Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD)
|
|
|
- Joseph Stanley
- 7 years ago
- Views:
Transcription
1 21 June 2012 EMA/CHMP/483572/2012 -corr 1 Respiratory Drafting Group Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD) Draft Agreed by Efficacy Working Party (EWP) 6 July 2010 Adopted by CHMP for release for consultation 22 July 2010 Start of public consultation 29 September 2010 End of consultation (deadline for comments) 1 March 2011 Agreed by Respiratory Drafting Group February 2012 Adopted by CHMP 9 July 2012 Date for coming into effect 1 September 2012 This guideline replaces Points to Consider CPMP/EWP/562/98, 19 May 1999 Keywords Chronic obstructive pulmonary disease (COPD), airflow obstruction, relief of symptoms, maintenance treatment, exacerbations. 1 correction related to a change on p. 4 7 Westferry Circus Canary Wharf London E14 4HB United Kingdom Telephone +44 (0) Facsimile +44 (0) info@ema.europa.eu Website An agency of the European Union European Medicines Agency, Reproduction is authorised provided the source is acknowledged.
2 Guideline on clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD) Table of contents Executive summary Introduction Scope Legal basis and relevant guidelines Main text Patient characteristics and selection of patients Methods to assess efficacy Efficacy endpoints Strategy and design of clinical trials Early studies Therapeutic confirmatory trials Safety Specific safety concerns Extent of exposure and long-term safety data /17
3 Executive summary This guideline is a revision of the CPMP Points to Consider on Clinical Investigation of Medicinal Products in the Chronic Treatment of Patients with Chronic Obstructive Pulmonary Disease (COPD) (CPMP/EWP562/98). It is intended to update this previous guidance with new scientific knowledge of the disease as stated in new and updated clinical guidelines and to revise the requirements for the clinical investigation of medicinal products for the treatment of COPD accordingly. The requirements for pivotal studies, consideration of the need for reversibility testing of pulmonary function in patients with COPD, primary and secondary efficacy variables in clinical studies and the potential role of biomarkers have been updated. 1. Introduction COPD is a preventable respiratory disorder characterised by airflow limitation, which is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response in the lungs to noxious particles or gases, primarily caused by cigarette smoking. Although COPD affects the lungs it is also associated with significant systemic consequences. It is estimated that approximately eight percent of the population have COPD and approximately ten percent of those over 40 years of age. However the true prevalence of the disease is likely to be higher than this due to under-diagnosis and diagnosis delayed until the disease becomes clinically apparent and is then moderately advanced. COPD is the fourth leading cause of death in Europe and is expected to rise to third by Worldwide, cigarette smoking (both current and past smoking) is the most commonly encountered risk factor for COPD. COPD is characterised by chronic inflammation associated with remodelling of the airway, lung parenchyma and pulmonary arteries, which in turn, give rise to the pathophysiological findings in COPD mucus hypersecretion and cilliary dysfunction, airflow limitation and hyperinflation, gas exchange abnormalities, pulmonary hypertension and systemic effects. The chronic airflow limitation seen in COPD is caused by a mixture of small airway disease (chronic obstructive bronchitis) and parenchymal destruction (emphysema), with the relative contribution of each varying from person to person. COPD is a heterogeneous disease in terms of its clinical presentation, disease severity and rate of disease progression. Some patients have few complaints but an extremely sedentary lifestyle; others describe chronic respiratory symptoms (e.g. dyspnoea on exertion and cough); some patients present with an acute exacerbation (e.g. wheezing, cough and dyspnoea). Intermittent exacerbations of COPD, which represent an exacerbation of the inflammatory response, can be caused by exposure to infection (viral, bacterial) or to environmental pollutants. Weight loss, nutritional abnormalities, skeletal muscle dysfunction, cardiovascular effects, anaemia, systemic inflammation, mental dysfunction are well-recognised extrapulmonary symptoms and signs of COPD. Patients with COPD are at increased risk of myocardial infarction, angina, respiratory infections, osteoporosis, bone fractures, diabetes, depression, sleep disorders, glaucoma and anaemia. COPD and its comorbidities cannot be cured and therefore must be treated on a chronic basis. The ultimate goal of treatment in COPD is to improve overall survival. Although much of the damage is irreversible at the time of clinical presentation, early diagnosis and appropriate management can prevent and improve symptoms (particularly dyspnoea), reduce the frequency and severity of exacerbations, improve health status and improve exercise capacity. At present no treatment has been shown to modify the rate of decline in lung function or to improve overall survival apart from smoking cessation. 3/17
4 Since the disease is usually progressive, the overall approach to managing stable COPD involves a stepwise increase in treatment, depending on the severity of the disease. The most important step in treating COPD is to encourage smoking cessation. Pharmacological therapies and non-pharmacological therapies should be added in a stepwise fashion depending on the severity of the disease and the clinical status of the patient. The mainstays of drug therapy for symptomatic relief in stable COPD are bronchodilators (primarily β 2 agonists, anticholinergics and less often theophylline) and mucolytics and in more severe cases, long-acting β 2 agonists (LABA) or long-acting muscarinic antagonists (LAMA) 2 with or without inhaled corticosteroids (ICS) concomitantly or in combination. Recently, antiinflammatory drugs with a new mechanism of action e.g. PDE-4 inhibitors, have been shown to have a role in severe COPD. Triple therapy (i.e. LABA + ICS + an anticholinergic) might also be considered for certain patients. Supplemental therapies, such as oxygen, pulmonary rehabilitation and physiotherapy, immunisations, nutrition and exercise also play an important role in the management of COPD. 2. Scope This document is intended to provide guidance for the clinical evaluation of new medicinal products for the treatment of COPD, new products which may provide symptomatic relief through improvement of airway obstruction, which may modify or prevent exacerbations or which may modify the course of the disease or modify disease progression. However specifically, this guideline will focus on the maintenance treatment of COPD. This guideline will not focus on treatment of exacerbations. 3. Legal basis and relevant guidelines This guideline has to be read in conjunction with the introduction and general principles (4) and parts I and II of the Annex I to Directive 2001/83/EC as amended. Applicants should also refer to other relevant European and ICH guidelines (in their current version) on the conduct of clinical development, especially those on: Dose-Response Information to Support Drug Registration CPMP/ICH/378/95 (ICH E4); Note for Guidance on Studies in Support of Special Populations: Geriatrics (ICH Topic E 7) and the Questions and Answers - EMEA/CHMP/ICH/604661/2009; Statistical Principles for Clinical Trials - CPMP/ICH/363/96 (ICH E9); Choice of Control Group in Clinical Trials - CPMP/ICH/364/96 (ICH E10); Population exposure: The Extent of Population Exposure to Assess Clinical Safety for Drugs - CPMP/ICH/375/95 (ICH E1A); Guideline on the investigation of drug interactions - CHMP/EWP/125211/2010 Guideline on the Requirements for Clinical Documentation for Orally Inhaled Products (OIP) Including the Requirements for Demonstration of Therapeutic Equivalence Between Two Inhaled Products for Use in the Treatment of Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Adults and For Use in the Treatment of Asthma in Children and Adolescents - CPMP/EWP/4151/00 Rev. 1; CPMP Note for Guidance on the Clinical Investigation of Medicinal Products in the Treatment of Asthma - CPMP/EWP/2922/01; 2 corr: a reference to LAMA has been included under the Introduction section as an alternative to LABA in combination with ICS for the most severe patients to make it consistent with the rest of the guideline. 4/17
5 Guideline on Missing Data in Confirmatory Clinical Trials - CPMP/EWP/1776/99 Rev.1-; Guideline on Clinical Development of Fixed Combination Medicinal Products - CPMP/EWP/240/05 Rev.1. This Guideline is intended to assist applicants during the clinical development of medicinal products. 4. Main text 4.1. Patient characteristics and selection of patients Diagnosis of COPD should be considered in any patient who has symptoms of cough, sputum production or dyspnoea or a history of exposure to risk factors for the disease, particularly tobacco smoking. COPD is confirmed when a patient who has persistent or recurrent symptoms that are compatible with COPD (i.e. chronic cough, chronic sputum production, dyspnoea) is found to have airflow obstruction that is not fully reversible (i.e. post-bronchodilator forced expiratory volume in one second to forced vital capacity ratio (FEV 1 /FVC) less than 0.70 (according to The Global Initiative for Chronic Obstructive Lung Disease (GOLD) or below the lower limit of normal (LLN) in patients over 60 years of age (according to ATS/ERS guidelines) and there is no alternative explanation for the symptoms and airflow obstruction. Alternatively, the FEV 1 to FEV 6 ratio below 0.73, or the LLN, can be used for diagnosis. Spirometry should be performed after the administration of an adequate dose of an inhaled bronchodilator in order to minimise variability. The following key aspects should be considered when selecting the target population: Characterisation of the population based on reversibility of chronic airflow limitation. Generally, patients with other causes of chronic airflow limitation should be excluded from clinical studies in COPD. Chest radiography can be of value in the differential diagnosis in suspected COPD. Patients with asthma or predominantly asthma, cystic fibrosis, bronchiolitis obliterans and fibrosis due to tuberculosis or very rarely α1-antityripsin deficiency should not be recruited to, and should be excluded from, clinical studies in COPD. Diagnosis should not rely on the presence or absence of reversibility of airflow obstruction. Nevertheless, the study population should be characterised with respect to the presence of airflow reversibility. Ideally the aim should be to study a homogenous population of patients with COPD and mainly irreversible airway function, which would allow for better characterisation of the effect of the drug. However it is accepted that up to 50% of patients with COPD do have some degree of reversibility of airflow obstruction and therefore some degree of freedom in respect of the inclusion of patients with differing degrees of airway reversibility, and the resultant need for sub-group analyses in respect of these differing degrees of airway reversibility, will be required. Consistency of effect across such subgroups should be demonstrated. If patients with reversibility are to be included, consideration should be given to the mechanism of action of the test drug and it must be ensured that the results are not driven by this subset of patients particularly when testing drugs with a bronchodilator mechanism of action. Disease severity 5/17
