PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks.
|
|
|
- Joan Howard
- 8 years ago
- Views:
Transcription
1 PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks. Trial Description Title STAR-TOR- Registry For The Evaluation Of The Safety, Tolerability And Efficacy Of Temsirolimus (Torisel ), Sunitinib (Sutent ), And Axiitnib (Inlyta ) For The Treatment Of Subjects With Advanced Renal Cell Carcinoma (mrcc), Mantle Cell Lymphoma (MCL), And Gastro-Intestinal Stroma Tumor (GIST) Trial Acronym STAR-TOR URL of the trial [---]* Brief Summary in Lay Language The purpose of this registry is to obtain a general view as regards efficacy, tolerability and safety issues of the Torisel, Sutent, and/or Inlyta therapies in patients with advanced renal cell carcinoma, recurrent / refractory mantle cell lymphoma (MCL) and gastro-intestinal stroma tumors (GIST) under the conditions of routine use Brief Summary in Scientific Language Survival time analysis Organizational Data DRKS-ID: DRKS Date of Registration in Partner Registry or other Primary Registry: 2008/06/13 Investigator Sponsored/Initiated Trial (IST/IIT): no Ethics Approval/Approval of the Ethics Committee: [---]* (leading) Ethics Committee Nr.: [---]* Page 1 of 7
2 Secondary IDs Primary Registry-ID: NCT (ClinicalTrials.gov) Sponsor-ID: 3066K (Pfizer) Other Secondary-ID: B Health condition or Problem studied Free text: Carcinoma, Renal Cell, Advanced Free text: Lymphoma, Mantle-Cell Free text: Gastrointestinal Stroma Tumors ICD10: C64 - Malignant neoplasm of kidney, except renal pelvis ICD10: C Other non-follicular lymphoma Interventions/Observational Groups Arm 1: Drug: Temsirolimus Arm 2: Drug: Temsirolimus Arm 3: Drug: Sunitinib Arm 4: Drug: Sunitinib Arm 5: Drug: Axitinib Characteristics Study Type: Non-interventional Study Type Non-Interventional: Observational study Allocation: [---]* Blinding: [---]* Who is blinded: [---]* Control: [---]* Purpose: [---]* Assignment: [---]* Phase: N/A Off-label use (Zulassungsüberschreitende Anwendung eines Arzneimittels): [---]* Page 2 of 7
3 Primary Outcome - Safety: absolute and relative incidence of adverse events; time frame: entire study - Efficacy: best response, progression-free survival, overall survival; time frame: entire study Secondary Outcome - Safety: absolute and relative incidence of therapy interruption; time frame: entire study Countries of recruitment DE Germany Locations of Recruitment Dr. med. Harald Held, Neumuenster Dr. med. Hans Wilhelm Duebbers, Ahaus Dr. Ludwig Fischer von Weikersthal, Amberg Studienzentrum Drs. Klausmann / Dr. Welslau, Haematologie-Onkologie-Diabetologie, Aschaffenburg Carsten Lange, Bernburg Dr. Jens-Uwe Krieger, Chemnitz Zeisigwaldklinikum Bethanien Chemnitz, Chemnitz Leonhard Stark, Deggendorf Prof. Dr. med. Udo Rebmann, Dessau Dr.med Johannes Mohm, Dresden Dr. med. Ralf Eckert, Eisleben Goebell, Erlangen Prof. Dr. med. Lothar Bergmann, Frankfurt am Main Dr. med. Gunter Derigs, Frankfurt am Main Hoffkes, Fulda PD Dr. Uwe Zimmermann, Greifswald Internistische Gemeinschaftspraxis, Guestrow Dr. med. Arne Strauss, Göttingen Page 3 of 7
4 Dr. med. Michael Rink, Hamburg Dr. med. Hanns-Detlev Harich, Hof Universitaetsklinikum des Saarlandes, Klinik fuer Urologie und Kinderurologie, Homburg/Saar Dr. med. Susan Foller, Jena Steinmetz, Koeln Klinisches Studienzentrum Urlogie, Koeln Dr. med. Martina Stauch, Kronach Dr. med. Ursula Vehling-Kaiser, Landshut Dr. Andreas Kohler, Langen Dietel, Leipzig Andreas Schwarzer, Leipzig Lutz Kamann, Leipzig Dr.med. Matthias Schulze, Markkleeberg Dr. med. Jan Klaus Schroder, Mulheim Dr.med. Wolfgang Abenhardt, München Herrmann, Münster Dr. med. Thomas Gehring, Neckarsulm Dres. Derouet Poenicke Becker, Neunkirchen Physician for Internal Medicine, Neuwied Dr. med. David Kunst, Nienburg Dr.med. Christian Linder, Nordhausen Dr. med. Joachim Zimber, Nürnberg Ralf-Bodo Kühn, Oldenburg Wolfgang Marz, Osnabruck Dr. med. Torsten Geyer, Ostfildern Dr. med. Ino Kietz, Parchim Office of Friedrich Overkamp, Oncologia Nova, Recklinghausen Andreas Hübner, Rostock Diakonie-Klinikum ggmbh, Schwäbisch Hall Dr. med. Thomas Geer, Schwäbisch Hall Office of Judith Franz-Werner, Speyer Dr. Matthias Groschek, Stolberg Page 4 of 7
