Pre-test / Post- test / Latest post-level of Copper micromol / 24 hr. 2#
|
|
|
- George Warren
- 7 years ago
- Views:
Transcription
1 Provocation tests for intoxication with Heavy s. Patient / ales = Heavy s Diaval-test nanool / litre 1# Copper icrool / 24 hr. 2# AK * f PD Cu,Hg ,1 5,7 16, , Yes? Yes /? AO 1996 PD 1 9 0,1 1, No - Yes /? BF * 2007 PD Cu,Hg , , Yes No Yes / No CK f 1999 PD Cu,Hg ,5 0,3 6,9 3, Yes No Yes / No CL 1996 PD Cu ,1 5,5 10, Partiel Yes? Yes /? DV 2001 PD Cu,Hg ,1 9,4 3, Yes No Yes / No DY * f 2001 PD Cu,Hg , , Yes Yes Yes / No EH 2003 PD Cu,Hg ,2 5, Yes No No / Yes FE f 2002 PD Cu,Hg ,1 5, No - Yes / Yes FI f 2001 PD Cu,Hg ,3 22,6 7, Yes? Yes /? JC * f 2006 PD Cu,Hg ,1 4, No? No /? HA f 2009 PD Cu,Hg ,2 15,6 7, Yes No Yes / No HBO 2005 PD Cu,Hg ,2 9, ? Yes Yes Yes / Yes OH 2007 PD Cu,Hg ,2 23,8 6, Yes No Yes / No OO * f 2010 PD Cu ,1 6, Yes - No / HJ 2001 PD Cu,Hg ,1 8, No? Yes / No JL f 2002 PD Cu,Hg ,9 5, Partiel Yes Yes /? KI f 2008 PD Cu,Hg ,3 13, Yes? Yes / No KH * f 1998 PD Cu,(Pb,Al) 6* ,1 3,1 3, Yes Yes Yes / Yes NL * 1995 PD ,1 2?? ?? No - - NR 2004 PD Cu,Hg ,4 9,9 16, Yes No Yes / Yes PU * f 2004 PD Cu,Hg , 2 6, No - Yes / No RK * f 6# 2009 PD Cu 1, ,2 4,0 2, Yes - Yes / No HO * 2003 PD Cu,Hg ,1 28, No - No / SB * f 7# 2005 PD Cu,Hg No No No /? SE f 2004 PD Cu ,5 8,8 3, Yes Yes Yes / No SG f 1998 PD Cu ,4 18,6 18, ? 2008 Yes Yes 11# Yes / Yes SS 2000 PD Cu,Hg ,1 9,6 8, No? Yes /? SSB * f 2000 PD Cu ,2 6, No Yes / No VU 2007 PD Cu,Hg ,5 6,6 5, Yes Yes No / No WN 2002 PD Cu ,2 10, No No Yes /?
2 ales = Heavy s Diaval-test µg / g creatinine 3# Copper µg / g creatinine 4# RK *f 6# 2009 PD Cu,Hg,Pb,Cd,Sb , Yes - Yes / No SF * 13# 2009 PD Cu,Hg , Yes - Yes /? HA f 2009 PD Cu,Hg,Sb , Yes No Yes / No
3 ales = Heavy s Atair-test µg / g creatinine 3# Copper µg / g creatinine 4# AJ f 2008 PD Cu,Pb 1,0 1, , No No / BH * 2005 PD Cu,Pb 1, ,001 1, Yes No Yes / No DA f 2001 PD? - 2, No - Yes / Yes EY f 1999 PD Cu,Hg,Pb 1,6 3,8 Not done 0, No Yes Yes / No EK * 2009 PD Cu 0,7 1,6 0,007 0, No? Yes / No GA f 1990 PD Cu,Hg - 4,6 0,017 0, Yes - No /? HH * f 2010 PD Cu,PB,Ba,Cs 3, ,1 O,013 0,5 0, Yes? No /? HL * f PD Cu,Hg,Pb 4 6,4 2,7 0,010 0, Yes JC * f 2005 PD Cu,Pb <dl 0,5 Not done 1, Yes JH * 2008 PD Cu,Hg, Al,As,Ni 1,3 3,0 2,0 0,006 0,66 0, Yes No Yes / No JI f 2003 PD Cu,Hg 1,1 4,6 2,3 Not done 0,85 0, No No Yes / No JNO 2007 PD Cu, Cd 0,9 1,3 0,7 0,011 0,63 0, Yes No Yes / No KE * f 1998 PD Cu 0,3 0,6 Not done 0, No - No / No KHe f 2004 PD Cu, Hg, Pb, 4,4 6,4 4, ,47 0, Yes - Yes / No KJ * f 2004 PD Cu, As 2,5 0,9 3,4 0,014 0,82 0, Yes No Yes / No LI * f 2009 PD Cu,Pb,Cs,Th,Tl, 0,9 1,9 0, Yes? - Yes / W LEN * 2004 PD Cu,Hg,Pb,Ni,Th 1,5 4,5 3,6 0,008 0,47 0, Yes No No / Yes MA * f 8# 2007 PD Cu,? 0,4 0.9 Not done 0,46 0, Yes No No MU * f 15# 2010 PD Cu,Pb,Ba, Gd 3.9 1,5 2,6 Not done 1,2 0, Yes Yes Yes /? OG * f 1997 PD Cu,Hg 4,9 3,1 4,9 Not done 0,413 0, Yes No Yes PA f 1999 PD Cu,Pb 1,2 3,1 Not done 1, Yes - No SEL 2006 PD Cu,Hg,Pb 1,2 2,1 0,5 0,009 0,424 0, Yes? Yes / Yes SI * f 2009 PD Cu,Hg,Pb,Ba,Ce Not done 2,5 3,7 Not done 0,58 0, Yes? Yes /? VU 2007 PD Cu, Hg,Pb 14# - 1,9 3,4 - Not done 0, Yes? Yes /? ZS * f 2004 PD Cu 0,7 1,5 1,5 0,002 0,58 0, Yes No No / No
4 ales = Heavy s DMSA-test µg / g creatinine 3 # Copper g / g creatinine 4 # health CM * f 2005 PD Hg,Pb Not done 6,7 Not done Not done Yes Yes Yes / DV 2001 PD Hg,Pb Not done 3,6 Not done 0, Yes No Yes / No EH 2003 PD Hg,Cu,Pb,Cs,W, Not done 4,1 Not done 0, Yes No No/ Yes U HK 2003 PD Cu,Hg,Pb 10# Not done 7 Not done 0, Yes Yes Yes / Yes KH * f 1998 PD (Cu),Pb,Al,Ba Not done 2,6 3,8 Not done 0,064 0, Yes Yes Yes / Yes NB f 2002 PD Hg,Pb Not done 9,4 Not done No No Yes / No NT * PD As,Cd,Pb,Ni,TI Not done 0,7 Not done 0, No No Yes / - QK f 2005 PD As,Pb,Th Not done 2,7 Not done 0, Yes? Yes / No WV 2005 PD As,Ba,Cd,Cs,Pb, TI 0,9 1,8 0,9 0,01 0, Yes Yes Yes /?
