Bone allograft in the UK: perceptions and realities
|
|
|
- Juniper Horn
- 8 years ago
- Views:
Transcription
1 Hip Int 2013; 23 ( 5 ): DOI: /hipint Review open access Bone allograft in the UK: perceptions and realities Richard Lomas 1, Akila Chandrasekar 1, Timothy N. Board 2 1 NHS Blood and Transplant Tissue Services, Liverpool - UK 2 Centre for Hip Surgery, Wrightington Hospital, Wigan - UK Bone allografts are widely used in the UK in joint revision surgery. Despite this widespread usage, there remain concerns among the surgical community regarding the safety of allografts, in terms of the risk of transmission of infection, together with a persistent misconception that allografts are in limited availability. In this paper we discuss the precautions taken to ensure that allografts are safe, and review the residual risks. We also demonstrate that the availability of allograft in the UK, both actual and potential, greatly exceeds the current clinical demand. Keywords: Allograft, Safety, Bone Accepted: November 22, 2012 INTRODUCTION Bone allografts are widely used by orthopaedic surgeons in the UK. The main clinical applications where bone allografts are used are for impaction grafting in joint revision surgery (1), and in spinal fusion surgery (2). For both these indications, the primary source of bone allograft is femoral heads, donated by patients undergoing hip arthroplasty. Bone grafts may also be donated by deceased donors, and for certain specialist procedures requiring massive bone allografts, such as reconstruction following tumour resection (3), grafts must be obtained from this source. However, we will focus for the purposes of this review on femoral head allografts donated by living donors. Following donation, the allografts are banked, either in local hospital based banks, or larger regional or national banks. They may be utilised either as unprocessed grafts or processed to remove donor marrow content (4), either by the providing tissue bank or in theatre immediately prior to use. Where bone grafting is required, it is established dogma that autograft is the gold standard, in terms of clinical performance. Autograft provides the structural properties of bone graft, together with autologous cells that are necessary for graft incorporation. The most common source of autograft bone is the patient s iliac crest. There are however significant drawbacks to using autograft. Firstly, there is a limited amount of material that can be safely removed, and this may be insufficient to provide the large volume of graft material required. Secondly, the harvest of autograft can cause long term donor site morbidity; for example, the use of iliac crest autograft for spinal fusion has been associated with chronic long term after effects at the donor site in a significant proportion of patients (5). Finally, the harvesting of autograft from a distal site increases the theatre time required to perform a procedure, which has resource implications for hospitals. For these reasons, allograft may be used to either replace or extend autograft where bone grafting is needed. Allograft has been shown to provide excellent clinical performance in different clinical indications (1, 2), in some cases equivalent to that provided by autograft. NHS Blood and Transplant (NHSBT) is a special health authority within the NHS, with responsibility for blood, organ and the bulk of tissue donation within the UK. In this role, we regularly meet with surgeons from different orthopaedic specialities to determine what they require in terms of allograft provision, and to ensure that these requirements are met. During these discussions, and notwithstanding 427
2 Bone allograft in the UK: perceptions and realities the widespread use of allograft in the UK, two concerns are consistently expressed: I) Allografts are unsafe; there is a risk of disease transmission from the donor to the recipient, or transmission of infection from the graft; II) There are not sufficient allografts available to meet clinical demand. In this review, we attempt address these concerns, and discuss whether or not they are justified. ARE ALLOGRAFTS SAFE? Any bone allograft has the potential to transmit disease to the recipient. This potential arises from either pre-existing diseases carried by the donor, or from contamination of the graft acquired during the donation process, or subsequent processing. The risk of disease transmission is a function principally of donor selection and screening, and of how the bone is processed and prepared, including any sterilisation and disinfection processes applied. While it is not, and will probably never be possible to state absolutely that there is no risk of disease transmission, the risks can be controlled and minimised to an acceptable level. The risk reduction process begins with donor selection. This is intended to screen out any potential donors who may harbour disease causing agents, or whose bone may not be suitable for transplantation for other reasons. For example it may be mechanically weak due to osteoporosis, or unsafe for transplantation due to a history of malignancy in the donor (there is a potential risk that malignant cells could be transferred to recipients by the graft). The UK guidelines for selection of living and deceased donors of bone are set by specialist committees working under the Joint UK Blood Transfusion Services/National Institute for Biological Standards and Control (NIBSC) Professional Advisory Committee (JPAC), and are freely available to view online (6). These committees are composed of clinical and scientific experts in the fields of blood, organ and tissue transplantation. The remits of JPAC and its subsidiary committees are to prepare detailed service guidelines for the UK Blood Transfusion Services (who are responsible for the majority of clinical tissue banking in the UK) and to provide advice to them as required. Other NHS tissue banks are free to adopt these guidelines. Donor selection has two distinct phases. The first is a thorough review of the donor s medical and behavioural history. The purpose of this is to identify any disease causing agents the donor may have been exposed to and harbour. These are summarised in Table I. Medical history is taken to identify any specific risks that the donor may harbour transmissible diseases. Behavioural history will address any aspects of the donor s activities that may increase their exposure to disease causing agents. This will include personal activities, such as intra-venous drug abuse, recent tattooing, or travel history to areas where transmissible disease causing agents (e.g. malaria) are endemic. This initial phase of donor screening is very effective; data show that after medical and behavioural history pre-screening, the risk of a donor subsequently testing positive for viral pathogens during the second phase of donor screening is less than 1 in 1,500 (Tab. II). TABLE I - POTENTIAL DONOR DERIVED DISEASE CAUSING AGENTS Class of agent Viruses Bacteria and fungi Prions Malignant cells Specific risk Transmission of specific viral diseases Transmission of sepsis, contamination of grafts with microbial toxins Transmission of diseases caused by prion agents (e.g. TSEs b ) Transmission of malignancies a Nucleic acid testing b Transmissible Spongiform Encephalopathies. Screening methodology Medical and behavioural history, serology screening, NAT a Medical history (e.g. recent, active infection) Medical history (e.g. unexplained dementia, or history of blood transfusion) Medical history (history of or active malignancy) Table II - LIVING BONE DONORS TESTED FOR TRANSMIS- SIBLE VIRAL INFECTIONS, (NHSBT/ HEALTH PROTECTION AGENCY SURVEILLANCE DATA (8)) Total No. of donors tested: 34,750 Hepatitis C positive 13 Hepatitis B positive 10 HIV positive 0 HTLV positive 0* * HTLV testing commenced in