6 The severity of the target COPD population should be defined a priori. Severity is classified on the basis of airflow limitation, symptoms, risk of exacerbation and presence of comorbidities (GOLD 2011). The most widely accepted classification of the spirometric severity of COPD is according to the Global Initiative for Chronic Obstructive Lung disease (GOLD). The GOLD classification is based on the degree of impairment of lung function and recognises four stages: Stage I: mild, Stage II: moderate, Stage III: severe and Stage IV: very severe. Furthermore, a combined COPD assessment based on GOLDstaging of airflow limitation, risk of exacerbation and current symptoms has been proposed (GOLD 2011). Alternative approaches to evaluate and stage disease severity can be used if validated, justified and generally accepted in clinical practice. Patients with mild COPD are generally treated with short-acting bronchodilators on demand with a firm recommendation for smoking cessation. Unless there is a specific need to study patients with mild disease, such patients would not normally be recruited into clinical studies assessing maintenance treatments for COPD. Clinical studies of medicinal products for maintenance treatment of COPD should see recruitment of patients with moderate to very severe disease. Baseline characteristics Patient and disease characteristics at baseline (i.e., demographic data, including age, sex, body mass index, pre- and post-bronchodilator FEV 1, reversibility of airflow limitation, dyspnoea scale, duration of disease, frequency, duration, severity and management of acute exacerbations in the last year prior to study inclusion, previous and concomitant therapies, and concomitant diseases including those specifically related to COPD such as weight loss and peripheral muscle wasting and dysfunction) should be well documented. Efforts to include a population representative of the target population should be made, including patients with relevant comorbidities. If possible patients are expected to remain clinically stable and free of exacerbations over a prespecified period prior to randomisation to study treatments. Criteria for defining stability should be stated in the study protocol. It should be ensured that treatment arms are balanced according to important predictors of outcome. Stratification according to relevant baseline characteristics, for example disease severity, number of exacerbations, could be considered. If the study includes a measure of exercise capacity or health status in the efficacy assessment, the documentation of baseline exercise capacity/health status is mandatory. Smoking history Special attention should be paid to current and/or past history of tobacco smoking (i.e. X pack years). In efficacy studies formal stratification of patients according to smoking status (non-smokers, current smokers, ex-smokers) could be carried out. Tobacco exposure should be monitored carefully throughout clinical studies in all patients and changes in smoking status documented and reported. The influence of this exposure on the estimates of efficacy should be evaluated by quantifying and illustrating any differences in tobacco exposure between the treatment groups and discussing the possible quantitative effect of these differences on outcome. Smoking cessation programmes and nicotine replacement therapy offered to smokers as aids to smoking cessation prior to randomisation should be carefully documented, as they may be confounders and may modify the treatment effect. Any effect of these aids on study outcome measures should be examined and documented and the possibility of pharmacokinetic interactions between the proposed new product and any replacement therapies should be investigated. Sub-populations 6/17
7 Relevant identified sub-populations should be justified and defined a priori in the study protocol. The following examples could be considered of interest: e.g. severity, degree of airflow obstruction reversibility, phenotype (i.e. chronic bronchitis versus emphysema), frequency of exacerbations (i.e. >2-3/year), degree of dyspnoea (e.g. MRC 2), requirement for oxygen therapy, exercise capacity, BMI (e.g. <21) and/or smoking status. The selection of the most relevant subpopulations should be made on a case by case basis. Consistent effects in relevant sub-populations should be shown Methods to assess efficacy Different types of drugs may be developed for COPD which may provide symptomatic relief through improvement of airway obstruction, which may modify or prevent exacerbations or which may modify the course of the disease or modify disease progression. The selection of endpoints will depend on the objective(s) of the clinical programme/clinical study. See further details in Section 4.3. Depending on the mechanism of action of the drug substance under evaluation, a complete characterisation of the effect of any therapy in COPD would require the inclusion of a number of different variables belonging to those domains expected to be affected by the study drug, because most treatments will produce benefits in more than one area. Comorbidities commonly present in patients with COPD, particularly heart failure, may impact on the clinical endpoints used in the assessment of medicinal products for the treatment of patients with COPD (e.g. symptom questionnaires, exercise capacity, specific disease index). The effect of comorbidities on some of these endpoints will need to be considered Efficacy endpoints Lung function Changes in spirometric parameters should be measured as a relevant part of the overall effect of any new therapy in the treatment of patients with COPD. Spirometry should be undertaken by trained healthcare professionals according to standardised methods. FEV 1 is the most extensively used parameter for adopting treatment strategies in COPD. FEV 1 is one of the most repeatable lung function parameters and in COPD is a measure of the obstructive element of the disease. If FEV 1 is the primary endpoint, the pre-bronchodilator FEV 1 is the preferred measure in the development of a new product for maintenance treatment, although depending on the mode of action other lung function parameters (e.g. post-bronchodilator FEV 1 ) could be the parameter of choice. Whether the preferred parameter is the pre-bronchodilator FEV 1 or the post-bronchodilator FEV 1 or other lung function parameters, this should be justified. It is recommended that FEV 1 is measured both pre- and post-bronchodilator, both at baseline and at repeated visits during each study treatment period. For a bronchodilator serial post-dose FEV 1 measurements should be carried out to characterise the time profile in order to determine time to effect and duration of effect, particularly in Phase II studies. The maintenance of the effect over time for any drug with an effect on lung function should also be assessed. A central quality assurance system is highly encouraged. The classification of lung function values as valid or invalid should be pre-specified and scientifically justified in the protocol according to 7/17
8 acceptable standards. It should be stated and justified how valid or invalid measurements will be used in the study analysis. A description of the quality achieved during spirometric testing should be provided in the study report by means of generally accepted parameters. Other measures of lung function which could also be recorded to characterise the effect of a new active substance include inspiratory capacity (IC), functional residual capacity (FRC), residual volume/total lung capacity (RV/TLC), forced vital capacity (FVC) and slow VC and the diffusing capacity of the lung for carbon monoxide (DLCO). Some of these measures of lung function may correlate better with improvements in symptoms and exercise tolerance than does FEV 1. They might be considered as appropriate alternative physiological endpoints if validated for use in COPD. Slow VC is preferred to FVC in some cases of severe airflow obstruction, particularly emphysema. Exacerbations Definitions of exacerbation and severity of the exacerbation need to be standardised to allow comparisons between different interventions in different settings. The proposed definition of an exacerbation of COPD is an acute event characterised by a worsening of the patient s respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication (GOLD 2011). Although criteria for medical interventions might be subject to local differences, the following classification of the severity of exacerbations is recommended for stable COPD patients: Mild: exacerbations described as an increase in respiratory symptoms that can be controlled by the patient with an increase in usual medication; Moderate: exacerbations that require treatment with systemic corticosteroids and/or antibiotics; Severe: exacerbations that require hospitalisation or result in death. The rate of moderate or severe exacerbations is a clinically relevant endpoint related to the associated morbidity and mortality and the usually significantly increased health-care requirement. The frequency and/or severity of exacerbations are important outcome measures that should be considered in clinical studies in COPD. Such measures can include reduction in the number of exacerbations, annual rate and severity of exacerbations. Time to first exacerbation might also be considered. If one of these measures is chosen as the primary efficacy endpoint, the others should be assessed also to ensure that improvement in one endpoint does not result in worsening in another. An evaluation of the frequency of exacerbations should normally be made over a period of at least one year due to seasonal variation in exacerbation rates. The timing of the study treatment may prove important (e.g. capturing the winter cold season in the majority of patients). There should be an established minimum time interval between exacerbations to consider them as different episodes. The end of an exacerbation has to be defined clearly so that a difference between the existing exacerbation and a new exacerbation can be measured. Evaluation by an external adjudication committee is encouraged. Patients and investigators reported outcomes The development of COPD may affect several aspects of a patient s health manifest by symptoms and physical limitations, and ultimately affecting general well-being and health perception. Disease-specific 8/17