5 Cu Hg Pb PD PD+ = Cobber- intoxication / - elevated = intoxication / -elevated = Lead intoxication / -elevated = Parkinson disease = Parkinson Plus disease Patients tested with Diaval are norally tested for and Copper only. 1# Note: Reference ranges 5-82 nanool / litre. 250 nanool / litre are absolutely a heavy intoxication. No lowest level is safe. (Ref. Range can be discussed). 2# Note: Reference ranges 0,1 1,3 icro ol / 24 hr. Chelated test ust be < 3,0 icro ol / 24 hr (Ref. Range can be discussed). 3# Note: Reference ranges < 3 µg / g creatinine. No lowest level is safe. 4# Note: Reference ranges µg / g creatinine. (Ref. Range can be discussed.) 5# Note: No patients, except SG and DA, have claied to get syptos when using safe reoval aalga by the rules recoended by IAOMT. 6# Note: The patient have been though soe treatent with chelating Agent before the chelating test. Test shows intoxication with Sb, Cs, Pt and TI too. 7# Note: Pre-test ercury 1,1 µg Hg / g creatinine. Post-test ercury 49 µg Hg / g creatinine. Post-test copper 431 µg / g creatinine. 8# Note: Test 1. and 2. done by noral doctor with subnoral dose Atair (750 g.). The test result indicates intoxication with copper. High in Nickel. 9# Note: Patient TA is acute intoxicated with and that is why the patient is not testes with. Copper is tested the ,6 µol / litre which is within reference ranges 10# Note: Urine saples collected 6 hours and not 24 hours as stated to the laboratory. 11# Note: Planed operation for PD has been cancelled after health iprove. 12# Note: Repeat test with fresh saple is recoended. 13# Note: Urine saple collected 6 hours 14# Note: The patient is intoxicated with several other heavy etals too. As, Be, Cs, Th. 15# Note: Gadoliniu pretest 70 µg / g creatinine. Posttest 50 µg / g creatinine Test urine collected only 16 hours. Patients tested with ore than one Agent: KH, HK, VU and NB In patients SEL and KH intoxication / elevated levels Aluiniu see to poop up after several treatents with. Pre- and Post-test is norally done the sae day or within few days The level or Copper could be fluctuating. This eans that the lowest level is not always the latest level. But in ost cases the level the Heavy s are decreased when treated. Note that the patient also could be re-intoxicated. Copper is said to be chelated before other heavy etals as ex. and Lead, which eans that a high level Copper could ask a higher level (or Lead) than seen.
B I N G O B I N G O. Hf Cd Na Nb Lr. I Fl Fr Mo Si. Ho Bi Ce Eu Ac. Md Co P Pa Tc. Uut Rh K N. Sb At Md H. Bh Cm H Bi Es. Mo Uus Lu P F.
 Hf Cd Na Nb Lr Ho Bi Ce u Ac I Fl Fr Mo i Md Co P Pa Tc Uut Rh K N Dy Cl N Am b At Md H Y Bh Cm H Bi s Mo Uus Lu P F Cu Ar Ag Mg K Thomas Jefferson National Accelerator Facility - Office of cience ducation
Hf Cd Na Nb Lr Ho Bi Ce u Ac I Fl Fr Mo i Md Co P Pa Tc Uut Rh K N Dy Cl N Am b At Md H Y Bh Cm H Bi s Mo Uus Lu P F Cu Ar Ag Mg K Thomas Jefferson National Accelerator Facility - Office of cience ducation
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers
 Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry
 Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
All answers must use the correct number of significant figures, and must show units!
 CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
DATING YOUR GUILD 1952-1960
 DATING YOUR GUILD 1952-1960 YEAR APPROXIMATE LAST SERIAL NUMBER PRODUCED 1953 1000-1500 1954 1500-2200 1955 2200-3000 1956 3000-4000 1957 4000-5700 1958 5700-8300 1959 12035 1960-1969 This chart displays
DATING YOUR GUILD 1952-1960 YEAR APPROXIMATE LAST SERIAL NUMBER PRODUCED 1953 1000-1500 1954 1500-2200 1955 2200-3000 1956 3000-4000 1957 4000-5700 1958 5700-8300 1959 12035 1960-1969 This chart displays
ELECTRON CONFIGURATION (SHORT FORM) # of electrons in the subshell. valence electrons Valence electrons have the largest value for "n"!
 179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
8. Relax and do well.
 CHEM 1314 3:30 pm Section Exam II ohn II. Gelder October 16, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic
CHEM 1314 3:30 pm Section Exam II ohn II. Gelder October 16, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic
Electronegativity and Polarity
 and Polarity N Goalby Chemrevise.org Definition: is the relative tendency of an atom in a molecule to attract electrons in a covalent bond to itself. is measured on the Pauling scale (ranges from 0 to
and Polarity N Goalby Chemrevise.org Definition: is the relative tendency of an atom in a molecule to attract electrons in a covalent bond to itself. is measured on the Pauling scale (ranges from 0 to
From Quantum to Matter 2006
 From Quantum to Matter 006 Why such a course? Ronald Griessen Vrije Universiteit, Amsterdam AMOLF, May 4, 004 vrije Universiteit amsterdam Why study quantum mechanics? From Quantum to Matter: The main
From Quantum to Matter 006 Why such a course? Ronald Griessen Vrije Universiteit, Amsterdam AMOLF, May 4, 004 vrije Universiteit amsterdam Why study quantum mechanics? From Quantum to Matter: The main
Victims Compensation Claim Status of All Pending Claims and Claims Decided Within the Last Three Years
 Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Put the human back in Human Resources.
