuringiensis-protected Plants
Regulatory Toxicology and Pharmacology 32, 156 173 (2000) doi:10.1006/rtph.2000.1426, available online at http://www.idealibrary.com on Safety and Advantages of Bacillus thuringiensis-protected Plants
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
MATERIAL FACT SHEET BACILLUS THURINGIENSIS
 MATERIAL FACT SHEET BACILLUS THURINGIENSIS MATERIAL NAME: Bacillus thuringiensis (Bt) MATERIAL TYPE: Microbial-derived U.S. EPA TOXICITY: Category: III, Caution USDA-NOP: considered nonsynthetic, allowed.
MATERIAL FACT SHEET BACILLUS THURINGIENSIS MATERIAL NAME: Bacillus thuringiensis (Bt) MATERIAL TYPE: Microbial-derived U.S. EPA TOXICITY: Category: III, Caution USDA-NOP: considered nonsynthetic, allowed.
- A9/1 - Format for the listing of end points to be included in the Tier III overall summary and assessments
 - A9/1 - - A9/1 - APPENDIX 9 FORMAT FOR THE LISTING OF END POINTS TO BE INCLUDED IN THE REASONED STATEMENT OF THE OVERALL CONCLUSIONS DRAWN BY THE REGULATORY AUTHORITY (LEVEL 2)8 General remark: Testing
- A9/1 - - A9/1 - APPENDIX 9 FORMAT FOR THE LISTING OF END POINTS TO BE INCLUDED IN THE REASONED STATEMENT OF THE OVERALL CONCLUSIONS DRAWN BY THE REGULATORY AUTHORITY (LEVEL 2)8 General remark: Testing
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Aliphatic Alcohols Facts
 United States Prevention, Pesticides EPA 738-F-07-004 Environmental Protection and Toxic Substances June 2007 Agency (7508P) Aliphatic Alcohols Facts Pesticide Registration All pesticides sold or distributed
United States Prevention, Pesticides EPA 738-F-07-004 Environmental Protection and Toxic Substances June 2007 Agency (7508P) Aliphatic Alcohols Facts Pesticide Registration All pesticides sold or distributed
R.E.D. FACTS. Chloroxylenol. Pesticide Reregistration. Use Profile. Regulatory History
 United States Prevention, Pesticides EPA-738-F-94-028 Environmental Protection And Toxic Substances September 1994 Agency (7508W) R.E.D. FACTS Pesticide Reregistration Use Profile Regulatory History Chloroxylenol
United States Prevention, Pesticides EPA-738-F-94-028 Environmental Protection And Toxic Substances September 1994 Agency (7508W) R.E.D. FACTS Pesticide Reregistration Use Profile Regulatory History Chloroxylenol
Overview on EFSA data requirements for the safety evaluation of food enzymes applications
 Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
BIOPESTICIDES REGISTRATION ACTION DOCUMENT
 BIOPESTICIDES REGISTRATION ACTION DOCUMENT Modified Cry3A Protein and the Genetic Material Necessary for its Production (Via Elements of pzm26) in Event MIR604 Corn SYN-IR604-8 U.S. Environmental Protection
BIOPESTICIDES REGISTRATION ACTION DOCUMENT Modified Cry3A Protein and the Genetic Material Necessary for its Production (Via Elements of pzm26) in Event MIR604 Corn SYN-IR604-8 U.S. Environmental Protection
TESTIMONY OF DR. STEVEN BRADBURY DIRECTOR, OFFICE OF PESTICIDE PROGRAMS U.S. ENVIRONMENTAL PROTECTION AGENCY
 TESTIMONY OF DR. STEVEN BRADBURY DIRECTOR, OFFICE OF PESTICIDE PROGRAMS U.S. ENVIRONMENTAL PROTECTION AGENCY BEFORE THE SUBCOMMITTEE ON NUTRITION AND HORTICULTURE OF THE AGRICULTURE COMMITTEE AND SUBCOMMITTEE
TESTIMONY OF DR. STEVEN BRADBURY DIRECTOR, OFFICE OF PESTICIDE PROGRAMS U.S. ENVIRONMENTAL PROTECTION AGENCY BEFORE THE SUBCOMMITTEE ON NUTRITION AND HORTICULTURE OF THE AGRICULTURE COMMITTEE AND SUBCOMMITTEE
DATA EVALUATION REPORT. STUDY TYPE: Acute Toxicity Study- Earthworm (OECD Guideline 207)
 Reviewer: Gail Tomimatsu, Ph.D. Date: Microbial Pesticides Branch Secondary Reviewer: Zigfridas Vaituzis, Ph.D. Microbial Pesticides Branch Date: Biopesticides and Pollution Prevention Division Phil Hutton,
Reviewer: Gail Tomimatsu, Ph.D. Date: Microbial Pesticides Branch Secondary Reviewer: Zigfridas Vaituzis, Ph.D. Microbial Pesticides Branch Date: Biopesticides and Pollution Prevention Division Phil Hutton,
1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Conclusion No cytotoxicity can be detected at concentrations up to 100%.
 Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
- A4/1 - APPENDIX 4. FORMAT FOR COMPILATION OF Tier I QUALITY CHECKS PART 1
 - A4/1 - APPENDIX 4 FORMAT FOR COMPILATION OF Tier I QUALITY CHECKS PART 1 SUMMARY REPORT - APPROPRIATE FOR STUDIES CONDUCTED IN ACCORDANCE WITH THE TEST GUIDELINES CURRENTLY SPECIFIED EXAMPLE 1 1. Data
- A4/1 - APPENDIX 4 FORMAT FOR COMPILATION OF Tier I QUALITY CHECKS PART 1 SUMMARY REPORT - APPROPRIATE FOR STUDIES CONDUCTED IN ACCORDANCE WITH THE TEST GUIDELINES CURRENTLY SPECIFIED EXAMPLE 1 1. Data
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Pest Control Products Board Nairobi, Kenya PESTICIDE REGISTRATION IN KENYA - BIOPESTICIDES. by P. N. Ngaruiya (Dr)
 Pest Control Products Board Nairobi, Kenya PESTICIDE REGISTRATION IN KENYA - BIOPESTICIDES by P. N. Ngaruiya (Dr) Introduction Pesticides, an indispensable tool in farming Chemical pesticides such as the
Pest Control Products Board Nairobi, Kenya PESTICIDE REGISTRATION IN KENYA - BIOPESTICIDES by P. N. Ngaruiya (Dr) Introduction Pesticides, an indispensable tool in farming Chemical pesticides such as the
Absorption of Drugs. Transport of a drug from the GI tract
 Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
Absorption of Drugs Absorption is the transfer of a drug from its site of administration to the bloodstream. The rate and efficiency of absorption depend on the route of administration. For IV delivery,
BIOTECHNOLOGY OPERATIONS
 BIOTECHNOLOGY OPERATIONS Principles and Practices Michael J. Roy TECHNISCHE INFORMATION SBIBLIOTHEK UNIVERSITATSBIBLIOTHEK HANNOVER CRC Press TaylorStFrancis Croup Boca Raton London New York CRC Press
BIOTECHNOLOGY OPERATIONS Principles and Practices Michael J. Roy TECHNISCHE INFORMATION SBIBLIOTHEK UNIVERSITATSBIBLIOTHEK HANNOVER CRC Press TaylorStFrancis Croup Boca Raton London New York CRC Press
WHY IS THIS IMPORTANT?
 CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
CHAPTER 1 WHAT IS MICROBIOLOGY AND WHY IS IT IMPORTANT? WHO / TDR / Crump WHY IS THIS IMPORTANT? Microbiology is more relevant than ever in today s world. Infectious diseases are a leading health-related
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
September 19, 1984 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM
 Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
Glyphosate Levels in Breakfast Foods: What is safe?
