The delivery of chemotherapy at home: an evidence synthesis
|
|
|
- Kerry Weaver
- 8 years ago
- Views:
Transcription
1 The delivery of chemotherapy at home: an evidence synthesis Mark Corbett, 1 Morag Heirs, 1 Micah Rose, 1 Alison Smith, 1 Lisa Stirk, 1 Gerry Richardson, 2 Daniel Stark, 3 Daniel Swinson, 4 Dawn Craig 1 and Alison Eastwood 1 * 1 Centre for Reviews and Dissemination, University of York, York, UK 2 Centre for Health Economics, University of York, York, UK 3 Leeds Institute of Cancer & Pathology, University of Leeds, Leeds, UK 4 St James s Institute of Oncology, Leeds Teaching Hospitals Foundation NHS Trust, Leeds, UK *Corresponding author Declared competing interests of authors: none Published April 2015 DOI: /hsdr03140 Scientific summary Chemotherapy at home: an evidence synthesis Health Services and Delivery Research 2015; Vol. 3: No. 14 DOI: /hsdr03140 NIHR Journals Library
2 SCIENTIFIC SUMMARY: CHEMOTHERAPY AT HOME: AN EVIDENCE SYNTHESIS Scientific summary Background Throughout the NHS there is an increasing focus on developing service models of care which meet the needs of patients, with care being delivered locally wherever possible to maximise convenience and centralised where necessary to improve outcomes. For cancer treatment, recent policy and guidance has focused on chemotherapy services being offered not only in cancer centres and cancer units but also in community and home settings, while maintaining safety and quality and delivering an efficient service. Many hospitals across England and Wales are delivering chemotherapy services at full capacity, with increasing demand for services putting a strain on NHS capacity resources. This can have a detrimental effect on patient experience as a result of longer waiting times. Delivering chemotherapy closer to home may be an approach by which the NHS could relieve demand for outpatient services while maintaining or even improving patient care. Nevertheless, the clinical and economic implications of delivering chemotherapy closer to home are uncertain. Objectives This aim of this study was to compare the impact of delivering intravenous chemotherapy in different settings (home, community and hospital outpatient) on a range of outcomes, including quality of life, safety and costs. Methods A systematic review of clinical effectiveness, qualitative and cost-effectiveness studies was undertaken. A decision model was developed to explore aspects of cost-effectiveness. Data from published and unpublished studies were sought systematically from 16 electronic databases (including MEDLINE, EMBASE and The Cochrane Library, searched from inception) in March 2013; updated searches of the most relevant databases were undertaken in October Reference lists and Google ( searches were used to identify any further studies. Studies of cancer patients receiving intravenous chemotherapy in two or more of the following settings were eligible: home, community based (e.g. general practitioner practice, mobile bus, or community hospital) and hospital outpatient. Within-setting comparisons were also eligible. Studies had to report at least one of the following outcomes: safety, quality of life, preference, satisfaction, social functioning, clinical outcomes (such as self-rated health), costs or resource/organisational issues. Any type of comparative design (including economic evaluation) was eligible. Single-setting studies were also identified and included, but were used in the review only where they might usefully supplement the comparative study evidence (this happened only for qualitative studies). Quality assessment tools, specific to particular study designs, were used to evaluate the validity of the included studies. Two reviewers independently screened all of the potentially relevant studies, and data extracted and quality assessed those included. Discrepancies were resolved by discussion. Clinical effectiveness and cost-effectiveness studies were summarised narratively; qualitative studies were synthesised using meta-ethnography. ii NIHR Journals Library
3 HEALTH SERVICES AND DELIVERY RESEARCH 2015 VOL. 3 NO. 14 (SCIENTIFIC SUMMARY) To supplement the published evidence and to gain insight into the variation in current NHS practice, a survey was undertaken, canvassing views from relevant professionals about their experience of providing home and community chemotherapy. The results of the survey were intended to help to describe the patient pathway and inform the development of a decision model. A lack of evidence led to a simple model based on one UK trial (OUTREACH) being developed. The aim of the model was to assess the cost-effectiveness of intravenous chemotherapy delivered in the home, community or outpatient setting in a population considered eligible for home treatment. The model was conducted from a NHS perspective using a 12-week time horizon. The summary measure of benefit was quality-adjusted life-years. Results The literature searches identified 4272 references and 245 potentially relevant full papers were screened. A total of 67 studies were included: 25 comparative studies and 42 single-setting studies. Of the 25 comparative studies, 10 were randomised controlled trials (RCTs) and 15 were non-randomised