Lipid-lowering therapies and liver enzymes
|
|
|
- Ira Whitehead
- 8 years ago
- Views:
Transcription
1 Lipid-lowering therapies and liver enzymes Delphine Stoll a,b, Roger Darioli a, Nicolas Rodondi a a b Consultation des lipides, Policlinique médicale universitaire, Université de Lausanne Service d endocrinologie, diabétologie et du métabolisme, CHUV, Université de Lausanne Summary Lipid-lowering drugs, especially HMGCoA reductase inhibitors, are widely used in the treatment and prevention of cardiovascular disease. They are generally well tolerated. The main, but uncommon, adverse effects of statins are myopathy and an increase in hepatic transaminases. This review focuses on concerns surrounding the safety of lipid-lowering therapy as it affects liver function. Asymptomatic statin-associated elevations in aminotransferases are common and doserelated, but they are not indicative of liver damage. Liver injury attributable to lipid-lowering therapies is very uncommon. Routine monitoring of liver function is not supported by the available evidence but is still recommended by the FDA. Decompensated cirrhosis, acute liver failure and significant cholestasis are contraindications for statin therapy, but not compensated chronic liver disease such as non-alcoholic fatty liver disease. Possible interactions (mainly with CYP3A4 inhibitors) deserve particular attention, and when statin therapy is needed the lowest effective dose should be prescribed in adults at risk for liver problems. Key words: lipid-lowering therapies; statins; liver function tests Introduction Financial disclosure: The authors have no financial conflicts of interest in the subject matter discussed in this review. Pr. R. Darioli recieves consulting fees from AstraZeneca, Pfizer, MSD, Sanofi-Aventis and research grant support from MSD, Sanofi-Aventis and Bioresearch. The HMGCoA reductase inhibitors (statins) have been widely used in the treatment of hypercholesterolaemia, since they have been shown to reduce cardiovascular morbidity and mortality in patients at risk for coronary heart disease (CHD) and those with CHD. Lipid-lowering therapy is generally well tolerated and the risk of clinically significant hepatic injury is rare [1]. However, potential hepatotoxicity remains an important concern for the health care providers when more aggressive management of dyslipidaemia is increasingly recommended [2]. Clinical case BC is a 63-year-old male with a medical history of liver transplantation 6 months previously for liver cirrhosis after chronic hepatitis B 16 years before. He also has a history of hypertension, type 2 diabetes mellitus treated by insulin, and type IIa hyperlipidaemia. Recurrence of non-icteric liver cirrhosis (Child-Pugh A) related to chronic viral infection was established by biopsy of graft in He was very concerned by persistent high levels of total cholesterol. Treatment of fluvastatin 80 mg daily was started in October 2005 due to persistent hypercholesterolaemia (CT 10.0, HDL 1.7, LDL 5.9, TG 1.8 mmol/l). One month later an increase in liver enzymes was observed (see fig. 1) and the fluvastat in treatment was discontinued. Despite dietary advice, plasma levels of total cholesterol remained elevated (8.2 and 8.6 mmol/l). In view of this patient s high cardiovascular risk a rechallenge with fluvastatin 80 mg/d was done three months later. Ezetimibe was then added and one year later fluvastatin was replaced by rosuvastatin 10 mg/d despite the increase in liver enzymes observed before statin therapy (fig. 1). During this period of statin therapy the patient s medication was the following: insulin, cyclosporine, valsartan, and lamivudine. A diagnosis procedure was performed to elucidate the cause of liver enzyme disturbances. Treatment of dyslipidaemia and liver tests HMGCoA reductase inhibitors (statins) The available statins exhibit major differences, including half-life, systemic exposure, maximum plasma concentration, bioavailability, protein binding, lipophilicity, metabolism, presence of active metabolites and excretion routes (table 1) [3]. The most common adverse effects of statins are gastrointestinal upsets, headache, rash and muscle aches: they are mild and transient. In the EXCEL study, a double-blind placebo-controlled trial evaluating the efficacy and tolerability of lovastatin, the incidence of these mild side effects was 1 15% including in the placebo group [4]. Statins rarely (ranging approximately from 1/ to 1/100, table 2) cause clinically significant liver injury, even in patients with un- Correspondence: PD Dr Nicolas Rodondi Consultation des lipides Policlinique médicale universitaire Bugnon 44 CH-1011 Lausanne 239
2 Figure 1 One case: Profile of liver enzymes observed from 2005 to derlying liver disease [5]. The symptoms of hepatitis induced by statins include fatigue, sluggishness, anorexia and weight loss, and resemble those of an influenza-like syndrome. Most often the serum aminotransferases are only moderately elevated. If the treatment is discontinued, symptoms subside almost overnight but the serum aminotransferase concentration may not return to normal for several weeks, depending on the degree of elevation [6]. Clinically significant elevation of aminotransferases resulting in liver failure is extremely rare (based on case reports) and hepatotoxicity related to statin has certainly been overstated. Generally mild (ALAT 2 3x) aminotransferase elevation associated with statin occurs within the first 12 weeks, is asymptomatic and improves spontaneously. The incidence ranges from 0% to 3% (table 2). Rates of moderate to severe (ALAT >3x) elevation are low and have not been shown to differ significantly from placebo; the incidence of liver function test elevation is 1.1% in statin users versus 1.1% in placebo group [7]. In a cohort of more than 2 million Dutch residents Goettsch showed that the incidence of rhabdomyolysis, myopathy, acute renal failure and hepatic impairment in statin users was less than 1 in 3000 person-years exposure to statins [8]. Non-alcoholic fatty liver disease (NAFLD) As the prevalences of obesity and non-alcoholic fatty liver disease are rising, we are often faced with the decision whether to start a statin in patients with metabolic syndrome and baseline aminotransferase elevations. Before starting lipidlowering agents, the cause of the liver enzyme elevation should be investigated [9]. The most common differential diagnoses are viral hepatitis, alcoholic liver disease, haemosiderosis and autoimmune hepatitis. Serology, CDT, iron measurements, autoantibodies and echography may be necessary. Alpha-1-antitrypsin deficiency and Wilson s disease are also possible causes of chronic liver disease that may need to be ruled out in rare cases. The prevalence of NAFLD is 20% and the prevalence of its progressive form, non-alcoholic steatohepatitis (NASH), is 2 3%. Liver biopsy is the only way of definitely confirming or ruling out the diagnosis of NASH. It may progress to fibrosis and cirrhosis even in individuals who are not heavy drinkers. No specific therapy has been approved for this condition. Metformin, thiazolidinediones, HMGCoA reductase inhibitors, antioxidants, pentoxifylline, lipoprotein lipase activators and low caloric diets have been tested without reaching any clearcut conclusion as to their benefits. Nevertheless there is increasing evidence that thiazolidinediones and medical and surgical treatments for obesity may confer a therapeutic benefit in NAFLD [10]. Risk factors for adverse effects of lipid-lowering agents Risk factors for adverse effects of statins are summarised in table 3. The adverse effects of statins on liver are more common within the first 3 months of treatment and occur more often in association with advanced age and chronic illness [11], and at higher dosage. Statin dose In a meta-analysis of 9 trials, K. Dale reported a dose-related increased risk of elevated AST/ALT with statin therapy (risk ratio 3.1, 95% confidence interval for high versus low intensity statin therapy) [12]. The frequency of aminotransferase elevation is 2/1000 patient-year with high doses and 1/1000 patient-year with low dose treatment [13]. In a retrospective analysis of data from 49 clinical trials, C. Newman found no differences between low-dose atorvastatin (10 mg) and placebo, but a slightly increased rate with high dose atorvastatin (80 mg) when examining AST/ALT elevation [14]. Analysing adverse events reported in 23 treatment arms in large prospective randomized statin trials, A. A. Alsheikh- Table 1 Pharmacokinetics of HMGCoA reductase inhibitors. Drug Metabolism T1/2 (h) Protein binding (%) Metabolites Urinary excretion (%) Faecal excretion (%) Lipophilicity Atorvastatin CYP3A Active 2 70 Yes Simvastatin CYP3A Active Yes Pravastatin Sulfation Inactive No Fluvastatin CYP2C Inactive 6 90 Yes Rosuvastatin Minimal by CYP Active No Adapted from [11, 34, 40]. 240