 Glyphosate Levels in Breakfast Foods: What is safe? The Alliance for Natural Health USA April 19, 2016 Glyphosate is the active ingredient in the world s most widely used herbicide, its use being largely
Glyphosate Levels in Breakfast Foods: What is safe? The Alliance for Natural Health USA April 19, 2016 Glyphosate is the active ingredient in the world s most widely used herbicide, its use being largely
Registration Decision. Sulfoxaflor
 Registration Decision RD2015-09 Sulfoxaflor (publié aussi en français) 8 June 2015 This document is published by the Health Canada Pest Management Regulatory Agency. For further information, please contact:
Registration Decision RD2015-09 Sulfoxaflor (publié aussi en français) 8 June 2015 This document is published by the Health Canada Pest Management Regulatory Agency. For further information, please contact:
FACULTY OF MEDICAL SCIENCE
 Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Bacillus thuringiensis israelensis (Bti) in drinking-water. Background document for development of WHO Guidelines for Drinking-water Quality
 Bacillus thuringiensis israelensis (Bti) in drinking-water Background document for development of WHO Guidelines for Drinking-water Quality 1 Bacillus thuringiensis israelensis (Bti) in drinking-water
Bacillus thuringiensis israelensis (Bti) in drinking-water Background document for development of WHO Guidelines for Drinking-water Quality 1 Bacillus thuringiensis israelensis (Bti) in drinking-water
Genetic Engineering and Biotechnology
 1 So, what is biotechnology?? The use of living organisms to carry out defined chemical processes for industrial or commercial application. The office of Technology Assessment of the U.S. Congress defines
1 So, what is biotechnology?? The use of living organisms to carry out defined chemical processes for industrial or commercial application. The office of Technology Assessment of the U.S. Congress defines
Module Three. Risk Assessment
 Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
Module Three Risk Assessment 136 Module Three Introduction to Risk Assessment Time Allotted: 90 Minutes Objectives: Upon completion of this module, the learner will be able to # Define and understand the
Chapter 12: SPECIFIC TARGET ORGAN SYSTEMIC TOXICITY (TOST) FOLLOWING A SINGLE EXPOSURE
 Chapter 12: SPECIFIC TARGET ORGAN SYSTEMIC TOXICITY (TOST) FOLLOWING A SINGLE EXPOSURE DEFINITIONS 1. Classification identifies the chemical substance as being a specific target organ/systemic toxicant
Chapter 12: SPECIFIC TARGET ORGAN SYSTEMIC TOXICITY (TOST) FOLLOWING A SINGLE EXPOSURE DEFINITIONS 1. Classification identifies the chemical substance as being a specific target organ/systemic toxicant
Lecture 13: DNA Technology. DNA Sequencing. DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology
 Lecture 13: DNA Technology DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology DNA Sequencing determine order of nucleotides in a strand of DNA > bases = A,
Lecture 13: DNA Technology DNA Sequencing Genetic Markers - RFLPs polymerase chain reaction (PCR) products of biotechnology DNA Sequencing determine order of nucleotides in a strand of DNA > bases = A,
The MON863 case - a chronicle of systematic deception
 The MON863 case - a chronicle of systematic deception August 13, 2002: The Monsanto company submits to the German authorities an application to import genetically engineered MON863 maize into the EU. This
The MON863 case - a chronicle of systematic deception August 13, 2002: The Monsanto company submits to the German authorities an application to import genetically engineered MON863 maize into the EU. This
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA
 CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
CHAPTER 6: RECOMBINANT DNA TECHNOLOGY YEAR III PHARM.D DR. V. CHITRA INTRODUCTION DNA : DNA is deoxyribose nucleic acid. It is made up of a base consisting of sugar, phosphate and one nitrogen base.the
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Safety Assessment of Bollgard II Cotton Event 15985
 Executive Summary Safety Assessment of Bollgard II Cotton Event 15985 Bollgard II cotton event 15985 was developed by Monsanto Company to produce the Cry2Ab2 insect control protein, which provides effective
Executive Summary Safety Assessment of Bollgard II Cotton Event 15985 Bollgard II cotton event 15985 was developed by Monsanto Company to produce the Cry2Ab2 insect control protein, which provides effective
Production of antigens and antibodies in plants: alternative technology?
 Production of antigens and antibodies in plants: alternative technology? George Lomonossoff John Innes Centre Norwich, UK ECOPA, Alicante 29 th Sept. 2006 Why use Plants as Biofactories? Produce large
Production of antigens and antibodies in plants: alternative technology? George Lomonossoff John Innes Centre Norwich, UK ECOPA, Alicante 29 th Sept. 2006 Why use Plants as Biofactories? Produce large
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Recombinant DNA and Biotechnology
 Recombinant DNA and Biotechnology Chapter 18 Lecture Objectives What Is Recombinant DNA? How Are New Genes Inserted into Cells? What Sources of DNA Are Used in Cloning? What Other Tools Are Used to Study
Recombinant DNA and Biotechnology Chapter 18 Lecture Objectives What Is Recombinant DNA? How Are New Genes Inserted into Cells? What Sources of DNA Are Used in Cloning? What Other Tools Are Used to Study
Adjuvants and Excipients
 Adjuvants and Excipients The Food Safety and Inspection Service (FSIS) transferred the official responsibility to evaluate adjuvants and excipients used in Veterinary Biologics back to the Animal and Plant
Adjuvants and Excipients The Food Safety and Inspection Service (FSIS) transferred the official responsibility to evaluate adjuvants and excipients used in Veterinary Biologics back to the Animal and Plant
A REVIEW OF GENERAL ACUTE TOXICITY STUDIES OF INCIDE PEST CONTROL INSULATION
 A REVIEW OF GENERAL ACUTE TOXICITY STUDIES OF INCIDE PEST CONTROL INSULATION Prepared For INCIDE TECHNOLOGIES, INC 50 North 41 st Ave. Phoenix, AZ 85009 June 8, 1998 Richard C. Pleus, Ph.D. INTERTOX, INC.