3 Lomas et al The second phase of donor screening is the testing of a blood sample collected at the time of donation for transmissible diseases. The first Technical Directive of the European Cell and Tissue Directive (2006/17/EC) defines the minimum screening requirements for all EU member states. They are predominantly viral markers, and comprise HIV 1 and 2, Hepatitis B and C, and syphilis. Individual member states, or tissue banking organisations within member states, can and do set more stringent criteria over and above EU directive requirements; for example in the UK, HTLV testing is also performed for all donors of tissue to blood service tissue banks. Blood can be tested by two basic techniques; serological screening, which detects antibodies or antigens, and Nucleic Acid Testing (NAT), which detects microbial DNA sequences. The advantage of serological screening for antibodies is that it can provide evidence of a past infection, even if the disease is no longer present - this may indicate a behavioural risk. The serology testing does however rely on the host response to infection, and in the very early stages of infection a potential donor may not have generated sufficient antibodies to be detected. The time between the onset of infection and the time when detection of infection is possible is termed the window period. During this window period, a donor is capable of transmitting an infection but may not test positive. Nucleic acid testing, which directly detects miniscule quantities of microbial DNA, is much more sensitive than serological testing, and reduces this window period. However, as it can only directly detect microorganisms, it will not indicate a cleared past infection. Therefore, the combination of the two techniques both greatly reduces the possibility of a false negative test result and provides an indication of any behavioural risk. With novel and emerging infections, as was the case with HIV and Hepatitis C, there will be a period where the pathogen is present in the donor population but not known about, and a subsequent period after the pathogen is identified but before a validated test is available. However, pathogen inactivation steps, such as processing and sterilisation help reduce the risk of transmission of both known and unknown pathogens. There are two core reasons why bone grafts are processed: I) To make them more clinically effective. There is evidence that the lipid components of donor marrow interfere with the revascularisation and incorporation of bone allografts, and that removing donor marrow can improve their osteoconductivity (9); II) To make them safer. Much of the potential infectivity of an allograft resides in its blood and bone marrow content. Removing this can reduce much of any potential infectivity (4). This is especially important for prions, for which there is no blood test available, and which are resistant to any sterilisation process we can use for bone allografts. The principle reason for processing a bone graft is to remove the bone marrow and cell content, leaving a minimally immunogenic bone matrix which is highly osteoconductive. This functions as a supportive scaffold, providing immediate biomechanical support, and which can be colonised by the recipient s cells, over time being remodelled and replaced by host bone. Donor bone marrow is not believed to contribute to this process, and may even retard it (7). Bone may be processed by the tissue bank providing the graft, or by the surgeon immediately prior to use. Marrow depletion protocols used by tissue banks comprise combinations of solvent and/or detergent cleaning, combined with the use of elevated temperatures and positive or negative pressure, whilst those used by surgeons in theatre are necessarily less complex, generally being restricted to the use of pressurised water jets and washing the bone in warm saline or water. Processing methodologies may also be antimicrobial, either via the removal of material that may be harbouring infectious agents, or through the direct antimicrobial effects of processing reagents or elevated temperatures. Currently in the UK, femoral head grafts from living donors are seldom if at all processed by the providing tissue bank due to cost and practicality. They are provided as unprocessed bone, but may undergo cutting, morsellisation and marrow depletion in theatre prior to implantation. In our experience, approximately 90% of femoral head grafts are morsellised prior to implantation, with the remainder being used as structural grafts. For surgeons wishing to use pre-processed bone, shaped or morsellised grafts from deceased tissue donors are available. Bone grafts, whether processed or not, may also be terminally sterilised before being implanted. For banked bone, is advisable that terminal sterilisation be performed if any processing has been done, as the bone will have been exposed to the risk of environmental contamination during processing. The most common methodology for terminally sterilising bone allografts is gamma irradiation, which has the advantage of being able to be applied after the bone has been processed and sealed in its final packaging. While irradiation can be relied upon to inactivate micro-organisms 429
4 Bone allograft in the UK: perceptions and realities such as viruses, bacteria and fungi, it is not effective at inactivating prions. There is currently no sterilisation process that can be applied to bone grafts to inactivate prions that will not also destroy the graft. Irradiation also weakens the structural matrix of bone grafts, and leads to a dose dependent reduction in their biomechanical properties. This reduction may or may not be clinical significant; there is some evidence that unprocessed femoral head allografts irradiated at high (50kGy) dose of irradiation do not perform as well clinically as un-irradiated grafts (9), while those irradiated at a lower (25kGy) dose perform equivalently to un-irradiated grafts (10). The efficacy of these precautions can be inferred by the fact that there has never been a case of donor to recipient disease transmission from a bone graft in the UK. Moreover, evidence suggests that the risk of deep seated infection (a rare but serious complication that affects primary and revision joint arthroplasty) is no higher for procedures utilising bone grafts than those that do not (12). CAN SUFFICIENT ALLOGRAFT BE PROVIDED TO MEET CLINICAL DEMAND? This is a concern that is often expressed by surgeons, and it is a common perception that insufficient bone allograft is available in the UK, or that there is a waiting list. To investigate this concern, it is first necessary to understand what the clinical demand for bone allograft is. It is well established that in the UK, the major use of bone allograft by bulk is in impaction grafting during joint (principally hip) revision. When using bone graft for this purpose, most surgeons prefer to utilise femoral head allografts from living donors, as this is a readily available graft, is easy to work with, and has good results in the orthopaedic literature (1). The essential question to ask therefore is how many joint revision procedures are performed using bone allograft, and how many primary hip replacements are performed over the same period? This analysis also needs to consider that hip revision procedures often require more than one femoral head; in our experience, the average number of femoral heads used per revision is Additionally, not all femoral heads removed during hip replacement will be available for transplantation; not all patients will consent or be medically suitable to donate their bone, and not all hospitals where hip replacement is performed will have donation programmes in place to enable femoral heads to be donated to a local, regional or national bank. In performing this analysis, the National Joint Registry (NJR) for England and Wales is a valuable tool. The NJR was established in 2002 to collect information on joint surgery and monitor the performance of joint replacement implants. It collects data on all hip, knee, ankle, elbow and shoulder replacement and revision surgery performed within the NHS and independent hospitals. Of particular interest to our analysis, the NJR dataset for revision arthroplasty