5 Dr. med. Heinz Kirchen, Trier Klinik für Urologie, Eberhard-Karls-Universitaet Tuebingen,, Tuebingen Klotz, Weiden Dr.med. Jan Janssen, Westerstede Universitaetsklinik Wuerzburg, Medizinische Poliklinik, Wuerzburg Jochen Gleissner, Wuppertal Mathias Schulze, Zittau Scheffler, Zwickau Recruitment Planned/Actual: [---]* (Anticipated or Actual) Date of First Enrollment: 2013/01/31 Target Sample Size: 1600 Monocenter/Multicenter trial: Multicenter trial National/International: [---]* Inclusion Criteria Gender: Both, male and female Minimum Age: 18 Years Maximum Age: 99 Years Additional Inclusion Criteria - Patients with proven tumor of RCC, MCL or GIST by histology. - Informed consent signed by patient. Exclusion criteria - Pregnancy Addresses Page 5 of 7
6 Primary Sponsor Pfizer Telephone: [---]* Contact for Scientific Queries Pfizer Pfizer CT.gov Call Center Telephone: [---]* Contact for Public Queries Pfizer CT.gov Call Center Telephone: Sources of Monetary or Material Support [---]* Bitte wenden Sie sich an den Sponsor / Please refer to primary sponsor Telephone: [---]* Status Recruitment Status: Recruiting ongoing Study Closing (LPLV): [---]* Trial Publications, Results and other documents Further trial documents To obtain contact information for a study center near you, click here. Page 6 of 7
7 The parameters in ClinicalTrials.gov and DRKS are not identical. Therefore the data import from ClinicalTrials.gov required adjustments. For full details please see the DRKS FAQs. - Translation on version: 8 - Last processed date by ClinicalTrials.gov: 2016/01/14 Please note: There are additional attributes available concerning this trial. To open an extended view please click here.
Study Details. Study Type: Observational Study Design: Observational Model: Cohort, Time Perspective: Prospective. Investigator Details
 Registry For Temsirolimus, Sunitinib, And Axitinib Treated Patients With Metastatic Renal Cell Carcinoma (mrcc), Mantle Cell Lymphoma (MCL), And Gastro-Intestinal Stroma Tumor (GIST) [STAR-TOR] Status:
Registry For Temsirolimus, Sunitinib, And Axitinib Treated Patients With Metastatic Renal Cell Carcinoma (mrcc), Mantle Cell Lymphoma (MCL), And Gastro-Intestinal Stroma Tumor (GIST) [STAR-TOR] Status:
Feasibility study to evaluate the usability of the newly developed health care management software ecare*seniors in primary care practices.
 Trial Description Title Patient-centered health care management in primary care: feasibility study evaluating the usability of the computer-based information system ecare*seniors for the individual health
Trial Description Title Patient-centered health care management in primary care: feasibility study evaluating the usability of the computer-based information system ecare*seniors for the individual health
Trial Description. DRKS-ID: DRKS00006862 Date of Registration in DRKS: 2015/03/06 Date of Registration in Partner Registry: [---]*
![Trial Description. DRKS-ID: DRKS00006862 Date of Registration in DRKS: 2015/03/06 Date of Registration in Partner Registry: [---]* Trial Description. DRKS-ID: DRKS00006862 Date of Registration in DRKS: 2015/03/06 Date of Registration in Partner Registry: [---]*](/thumbs/39/18699142.jpg) PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Treatment with new oral anticoagulants in primary care practices Trial Acronym [---]* URL of the trial [---]* Brief
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Treatment with new oral anticoagulants in primary care practices Trial Acronym [---]* URL of the trial [---]* Brief
Trial Description. Organizational Data. Secondary IDs
 Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
 PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Investigation of the effect of isomaltulose (PalatinoseTM) on metabolic parameters in subjects with Type 2 Diabetes.