 Put the human back in Human Resources A Co m p l et e Hu m a n Ca p i t a l Ma n a g em en t So l u t i o n t h a t em p o w er s HR p r o f essi o n a l s t o m eet t h ei r co r p o r a t e o b j ect
Put the human back in Human Resources A Co m p l et e Hu m a n Ca p i t a l Ma n a g em en t So l u t i o n t h a t em p o w er s HR p r o f essi o n a l s t o m eet t h ei r co r p o r a t e o b j ect
SCO TT G LEA SO N D EM O Z G EB R E-
 SCO TT G LEA SO N D EM O Z G EB R E- EG Z IA B H ER e d it o r s N ) LICA TIO N S A N D M ETH O D S t DVD N CLUDED C o n t e n Ls Pr e fa c e x v G l o b a l N a v i g a t i o n Sa t e llit e S y s t e
SCO TT G LEA SO N D EM O Z G EB R E- EG Z IA B H ER e d it o r s N ) LICA TIO N S A N D M ETH O D S t DVD N CLUDED C o n t e n Ls Pr e fa c e x v G l o b a l N a v i g a t i o n Sa t e llit e S y s t e
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
Opis przedmiotu zamówienia - zakres czynności Usługi sprzątania obiektów Gdyńskiego Centrum Sportu
 O p i s p r z e d m i o t u z a m ó w i e n i a - z a k r e s c z y n n o c i f U s ł u i s p r z» t a n i a o b i e k t ó w G d y s k i e C eo n t r u m S p o r t us I S t a d i o n p i ł k a r s k i
O p i s p r z e d m i o t u z a m ó w i e n i a - z a k r e s c z y n n o c i f U s ł u i s p r z» t a n i a o b i e k t ó w G d y s k i e C eo n t r u m S p o r t us I S t a d i o n p i ł k a r s k i
1.- L a m e j o r o p c ió n e s c l o na r e l d i s co ( s e e x p li c a r á d es p u é s ).
 PROCEDIMIENTO DE RECUPERACION Y COPIAS DE SEGURIDAD DEL CORTAFUEGOS LINUX P ar a p od e r re c u p e ra r nu e s t r o c o rt a f u e go s an t e un d es a s t r e ( r ot u r a d e l di s c o o d e l a
PROCEDIMIENTO DE RECUPERACION Y COPIAS DE SEGURIDAD DEL CORTAFUEGOS LINUX P ar a p od e r re c u p e ra r nu e s t r o c o rt a f u e go s an t e un d es a s t r e ( r ot u r a d e l di s c o o d e l a
Sustainable energy products Simulation based design for recycling
 Sustainable energy products Simulation based design for recycling Markus A. Reuter (Prof. Dr. Dr. hc) Director: Technology Management, Outotec Oyj Aalto University (Finland), Central South University (China),
Sustainable energy products Simulation based design for recycling Markus A. Reuter (Prof. Dr. Dr. hc) Director: Technology Management, Outotec Oyj Aalto University (Finland), Central South University (China),
 C o a t i a n P u b l i c D e b tm a n a g e m e n t a n d C h a l l e n g e s o f M a k e t D e v e l o p m e n t Z a g e bo 8 t h A p i l 2 0 1 1 h t t pdd w w wp i j fp h D p u b l i c2 d e b td S t
C o a t i a n P u b l i c D e b tm a n a g e m e n t a n d C h a l l e n g e s o f M a k e t D e v e l o p m e n t Z a g e bo 8 t h A p i l 2 0 1 1 h t t pdd w w wp i j fp h D p u b l i c2 d e b td S t
S e w i n g m a c h i n e s for but t - seams. - c o m p l e t e b r o c h u r e -
 S e w i n g m a c h i n e s for but t - seams - c o m p l e t e b r o c h u r e - D o h l e s e w i n g m a c h i n e s f o r b u t t - s e a m s Head Office D o h l e m a n u f a c t u re b u t t s e
S e w i n g m a c h i n e s for but t - seams - c o m p l e t e b r o c h u r e - D o h l e s e w i n g m a c h i n e s f o r b u t t - s e a m s Head Office D o h l e m a n u f a c t u re b u t t s e
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
1332 CHAPTER 18 Sample Questions
 1332 CHAPTER 18 Sample Questions Couple E 0 Couple E 0 Br 2 (l) + 2e 2Br (aq) +1.06 V AuCl 4 + 3e Au + 4Cl +1.00 V Ag + + e Ag +0.80 V Hg 2+ 2 + 2e 2 Hg +0.79 V Fe 3+ (aq) + e Fe 2+ (aq) +0.77 V Cu 2+
1332 CHAPTER 18 Sample Questions Couple E 0 Couple E 0 Br 2 (l) + 2e 2Br (aq) +1.06 V AuCl 4 + 3e Au + 4Cl +1.00 V Ag + + e Ag +0.80 V Hg 2+ 2 + 2e 2 Hg +0.79 V Fe 3+ (aq) + e Fe 2+ (aq) +0.77 V Cu 2+
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
It takes four quantum numbers to describe an electron. Additionally, every electron has a unique set of quantum numbers.
 So, quantum mechanics does not define the path that the electron follows; rather, quantum mechanics works by determining the energy of the electron. Once the energy of an electron is known, the probability
So, quantum mechanics does not define the path that the electron follows; rather, quantum mechanics works by determining the energy of the electron. Once the energy of an electron is known, the probability
Mr. Bracken. Multiple Choice Review: Thermochemistry
 Mr. Bracken AP Chemistry Name Period Multiple Choice Review: Thermochemistry 1. If this has a negative value for a process, then the process occurs spontaneously. 2. This is a measure of how the disorder
Mr. Bracken AP Chemistry Name Period Multiple Choice Review: Thermochemistry 1. If this has a negative value for a process, then the process occurs spontaneously. 2. This is a measure of how the disorder
Exam 1. Spring 2012/13 CHE 140 Section: 5701 & 5702 100 total points Date: Mon. Feb. 11 & Tue. Feb. 12, 2013
 + 80 points Exam 1 Spring 2012/13 Name: CHE 140 Section: 5701 & 5702 100 total points Date: Mon. Feb. 11 & Tue. Feb. 12, 2013 Directions: Answer the following questions completely. For multiple choice
+ 80 points Exam 1 Spring 2012/13 Name: CHE 140 Section: 5701 & 5702 100 total points Date: Mon. Feb. 11 & Tue. Feb. 12, 2013 Directions: Answer the following questions completely. For multiple choice
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Future Trends in Airline Pricing, Yield. March 13, 2013
 Future Trends in Airline Pricing, Yield Management, &AncillaryFees March 13, 2013 THE OPPORTUNITY IS NOW FOR CORPORATE TRAVEL MANAGEMENT BUT FIRST: YOU HAVE TO KNOCK DOWN BARRIERS! but it won t hurt much!