includes a question whether or not bone graft was used during the procedure. It should be noted that the definition of bone graft in this context could include allograft, autograft or artificial bone substitute; estimates of the proportion of these cases that utilise allograft range from the vast majority to approximately 50%. To facilitate the analysis, a custom dataset extract was kindly provided by the NJR, summarising the number of primary hip replacements and hip revisions performed during the calendar year 2010 (Tab. III). This revealed that in 2010, 6,727 revision hip arthroplasties were performed in England and Wales, 1,871 (29.8%) of which used bone graft. There was considerable variation between the percentage of revision arthroplasties using bone graft amongst individual hospitals (Tab. II), ranging from 1.1% to 47.4% amongst those hospital trusts performing the most hip revision procedures. If, to take the highest demand scenario, we assume that the potential demand for bone allograft is equal to that hospital trust utilising the highest percentage of bone graft in hip revision, and that every revision arthroplasty utilises allograft rather than autograft or synthetic bone substitute, we can estimate the total demand for bone graft, in hip revision, to be 7,749 femoral heads per annum. We also know from the NJR data that over this same period, 70,669 primary hip replacements were performed. In our experience, a proportion of these femoral heads will not be suitable or available for donation, principally due to the donors not meeting our medical acceptance criteria for bone donation: we find that approximately 35% of potential donors fall into this category. However, this still leaves a donor pool of up to 45,934 grafts per annum, six times our worst case clinical demand assumption. The limits of this very basic estimation must be acknowledged; we have only considered one potential use of bone allograft, that of hip revision, and not others such as knee revision, or spinal fusion. However, we can state with confidence that all other uses of femoral head allograft put 430
5 Lomas et al TABLE III - UK HOSPITAL TRUSTS PERFORMING MOST HIP REVISIONS, AND PERCENTAGE USING BONE GRAFT (2010 DATA) Name of trust No. hip revision procedures No. using bone graft % using bone graft Wrightington Wigan And Leigh NHS Foundation Trust Nuffield Orthopaedic Centre NHS Trust Robert Jones And Agnes Hunt Orthopaedic And District Hospital NHS Trust The Royal Orthopaedic Hospital NHS Foundation Trust North Bristol NHS Trust Norfolk And Norwich University Hospitals NHS Foundation Trust Sheffield Teaching Hospitals NHS Foundation Trust East Kent Hospitals University NHS Foundation Trust Nottingham University Hospitals NHS Trust Cardiff and Vale University Local Health Board Abertawe Bro Morgannwg University Local Health Board North Tees And Hartlepool NHS Foundation Trust Taunton And Somerset NHS Foundation Trust Royal Devon And Exeter NHS Foundation Trust Royal National Orthopaedic Hospital NHS Trust Northumbria Healthcare NHS Foundation Trust Aneurin Bevan Local Health Board York Teaching Hospital NHS Foundation Trust Gloucestershire Hospitals NHS Foundation Trust East Sussex Hospitals NHS Trust TABLE IV - NUMBER OF FEMORAL HEADS BANKED AND ISSUED ANNUALLY, (NHSBT) Year No. grafts banked No. grafts issued ,313 3, ,761 2, ,806 2, ,083 2, ,410 2,642 together only amount to a small proportion of that used for hip revision. We have also based our estimation of clinical demand on the top end of the usage scale, a hospital trust that uses bone allograft in 47.4% of its hip revision procedures. This is far higher than the average percentage use, 29.8%. However, even accounting for these, there is obviously a large excess potential supply of bone available. Presently, bone may be banked either by the trust themselves, regional banks collecting bone from more than one local hospital, or by NHSBT s national banking service. The total number of femoral heads banked by these three sources is only a fraction of the potential amount available; for example, over the time period in question NHSBT banked 4,083 femoral heads. The number of femoral heads banked elsewhere is not known, but is unlikely to be more than a fraction of that banked by NHSBT. One factor which renders it difficult for individual hospitals to establish a bone banking programme is regulatory requirement imposed by the Human Tissue Authority. There is a financial burden due to the cost of the licence itself, plus human resource commitments required to meet the requirements of the licence and inspections. Participating in NHSBT s banking service has the advantage of NHSBT assuming reponsibility for these issues, and providing participating hospitals with satellite site licences and staff training permitting them to collect femoral heads for NHSBT s centralised banking service. Table IV summarises the collection and provision of femoral head allografts from living donors by NHSBT during the calendar years 2007 to Between 2007 to 2009, 431
6 Bone allograft in the UK: perceptions and realities clinical demand closely approximated the number of grafts available, and may in reality have been restricted by limited availability. It was common during this period for there to be a short waiting list for grafts, and this may have contributed to the perception amongst surgeons that there was limited availability. This was addressed through improving the efficiency of NHSBTs collection services, by concentrating resources in hospitals with the potential to donate large numbers of femoral heads, and improving the efficiency of collection therein, and closing down donation programmes in smaller donation centres. This resulted in a significant increase in the number of grafts banked, and in 2010 and 2011 the number of grafts banked greatly exceeded the number of grafts supplied. This has enabled the implementation of a stock management system to more closely match banking to clinical requirement. However, should clinical requirements increase, the number of collection sites can easily be increased through the provision of satellite licences and training of hospital staff in the correct protocols and procedures for the collection of bone grafts. DISCUSSION This purpose of this paper is to address two concerns commonly expressed by surgeons regarding the provision of bone allograft. Firstly, we have discussed the risk of disease transmission via bone allograft, and the precautions in place to prevent this happening. While bone allograft (or any other form of allograft) can never be stated to be 100% safe, the risks of disease transmission are extremely low; indeed, there has never been a recorded case of disease transmission from a bone allograft in the UK. Surgeons will often ask for a precise assessment of risk, even if it is very low, in terms that they can express to patients. This is of course difficult to provide for an event that has never occurred. However, based on surveillance data from the screening of living bone donors (11), it has been calculated that the residual risk of transmitting Hepatitis B or C through a bone graft after donor selection and screening is less than 1 in 2.3 million; at current rates of bone graft use, this equates to one potential transmission every 500 years. Secondly, we have addressed the perception that bone allograft is limited in supply, and have established not only that there is more than enough allograft currently available to meet clinical requirements, but also there is a large additional supply of allograft that is not currently banked, but could be if clinical requirement were to increase. ACKNOWLEDGEMENTS We would like to thank Mrs Anita Ambrose and Ms Emma Fitzgerald for kindly providing data used in the preparation of this manuscript. Financial Support: None. Conflict of Interest: RL and AC are employees of NHSBT-Tissue Services, which provides tissue banking services to the NHS. TB is a paid consultant to NHSBT Tissue Services. Address for correspondence: Dr Richard Lomas Senior Clinical Development Scientist NHS Blood and Transplant Tissue Services 14 Estuary Banks Speke Liverpool Merseyside, L248RB, UK richard.lomas@nhsbt.nhs.uk REFERENCES 1. McNamara IR. Impaction bone grafting in revision hip surgery: past, present and future. Cell Tissue Bank. 2010;11(1): Gibson S, McLeod I, Wardlaw D, Urbaniak S. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: a randomized control trial. Spine. 2002;27(15): Rödl RW, Ozaki T, Hoffmann C, Böttner F, Lindner N, Winkelmann W. Osteoarticular allograft in surgery for highgrade malignant tumours of bone. J Bone Joint Surg Br. 2000;82(7): Lomas R, Drummond O, Kearney JN. Processing of whole femoral head allografts: a method for improving clinical efficacy and safety. Cell Tissue Bank. 2000;1(3): Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28(2): UK Blood Transfusion and Tissue Transplantation Services, guidelines for live and deceased donors of tissue. Available at: Accessed September 24,
