CONSENT TO PARTICIPATE IN A CLINICAL. STUDY NUMBER: 11-E-0072 PRINCIPAL INVESTIGATOR: Frederick Miller, M.D., Ph.D.
|
|
|
- Edward Stephens
- 8 years ago
- Views:
Transcription
1 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient INSTITUTE: National Institute of Environmental Health Sciences STUDY NUMBER: 11-E-0072 PRINCIPAL INVESTIGATOR: Frederick Miller, M.D., Ph.D. STUDY TITLE: Environmental Risk Factors for the Anti-Synthetase Syndrome Initial Review Approved by the IRB on 09/28/10 Amendment Approved by the IRB on 02/04/11 (A) Posted to Web: 02/11/11 Adult NIH INTRODUCTION We invite you to take part in a research study at the National Institutes of Health (NIH). First, we want you to know that: Taking part in NIH research is entirely voluntary. You may choose not to take part, or you may withdraw from the study at any time. In either case, you will not lose any benefits to which you are otherwise entitled. However, to receive care at the NIH, you must be taking part in a study or be under evaluation for study participation. You may receive no benefit from taking part. The research may give us knowledge that may help people in the future. Second, some people have personal, religious or ethical beliefs that may limit the kinds of medical or research treatments they would want to receive (such as blood transfusions). If you have such beliefs, please discuss them with your NIH doctors or research team before you agree to the study. Now we will describe this research study. Before you decide to take part, please take as much time as you need to ask any questions and discuss this study with anyone at NIH, or with family, friends or your personal physician or other health professional. THE PURPOSE OF THIS STUDY If you are the parent of a child enrolling in the study you are reading this consent form for your child, who will be the participant in the study. Researchers at the National Institute of Environmental Health Sciences (NIEHS), in collaboration with other researchers, are conducting a study to try to understand why some persons develop certain diseases while other persons do not. Scientists believe that differences in exposures, or people s responses to certain exposures in the environment as a result of differences in the genetic makeup of the person, may determine who develops certain diseases. CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient NIH (07-09) File in Section 4: Protocol Consent (1)
2 STUDY NUMBER: 11-E-0072 CONTINUATION: page 2 of 11 pages You are being asked to enroll in this study because you either have recently developed a form of muscle disease called myositis or because you are healthy and have volunteered to be in a comparison group of people who do not have a form of myositis or any other autoimmune disease. The information and samples from myositis patients cannot be analyzed or fully understood unless it is compared to similar information and samples from healthy people. The primary group being studied consists of persons with myositis who also produce a protein in the blood that reacts with the person s tissues called an autoantibody. These autoantibodies bind to certain parts of the person s own cells, called synthetases, that are involved in making new proteins. They are therefore called anti-synthetase autoantibodies. A syndrome called the antisynthetase syndrome, which includes myositis and lung disease, is associated with having the anti-synthetase autoantibodies. This study is being conducted to determine if persons with the anti-synthetase syndrome have had different environmental exposures before disease onset compared to other patients with myositis who do not have these autoantibodies and compared to healthy individuals who do not have an autoimmune disease. About 150 myositis subjects with anti-synthetase syndrome will be compared to 150 matched persons who are their friends, cousins or other volunteers, who do not have myositis or another autoimmune disease. The same 150 myositis subjects with the antisynthetase syndrome will also be compared to 150 myositis patients without the anti-synthetase syndrome. You will not be in a treatment program for your medical conditions at NIH in this study, but we will report laboratory and other results that may be important to your health to you and your health care provider. RESEARCH TESTS OR PROCEDURES FOR THIS STUDY If you agree to participate in this study, doctors involved in this protocol will see you at the NIH Clinical Center where you will have a thorough medical evaluation. This evaluation will involve obtaining your medical records, answering questions about your medical history, completing written questionnaires, undergoing a physical examination and donating blood, urine and possibly other clinical specimens for research purposes. The specific tests that you will have depend on whether you have myositis or are a volunteer without myositis or any other autoimmune disease. TESTS THAT ALL SUBJECTS WILL HAVE All subjects will be asked to do the following in this study, which should take about three to four hours: 1) Allow us to obtain and review your past medical records from your doctors or other health care providers. The information from your records will be kept strictly confidential. 2) You will spend about 1-2 hours completing forms about your past medical history and the types of exposures you have had at work, at home and elsewhere. If you have myositis you will also be asked if you have had certain infections, engaged in heavy exercise or physical exertion, reacted to the sun in a particular way, used tobacco or alcohol, or experienced stressful events in your life before the diagnosis of your disease. If you are a healthy volunteer you will be asked if you had the same exposures before the date of diagnosis of disease of the myositis subject to which you are matched, which will be printed on your questionnaire. 3) You will be given a kit that contains instructions and a filter to be put onto your vacuum cleaner to collect house dust in your bedroom that will be stored for possible future analyses of infectious or toxic agents based on the other results from the study. 4) You will undergo a medical history and physical examination by your physician who will also complete forms describing the findings from this evaluation. This evaluation is performed to document what features of myositis you have or to
3 STUDY NUMBER: 11-E-0072 CONTINUATION: page 3 of 11 pages confirm that you do not have these illnesses. If any abnormalities or diseases are detected that your health care provider did not previously identify, additional testing may be performed or recommended for clinical care purposes only. 5) If you are an adult you will have about 8 tablespoons (120 milliliters) of blood collected for a number of clinical and research tests. If the subject is a child the amount of blood drawn will be less and will depend on the size of the child. Blood drawing involves cleaning the skin of the arm with alcohol. A needle is then inserted into the vein. Blood is then quickly withdrawn using a special tube or syringe. The following tests will be performed on the blood samples: a) To confirm that you do not have certain diseases or medical problems that you are unaware of, a blood specimen will be collected for routine clinical chemistry and other blood studies, including tests for autoantibodies found in patients with rheumatic diseases. b) Because certain environmental exposures may cause certain diseases, we will be testing your blood for evidence of past exposures and if you have had certain infections. The NIH laboratories will perform some of these tests but our collaborators will also perform some of these tests in their laboratories. c) The blood testing you will undergo includes tests of muscle enzymes and other blood chemistries that may reflect ongoing inflammation. d) As part of this study, we will test you for infection with the human immunodeficiency virus (HIV), the virus that causes AIDS. If you are infected with HIV you will still be able to participate in this study. We will tell you what the results mean, how to find care, how to avoid infecting others, how we report newly diagnosed HIV infection, and the importance of informing your partners at possible risk because of your HIV infection. e) The hereditary factors that determine many of our physical characteristics and may predispose to disease are called genes. To identify which genes may be related to disease, DNA, the chemical substance that contains the genes, is extracted from blood or tissues from healthy persons and from those with disease and compared using statistical methods. We will be testing your blood for the genes for human leukocyte antigens (HLA) and for other genes that may be important in regulating the immune system. This will allow us to determine if certain forms of these genes are more common in patients with the anti-synthetase syndrome. f) We will be comparing the metabolism in your blood cells with the metabolism in blood cells from other groups of subjects to see if persons with the anti-synthetase syndrome have a different metabolism. The metabolism in your blood cells is controlled by how some genes are activated (turned on) and some are deactivated (turned off) and this determines which proteins are made. Because this pattern of proteins and gene activation may help us understand how diseases occur, research tests on blood samples will determine the types of genes and proteins that are active in your blood cells. g) We are asking you to let us have samples of your blood and urine to store for future research on the causes of autoimmune diseases and their complications. We will also ask to collect any past blood or tissue biopsy specimens that are no longer needed for your clinical care, for research purposes. Your blood samples will be processed in the NIH and by our contractors for testing and for storage in freezers. We will store all your samples with a code and not with your name. Coding is done to protect your identity and only those researchers closely involved with the progress of the study will have access to the locked files that can link the code to your name or