9 questionnaires, dyspnoea and symptom scales are considered relevant outcomes for the characterisation of response to treatment. Health status and Health Related Quality of Life (HRQoL) The impact of disease on a patient s daily life, activity and well-being should be assessed at regular intervals. There is a wide range of questionnaires available. Disease-specific questionnaires (e.g. the Chronic Respiratory Questionnaire (CRQ) and the St George s Respiratory Questionnaire (SGRQ)) cover different health related domains. Disease-specific instruments tend to be more sensitive to changes and therefore better suited to measure treatment effects in COPD than generic instruments. More recently new tools have been introduced into clinical trials. Among them, the COPD Assessment Test (CAT), a patient and clinician rating scale, deserves some consideration for its easy management and its good correlation with the SGRQ. General questionnaires (e.g. SF-36) and questionnaires with a narrower perspective such as the activity of daily living questionnaires (Nottingham Extended Activity of Daily Living (EADL) or London EADL) or the functional status questionnaires can also provide relevant information, focusing on the number of activities that a patient can perform. Other health-related questionnaires, specific or generic, can be utilised if sufficiently validated and extensively used. Dyspnoea Instruments used to measure dyspnoea should rely on patient-reported outcomes and be multidimensional whenever possible. Dyspnoea can be measured using clinical ratings based on activities of daily living and ratings during an exercise task. The Baseline and Transition Dyspnoea Indices (clinician rated scales, BDI and TDI, respectively) and the dyspnoea component of the CRQ (patient rated scale) are examples of clinical ratings extensively used in randomised controlled trials. The BDI/TDI is a validated instrument developed to measure the impact of dyspnoea on three domains functional impairment, magnitude of task and magnitude of effort. Alternatively, there are a number of methods for patients with COPD to rate their dyspnoea during an exercise test such as cycle ergometry or treadmill walking. The two more commonly used methods are the Borg Category Rating Dysnea Score (CR10), which is preferred, and visual analogue scales (VAS). COPD symptom scales According to widely accepted COPD treatment guidelines, (ERS, ATS, GOLD), the three cardinal symptoms of COPD are dyspnoea, sputum production and cough. The symptoms can be evaluated over the course of the clinical study by use of patient diaries. Improvements in these symptoms are to be expected with most drugs, but the magnitude of improvement is difficult to estimate and a clinically relevant standard for improvement has not yet been established. This needs to be discussed on a study by study basis. Symptoms to be recorded should include night-time symptoms, night-time awakening, daytime symptoms, cough, wheezing, dyspnoea, sputum production, etc. Patients questionnaires or diary cards Questionnaires or diary cards should be provided, one for the patient to capture the unreported exacerbations (mild exacerbations) and another for the investigator to collect the reported (moderate- 9/17
10 severe) exacerbations. Diary entries may be entered into an electronic diary which, in addition to recording exacerbation data, may also capture symptoms Exercise capacity In patients with COPD exercise testing is useful in the clinical setting to assess the degree of impairment, prognosis and the effects of interventions. Several methods for evaluating exercise capacity have been developed. The severity and cause of exercise intolerance are best assessed by conducting standardised laboratory exercise testing in which detailed physiological respiratory/ metabolic measurements are made while patients perform cycle ergometry or walk at a specific speed on the treadmill. Laboratory test protocols can be either constant ( endurance ) or incremental work rate tests. Endurance tests rather than incremental testing have been more extensively used in COPD. Cycle and treadmill exercise have been used interchangeably although the former has been used more commonly in clinical studies in COPD, as the work rate for endurance and incremental tests is easier to quantify. Simpler tests can also be used, although the information gathered is more limited. The six-minute walking distance (6-MWD) is a relatively simple test that has been used extensively in studies to evaluate possible benefits of pharmacological intervention; the shuttle walking test, a better standardised and simpler field test, is also widely used. Rescue medication The use of rescue medication (e.g. β 2 agonist, reliever inhaler) reflects effects on symptoms and therefore can be considered as a clinical endpoint. Both the number of times that rescue medication is required during the day and at night and the number of puffs used on each occasion should be recorded; the number of times that rescue medication is used is the more relevant measure. Composite scores Changes in the BODE-Index are considered of interest. The BODE-Index is a composite index based on body mass index, airflow obstruction as measured by FEV 1, dyspnoea assessed by the Medical Research Council (MRC) Dyspnoea Scale, and exercise capacity measured by the 6-minute walking distance. Other composite scores might be used if validated and generally accepted. Imaging Computed tomography (CT) imaging can accurately characterise lung parenchymal changes and facilitate quantitative assessment. Although in clinical practice plain radiography still has an important role in the evaluation of COPD, CT densitometric evaluation might have a role in the assessment of the progression of emphysema and the evaluation of airway wall thickening. As yet the use of CT imaging is not fully validated and therefore is not appropriate for use in clinical studies as a primary or important secondary endpoint. However to explore the possible role that CT imaging might have in clinical studies in COPD, its inclusion as a secondary endpoint should be considered. Other important considerations when using CT imaging concern the total exposure to radiation. If changes in lung structure are to be assessed it should be demonstrated that the observed changes in lung tissue are linked to functional changes which provide clinically meaningful benefit to the patient. Other potential endpoints Physical activity should be considered as a potential secondary endpoint. 10/17
11 The value of biomarkers of systemic inflammation in COPD is not yet established. To explore the possible role that they might have in clinical studies in COPD, their inclusion as secondary endpoints should be considered Strategy and design of clinical trials Early studies When a new chemical entity is being developed full pharmacokinetic/pharmacodynamic documentation is required. Pharmacodynamic studies The mechanism of action should be characterised and the selection of the relevant pharmacodynamic endpoints justified. First studies in man should provide preliminary safety data and should determine the dose range to be studied in the therapeutic programme. Pharmacokinetic studies The pharmacokinetics of the product should be described and absorption, bioavailability, distribution, metabolism and elimination characterised. For orally inhaled drugs the extent of systemic absorption due to pulmonary absorption and gastrointestinal absorption should be distinguished (e.g. in studies utilising an active charcoal blockade). Pressurised and non-pressurised metered dose inhalers, dry powder inhalers and nebulisers have different flow-dependent pulmonary deposition patterns and pulmonary deposition of drug following inhalation from these different inhalation devices may also be dependent on the severity of the disease/inhalation capacity of the patient (e.g. dry powder inhalers) and/or the patient s ability to coordinate actuation of the inhalation device with inspiration of breath (e.g. pressurised metered dose inhalers). Consequently, variability in performance should be investigated through pharmacokinetic studies, possibly supported by scintigraphic lung deposition studies, in order to select the patient population able to use the device appropriately or select the dose for each patient group to achieve the required pulmonary deposition. The use of spacing devices to improve a patient s ability to co-ordinate actuation of the pressurised metered dose inhaler with inspiration of breath has to be supported by appropriate in vitro data, pulmonary deposition data and/or clinical data. Therapeutic exploratory studies Specific dose response studies should be performed. Extrapolation from previous dose finding studies in related diseases such as asthma are only of limited value as there is no certainty that both asthma and COPD would respond in a similar way to the same dose. The choice of the population will depend mainly on the mechanism of action of the products and on the intended target population. The dose related benefit and adverse effects should be characterised in double blind, randomised, parallel group, placebo controlled studies. The aim of dose response studies is to define the dose with the best benefit/risk balance and dosing schedule for confirmatory studies. The final study design will depend on the pharmacology of the test product. 11/17