7 Lomas et al 7. Thorén K, Aspenberg P, Thorngren KG. Lipid extracted bank bone. Bone conductive and mechanical properties. Clin Orthop Relat Res. 1995;(311): Safe Supplies: Focusing on epidemiology. Annual review from the NHSBT/HPA Colindale Epidemiology Unit Available at: C/ Accessed September 24, Hassaballa M, Mehendale S, Poniatowski S, Kalantzis G, Smith E, Learmonth ID. Subsidence of the stem after impaction bone grafting for revision hip replacement using irradiated bone. J Bone Joint Surg Br. 2009;91(1): Mehendale S, Learmonth ID, Smith EJ, Nedungayil S, Maheshwari R, Hassaballa MA. Use of irradiated bone graft for impaction grafting in acetabular revision surgery: a review of fifty consecutive cases. Hip Int. 2009;19(2): Kwong FN, Ibrahim T, Power RA. Incidence of infection with the use of non-irradiated morcellised allograft bone washed at the time of revision arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(11): Brant LJ, Davison KL, Vox Sang. Infections detected in English surgical bone and deceased donors ( ) and estimated risk of undetected hepatitis B and hepatitis C virus. 2008;95(4):
Dental Bone Grafting Options. A review of bone grafting options for patients needing more bone to place dental implants
 Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Viral Safety of Plasma-Derived Products
 Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
Viral Safety of Plasma-Derived Products SLIDE 1 This presentation will cover viral validation studies for plasma-derived products. FDA requires that the manufacturing process for biopharmaceutical products
CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS
 CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS Precision Machined Tissue Medtronic Sofamor Danek established itself as a leader in the machined allograft tissue market with the introduction of the CORNERSTONE-SR
CORNERSTONE TISSUE PARALLEL TISSUE OFFERINGS Precision Machined Tissue Medtronic Sofamor Danek established itself as a leader in the machined allograft tissue market with the introduction of the CORNERSTONE-SR
Information for people who have an increased risk of Creutzfeldt-Jakob disease (CJD)
 Information for people who have an increased risk of Creutzfeldt-Jakob disease (CJD) There are several types of Creutzfeldt-Jakob disease (CJD). In this leaflet the term CJD covers all types unless a particular
Information for people who have an increased risk of Creutzfeldt-Jakob disease (CJD) There are several types of Creutzfeldt-Jakob disease (CJD). In this leaflet the term CJD covers all types unless a particular
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
L 38/40 Official Journal of the European Union 9.2.2006
 L 38/40 Official Journal of the European Union 9.2.2006 COMMISSION DIRECTIVE 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain
L 38/40 Official Journal of the European Union 9.2.2006 COMMISSION DIRECTIVE 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain
BLOOD TRANSFUSION SAFETY
 BLOOD TRANSFUSION SAFETY Estimated use of red cell transfusion in developed countries 35% 15% 6 3 7% 34% Pregnancy-related 6% Children 3% Surgery 34% Trauma 7% Medical 35% Haematological 15% blood saves
BLOOD TRANSFUSION SAFETY Estimated use of red cell transfusion in developed countries 35% 15% 6 3 7% 34% Pregnancy-related 6% Children 3% Surgery 34% Trauma 7% Medical 35% Haematological 15% blood saves
ADVISORY COMMITTEE ON DANGEROUS PATHOGENS Annex M guidance
 ACDP/98/P8 Open Government Status: Open ADVISORY COMMITTEE ON DANGEROUS PATHOGENS Annex M guidance Issue The ACDP TSE Risk Management Sub Group has produced some new guidelines for managing variant Creutzfeldt-Jakob
ACDP/98/P8 Open Government Status: Open ADVISORY COMMITTEE ON DANGEROUS PATHOGENS Annex M guidance Issue The ACDP TSE Risk Management Sub Group has produced some new guidelines for managing variant Creutzfeldt-Jakob
Guide to Regulatory Requirements for the Procurement of Human Tissues and Cells intended for Human Application
 Guide to Regulatory Requirements for the Procurement of Human Tissues and Cells intended for Human AUT-G0102-1 3 JANUARY 2013 This guide does not purport to be an interpretation of law and/or regulations
Guide to Regulatory Requirements for the Procurement of Human Tissues and Cells intended for Human AUT-G0102-1 3 JANUARY 2013 This guide does not purport to be an interpretation of law and/or regulations
BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011. 1. ABO Typing: Performed each time with each donation
 Testing Performed: BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011 1. ABO Typing: Performed each time with each donation 2. Rh Typing: o Performed along with ABO typing to determine
Testing Performed: BLOOD DONOR TESTING & LOOKBACK STUDIES Shan Yuan, MD Last Updated 2/8/2011 1. ABO Typing: Performed each time with each donation 2. Rh Typing: o Performed along with ABO typing to determine
Need for Expansion of BTS. Insufficient Space
 For discussion on 17 November 2014 LC Paper No. CB(2)242/14-15(07) Legislative Council Panel on Health Services Expansion of the Hong Kong Red Cross Blood Transfusion Service Headquarters Purpose This
For discussion on 17 November 2014 LC Paper No. CB(2)242/14-15(07) Legislative Council Panel on Health Services Expansion of the Hong Kong Red Cross Blood Transfusion Service Headquarters Purpose This
Executive summary. Executive summary 8
 Executive summary Q fever is a zoonosis an infectious disease that can be transmitted from animals to humans caused by the bacterium Coxiella burnetii (C. burnetii). Until 2006, Q fever was a rare disease
Executive summary Q fever is a zoonosis an infectious disease that can be transmitted from animals to humans caused by the bacterium Coxiella burnetii (C. burnetii). Until 2006, Q fever was a rare disease
Mother s blood test to check her unborn baby s blood group
 Mother s blood test to check her unborn baby s blood group This leaflet explains why it is important to have a blood test to check the baby s blood group, so that only those who need it, receive anti-d
Mother s blood test to check her unborn baby s blood group This leaflet explains why it is important to have a blood test to check the baby s blood group, so that only those who need it, receive anti-d
1) Siderophores are bacterial proteins that compete with animal A) Antibodies. B) Red blood cells. C) Transferrin. D) White blood cells. E) Receptors.
 Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Prof. Lester s BIOL 210 Practice Exam 4 (There is no answer key. Please do not email or ask me for answers.) Chapters 15, 16, 17, 19, HIV/AIDS, TB, Quorum Sensing 1) Siderophores are bacterial proteins
Chapter 3. Immunity and how vaccines work
 Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
Chapter 3 Immunity and how vaccines work 3.1 Objectives: To understand and describe the immune system and how vaccines produce immunity To understand the differences between Passive and Active immunity
ATBF Comment to. February 2010. This response only includes those items wherein the ATBF wishes to submit comments at this time.