4 STUDY NUMBER: 11-E-0072 CONTINUATION: page 4 of 11 pages other private information. These coded samples will be used to complete studies described in this consent form by investigators involved in this protocol. The investigators conducting this study do not plan to provide you with the results of the research tests mentioned above because further research may be necessary before the results are meaningful. If meaningful information is developed from this study that may become important for your health, however, you and your health care provider will be informed when it becomes available. By agreeing to participate in this study, you do not waive any rights that you may have regarding access to and disclosure of your records. For further information on those rights, please contact Drs. Frederick Miller or Lisa Rider. TESTS THAT ONLY MYOSITIS SUBJECTS WILL HAVE If you have myositis and your doctor believes additional tests are needed to better understand your disease and determine your treatment, you may have some of the tests listed below: Electrocardiogram: This is a test measuring the electrical activity of your heart. Wires are attached to the chest wall, arms and legs using a sticky paste which takes only a few minutes. There is no discomfort from this procedure. If the electrocardiogram shows any abnormalities, an echocardiogram, or sound-wave study of your heart function would also be done. No harmful consequences of this procedure are known. Pulmonary Function Tests: Your lung function and breathing would be assessed by a pulmonary function test if you are having any difficulties with breathing. You would have to blow into a tube for a few seconds. If this test shows abnormalities, a chest x-ray or chest CT scan may also be done. Chest X-ray: This test will be done only if your doctor decides it is needed for your care. A chest X-ray uses a small amount of radiation to determine if there are any abnormalities in your lungs or other chest tissues. Please, however, notify your doctor if you have had any other recent x-ray studies since effects of radiation can be additive. If you are pregnant you will not be permitted to participate in this aspect of the study since it is best to avoid radiation to the human embryo, so if you are a woman of child-bearing potential you will have a pregnancy test before having a chest X-ray. Chest CT scan: If your doctor decides it is needed for your care because your chest x-ray is abnormal or you have significant breathing problems that need further evaluation, you may have a CT scan of the chest. This test involves having you lie on a padded table, which is slowly moved into a large tube inside the machine where multiple X-rays will be taken. Please notify your doctor, however, if you have had any other recent x-ray studies because the effects of radiation can be additive. If you are pregnant you will not be permitted to participate in this aspect of the study because it is best to avoid radiation to the human embryo, so if you are a woman of childbearing potential you will have a pregnancy test before having a chest CT scan. Magnetic Resonance Imaging Scan of the Lower Extremities: This study produces a picture of your muscles or the tissue under the skin (subcutaneous tissue) using magnetism. The test involves having you lie on a padded table, which is slowly moved into a large tube inside the machine where there is a powerful magnet. Watches, keys, coins, bank and credit cards, and certain types of jewelry cannot be brought into the room with the machine because of the power of the magnet. You will have to lie very still for the test, approximately 30 minutes. This test involves no x-rays and there are no known risks from the test, although some people may feel claustrophobic. You can communicate with the MRI technician during this test and the procedure will be stopped if you cannot tolerate it. If you are unable to lie still for 30 minutes for this test, you will not be able to undergo this evaluation, but may participate in other aspects of the study. You will sign a separate consent form for the
5 STUDY NUMBER: 11-E-0072 CONTINUATION: page 5 of 11 pages MRI that will explain that you cannot have this procedure if you have a pacemaker, defibrillator, certain ear implants, or some types of clips used on brain aneurysms. Other Procedures: When clinically indicated, additional testing may be recommended for you, including an electromyography study or a muscle or lung biopsy. These procedures require separate informed consent and will be further explained to you if they are recommended. Also, because the risk of cancer is increased in adults with myositis, if you have myositis it is important for you to be carefully evaluated for cancer. Therefore, you may have additional testing for cancer, if clinically indicated, based on your age, sex or other risk factors. These tests could include certain blood tests to assess the possibility of prostate cancer, a pelvic exam to assess the possibility of cervical or other pelvic cancer, stool tests to look for blood or an abdominal CT scan similar to the chest CT scan above to evaluate cancers in the abdomen or pelvis. Children may rarely undergo other recommended tests to check for cancer. QUESTIONNAIRES AND FOLLOW-UP CONTACTS In order to determine if you have had certain exposures in the past, we will ask about your past occupations, hobbies, other activities, infections, stressful life events, and other exposures of interest to the study by having you complete questionnaires. Depending on the answers to these questions, you may be contacted by phone by persons involved in this study to clarify certain answers and to obtain more detailed information about your occupations or if additional samples or information is needed in future aspects of the study. You or your doctor may be also contacted in the future for additional information or to give additional blood samples, and you may be asked to consider participating in future studies. Your participation in this and all future studies is completely voluntary. You may withdraw from this study at any time, and you may decline to participate in any follow-up studies without in any way affecting your eligibility for participation in future research at the NIH. RISKS OR DISCOMFORTS TO YOU IF YOU TAKE PART IN THIS STUDY You may reasonably expect to experience the following risks and/or discomforts. The major risks of blood drawing involve the pain of the needle puncturing the skin and the risk of getting a bruise. There is also a small chance of infection or bleeding around the spot where the blood was drawn and a very few persons may faint during blood drawing. You will receive appropriate treatment for any complications of this sort. Some people are concerned that research about genetic causes of illness may give information that is not only about themselves, but also about their relatives and other groups of people who are like them. Because the diseases we are studying result from many genes and exposures, it is unlikely, although possible, that we will learn genetic information in this study that can be used to diagnose or predict autoimmune diseases in you or your family. The researchers will learn new medical information about you from the testing as well as new details about your cells and your DNA, and it is possible that this information, or the changes seen in your blood samples, may be associated with a specific disease and this could affect your ability to obtain health insurance in the future. NIH and the Clinical Center, like other hospitals, may be required to release such information to insurance companies if you have signed a release of information form. However, as explained below, a new Federal law, called the Genetic Information Nondiscrimination Act (GINA), generally makes it illegal for health insurance companies, group health plans, and most employers to discriminate against you based on your genetic information.