12 Studies of short duration, the duration depending on the mechanism of action of the drug and the selected endpoints, may be sufficient, for example, for bronchodilators 6-12-week studies may be acceptable. If an anti-inflammatory effect and/or an effect on exacerbations is being explored a longer duration of study will be needed. The effect on lung function and patient reported outcomes such as symptoms and health status are appropriate measures for exploratory studies Therapeutic confirmatory trials The selection of patients for confirmatory studies will depend on the type of drug and its intended place in the treatment of COPD. Patients should always be treated in line with international clinical management recommendations. Choice of comparator Selecting an appropriate comparator is difficult as drugs with differing modes of action and combinations of treatments are used in the management and treatment of COPD. For symptomatic treatment, the most useful comparators are either a placebo and an active comparator or an active comparator alone depending on the type of drug being studied and its place in the therapeutic armamentarium. Placebo-controlled studies might be acceptable but only in certain circumstances. The use of placebo might raise ethical issues especially in patients with moderate to severe disease unless it is added to the best standard of care. In all studies adequate rescue measures must be available. The choice of the comparator will depend also on the severity of COPD and the type of study design (i.e. substitution of standard therapy, add-on therapy or combination therapy). Conventional pharmacological treatment of COPD depends on the severity of the disease. Ultimately, in the selection of the comparator consideration must be given to current clinical treatment recommendations. Choice of minimal important difference The minimally important difference should be defined in the protocol and needs to be related to the stage of disease and the characteristics of the population and be justified through literature. Blinding/Masking Double blinding is preferred whenever possible. When a double blind study is not possible (e.g. some inhalers are difficult to blind), a three-arm study comparing the new drug with placebo (blinded comparison), and including an active comparator in the third arm of the study (unblinded comparison) as another control arm, would be preferred. If an unblinded trial is conducted, a blinded evaluation is necessary. Study design The therapeutic management of COPD is concerned with either the provision of symptomatic relief which may modify or prevent exacerbations or modification of the course of the disease and disease progression. 12/17
13 Symptomatic treatment of COPD Population Clinical studies of medicinal products for maintenance treatment of COPD should recruit patients with moderate to very severe disease. Primary endpoint Efficacy in the symptomatic treatment of COPD can be demonstrated either through a single primary endpoint or through co-primary endpoints, depending on the endpoints selected. The choice of endpoint might be influenced by the mechanism of action of the test product. Whichever endpoints are chosen must be thoroughly justified. Measurement of lung function parameters alone is considered to be insufficient in the assessment of therapeutic effect. If lung function is selected as a primary endpoint (FEV 1 would be the parameter of choice), additional evidence of efficacy must be demonstrated through the use of a co-primary endpoint, which should either be a symptom-based endpoint or a patient-related endpoint. In moderate/severe COPD this might be the number of exacerbations and/or symptoms such as dyspnoea on exertion, or health status assessed through the use of a disease-specific questionnaire such as the St George s Respiratory Questionnaire (SGRQ) and/or assessment of exercise capacity. Efficacy should be demonstrated convincingly for both co-primary endpoints and improvements seen in these endpoints must be statistically significant and clinically relevant. In certain circumstances clinically relevant and patient-relevant endpoints of efficacy (rather than physiological measures of lung function) may be acceptable as the single primary endpoint in the assessment of efficacy provided that the chosen endpoint is valid and is a generally accepted tool with which to monitor treatment effect, e.g. exacerbations. In such cases, further support from relevant/key secondary endpoints of function and symptoms or health status, will be required. New drugs intended as alternatives to well known and well-accepted therapies such as bronchodilators or inhaled glucocorticosteroids All patients entered into clinical studies should receive adequate background/maintenance therapy according to the severity of their disease. The appropriate study design would be either a study in which patients receive the new drug (the test product) in one arm, an established comparator in the second arm and placebo in the third (preferred option), or would be a study comparing the new drug with the established active comparator only. The three-arm placebo- and active-controlled study would aim to demonstrate that the test product is superior to placebo and allow putting results into perspective; the two-arm study would aim to demonstrate that the test product is at least non-inferior to the active comparator. Active control non-inferiority studies must be sensitive enough to be able to discriminate between the new product and the active control product and to be able to pick up clinically relevant differences, which might exist between the two active products. A clinically relevant response to treatment must be demonstrated. Such comparative studies must have assay sensitivity. If only a comparison with placebo is available or appropriate, the effect of the new drug must demonstrate clear statistically significant and clinically relevant benefit over placebo and the safety profile must be examined and described carefully to ensure that the benefit/risk balance is acceptable. 13/17
14 Add-on therapy Most patients with moderate to severe COPD are treated with bronchodilators alone or bronchodilators plus inhaled corticosteroids. For drugs to be used as add-on therapy, a placebo comparison must be carried out providing that all patients receive optimised background therapy (for example, a longacting β 2 agonist alone or a long-acting β 2 agonist plus an inhaled corticosteroid). Study duration Study duration will depend on the primary endpoints chosen. The effect on lung function parameters and symptoms might be demonstrated in 12 to 24 weeks; demonstration of efficacy through reduction in exacerbations will require studies of longer duration, at least one year. COPD is a chronic disease and symptomatic benefit is expected to be maintained long-term. Therefore, although efficacy may be demonstrated in controlled clinical studies over 12 to 24 weeks, demonstration of maintenance of effect in longer extension studies to at least one year, blinded whenever possible, is required. Medicinal products aimed to modify the course of the disease and/or disease progression. To date, no treatment has demonstrated an effect on disease progression apart from smoking cessation. There is limited experience in these types of studies. In principle, clinical studies of medicinal products that might modify the course of the disease or might slow or prevent disease progression in COPD should preferentially recruit early-stage patients. Studies to demonstrate the effect of the new drug on prevention of disease progression, potential disease modification, etc should be parallel group and controlled (and if possible double blind) studies. Prevention of the disease progression in COPD should be demonstrated by modifying the long-term decline in lung function. A possible effect of any treatment in the prevention of disease progression may be assessed by means of periodical measurements of FEV 1 over time, comparing the difference in the decline in FEV 1 as measured by the slope of the FEV 1 curve between treatment groups. Alternative parameters might be used if justified and properly validated. Because of the variability shown in longitudinal studies, confident assessment of the rate of decline in an individual patient requires a sufficiently long period of study, at least 3-5 years. Concomitant therapy Medications that are permitted during clinical studies, including rescue medication, should be prespecified in the protocol and their use recorded accurately. The possible influence of non-pharmaceutical management of COPD on pharmaceutical intervention, e.g. smoking cessation, surgical treatment, oxygen, physiotherapy, exercise, etc, should be assessed. Nutritional status and nutrient supply during the pharmaceutical intervention should also be controlled. The use of all concomitant therapies should be accurately recorded and balanced across treatment groups at baseline. A run-in period to standardise concomitant medications is recommended. The use of rescue medication (short-acting bronchodilators) should be standardised wherever possible to minimise confounding of the results, should be recorded carefully and should be analysed. 14/17