Dopaminergic and cholinergic modulation of oculomotor control during saccades
 Trial Description Title Dopaminergic and cholinergic modulation of oculomotor control during saccades Trial Acronym URL of the trial Brief Summary in Lay Language In this basic science study we investigate
Trial Description Title Dopaminergic and cholinergic modulation of oculomotor control during saccades Trial Acronym URL of the trial Brief Summary in Lay Language In this basic science study we investigate
Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL
 Trial Description Title Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL Trial Acronym SUPRATOL URL of the trial [---]*
Trial Description Title Prospective multicenter study for functional evaluation of transoral laser microsurgery (TLM) for supraglottic carcinomas - SUPRATOL Trial Acronym SUPRATOL URL of the trial [---]*
PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks.
 PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks. Trial Description Title A Phase IIIb, Multicentre, Open-label Study of Nilotinib in Adult Patients With
PLEASE NOTE: This study has been imported from ClinicalTrials.gov without additional data checks. Trial Description Title A Phase IIIb, Multicentre, Open-label Study of Nilotinib in Adult Patients With
Raman spectroscopy: Setup of an experimental system for analysis of urinary stone composition
 PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Raman spectroscopy: Setup of an experimental system for analysis of urinary stone composition Trial Acronym Ramalith
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Raman spectroscopy: Setup of an experimental system for analysis of urinary stone composition Trial Acronym Ramalith
Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Germany
 Trial Description Title Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Trial Acronym ERGO-Study URL of the trial [---]* Brief Summary
Trial Description Title Work-related diseases of occupational therapists - pilot study for creating an "occupational therapist-cohort" in Trial Acronym ERGO-Study URL of the trial [---]* Brief Summary
Evaluation of a patient's ressource srengthening psychotherapeutic intervention in treatment of residual depression symptoms
 PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Euthymic Therapy: a Randomised Controlled Trial Trial Acronym [---]* URL of the trial [---]* Brief Summary in Lay Language
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title Euthymic Therapy: a Randomised Controlled Trial Trial Acronym [---]* URL of the trial [---]* Brief Summary in Lay Language
Trial Description. DRKS-ID: DRKS00006819 Date of Registration in DRKS: 2014/10/31 Date of Registration in Partner Registry: [---]* Title
![Trial Description. DRKS-ID: DRKS00006819 Date of Registration in DRKS: 2014/10/31 Date of Registration in Partner Registry: [---]* Title Trial Description. DRKS-ID: DRKS00006819 Date of Registration in DRKS: 2014/10/31 Date of Registration in Partner Registry: [---]* Title](/thumbs/36/17491077.jpg) Trial Description Title SCHILD - protective intervention for longtime course in depression Trial Acronym SCHILD URL of the trial [---]* Brief Summary in Lay Language Even if depression is treated appropriate
Trial Description Title SCHILD - protective intervention for longtime course in depression Trial Acronym SCHILD URL of the trial [---]* Brief Summary in Lay Language Even if depression is treated appropriate
WAKE-Up Pilot Study, Mental Training for patients with relapse-remitting multiple sclerosis and fatigue
 PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title WAKE-Up Pilot Study, Mental Training for patients with relapse-remitting multiple sclerosis and fatigue Trial Acronym
PLEASE NOTE: This trial has been registered retrospectively. Trial Description Title WAKE-Up Pilot Study, Mental Training for patients with relapse-remitting multiple sclerosis and fatigue Trial Acronym
In this observational study, the effect of dabigatran and rivaroxaban on platelets will be evaluated.