6 STUDY NUMBER: 11-E-0072 CONTINUATION: page 6 of 11 pages BENEFITS TO YOU OF TAKING PART IN THIS STUDY This is a research study. By donating information about your health and exposures, as well as your blood or biopsy samples you may not personally benefit from this study, but you may be helping scientists discover genetic differences in our cells that make some people more sensitive to environmental factors or in understanding which environmental exposures may be related to disease. The physicians involved in your care at the NIH will not take over your clinical care. We will, however, work with your referring physician and make treatment recommendations to your doctor if needed. If you have myositis, you may potentially benefit directly from participation in this study by undergoing a thorough clinical evaluation, the results of which will be shared with your doctor in order to help them plan the most appropriate treatments for you. If we discover any new information during the study that might affect your health, we will notify you and your health care provider immediately. WHAT OTHER CHOICES YOU HAVE BESIDES TAKING PART IN THIS STUDY You have the alternative of not participating in this study. WHAT WILL HAPPEN TO THE SAMPLES OR INFORMATION THAT ARE COLLECTED FROM THIS STUDY? Your DNA/blood/cell/other samples/study records will remain stored indefinitely in order to allow for the studies to be completed and to allow for retesting of your samples as necessary. Your DNA/blood/cell/and other samples will be stored at two sites: in the laboratory of Dr. Frederick Miller in Bethesda, MD, and in the repository of the National Institute of Environmental Health Sciences. The reason for duplication of long-term storage is to insure against accidental loss of frozen samples. All stored DNA/blood/cell samples and information generated from this study will be identified by a code and not your name. This code will be kept secure in a locked area or in computer files that only Dr. Miller and a few specific investigators or their designees in this study can access with a password. As part of this protocol, some of the data are stored coded in controlled access databases in which protocol investigators with approval have access to the data; however, your information is coded and you cannot be personally identified. When data are transferred to investigators with approved projects no identifying information about you will be provided to these investigators. Your coded information or samples may be sent to other investigators involved in this protocol for research purposes, as defined in this protocol. These investigators will not know your name and will not know which samples are yours. The collaborating investigators may also collect information and samples from study subjects and are located at: the National Institutes of Health in Bethesda, MD and Research Triangle Park, NC; the Johns Hopkins University, Baltimore, MD; the Brigham and Women's Hospital, Boston, MA; the Medical University of South Carolina, Charleston, SC; the University of Pittsburgh, Pittsburgh, PA; and The Mayo Clinic, Rochester, MN. Your samples will not be available for routine care or commercial diagnostic testing. It is possible that your samples or study records may be shared anonymously with other investigators for other research use beyond the scope of this study. Such usage will be strictly anonymous, in that no identifying information about you, including your name, will be provided to the researcher, and there will be no way for the researchers to link these samples back to you.
7 STUDY NUMBER: 11-E-0072 CONTINUATION: page 7 of 11 pages WHAT TO DO IF YOU DECIDE TO WITHDRAW FROM THIS STUDY You may withdraw from this study at any time by providing written notification to your primary NIH doctor. You may ask to no longer be contacted by us and to not return to the NIH. In this case, we will no longer contact you by phone or mail. If you decide to withdraw from this study, it will not in any way affect your eligibility for medical care or participation in future research at the NIH. COMPENSATION You and your referring health care provider will each be paid $100, for the time, expense and inconvenience involved in participation, after your enrollment into the study. In some cases, you may be asked to return for one or more additional visits to repeat some of the research tests and, if this occurs, you and your local health care provider will again each be paid $100. CONFLICT OF INTEREST STATEMENT 1. The National Institutes of Health reviews NIH staff researchers at least yearly for conflicts of interest. The following link contains details on this process You may ask your research team for additional information or a copy of the Protocol Review Guide. 2. This protocol has investigators(s) who are not NIH employees. They are expected to comply with their Institution s conflict of interest policies.
8 STUDY NUMBER: 11-E-0072 CONTINUATION: page 8 of 11 pages CONSENT TO STORE YOUR INFORMATION AND SAMPLES FROZEN FOR POSSIBLE FUTURE RESEARCH Thank you for agreeing to participate in this study. Now we would like your permission for the NIH to store the remainder of your blood and other samples, as well as your coded information, for possible future research. Your information and frozen samples will be stored under a number code. Only the NIH study researchers will be able to match this code with your name. The remainder of your coded information and specimens may be used for additional studies of autoimmune diseases and their causes. Your remaining coded information and samples may also be used to study disorders unrelated to the diseases being studied in this research. The researchers will not have access to your name or any identifying information about you. Your consent to frozen storage of your samples does not affect your ability to participate in this study. I AGREE to frozen storage of my samples and information for possible future research I DO NOT agree to this Signature of Adult Patient/Parent/Legal Representative Signature of Witness Signature of Investigator
9 STUDY NUMBER: 11-E-0072 CONTINUATION: page 9 of 11 pages CONSENT TO USE YOUR SAMPLES FOR GENOMIC RESEARCH The researchers would like permission to use your DNA samples for more extensive genetic testing called genome-wide association studies that are trying to determine the genetic risk and protective factors for myositis. This test analyzes a large number of parts of your DNA and allows researchers to see if genes not previously known to be associated with disease are important in developing some diseases. We are also asking your permission to put your coded information from your DNA testing and certain medical record information in a government health research database, available on the internet. Aggregate data that combines all participants in this study would be available to anyone on the internet. Your individual coded medical information and information from more detailed analyses of the coded samples will be put in a controlled-access database. The information in this database will be available in a secured manner only to qualified researchers who have received approval from an NIH Data Access Committee. Please note that traditionally-used identifying information about you, such as your name, address, telephone number, or social security number, will not be put into the public genetic databases for this project and will also not be available to these approved researchers. Your privacy is very important to us and we will use many safety measures to protect your privacy. However, in spite of all of the safety measures that we will use, we cannot guarantee that your identity will never become known. Under Federal law, anyone has a right to request the release of an individual s coded data (data that does not contain personally identifiable information) from the genome-wide database, including members of the public, insurers, employers and law enforcement agencies. NIH would take a legal position that this data is not releasable, but it is unclear that this would be upheld in court. Although your genetic information is unique to you, you share some genetic information with your children, brothers, sisters, and other blood relatives. Consequently, it may be possible that genetic information from them could be used to help identify you. Similarly, it may be possible that genetic information from you could be used to help identify them. While the public genetic databases developed for this project will not contain information that is traditionally used to identify you, such as your name, address, telephone number, or social security number, it is possible using sophisticated laboratory techniques to link your genetic information in these databases back to you. For example, someone could compare information in these genetic databases with information from you (or a blood relative) in another database and be able to identify you (or your blood relative). Since some genetic variations can help to predict the future health problems of yourself and your blood relatives, this information might be of interest to employers, health providers, insurance companies, and others. Patterns of genetic variation also can be used by law enforcement agencies to identify a person or his/her blood relatives. Therefore, your genetic information potentially could be used in ways that could cause you or your family distress, such as by revealing that you (or a blood relative) carry a genetic disease. It also is possible that there could be violations to the security of the computer systems used to store the codes linking the genetic and medical information to you. There also may be other privacy risks that we have not foreseen. While we believe that the risks to you and your family are very low, we are unable to tell you exactly what all of the risks are. A new Federal law, called the Genetic Information Nondiscrimination Act (GINA), generally makes it illegal for health insurance companies, group health plans, and most employers to discriminate against you based on your genetic information. According to this law, health insurance companies or group health plans (as of May 21, 2010) cannot request your genetic information or use it to make decisions about your eligibility or premiums; and employers cannot use it in deciding to hire, promote, or fire you or in setting the terms of your employment (as of Nov 21, 2009). Be aware that this new Federal law does not protect you against genetic discrimination by companies that sell life insurance, disability insurance, or long-term care insurance.