15 Handling of withdrawals Handling of missing data should be in line with the Guideline on Missing data in Confirmatory Clinical Trials (CPMP/EWP/1776/99 Rev1). Additional statistical methods should be implemented to take into account the potential for over dispersion due to the variability in exacerbation rates between subjects Safety Specific safety concerns In COPD reduction of therapy once symptom control has been achieved is not normally possible. Moreover, continuing deterioration of lung function usually requires the progressive introduction of more treatments to limit the impact of such deterioration. Therefore a complete safety evaluation, as the primary safety assessment, is required for any treatment for COPD and requires focus on the occurrence of adverse events for each individual component and adverse events known to occur with the particular combination of active drugs. This means that the assessment of adverse events related to a specific treatment or therapeutic group, for example inhaled corticosteroids, would require assessment of both local effects, such as oropharyngeal examination, and systemic effects, such as assessment of hypothalamic pituitary adrenocortical (HPA) axis function, assessment of bone mineral density and ophthalmological assessments for determination of new onset lens opacities and/or increased ocular pressure; the assessment of adverse events related to a particular combination of active drugs, for example long-acting β 2 agonist and inhaled corticosteroid combinations, would require assessment of any increased incidence of pneumonia or lower respiratory tract infections. A particular safety concern for any immuno-modulatory compound is the long-term effect on host defence, cancer defence, wound healing or response to vaccination. Any dossier submitted should address such concerns. The incidence of upper respiratory tract infections, sinusitis, bronchitis, pneumonia and tuberculosis in controlled trials is of particular interest. Cardiovascular adverse effects (myocardial infarction, angina, hypertension, atrial fibrillation, cardiac failure, cardiovascular death, QT prolongation, systemic embolism, stroke, etc.) are of particular concern and should be closely monitored, assessed and described, particularly when using combinations of long-acting β 2 agonists + anticholinergics. Indications of increased risk of cardiovascular adverse events are an important concern and may trigger the need for additional studies to exclude an unacceptable increase in cardiovascular risk associated with the new drug substance. Weight loss and any other potentially relevant identified AEs should be addressed during clinical development. Special attention should be paid to any potential deleterious effect on body mass index. Any other safety concern, potential or identified during pre-clinical or clinical development should be adequately addressed in subsequent studies. Specific safety studies to address potential or identified risks may be needed. All cause-mortality should be considered a relevant safety endpoint. This should always be linked to an assessment of the potential relationship with COPD. A causality assessment of all deaths should be made. Changes in the BODE Index might be predictive of mortality in patients with severe COPD; its assessment is highly recommended. 15/17
16 Extent of exposure and long-term safety data Chronic obstructive pulmonary disease is a chronic disease and therefore robust prospective safety data from at least 1 year of treatment are required. An adequate representation of patients with COPD with common characteristics and/or common concomitant diseases (i.e., the elderly, patients with renal or hepatic impairment or patients with heart failure) is required in such studies. 16/17
17 LIST OF ABBREVIATIONS ATS/ERS BDI/TDI COPD CRQ CR10 CT DLCO FEV 1 FEV 6 FRC FVC GOLD HRQoL IC LABA LLN MRC SGRQ RV/TLC 6MWD VAS VC American Thoracic Society/European Respiratory Society Baseline and Transition Dyspnoea Indices Chronic Obstructive Pulmonary Disease Chronic Respiratory Questionnaire Borg Category Rating Dyspnoea Score Computed Tomography Diffusing capacity of the Lung for Carbon Monoxide Forced Expiratory Volume in one second Forced Expiratory Volume in six seconds Functional Residual Capacity Forced Vital Capacity Global Initiative for Chronic Obstructive Lung Disease Heath Related Quality of Life Inspiratory Capacity Long-acting β 2 Agonist Lower Limit of Normal Medical Research Council St George s Respiratory Questionnaire Residual Volume / Total Lung Capacity Six Minute Walking Distance Visual Analogue Scale Vital Capacity 17/17
Guideline on the clinical investigation of medicinal products for the treatment of asthma
 22 October 2015 CHMP/EWP/2922/01 Rev.1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the clinical investigation of medicinal products for the treatment of Draft Agreed by Respiratory
22 October 2015 CHMP/EWP/2922/01 Rev.1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the clinical investigation of medicinal products for the treatment of Draft Agreed by Respiratory
COPD and Asthma Differential Diagnosis
 COPD and Asthma Differential Diagnosis Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death in America. Learning Objectives Use tools to effectively diagnose chronic obstructive
COPD and Asthma Differential Diagnosis Chronic Obstructive Pulmonary Disease (COPD) is the third leading cause of death in America. Learning Objectives Use tools to effectively diagnose chronic obstructive
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Note for guidance on clinical investigation of medicinal products for treatment of asthma
 1 2 3 27 June 2013 CHMP/EWP/2922/01 Rev.1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Note for guidance on clinical investigation of medicinal products for treatment of asthma Draft Draft
1 2 3 27 June 2013 CHMP/EWP/2922/01 Rev.1 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 Note for guidance on clinical investigation of medicinal products for treatment of asthma Draft Draft
Bronchodilators in COPD
 TSANZSRS Gold Coast 2015 Can average outcomes in COPD clinical trials guide treatment strategies? Long live the FEV1? Christine McDonald Dept of Respiratory and Sleep Medicine Austin Health Institute for
TSANZSRS Gold Coast 2015 Can average outcomes in COPD clinical trials guide treatment strategies? Long live the FEV1? Christine McDonald Dept of Respiratory and Sleep Medicine Austin Health Institute for
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 19 March 2003 CPMP/EWP/785/97 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 19 March 2003 CPMP/EWP/785/97 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER
Chronic Obstructive Pulmonary Disease: Developing Drugs for Treatment Guidance for Industry
 Chronic Obstructive Pulmonary Disease: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Chronic Obstructive Pulmonary Disease: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN THE TREATMENT OF DEPRESSION
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 25 April 2002 CPMP/EWP/518/97, Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 25 April 2002 CPMP/EWP/518/97, Rev. 1 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 London, 22 October 2009 Doc. Ref. EMEA/CHMP/EWP/692702/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) REFLECTION PAPER ON THE EXTRAPOLATION OF RESULTS FROM CLINICAL STUDIES CONDUCTED OUTSIDE
London, 22 October 2009 Doc. Ref. EMEA/CHMP/EWP/692702/2008 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) REFLECTION PAPER ON THE EXTRAPOLATION OF RESULTS FROM CLINICAL STUDIES CONDUCTED OUTSIDE
ICH Topic E 8 General Considerations for Clinical Trials. Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95)
 European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
Adoption by CHMP for release for consultation November 2010. End of consultation (deadline for comments) 31 March 2011
 1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Reflection paper on clinical aspects related to tissue engineered products
 1 2 3 19 March 2012 EMA/CAT/CPWP/573420/2009 Committee for Advanced Therapies (CAT) 4 5 6 Reflection paper on clinical aspects related to tissue engineered products Draft Draft agreed by CPWP 7 October
1 2 3 19 March 2012 EMA/CAT/CPWP/573420/2009 Committee for Advanced Therapies (CAT) 4 5 6 Reflection paper on clinical aspects related to tissue engineered products Draft Draft agreed by CPWP 7 October
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
Marilyn Borkgren-Okonek, APN, CCNS, RN, MS Suburban Lung Associates, S.C. Elk Grove Village, IL
 Marilyn Borkgren-Okonek, APN, CCNS, RN, MS Suburban Lung Associates, S.C. Elk Grove Village, IL www.goldcopd.com GLOBAL INITIATIVE FOR CHRONIC OBSTRUCTIVE LUNG DISEASE GLOBAL STRATEGY FOR DIAGNOSIS, MANAGEMENT
Marilyn Borkgren-Okonek, APN, CCNS, RN, MS Suburban Lung Associates, S.C. Elk Grove Village, IL www.goldcopd.com GLOBAL INITIATIVE FOR CHRONIC OBSTRUCTIVE LUNG DISEASE GLOBAL STRATEGY FOR DIAGNOSIS, MANAGEMENT
NICE Pathways bring together all NICE guidance, quality standards and other NICE information on a specific topic.
 bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 22 January 2009 Doc. Ref. CPMP/EWP/4151/00 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 22 January 2009 Doc. Ref. CPMP/EWP/4151/00 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Chronic obstructive pulmonary disease (COPD)
 Chronic obstructive pulmonary disease (COPD) Chronic obstructive pulmonary disease (COPD) is the name for a group of lung diseases including chronic bronchitis, emphysema and chronic obstructive airways
Chronic obstructive pulmonary disease (COPD) Chronic obstructive pulmonary disease (COPD) is the name for a group of lung diseases including chronic bronchitis, emphysema and chronic obstructive airways
Pharmacology of the Respiratory Tract: COPD and Steroids
 Pharmacology of the Respiratory Tract: COPD and Steroids Dr. Tillie-Louise Hackett Department of Anesthesiology, Pharmacology and Therapeutics University of British Columbia Associate Head, Centre of Heart
Pharmacology of the Respiratory Tract: COPD and Steroids Dr. Tillie-Louise Hackett Department of Anesthesiology, Pharmacology and Therapeutics University of British Columbia Associate Head, Centre of Heart
Pathway for Diagnosing COPD
 Pathway for Diagnosing Visit 1 Registry Clients at Risk Patient presents with symptoms suggestive of Exertional breathlessness Chronic cough Regular sputum production Frequent bronchitis ; wheeze Occupational
Pathway for Diagnosing Visit 1 Registry Clients at Risk Patient presents with symptoms suggestive of Exertional breathlessness Chronic cough Regular sputum production Frequent bronchitis ; wheeze Occupational
An Overview of Asthma - Diagnosis and Treatment
 An Overview of Asthma - Diagnosis and Treatment Asthma is a common chronic disorder of the airways that is complex and characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness,
An Overview of Asthma - Diagnosis and Treatment Asthma is a common chronic disorder of the airways that is complex and characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness,
Department of Surgery
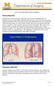 What is emphysema? 2004 Regents of the University of Michigan Emphysema is a chronic disease of the lungs characterized by thinning and overexpansion of the lung-like blisters (bullae) in the lung tissue.
What is emphysema? 2004 Regents of the University of Michigan Emphysema is a chronic disease of the lungs characterized by thinning and overexpansion of the lung-like blisters (bullae) in the lung tissue.