 Trial Description Title Effect of dabigatran and rivaroxaban on Platelets Trial Acronym BIG-ROX-P URL of the trial [---]* Brief Summary in Lay Language In this observational study, the effect of dabigatran
Trial Description Title Effect of dabigatran and rivaroxaban on Platelets Trial Acronym BIG-ROX-P URL of the trial [---]* Brief Summary in Lay Language In this observational study, the effect of dabigatran
Changes in liver volume as determined by MRI scans after two weeks of a low energy diet (t0: before start of the diet; t1: after two weeks of diet)
 Trial Description Title Influence of different preoperative low energy diets on changes in liver volume, liver fat content, visceral fat and body composition as well as liver and adipose tissue mrna expression
Trial Description Title Influence of different preoperative low energy diets on changes in liver volume, liver fat content, visceral fat and body composition as well as liver and adipose tissue mrna expression
Navigating GIST. The Life Raft Group June 12, 2008
 Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Navigating GIST Clinical Trials The Life Raft Group June 12, 2008 Some observations: Annually, only 3% of adult patients participate in cancer clinical trials. Lara et. al; Evaluation of factors affecting
Study on Melanoma - New Research for Cancer Patients
 A service of the U.S. National Institutes of Health Trial record 7 of 203 for: Melanoma Analysis Previous Study Return to List Next Study Expression Analysis of Specific Markers in Non-small Cell Lung
A service of the U.S. National Institutes of Health Trial record 7 of 203 for: Melanoma Analysis Previous Study Return to List Next Study Expression Analysis of Specific Markers in Non-small Cell Lung
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Guidance for Industry Information Program on Clinical Trials for Serious or Life-Threatening Diseases and Conditions
 Guidance for Industry Information Program on Clinical Trials for Serious or Life-Threatening Diseases and Conditions U.S. Department of Health and Human Services Food and Drug Administration Center for
Guidance for Industry Information Program on Clinical Trials for Serious or Life-Threatening Diseases and Conditions U.S. Department of Health and Human Services Food and Drug Administration Center for
Stage IV Renal Cell Carcinoma. Changing Management in A Comprehensive Community Cancer Center. Susquehanna Health Cancer Center
 Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF)
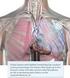 Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF) Michael Böhm, Helmut Drexler, Hanno Oswald, Karin Rybak, Ralph Bosch,
Effect of implanted device-based impedance monitoring with telemedicine alerts on mortality and morbidity in heart failure (OptiLink HF) Michael Böhm, Helmut Drexler, Hanno Oswald, Karin Rybak, Ralph Bosch,
MOLOGEN AG. Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer. Berlin, 12 May 2015
 Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
Q1 Results 2015 Conference Call Dr. Matthias Schroff Chief Executive Officer Berlin, 12 May 2015 V1-6 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future
NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA)
 NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA) Merat Esfahani, MD Medical Oncologist, Hematologist Cancer Liaison Physician SwedishAmerican Regional Cancer Center
NEOPLASMS OF KIDNEY (RENAL CELL CARCINOMA) And RENAL PELVIS (TRANSITIONAL CELL CARCINOMA) Merat Esfahani, MD Medical Oncologist, Hematologist Cancer Liaison Physician SwedishAmerican Regional Cancer Center
The New Kid on the Block for Advanced Renal Cell Carcinoma
 The New Kid on the Block for Advanced Renal Cell Carcinoma Wyeth Pharmaceuticals recently launched Torisel (temsirolimus), a targeted, first-in-class mtor inhibitor. This new treatment for metastatic renal
The New Kid on the Block for Advanced Renal Cell Carcinoma Wyeth Pharmaceuticals recently launched Torisel (temsirolimus), a targeted, first-in-class mtor inhibitor. This new treatment for metastatic renal
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance
 Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Basic Results Database
 Basic Results Database Deborah A. Zarin, M.D. ClinicalTrials.gov December 2008 1 ClinicalTrials.gov Overview and PL 110-85 Requirements Module 1 2 Levels of Transparency Prospective Clinical Trials Registry
Basic Results Database Deborah A. Zarin, M.D. ClinicalTrials.gov December 2008 1 ClinicalTrials.gov Overview and PL 110-85 Requirements Module 1 2 Levels of Transparency Prospective Clinical Trials Registry
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics
 Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Registries: An alternative for clinical trials?
 Registries: An alternative for clinical trials? Prof. Dr. Joerg Hasford, M.D., Ph.D. Department of Medical Informatics, Biometry and Epidemiology Ludwig-Maximilians-Universität Email: has@ibe.med.uni-muenchen.de
Registries: An alternative for clinical trials? Prof. Dr. Joerg Hasford, M.D., Ph.D. Department of Medical Informatics, Biometry and Epidemiology Ludwig-Maximilians-Universität Email: has@ibe.med.uni-muenchen.de
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009
 Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
PARTICIPATING IN CLINICAL TRIALS A GUIDE FOR PEOPLE WITH MS
 PARTICIPATING IN CLINICAL TRIALS A GUIDE FOR PEOPLE WITH MS If you have ever taken a medication or received rehabilitative physical therapy, then you have experienced the benefits of clinical research.