10 STUDY NUMBER: 11-E-0072 CONTINUATION: page 10 of 11 pages The following link contains details on this policy: You may ask your research team for additional information or a copy of The Genetic Information Nondiscrimination Act of 2008 informational document. We believe that the benefits of learning more about myositis outweigh these potential risks. There is a great potential for researchers and health professionals to learn a great deal about the causes of these diseases and from that, to develop better ways to prevent, detect, treat, and cure these illnesses. We will make every attempt to protect your confidentiality and to make sure that your personal identity does not become known. You may still participate in the study even if you do not choose to have genome-wide testing and allow your health information to be placed in the government database. I AGREE to genome-wide association studies of my DNA and inclusion of the results into a government database I DO NOT agree to this Signature of Adult Patient/Parent/Legal Representative Signature of Witness Signature of Investigator
11 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient STUDY NUMBER: 11-E-0072 CONTINUATION: page 11 of 11 pages OTHER PERTINENT INFORMATION 1. Confidentiality. When results of an NIH research study are reported in medical journals or at scientific meetings, the people who take part are not named and identified. In most cases, the NIH will not release any information about your research involvement without your written permission. However, if you sign a release of information form, for example, for an insurance company, the NIH will give the insurance company information from your medical record. This information might affect (either favorably or unfavorably) the willingness of the insurance company to sell you insurance. The Federal Privacy Act protects the confidentiality of your NIH medical records. However, you should know that the Act allows release of some information from your medical record without your permission, for example, if it is required by the Food and Drug Administration (FDA), members of Congress, law enforcement officials, or authorized hospital accreditation organizations. 2. Policy Regarding Research-Related Injuries. The Clinical Center will provide short-term medical care for any injury resulting from your participation in research here. In general, no long-term medical care or financial compensation for research-related injuries will be provided by the National Institutes of Health, the Clinical Center, or the Federal Government. However, you have the right to pursue legal remedy if you believe that your injury justifies such action. 3. Payments. The amount of payment to research volunteers is guided by the National Institutes of Health policies. In general, patients are not paid for taking part in research studies at the National Institutes of Health. Reimbursement of travel and subsistence will be offered consistent with NIH guidelines. 4. Problems or Questions. If you have any problems or questions about this study, or about your rights as a research participant, or about any research-related injury, contact the Principal Investigator, Dr. Frederick W. Miller; NIH Building 10, Room , Telephone: or toll free at Other researchers you may call at are: Dr. Lisa Rider, Dr. Irene Whitt or Anna Jansen. You may also call the Clinical Center Patient Representative at Consent Document. Please keep a copy of this document in case you want to read it again. COMPLETE APPROPRIATE ITEM(S) BELOW: A. Adult Patient s Consent B. Parent s Permission for Minor Patient. I have read the explanation about this study and have been given the I have read the explanation about this study and have been given the opportunity to discuss it and to ask questions. I hereby consent to take opportunity to discuss it and to ask questions. I hereby give permission part in this study. for my child to take part in this study. (Attach NIH , Minor s Assent, if applicable.) Signature of Adult Patient/Legal Representative Signature of Parent(s)/Guardian Print Name Print Name C. Child s Verbal Assent (If Applicable) The information in the above consent was described to my child and my child agrees to participate in the study. Signature of Parent(s)/Guardian Print Name THIS CONSENT DOCUMENT HAS BEEN APPROVED FOR USE FROM SEPTEMBER 28, 2010 THROUGH SEPTEMBER 27, Signature of Investigator Signature of Witness Print Name Print Name CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY (Continuation Sheet) Adult Patient or Parent, for Minor Patient NIH (07-09) File in Section 4: Protocol Consent
If you are signing for a minor child, you refers to your child throughout the consent document.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
Consent Form: Example 2 (DNA Sequencing)
 Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry
 National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
1. General Information About The Mitochondrial Disease Biobank
 Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
Pl"OtocolDirector: Iris Schrijver _ IRB Approval Date: _June 20 2006 IRE Expiration Date: June 19, 2007 _ STANFORD SAMPLE CONSENT FORM
 Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
CONSENT FORM TEMPLATE. Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens
 CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
RESEARCH SUBJECT INFORMATION AND CONSENT FORM
 1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY
 The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
CORD BLOOD TRANSPLANTATION STUDY EXPANDED ACCESS PROTOCOL APPENDIX A SAMPLE CONSENT FORM
 APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION
 PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH
 The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH Title of Research: Funding Agency/Sponsor: Study Doctors: Research Personnel:
The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH Title of Research: Funding Agency/Sponsor: Study Doctors: Research Personnel:
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
The Liver and Alpha-1. Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION
 The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION What Is Alpha-1 Antitrypsin Deficiency? Alpha-1 is a condition that may result in serious lung disease in adults and/or liver
The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION What Is Alpha-1 Antitrypsin Deficiency? Alpha-1 is a condition that may result in serious lung disease in adults and/or liver
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
Guidance on the Genetic Information Nondiscrimination Act: Implications for Investigators and Institutional Review Boards
 Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on the Genetic Information Nondiscrimination Act: Implications for Investigators and Institutional Review
Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on the Genetic Information Nondiscrimination Act: Implications for Investigators and Institutional Review
Genes for Good Consent Form
 Genes for Good Consent Form Version 2.1 The next few screens contain information about Genes for Good and the benefits and risks of participating. This is called "informed consent", because we want you
Genes for Good Consent Form Version 2.1 The next few screens contain information about Genes for Good and the benefits and risks of participating. This is called "informed consent", because we want you
Patient Information Leaflet: Part 1 select-d
 Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
1 ALPHA-1. Am I an Alpha-1 Carrier? FOUNDATION FOUNDATION. Learn how being an Alpha-1 carrier can affect you and your family
 Am I an Alpha-1 Carrier? 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people affected by Alpha-1 worldwide.