Future Trends in Airline Pricing, Yield Management, &AncillaryFees March 13, 2013 THE OPPORTUNITY IS NOW FOR CORPORATE TRAVEL MANAGEMENT BUT FIRST: YOU HAVE TO KNOCK DOWN BARRIERS! but it won t hurt much!
Chemistry 122 Mines, Spring 2014
 Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
CHEM 107 (Spring-2005) Final Exam (100 pts)
 CHEM 107 (Spring-2005) Final Exam (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
CHEM 107 (Spring-2005) Final Exam (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
2. Write the chemical formula(s) of the product(s) and balance the following spontaneous reactions.
 1. Using the Activity Series on the Useful Information pages of the exam write the chemical formula(s) of the product(s) and balance the following reactions. Identify all products phases as either (g)as,
1. Using the Activity Series on the Useful Information pages of the exam write the chemical formula(s) of the product(s) and balance the following reactions. Identify all products phases as either (g)as,
REVIEW QUESTIONS Chapter 8
 Chemistry 101 ANSWER KEY REVIEW QUESTIONS Chapter 8 Use only a periodic table to answer the following questions. 1. Write complete electron configuration for each of the following elements: a) Aluminum
Chemistry 101 ANSWER KEY REVIEW QUESTIONS Chapter 8 Use only a periodic table to answer the following questions. 1. Write complete electron configuration for each of the following elements: a) Aluminum
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
K + Cl - Metal M. Zinc 1.0 M M(NO
 Redox and Electrochemistry This section should be fresh in your minds because we just did this section in the text. Closely related to electrochemistry is redox chemistry. Count on at least one question
Redox and Electrochemistry This section should be fresh in your minds because we just did this section in the text. Closely related to electrochemistry is redox chemistry. Count on at least one question
Role of Hydrogen Bonding on Protein Secondary Structure Introduction
 Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
ORTEC DET-SW-UPG. Latest Software Features. Ease of Use. Source Location with the Detective V3 Software
 ORTEC DET-SW-UPG Latest Software Features Three Search Modes: Gamma/Neutron total count rate. SNM search mode. Sliding average "monitor" mode. (NEW) User choice of identification schemes: Classify mode
ORTEC DET-SW-UPG Latest Software Features Three Search Modes: Gamma/Neutron total count rate. SNM search mode. Sliding average "monitor" mode. (NEW) User choice of identification schemes: Classify mode
Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material
 Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material W. Wolfe 1, C.-M. Cheng 1, R. Baker 1, T. Butalia 1, J. Massey-Norton 2 1 The Ohio State University, 2 American Electric
Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material W. Wolfe 1, C.-M. Cheng 1, R. Baker 1, T. Butalia 1, J. Massey-Norton 2 1 The Ohio State University, 2 American Electric
Electrochemistry Worksheet
 Electrochemistry Worksheet 1. Assign oxidation numbers to each atom in the following: a. P 4 O 6 b. BiO 3 c. N 2 H 4 d. Mg(BrO 4 ) 2 e. MnSO 4 f. Mn(SO 4 ) 2 2. For each of the reactions below identify
Electrochemistry Worksheet 1. Assign oxidation numbers to each atom in the following: a. P 4 O 6 b. BiO 3 c. N 2 H 4 d. Mg(BrO 4 ) 2 e. MnSO 4 f. Mn(SO 4 ) 2 2. For each of the reactions below identify
Campus Sustainability Assessment and Related Literature
 Campus Sustainability Assessment and Related Literature An Annotated Bibliography and Resource Guide Andrew Nixon February 2002 Campus Sustainability Assessment Review Project Telephone: (616) 387-5626
Campus Sustainability Assessment and Related Literature An Annotated Bibliography and Resource Guide Andrew Nixon February 2002 Campus Sustainability Assessment Review Project Telephone: (616) 387-5626
Standard Operation Procedure. Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry
 Standard Operation Procedure Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry Soil & Plant Analysis Laboratory University of Wisconsin Madison http://uwlab.soils.wisc.edu
Standard Operation Procedure Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry Soil & Plant Analysis Laboratory University of Wisconsin Madison http://uwlab.soils.wisc.edu
Chapter 8 - Chemical Equations and Reactions
 Chapter 8 - Chemical Equations and Reactions 8-1 Describing Chemical Reactions I. Introduction A. Reactants 1. Original substances entering into a chemical rxn B. Products 1. The resulting substances from
Chapter 8 - Chemical Equations and Reactions 8-1 Describing Chemical Reactions I. Introduction A. Reactants 1. Original substances entering into a chemical rxn B. Products 1. The resulting substances from
 W Cisco Kompetanse eek end 2 0 0 8 SMB = Store Mu ll ii gg hh eter! Nina Gullerud ng ulleru@ c is c o. c o m 1 Vår E n t e r p r i s e e r f a r i n g... 2 S m å o g M e llo m s t o r e B e d r i f t e
W Cisco Kompetanse eek end 2 0 0 8 SMB = Store Mu ll ii gg hh eter! Nina Gullerud ng ulleru@ c is c o. c o m 1 Vår E n t e r p r i s e e r f a r i n g... 2 S m å o g M e llo m s t o r e B e d r i f t e
Transient Voltage Suppressor SMBJ5.0 - SMBJ440CA
 Features: Glass passivated junction Low incremental surge resistance, excellent clamping capability 600W peak pulse power capability with a 10/1,000μs waveform, repetition rate (duty cycle): 0.01% Very
Features: Glass passivated junction Low incremental surge resistance, excellent clamping capability 600W peak pulse power capability with a 10/1,000μs waveform, repetition rate (duty cycle): 0.01% Very
CHEM 110: CHAPTER 3: STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS
 1 CHEM 110: CHAPTER 3: STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS The Chemical Equation A chemical equation concisely shows the initial (reactants) and final (products) results of
1 CHEM 110: CHAPTER 3: STOICHIOMETRY: CALCULATIONS WITH CHEMICAL FORMULAS AND EQUATIONS The Chemical Equation A chemical equation concisely shows the initial (reactants) and final (products) results of
Ionizing Radiation, Czech Republic, CMI (Czech Metrology Institute)
 Ionizing Radiation, Czech Republic, (Czech Metrology Institute) Calibration or Measurement RADIOACTIVITY 1.0E+00 1.0E+02 Bq cm -2 C-14 1.0E+01 1.0E+02 Bq cm -2 Co-60 1.0E+01 1.0E+02 Bq cm -2 Sr-90 1.0E+01
Ionizing Radiation, Czech Republic, (Czech Metrology Institute) Calibration or Measurement RADIOACTIVITY 1.0E+00 1.0E+02 Bq cm -2 C-14 1.0E+01 1.0E+02 Bq cm -2 Co-60 1.0E+01 1.0E+02 Bq cm -2 Sr-90 1.0E+01
CHAPTER 21 ELECTROCHEMISTRY
 Chapter 21: Electrochemistry Page 1 CHAPTER 21 ELECTROCHEMISTRY 21-1. Consider an electrochemical cell formed from a Cu(s) electrode submerged in an aqueous Cu(NO 3 ) 2 solution and a Cd(s) electrode submerged
Chapter 21: Electrochemistry Page 1 CHAPTER 21 ELECTROCHEMISTRY 21-1. Consider an electrochemical cell formed from a Cu(s) electrode submerged in an aqueous Cu(NO 3 ) 2 solution and a Cd(s) electrode submerged
WASTE STREAM 2Y51 Analytical Services Process Facilities - North Labs
 WASTE STREAM 2Y51 Analytical Services Process Facilities North Labs SITE SITE OWNER WASTE CUSTODIAN WASTE TYPE Sellafield Nuclear Decommissioning Authority Sellafield Limited LLW WASTE VOLUMES Stocks:
WASTE STREAM 2Y51 Analytical Services Process Facilities North Labs SITE SITE OWNER WASTE CUSTODIAN WASTE TYPE Sellafield Nuclear Decommissioning Authority Sellafield Limited LLW WASTE VOLUMES Stocks:
Name Electrochemical Cells Practice Exam Date:
 Name Electrochemical Cells Practice Exam Date: 1. Which energy change occurs in an operating voltaic cell? 1) chemical to electrical 2) electrical to chemical 3) chemical to nuclear 4) nuclear to chemical
Name Electrochemical Cells Practice Exam Date: 1. Which energy change occurs in an operating voltaic cell? 1) chemical to electrical 2) electrical to chemical 3) chemical to nuclear 4) nuclear to chemical
The Role of Triads in the Evolution of the Periodic Table: Past and Present
 The Role of Triads in the Evolution of the Periodic Table: Past and Present Eric Scerri Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095; scerri@chem.ucla.edu
The Role of Triads in the Evolution of the Periodic Table: Past and Present Eric Scerri Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095; scerri@chem.ucla.edu
Nuclear ZPE Tapping. Horace Heffner May 2007
 ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
 FORT WAYNE COMMUNITY SCHOOLS 12 00 SOUTH CLINTON STREET FORT WAYNE, IN 468 02 6:02 p.m. Ma r c h 2 3, 2 015 OFFICIAL P ROCEED ING S Ro l l Ca l l e a r d o f h o o l u e e o f t h e r t y m m u t y h o
FORT WAYNE COMMUNITY SCHOOLS 12 00 SOUTH CLINTON STREET FORT WAYNE, IN 468 02 6:02 p.m. Ma r c h 2 3, 2 015 OFFICIAL P ROCEED ING S Ro l l Ca l l e a r d o f h o o l u e e o f t h e r t y m m u t y h o
Chapter 2 Lecture Notes: Atoms
 Educational Goals Chapter 2 Lecture Notes: Atoms 1. Describe the subatomic structure of an atom. 2. Define the terms element and atomic symbol. 3. Understand how elements are arranged in the periodic table
Educational Goals Chapter 2 Lecture Notes: Atoms 1. Describe the subatomic structure of an atom. 2. Define the terms element and atomic symbol. 3. Understand how elements are arranged in the periodic table
CODES FOR PHARMACY ONLINE CLAIMS PROCESSING
 S FOR PHARMACY ONLINE CLAIMS PROCESSING The following is a list of error and warning codes that may appear when processing claims on the online system. The error codes are bolded. CODE AA AB AI AR CB CD
S FOR PHARMACY ONLINE CLAIMS PROCESSING The following is a list of error and warning codes that may appear when processing claims on the online system. The error codes are bolded. CODE AA AB AI AR CB CD
Payor Sheet for Medicare Part D/ PDP and MA-PD
 Payor Specification Sheet for MEDICARE PART D/PDP AND MA-PD PRIME THERAPEUTICS LLC CLIENTS JANUARY 1, 2006 (Page 1 of 8) BIN: PCN: See BINs on page 2 (in bold red type) See PCNs on page 2 (in bold red
Payor Specification Sheet for MEDICARE PART D/PDP AND MA-PD PRIME THERAPEUTICS LLC CLIENTS JANUARY 1, 2006 (Page 1 of 8) BIN: PCN: See BINs on page 2 (in bold red type) See PCNs on page 2 (in bold red
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
JCUT-3030/6090/1212/1218/1325/1530
 JCUT CNC ROUTER/CNC WOODWORKING MACHINE JCUT-3030/6090/1212/1218/1325/1530 RZNC-0501 Users Guide Chapter I Characteristic 1. Totally independent from PC platform; 2. Directly read files from U Disk; 3.
JCUT CNC ROUTER/CNC WOODWORKING MACHINE JCUT-3030/6090/1212/1218/1325/1530 RZNC-0501 Users Guide Chapter I Characteristic 1. Totally independent from PC platform; 2. Directly read files from U Disk; 3.
Excel Invoice Format. SupplierWebsite - Excel Invoice Upload. Data Element Definition UCLA Supplier website (Rev. July 9, 2013)
 Excel Invoice Format Excel Column Name Cell Format Notes Campus* Supplier Number* Invoice Number* Order Number* Invoice Date* Total Invoice Amount* Total Sales Tax Amount* Discount Amount Discount Percent
Excel Invoice Format Excel Column Name Cell Format Notes Campus* Supplier Number* Invoice Number* Order Number* Invoice Date* Total Invoice Amount* Total Sales Tax Amount* Discount Amount Discount Percent
The Lewis structure is a model that gives a description of where the atoms, charges, bonds, and lone pairs of electrons, may be found.