PARTICIPATING IN CLINICAL TRIALS A GUIDE FOR PEOPLE WITH MS If you have ever taken a medication or received rehabilitative physical therapy, then you have experienced the benefits of clinical research.
A Phase 2 Study of Interferon Beta-1a (Avonex ) in Ulcerative Colitis
 A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center
 What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
Renal Cell Carcinoma (Event Driven)
 Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
Clinical Trials. Helpful information and answers for patients
 Clinical Trials Helpful information and answers for patients I joined a clinical trial because I want to make it a better world for my daughter and grandchildren. Hopefully, by the time they re old enough
Clinical Trials Helpful information and answers for patients I joined a clinical trial because I want to make it a better world for my daughter and grandchildren. Hopefully, by the time they re old enough
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes
 Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text)
 Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
Master Online Study Program @dvanced Oncology. Study part time team up internationally!
 Master Online Study Program @dvanced Oncology Study part time team up internationally! Ulm University is developing an innovative international postgraduate study program @dvanced Oncology addressing clinical
Master Online Study Program @dvanced Oncology Study part time team up internationally! Ulm University is developing an innovative international postgraduate study program @dvanced Oncology addressing clinical
Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta
 Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta S.C. di Oncologia Medica, Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia Non-Clear Cell Renal Cell Carcinoma (nccrcc) nccrcc
Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta S.C. di Oncologia Medica, Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia Non-Clear Cell Renal Cell Carcinoma (nccrcc) nccrcc
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
What is New in Oncology. Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals
 What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
Ask Us About Clinical Trials
 Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
http://clinicaltrials.gov/ct2/show/nct01462513
 Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies L-BLP25 in Patients With Colorectal Carcinoma After Curative Resection of Hepatic
Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies L-BLP25 in Patients With Colorectal Carcinoma After Curative Resection of Hepatic
What s Next for Data Sharing: Insight from the NIH Experience
 What s Next for Data Sharing: Insight from the NIH Experience Jerry Sheehan Assistant Director for Policy Development National Library of Medicine National Institutes of Health SHARE In-Person Meeting
What s Next for Data Sharing: Insight from the NIH Experience Jerry Sheehan Assistant Director for Policy Development National Library of Medicine National Institutes of Health SHARE In-Person Meeting
REF/2011/06/002450 CTRI Website URL - http://ctri.nic.in
 Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 07:52:01 GMT) CTRI Number Last Modified On 08/07/2013 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
CURRICULUM VITAE. M. Sc. Anne-Katharina Schiefele
 CURRICULUM VITAE Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 2882 E-mail: schiefele@uni-trier.de Birthday: November 30, 1987
CURRICULUM VITAE Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 2882 E-mail: schiefele@uni-trier.de Birthday: November 30, 1987
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba
 Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ASCO Initiatives in Personalized Medicine. Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology
 ASCO Initiatives in Personalized Medicine Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology Financial Disclosures No financial relationships to disclose.
ASCO Initiatives in Personalized Medicine Richard L. Schilsky, MD, FACP, FASCO Chief Medical Officer American Society of Clinical Oncology Financial Disclosures No financial relationships to disclose.
BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED
 ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products
 Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry FDA Approval of New Cancer Treatment Uses for Marketed Drug and Biological Products U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Clinical trial enrollment among older cancer patients
 Clinical trial enrollment among older cancer patients Sharon H. Giordano MD, MPH Professor, Health Services Research Mariana Chavez Mac Gregor MD, MSc Assistant Professor, Breast Medical Oncology Department
Clinical trial enrollment among older cancer patients Sharon H. Giordano MD, MPH Professor, Health Services Research Mariana Chavez Mac Gregor MD, MSc Assistant Professor, Breast Medical Oncology Department
Advancing research: a physician s guide to clinical trials
 Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
NSW Cancer Trials Network Network Portfolio Policy October 2012
 NSW Cancer Trials Network Network Portfolio Policy October 2012 E12/18446 Cancer Institute NSW Page 1 of 7 Contents BACKGROUND... 3 NSW CANCER TRIALS NETWORK PORTFOLIO KEY PRINCIPLES... 3 PORTFOLIO COMPLIANCE
NSW Cancer Trials Network Network Portfolio Policy October 2012 E12/18446 Cancer Institute NSW Page 1 of 7 Contents BACKGROUND... 3 NSW CANCER TRIALS NETWORK PORTFOLIO KEY PRINCIPLES... 3 PORTFOLIO COMPLIANCE
Presenting the SUTENT Patient Call Center.
 Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
Presenting the SUTENT Patient Call Center. Please see patient Medication Guide and full prescribing information attached. We re here to support you. Dealing with cancer is a journey. Along the way, you
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
30th GMS. Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte
 30th GMS Symposium on Vascular Surgery Berlin 5 7 November 2015 Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte Scientific direction: PD Dr. med. R. I. Rückert Dr.
30th GMS Symposium on Vascular Surgery Berlin 5 7 November 2015 Russisches Haus der Wissenschaft und Kultur Friedrichstraße 176-179 10117 Berlin-Mitte Scientific direction: PD Dr. med. R. I. Rückert Dr.
Accreditation, Certification and Quality Assurance Institute
 Accreditation, Certification and Quality Assurance Institute 1 Contents By HEIs for HEIs 4 Members 5 Organisational Structure 6 Objectives 8 The Quality Control Cycle 10 Further Development of the Accreditation
Accreditation, Certification and Quality Assurance Institute 1 Contents By HEIs for HEIs 4 Members 5 Organisational Structure 6 Objectives 8 The Quality Control Cycle 10 Further Development of the Accreditation
News Release. Merck KGaA, Darmstadt, Germany, and Pfizer Receive FDA Breakthrough Therapy Designation for Avelumab in Metastatic Merkel Cell Carcinoma
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
REF/2011/03/002226 CTRI Website URL - http://ctri.nic.in
 Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 15:20:58 GMT) CTRI Number Last Modified On 28/01/2014 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Thu, 14 Jul 2016 15:20:58 GMT) CTRI Number Last Modified On 28/01/2014 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Curriculum Vitae - Rubel, J. 1
 Curriculum Vitae - Rubel, J. 1 CURRICULUM VITAE Julian Rubel Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 3181. FAX 0049 (0)651
Curriculum Vitae - Rubel, J. 1 CURRICULUM VITAE Julian Rubel Address: Department of Clinical Psychology and Psychotherapy, University of Trier, 54286 Trier, Germany TEL 0049 (0)651 201 3181. FAX 0049 (0)651
Multiple Primary and Histology Site Specific Coding Rules KIDNEY. FLORIDA CANCER DATA SYSTEM MPH Kidney Site Specific Coding Rules
 Multiple Primary and Histology Site Specific Coding Rules KIDNEY 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways to view the Multiple l Primary/Histology
Multiple Primary and Histology Site Specific Coding Rules KIDNEY 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways to view the Multiple l Primary/Histology
5 th Heidelberg Myeloma Workshop
 Scientific program 5 th Heidelberg Myeloma Workshop Current status and developments in diagnosis and therapy of Multiple Myeloma Congress venue: Lecture hall, Hospital for Internal Medicine Friday, 24
Scientific program 5 th Heidelberg Myeloma Workshop Current status and developments in diagnosis and therapy of Multiple Myeloma Congress venue: Lecture hall, Hospital for Internal Medicine Friday, 24
Practical aspects of early phase oncology trials the oncologists view
 Practical aspects of early phase oncology trials the oncologists view S. E. Al-Batran, MD Head, Institute of Clinical Cancer Research (IKF) University Cancer Center (UCT) Nordwest Hospital Frankfurt Outline
Practical aspects of early phase oncology trials the oncologists view S. E. Al-Batran, MD Head, Institute of Clinical Cancer Research (IKF) University Cancer Center (UCT) Nordwest Hospital Frankfurt Outline
See you at the ACHEMA 2015 15. - 19.06.2015
 Sprache/ Language 15. 16. 17. 18. 19. Markus Dönni Geschäftsführer/ Managing Director Johannes Fliegen Geschäftsführer/ Managing Director Jochen Lindenberg Geschäftsführer/ Managing Director Christian
Sprache/ Language 15. 16. 17. 18. 19. Markus Dönni Geschäftsführer/ Managing Director Johannes Fliegen Geschäftsführer/ Managing Director Jochen Lindenberg Geschäftsführer/ Managing Director Christian
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Principal Investigator and Sub Investigator Responsibilities
 Principal Investigator and Sub Investigator Responsibilities I. Purpose To define the roles and responsibilities of Principal Investigators conducting research at GRU. II. Definition The term Principal
Principal Investigator and Sub Investigator Responsibilities I. Purpose To define the roles and responsibilities of Principal Investigators conducting research at GRU. II. Definition The term Principal
Gruppo di lavoro: Malattie Tromboemboliche
 Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
New Hampshire Childhood Cancer
 Introduction: New Hampshire Childhood Cancer New Hampshire, Childhood Cancer, January 2009 Issue Brief Cancer in children is relatively uncommon, impacting fewer than twenty two of every 100,000 children
Introduction: New Hampshire Childhood Cancer New Hampshire, Childhood Cancer, January 2009 Issue Brief Cancer in children is relatively uncommon, impacting fewer than twenty two of every 100,000 children
Health Disparities in Multiple Myeloma. Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc.