3 Table 2 Incidence rate of liver enzyme increase (>3x ULN or >120 U/l) in participants of randomised controlled trials of statins. Participants (N) Type of statins Duration (yr) Single measure (%) 2 consecutive measures (%) Trials Statin Placebo Statin Placebo Statin Placebo HPS Simva EXCEL Lova ASCOT Atorva 3.3 LIPID Prava AFCAPS/ TexCAPS Lova WOSCOPS Prava PROSPER Prava S Simva CARE Prava MIRACL Atorva LIPS Fluva GREACE Atorva 3.0 PMSG Prava ACAPS Lova REGRESS Prava FLARE Fluva KAPS Prava LRT Lova MAAS Simva Riegger et al Fluva LCAS Fluva Total Incidence per 1000 person-yr Where no data, no increase in liver enzymes. Adapted from [13]. Table 3 Risk factors for adverse effects of statins. High dose Advanced age and chronic illness More common within the first 3 months Drugs: Cytochrome P450 3A4 inhibitors: antibiotics, antifungal drugs, antidepressants, protease inhibitors, antiarrythmics, immunosuppressive drugs Cytochrome P450 2C9 inhibitors: antifungal drugs Other lipid-lowering agents: niacin, fibrates (gemfibrozil> bezafibrate=fenofibrate) Adapted from [11]. Ali found that drug and dose-specific effects were more important determinants of liver toxicity than magnitude of LDL lowering [15]. From a pooled analysis of all statin New Drug Application Data, it is clear that the highest doses of statins have higher rates of transaminase elevation, but that there is no correlation between a persistent increase in aminotransferases and statin-induced lowering of LDL [16]. Hydrophilicity In his meta-analysis, K. Dale found that higher intensity hydrophilic statin therapy may increase the risk of elevated AST/ALT (RR 3.54 [95% CI, ]), but not higher intensity lipophilic therapy (RR 1.58 [95% CI, ]). However, this meta-analysis was based on limited trial data (only 9 trials included), rosuvastatin was not included (only pravastatin as hydrophilic) and no clear physiological explanation was provided. It is not certain that this was a real effect rather than a chance finding. Statin dosage is probably a more important factor, as described above. Drug interaction (table 4) Hepatotoxicity is more common among patients receiving drugs that are metabolised by the cytochrome P450 enzyme systems [11]. Studies compared the interaction profiles of pravastatin, simvastatin and atorvastatin when coadministrated with cytochrome P450 3A4 inhibitors in healthy subjects: comparing with pravastatin alone, co-administration of 241
4 Table 4 Common drugs that may interact with statins. Drugs that may increase statin concentration Macrolides (azithromycin, clarithromycin, erythromycin, telithromycin) HIV protease inhibitors (lopinavir, ritonavir, saquinavir, amprenavir, atazanavir, fosamprenavir, indinavir, nelfinavir, tipranavir, darunavir) Azole antifungals (itraconazole, ketoconazole, miconazole, posaconazole, voriconazole) Diclofenac Nefazodone Calcium antagonists (diltiazem, verapamil) Amiodarone Cyclosporine, tacrolimus Drugs whose concentration may be increased by statin Digoxine Coumarin anticoagulants* Oral contraceptives Drugs with similar side effects Fibrates (gemfibrozil>bezafibrate=fenofibrate) Niacin * INR increased by about 0.3. Adapted from [11]. Table 5 Monitoring of liver function tests with statin therapy. National Lipid Association Statin Safety Assessement Task Force [9]: If ALAT <3x: no need to discontinue statin If ALAT >3x: repeat test, find aetiology, 4dose or change therapy Bilirubin better indicator of liver injury than aminotransferases Liver Expert Panel [30, 32, 33]: Asymptomatic increases in ASAT & ALAT: class effect of statin, uncommon liver dysfunction No routine monitoring of LFTs, measure only if risks (chronic hepatitis, OH, interaction) Statins can be used in patients with NAFLD or NASH Chronic liver disease and Child s A cirrhosis: not a contraindication for statin use Contraindication for statin: decompensated cirrhosis or acute liver failure or significant cholestasis FDA: pravastatin with verapamil or itraconazole found no significant change in pravastatin pharmacokinetics. However, concomitant verapamil increased the simvastatin concentration, itraconazole the atorvastatin concentration and clarithromycin all three statins concentrations [17]. With the exception of pravastatin (transformed enzymatically into liver cytosol) and rosuvastatin (minimal cytochrome metabolism), Initial LFTs, then 2 12 weeks and every 6 months and at any drug increase Inform patients of possible drug drug interactions all statins undergo metabolism by cytochrome P450 (table 1). Theoretically all drugs that inhibit cytochrome P450 3A4 may increase the serum concentration of atorvastatin and simvastatin, and drugs that inhibit cytochrome P450 2C9 may increase the concentration of fluvastatin. It is suggested that cyclosporine may interact with statins via mechanisms not limited to CYP450 3A4 inhibition, since an increase in pravastatin bioavailability has been reported in its presence [11]. In the ALERT trial, fluvastatin was shown to be very safe in renal transplant patients receiving cyclosporine [18]. However, severe adverse effects, such as rhabdomyolysis, have also been reported from statins not metabolised by cytochrome P450 [19]. Although theoretically plausible, the clinical relevance of these differences between statins is uncertain. Differences between lipid-lowering agents for effects on liver Statins Irreversible liver damage leading to death or liver transplantation appears to be extremely uncommon with statins [5]. Asymptomatic elevation of aminotransferases under statins has been found in trials but does not necessarily indicate hepatic damage, especially when seen in the setting of a normal bilirubin level (without significant cholestasis) [9]. The increase in transaminases is a dose-related phenomenon and the degree of elevation does not reflect the amount of liver injury [20]. Clinically significant acute liver injury is very rare and may be seen in combination with other medications. Fulminant hepatic failure is extremely rare and estimated to be 2 in one million. Autoimmune hepatitis may be induced in genetically susceptible individuals (case reports). The rate of acute liver failure associated with lovastatin is one per million patient-treatment years, which is the same as the background rate of idiopathic acute liver failure [7]. Niacin Nicotinic acid is used primarily to increase HDL. Acute hepatic failure has been reported [21], but is very rare. The typical pattern of injury involves elevation of aminotransferase levels although a mixed pattern of hepatocellular and cholestatic injury can be seen. This potential hepatotoxicity is common with sustained-release formulations (SR) but rare with immediate-release or extended-release (ER). The increase in liver toxicity with SR niacin chiefly occurred with doses >1500 mg/day. In contrast to dietary-supplement SR niacin formulations, an ER formulation available only on prescription has shown a notable rarity of hepatotoxicity in large clinical trials submitted to the US Food and Drug Administration (FDA) [22]. Fibrates Some case reports have found that gemfibrozil results in cholestatic hepatitis and other rare reported cases of hepatocellular injury [23]. Fenofibrate was reported to be potentially involved in a case of hepatitis and fibrosis that was possibly increased due to use in combination with statins [24]. As a general rule, high doses of statin should be avoided in patients who are taking a fibrate. The risk of fibrate toxicity is higher in patients with impaired renal function, because these drugs are largely excreted by the kidney. 242