 CEM110 Week 12 Notes (Chemical Bonding) Page 1 of 8 To help understand molecules (or radicals or ions), VSEPR shapes, and properties (such as polarity and bond length), we will draw the Lewis (or electron
CEM110 Week 12 Notes (Chemical Bonding) Page 1 of 8 To help understand molecules (or radicals or ions), VSEPR shapes, and properties (such as polarity and bond length), we will draw the Lewis (or electron
CHARLIE S CHALLENGE: At Home Invention Kit
 HARLIE S HALLENGE: A H Ivi Ki GET! R O F DON T i E A ii.. T I ii i. JUNE INE: 6 L DEAD I i i. ii i iv i b i iii F iv f f i B f i ii i i T fi i i i. i f i i iv i iv iv f i f i i fi i ii T b. I. Ev i 5-15
HARLIE S HALLENGE: A H Ivi Ki GET! R O F DON T i E A ii.. T I ii i. JUNE INE: 6 L DEAD I i i. ii i iv i b i iii F iv f f i B f i ii i i T fi i i i. i f i i iv i iv iv f i f i i fi i ii T b. I. Ev i 5-15
EXPERIMENT 8: Activity Series (Single Displacement Reactions)
 EPERIMENT 8: Activity Series (Single Displacement Reactions) PURPOSE a) Reactions of metals with acids and salt solutions b) Determine the activity of metals c) Write a balanced molecular equation, complete
EPERIMENT 8: Activity Series (Single Displacement Reactions) PURPOSE a) Reactions of metals with acids and salt solutions b) Determine the activity of metals c) Write a balanced molecular equation, complete
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes.
 1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
1 PERIODIC TABLE OF GROUPS OF ELEMENTS Elements can be classified using two different schemes. Metal Nonmetal Scheme (based on physical properties) Metals - most elements are metals - elements on left
TRADING HOURS EURONEXT CASH MARKETS: 24 DECEMBER 2013 TO 1 JANUARY 2014
 DATE: 21 NOVEMBER 2013 TRADING HOURS EURONEXT CASH MARKETS: 24 DECEMBER 2013 TO 1 JANUARY 2014 Executive Summary Trading arrangements for the period 24 December 2013 to 1 January 2014, inclusive, for the
DATE: 21 NOVEMBER 2013 TRADING HOURS EURONEXT CASH MARKETS: 24 DECEMBER 2013 TO 1 JANUARY 2014 Executive Summary Trading arrangements for the period 24 December 2013 to 1 January 2014, inclusive, for the
SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS. Oxidation-Reduction
 SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS OxidationReduction Oxidation is loss of electrons. (Oxygen is EN enough to grab e away from most elements, so the term originally meant
SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS OxidationReduction Oxidation is loss of electrons. (Oxygen is EN enough to grab e away from most elements, so the term originally meant
Earthquake Hazard Zones: The relative risk of damage to Canadian buildings
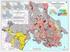 Earthquake Hazard Zones: The relative risk of damage to Canadian buildings by Paul Kovacs Executive Director, Institute for Catastrophic Loss Reduction Adjunct Research Professor, Economics, Univ. of Western
Earthquake Hazard Zones: The relative risk of damage to Canadian buildings by Paul Kovacs Executive Director, Institute for Catastrophic Loss Reduction Adjunct Research Professor, Economics, Univ. of Western
INTERACTIVE MAP EGYPT
 Multi-criteria Analysis for lanning Renewable Energy (MapRE) INTERACTIVE MA EGYT Of Southern and Eastern Africa Renewable Energy Zones (SEAREZs) This interactive DF map contains locations of high quality
Multi-criteria Analysis for lanning Renewable Energy (MapRE) INTERACTIVE MA EGYT Of Southern and Eastern Africa Renewable Energy Zones (SEAREZs) This interactive DF map contains locations of high quality
I n la n d N a v ig a t io n a co n t r ib u t io n t o eco n o m y su st a i n a b i l i t y
 I n la n d N a v ig a t io n a co n t r ib u t io n t o eco n o m y su st a i n a b i l i t y and KB rl iak s iol mi a, hme t a ro cp hm a5 a 2k p0r0o 9f i,e ls hv oa nr t ds eu rmv oedye l o nf dae cr
I n la n d N a v ig a t io n a co n t r ib u t io n t o eco n o m y su st a i n a b i l i t y and KB rl iak s iol mi a, hme t a ro cp hm a5 a 2k p0r0o 9f i,e ls hv oa nr t ds eu rmv oedye l o nf dae cr
 How to Subnet a Network How to use this paper Absolute Beginner: Read all Sections 1-4 N eed a q uick rev iew : Read Sections 2-4 J ust need a little h elp : Read Section 4 P a r t I : F o r t h e I P
How to Subnet a Network How to use this paper Absolute Beginner: Read all Sections 1-4 N eed a q uick rev iew : Read Sections 2-4 J ust need a little h elp : Read Section 4 P a r t I : F o r t h e I P
2. The percent yield is the maximum amount of product that can be produced from the given amount of limiting reactant.
 UNIT 6 stoichiometry practice test True/False Indicate whether the statement is true or false. moles F 1. The mole ratio is a comparison of how many grams of one substance are required to participate in
UNIT 6 stoichiometry practice test True/False Indicate whether the statement is true or false. moles F 1. The mole ratio is a comparison of how many grams of one substance are required to participate in
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Sample Analysis Design Isotope Dilution
 Isotope Dilution Most accurate and precise calibration method available Requires analyte with two stable isotopes Monoisotopic elements cannot be determined via isotope dilution Spike natural sample with
Isotope Dilution Most accurate and precise calibration method available Requires analyte with two stable isotopes Monoisotopic elements cannot be determined via isotope dilution Spike natural sample with
The Dozenal Society of America
 The Dozenal Society of America Multiplication Tables of Various Bases by Michael Thomas De Vlieger Ever wonder what multiplication tables might look like in alternative bases? The dsa Updated 6 February
The Dozenal Society of America Multiplication Tables of Various Bases by Michael Thomas De Vlieger Ever wonder what multiplication tables might look like in alternative bases? The dsa Updated 6 February
BLADE 12th Generation. Rafał Olszewski. Łukasz Matras
 BLADE 12th Generation Rafał Olszewski Łukasz Matras Jugowice, 15-11-2012 Gl o b a l M a r k e t i n g Dell PowerEdge M-Series Blade Server Portfolio M-Series Blades couple powerful computing capabilities
BLADE 12th Generation Rafał Olszewski Łukasz Matras Jugowice, 15-11-2012 Gl o b a l M a r k e t i n g Dell PowerEdge M-Series Blade Server Portfolio M-Series Blades couple powerful computing capabilities
INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION. 37 74 20 40 60 80 m/e
 CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
CHM111(M)/Page 1 of 5 INTI COLLEGE MALAYSIA A? LEVEL PROGRAMME CHM 111: CHEMISTRY MOCK EXAMINATION: DECEMBER 2000 SESSION SECTION A Answer ALL EIGHT questions. (52 marks) 1. The following is the mass spectrum
Standard Free Energies of Formation at 298 K. Average Bond Dissociation Energies at 298 K
 1 Thermodynamics There always seems to be at least one free response question that involves thermodynamics. These types of question also show up in the multiple choice questions. G, S, and H. Know what
1 Thermodynamics There always seems to be at least one free response question that involves thermodynamics. These types of question also show up in the multiple choice questions. G, S, and H. Know what
SUS. Company Profile. Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0)208/658535 Fax 0049(0)208/658536
 SUS Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0208/658535 Fax 0049(0208/658536 Company Profile SUS was founded in 1986 in Oberhausen in the Ruhr area (close to Düsseldorf, in order to meet
SUS Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0208/658535 Fax 0049(0208/658536 Company Profile SUS was founded in 1986 in Oberhausen in the Ruhr area (close to Düsseldorf, in order to meet
english parliament of finland
 gh f fd 213 P cvd f h f y f h g 4 Fby 213. E H (Sc Dcc Py) w -cd S, P Rv (N C Py) F Dy S d A Jh (Th F Py) Scd Dy S. Th g c c 5 Fby, wh Pd f h Rbc S Nö d P f h f fwg h c 212. P g dc c 12 Fby h b f P M c.