 Health Disparities in Multiple Myeloma Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc. Multiple Myeloma Overview Multiple myeloma (MM) is a type of blood cancer that develops
Health Disparities in Multiple Myeloma Kenneth R. Bridges, M.D. Senior Medical Director Onyx Pharmaceuticals, Inc. Multiple Myeloma Overview Multiple myeloma (MM) is a type of blood cancer that develops
Lung Cancer. Public Outcomes Report. Submitted by Omar A. Majid, MD
 Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Functional Specifications of the eztrial Clinical Trial Information Management System
 TR-IIS-10-004 Functional Specifications of the eztrial Clinical Trial Information Management System Hong-Yi Chen, Chung-Tsuo Lin, Chia-Hui Chang, Yi-Ru Cian, Yi-Fang Lee, and Chun-Nan Hsu June, 15 2010
TR-IIS-10-004 Functional Specifications of the eztrial Clinical Trial Information Management System Hong-Yi Chen, Chung-Tsuo Lin, Chia-Hui Chang, Yi-Ru Cian, Yi-Fang Lee, and Chun-Nan Hsu June, 15 2010
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Health Care Job Information Sheet #20. Clinical Research
 Health Care Job Information Sheet #20 Clinical Research A. Background B. Occupations 1) Clinical Research Associate (Study Monitor) 2) Clinical Research Coordinator 3) Other positions in the field C. Labour
Health Care Job Information Sheet #20 Clinical Research A. Background B. Occupations 1) Clinical Research Associate (Study Monitor) 2) Clinical Research Coordinator 3) Other positions in the field C. Labour
How to search the EU Clinical Trials Register
 28 April 2014 Information and Communications Technology Contents Contents... 1 1. Searching the EU Clinical Trials Register... 1 1.1. Basic search... 2 1.2. Search for multiple words (AND operator)...
28 April 2014 Information and Communications Technology Contents Contents... 1 1. Searching the EU Clinical Trials Register... 1 1.1. Basic search... 2 1.2. Search for multiple words (AND operator)...
REGISTRY OF IRREVERSIBLE ELECTROPORATION FOR THE ABLATION OF PROSTATE CANCER WITH USE OF NANOKNIFE DEVICE
 REGISTRY OF IRREVERSIBLE ELECTROPORATION FOR THE ABLATION OF PROSTATE CANCER WITH USE OF NANOKNIFE DEVICE A Multi-Center, International Registry to evaluate the treatment of Prostate Cancer in terms of
REGISTRY OF IRREVERSIBLE ELECTROPORATION FOR THE ABLATION OF PROSTATE CANCER WITH USE OF NANOKNIFE DEVICE A Multi-Center, International Registry to evaluate the treatment of Prostate Cancer in terms of
Molecular markers and clinical trial design parallels between oncology and rare diseases?
 Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data)
 Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data) 3rd EUDAT Conference 25 September 2014 Amsterdam, Netherlands Prof. Dr. C. Ohmann ECRIN-ERIC Major
Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data) 3rd EUDAT Conference 25 September 2014 Amsterdam, Netherlands Prof. Dr. C. Ohmann ECRIN-ERIC Major
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013. National Registry of Diseases Office (NRDO)
 Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Clinical Trial Transparency. What is available?