5 Ezetimibe This drug inhibits intestinal absorption of cholesterol, but it enters the circulation and some authors found that it may in rare cases cause hepatotoxicity in the form of severe cholestatic hepatitis and acute autoimmune hepatitis [25]. In some case reports it was noted that the frequency of increased transaminases was potentially higher in patients receiving ezetimibe when associated with statins [26]. Combinations Combination therapy of statins and fibrates may be needed to treat mixed hyperlipidaemia. In a meta-analysis covering 36 clinical trials and 29 case reports, A. Shek found that this combination increased the risk of myopathy but was unable to conclude on an elevated risk of hepatotoxicity [27]. The combination of statin and gemfibrozil is contraindicated; in a retrospective observational study using an administrative claims database, M. J. Cziraky showed that this association increases the relative risk of hepatic adverse medical events [28]. The FIELD study [29], despite its mixed results concerning the effect of fenofibrate on cardiovascular outcomes in individuals with type 2 diabetes, found that fenofibrate was well tolerated when used alone or in combination with statin. ACCORD includes a treatment arm with combined treatment of atorvastatin and fenofibrate in diabetic patients. The results, expected in the next few years, will certainly be of help in balancing the risks and benefits of such a combination in this high cardiovascular risk population. When to measure liver function tests? There is controversy on whether to do initial laboratory tests and monitoring in patients receiving statins (table 5). For hepatologists, statin-associated elevations in aminotransferase levels are not indicative of liver damage or dysfunction, and liver function tests should not be monitored in patients receiving longterm statin therapy [30]. The FDA still recommends initial measurements before therapy, after 2 to 12 weeks of treatment and every 6 months during longterm treatment and at any drug increase [31]. A more common approach is to measure aminotransferases, especially when there is increased risk for the liver, i.e., chronic hepatitis, excessive alcohol consumption or potential drug interaction [32]. Baseline values may also be useful for the purpose of comparison. Routine monitoring probably adds little information when prescribing low doses in otherwise healthy individuals not taking multiple medications, but it is probably more useful to test when symptoms occur. In the case of hepatotoxicity many adverse reactions are cholestatic, generally benign and, due to pruritus or jaundice, easily detected before serious liver injury occurs. Patients should be aware of possible drug to drug interactions and the physician s judgment must prevail in regard to monitoring [33]. If an aminotransferase increase occurs under statin therapy, it is recommended that the reason first be found, knowing that it is not always drug induced. If ALT is more than 3 times the baseline value on two different blood tests, it is recommended that the dose be lowered or the therapy changed [34]. The National Lipid Association Statin Safety Task Force made a list of recommendations for the monitoring of liver, muscle and kidney toxicity in statin users, based on a review of data from numerous different sources [9]. Regarding the liver, their cautious recommendation was to continue routine liver function monitoring until there is a change in the FDA-approved prescribing information for statins for medico-legal issues. Use of statins in chronic liver disease The data regarding the use of statin in chronic liver disease are limited, but several recent studies have investigated whether patients with underlying liver disease are at increased risk for statin hepatotoxicity. Investigators have recently shown that patients with chronic hepatitis C are not at higher risk for statin hepatotoxicity than hyperlipidaemic patients with no hepatitis [35]. In a trial comparing pravastatin 80 mg or placebo in patients with chronic liver disease (NASH or hepatitis C), the rate of aminotransferase elevation was low in the group receiving pravastatin and not different from placebo, and none of the patients had an exacerbation of their underlying liver disease [36]. Another study showed that hyperlipidaemic individuals with elevated baseline liver enzymes did not have a higher incidence of simvastatin or atorvastatin hepatotoxicity than those with normal liver enzymes [37]. Emerging data even suggest that statin may confer benefits in patients with underlying liver disease, such as NASH [38]. One issue in clinical practice is that patients with underlying liver disease exhibit regular fluctuations in their liver tests, and it may therefore be very difficult to determine whether the cause of the variation in liver function tests is the underlying liver disease or the lipidlowering medication. In the case of liver disease the recommendation is to avoid statin therapy in patients with significant cholestasis, acute liver failure or decompensated cirrhosis [30]. Child s A cirrhosis is not a contraindication to lipid-lowering therapy, and statins can be used in NAFLD and NASH. Indeed, patients with NAFLD may benefit from statins due to their high risk of cardiovascular disease [39]. In the case of chronic liver disease and dyslipidaemia, if the patient can completely abstain from alcohol a low dose statin may be used and, if necessary and with appropriate caution, combined therapy [36]. Back to the case The evolution of the liver enzymes under lipid-lowering therapy was marked by episodes of acute elevation of gamma GT, although without significant worsening of alkaline phosphatase and transaminase (figure 1) or elevation of bilirubin levels, in the absence of alcohol 243
6 abuse. Without changes in treatment the liver enzymes returned to a level similar to that in 2005 before the introduction of fluvastatin. For these reasons there was little evidence of drug toxicity. In 2008, liver biopsy confirmed the liver cirrhosis (Child-Pugh A) of the graft, with only mild signs of inflammation. CT scan in 2008 revealed the presence of occlusive thrombosis of the portal vein. In the absence of other common causes of gamma GT elevation, such as alcohol abuse, new viral hepatitis or NASH, we assumed that this transient worsening was related to acute occlusive portal vein thrombosis. Anticoagulation with warfarin was started immediately. Under lipid-lowering therapy the LDLchol levels oscillated between 2.5 and 3.4 mmol/l. A case of this nature suggests that in the presence of liver disease drugs must be introduced with caution. In this case of hypercholesterolaemia, combined with other cardiovascular risk factors in a diabetic patient, the role of the clinician is to evaluate the risk benefit ratio of lipid-lowering therapy in the light of the available scientific data. Further, the worsening of liver parameters should not be immediately interpreted as adverse effects