A REVIEW OF GENERAL ACUTE TOXICITY STUDIES OF INCIDE PEST CONTROL INSULATION Prepared For INCIDE TECHNOLOGIES, INC 50 North 41 st Ave. Phoenix, AZ 85009 June 8, 1998 Richard C. Pleus, Ph.D. INTERTOX, INC.
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
DNA Technology Mapping a plasmid digesting How do restriction enzymes work?
 DNA Technology Mapping a plasmid A first step in working with DNA is mapping the DNA molecule. One way to do this is to use restriction enzymes (restriction endonucleases) that are naturally found in bacteria
DNA Technology Mapping a plasmid A first step in working with DNA is mapping the DNA molecule. One way to do this is to use restriction enzymes (restriction endonucleases) that are naturally found in bacteria
One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 1640 1644, March 1997 Agricultural Sciences One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins BRUCE E. TABASHNIK*, YONG-BIAO
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 1640 1644, March 1997 Agricultural Sciences One gene in diamondback moth confers resistance to four Bacillus thuringiensis toxins BRUCE E. TABASHNIK*, YONG-BIAO
US Environmental Protection Agency Office of Pesticide Programs
 US Environmental Protection Agency Office of Pesticide Programs BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene (PC Code 224459) BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene
US Environmental Protection Agency Office of Pesticide Programs BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene (PC Code 224459) BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
402 Adopted: 24 Feb 1987
 OECD GUIDELINE FOR TESTING OF CHEMICALS Adopted: 24 Feb 1987 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid or liquid test substance Chemical identification of test substance
OECD GUIDELINE FOR TESTING OF CHEMICALS Adopted: 24 Feb 1987 1. I N T R O D U C T O R Y I N F O R M A T I O N P r e r e q u i s i t e s Solid or liquid test substance Chemical identification of test substance
Pest Management Regulatory Agency
 Pest Management Regulatory Agency Overview Document Introduction The Pest Management Regulatory Agency (PMRA) was established in April 1995 in response to the recommendations of the Pesticide Registration
Pest Management Regulatory Agency Overview Document Introduction The Pest Management Regulatory Agency (PMRA) was established in April 1995 in response to the recommendations of the Pesticide Registration
Integrated Pest Management
 Chapter 2 Integrated Pest Management In This Chapter Keywords After learning the information in this chapter, you will be able to: 1. Define Integrated Pest Management (IPM). 2. List and describe the 5
Chapter 2 Integrated Pest Management In This Chapter Keywords After learning the information in this chapter, you will be able to: 1. Define Integrated Pest Management (IPM). 2. List and describe the 5
OFF! CLIP-ON MOSQUITO REPELLENT
 1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and Son, Limited 1 Webster Street Brantford ON N3T 5R1
1. PRODUCT AND COMPANY IDENTIFICATION Product information Trade name : Use of the : Insect Repellent Substance/Preparation Company : S.C. Johnson and Son, Limited 1 Webster Street Brantford ON N3T 5R1
Manufacturing process of biologics
 Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
Manufacturing process of biologics K. Ho Afssaps, France 2011 ICH International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2011 ICH 1 Disclaimer:
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Recommended Dose Volumes for Common Laboratory Animals IQ 3Rs Leadership Group - Contract Research Organization Working Group
 NOTE: This document includes dose volume guidelines that have been researched and published as well as standards that have gained acceptance through empirical use across multiple members of the IQ 3Rs
NOTE: This document includes dose volume guidelines that have been researched and published as well as standards that have gained acceptance through empirical use across multiple members of the IQ 3Rs
POTENTIAL HAZARD INFORMATION & SIGNATURE SHEET
 POTENTIAL HAZARD INFORMATION & SIGNATURE SHEET Scientific research involves exposure to various hazards. When deciding to allow your child to participate in research projects conducted in University of
POTENTIAL HAZARD INFORMATION & SIGNATURE SHEET Scientific research involves exposure to various hazards. When deciding to allow your child to participate in research projects conducted in University of
Biotechnology and its Applications
 Chapter 12 Biotechnology and its Applications IMPORTANT TERMS 1. Biotechnology: It is a branch of science that deals with industrial scale production of biopharmaceuticals and biological using genetically
Chapter 12 Biotechnology and its Applications IMPORTANT TERMS 1. Biotechnology: It is a branch of science that deals with industrial scale production of biopharmaceuticals and biological using genetically
The Science of Chemical Safety Essential Toxicology - 4. Hazard and Risk. John Duffus & Howard Worth. IUPAC Educators Resource Material IUPAC
 The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (JECFA)
 Food and Agriculture Organization of the United Nations World Health Organization JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (JECFA) PROCEDURES FOR RECOMMENDING MAXIMUM RESIDUE LIMITS- RESIDUES OF
Food and Agriculture Organization of the United Nations World Health Organization JOINT FAO/WHO EXPERT COMMITTEE ON FOOD ADDITIVES (JECFA) PROCEDURES FOR RECOMMENDING MAXIMUM RESIDUE LIMITS- RESIDUES OF
Principles of Disease and Epidemiology. Copyright 2010 Pearson Education, Inc.
 Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
Probiotics for the Treatment of Adult Gastrointestinal Disorders
 Probiotics for the Treatment of Adult Gastrointestinal Disorders Darren M. Brenner, M.D. Division of Gastroenterology Northwestern University, Feinberg School of Medicine Chicago, Illinois What are Probiotics?
Probiotics for the Treatment of Adult Gastrointestinal Disorders Darren M. Brenner, M.D. Division of Gastroenterology Northwestern University, Feinberg School of Medicine Chicago, Illinois What are Probiotics?
How To Use An Antibody
 GUIDELINES FOR THE PRODUCTION OF ANTIBODIES IN LABORATORY ANIMALS Table of Contents 1. Purpose 2. Choice of Species and Strain 3. Immunizing Antigen 4. Procedures for Polyclonal Antibody Production 5.
GUIDELINES FOR THE PRODUCTION OF ANTIBODIES IN LABORATORY ANIMALS Table of Contents 1. Purpose 2. Choice of Species and Strain 3. Immunizing Antigen 4. Procedures for Polyclonal Antibody Production 5.
Taming Major Maize Field Pests in Kenya: The Role of Biotechnology
 Taming Major Maize Field Pests in Kenya: The Role of Biotechnology Stephen Mugo and Simon Gichuki Presentation to OFAB, Jacaranda Hotel, Nairobi, Kenya, 30 th July 2009 Presentation made to STAK in support
Taming Major Maize Field Pests in Kenya: The Role of Biotechnology Stephen Mugo and Simon Gichuki Presentation to OFAB, Jacaranda Hotel, Nairobi, Kenya, 30 th July 2009 Presentation made to STAK in support
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Background Note on Bt Cotton Cultivation in India
 Background Note on Bt Cotton Cultivation in India Under the Environment Protection Act (1986), the Ministry of Environment & Forests, has notified the Rules for the Manufacture, Use, Import, Export and
Background Note on Bt Cotton Cultivation in India Under the Environment Protection Act (1986), the Ministry of Environment & Forests, has notified the Rules for the Manufacture, Use, Import, Export and
Potential synergies that can enhance Bt toxicity in SmartStax
 TESTBIOTECH Background 28-6 - 2011 Potential synergies that can enhance Bt toxicity in SmartStax Analyses of Levine et al., 2008a and MacRae 2008 Report Number MSL0021104 and MSL 0020554 Christoph Then
TESTBIOTECH Background 28-6 - 2011 Potential synergies that can enhance Bt toxicity in SmartStax Analyses of Levine et al., 2008a and MacRae 2008 Report Number MSL0021104 and MSL 0020554 Christoph Then
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
May 31, 2002 CERTIFIED MAIL. Ronald Landis, Ph.D. Landis International 3185 Madison Highway PO Box 5126 Valdosta, GA 31603-5126. Dear Mr.