 Clinical Trial Transparency What is available? 1 Outline Clinical Trial Data Collection and Reporting Data Definitions Clinical Trial Transparency Summary Report Requirements Example of Results Posting
Clinical Trial Transparency What is available? 1 Outline Clinical Trial Data Collection and Reporting Data Definitions Clinical Trial Transparency Summary Report Requirements Example of Results Posting
Individual Mobility Services: Closing the Gap between Public and Private Transport
 1st Eastern Mediterranean Conference on Intermodal Passenger Travel Individual Mobility Services: Closing the Gap between Public and Private Transport Christian Maertins & Dr. Hinrich Schmöe Nicosia, 14/11/2008
1st Eastern Mediterranean Conference on Intermodal Passenger Travel Individual Mobility Services: Closing the Gap between Public and Private Transport Christian Maertins & Dr. Hinrich Schmöe Nicosia, 14/11/2008
Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations
 Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations Clinical Trials Registry S. 1082 (Title II, Subtitle C) H.R.
Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations Clinical Trials Registry S. 1082 (Title II, Subtitle C) H.R.
Secondary Use of the EHR via Pseudonymisation
 Secondary Use of the EHR via Klaus POMMERENING Institut für Medizinische Biometrie, Epidemiologie und Informatik Johannes-Gutenberg-Universität D-55101 Mainz, Germany Michael RENG Klinik und Poliklinik
Secondary Use of the EHR via Klaus POMMERENING Institut für Medizinische Biometrie, Epidemiologie und Informatik Johannes-Gutenberg-Universität D-55101 Mainz, Germany Michael RENG Klinik und Poliklinik
Number. Source: Vital Records, M CDPH
 Epidemiology of Cancer in Department of Public Health Revised April 212 Introduction The general public is very concerned about cancer in the community. Many residents believe that cancer rates are high
Epidemiology of Cancer in Department of Public Health Revised April 212 Introduction The general public is very concerned about cancer in the community. Many residents believe that cancer rates are high
GOOD CLINICAL PRACTICE PROGRAM. Workshop 2006 DC THERA. November 27th 28th 2006, Dorint Hotel Erlangen, Germany
 DC THERA Dendritic Cells & New Immunotherapies EU Sixth Framework Programme Life Science, Genomics and Biotechnology for Health DC-THERA Dendritic Cells & New Immunotherapies Network of Excellence, Project
DC THERA Dendritic Cells & New Immunotherapies EU Sixth Framework Programme Life Science, Genomics and Biotechnology for Health DC-THERA Dendritic Cells & New Immunotherapies Network of Excellence, Project
FACT SHEET (Available at http://prsinfo.clinicaltrials.gov/) Registration at ClinicalTrials.gov: As required by Public Law 110-85, Title VIII
 10/27/09 NOTE: Section 2, Timing of Registration at ClinicalTrials.gov, was revised on 10/23/09. This document supersedes the Fact Sheet issued on 11/09/07. Note that the new text is highlighted below.
10/27/09 NOTE: Section 2, Timing of Registration at ClinicalTrials.gov, was revised on 10/23/09. This document supersedes the Fact Sheet issued on 11/09/07. Note that the new text is highlighted below.
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche
 Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche Andrea Busolo U.O. di Radiologia Rocco De Vivo U.O. di Oncologia Medica ULSS 6 - Vicenza Activity and efficacy
Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche Andrea Busolo U.O. di Radiologia Rocco De Vivo U.O. di Oncologia Medica ULSS 6 - Vicenza Activity and efficacy
Singapore Clinical Trials Register. Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY
 Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
The Role Of Industry in the Australian Clinical Trials Sector
 Mitch Kirkman, Development QA Manager Australian Clinical Trials Alliance Symposium 09 October 2015 CORP0270 September 2015 Global Clinical Development Local Clinical Trials Australian Medical Research
Mitch Kirkman, Development QA Manager Australian Clinical Trials Alliance Symposium 09 October 2015 CORP0270 September 2015 Global Clinical Development Local Clinical Trials Australian Medical Research
List of participating Local Clinical Centers (LCCs) As of September 2013
 List of participating Local Clinical Centers (LCCs) As of September 2013 GERMANY sorted by Federal State Baden-Wurttemberg LCC Ulm Universitätsklinikum Ulm Studienzentrale Innere II Albert-Einstein-Allee
List of participating Local Clinical Centers (LCCs) As of September 2013 GERMANY sorted by Federal State Baden-Wurttemberg LCC Ulm Universitätsklinikum Ulm Studienzentrale Innere II Albert-Einstein-Allee
The Clinical Trials Process an educated patient s guide
 The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-
The Clinical Trials Process an educated patient s guide Gwen L. Nichols, MD Site Head, Oncology Roche TCRC, Translational and Clinical Research Center New York DISCLAIMER I am an employee of Hoffmann-