of statins, but should drive a diagnostic search for its aetiology. Finally, according to current scientific evidence statin therapy should not be regarded as contraindicated in patients with chronic liver disease or compensated liver cirrhosis. However, strict clinical and biochemical monitoring of therapy is required and counselling by specialists recommended. Conclusion In many cases elevation of aminotransferase levels under statins is not indicative of liver damage or dysfunction, but related to other causes such as NAFLD, NASH or viral hepatitis. This elevation of liver enzymes is correlated with dose. In some cases statin therapy has been reported to increase the risk of dramatic liver effects such as acute liver failure and death, but this is an extremely rare complication, uncommon and often asymptomatic. Liver function tests should not be routinely monitored in patients receiving long-term statin therapy. If NAFLD, NASH, compensated cirrhosis and chronic liver disease are not contraindications for statin therapy, significant cholestasis, acute liver failure or decompensated cirrhosis are. The best options in reducing the risks of adverse liver effects are caution in patients at risk of liver dysfunction, in particular with statin dose, observance of contraindications for the use of lipid-lowering agents, and careful attention to drug interactions. References 1 Anonymous, Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934): Grundy SM, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3): Corsini A, et al. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84(3): Bradford RH, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results: two-year efficacy and safety follow-up. Am J Cardiol. 1994;74(7): Vuppalanchi R, Chalasani N. Statins for hyperlipidemia in patients with chronic liver disease: are they safe? Clin Gastroenterol Hepatol.2006;4(7): Kinnman N, Hultcrantz R. Lipid lowering medication and hepatotoxicity. J Intern Med. 2001;250(3): Chang CY, Schiano TD. Review article: drug hepatotoxicity. Aliment Pharmacol Ther. 2007;25(10): Goettsch WG, et al. Results from a rosuvastatin historical cohort study in more than 45,000 Dutch statin users, a PHARMO study. Pharmacoepidemiol Drug Saf. 2006;15(7): McKenney JM, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97 (8A):89C 94C. 10 Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109(23 Suppl 1):III Dale KM, et al. Impact of statin dosing intensity on transaminase and creatine kinase. Am J Med. 2007;120(8): Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C 60C. 14 Newman C, et al. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97(1): Alsheikh-Ali AA, et al. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50(5): Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. Am J Cardiol. 2006;97(8A):44C 51C. 17 Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94(9): Holdaas H, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361(9374): Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289(13): Jacobson TA, et al. Safety considerations with gastrointestinally active lipidlowering drugs. Am J Cardiol. 2007;99(6A):47C 55C. 21 Hodis HN. Acute hepatic failure associated with the use of low-dose sustainedrelease niacin. JAMA. 1990;264(2): Guyton JR, Bays HE. Safety considerations with niacin therapy. Am J Cardiol. 2007;99(6A):22C 31C. 23 de Diego Lorenzo A, et al. Cholestatic hepatitis caused by gemfibrozil. Rev Esp Enferm Dig. 2001;93(9): Ho CY, et al. Fenofibrate-induced acute cholestatic hepatitis. J Chin MedAssoc. 2004;67(5): Stolk MF, et al. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol. 2006;4(7): Cruz-Fernandez JM, et al. Efficacy and safety of ezetimibe co-administered with ongoing atorvastatin therapy in achieving low-density lipoprotein goal in patients with hypercholesterolemia and coronary heart disease. Int J Clin Pract. 2005;59(6): Shek A, Ferrill MJ. Statin-fibrate combination therapy. Ann Pharmacother. 2001;35(7-8): Cziraky MJ, et al. Statin safety: an assessment using an administrative claims database. Am J Cardiol. 2006;97(8A):61C 68C. 29 KeechA, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500): Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97(8A):77C 81C. 31 FDA. [cited; Available from: < 32 Bradford RH, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med. 1991;151(1): Gotto AM Jr. Safety and statin therapy: reconsidering the risks and benefits. Arch Intern Med. 2003;163(6): Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341(7): Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4(7):902 7; quiz Lewis JH, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5): Chalasani N, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5): Georgescu EF, Georgescu M. Therapeutic options in non-alcoholic steatohepatitis (NASH). Are all agents alike? Results of a preliminary study. J Gastrointestin Liver Dis. 2007;16(1): Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4): Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41(5):
LIVER FUNCTION TESTS AND STATINS
 LIVER FUNCTION TESTS AND STATINS Philippe J. Zamor and Mark W. Russo Current Opinion in Cardiology 2011,26:338 341 SUMMARY Purpose of review: To discuss recent data on statins in patients with elevated
LIVER FUNCTION TESTS AND STATINS Philippe J. Zamor and Mark W. Russo Current Opinion in Cardiology 2011,26:338 341 SUMMARY Purpose of review: To discuss recent data on statins in patients with elevated
Primary Care Management of Women with Hyperlipidemia. Julie Marfell, DNP, BC, FNP, Chairperson, Department of Family Nursing
 Primary Care Management of Women with Hyperlipidemia Julie Marfell, DNP, BC, FNP, Chairperson, Department of Family Nursing Objectives: Define dyslipidemia in women Discuss the investigation process leading
Primary Care Management of Women with Hyperlipidemia Julie Marfell, DNP, BC, FNP, Chairperson, Department of Family Nursing Objectives: Define dyslipidemia in women Discuss the investigation process leading
High Blood Cholesterol
 National Cholesterol Education Program ATP III Guidelines At-A-Glance Quick Desk Reference 1 Step 1 2 Step 2 3 Step 3 Determine lipoprotein levels obtain complete lipoprotein profile after 9- to 12-hour
National Cholesterol Education Program ATP III Guidelines At-A-Glance Quick Desk Reference 1 Step 1 2 Step 2 3 Step 3 Determine lipoprotein levels obtain complete lipoprotein profile after 9- to 12-hour
Patient Information VYTORIN (VI-tor-in) (ezetimibe and simvastatin) Tablets
 Patient Information VYTORIN (VI-tor-in) (ezetimibe and simvastatin) Tablets Read this Patient Information carefully before you start taking VYTORIN and each time you get a refill. There may be new information.
Patient Information VYTORIN (VI-tor-in) (ezetimibe and simvastatin) Tablets Read this Patient Information carefully before you start taking VYTORIN and each time you get a refill. There may be new information.
Statins for Hyperlipidemia (High Cholesterol)
 Statins for Hyperlipidemia (High Cholesterol) Examples of statin drugs Brand Name Mevacor Pravachol Zocor Lescol, Lescol XL Lipitor Crestor Chemical Name lovastatin pravastatin sodium simvastatin fluvastatin
Statins for Hyperlipidemia (High Cholesterol) Examples of statin drugs Brand Name Mevacor Pravachol Zocor Lescol, Lescol XL Lipitor Crestor Chemical Name lovastatin pravastatin sodium simvastatin fluvastatin
PRESCRIBING INFORMATION Refer to Summary of Product Characteristics (SmPC) before Prescribing
 EZETROL (ezetimibe) PRESCRIBING INFORMATION Refer to Summary of Product Characteristics (SmPC) before Prescribing Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
EZETROL (ezetimibe) PRESCRIBING INFORMATION Refer to Summary of Product Characteristics (SmPC) before Prescribing Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Management of Lipids in 2015: Just Give them a Statin?