 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES May 31, 2002 CERTIFIED MAIL Ronald Landis, Ph.D. Landis International 3185 Madison
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES May 31, 2002 CERTIFIED MAIL Ronald Landis, Ph.D. Landis International 3185 Madison
Guide to U.S. Regulation of Genetically Modified Food and Agricultural Biotechnology Products
 Pew Initiative on Food and Biotechnology Executive Summary Guide to U.S. Regulation of Genetically Modified Food and Agricultural Biotechnology Products The products of biotechnology 1 are regulated under
Pew Initiative on Food and Biotechnology Executive Summary Guide to U.S. Regulation of Genetically Modified Food and Agricultural Biotechnology Products The products of biotechnology 1 are regulated under
7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology)
 7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 44 credit hours: 1- Compulsory courses: 14 credit hours. 1504801, 1504802, 1504803,
7- Doctoral Degree in Public Health and Public Health Sciences (Majoring Microbiology) Students should fulfill a total of 44 credit hours: 1- Compulsory courses: 14 credit hours. 1504801, 1504802, 1504803,
8. REGULATIONS AND ADVISORIES
 ARSENIC 395 The international and national regulations and guidelines pertaining to arsenic and its metabolites in air, water, and other media are summarized in Table 8-1. ATSDR has not derived inhalation
ARSENIC 395 The international and national regulations and guidelines pertaining to arsenic and its metabolites in air, water, and other media are summarized in Table 8-1. ATSDR has not derived inhalation
Tungsten Heavy Alloy. Densimet, Mallory Metal, Densalloy, High Density Tungsten Alloy
 Safety Data Sheet SECTION 1: IDENTIFICATION Chemical Name: Synonyms: CAS #: Tungsten Heavy Alloy Densimet, Mallory Metal, Densalloy, High Density Tungsten Alloy 7440-33-7 (Tungsten), 7440-02-0 (Nickel),
Safety Data Sheet SECTION 1: IDENTIFICATION Chemical Name: Synonyms: CAS #: Tungsten Heavy Alloy Densimet, Mallory Metal, Densalloy, High Density Tungsten Alloy 7440-33-7 (Tungsten), 7440-02-0 (Nickel),
The US EPA s analysis of Bt crops finds that they pose no significant risk to the environment or to human health.
 Are Bt crops safe? Mike Mendelsohn, John Kough, Zigfridais Vaituzis & Keith Matthews The US EPA s analysis of Bt crops finds that they pose no significant risk to the environment or to human health. Bacillus
Are Bt crops safe? Mike Mendelsohn, John Kough, Zigfridais Vaituzis & Keith Matthews The US EPA s analysis of Bt crops finds that they pose no significant risk to the environment or to human health. Bacillus
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use EMEA/MRL/717/99-FINAL December 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TOLDIMFOS SUMMARY
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use EMEA/MRL/717/99-FINAL December 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TOLDIMFOS SUMMARY
2) Macrophages function to engulf and present antigen to other immune cells.
 Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
Immunology The immune system has specificity and memory. It specifically recognizes different antigens and has memory for these same antigens the next time they are encountered. The Cellular Components
The Use of Enzymes in Dietary Supplements USP Enzyme Workshop July 8, 2009
 The Use of Enzymes in Dietary Supplements USP Enzyme Workshop July 8, 2009 Nena Dockery Introduction and History of Use Commercial production of enzymes for use as digestive aids has an extensive history
The Use of Enzymes in Dietary Supplements USP Enzyme Workshop July 8, 2009 Nena Dockery Introduction and History of Use Commercial production of enzymes for use as digestive aids has an extensive history
Risk Assessment in Chemical Food Safety. Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/
 Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Safety Data Sheet Deodorant Roll-On Aluminium Free. 1. Identification
 1. Identification 1.1. Product identifier Product Identity Alternate Names Product Code: 754 1.2. Relevant identified uses of the substance or mixture and uses advised against Intended use Deodorant Application
1. Identification 1.1. Product identifier Product Identity Alternate Names Product Code: 754 1.2. Relevant identified uses of the substance or mixture and uses advised against Intended use Deodorant Application
Risk Assessment for Food Safety in Hong Kong
 TECHNICAL TRAINING ON RISK ANALYSIS FOR Delhi, SAARC India, COUNTRIES June 17-21, 2013 FAO RAP, Bangkok, Thailand Quality Council of India Risk Assessment for Food Safety in Hong Kong Dr CHOW chor-yiu
TECHNICAL TRAINING ON RISK ANALYSIS FOR Delhi, SAARC India, COUNTRIES June 17-21, 2013 FAO RAP, Bangkok, Thailand Quality Council of India Risk Assessment for Food Safety in Hong Kong Dr CHOW chor-yiu
Medical Microbiology Culture Media :
 Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
Lecture 3 Dr. Ismail I. Daood Medical Microbiology Culture Media : Culture media are used for recognition and identification (diagnosis) of microorganisms. The media are contained in plates (Petri dishes),
Omega-3 fatty acids improve the diagnosis-related clinical outcome. Critical Care Medicine April 2006;34(4):972-9
 Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
U.S. Environmental Protection Agency 1. Tom Moriarty Office of Pesticide Programs U. S. Environmental Protection Agency.