 ATBF Comment to THERAPEUTIC GOODS ORDER NO. XX - Standards for minimising infectious disease transmission via therapeutic goods that are human blood and blood components, human tissues and human cellular
ATBF Comment to THERAPEUTIC GOODS ORDER NO. XX - Standards for minimising infectious disease transmission via therapeutic goods that are human blood and blood components, human tissues and human cellular
Blood, Plasma, and Cellular Blood Components INTRODUCTION
 Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
Blood, Plasma, and Cellular Blood Components INTRODUCTION This chapter of the Guideline provides recommendations to Sponsors of Requests for Revision for new monographs for blood, plasma, and cellular
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice
 Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
Beginner's guide to Hepatitis C testing and immunisation against hepatitis A+B in general practice Dr Chris Ford GP & SMMGP Clinical Lead Kate Halliday Telford & Wrekin Shared Care Coordinator Aims Discuss:
CAREERS IN BIOMEDICAL SCIENCE & THE IBMS. Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire
 CAREERS IN BIOMEDICAL SCIENCE & THE IBMS Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire What is a biomedical scientist? Biomedical scientists carry out investigations
CAREERS IN BIOMEDICAL SCIENCE & THE IBMS Betty Kyle Scottish Regional Representative IBMS Lead Biomedical Scientist NHS Lanarkshire What is a biomedical scientist? Biomedical scientists carry out investigations
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE
 FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
FACTS AND FIGURES JUNE 2004 NO 2 THE PREPARATION OF SINGLE DONOR CRYOPRECIPITATE Revised Edition Shân Lloyd National Blood Transfusion Service Zimbabwe Published by the World Federation of Hemophilia (WFH);
Department of Transfusion Medicine and Immunohematology
 Department of Transfusion Medicine and Immunohematology Activities Transfusion medicine is a multidisciplinary area concerned with the proper use of blood and blood components in the treatment of human
Department of Transfusion Medicine and Immunohematology Activities Transfusion medicine is a multidisciplinary area concerned with the proper use of blood and blood components in the treatment of human
Viral hepatitis. Report by the Secretariat
 SIXTY-THIRD WORLD HEALTH ASSEMBLY A63/15 Provisional agenda item 11.12 25 March 2010 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses (hepatitis A, B, C, D and
SIXTY-THIRD WORLD HEALTH ASSEMBLY A63/15 Provisional agenda item 11.12 25 March 2010 Viral hepatitis Report by the Secretariat THE DISEASES AND BURDEN 1. The group of viruses (hepatitis A, B, C, D and
Blood & Marrow Transplant Glossary. Pediatric Blood and Marrow Transplant Program Patient Guide
 Blood & Marrow Transplant Glossary Pediatric Blood and Marrow Transplant Program Patient Guide Glossary Absolute Neutrophil Count (ANC) -- Also called "absolute granulocyte count" amount of white blood
Blood & Marrow Transplant Glossary Pediatric Blood and Marrow Transplant Program Patient Guide Glossary Absolute Neutrophil Count (ANC) -- Also called "absolute granulocyte count" amount of white blood
AUTOLOGOUS BLOOD DONATION
 AUTOLOGOUS BLOOD DONATION UCLA Blood and Platelet Center 1045 Gayley Avenue Los Angeles, California 90024-3401 phone: 310.794.7207 website: www.gotblood.ucla.edu ----------------------------------------------------------------------------------------------------------------------------
AUTOLOGOUS BLOOD DONATION UCLA Blood and Platelet Center 1045 Gayley Avenue Los Angeles, California 90024-3401 phone: 310.794.7207 website: www.gotblood.ucla.edu ----------------------------------------------------------------------------------------------------------------------------
Reference: NHS England B04/P/a
 Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation (HSCT) (All Ages): Revised Reference: NHS England B04/P/a 1 NHS England Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation
Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation (HSCT) (All Ages): Revised Reference: NHS England B04/P/a 1 NHS England Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation
Managed Services Case Study
 Managed Services Case Study March 2015 You may re-use the text of this document (not including logos) free of charge in any format or medium, under the terms of the Open Government Licence. To view this
Managed Services Case Study March 2015 You may re-use the text of this document (not including logos) free of charge in any format or medium, under the terms of the Open Government Licence. To view this
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance
 University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
University of Colorado Hospital Policy and Procedure Transplant and Implant Tissue Storage and Issuance Related Policies and Procedures: Temperature Controlled Storage Unit Monitoring Hospital Infection
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND
 EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
EPIDEMIOLOGY OF HEPATITIS B IN IRELAND Table of Contents Acknowledgements 3 Summary 4 Introduction 5 Case Definitions 6 Materials and Methods 7 Results 8 Discussion 11 References 12 Epidemiology of Hepatitis
*6816* 6816 CONSENT FOR DECEASED KIDNEY DONOR ORGAN OPTIONS
 The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
The shortage of kidney donors and the ever-increasing waiting list has prompted the transplant community to look at different types of organ donors to meet the needs of our patients on the waiting list.
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy
 New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
New York State Department of Health Immunization Program Combined Hepatitis A and B Vaccine Dosing Schedule Policy Policy Statement The accelerated four-dose schedule for combined hepatitis A and B vaccine
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES
 THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
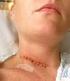
Monitoring surgical wounds for infection
 Monitoring surgical wounds for infection Information for patients This leaflet explains surgical wound infection and the national programme for monitoring infections acquired in hospitals This hospital
Monitoring surgical wounds for infection Information for patients This leaflet explains surgical wound infection and the national programme for monitoring infections acquired in hospitals This hospital
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor. Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015
 Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
Determining Donor Eligibility Blood Donor vs. Stem Cell Donor Wanda Koetz, RN, HPC Clinical Nurse Lead, Memorial Blood Centers ASFA - May 7, 2015 Objectives The learner will be able to: Define donor eligibility
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery
 Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Spine Clinic Neurospine Specialists, Orthopaedics and Neurosurgery REVISION SPINE SURGERY Revision surgery is a very complex field which requires experience, training and evaluation in a very individual
Cord blood banking: information for parents
 Cord blood banking: information for parents Published August 2006 by the RCOG Contents Page number Key points 1 About this information 2 What is cord blood? 2 Why is cord blood useful? 3 How is cord blood
Cord blood banking: information for parents Published August 2006 by the RCOG Contents Page number Key points 1 About this information 2 What is cord blood? 2 Why is cord blood useful? 3 How is cord blood
National Health Burden of CLD in Italy
 National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
National Health Burden of CLD in Italy 11,000 deaths due to liver cirrhosis or HCC in 2006 Direct costs for the National Health System for treating CLD patients: 420 M / year for hospital care 164 M /
Blood cells are vital to the human body
 Candide FONT-SALA Blood cells are vital to the human body Red cells transport oxygen From http://www.english-online.at/biology/blood/blood-supply-and-blood-diseases.htm Red cell life span 120 days 200
Candide FONT-SALA Blood cells are vital to the human body Red cells transport oxygen From http://www.english-online.at/biology/blood/blood-supply-and-blood-diseases.htm Red cell life span 120 days 200
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Heart transplantation
 Heart transplantation A patient s guide 1 Heart transplantation Heart transplantation has the potential to significantly improve the length and quality of life for patients with severe heart failure.
Heart transplantation A patient s guide 1 Heart transplantation Heart transplantation has the potential to significantly improve the length and quality of life for patients with severe heart failure.