 Management of Lipids in 2015: Just Give them a Statin? James H. Stein, M.D. Division of Cardiovascular Medicine University of Wisconsin School of Medicine and Public Health Stone NJ, et al. Circulation
Management of Lipids in 2015: Just Give them a Statin? James H. Stein, M.D. Division of Cardiovascular Medicine University of Wisconsin School of Medicine and Public Health Stone NJ, et al. Circulation
MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES
 MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest EXPLAINING
MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest EXPLAINING
Statin Template Guidance Use of statins in primary and secondary prevention of vascular disease Endorsed by ABHB MTC for use in Gwent (October 2012)
 Statin Template Guidance Use of statins in primary and secondary prevention of vascular disease Endorsed by ABHB MTC for use in Gwent (October 2012) Notes relating to this guidance This guidance serves
Statin Template Guidance Use of statins in primary and secondary prevention of vascular disease Endorsed by ABHB MTC for use in Gwent (October 2012) Notes relating to this guidance This guidance serves
Alanine aminotransferase (serum, plasma)
 Alanine aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Alanine aminotransferase (ALT) 1.2 Alternative names Systematic name L alanine:2 oxoglutarate aminotransferase
Alanine aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Alanine aminotransferase (ALT) 1.2 Alternative names Systematic name L alanine:2 oxoglutarate aminotransferase
ORIGINAL INVESTIGATION. Screening for Statin-Related Toxicity. The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting
 ORIGINAL INVESTIGATION Screening for Statin-Related Toxicity The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting C. Christopher Smith, MD; Lana I. Bernstein, MD; Roger
ORIGINAL INVESTIGATION Screening for Statin-Related Toxicity The Yield of Transaminase and Creatine Kinase Measurements in a Primary Care Setting C. Christopher Smith, MD; Lana I. Bernstein, MD; Roger
NP/PA Clinical Hepatology Fellowship Summary of Year-Long Curriculum
 OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
HYPERCHOLESTEROLAEMIA STATIN AND BEYOND
 HYPERCHOLESTEROLAEMIA STATIN AND BEYOND Andrea Luk Division of Endocrinology Department of Medicine & Therapeutics The Chinese University of Hong Kong HA Convention 4 May 2016 Statins reduce CVD and all-cause
HYPERCHOLESTEROLAEMIA STATIN AND BEYOND Andrea Luk Division of Endocrinology Department of Medicine & Therapeutics The Chinese University of Hong Kong HA Convention 4 May 2016 Statins reduce CVD and all-cause
PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION
 Hull & East Riding Prescribing Committee PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION For guidance on Primary Prevention please see NICE guidance http://www.nice.org.uk/guidance/cg181
Hull & East Riding Prescribing Committee PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION For guidance on Primary Prevention please see NICE guidance http://www.nice.org.uk/guidance/cg181
Cardiovascular Risk and Dyslipidemia Management Clinician Guide MAY 2015
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Cardiovascular Risk and Dyslipidemia Management Clinician Guide MAY 2015 Introduction This Clinician Guide was developed to assist Primary Care physicians
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Cardiovascular Risk and Dyslipidemia Management Clinician Guide MAY 2015 Introduction This Clinician Guide was developed to assist Primary Care physicians
The incidence of true liver injury
 Considerations for Safe Use of Statins: Liver Enzyme Abnormalities and Muscle Toxicity R. CLARK GILLETT, JR., MD, and ANGELICA NORRELL, PharmD, Columbus Regional Healthcare System, The Medical Center,
Considerations for Safe Use of Statins: Liver Enzyme Abnormalities and Muscle Toxicity R. CLARK GILLETT, JR., MD, and ANGELICA NORRELL, PharmD, Columbus Regional Healthcare System, The Medical Center,
Omega-3 Fatty Acid Products
 Omega-3 Fatty Acid Products Policy Number: 5.01.563 Last Review: 7/2016 Origination: 6/2014 Next Review: 7/2017 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for the
Omega-3 Fatty Acid Products Policy Number: 5.01.563 Last Review: 7/2016 Origination: 6/2014 Next Review: 7/2017 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for the
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
2.1 AST can be measured in heparin plasma or serum. 3 Summary of clinical applications and limitations of measurements
 Aspartate aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Aspartate aminotransferase (AST) 1.2 Alternative names Systematic name L aspartate:2 oxoglutarate aminotransferase
Aspartate aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Aspartate aminotransferase (AST) 1.2 Alternative names Systematic name L aspartate:2 oxoglutarate aminotransferase
Primary Care Guidance Program: Non-Alcohol related Fatty Liver Disease (NAFLD) Guidance on Management in Primary Care
 Primary Care Guidance Program: Non-Alcohol related Fatty Liver Disease (NAFLD) Guidance on Management in Primary Care This advice has been developed to help GPs with shared care of patients with Non- Alcohol
Primary Care Guidance Program: Non-Alcohol related Fatty Liver Disease (NAFLD) Guidance on Management in Primary Care This advice has been developed to help GPs with shared care of patients with Non- Alcohol
Approach to Abnormal Liver Tests
 Approach to Abnormal Liver Tests Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of Gastroenterology and Hepatology Indiana University School
Approach to Abnormal Liver Tests Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of Gastroenterology and Hepatology Indiana University School
REYNOLDS QUESTION OF THE MONTH
 Arizona Geriatrics Society Vol. 15 No.1 REYNOLDS QUESTION OF THE MONTH Prescribing Statins for Older Adults Is one statin superior to the others for use in the elderly? Do older adults have a higher incidence
Arizona Geriatrics Society Vol. 15 No.1 REYNOLDS QUESTION OF THE MONTH Prescribing Statins for Older Adults Is one statin superior to the others for use in the elderly? Do older adults have a higher incidence
Welchol (colesevelam HCl) Receives FDA Approval to Reduce Blood Glucose in Adults with Type 2 Diabetes
 For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Takashi Shoda, President and Representative Director (Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Takashi Shoda, President and Representative Director (Code no.: 4568, First Section, Tokyo, Osaka and Nagoya Stock Exchanges)
Lipid Management in Diabetic Patients (June 2014 version)
 (June 2014 version) Background Accelerated atherosclerosis is multifactorial and begins years/decades prior to the diagnosis of type 2 diabetes: Risk for atherosclerotic events is two to four-fold greater
(June 2014 version) Background Accelerated atherosclerosis is multifactorial and begins years/decades prior to the diagnosis of type 2 diabetes: Risk for atherosclerotic events is two to four-fold greater
Which drugs should be used to treat diabetes in cirrhotic patients?
 Which drugs should be used to treat diabetes in cirrhotic patients? Frankfurt am Main 10-12 September 2015 Jörg Bojunga Medizinische Klinik I Johann Wolfgang Goethe-Universität Frankfurt am Main Significance
Which drugs should be used to treat diabetes in cirrhotic patients? Frankfurt am Main 10-12 September 2015 Jörg Bojunga Medizinische Klinik I Johann Wolfgang Goethe-Universität Frankfurt am Main Significance
Lipid-lowering: Can ezetimibe help close the treatment gap?
 REVIEW CME CREDIT RYAN C. NEAL, MD* Assistant Professor of Medicine, Baylor College of Medicine, Houston, Texas PETER H. JONES, MD Associate Professor of Medicine, Baylor College of Medicine, Houston,
REVIEW CME CREDIT RYAN C. NEAL, MD* Assistant Professor of Medicine, Baylor College of Medicine, Houston, Texas PETER H. JONES, MD Associate Professor of Medicine, Baylor College of Medicine, Houston,
Cirrhosis and HCV. Jonathan Israel M.D.
 Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Abnormal Liver Function. Dr William Alazawi MA(Cantab) PhD MRCP Senior Lecturer and Consultant in Hepatology Queen Mary, University of London
 Abnormal Liver Function Dr William Alazawi MA(Cantab) PhD MRCP Senior Lecturer and Consultant in Hepatology Queen Mary, University of London Does Liver Disease Matter? Mortality in England & Wales Liver-related
Abnormal Liver Function Dr William Alazawi MA(Cantab) PhD MRCP Senior Lecturer and Consultant in Hepatology Queen Mary, University of London Does Liver Disease Matter? Mortality in England & Wales Liver-related
Anti-Atheroscrerotic Drugs
 Anti-Atheroscrerotic Drugs Masuko Ushio-Fukai, PhD, FAHA Dept. of Pharmacology University of Illinois at Chicago Anti-Atherogenic Drugs: Treatment of Hyperlipidemias Knowledge Objectives: 1) Know the mechanism
Anti-Atheroscrerotic Drugs Masuko Ushio-Fukai, PhD, FAHA Dept. of Pharmacology University of Illinois at Chicago Anti-Atherogenic Drugs: Treatment of Hyperlipidemias Knowledge Objectives: 1) Know the mechanism
BACKGROUND MEDIA INFORMATION Fast facts about liver disease
 BACKGROUND MEDIA INFORMATION Fast facts about liver disease Liver, or hepatic, disease comprises a wide range of complex conditions that affect the liver. Liver diseases are extremely costly in terms of
BACKGROUND MEDIA INFORMATION Fast facts about liver disease Liver, or hepatic, disease comprises a wide range of complex conditions that affect the liver. Liver diseases are extremely costly in terms of
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI)
 Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Novartis Gilenya FDO Program Clinical Protocol and Highlights from Prescribing Information (PI) Highlights from Prescribing Information - the link to the full text PI is as follows: http://www.pharma.us.novartis.com/product/pi/pdf/gilenya.pdf
Evaluation of Liver Function tests in Primary Care. Abid Suddle Institute of Liver Studies, KCH
 Evaluation of Liver Function tests in Primary Care Abid Suddle Institute of Liver Studies, KCH Liver Function tests Markers of hepatocellular damage Cholestasis Liver synthetic function Markers of Hepatocellular
Evaluation of Liver Function tests in Primary Care Abid Suddle Institute of Liver Studies, KCH Liver Function tests Markers of hepatocellular damage Cholestasis Liver synthetic function Markers of Hepatocellular
Prof. of Tropical Medicine Faculty of Medicine Alexandria University
 prof. Dr. Ali El-Kady (MD) Prof. of Tropical Medicine Faculty of Medicine Alexandria University DRUGS THAT MAY CAUSE LIVER DYSFUNCTION DAMAGE The liver is the principal organ that is capable of converting
prof. Dr. Ali El-Kady (MD) Prof. of Tropical Medicine Faculty of Medicine Alexandria University DRUGS THAT MAY CAUSE LIVER DYSFUNCTION DAMAGE The liver is the principal organ that is capable of converting
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT
 PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
PRIOR AUTHORIZATION PROTOCOL FOR HEPATITIS C TREATMENT HARVONI (90mg ledipasvir/400mg sofosbuvir): tablet (PREFERRED AGENT) SOVALDI (sofosbuvir ): 400mg tablets (PREFERRED AGENT ) OLYSIO (simeprivir) PEG-INTRON
JNC-8 Blood Pressure and ACC/AHA Cholesterol Guideline Updates. January 30, 2014
 JNC-8 Blood Pressure and ACC/AHA Cholesterol Guideline Updates January 30, 2014 GOALS Review key recommendations from recently published guidelines on blood pressure and cholesterol management Discuss
JNC-8 Blood Pressure and ACC/AHA Cholesterol Guideline Updates January 30, 2014 GOALS Review key recommendations from recently published guidelines on blood pressure and cholesterol management Discuss
HYPERTENSION ASSOCIATED WITH RENAL DISEASES
 RENAL DISEASE v Patients with renal insufficiency should be encouraged to reduce dietary salt and protein intake. v Target blood pressure is less than 135-130/85 mmhg. If patients have urinary protein
RENAL DISEASE v Patients with renal insufficiency should be encouraged to reduce dietary salt and protein intake. v Target blood pressure is less than 135-130/85 mmhg. If patients have urinary protein
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins)
 Drug Class Review on HMG-CoA Reductase Inhibitors (Statins) Final Report September 2005 The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins) Final Report September 2005 The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles
Management of hepatitis C: pre- and post-liver transplantation. Piyawat Komolmit Bangkok
 Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
Management of hepatitis C: pre- and post-liver transplantation Piyawat Komolmit Bangkok Liver transplantation and CHC Cirrhosis secondary to HCV is the leading cause of liver transplantation in the US
**Form 1: - Consultant Copy** Telephone Number: Fax Number: Email: Author: Dr Bernard Udeze Pharmacist: Claire Ault Date of issue July 2011
 Effective Shared Care Agreement for the treatment of Dementia in Alzheimer s Disease Donepezil tablets / orodispersible tablets (Aricept / Aricept Evess ) These forms (1 and 2) are to be completed by both
Effective Shared Care Agreement for the treatment of Dementia in Alzheimer s Disease Donepezil tablets / orodispersible tablets (Aricept / Aricept Evess ) These forms (1 and 2) are to be completed by both
Non-alcoholic fatty liver disease: Prognosis and Treatment
 Non-alcoholic fatty liver disease: Prognosis and Treatment Zachary Henry, M.D. Assistant Professor UVA Gastroenterology & Hepatology October 28, 2015 Overview Case Presentation Prognosis Effects of fibrosis
Non-alcoholic fatty liver disease: Prognosis and Treatment Zachary Henry, M.D. Assistant Professor UVA Gastroenterology & Hepatology October 28, 2015 Overview Case Presentation Prognosis Effects of fibrosis
Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease. From : New England Journal of Medicine
 Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease From : New England Journal of Medicine Volume 351:1521-1531, Number 15, Oct 7, 2004 馬 偕 紀 念 醫 院 一 般 內 科, 肝 膽 腸 胃 科 新 竹 分 院 陳 重
Lamivudine for Patients with hronic Hepatitis B and Advanced Liver Disease From : New England Journal of Medicine Volume 351:1521-1531, Number 15, Oct 7, 2004 馬 偕 紀 念 醫 院 一 般 內 科, 肝 膽 腸 胃 科 新 竹 分 院 陳 重
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
ELEMENTS FOR A PUBLIC SUMMARY. Overview of disease epidemiology. Summary of treatment benefits
 VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
Statins and Risk for Diabetes Mellitus. Background
 Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
Strategies to Improve Model-based Decision-making During Clinical Development
 Strategies to Improve Model-based Decision-making During Clinical Development David Hermann Pfizer Global Research and Development, Ann Arbor, USA Wenping Wang formerly with Pharsight Corporation, now
Strategies to Improve Model-based Decision-making During Clinical Development David Hermann Pfizer Global Research and Development, Ann Arbor, USA Wenping Wang formerly with Pharsight Corporation, now
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation
 NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 06/06/2012 Review Date 06/06/2014 Version 1.1 EQIA Yes /
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 06/06/2012 Review Date 06/06/2014 Version 1.1 EQIA Yes /
嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯
 The Clinical Efficacy and Safety of Sodium Glucose Cotransporter-2 (SGLT2) Inhibitors in Adults with Type 2 Diabetes Mellitus 嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯 Diabetes Mellitus : A group of diseases characterized
The Clinical Efficacy and Safety of Sodium Glucose Cotransporter-2 (SGLT2) Inhibitors in Adults with Type 2 Diabetes Mellitus 嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯 Diabetes Mellitus : A group of diseases characterized
THE THIRD REPORT OF THE EXpert
 SPECIAL COMMUNICATION Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults
SPECIAL COMMUNICATION Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults
GT-020 Phase 1 Clinical Trial: Results of Second Cohort
 GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
GT-020 Phase 1 Clinical Trial: Results of Second Cohort July 29, 2014 NASDAQ: GALT www.galectintherapeutics.com 2014 Galectin Therapeutics inc. Forward-Looking Statement This presentation contains, in
1. PATHOPHYSIOLOGY OF METABOLIC SYNDROME
 1. PATHOPHYSIOLOGY OF METABOLIC SYNDROME Izet Aganović, Tina Dušek Department of Internal Medicine, Division of Endocrinology, University Hospital Center Zagreb, Croatia 1 Introduction The metabolic syndrome
1. PATHOPHYSIOLOGY OF METABOLIC SYNDROME Izet Aganović, Tina Dušek Department of Internal Medicine, Division of Endocrinology, University Hospital Center Zagreb, Croatia 1 Introduction The metabolic syndrome
Cardiovascular Risk in Diabetes
 Cardiovascular Risk in Diabetes Lipids Hypercholesterolaemia is an important reversible risk factor for cardiovascular disease and should be tackled aggressively in all diabetic patients. In Type 1 patients,
Cardiovascular Risk in Diabetes Lipids Hypercholesterolaemia is an important reversible risk factor for cardiovascular disease and should be tackled aggressively in all diabetic patients. In Type 1 patients,
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
10/11/2014. Laura C. Halder, Pharm.D. Postgraduate Year Two Pharmacy Resident Cardiology Abbott Northwestern Hospital Allina Health October 30, 2014
 Laura C. Halder, Pharm.D. Postgraduate Year Two Pharmacy Resident Cardiology Abbott Northwestern Hospital Allina Health October 30, 2014 1 1. List two major changes to the 2013 cholesterol treatment guidelines.