 U.S. Environmental Protection Agency 1 Tom Moriarty Office of Pesticide Programs U. S. Environmental Protection Agency. 1 U.S. Environmental Protection Agency 2 Bee Health Multiple federal reports have
U.S. Environmental Protection Agency 1 Tom Moriarty Office of Pesticide Programs U. S. Environmental Protection Agency. 1 U.S. Environmental Protection Agency 2 Bee Health Multiple federal reports have
CUSTOM ANTIBODIES. Fully customised services: rat and murine monoclonals, rat and rabbit polyclonals, antibody characterisation, antigen preparation
 CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
GENETICALLY MODIFIED ORGANISMS: THE FACTS
 GENETICALLY MODIFIED ORGANISMS: THE FACTS Sydney Hayter February 28, 2015 What is today about? Clearly define genetic modification Public perception State facts on genetically modified organisms Unbiased
GENETICALLY MODIFIED ORGANISMS: THE FACTS Sydney Hayter February 28, 2015 What is today about? Clearly define genetic modification Public perception State facts on genetically modified organisms Unbiased
Hapten - a small molecule that is antigenic but not (by itself) immunogenic.
 Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
Chapter 3. Antigens Terminology: Antigen: Substances that can be recognized by the surface antibody (B cells) or by the TCR (T cells) when associated with MHC molecules Immunogenicity VS Antigenicity:
Mammalian digestive tracts
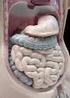 Mammalian digestive tracts Mouth: mastication, some digestive enzymes Esophagus: simple transport tube Stomach: most initial digestion, some physical processing Small intestine: digestion continues, some
Mammalian digestive tracts Mouth: mastication, some digestive enzymes Esophagus: simple transport tube Stomach: most initial digestion, some physical processing Small intestine: digestion continues, some
Procedure for Visitors in UIC Laboratories
 Procedure for Visitors in UIC Laboratories 1129 S. Hermitage, Chicago, IL 60612-7217 Office: (312) 996-7411 OBJECTIVE In order to protect the Principal Investigator (PI) and the University of Illinois
Procedure for Visitors in UIC Laboratories 1129 S. Hermitage, Chicago, IL 60612-7217 Office: (312) 996-7411 OBJECTIVE In order to protect the Principal Investigator (PI) and the University of Illinois
What are the causes of air Pollution
 What are the causes of air Pollution Pollutant Particulate Matter (PM-PM 10 and PM 2.5 ) Description and main UK sources Particulate Matter is generally categorised on the basis of the size of the particles
What are the causes of air Pollution Pollutant Particulate Matter (PM-PM 10 and PM 2.5 ) Description and main UK sources Particulate Matter is generally categorised on the basis of the size of the particles
GENE CLONING AND RECOMBINANT DNA TECHNOLOGY
 GENE CLONING AND RECOMBINANT DNA TECHNOLOGY What is recombinant DNA? DNA from 2 different sources (often from 2 different species) are combined together in vitro. Recombinant DNA forms the basis of cloning.
GENE CLONING AND RECOMBINANT DNA TECHNOLOGY What is recombinant DNA? DNA from 2 different sources (often from 2 different species) are combined together in vitro. Recombinant DNA forms the basis of cloning.
MATERIAL SAFETY DATA SHEET MSDS No.: 007
 PRODUCT AND COMPANY INFORMATION SECTION 1 Water-Jel Technologies 50 Broad Street Manufacturer/Distributor Carlstadt, NJ 07072 201-507-8300 800-275-3433 Product Name: Synonyms: Topical Cream, Burn Cream
PRODUCT AND COMPANY INFORMATION SECTION 1 Water-Jel Technologies 50 Broad Street Manufacturer/Distributor Carlstadt, NJ 07072 201-507-8300 800-275-3433 Product Name: Synonyms: Topical Cream, Burn Cream
Formulation of bio-pesticides and mass culture of natural enemies for pest management. D. Ahangama
 Formulation of bio-pesticides and mass culture of natural enemies for pest management D. Ahangama Bio-pesticides Microbial pesticides Fungi, Bacteria, Viruses, Protozoa, Nematodes Biochemical Substances
Formulation of bio-pesticides and mass culture of natural enemies for pest management D. Ahangama Bio-pesticides Microbial pesticides Fungi, Bacteria, Viruses, Protozoa, Nematodes Biochemical Substances
Enzymes: Practice Questions #1
 Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
Enzymes: Practice Questions #1 1. Compound X increases the rate of the reaction below. Compound X is most likely A. an enzyme B. a lipid molecule C. an indicator D. an ADP molecule 2. The equation below
Bt toxin is released in root exudates from 12 transgenic corn hybrids representing three transformation events
 Soil Biology & Biochemistry 34 (2002) 133±137 Short communication Bt toxin is released in root exudates from 12 transgenic corn hybrids representing three transformation events D. Saxena a, S. Flores b,
Soil Biology & Biochemistry 34 (2002) 133±137 Short communication Bt toxin is released in root exudates from 12 transgenic corn hybrids representing three transformation events D. Saxena a, S. Flores b,