Blood Transfusion. There are three types of blood cells: Red blood cells. White blood cells. Platelets.
 Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Guidance Document Infectious Substances
 Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.
 Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.CA WWW This guide is intended for patients in need of an unrelated volunteer
Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.CA WWW This guide is intended for patients in need of an unrelated volunteer
Transplant Coordinator
 Have you signed your organ donor card? Hearts, kidneys, livers, and lungs can all be transplanted. When you die, one of your organs could help a critically ill patient live a longer life. What I do every
Have you signed your organ donor card? Hearts, kidneys, livers, and lungs can all be transplanted. When you die, one of your organs could help a critically ill patient live a longer life. What I do every
APPENDIX A GUIDANCE DOCUMENT
 APPENDIX A GUIDANCE DOCUMENT Infectious Substances International Civil Aviation Organization Technical Instructions for the Safe Transport of Dangerous Goods by Air, 2005-2006 Note: This guidance document
APPENDIX A GUIDANCE DOCUMENT Infectious Substances International Civil Aviation Organization Technical Instructions for the Safe Transport of Dangerous Goods by Air, 2005-2006 Note: This guidance document
What does Hepatitis C mean to me as a CKD Patient?
 What does Hepatitis C mean to me as a CKD Patient? Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation and Treatment of Hepatitis C in CKD Introduction Worldwide, about 170 million people
What does Hepatitis C mean to me as a CKD Patient? Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation and Treatment of Hepatitis C in CKD Introduction Worldwide, about 170 million people
AMEX International Healthcare Plan Benefits schedule
 Quality health plans & benefits Healthier living Financial well-being Intelligent solutions AMEX International Healthcare Plan Benefits schedule Effective 1 April 2015 46.06.933.1-EUAM D (4/15) Your flexible
Quality health plans & benefits Healthier living Financial well-being Intelligent solutions AMEX International Healthcare Plan Benefits schedule Effective 1 April 2015 46.06.933.1-EUAM D (4/15) Your flexible
Information for Our Patients Regarding Metal-on-Metal (MoM) Hip Replacements
 Information for Our Patients Regarding Metal-on-Metal (MoM) Hip Replacements The American Association of Hip and Knee Surgeons, The Hip Society and the American Academy of Orthopaedic Surgeons Concerns
Information for Our Patients Regarding Metal-on-Metal (MoM) Hip Replacements The American Association of Hip and Knee Surgeons, The Hip Society and the American Academy of Orthopaedic Surgeons Concerns
LifeNet Health Presented by James Clagett, PhD., Chief Science Officer LifeNet Health - The Best Keep Secret in Regenerative Medicine
 Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
CLIENT AGREEMENT. Copyright 2014 MiracleCord Inc. All rights reserved.
 CLIENT AGREEMENT MiracleCord, Inc. is a provider of services for the collecting, testing, processing, cryopreserving and storing of cells collected from a newbornʼs umbilical cord blood ( Cord Blood )
CLIENT AGREEMENT MiracleCord, Inc. is a provider of services for the collecting, testing, processing, cryopreserving and storing of cells collected from a newbornʼs umbilical cord blood ( Cord Blood )
Exeter. Surgical Technique. V40 Stem Cement-in-Cement. Orthopaedics
 Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Medical Questionnaire and Informed Consent Public Cord Blood Bank Switzerland
 Medical Questionnaire and Informed Consent Public Cord Blood Bank Switzerland You have just read the information sheet for cord blood donors and you would like to donate cord blood. We thank you for answering
Medical Questionnaire and Informed Consent Public Cord Blood Bank Switzerland You have just read the information sheet for cord blood donors and you would like to donate cord blood. We thank you for answering
Human Tissue Authority
 Human Tissue Authority Guidance document for establishments working with Umbilical cord blood 29 November 2010 Contents Introduction Paragraph 1 The umbilical cord blood sector and the Role of the Designated
Human Tissue Authority Guidance document for establishments working with Umbilical cord blood 29 November 2010 Contents Introduction Paragraph 1 The umbilical cord blood sector and the Role of the Designated
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
SPINAL FUSION. North American Spine Society Public Education Series
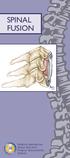 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
Red Blood Cell Transfusions for Sickle Cell Disease
 Red Blood Cell Transfusions for Sickle Cell Disease Red Blood Cell Transfusions for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital, Departments of Hematology, Patient Education,
Red Blood Cell Transfusions for Sickle Cell Disease Red Blood Cell Transfusions for Sickle Cell Disease 1 Produced by St. Jude Children s Research Hospital, Departments of Hematology, Patient Education,
Where Will my New Kidney Come From?
 Where Will my New Kidney Come From? The Organ Shortage There is a severe shortage of organs for transplant. This means that the wait for a kidney transplant can be many years. The UW Transplant Program
Where Will my New Kidney Come From? The Organ Shortage There is a severe shortage of organs for transplant. This means that the wait for a kidney transplant can be many years. The UW Transplant Program
The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings. Prof. Tony Pagliuca
 The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings Prof. Tony Pagliuca UK STEM CELL STRATEGIC FORUM The future of unrelated donor SCT in the UK Antonio Pagliuca, Transplant
The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings Prof. Tony Pagliuca UK STEM CELL STRATEGIC FORUM The future of unrelated donor SCT in the UK Antonio Pagliuca, Transplant
How To Inspect A Blood Bank
 Site visit inspection report on compliance with HTA minimum standards Belfast Cord Blood Bank HTA licensing number 11077 Licensed for the procurement, processing, testing, storage, distribution and import/export
Site visit inspection report on compliance with HTA minimum standards Belfast Cord Blood Bank HTA licensing number 11077 Licensed for the procurement, processing, testing, storage, distribution and import/export
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Living Donor Paired Exchange Registry. What is Living Kidney Donor Paired Exchange?