Am I an Alpha-1 Carrier? 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people affected by Alpha-1 worldwide.
1 ALPHA-1. The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) FOUNDATION FOUNDATION. A patient s guide to Alpha-1 liver disease
 The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people
The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people
Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT
 Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT Regulatory changes will occur for investigators studying human DNA The recent acceleration and widening
Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT Regulatory changes will occur for investigators studying human DNA The recent acceleration and widening
Saint Francis Kidney Transplant Program Issue Date: 6/9/15
 Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
PATIENT INFORMATION INSURANCE INFORMATION
 (mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
(mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
Acute Myeloid Leukemia
 Acute Myeloid Leukemia Introduction Leukemia is cancer of the white blood cells. The increased number of these cells leads to overcrowding of healthy blood cells. As a result, the healthy cells are not
Acute Myeloid Leukemia Introduction Leukemia is cancer of the white blood cells. The increased number of these cells leads to overcrowding of healthy blood cells. As a result, the healthy cells are not
CONSENT TO PARTICIPATE IN A CLINICAL. STUDY NUMBER: 08-N-0215 PRINCIPAL INVESTIGATOR: Mark Hallett, M.D.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient INSTITUTE: National Institute of Neurological Disorders and Stroke STUDY NUMBER: 08-N-0215
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient INSTITUTE: National Institute of Neurological Disorders and Stroke STUDY NUMBER: 08-N-0215
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet
 U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
Mesothelioma. 1995-2013, The Patient Education Institute, Inc. www.x-plain.com ocft0101 Last reviewed: 03/21/2013 1
 Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
#3: SAMPLE CONSENT FORM
 #3: SAMPLE CONSENT FORM [Key Element #3: Who is conducting the study] UPMC University of Pittsburgh Medical Center Western Psychiatric Institute and Clinic CONSENT TO ACT AS A PARTICIPANT IN A RESEARCH
#3: SAMPLE CONSENT FORM [Key Element #3: Who is conducting the study] UPMC University of Pittsburgh Medical Center Western Psychiatric Institute and Clinic CONSENT TO ACT AS A PARTICIPANT IN A RESEARCH
How To Treat Leukaemia With Cord Blood Stem Cell
 Cord blood for the treatment of acute lymphoblastic leukemia in young children By Caitlin McGreevy Kiara Paramjothy Pass with Merit RESEARCH PAPER BASED ON PATHOLOGY LECTURES AT MEDLINK 2011 1 Abstract:
Cord blood for the treatment of acute lymphoblastic leukemia in young children By Caitlin McGreevy Kiara Paramjothy Pass with Merit RESEARCH PAPER BASED ON PATHOLOGY LECTURES AT MEDLINK 2011 1 Abstract:
ASSESSMENT OF MAGNETIC RESONANCE IMAGING PROTOCOLS WITH HEALTHY VOLUNTEERS
 SHEFC BRAIN IMAGING RESEARCH CENTRE FOR SCOTLAND Department of Clinical Neurosciences The University of Edinburgh Western General Hospital Crewe Road Edinburgh EH4 2XU Tel: 0131 537 2664 Fax: 0131 537
SHEFC BRAIN IMAGING RESEARCH CENTRE FOR SCOTLAND Department of Clinical Neurosciences The University of Edinburgh Western General Hospital Crewe Road Edinburgh EH4 2XU Tel: 0131 537 2664 Fax: 0131 537
INTRODUCTION Thrombophilia deep vein thrombosis DVT pulmonary embolism PE inherited thrombophilia
 INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form
 SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form 1. This Medical Power of Attorney (also known as the Health Care Agent Designation Form) allows you to
SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form 1. This Medical Power of Attorney (also known as the Health Care Agent Designation Form) allows you to
RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM
 If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
Clinical Trials: Questions and Answers
 Clinical Trials: Questions and Answers Key Points Clinical trials are research studies that test how well new medical approaches work in people (see Question 1). Every clinical trial has a protocol, which
Clinical Trials: Questions and Answers Key Points Clinical trials are research studies that test how well new medical approaches work in people (see Question 1). Every clinical trial has a protocol, which
Multiple Myeloma. This reference summary will help you understand multiple myeloma and its treatment options.
 Multiple Myeloma Introduction Multiple myeloma is a type of cancer that affects white blood cells. Each year, thousands of people find out that they have multiple myeloma. This reference summary will help
Multiple Myeloma Introduction Multiple myeloma is a type of cancer that affects white blood cells. Each year, thousands of people find out that they have multiple myeloma. This reference summary will help
Smoking and misuse of certain pain medicines can affect the risk of developing renal cell cancer.
 Renal cell cancer Renal cell cancer is a disease in which malignant (cancer) cells form in tubules of the kidney. Renal cell cancer (also called kidney cancer or renal adenocarcinoma) is a disease in which
Renal cell cancer Renal cell cancer is a disease in which malignant (cancer) cells form in tubules of the kidney. Renal cell cancer (also called kidney cancer or renal adenocarcinoma) is a disease in which
Recruiting now. Could you help by joining this study?
 Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
JOHNS HOPKINS NOTICE OF PRIVACY PRACTICES FOR HOPKINS ELDERPLUS/PROGRAM OF ALL-INCLUSIVE CARE FOR THE ELDERLY (PACE)
 JOHNS HOPKINS NOTICE OF PRIVACY PRACTICES FOR HOPKINS ELDERPLUS/PROGRAM OF ALL-INCLUSIVE CARE FOR THE ELDERLY (PACE) Effective Date: September 1, 2013 THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT
JOHNS HOPKINS NOTICE OF PRIVACY PRACTICES FOR HOPKINS ELDERPLUS/PROGRAM OF ALL-INCLUSIVE CARE FOR THE ELDERLY (PACE) Effective Date: September 1, 2013 THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT
Research Ethics Review Committee (WHO ERC)
 Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
INTRODUCTION Thrombophilia deep vein thrombosis DVT pulmonary embolism PE inherited thrombophilia
 INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM
 GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
Imaging Markers of Brain Network Dysfunction in Multiple Sclerosis
 Faculty of Medicine & Health Sciences School of Medicine Radiological Sciences Research Group The University of Nottinham University Park Nottingham NG7 2RD t: +44 (0)115 823 0018 f: +44 (0)115 823 0004
Faculty of Medicine & Health Sciences School of Medicine Radiological Sciences Research Group The University of Nottinham University Park Nottingham NG7 2RD t: +44 (0)115 823 0018 f: +44 (0)115 823 0004
Power of Attorney for Health Care For
 Power of Attorney for Health Care For Name: Date of Birth: Address: Telephone: This document is on file at Copies of this document have been given to my health care agent(s) and: 1. 2. 3. 4. 5. Courtesy
Power of Attorney for Health Care For Name: Date of Birth: Address: Telephone: This document is on file at Copies of this document have been given to my health care agent(s) and: 1. 2. 3. 4. 5. Courtesy
NOTICE OF PRIVACY PRACTICES
 THE PHYSICIAN PRACTICE, P.A. NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW
THE PHYSICIAN PRACTICE, P.A. NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW
Ambassador Application
 Ambassador Application Dear Applicant, Thank you for your interest in Dallas Medical Center s Ambassador Program! Your willingness to invest a few hours each week is greatly appreciated. I believe you
Ambassador Application Dear Applicant, Thank you for your interest in Dallas Medical Center s Ambassador Program! Your willingness to invest a few hours each week is greatly appreciated. I believe you