10. T and B cells are types of a. endocrine cells. c. lymphocytes. b. platelets. d. complement cells.
 Virus and Immune System Review Directions: Write your answers on a separate piece of paper. 1. Why does a cut in the skin threaten the body s nonspecific defenses against disease? a. If a cut bleeds, disease-fighting
Virus and Immune System Review Directions: Write your answers on a separate piece of paper. 1. Why does a cut in the skin threaten the body s nonspecific defenses against disease? a. If a cut bleeds, disease-fighting
DIGESTION is the physical and
 Digestion DIGESTION is the physical and chemical breakdown of feeds as they pass through the gastrointestinal tract. The structures of the gastrointestinal tract include the mouth, the esophagus, the stomach,
Digestion DIGESTION is the physical and chemical breakdown of feeds as they pass through the gastrointestinal tract. The structures of the gastrointestinal tract include the mouth, the esophagus, the stomach,
PURDUE PESTICIDE PROGRAMS
 PPP-40 PURDUE PESTICIDE PROGRAMS Purdue University Cooperative Extension Service PESTICIDE TOXICOLOGY Evaluating Safety and Risk Fred Whitford, Coordinator, Purdue Pesticide Programs Tom Fuhremann, Director
PPP-40 PURDUE PESTICIDE PROGRAMS Purdue University Cooperative Extension Service PESTICIDE TOXICOLOGY Evaluating Safety and Risk Fred Whitford, Coordinator, Purdue Pesticide Programs Tom Fuhremann, Director
Bifenthrin 41 5.2 BIFENTHRIN (178)
 Bifenthrin 41 5.2 BIFENTHRIN (178) TOXICOLOGY Bifenthrin is the International Organization for Standardization (ISO) approved name for 2-methyl-3- phenylphenyl) methyl (1RS, 3RS)-3-[(Z)-2-chloro-3, 3,
Bifenthrin 41 5.2 BIFENTHRIN (178) TOXICOLOGY Bifenthrin is the International Organization for Standardization (ISO) approved name for 2-methyl-3- phenylphenyl) methyl (1RS, 3RS)-3-[(Z)-2-chloro-3, 3,
Why Genetically Engineered (GE) Foods should be Labeled: Inadequate Regulations, Unanswered Safety Questions
 Why Genetically Engineered (GE) Foods should be Labeled: Inadequate Regulations, Unanswered Safety Questions Michael Hansen, Ph.D. Senior Scientist, Consumers Union Consumer Issues Conference 2014 Food:
Why Genetically Engineered (GE) Foods should be Labeled: Inadequate Regulations, Unanswered Safety Questions Michael Hansen, Ph.D. Senior Scientist, Consumers Union Consumer Issues Conference 2014 Food:
MATERIAL SAFETY DATA SHEET Utrecht Gesso Painting Grounds. Section 2 Hazard Identification (composition / information on ingredients)
 Page 1 of 6 MATERIAL SAFETY DATA SHEET Utrecht Gesso Painting Grounds MSDS 908.4 Date: April 27, 2014 Information: 800-223-9132 or: 609-409-8001 Section 1 Company and Product Identification Utrecht Art
Page 1 of 6 MATERIAL SAFETY DATA SHEET Utrecht Gesso Painting Grounds MSDS 908.4 Date: April 27, 2014 Information: 800-223-9132 or: 609-409-8001 Section 1 Company and Product Identification Utrecht Art
Animal Pharming: The Industrialization of Transgenic Animals December 1999
 Animal Pharming: The Industrialization of Transgenic Animals December 1999 Animal pharming, the process of using transgenic animals to produce human drugs, is staking its claim in a lucrative world market.
Animal Pharming: The Industrialization of Transgenic Animals December 1999 Animal pharming, the process of using transgenic animals to produce human drugs, is staking its claim in a lucrative world market.