Laura C. Halder, Pharm.D. Postgraduate Year Two Pharmacy Resident Cardiology Abbott Northwestern Hospital Allina Health October 30, 2014 1 1. List two major changes to the 2013 cholesterol treatment guidelines.
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT )
 BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
BCCA Protocol Summary for Palliative Treatment of Advanced Pancreatic Neuroendocrine Tumours using SUNItinib (SUTENT ) Protocol Code Tumour Group Contact Physician UGIPNSUNI Gastrointestinal Dr. Hagen
Managing potential drug-drug interactions to achieve success in HCV triple therapy. David Back University of Liverpool
 Managing potential drug-drug interactions to achieve success in HCV triple therapy David Back University of Liverpool Understanding the metabolic pathways of DAAs Drug CYP 3A4 P-gp Telaprevir Boceprevir
Managing potential drug-drug interactions to achieve success in HCV triple therapy David Back University of Liverpool Understanding the metabolic pathways of DAAs Drug CYP 3A4 P-gp Telaprevir Boceprevir
IS VITAMIN E SAFE TO USE?
 Vitamin E & Fatty Liver IS VITAMIN E SAFE TO USE? Nonalcoholic Fatty Liver Disease (NAFLD) & Nonalcoholic Steatohepatitis (NASH) Prevalence: 5.7-16.5% 57 5 in US Usually diagnosed in 40-60 y/o s Non-significant
Vitamin E & Fatty Liver IS VITAMIN E SAFE TO USE? Nonalcoholic Fatty Liver Disease (NAFLD) & Nonalcoholic Steatohepatitis (NASH) Prevalence: 5.7-16.5% 57 5 in US Usually diagnosed in 40-60 y/o s Non-significant
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Your healthcare provider has ordered a Boston Heart Cardiac Risk Assessment
 Your healthcare provider has ordered a Boston Heart Cardiac Risk Assessment What does that mean for you? Your healthcare provider has determined that you may be at risk for cardiovascular disease (CVD).
Your healthcare provider has ordered a Boston Heart Cardiac Risk Assessment What does that mean for you? Your healthcare provider has determined that you may be at risk for cardiovascular disease (CVD).
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation
 NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016 Version 1.4 EQIA Yes 01/06/2012
NHS FORTH VALLEY Rivaroxaban for Stroke Prevention in Atrial Fibrillation Date of First Issue 06/06/2012 Approved 06/06/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016 Version 1.4 EQIA Yes 01/06/2012
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
High Cholesterol and Heart Disease
 Evaluating statin drugs to treat: High Cholesterol and Heart Disease Comparing Effectiveness, Safety, and Price Contents 2: Our recommendations 4: Welcome 7: Who should take a statin drug? 10: Choosing
Evaluating statin drugs to treat: High Cholesterol and Heart Disease Comparing Effectiveness, Safety, and Price Contents 2: Our recommendations 4: Welcome 7: Who should take a statin drug? 10: Choosing
Making Sense of the New Statin guidelines. They are more than just lowering your cholesterol!
 Making Sense of the New Statin guidelines They are more than just lowering your cholesterol! No Disclosures Margaret (Peg) O Donnell DNPs, FNP, ANP B-C, FAANP Senior Nurse Practitioner South Nassau Communities
Making Sense of the New Statin guidelines They are more than just lowering your cholesterol! No Disclosures Margaret (Peg) O Donnell DNPs, FNP, ANP B-C, FAANP Senior Nurse Practitioner South Nassau Communities
gemfibrozil, cyclosporine, danazol Verapamil, diltiazem, Do not exceed 10 mg simvastatin dronedarone daily
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZOCOR safely and effectively. See full prescribing information for ZOCOR. ZOCOR () Tablets Initial
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ZOCOR safely and effectively. See full prescribing information for ZOCOR. ZOCOR () Tablets Initial
Patterns of abnormal LFTs and their differential diagnosis
 Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT
 Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
Page 1 of 6 TITLE: PATIENTS CANDIDA INFECTIONS - TREATMENT GUIDELINES IN ADULT GUIDELINES: These are the 2011 Guidelines for the Treatment of Candida species infections in adult patients. These recommendations
TACROLIMUS LEVELS IN LIVER TRANSPLANT; INDIAN STUDY
 TACROLIMUS LEVELS IN LIVER TRANSPLANT; INDIAN STUDY PRADEEP NAIK*, DHARMESH KAPOOR**, DCS REDDY** *Dept. of Biochemistry, ** Dept. of Hepatology Global Hospital, lakdi ka pool, Hyderabad, 500004. Introduction:
TACROLIMUS LEVELS IN LIVER TRANSPLANT; INDIAN STUDY PRADEEP NAIK*, DHARMESH KAPOOR**, DCS REDDY** *Dept. of Biochemistry, ** Dept. of Hepatology Global Hospital, lakdi ka pool, Hyderabad, 500004. Introduction:
ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE
 ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE I- BACKGROUND: Coronary artery disease and stoke are the major killers in the United States.
ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE I- BACKGROUND: Coronary artery disease and stoke are the major killers in the United States.
BMC Med 7/19/2007; Simvastatin linked to reduced incidence of dementia, Parkinson s disease.
 March 3, 2012 BD Response to FDA statement regarding Statins The Food and Drug Administration announced on Tuesday (February 28, 2012) the changes to the safety information on the labels of statins regarding
March 3, 2012 BD Response to FDA statement regarding Statins The Food and Drug Administration announced on Tuesday (February 28, 2012) the changes to the safety information on the labels of statins regarding
After the Cure: Long-Term Management of HCV Liver Disease Norah A. Terrault, MD, MPH
 After the Cure: Long-Term Management of HCV Liver Disease Norah A. Terrault, MD, MPH Professor of Medicine Department of Gastroenterology Director, Viral Hepatitis Center University of California San Francisco
After the Cure: Long-Term Management of HCV Liver Disease Norah A. Terrault, MD, MPH Professor of Medicine Department of Gastroenterology Director, Viral Hepatitis Center University of California San Francisco
1/7/2012. Objectives. Epidemiology of Atrial Fibrillation(AF) Stroke in AF. Stroke Risk Stratification in AF
 Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Objectives Atrial Fibrillation and Prevention of Thrombotic Complications: Therapeutic Update Andrea C. Flores Pharm.D Pharmacy Resident at the Miami VA Healthcare System Review the epidemiology, pathophysiology
Anticoagulation at the end of life. Rhona Maclean Rhona.maclean@sth.nhs.uk
 Anticoagulation at the end of life Rhona Maclean Rhona.maclean@sth.nhs.uk Content Anticoagulant Therapies Indications for anticoagulation Venous thromboembolism (VTE) Atrial Fibrillation Mechnical Heart
Anticoagulation at the end of life Rhona Maclean Rhona.maclean@sth.nhs.uk Content Anticoagulant Therapies Indications for anticoagulation Venous thromboembolism (VTE) Atrial Fibrillation Mechnical Heart
TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION. Robert Dobbins, M.D. Ph.D.
 TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION Robert Dobbins, M.D. Ph.D. Learning Objectives Recognize current trends in the prevalence of type 2 diabetes. Learn differences between type 1 and type
TYPE 2 DIABETES MELLITUS: NEW HOPE FOR PREVENTION Robert Dobbins, M.D. Ph.D. Learning Objectives Recognize current trends in the prevalence of type 2 diabetes. Learn differences between type 1 and type
Cholesterol and Triglycerides What You Should Know
 Cholesterol and Triglycerides What You Should Know Michael T. McDermott MD Professor of Medicine Endocrinology Practice Director Division of Endocrinology, Metabolism and Diabetes University of Colorado
Cholesterol and Triglycerides What You Should Know Michael T. McDermott MD Professor of Medicine Endocrinology Practice Director Division of Endocrinology, Metabolism and Diabetes University of Colorado
Education. Panel. Triglycerides & HDL-C
 Triglycerides & HDL-C Thomas Dayspring, MD, ACP Clinical Assistant Professor of Medicine University of Medicine and Dentistry of New Jersey Attending in Medicine: St Joseph s s Hospital, Paterson, NJ Certified
Triglycerides & HDL-C Thomas Dayspring, MD, ACP Clinical Assistant Professor of Medicine University of Medicine and Dentistry of New Jersey Attending in Medicine: St Joseph s s Hospital, Paterson, NJ Certified
FDA Approved Oral Anticoagulants
 FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS
 Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Adams Memorial Hospital Decatur, Indiana EXPLANATION OF LABORATORY TESTS Your health is important to us! The test descriptions listed below are for educational purposes only. Laboratory test interpretation
Update on Hepatitis C. Sally Williams MD
 Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
Update on Hepatitis C Sally Williams MD Hep C is Everywhere! Hepatitis C Magnitude of the Infection Probably 8 to 10 million people in the U.S. are infected with Hep C 30,000 new cases are diagnosed annually;
MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol)
 MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol) 1. Hepatotoxicity: In Active TB Disease a. Background: 1. Among the 4 standard anti-tb drugs,
MANAGEMENT OF COMMON SIDE EFFECTS of INH (Isoniazid), RIF (Rifampin), PZA (Pyrazinamide), and EMB (Ethambutol) 1. Hepatotoxicity: In Active TB Disease a. Background: 1. Among the 4 standard anti-tb drugs,
What to Do with the Patient With Abnormal Liver Enzymes? Nizar N. Zein, M.D. The Cleveland Clinic
 What to Do with the Patient With Abnormal Liver Enzymes? Nizar N. Zein, M.D. The Cleveland Clinic Introduction Elevated liver enzymes is often not a clinical problem by itself. However it is a warning
What to Do with the Patient With Abnormal Liver Enzymes? Nizar N. Zein, M.D. The Cleveland Clinic Introduction Elevated liver enzymes is often not a clinical problem by itself. However it is a warning
See 17 for PATIENT COUNSELING INFORMATION CYP3A4 inhibitors (e.g., clarithromycin, itraconazole, HIV protease
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LIPITOR safely and effectively. See full prescribing information for LIPITOR. LIPITOR (atorvastatin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LIPITOR safely and effectively. See full prescribing information for LIPITOR. LIPITOR (atorvastatin
Clinical Study Synopsis for Public Disclosure
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis - which is part of the clinical
More information for patients and caregivers can be accessed at http://www.xarelto-us.com/.
 Janssen Research & Development Submits Application to U.S. FDA for XARELTO (rivaroxaban) to Reduce Secondary Cardiovascular Events in Patients with Acute Coronary Syndrome RARITAN, DECEMBER 29, 2011 -
Janssen Research & Development Submits Application to U.S. FDA for XARELTO (rivaroxaban) to Reduce Secondary Cardiovascular Events in Patients with Acute Coronary Syndrome RARITAN, DECEMBER 29, 2011 -
Role of Body Weight Reduction in Obesity-Associated Co-Morbidities
 Obesity Role of Body Weight Reduction in JMAJ 48(1): 47 1, 2 Hideaki BUJO Professor, Department of Genome Research and Clinical Application (M6) Graduate School of Medicine, Chiba University Abstract:
Obesity Role of Body Weight Reduction in JMAJ 48(1): 47 1, 2 Hideaki BUJO Professor, Department of Genome Research and Clinical Application (M6) Graduate School of Medicine, Chiba University Abstract:
NEW GUIDELINES FOR CHOLESTEROL MANAGEMENT: WHAT HAS CHANGED? LAUREN HYNICKA, PHARMD, BCPS MOHAMED SARG, PHARMD, BCPS
 NEW GUIDELINES FOR CHOLESTEROL MANAGEMENT: WHAT HAS CHANGED? LAUREN HYNICKA, PHARMD, BCPS MOHAMED SARG, PHARMD, BCPS NEW GUIDELINES FOR CHOLESTEROL MANAGEMENT: WHAT HAS CHANGED? ACTIVITY DESCRIPTION Cholesterol
NEW GUIDELINES FOR CHOLESTEROL MANAGEMENT: WHAT HAS CHANGED? LAUREN HYNICKA, PHARMD, BCPS MOHAMED SARG, PHARMD, BCPS NEW GUIDELINES FOR CHOLESTEROL MANAGEMENT: WHAT HAS CHANGED? ACTIVITY DESCRIPTION Cholesterol
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
Liver Diseases. An Essential Guide for Nurses and Health Care Professionals
 Brochure More information from http://www.researchandmarkets.com/reports/1047385/ Liver Diseases. An Essential Guide for Nurses and Health Care Professionals Description: Liver disease is a rapidly growing
Brochure More information from http://www.researchandmarkets.com/reports/1047385/ Liver Diseases. An Essential Guide for Nurses and Health Care Professionals Description: Liver disease is a rapidly growing
Amylase and Lipase Tests
 Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
NASH: It is not JUST a Fatty Liver. Karen F. Murray, M.D. Director of Hepatobiliary Program Children s Hospital and Regional Medical Center
 NASH: It is not JUST a Fatty Liver Karen F. Murray, M.D. Director of Hepatobiliary Program Children s Hospital and Regional Medical Center Stages of Fatty Liver Disorders Fatty Liver 16-35% of Western
NASH: It is not JUST a Fatty Liver Karen F. Murray, M.D. Director of Hepatobiliary Program Children s Hospital and Regional Medical Center Stages of Fatty Liver Disorders Fatty Liver 16-35% of Western
Drug/Drug and Drug/Food Interactions with Target-Specific Oral Anticoagulants
 Drug/Drug and Drug/Food Interactions with Target-Specific Oral Anticoagulants Sara R. Vazquez, PharmD, BCPS, CACP Clinical Pharmacist University of Utah Health Care Thrombosis Service Nothing to disclose
Drug/Drug and Drug/Food Interactions with Target-Specific Oral Anticoagulants Sara R. Vazquez, PharmD, BCPS, CACP Clinical Pharmacist University of Utah Health Care Thrombosis Service Nothing to disclose
How To Treat Dyslipidemia
 An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS
 NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS Date of First Issue 01/12/ 2012 Approved 15/11/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016
NHS FORTH VALLEY RIVAROXABAN AS TREATMENT FOR DEEP VEIN THROMBOSIS AND PULMONARY EMBOLISM IN ADULTS Date of First Issue 01/12/ 2012 Approved 15/11/2012 Current Issue Date 29/10/2014 Review Date 29/10/2016
Many asymptomatic individuals
 Facts, myths and misconceptions about LDL-C and HDL-C By Michael B. Clearfield, DO Many asymptomatic individuals will succumb to cardiovascular disease (CVD), which is the leading cause of death and loss
Facts, myths and misconceptions about LDL-C and HDL-C By Michael B. Clearfield, DO Many asymptomatic individuals will succumb to cardiovascular disease (CVD), which is the leading cause of death and loss
Initiation and Adjustment of Insulin Regimens
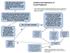 Start with bedtime intermediateacting insulin or bedtime or morning long-acting insulin (can initiate with 10 units or 0.2 units per kg) Initiation and Adjustment of Insulin Regimens Insulin regimens should
Start with bedtime intermediateacting insulin or bedtime or morning long-acting insulin (can initiate with 10 units or 0.2 units per kg) Initiation and Adjustment of Insulin Regimens Insulin regimens should