1. Study title Is the title self explanatory to a layperson? If not, a simplified title should be included.
 These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
These guidelines apply to all research projects where human subjects are involved in the study GUIDELINES FOR RESEARCHERS PATIENT INFORMATION SHEET & CONSENT FORM The guidance, which follows, applies primarily
What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide
 What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide A M E R I C A N C O L L E G E O F C H E S T P H Y S I C I A N S Lung cancer is one of the most common cancers. About 170,000
What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide A M E R I C A N C O L L E G E O F C H E S T P H Y S I C I A N S Lung cancer is one of the most common cancers. About 170,000
Participant Invitation and Information Sheet MRI Test Run
 ON QE TRUST HEADED PAPER A randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of Idiopathic Intracranial Hypertension: the IIH:WT Trial
ON QE TRUST HEADED PAPER A randomised controlled trial of bariatric surgery versus a community weight loss programme for the sustained treatment of Idiopathic Intracranial Hypertension: the IIH:WT Trial
Blood Transfusion. Red Blood Cells White Blood Cells Platelets
 Blood Transfusion Introduction Blood transfusions are very common. Each year, almost 5 million Americans need a blood transfusion. Blood transfusions are given to replace blood lost during surgery or serious
Blood Transfusion Introduction Blood transfusions are very common. Each year, almost 5 million Americans need a blood transfusion. Blood transfusions are given to replace blood lost during surgery or serious
Understanding Endometriosis - Information Pack
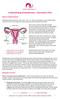 What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
What is endometriosis? Endometriosis (pronounced en- doh mee tree oh sis) is the name given to the condition where cells like the ones in the lining of the womb (uterus) are found elsewhere in the body.
Participating in Alzheimer s Disease Clinical Trials and Studies
 Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
Prostate Cancer Screening. A Decision Guide for African Americans
 Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
1 ALPHA-1. What is Alpha-1? A family history... of lung disease? of liver disease? FOUNDATION
 What is Alpha-1? A family history... of lung disease? of liver disease? What you need to know about Alpha-1 Antitrypsin Deficiency 1 ALPHA-1 FOUNDATION What is Alpha-1? Alpha-1 Antitrypsin Deficiency (Alpha-1)
What is Alpha-1? A family history... of lung disease? of liver disease? What you need to know about Alpha-1 Antitrypsin Deficiency 1 ALPHA-1 FOUNDATION What is Alpha-1? Alpha-1 Antitrypsin Deficiency (Alpha-1)
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
KUTTEH KE FERTILITY ASSOCIATES OF MEMPHIS, PLLC AND MEMPHIS FERTILITY LABORATORY, INC.
 1 of 6 KUTTEH KE FERTILITY ASSOCIATES OF MEMPHIS, PLLC 80 Humphreys Center, Suite 307 Memphis, Tennessee 38120-2363 (901) 747-2229 AND MEMPHIS FERTILITY LABORATORY, INC. IN VITRO FERTILIZATION/EMBRYO TRANSFER
1 of 6 KUTTEH KE FERTILITY ASSOCIATES OF MEMPHIS, PLLC 80 Humphreys Center, Suite 307 Memphis, Tennessee 38120-2363 (901) 747-2229 AND MEMPHIS FERTILITY LABORATORY, INC. IN VITRO FERTILIZATION/EMBRYO TRANSFER
Information for patients and the public and patient information about DNA / Biobanking across Europe
 Information for patients and the public and patient information about DNA / Biobanking across Europe BIOBANKING / DNA BANKING SUMMARY: A biobank is a store of human biological material, used for the purposes
Information for patients and the public and patient information about DNA / Biobanking across Europe BIOBANKING / DNA BANKING SUMMARY: A biobank is a store of human biological material, used for the purposes
Report from NHRPAC. The Common Rule for the protection of human subjects of research (45 CFR 46) includes a definition of minimal risk:
 The content of this document does not represent the official views or policies of the Office for Human Research Protections (OHRP) nor of the Department of Health and Human Services (HHS). The content
The content of this document does not represent the official views or policies of the Office for Human Research Protections (OHRP) nor of the Department of Health and Human Services (HHS). The content
RIDGE PHYSICAL THERAPY & WELLNESS CENTER. Intake Form
 Intake Form : Personal Information please print clearly Name: last first middle initial Home Address: Home Telephone: ( ) Cell Phone: E-Mail Address: Social Security #: of Birth: Age: Sex: M F Marital
Intake Form : Personal Information please print clearly Name: last first middle initial Home Address: Home Telephone: ( ) Cell Phone: E-Mail Address: Social Security #: of Birth: Age: Sex: M F Marital
Thymus Cancer. This reference summary will help you better understand what thymus cancer is and what treatment options are available.
 Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
Who to call for an emergency: Name: Relationship: Home Phone: ( ) - Work Phone: ( ) - Cell Phone: ( ) -
 4425 Ponce de Leon Blvd., Suite 115 Email:info@ Dr. Mercedes Gonzalez, Pediatric Dermatologist Patient Information: Patient Name: Social Security Number: / / Date of Birth: / / Sex: M / F (Circle one)
4425 Ponce de Leon Blvd., Suite 115 Email:info@ Dr. Mercedes Gonzalez, Pediatric Dermatologist Patient Information: Patient Name: Social Security Number: / / Date of Birth: / / Sex: M / F (Circle one)
IOWA DEPARTMENT OF JUSTICE ATTORNEY GENERAL'S OFFICE CRIME VICTIM ASSISTANCE DIVISION SEXUAL ABUSE EXAMINATION PAYMENT PROGRAM
 IOWA DEPARTMENT OF JUSTICE ATTORNEY GENERAL'S OFFICE CRIME VICTIM ASSISTANCE DIVISION SEXUAL ABUSE EXAMINATION PAYMENT PROGRAM The Attorney General's Crime Victim Assistance Division administers the Sexual
IOWA DEPARTMENT OF JUSTICE ATTORNEY GENERAL'S OFFICE CRIME VICTIM ASSISTANCE DIVISION SEXUAL ABUSE EXAMINATION PAYMENT PROGRAM The Attorney General's Crime Victim Assistance Division administers the Sexual
TEXAS MEDICAL POWER OF ATTORNEY
 TEXAS MEDICAL POWER OF ATTORNEY NAME DATE BHS 80000246 v1 11/13 baptisthealthsystem.com That s Baptist Care. Page 1 of 5 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL
TEXAS MEDICAL POWER OF ATTORNEY NAME DATE BHS 80000246 v1 11/13 baptisthealthsystem.com That s Baptist Care. Page 1 of 5 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL
Lung Cancer. This reference summary will help you better understand lung cancer and the treatment options that are available.
 Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
PATIENT INFORMATION SHEET KEY FACTS
 PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
Patient Information Sheet
 Healthcare Worker exposure to a patient s blood What is a healthcare worker exposure? Patient Information Sheet Occasionally, health care workers come into contact with the blood or body fluids of their
Healthcare Worker exposure to a patient s blood What is a healthcare worker exposure? Patient Information Sheet Occasionally, health care workers come into contact with the blood or body fluids of their
City of New London. Access to Employee. Records. Policy
 City of New London Access to Employee Medical and Exposure Records Policy 8/2003 I. PURPOSE The purpose of this policy is to establish procedures to insure that employee medical and exposure records of
City of New London Access to Employee Medical and Exposure Records Policy 8/2003 I. PURPOSE The purpose of this policy is to establish procedures to insure that employee medical and exposure records of
BOWLING GREEN INTERNAL MEDICINE AND PEDIATRICS ASSOCIATES TREATMENT AUTHORIZATIONS AND FINANCIAL POLICIES
 BOWLING GREEN INTERNAL MEDICINE AND PEDIATRICS ASSOCIATES TREATMENT AUTHORIZATIONS AND FINANCIAL POLICIES Patient Name: Date: FINANCIAL POLICY FOR PATIENTS Effective July 10, 2000 our office has established
BOWLING GREEN INTERNAL MEDICINE AND PEDIATRICS ASSOCIATES TREATMENT AUTHORIZATIONS AND FINANCIAL POLICIES Patient Name: Date: FINANCIAL POLICY FOR PATIENTS Effective July 10, 2000 our office has established
Consent for Frozen Donor Oocyte In Vitro Fertilization and Embryo Transfer (Recipient)
 Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
Legal Issues for People with HIV
 Legal Issues for People with HIV Duke Legal Project Box 90360 Durham, NC 27708-0360 (919) 613-7169 (888) 600-7274 Duke Legal Project is a clinical legal education program of Duke Law School. Legal Representation
Legal Issues for People with HIV Duke Legal Project Box 90360 Durham, NC 27708-0360 (919) 613-7169 (888) 600-7274 Duke Legal Project is a clinical legal education program of Duke Law School. Legal Representation
Clinical Trials Network
 National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
INDIVIDUAL LIFE INSURANCE APPLICATION PART II - MEDICAL EXAMINATION
 INDIVIDUAL LIFE INSURANCE APPLICATION PART II - MEDICAL EXAMINATION ReliaStar Life Insurance Company, 20 Washington Avenue South, Minneapolis, MN 55401 Security Life of Denver Insurance Company, 1290 Broadway,
INDIVIDUAL LIFE INSURANCE APPLICATION PART II - MEDICAL EXAMINATION ReliaStar Life Insurance Company, 20 Washington Avenue South, Minneapolis, MN 55401 Security Life of Denver Insurance Company, 1290 Broadway,
HIPAA Privacy Rule CLIN-203: Special Privacy Considerations
 POLICY HIPAA Privacy Rule CLIN-203: Special Privacy Considerations I. Policy A. Additional Privacy Protection for Particularly Sensitive Health Information USC 1 recognizes that federal and California
POLICY HIPAA Privacy Rule CLIN-203: Special Privacy Considerations I. Policy A. Additional Privacy Protection for Particularly Sensitive Health Information USC 1 recognizes that federal and California
How To Participate In A Bladder Cancer Study
 SCOTTSDALE HEALTHCARE INSTITUTIONAL REVIEW BOARD Consent to Participate in Research Protocol Name: A Study to Compare the Study Drug, Erlotinib or Polyphenon E, to a Placebo (a substance that looks like
SCOTTSDALE HEALTHCARE INSTITUTIONAL REVIEW BOARD Consent to Participate in Research Protocol Name: A Study to Compare the Study Drug, Erlotinib or Polyphenon E, to a Placebo (a substance that looks like
You know them well They know you well You trust them to do what you desire And, you trust them to do what is best for you.
 Advanced Directive Act of Texas Part III Medical Power of Attorney and Content of a DNR By James L. Holly, MD Your Life Your Health The Examiner April 21, 2005 There are many decisions which are made in
Advanced Directive Act of Texas Part III Medical Power of Attorney and Content of a DNR By James L. Holly, MD Your Life Your Health The Examiner April 21, 2005 There are many decisions which are made in
Blood Transfusion. There are three types of blood cells: Red blood cells. White blood cells. Platelets.
 Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Cancer of the Cervix
 Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
PPS UNDERWRITING GUIDE FOR APPLICANTS
 PPS UNDERWRITING GUIDE FOR APPLICANTS UNDERWRITING guide 2013 WHAT HAPPENS WHEN YOU SUBMIT YOUR APPLICATION FOR INSURANCE? Once an application is submitted it is put through a number of processes to ensure
PPS UNDERWRITING GUIDE FOR APPLICANTS UNDERWRITING guide 2013 WHAT HAPPENS WHEN YOU SUBMIT YOUR APPLICATION FOR INSURANCE? Once an application is submitted it is put through a number of processes to ensure
www.irishheart.ie CARDIOMYOPATHY SUPPORT GROUP IRELAND
 www.irishheart.ie CARDIOMYOPATHY SUPPORT GROUP IRELAND Cardiomyopathy Support Group This is a voluntary group of people, all of whom have cardiomyopathy. It was set up in association with the Irish Heart
www.irishheart.ie CARDIOMYOPATHY SUPPORT GROUP IRELAND Cardiomyopathy Support Group This is a voluntary group of people, all of whom have cardiomyopathy. It was set up in association with the Irish Heart
Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.
 Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.CA WWW This guide is intended for patients in need of an unrelated volunteer
Helping you find the one match.. Guide for Unrelated Stem Cell Transplant Patients OneMatch Stem Cell and Marrow Network BLOOD.CA WWW This guide is intended for patients in need of an unrelated volunteer
JUST 5 EASY STEPS FOR CORD BLOOD DONATION...