studies; nine of the comparative studies included a concurrent full economic evaluation. Most studies evaluated adult populations and compared home and hospital outpatient settings. The 10 randomised trials recruited 482 participants in total. Several trials were appropriately designed to minimise avoidable biases. However, slow recruitment rates and the non-participation of eligible patients for setting-related reasons meant that trial sample sizes were small and populations were inherently biased to favour the home or community settings. This bias was evident in the results for the preference and satisfaction outcomes, although these data were limited as only one trial studied strength of preference. Perhaps surprisingly, there was little evidence to suggest differences between settings in terms of quality of life, clinical outcomes or psychological outcomes. Adverse event data also did not suggest any important differences between settings; these data were limited by the small study sizes. The 15 non-randomised studies added little to the randomised trial evidence: the main limitations were the small populations and a high risk that the study results were biased as a result of confounding. All nine of the economic evaluations were judged as being of low or uncertain quality. Most were cost consequence analyses, which presented cost outcomes alongside clinical trial results but derived no summary measure of benefit. Only one evaluation assessed patient health-related quality of life and reported utility outputs (many studies used patient preference as an outcome measure). Poor reporting of resource use and use of different perspectives across different settings made the results difficult to compare. High levels of uncertainty made it difficult to ascertain whether or not costs or outcomes differed between settings. In general, these studies provided limited evidence from which to draw an overall conclusion regarding cost-effectiveness or to inform or populate a decision model. The 17 qualitative studies evaluated the opinions and experiences of more than 450 participants in total, including patients, family members and health-care professionals. Generally, study quality was moderate to good but most studies did not appear to consider the impact of the researcher on data collection and analysis. Overall, data were grouped under three main lines of argument: barriers to service provision, satisfaction with chemotherapy and making compromises to maintain normality. The last of these was seen as key to being able to survive a difficult time and look forward. Most patients made explicit trade-offs to maximise their resources (such as time, money and energy). Normality was maintained more easily when family life was minimally interrupted, the impact of cancer on daily life and family members was controllable, and patients were able to participate in activities of value. Time spent travelling and waiting for treatment meant less time and energy for normal life. Outpatient settings were most often associated with increased confidence in staff ability to deal with adverse reactions, but there was evidence that good, visible communication between an expert centre and a community or home location could alleviate some safety concerns. Based on available data, the time and energy consumed by outpatient treatment reduced overall quality of life such that patients preferred Queen s Printer and Controller of HMSO This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. iii
4 SCIENTIFIC SUMMARY: CHEMOTHERAPY AT HOME: AN EVIDENCE SYNTHESIS alternative treatment settings. These themes were particularly evident in accounts from patients receiving palliative treatment and from parents of children with cancer. We circulated the survey widely and it was passed on further by initial contacts. This made it impossible to calculate a response rate. Twenty-two NHS organisations (all in England) and nine private providers responded to the survey. The results suggested wide variation in the ways in which home and community chemotherapy was delivered. It was evident that more patients were eligible for community treatment than home treatment and that chemotherapy regimen and patient performance were important determinants of eligibility. Private providers were frequently used to deliver treatment in the home setting and appeared to use more selective eligibility criteria (e.g. treating patients only after two or more cycles had been delivered in hospital). Several NHS organisations highlighted that value-added tax savings associated with home chemotherapy were a significant motivator for providing such a service. We anticipated that we would be able to develop and populate a robust decision model through combination of the published evidence and the survey. However, limitations of the available data meant that results from the cost-effectiveness model were highly unstable and should be viewed as exploratory rather than robust. In the base-case analysis, intravenous chemotherapy in the community setting was the most cost-effective option, but none of the settings had a high probability of being the most cost-effective. Sensitivity analyses highlighted the fragility of the results to parameter changes. Adjusting