gh f fd 213 P cvd f h f y f h g 4 Fby 213. E H (Sc Dcc Py) w -cd S, P Rv (N C Py) F Dy S d A Jh (Th F Py) Scd Dy S. Th g c c 5 Fby, wh Pd f h Rbc S Nö d P f h f fwg h c 212. P g dc c 12 Fby h b f P M c.
Cl1'15 Charlotte-Mecklenburg Schools
 ~ Cl1'15 Charlotte-Mecklenburg Schools Starmount e Elementary -- Streets (proposed reopening 2015-2016) Schools ~ Ra ilroad -- water Bodies Home School Areas D NATIONS FORD D MONTCLAIRE D HUNTINGTOWNE
~ Cl1'15 Charlotte-Mecklenburg Schools Starmount e Elementary -- Streets (proposed reopening 2015-2016) Schools ~ Ra ilroad -- water Bodies Home School Areas D NATIONS FORD D MONTCLAIRE D HUNTINGTOWNE
Using a Balanced Scorecard to Tie the Results Act to Your Day-to-Day Operational Priorities
 Using a Balanced Scorecard to Tie the Results Act to Your Day-to-Day Operational Priorities September 2004 Institute for the Study of Public Policy Implementation Overview Background Behind the Results
Using a Balanced Scorecard to Tie the Results Act to Your Day-to-Day Operational Priorities September 2004 Institute for the Study of Public Policy Implementation Overview Background Behind the Results
High-tech recycling of critical metals: Opportunities and challenges
 High-tech recycling of critical metals: Opportunities and challenges Application know-how Metals Chemistry material Material science solutions Metallurgy Material solutions Recycling Christina Meskers
High-tech recycling of critical metals: Opportunities and challenges Application know-how Metals Chemistry material Material science solutions Metallurgy Material solutions Recycling Christina Meskers
AP Chemistry 2009 Free-Response Questions Form B
 AP Chemistry 009 Free-Response Questions Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
AP Chemistry 009 Free-Response Questions Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
DHL EXPRESS CANADA E-BILL STANDARD SPECIFICATIONS
 DHL EXPRESS CANADA E-BILL STANDARD SPECIFICATIONS 1 E-Bill Standard Layout A B C D E F G Field/ DHL Account Number Billing Customer Name Billing Customer Address Billing Customer City Billing Customer
DHL EXPRESS CANADA E-BILL STANDARD SPECIFICATIONS 1 E-Bill Standard Layout A B C D E F G Field/ DHL Account Number Billing Customer Name Billing Customer Address Billing Customer City Billing Customer
Chemistry Themed. Types of Reactions
 Chemistry Themed Types of Reactions 1 2 Chemistry in the Community-2015-2016 Types of Reactions Date In-Class Assignment Homework T 10/20 TEST on Reactivity of Metals and Redox None W 10/21 Late Start
Chemistry Themed Types of Reactions 1 2 Chemistry in the Community-2015-2016 Types of Reactions Date In-Class Assignment Homework T 10/20 TEST on Reactivity of Metals and Redox None W 10/21 Late Start
WASTE STREAM 2F35 Excellox-Type Transport Flasks and French-Design Dry Flasks
 SITE SITE OWR WASTE CUSTODIAN WASTE TYPE Sellafield Nuclear Decommissioning Authority Sellafield Limited LLW WASTE VOLUMES Stocks: At 1.4.2013... Future arisings - Total future arisings: 45.4 m³ Comment
SITE SITE OWR WASTE CUSTODIAN WASTE TYPE Sellafield Nuclear Decommissioning Authority Sellafield Limited LLW WASTE VOLUMES Stocks: At 1.4.2013... Future arisings - Total future arisings: 45.4 m³ Comment
Special Advertising Section
 S Ad S Nw Yk H F S B R, f d -kd f kwd wkf, h G Rh, Nw Yk R d k d b. By S H. B Th d f h -y G Rh, Nw Yk R f: 18 d d, xy j k, f h w hh- d 7% f h wd y f fh w. By y, h wh h d f b. Y h f y f, y d b k d h I Dy
S Ad S Nw Yk H F S B R, f d -kd f kwd wkf, h G Rh, Nw Yk R d k d b. By S H. B Th d f h -y G Rh, Nw Yk R f: 18 d d, xy j k, f h w hh- d 7% f h wd y f fh w. By y, h wh h d f b. Y h f y f, y d b k d h I Dy
Electrochemistry Voltaic Cells
 Electrochemistry Voltaic Cells Many chemical reactions can be classified as oxidation-reduction or redox reactions. In these reactions one species loses electrons or is oxidized while another species gains
Electrochemistry Voltaic Cells Many chemical reactions can be classified as oxidation-reduction or redox reactions. In these reactions one species loses electrons or is oxidized while another species gains
APPENDIX B: EXERCISES
 BUILDING CHEMISTRY LABORATORY SESSIONS APPENDIX B: EXERCISES Molecular mass, the mole, and mass percent Relative atomic and molecular mass Relative atomic mass (A r ) is a constant that expresses the ratio
BUILDING CHEMISTRY LABORATORY SESSIONS APPENDIX B: EXERCISES Molecular mass, the mole, and mass percent Relative atomic and molecular mass Relative atomic mass (A r ) is a constant that expresses the ratio
1. How could I possibly display the list of all carriers with Interline Ticketing Agreement?
 1. How could I possibly display the list of all carriers with Interline Ticketing Agreement? To display a master list of all carriers for which Amadeus maintains interline ticketing agreements, enter TGAD.