 Living Paired Exchange Registry What is Living Kidney Paired Exchange? The Living Paired Exchange Registry. At Canadian Blood Services, we are committed to helping Canadians help each other. By building
Living Paired Exchange Registry What is Living Kidney Paired Exchange? The Living Paired Exchange Registry. At Canadian Blood Services, we are committed to helping Canadians help each other. By building
Calculation of Alert Levels for Assessing Collection Center Donor Quality for PMF Evaluation
 7 September 2010 Reference: EPI TF 10015 Calculation of Alert Levels for Assessing Collection Center Donor Quality for PMF Evaluation Background The quality and safety of plasma protein therapies and the
7 September 2010 Reference: EPI TF 10015 Calculation of Alert Levels for Assessing Collection Center Donor Quality for PMF Evaluation Background The quality and safety of plasma protein therapies and the
Human Tissue Authority. Guide to Quality and Safety Assurance for Human Tissues and Cells for Patient Treatment
 Human Tissue Authority Guide to Quality and Safety Assurance for Human Tissues and Cells for Patient Treatment 12 November 2010 Preamble This guide explains the requirements for licences which store tissues
Human Tissue Authority Guide to Quality and Safety Assurance for Human Tissues and Cells for Patient Treatment 12 November 2010 Preamble This guide explains the requirements for licences which store tissues
Stemlife tomorrow s lifeline
 StemlifeTM Frequently Asked Questions 1300 553 474 www.stemlife.com.au TM Stemlife 1. COLLECTION OF CORD STEM CELLS Does Stemlife collect Cord Tissue? Absolutely. In fact, Stemlife provides the most advanced
StemlifeTM Frequently Asked Questions 1300 553 474 www.stemlife.com.au TM Stemlife 1. COLLECTION OF CORD STEM CELLS Does Stemlife collect Cord Tissue? Absolutely. In fact, Stemlife provides the most advanced
Directed/Related Cord Blood Collection by NHS Blood and Transplant
 Directed/Related Cord Blood Collection by Jacqui Thompson Clinical Scientist Section Head of Specialist Procedures Stem Cells and Immunotherapies Department National Blood Service, NHSBT Birmingham Why
Directed/Related Cord Blood Collection by Jacqui Thompson Clinical Scientist Section Head of Specialist Procedures Stem Cells and Immunotherapies Department National Blood Service, NHSBT Birmingham Why
Precise control of precursor material properties essential; temperatures involved; Powdery surface finish; Completed part may contain trapped powder
 The creation of substitutes to repair damaged tissues and organs dates back to the beginning of recorded history [1]. Several ancient civilizations dabbled in tissue repair; for example, Indian physicians
The creation of substitutes to repair damaged tissues and organs dates back to the beginning of recorded history [1]. Several ancient civilizations dabbled in tissue repair; for example, Indian physicians
Stem Cell Therapy. Treat your pain, improve your lifestyle and look after your wellbeing. What are stem cells?
 (02) 9824 3044 LIVERPOOL NSW 2170 Stem Cell Therapy Treat your pain, improve your lifestyle and look after your wellbeing What are stem cells? Stem cells are cells that can differentiate into other types
(02) 9824 3044 LIVERPOOL NSW 2170 Stem Cell Therapy Treat your pain, improve your lifestyle and look after your wellbeing What are stem cells? Stem cells are cells that can differentiate into other types
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
20.3 Diseases Caused by Bacteria and Viruses
 20.3 Diseases Caused by Bacteria and Viruses Lesson Objectives Explain how bacteria cause disease. Explain how viruses cause disease. Define emerging disease and explain why emerging diseases are a threat
20.3 Diseases Caused by Bacteria and Viruses Lesson Objectives Explain how bacteria cause disease. Explain how viruses cause disease. Define emerging disease and explain why emerging diseases are a threat
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY. Agenda item 12.3 24 May 2014. Hepatitis
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY WHA67.6 Agenda item 12.3 24 May 2014 Hepatitis The Sixty-seventh World Health Assembly, Having considered the report on hepatitis; 1 Reaffirming resolution WHA63.18,
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY WHA67.6 Agenda item 12.3 24 May 2014 Hepatitis The Sixty-seventh World Health Assembly, Having considered the report on hepatitis; 1 Reaffirming resolution WHA63.18,
Site visit inspection report on compliance with HTA minimum standards. Belfast Cord Blood Bank. HTA licensing number 11077.
 Site visit inspection report on compliance with HTA minimum standards Belfast Cord Blood Bank HTA licensing number 11077 Licensed for the procurement, processing, testing, storage, distribution and import/export
Site visit inspection report on compliance with HTA minimum standards Belfast Cord Blood Bank HTA licensing number 11077 Licensed for the procurement, processing, testing, storage, distribution and import/export
Draft guidance for registered pharmacies preparing unlicensed medicines
 Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
Draft guidance for registered pharmacies preparing unlicensed medicines January 2014 1 The General Pharmaceutical Council is the regulator for pharmacists, pharmacy technicians and registered pharmacies
(EMEA/CHMP/BWP/298390/2005)
 9 February 2006 Reference: EMEA 06004 PPTA s comments on the proposed Guidelines on Validation of immunoassays for the detection of Hepatitis B Virus surface antigen (HBsAg) (EMEA/CHMP/BWP/298390/2005)
9 February 2006 Reference: EMEA 06004 PPTA s comments on the proposed Guidelines on Validation of immunoassays for the detection of Hepatitis B Virus surface antigen (HBsAg) (EMEA/CHMP/BWP/298390/2005)
4. All cord blood banks should be subject to the same standards, regulations and accreditation requirements.
 WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
it will set at body temperature, and it will grow. UNIQUE FORMULATIONS
 BETTER BONE GRAFT FORMULATION. SUPERIOR BONE GRAFT HANDLING. When choosing a Demineralized Bone Matrix, remember that not all products are created equal. For excellent clinical performance and patient
BETTER BONE GRAFT FORMULATION. SUPERIOR BONE GRAFT HANDLING. When choosing a Demineralized Bone Matrix, remember that not all products are created equal. For excellent clinical performance and patient
What is a Stem Cell Transplantation?
 What is a Stem Cell Transplantation? Guest Expert: Stuart, MD Associate Professor, Medical Oncology www.wnpr.org www.yalecancercenter.org Welcome to Yale Cancer Center Answers with Drs. Ed and Ken. I am
What is a Stem Cell Transplantation? Guest Expert: Stuart, MD Associate Professor, Medical Oncology www.wnpr.org www.yalecancercenter.org Welcome to Yale Cancer Center Answers with Drs. Ed and Ken. I am
Pros and Cons of Stem Cell Sources and their availability in Africa. Dr Jaimendra Singh Inkosi Albert Luthuli Central Hospital Durban, South Africa
 Pros and Cons of Stem Cell Sources and their availability in Africa Dr Jaimendra Singh Inkosi Albert Luthuli Central Hospital Durban, South Africa Introduction The ability to perform a haematopoietic stem
Pros and Cons of Stem Cell Sources and their availability in Africa Dr Jaimendra Singh Inkosi Albert Luthuli Central Hospital Durban, South Africa Introduction The ability to perform a haematopoietic stem
FAQs HIV & AIDS. What is HIV? A virus that reduces the effectiveness of your immune system, meaning you are less protected against disease.