Prevention of Acute COPD exacerbations
 December 3, 2015 Prevention of Acute COPD exacerbations George Pyrgos MD 1 Disclosures No funding received for this presentation I have previously conducted clinical trials with Boehringer Ingelheim. Principal
December 3, 2015 Prevention of Acute COPD exacerbations George Pyrgos MD 1 Disclosures No funding received for this presentation I have previously conducted clinical trials with Boehringer Ingelheim. Principal
COPD PROTOCOL CELLO. Leiden
 COPD PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives and medication of COPD. The Cello way of working can be viewed on
COPD PROTOCOL CELLO Leiden May 2011 1 Introduction This protocol includes an explanation of the clinical picture, diagnosis, objectives and medication of COPD. The Cello way of working can be viewed on
Understanding COPD. Carolinas Healthcare System
 Understanding COPD Carolinas Healthcare System 2013 This self-directed learning module contains information about the pathophysiology, diagnosis, and treatment of COPD. Target Audience: All RNs and LPNs
Understanding COPD Carolinas Healthcare System 2013 This self-directed learning module contains information about the pathophysiology, diagnosis, and treatment of COPD. Target Audience: All RNs and LPNs
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Pulmonary Disorders. Chronic Obstructive Pulmonary Disease (COPD) Chronic Obstructive Pulmonary Disease (COPD)
 RCS 6080 Medical and Psychosocial Aspects of Rehabilitation Counseling Pulmonary Disorders Chronic Obstructive Pulmonary Disease (COPD) Characterized by decreased expiratory airflow Reduction in expiratory
RCS 6080 Medical and Psychosocial Aspects of Rehabilitation Counseling Pulmonary Disorders Chronic Obstructive Pulmonary Disease (COPD) Characterized by decreased expiratory airflow Reduction in expiratory
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)
 Guidance for Industry Acute Bacterial Exacerbations of Chronic Bronchitis in Patients With Chronic Obstructive Pulmonary Disease: Developing Antimicrobial Drugs for Treatment U.S. Department of Health
Guidance for Industry Acute Bacterial Exacerbations of Chronic Bronchitis in Patients With Chronic Obstructive Pulmonary Disease: Developing Antimicrobial Drugs for Treatment U.S. Department of Health
Tests. Pulmonary Functions
 Pulmonary Functions Tests Static lung functions volumes Dynamic lung functions volume and velocity Dynamic Tests Velocity dependent on Airway resistance Resistance of lung tissue to change in shape Dynamic
Pulmonary Functions Tests Static lung functions volumes Dynamic lung functions volume and velocity Dynamic Tests Velocity dependent on Airway resistance Resistance of lung tissue to change in shape Dynamic
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 20 September 2001 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 20 September 2001 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON CLINICAL
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 17 December 2003 CPMP/EWP/556/95 rev 1/Final COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
Interpretation of Pulmonary Function Tests
 Interpretation of Pulmonary Function Tests Dr. Sally Osborne Cellular & Physiological Sciences University of British Columbia Room 3602, D.H Copp building 604 822-3421 sally.osborne@ubc.ca www.sallyosborne.com
Interpretation of Pulmonary Function Tests Dr. Sally Osborne Cellular & Physiological Sciences University of British Columbia Room 3602, D.H Copp building 604 822-3421 sally.osborne@ubc.ca www.sallyosborne.com
National Learning Objectives for COPD Educators
 National Learning Objectives for COPD Educators National Learning Objectives for COPD Educators The COPD Educator will be able to achieve the following objectives. Performance objectives, denoted by the
National Learning Objectives for COPD Educators National Learning Objectives for COPD Educators The COPD Educator will be able to achieve the following objectives. Performance objectives, denoted by the
Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease
 Page 1 of 67 AJRCCM Articles in Press. Published on August 9, 2012 as doi:10.1164/rccm.201204-0596pp GOLD Executive Summary Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive
Page 1 of 67 AJRCCM Articles in Press. Published on August 9, 2012 as doi:10.1164/rccm.201204-0596pp GOLD Executive Summary Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive
Longitudinal Modeling of Lung Function in Respiratory Drug Development
 Longitudinal Modeling of Lung Function in Respiratory Drug Development Fredrik Öhrn, PhD Senior Clinical Pharmacometrician Quantitative Clinical Pharmacology AstraZeneca R&D Mölndal, Sweden Outline A brief
Longitudinal Modeling of Lung Function in Respiratory Drug Development Fredrik Öhrn, PhD Senior Clinical Pharmacometrician Quantitative Clinical Pharmacology AstraZeneca R&D Mölndal, Sweden Outline A brief
understanding the professional guidelines
 SEVERE ASTHMA understanding the professional guidelines This guide includes information on what the European Respiratory Society (ERS) and the American Thoracic Society (ATS) have said about severe asthma.
SEVERE ASTHMA understanding the professional guidelines This guide includes information on what the European Respiratory Society (ERS) and the American Thoracic Society (ATS) have said about severe asthma.
PULMONARY FUNCTION TESTS A Workshop on Simple Spirometry & Flow Volume Loops
 PULMONARY FUNCTION TESTS A Workshop on Simple Spirometry & Flow Volume Loops YOU SHOULD READ THE FOLLOWING MATERIAL BEFORE Tuesday March 30 Interpretation of PFTs Learning Objectives 1. Specify the indications
PULMONARY FUNCTION TESTS A Workshop on Simple Spirometry & Flow Volume Loops YOU SHOULD READ THE FOLLOWING MATERIAL BEFORE Tuesday March 30 Interpretation of PFTs Learning Objectives 1. Specify the indications
Exploratory data: COPD and blood eosinophils. David Price: 9.23-9.35am
 Exploratory data: COPD and blood eosinophils David Price: 9.23-9.35am Blood Eosinophilia in COPD The reliability and utility of blood eosinophils as a marker of disease burden, healthcare resource utilisation
Exploratory data: COPD and blood eosinophils David Price: 9.23-9.35am Blood Eosinophilia in COPD The reliability and utility of blood eosinophils as a marker of disease burden, healthcare resource utilisation
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
Management of exacerbations in chronic obstructive pulmonary disease in Primary Care
 Management of exacerbations in chronic obstructive pulmonary disease in Primary Care Acute exacerbations of chronic obstructive pulmonary disease (COPD) are associated with significant morbidity and mortality.
Management of exacerbations in chronic obstructive pulmonary disease in Primary Care Acute exacerbations of chronic obstructive pulmonary disease (COPD) are associated with significant morbidity and mortality.
Prof. Florian Gantner. Vice President Respiratory Diseases Research Boehringer Ingelheim
 Prof. Florian Gantner Vice President Respiratory Diseases Research Boehringer Ingelheim Research and Development in Practice: COPD Chronic Obstructive Pulmonary Disease (COPD) Facts Main cause of COPD
Prof. Florian Gantner Vice President Respiratory Diseases Research Boehringer Ingelheim Research and Development in Practice: COPD Chronic Obstructive Pulmonary Disease (COPD) Facts Main cause of COPD
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
Addendum to the Guideline on antiarrhythmics on atrial fibrillation and atrial flutter
 22 July 2010 EMA/CHMP/EWP/213056/2010 Addendum to the Guideline on antiarrhythmics on atrial fibrillation and atrial flutter Draft Agreed by Efficacy Working Party July 2008 Adoption by CHMP for release
22 July 2010 EMA/CHMP/EWP/213056/2010 Addendum to the Guideline on antiarrhythmics on atrial fibrillation and atrial flutter Draft Agreed by Efficacy Working Party July 2008 Adoption by CHMP for release
Compare the physiologic responses of the respiratory system to emphysema, chronic bronchitis, and asthma
 Chapter 31 Drugs Used to Treat Lower Respiratory Disease Learning Objectives Describe the physiology of respirations Compare the physiologic responses of the respiratory system to emphysema, chronic bronchitis,
Chapter 31 Drugs Used to Treat Lower Respiratory Disease Learning Objectives Describe the physiology of respirations Compare the physiologic responses of the respiratory system to emphysema, chronic bronchitis,
COPD It Can Take Your Breath Away www.patientedu.org
 written by Harvard Medical School COPD It Can Take Your Breath Away www.patientedu.org What Is COPD? COPD stands for chronic obstructive pulmonary disease. There are 2 major diseases included in COPD:
written by Harvard Medical School COPD It Can Take Your Breath Away www.patientedu.org What Is COPD? COPD stands for chronic obstructive pulmonary disease. There are 2 major diseases included in COPD:
Pulmonary Rehabilitation in Newark and Sherwood
 Pulmonary Rehabilitation in Newark and Sherwood With exception of smoking cessation pulmonary rehabilitation is the single most effective intervention for any patient with COPD. A Cochrane review published
Pulmonary Rehabilitation in Newark and Sherwood With exception of smoking cessation pulmonary rehabilitation is the single most effective intervention for any patient with COPD. A Cochrane review published
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 19 July 2007 Doc. Ref: EMEA/27170/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON COMPASSIONATE USE OF MEDICINAL
European Medicines Agency Evaluation of Medicines for Human Use London, 19 July 2007 Doc. Ref: EMEA/27170/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON COMPASSIONATE USE OF MEDICINAL
Drug therapy SHORT-ACTING BETA AGONISTS SHORT-ACTING ANTICHOLINERGICS LONG-ACTING BETA AGONISTS LONG-ACTING ANTICHOLINERGICS
 Drug therapy 6 6.1 What is the role of bronchodilators in COPD? 52 SHORT-ACTING BETA AGONISTS 6.2 How do short-acting beta agonists work? 52 6.3 What are the indications for their use? 52 6.4 What is the
Drug therapy 6 6.1 What is the role of bronchodilators in COPD? 52 SHORT-ACTING BETA AGONISTS 6.2 How do short-acting beta agonists work? 52 6.3 What are the indications for their use? 52 6.4 What is the
written by Harvard Medical School COPD It Can Take Your Breath Away www.patientedu.org/copd
 written by Harvard Medical School COPD It Can Take Your Breath Away www.patientedu.org/copd What Is COPD? COPD stands for chronic obstructive pulmonary disease. There are two major diseases included in
written by Harvard Medical School COPD It Can Take Your Breath Away www.patientedu.org/copd What Is COPD? COPD stands for chronic obstructive pulmonary disease. There are two major diseases included in
Your Go-to COPD Guide
 Your Go-to COPD Guide Learning how to live with chronic obstructive pulmonary disease (COPD) Inside, you ll learn: COPD facts COPD symptoms and triggers How to talk with your doctor Different treatment
Your Go-to COPD Guide Learning how to live with chronic obstructive pulmonary disease (COPD) Inside, you ll learn: COPD facts COPD symptoms and triggers How to talk with your doctor Different treatment
Background information
 Background information Asthma Asthma is a complex disease affecting the lungs that can be managed but cannot be cured. 1 Asthma can be controlled well in most people most of the time, although some people
Background information Asthma Asthma is a complex disease affecting the lungs that can be managed but cannot be cured. 1 Asthma can be controlled well in most people most of the time, although some people
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
Medicines Use Review Supporting Information for Asthma Patients
 Medicines Use Review Supporting Information for Asthma Patients What is asthma? Asthma is a chronic inflammatory disorder of the airways. The inflammation causes an associated increase in airway hyper-responsiveness,
Medicines Use Review Supporting Information for Asthma Patients What is asthma? Asthma is a chronic inflammatory disorder of the airways. The inflammation causes an associated increase in airway hyper-responsiveness,
Cost-effectiveness of Pirfenidone (Esbriet ) for the treatment of Idiopathic Pulmonary Fibrosis.