1. How could I possibly display the list of all carriers with Interline Ticketing Agreement? To display a master list of all carriers for which Amadeus maintains interline ticketing agreements, enter TGAD.
english parliament of finland
 gh f fd 2012 P cvd f h f y f h g Mdy, 6 Fby 2012. A h d MP, K T hd h ch h c f h S. E H (Sc Dcc Py) w -cd S, P Rv (N C Py) F Dy S d A Jh (F Py) Scd Dy S. Th g c c Tdy, 7 Fby, whch Pd f h Rbc Tj H d P f
gh f fd 2012 P cvd f h f y f h g Mdy, 6 Fby 2012. A h d MP, K T hd h ch h c f h S. E H (Sc Dcc Py) w -cd S, P Rv (N C Py) F Dy S d A Jh (F Py) Scd Dy S. Th g c c Tdy, 7 Fby, whch Pd f h Rbc Tj H d P f
Using Predictive Modeling to Reduce Claims Losses in Auto Physical Damage
 Using Predictive Modeling to Reduce Claims Losses in Auto Physical Damage CAS Loss Reserve Seminar 23 Session 3 Private Passenger Automobile Insurance Frank Cacchione Carlos Ariza September 8, 23 Today
Using Predictive Modeling to Reduce Claims Losses in Auto Physical Damage CAS Loss Reserve Seminar 23 Session 3 Private Passenger Automobile Insurance Frank Cacchione Carlos Ariza September 8, 23 Today
Gold Refining and Coin Manufacturing at the Royal Canadian Mint
 INTERNET Sub title will go here The Application of XRF to Gold Refining and Coin Manufacturing at the Royal Canadian Mint Michael W. Hinds, Ph.D. The Business of the Mint Circulation Coins (Canada & Other
INTERNET Sub title will go here The Application of XRF to Gold Refining and Coin Manufacturing at the Royal Canadian Mint Michael W. Hinds, Ph.D. The Business of the Mint Circulation Coins (Canada & Other
Elements from Another Universe: Understanding the Beauty of the Periodic Table
 Elements from Another Universe: Understanding the Beauty of the Periodic Table Learning Objectives: The students will examine the properties of make believe elements, arrange these elements so as to create
Elements from Another Universe: Understanding the Beauty of the Periodic Table Learning Objectives: The students will examine the properties of make believe elements, arrange these elements so as to create
We emphasize Lewis electron dot structures because of their usefulness in explaining structure of covalent molecules, especially organic molecules.
 Chapter 10 Bonding: Lewis electron dot structures and more Bonding is the essence of chemistry! Not just physics! Chemical bonds are the forces that hold atoms together in molecules, in ionic compounds,
Chapter 10 Bonding: Lewis electron dot structures and more Bonding is the essence of chemistry! Not just physics! Chemical bonds are the forces that hold atoms together in molecules, in ionic compounds,
Nomenclature and Formulas of Ionic Compounds. Section I: Writing the Name from the Formula
 Purpose: Theory: Nomenclature and Formulas of Ionic Compounds 1. To become familiar with the rules of chemical nomenclature, based on the classification of compounds. 2. To write the proper name of the
Purpose: Theory: Nomenclature and Formulas of Ionic Compounds 1. To become familiar with the rules of chemical nomenclature, based on the classification of compounds. 2. To write the proper name of the
KM client format supported by KB valid from 26 March 2007
 supported by KB valid from 26 March 2007 1/15 VRSION 1.2. UPDATD: 13.12.2005. 1 Introduction...2 1.1 Purpose of this document...2 1.2 Characteristics of the KM format...2 2 Formal check of KM format...3
supported by KB valid from 26 March 2007 1/15 VRSION 1.2. UPDATD: 13.12.2005. 1 Introduction...2 1.1 Purpose of this document...2 1.2 Characteristics of the KM format...2 2 Formal check of KM format...3
Chemical Equations. Chemical Equations. Chemical reactions describe processes involving chemical change
 Chemical Reactions Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into new pure
Chemical Reactions Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into new pure
Analyses on copper samples from Micans
 PDF rendering: DokumentID 1473479, Version 1., Status Godkänt, Sekretessklass Öppen Analyses on copper samples from Micans P. Berastegui, M. Hahlin, M. Ottosson, M. Korvela, Y. Andersson, R. Berger and
PDF rendering: DokumentID 1473479, Version 1., Status Godkänt, Sekretessklass Öppen Analyses on copper samples from Micans P. Berastegui, M. Hahlin, M. Ottosson, M. Korvela, Y. Andersson, R. Berger and
NET IONIC EQUATIONS. A balanced chemical equation can describe all chemical reactions, an example of such an equation is:
 NET IONIC EQUATIONS A balanced chemical equation can describe all chemical reactions, an example of such an equation is: NaCl + AgNO 3 AgCl + NaNO 3 In this case, the simple formulas of the various reactants
NET IONIC EQUATIONS A balanced chemical equation can describe all chemical reactions, an example of such an equation is: NaCl + AgNO 3 AgCl + NaNO 3 In this case, the simple formulas of the various reactants
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Given: 4 NO2(g) + O2(g) 2 N2O5(g) ΔH = -110.2 kj find ΔH for N2O5(g) 2 NO2(g) + 1/2 O2(g).
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Given: 4 NO2(g) + O2(g) 2 N2O5(g) ΔH = -110.2 kj find ΔH for N2O5(g) 2 NO2(g) + 1/2 O2(g).
Manpower Codes Lookup
 00 NO PREFERENCE RECORDED 01 NO RELIGIOUS PREFERENCE 02 SEVENTH-DAY ADVENTIST 04 ASSEMBLIES OF GOD 05 GRACE GOSPEL FELLOWSHIP 06 AMERICAN BAPTIST CHURCHES 07 INDEPENDENT BAPTIST BIBLE MISSION 08 SOUTHERN
00 NO PREFERENCE RECORDED 01 NO RELIGIOUS PREFERENCE 02 SEVENTH-DAY ADVENTIST 04 ASSEMBLIES OF GOD 05 GRACE GOSPEL FELLOWSHIP 06 AMERICAN BAPTIST CHURCHES 07 INDEPENDENT BAPTIST BIBLE MISSION 08 SOUTHERN