cost values within plausible ranges also altered the preferred treatment setting. There was significant uncertainty over which treatment settings were cost-effective. Robust data to inform cost-effectiveness modelling would be needed to resolve this uncertainty, as well as further consideration of service configuration and appropriate patient pathways. Conclusions The results of this study highlighted not only avoidable study design and reporting limitations but also inherent and sometimes unavoidable difficulties that arise during primary studies of chemotherapy settings. Several studies were designed appropriately to minimise avoidable biases but implementing randomised trials in this area appears difficult in terms of patient accrual and recruiting unbiased populations. These issues impacted on the concurrent economic evaluations and were further compounded by poor reporting of cost and resource data. Consequently, few robust conclusions can be made about the clinical effectiveness and cost-effectiveness of different settings. High uncertainty remains owing to trial sizes that were potentially too small to detect effects reliably. It was unclear whether or not the quality-of-life instruments used in the studies were sensitive enough to detect differences in quality of life between chemotherapy settings. Accordingly, the results of the exploratory cost-effectiveness model based on the OUTREACH trial were not robust and the cost-effectiveness results of the model should be interpreted with caution. Qualitative studies were more informative. They indicated that decisions and preferences about intravenous chemotherapy treatment setting are strongly influenced by a desire to maintain normality. Patient time and energy required for outpatient chemotherapy reduces overall quality of life enough for patients to prefer alternative treatment settings. However, compromises were needed to balance competing factors and patient preference for specific locations reflecting individual situations. Limitations of the qualitative studies were that all evaluated a new or proposed service against an existing (perhaps struggling) hospital outpatient setting, and participants were drawn from biased samples. iv NIHR Journals Library
5 HEALTH SERVICES AND DELIVERY RESEARCH 2015 VOL. 3 NO. 14 (SCIENTIFIC SUMMARY) Implications for research Considering the likely challenges involved in performing further RCTs using conventional study designs, a better design might be to nest a RCT within a larger observational cohort of patients: ambivalent patients could be randomised and patients with preferences could receive their preferred setting. Such a study might also incorporate (into questionnaires) the qualitative data themes identified in this review. Efficacy estimates would result from the randomised component of the study. Any additional influence of motivational factors could be studied by comparing randomised and non-randomised patients treated in the same setting. The results from this nested design should also indicate whether or not there are any clinical or demographic differences between the different populations at baseline, and produce estimates of likely rates of uptake of the different settings, to help inform future service provision. Such a study should more clearly identify and quantify issues such as setting-related adverse events, waiting times, anxiety and transport problems, and indicate how their prevalence and impact might vary according to patient characteristics. For an economic evaluation to be reliable, detailed patient characteristics, resource use, cost and quality-of-life data are needed. The ideal collection method for these parameters is a large multicentre RCT that incorporates a wide variety of providers. However, a key theme that emerged from the review and survey concerned a high level of variation in current practice in the NHS. This variation makes it unlikely that a RCT will provide sufficient evidence for a broad economic evaluation. We surmise that, in order to explore this variation and mitigate generalisability issues, broad observational data will be necessary. Information from large observational data sets such as the Systemic Anti-Cancer Therapy and the General Practice Research Database could be linked to provide a clearer portrait of current provision. Study registration This study is registered as PROSPERO CRD Funding Funding for this study was provided by the Health Services and Delivery Research programme of the National Institute for Health Research. Queen s Printer and Controller of HMSO This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. v
6