 Cost-effectiveness of Pirfenidone (Esbriet ) for the treatment of Idiopathic Pulmonary Fibrosis. March 2013 1. Pirfenidone is indicated in adults for the treatment of mild to moderate Idiopathic Pulmonary
Cost-effectiveness of Pirfenidone (Esbriet ) for the treatment of Idiopathic Pulmonary Fibrosis. March 2013 1. Pirfenidone is indicated in adults for the treatment of mild to moderate Idiopathic Pulmonary
Oxygen Therapy. Oxygen therapy quick guide V3 July 2012.
 PRESENTATION Oxygen (O 2 ) is a gas provided in a compressed form in a cylinder. It is also available in a liquid form. It is fed via a regulator and flow meter to the patient by means of plastic tubing
PRESENTATION Oxygen (O 2 ) is a gas provided in a compressed form in a cylinder. It is also available in a liquid form. It is fed via a regulator and flow meter to the patient by means of plastic tubing
COPD. Information brochure for chronic obstructive pulmonary disease.
 COPD Information brochure for chronic obstructive pulmonary disease. CONTENTS What does COPD mean?...04 What are the symptoms of COPD?...06 What causes COPD?...09 Treating COPD...10 Valve therapy in COPD...12
COPD Information brochure for chronic obstructive pulmonary disease. CONTENTS What does COPD mean?...04 What are the symptoms of COPD?...06 What causes COPD?...09 Treating COPD...10 Valve therapy in COPD...12
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May 2011
 TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
2. Background This drug had not previously been considered by the PBAC.
 PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
PUBLIC SUMMARY DOCUMENT Product: Ambrisentan, tablets, 5 mg and 10 mg, Volibris Sponsor: GlaxoSmithKline Australia Pty Ltd Date of PBAC Consideration: July 2009 1. Purpose of Application The submission
Guideline on Process Validation
 1 2 3 4 29 March 2012 EMA/CHMP/CVMP/QWP/70278/2012-Rev1 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) 5 6 Draft Draft Agreed by CHMP /
1 2 3 4 29 March 2012 EMA/CHMP/CVMP/QWP/70278/2012-Rev1 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) 5 6 Draft Draft Agreed by CHMP /
Insights for Improvement: Advancing COPD Care Through Quality Measurement. An NCQA Insights for Improvement Publication
 2009 Insights for Improvement: Advancing COPD Care Through Quality Measurement An NCQA Insights for Improvement Publication Chronic obstructive pulmonary disease (COPD) includes chronic bronchitis, emphysema,
2009 Insights for Improvement: Advancing COPD Care Through Quality Measurement An NCQA Insights for Improvement Publication Chronic obstructive pulmonary disease (COPD) includes chronic bronchitis, emphysema,
Clinical Guideline. Recommendation 3: For stable COPD patients with respiratory symptoms
 Clinical Guideline Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians,
Clinical Guideline Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians,
COPD Prescribing Guidelines
 South Staffordshire Area Prescribing Group COPD Prescribing Guidelines Inhaler choices in this guideline are different from previous versions produced by the APG. It is not expected patients controlled
South Staffordshire Area Prescribing Group COPD Prescribing Guidelines Inhaler choices in this guideline are different from previous versions produced by the APG. It is not expected patients controlled
James F. Kravec, M.D., F.A.C.P
 James F. Kravec, M.D., F.A.C.P Chairman, Department of Internal Medicine, St. Elizabeth Health Center Chair, General Internal Medicine, Northeast Ohio Medical University Associate Medical Director, Hospice
James F. Kravec, M.D., F.A.C.P Chairman, Department of Internal Medicine, St. Elizabeth Health Center Chair, General Internal Medicine, Northeast Ohio Medical University Associate Medical Director, Hospice
CLINICAL PATHWAY. Acute Medicine. Chronic Obstructive Pulmonary Disease
 CLINICAL PATHWAY Acute Medicine Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease Table of Contents (tap to jump to page) INTRODUCTION 1 Scope of this Pathway 1 Pathway Contacts
CLINICAL PATHWAY Acute Medicine Chronic Obstructive Pulmonary Disease Chronic Obstructive Pulmonary Disease Table of Contents (tap to jump to page) INTRODUCTION 1 Scope of this Pathway 1 Pathway Contacts
Clinical guideline Published: 23 June 2010 nice.org.uk/guidance/cg101
 Chronic obstructive pulmonary disease in over 16s: diagnosis and management Clinical guideline Published: 23 June 2010 nice.org.uk/guidance/cg101 NICE 2010. All rights reserved. Your responsibility The
Chronic obstructive pulmonary disease in over 16s: diagnosis and management Clinical guideline Published: 23 June 2010 nice.org.uk/guidance/cg101 NICE 2010. All rights reserved. Your responsibility The
Clinical Guideline. Recommendation 3: For stable COPD patients with respiratory symptoms
 Clinical Guideline Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians,
Clinical Guideline Diagnosis and Management of Stable Chronic Obstructive Pulmonary Disease: A Clinical Practice Guideline Update from the American College of Physicians, American College of Chest Physicians,
Idiopathic Pulmonary Fibrosis
 Idiopathic Pulmonary Fibrosis What is Idiopathic Pulmonary Fibrosis? Idiopathic pulmonary fibrosis (IPF) is a condition that causes persistent and progressive scarring of the tiny air sacs (alveoli) in
Idiopathic Pulmonary Fibrosis What is Idiopathic Pulmonary Fibrosis? Idiopathic pulmonary fibrosis (IPF) is a condition that causes persistent and progressive scarring of the tiny air sacs (alveoli) in
Irish Association for Emergency Medicine (IAEM) submission to the National COPD Strategy
 31 st Irish Association for Emergency Medicine (IAEM) submission to the National COPD Strategy 1 Introduction Chronic obstructive pulmonary disease (COPD) is an important disease for patients, the health
31 st Irish Association for Emergency Medicine (IAEM) submission to the National COPD Strategy 1 Introduction Chronic obstructive pulmonary disease (COPD) is an important disease for patients, the health
Riociguat Clinical Trial Program
 Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Lothian Guideline for Domiciliary Oxygen Therapy Service for COPD
 Lothian Guideline for Domiciliary Oxygen Therapy Service for COPD This document describes the standard for clinical assessment, prescription, optimal management and follow-up of patients receiving domiciliary
Lothian Guideline for Domiciliary Oxygen Therapy Service for COPD This document describes the standard for clinical assessment, prescription, optimal management and follow-up of patients receiving domiciliary
COPD MANAGEMENT PROTOCOL STANFORD COORDINATED CARE
 I. PURPOSE To establish guidelines f the collabative management of patients with a diagnosis of chronic obstructive pulmonary disease (COPD) who are not adequately controlled and to define the roles and
I. PURPOSE To establish guidelines f the collabative management of patients with a diagnosis of chronic obstructive pulmonary disease (COPD) who are not adequately controlled and to define the roles and
Summary of the risk management plan (RMP) for Ofev (nintedanib)
 EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON DATA MONITORING COMMITTEES
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
COPD Intervention. Components:
 COPD Intervention 1. Primary disease education Member will have an increased understanding of chronic obstructive pulmonary disease, the causes, risks and complications. Explain COPD Explain how COPD is
COPD Intervention 1. Primary disease education Member will have an increased understanding of chronic obstructive pulmonary disease, the causes, risks and complications. Explain COPD Explain how COPD is
Spirometry Workshop for Primary Care Nurse Practitioners
 Spirometry Workshop for Primary Care Nurse Practitioners Catherine Casey S. Jones PhD, RN, AE-C, ANP-C Certified Adult Nurse Practitioner Texas Pulmonary & Critical Care Consultants P.A. and Visiting Assistant
Spirometry Workshop for Primary Care Nurse Practitioners Catherine Casey S. Jones PhD, RN, AE-C, ANP-C Certified Adult Nurse Practitioner Texas Pulmonary & Critical Care Consultants P.A. and Visiting Assistant