 HIV & AIDS What is HIV? A virus that reduces the effectiveness of your immune system, meaning you are less protected against disease. What does HIV stand for? Human Immunodeficiency Virus Where did HIV
HIV & AIDS What is HIV? A virus that reduces the effectiveness of your immune system, meaning you are less protected against disease. What does HIV stand for? Human Immunodeficiency Virus Where did HIV
Donating Life. The Workplace Partnership for Life Program
 The Workplace Partnership for Life Program Every day about 63 people receive an organ transplant, but another 16 people on the waiting list die because not enough organs are available. Approximately 80,000
The Workplace Partnership for Life Program Every day about 63 people receive an organ transplant, but another 16 people on the waiting list die because not enough organs are available. Approximately 80,000
NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME
 NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME CERVICAL CANCER Introduction Cancer of the cervix is the second most common form of cancer amongst South African women. Approximately one in every
NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME CERVICAL CANCER Introduction Cancer of the cervix is the second most common form of cancer amongst South African women. Approximately one in every
Cytomegalovirus (HHV5/CMV) Roseolovirus (HHV6 & 7)
 Betaherpesvirinae Cytomegalovirus (HHV5/CMV) Roseolovirus (HHV6 & 7) CYTOMEGALOVIRUS CMV is thought to be amongst the oldest type of herpesvirus in evolutionary terms. CMV is the prototype of beta-herpesviruses
Betaherpesvirinae Cytomegalovirus (HHV5/CMV) Roseolovirus (HHV6 & 7) CYTOMEGALOVIRUS CMV is thought to be amongst the oldest type of herpesvirus in evolutionary terms. CMV is the prototype of beta-herpesviruses
HSA Consumer Guide. Understanding Vaccines, Vaccine Development and Production. www.hsa.gov.sg November 2009. How a Vaccine Works.
 November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
CPT Codes for Bone Marrow Transplant January 2015 James L. Gajewski, MD
 The blood and marrow transplant field has 15 dedicated CPT codes. These CPT codes can be categorized into three groups: 1. Collection Codes 2. Cell Processing Codes 3. Cell Infusion Codes Collection Codes
The blood and marrow transplant field has 15 dedicated CPT codes. These CPT codes can be categorized into three groups: 1. Collection Codes 2. Cell Processing Codes 3. Cell Infusion Codes Collection Codes
Narrator: Transplants using stem cells from the blood, bone marrow or umbilical cord blood
 [Track 2: What Is a Transplant?] Narrator: Transplants using stem cells from the blood, bone marrow or umbilical cord blood can be an effective treatment for people with blood cancers such as leukemia,
[Track 2: What Is a Transplant?] Narrator: Transplants using stem cells from the blood, bone marrow or umbilical cord blood can be an effective treatment for people with blood cancers such as leukemia,
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute
 chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
Cell Bank Safety Testing for Cellular Therapeutics. Christopher A Bravery
 Cell Bank Safety Testing for Cellular Therapeutics Christopher A Bravery cbravery@advbiols.com 1 Introduction Definitions used in this talk Cellular therapeutics (CTP) EU: Advanced Therapy Medicinal Products
Cell Bank Safety Testing for Cellular Therapeutics Christopher A Bravery cbravery@advbiols.com 1 Introduction Definitions used in this talk Cellular therapeutics (CTP) EU: Advanced Therapy Medicinal Products
healthcare associated infection 1.2
 healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
healthcare associated infection A C T I O N G U I D E 1.2 AUSTRALIAN SAFETY AND QUALITY GOALS FOR HEALTH CARE What are the goals? The Australian Safety and Quality Goals for Health Care set out some important
The Immune System. 2 Types of Defense Mechanisms. Lines of Defense. Line of Defense. Lines of Defense
 The Immune System 2 Types of Defense Mechanisms Immune System the system that fights infection by producing cells to inactivate foreign substances to avoid infection and disease. Immunity the body s ability
The Immune System 2 Types of Defense Mechanisms Immune System the system that fights infection by producing cells to inactivate foreign substances to avoid infection and disease. Immunity the body s ability
NORTHWEST TRANSFUSION SYMPOSIUM. Final Program. Tacoma, Washington 9/12/2015
 2015 NORTHWEST TRANSFUSION SYMPOSIUM Final Program Tacoma, Washington 9/12/2015 Table of Contents Learning Objectives...2 Accreditation...3 Disclosure Policy...3 Committee Members...4 Speaker Schedule...5
2015 NORTHWEST TRANSFUSION SYMPOSIUM Final Program Tacoma, Washington 9/12/2015 Table of Contents Learning Objectives...2 Accreditation...3 Disclosure Policy...3 Committee Members...4 Speaker Schedule...5
Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC
 Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC August 2013 Contents 1 Introduction...3 2 Scope of the directive...3 2.1 What is an in vitro diagnostic medical device?... 3 2.2 Specimen
Guidance on the In Vitro Diagnostic Medical Devices Directive 98/79/EC August 2013 Contents 1 Introduction...3 2 Scope of the directive...3 2.1 What is an in vitro diagnostic medical device?... 3 2.2 Specimen
Core Topic 2. The immune system and how vaccines work
 Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Core Topic 2 The immune system and how vaccines work Learning outcome To be able to describe in outline the immune system and how vaccines work in individuals and populations Learning objectives Explain
Learning about Hepatitis C and Chronic Kidney Disease
 Learning about Hepatitis C and Chronic Kidney Disease Hepatitis C and Chronic Kidney Disease If you have chronic kidney disease (CKD), you want to learn all you can about your disease and what you can
Learning about Hepatitis C and Chronic Kidney Disease Hepatitis C and Chronic Kidney Disease If you have chronic kidney disease (CKD), you want to learn all you can about your disease and what you can
Use of Nucleic Acid Tests to Reduce the Risk of Transmission of Hepatitis B Virus from Donors
 This document is scheduled to be published in the Federal Register on 01/08/2016 and available online at http://federalregister.gov/a/2016-00149, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/08/2016 and available online at http://federalregister.gov/a/2016-00149, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Recruitment Start date: April 2010 End date: Recruitment will continue until enrolment is fully completed
 Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
Patient Information Booklet Kidney Transplantation. Cardiff Transplant Unit
 Patient Information Booklet Kidney Transplantation Cardiff Transplant Unit Introduction You have been given this information booklet because your kidney specialist thinks you may benefit from a kidney
Patient Information Booklet Kidney Transplantation Cardiff Transplant Unit Introduction You have been given this information booklet because your kidney specialist thinks you may benefit from a kidney
Bloodborne Pathogens (HIV, HBV, and HCV) Exposure Management
 Bloodborne Pathogens Exposure Policy and Procedures Employees of the State of South Dakota Department of Health Bloodborne Pathogens (HIV, HBV, and HCV) Exposure Management PEP Hotline 1-888-448-4911 DOH
Bloodborne Pathogens Exposure Policy and Procedures Employees of the State of South Dakota Department of Health Bloodborne Pathogens (HIV, HBV, and HCV) Exposure Management PEP Hotline 1-888-448-4911 DOH
Opinion On Quality and Safety of Blood SCIENTIFIC COMMITTEE ON MEDICINAL PRODUCTS AND MEDICAL DEVICES
 EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate B - Scientific Health Opinions Unit B2 - Management of scientific committees I SCIENTIFIC COMMITTEE ON MEDICINAL PRODUCTS
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate B - Scientific Health Opinions Unit B2 - Management of scientific committees I SCIENTIFIC COMMITTEE ON MEDICINAL PRODUCTS
Technology Breakthrough in Spinal Implants (Technical Insights)
 Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