7 Health Services and Delivery Research ISSN (Print) ISSN (Online) This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) ( Editorial contact: nihredit@southampton.ac.uk The full HS&DR archive is freely available to view online at Print-on-demand copies can be purchased from the report pages of the NIHR Journals Library website: Criteria for inclusion in the Health Services and Delivery Research journal Reports are published in Health Services and Delivery Research (HS&DR) if (1) they have resulted from work for the HS&DR programme or programmes which preceded the HS&DR programme, and (2) they are of a sufficiently high scientific quality as assessed by the reviewers and editors. HS&DR programme The Health Services and Delivery Research (HS&DR) programme, part of the National Institute for Health Research (NIHR), was established to fund a broad range of research. It combines the strengths and contributions of two previous NIHR research programmes: the Health Services Research (HSR) programme and the Service Delivery and Organisation (SDO) programme, which were merged in January The HS&DR programme aims to produce rigorous and relevant evidence on the quality, access and organisation of health services including costs and outcomes, as well as research on implementation. The programme will enhance the strategic focus on research that matters to the NHS and is keen to support ambitious evaluative research to improve health services. For more information about the HS&DR programme please visit the website: This report The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 12/5001/67. The contractual start date was in March The final report began editorial review in May 2014 and was accepted for publication in October The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report. This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health. Queen s Printer and Controller of HMSO This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. Published by the NIHR Journals Library ( produced by Prepress Projects Ltd, Perth, Scotland (
8 Health Services and Delivery Research Editor-in-Chief Professor Ray Fitzpatrick Professor of Public Health and Primary Care, University of Oxford, UK NIHR Journals Library Editor-in-Chief Professor Tom Walley Director, NIHR Evaluation, Trials and Studies and Director of the HTA Programme, UK NIHR Journals Library Editors Professor Ken Stein Chair of HTA Editorial Board and Professor of Public Health, University of Exeter Medical School, UK Professor Andree Le May Chair of NIHR Journals Library Editorial Group (EME, HS&DR, PGfAR, PHR journals) Dr Martin Ashton-Key Consultant in Public Health Medicine/Consultant Advisor, NETSCC, UK Professor Matthias Beck Chair in Public Sector Management and Subject Leader (Management Group), Queen s University Management School, Queen s University Belfast, UK Professor Aileen Clarke Professor of Public Health and Health Services Research, Warwick Medical School, University of Warwick, UK Dr Tessa Crilly Director, Crystal Blue Consulting Ltd, UK Dr Peter Davidson Director of NETSCC, HTA, UK Ms Tara Lamont Scientific Advisor, NETSCC, UK Professor Elaine McColl Director, Newcastle Clinical Trials Unit, Institute of Health and Society, Newcastle University, UK Professor William McGuire Professor of Child Health, Hull York Medical School, University of York, UK Professor Geoffrey Meads Professor of Health Sciences Research, Faculty of Education, University of Winchester, UK Professor John Powell Consultant Clinical Adviser, National Institute for Health and Care Excellence (NICE), UK Professor James Raftery Professor of Health Technology Assessment, Wessex Institute, Faculty of Medicine, University of Southampton, UK Dr Rob Riemsma Reviews Manager, Kleijnen Systematic Reviews Ltd, UK Professor Helen Roberts Professor of Child Health Research, UCL Institute of Child Health, UK Professor Helen Snooks Professor of Health Services Research, Institute of Life Science, College of Medicine, Swansea University, UK Please visit the website for a list of members of the NIHR Journals Library Board: Editorial contact: nihredit@southampton.ac.uk
Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation
 Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation Mark A Williams, 1* Esther M Williamson, 1 Peter J Heine, 1 Vivien Nichols,
Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomised controlled trial and economic evaluation Mark A Williams, 1* Esther M Williamson, 1 Peter J Heine, 1 Vivien Nichols,
Scientific summary. Evaluating a major innovation in hospital design Health Services and Delivery Research 2015; Vol. 3: No. 3 DOI: 10.
 Evaluating a major innovation in hospital design: workforce implications and impact on patient and staff experiences of all single room hospital accommodation Jill Maben, 1* Peter Griffiths, 2 Clarissa
Evaluating a major innovation in hospital design: workforce implications and impact on patient and staff experiences of all single room hospital accommodation Jill Maben, 1* Peter Griffiths, 2 Clarissa
Variation in compulsory psychiatric inpatient admission in England: a cross-sectional, multilevel analysis
 Variation in compulsory psychiatric inpatient admission in England: a cross-sectional, multilevel analysis Scott Weich, 1 * Orla McBride, 2 Liz Twigg, 3 Patrick Keown, 4 Eva Cyhlarova, 5 David Crepaz-Keay,
Variation in compulsory psychiatric inpatient admission in England: a cross-sectional, multilevel analysis Scott Weich, 1 * Orla McBride, 2 Liz Twigg, 3 Patrick Keown, 4 Eva Cyhlarova, 5 David Crepaz-Keay,
Systematic review of the effects of schools and school environment interventions on health: evidence mapping and synthesis
 Systematic review of the effects of schools and school environment interventions on health: evidence mapping and synthesis C Bonell, 1 * F Jamal, 2 A Harden, 2 H Wells, 3 W Parry, 4 A Fletcher, 5 M Petticrew,