Pre-Operative Services Teaching Rounds 2 Jan 2011
 Pre-Operative Services Teaching Rounds 2 Jan 2011 Deborah Richman MBChB FFA(SA) Director Pre-Operative Services Department of Anesthesia Stony Brook University Medical Center, NY drichman@notes.cc.sunysb.edu
Pre-Operative Services Teaching Rounds 2 Jan 2011 Deborah Richman MBChB FFA(SA) Director Pre-Operative Services Department of Anesthesia Stony Brook University Medical Center, NY drichman@notes.cc.sunysb.edu
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL MEDICINAL PRODUCTS INTENDED FOR THE TREATMENT OF NEUROPATHIC PAIN
 European Medicines Agency Evaluation of Medicines for Human Use London, 24 January 2007 Doc. Ref. CPMP/EWP/252/03 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL MEDICINAL
European Medicines Agency Evaluation of Medicines for Human Use London, 24 January 2007 Doc. Ref. CPMP/EWP/252/03 Rev. 1 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL MEDICINAL
Questions and answers on post approval change management protocols
 30 March 2012 EMA/CHMP/CVMP/QWP/586330/2010 Committee for Medicinal Products for Human Use (CHMP) Questions and answers on post approval change management protocols Draft agreed by CHMP / CVMP Quality
30 March 2012 EMA/CHMP/CVMP/QWP/586330/2010 Committee for Medicinal Products for Human Use (CHMP) Questions and answers on post approval change management protocols Draft agreed by CHMP / CVMP Quality
Classifying Asthma Severity and Initiating Treatment in Children 0 4 Years of Age
 Classifying Asthma Severity and Initiating Treatment in Children 0 4 Years of Age Components of Severity Symptoms Intermittent 2 days/week Classification of Asthma Severity (0 4 years of age) Persistent
Classifying Asthma Severity and Initiating Treatment in Children 0 4 Years of Age Components of Severity Symptoms Intermittent 2 days/week Classification of Asthma Severity (0 4 years of age) Persistent
Guideline on clinical investigation of medicinal products, including depot preparations in the treatment of schizophrenia
 20 September 2012 EMA/CHMP/40072/2010 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products, including depot preparations in the treatment
20 September 2012 EMA/CHMP/40072/2010 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products, including depot preparations in the treatment
American Thoracic Society Documents
 American Thoracic Society Documents An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations Standardizing Endpoints for Clinical Asthma Trials and
American Thoracic Society Documents An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations Standardizing Endpoints for Clinical Asthma Trials and
Emphysema. Introduction Emphysema is a type of chronic obstructive pulmonary disease, or COPD. COPD affects about 64 million people worldwide.
 Emphysema Introduction Emphysema is a type of chronic obstructive pulmonary disease, or COPD. COPD affects about 64 million people worldwide. Emphysema involves damage to the air sacs in the lungs. This
Emphysema Introduction Emphysema is a type of chronic obstructive pulmonary disease, or COPD. COPD affects about 64 million people worldwide. Emphysema involves damage to the air sacs in the lungs. This
An Outcomes Strategy for COPD and Asthma: NHS Companion Document
 An Outcomes Strategy for COPD and Asthma: NHS Companion Document 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
An Outcomes Strategy for COPD and Asthma: NHS Companion Document 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
EUROPEAN LUNG FOUNDATION
 PULMONARY REHABILITATION understanding the professional guidelines This guide includes information on what the European Respiratory Society and the American Thoracic Society have said about pulmonary rehabilitation.
PULMONARY REHABILITATION understanding the professional guidelines This guide includes information on what the European Respiratory Society and the American Thoracic Society have said about pulmonary rehabilitation.
Hospital-based SNF Coding Tip Sheet: Top 25 codes and ICD-10 Chapter Overview
 Hospital-based SNF Coding Tip Sheet: Top 25 codes and Chapter Overview Chapter 5 - Mental, Behavioral and Neurodevelopmental Disorders (F00-F99) Classification improvements (different categories) expansions:
Hospital-based SNF Coding Tip Sheet: Top 25 codes and Chapter Overview Chapter 5 - Mental, Behavioral and Neurodevelopmental Disorders (F00-F99) Classification improvements (different categories) expansions:
RESPIRATORY VENTILATION Page 1
 Page 1 VENTILATION PARAMETERS A. Lung Volumes 1. Basic volumes: elements a. Tidal Volume (V T, TV): volume of gas exchanged each breath; can change as ventilation pattern changes b. Inspiratory Reserve
Page 1 VENTILATION PARAMETERS A. Lung Volumes 1. Basic volumes: elements a. Tidal Volume (V T, TV): volume of gas exchanged each breath; can change as ventilation pattern changes b. Inspiratory Reserve
Better Breathing with COPD
 Better Breathing with COPD People with Chronic Obstructive Pulmonary Disease (COPD) often benefit from learning different breathing techniques. Pursed Lip Breathing Pursed Lip Breathing (PLB) can be very
Better Breathing with COPD People with Chronic Obstructive Pulmonary Disease (COPD) often benefit from learning different breathing techniques. Pursed Lip Breathing Pursed Lip Breathing (PLB) can be very
Electronic patient diaries in clinical research
 Topics Electronic diaries in Clinical Trials Electronic diaries versus Paper Electronic patient diaries in clinical research Case Study: Novel detection of exacerbations of COPD with patient reported outcome
Topics Electronic diaries in Clinical Trials Electronic diaries versus Paper Electronic patient diaries in clinical research Case Study: Novel detection of exacerbations of COPD with patient reported outcome
Pulmonary Diseases. Lung Disease: Pathophysiology, Medical and Exercise Programming. Overview of Pathophysiology
 Lung Disease: Pathophysiology, Medical and Exercise Programming Overview of Pathophysiology Ventilatory Impairments Increased airway resistance Reduced compliance Increased work of breathing Ventilatory
Lung Disease: Pathophysiology, Medical and Exercise Programming Overview of Pathophysiology Ventilatory Impairments Increased airway resistance Reduced compliance Increased work of breathing Ventilatory
Respiratory Concerns in Children with Down Syndrome
 Respiratory Concerns in Children with Down Syndrome Paul E. Moore, M.D. Associate Professor of Pediatrics and Pharmacology Director, Pediatric Allergy, Immunology, and Pulmonary Medicine Vanderbilt University
Respiratory Concerns in Children with Down Syndrome Paul E. Moore, M.D. Associate Professor of Pediatrics and Pharmacology Director, Pediatric Allergy, Immunology, and Pulmonary Medicine Vanderbilt University
Respiratory Care. A Life and Breath Career for You!
 Respiratory Care A Life and Breath Career for You! Respiratory Care Makes a Difference At 9:32 am, Lori Moreno brought a newborn baby struggling to breathe back to life What have you accomplished today?
Respiratory Care A Life and Breath Career for You! Respiratory Care Makes a Difference At 9:32 am, Lori Moreno brought a newborn baby struggling to breathe back to life What have you accomplished today?
5. Treatment of Asthma in Children
 Treatment of sthma in hildren 5. Treatment of sthma in hildren 5.1 Maintenance Treatment 5.1.1 rugs Inhaled Glucocorticoids. Persistent wheezing in children under the age of three can be controlled with
Treatment of sthma in hildren 5. Treatment of sthma in hildren 5.1 Maintenance Treatment 5.1.1 rugs Inhaled Glucocorticoids. Persistent wheezing in children under the age of three can be controlled with
Abnormalities Consistent with Asbestos-Related Disease Among Long-Term Demolition Workers
 Abnormalities Consistent with Asbestos-Related Disease Among Long-Term Demolition Workers Stephen M. Levin, M.D. Mount Sinai School of Medicine New York, New York November 1994 The Center to Protect Workers
Abnormalities Consistent with Asbestos-Related Disease Among Long-Term Demolition Workers Stephen M. Levin, M.D. Mount Sinai School of Medicine New York, New York November 1994 The Center to Protect Workers