 JUST 5 EASY STEPS FOR CORD BLOOD DONATION... STEP 1 Read this Information Kit. STEP 2 Print and fill in the forms. STEP 3 Give completed forms to your healthcare provider or to your hospital RN on day
JUST 5 EASY STEPS FOR CORD BLOOD DONATION... STEP 1 Read this Information Kit. STEP 2 Print and fill in the forms. STEP 3 Give completed forms to your healthcare provider or to your hospital RN on day
The degree of liver inflammation or damage (grade) Presence and extent of fatty liver or other metabolic liver diseases
 ilearning about your health Liver Biopsy www.cpmc.org/learning What is a Liver Biopsy? A liver biopsy is a procedure where a specially trained doctor (typically a hepatologist, radiologist, or gastroenterologist)
ilearning about your health Liver Biopsy www.cpmc.org/learning What is a Liver Biopsy? A liver biopsy is a procedure where a specially trained doctor (typically a hepatologist, radiologist, or gastroenterologist)
patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015
 patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015 BRCA1 and BRCA2 Mutations Cancer is a complex disease thought to be caused by several different factors. A few types of cancer
patient education Fact Sheet PFS007: BRCA1 and BRCA2 Mutations MARCH 2015 BRCA1 and BRCA2 Mutations Cancer is a complex disease thought to be caused by several different factors. A few types of cancer
SPORTS INSURANCE PROPOSAL FORM (All questions must be answered in ink)
 SPORTS INSURANCE PROPOSAL FORM (All questions must be answered in ink) Hanleigh Management Inc. Hanleigh Management, Inc., Hanleigh General Agency, Inc. 50 Tice Blvd., Suite 122, Woodcliff Lake, New Jersey
SPORTS INSURANCE PROPOSAL FORM (All questions must be answered in ink) Hanleigh Management Inc. Hanleigh Management, Inc., Hanleigh General Agency, Inc. 50 Tice Blvd., Suite 122, Woodcliff Lake, New Jersey
PICC- Peripherally Inserted Central Catheter PROCEDURAL CONSENT FORM. A. Interpreter / cultural needs. B. Procedure. C. Risks of the procedure
 DO NOT WRITE IN THIS BINDING MARGIN v3.00-03/2011 SW9266 Facility: PICC- Peripherally Inserted Central Catheter A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
DO NOT WRITE IN THIS BINDING MARGIN v3.00-03/2011 SW9266 Facility: PICC- Peripherally Inserted Central Catheter A. Interpreter / cultural needs An Interpreter Service is required? Yes No If Yes, is a qualified
Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591
 Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591 Andres U. Katz, M.D. Richard S. Anderson, M.D. G. Thomas
Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591 Andres U. Katz, M.D. Richard S. Anderson, M.D. G. Thomas
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
SAVE A LIFE... BY GIVING LIFE!
 SAVE A LIFE... BY GIVING LIFE! FOLLOW US ON: HÉMA-QUÉBEC PUBLIC CORD BLOOD BANK www.hema-quebec.qc.ca Scan this code with your smart phone to access the page Register to the Public Cord Blood Bank on the
SAVE A LIFE... BY GIVING LIFE! FOLLOW US ON: HÉMA-QUÉBEC PUBLIC CORD BLOOD BANK www.hema-quebec.qc.ca Scan this code with your smart phone to access the page Register to the Public Cord Blood Bank on the
Clinical Trials and Screening: What You Need to Know
 Scan for mobile link. Clinical Trials and Screening: What You Need to Know What is a Clinical Trial? At A Glance A clinical trial is a research study that tests how well new medical techniques work in
Scan for mobile link. Clinical Trials and Screening: What You Need to Know What is a Clinical Trial? At A Glance A clinical trial is a research study that tests how well new medical techniques work in
THORACIC DIAGNOSTIC ASSESMENT PROGRAM (DAP) PATIENT INFORMATION FOR:
 central east regional cancer program in partnership with cancer care ontario THORACIC DIAGNOSTIC ASSESMENT PROGRAM (DAP) PATIENT INFORMATION FOR: Thoracic dap booklet March2012.indd 1 SCHEDULED TESTS YOUR
central east regional cancer program in partnership with cancer care ontario THORACIC DIAGNOSTIC ASSESMENT PROGRAM (DAP) PATIENT INFORMATION FOR: Thoracic dap booklet March2012.indd 1 SCHEDULED TESTS YOUR
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
R 3160 PHYSICAL EXAMINATION
 TEACHING STAFF EBERS PHYSICAL EXAINATION R 3160/Page 1 of 6 A. Definitions R 3160 PHYSICAL EXAINATION 1. Employee assurance statement means a statement signed by the employee certifying that information
TEACHING STAFF EBERS PHYSICAL EXAINATION R 3160/Page 1 of 6 A. Definitions R 3160 PHYSICAL EXAINATION 1. Employee assurance statement means a statement signed by the employee certifying that information
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
INFORMATION LEAFLET. If anything is not clear, or if you would like more information, please
 BIO INFOBK 1492 0410:Layout 1 21/04/2010 15:24 Page 1 INFORMATION LEAFLET You are being invited to take part in a major medical research project called UK Biobank. The purpose of UK Biobank is to set up
BIO INFOBK 1492 0410:Layout 1 21/04/2010 15:24 Page 1 INFORMATION LEAFLET You are being invited to take part in a major medical research project called UK Biobank. The purpose of UK Biobank is to set up
INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM
 INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM A. GENERAL GUIDELINES AND COMMENTS Purpose and Use of the Model Form This is a guide for practitioners to using the Model Informed
INFORMED CONSENT FOR EGG DONORS PRACTITIONER S GUIDE TO USING THE MODEL FORM A. GENERAL GUIDELINES AND COMMENTS Purpose and Use of the Model Form This is a guide for practitioners to using the Model Informed
INFORMED CONSENT FOR SLEEVE GASTRECTOMY
 INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric surgery: correlation with pulmonary function.
 Version 2. 8.09.2011 Hammersmith Hospital Du Cane Rd London W12 0HS Tel: 020 8383 1000 www.imperial.nhs.uk PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric
Version 2. 8.09.2011 Hammersmith Hospital Du Cane Rd London W12 0HS Tel: 020 8383 1000 www.imperial.nhs.uk PATIENT INFORMATION SHEET Study name: CT morphology of lung parenchyma pre and post bariatric
PATIENT INFORMATION FORM
 737 Pearl Street, Suite 108 Phone: 858.456.2114 Fax: 858.456.2103 www.abilityrehabsd.com PATIENT INFORMATION FORM Please print and complete ALL items. If an item doesn t apply, put N/A Patient Name: Age:
737 Pearl Street, Suite 108 Phone: 858.456.2114 Fax: 858.456.2103 www.abilityrehabsd.com PATIENT INFORMATION FORM Please print and complete ALL items. If an item doesn t apply, put N/A Patient Name: Age:
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167
 APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