Systematic review of the effects of schools and school environment interventions on health: evidence mapping and synthesis C Bonell, 1 * F Jamal, 2 A Harden, 2 H Wells, 3 W Parry, 4 A Fletcher, 5 M Petticrew,
Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries
 Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries A randomised controlled trial of treatments for whiplash injuries SE Lamb, 1 * MA Williams,
Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries A randomised controlled trial of treatments for whiplash injuries SE Lamb, 1 * MA Williams,
Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review
 Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review DA Lane, 1 * S Raichand, 2 D Moore, 2 M Connock, 2 A Fry-Smith 2 and DA Fitzmaurice
Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review DA Lane, 1 * S Raichand, 2 D Moore, 2 M Connock, 2 A Fry-Smith 2 and DA Fitzmaurice
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 54 DECEMBER 2014 ISSN 2050-4349 Reducing Care Utilisation through Self-management Interventions (RECURSIVE): a systematic review and meta-analysis Maria
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 54 DECEMBER 2014 ISSN 2050-4349 Reducing Care Utilisation through Self-management Interventions (RECURSIVE): a systematic review and meta-analysis Maria
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 21 JULY 2014 ISSN 2050-4349 Initiatives to reduce length of stay in acute hospital settings: a rapid synthesis of evidence relating to enhanced recovery
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 21 JULY 2014 ISSN 2050-4349 Initiatives to reduce length of stay in acute hospital settings: a rapid synthesis of evidence relating to enhanced recovery
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 43 NOVEMBER 2014 ISSN 2050-4349 What evidence is there for a relationship between organisational features and patient outcomes in congenital heart disease
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 43 NOVEMBER 2014 ISSN 2050-4349 What evidence is there for a relationship between organisational features and patient outcomes in congenital heart disease
Rituximab for the first-line treatment of stage III IV. D Papaioannou,* R Rafia, J Rathbone, M Stevenson, H Buckley Woods and J Stevens
 Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
Rituximab for the first-line treatment of stage III IV follicular lymphoma Rituximab for the first-line treatment of stage III IV follicular lymphoma (review of Technology Appraisal No. 110): a systematic
The clinical effectiveness and costeffectiveness surgery for obesity: a systematic review and economic evaluation
 The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 14 MAY 2014 ISSN 2050-4349 Being a manager, becoming a professional? A case study and interview-based exploration of the use of management knowledge
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 14 MAY 2014 ISSN 2050-4349 Being a manager, becoming a professional? A case study and interview-based exploration of the use of management knowledge
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 55 DECEMBER 2014 ISSN 2050-4349 Learning for the NHS on procurement and supply chain management: a rapid evidence assessment Saba Hinrichs, Deepa Jahagirdar,
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 55 DECEMBER 2014 ISSN 2050-4349 Learning for the NHS on procurement and supply chain management: a rapid evidence assessment Saba Hinrichs, Deepa Jahagirdar,
NHS commissioning practice and health system governance: a mixed-methods realistic evaluation
 NHS commissioning practice and health system governance: a mixed-methods realistic evaluation Rod Sheaff, 1 * Nigel Charles, 1 Ann Mahon, 2 Naomi Chambers, 2 Verdiana Morando, 3 Mark Exworthy, 4 Richard
NHS commissioning practice and health system governance: a mixed-methods realistic evaluation Rod Sheaff, 1 * Nigel Charles, 1 Ann Mahon, 2 Naomi Chambers, 2 Verdiana Morando, 3 Mark Exworthy, 4 Richard
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 20 JULY 2014 ISSN 2050-4349 An exploration of the implementation of open disclosure of adverse events in the UK: a scoping review and qualitative exploration
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 20 JULY 2014 ISSN 2050-4349 An exploration of the implementation of open disclosure of adverse events in the UK: a scoping review and qualitative exploration
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 39 JUNE 2014 ISSN 1366-5278 A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 39 JUNE 2014 ISSN 1366-5278 A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions
PUBLIC HEALTH RESEARCH
 PUBLIC HEALTH RESEARCH VOLUME 3 ISSUE 14 NOVEMBER 2015 ISSN 2050-4381 A cluster randomised controlled trial comparing the effectiveness and cost-effectiveness of a school-based cognitive behavioural therapy
PUBLIC HEALTH RESEARCH VOLUME 3 ISSUE 14 NOVEMBER 2015 ISSN 2050-4381 A cluster randomised controlled trial comparing the effectiveness and cost-effectiveness of a school-based cognitive behavioural therapy
HowHow to Find the Best Online Stock Broker for You
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 50 DECEMBER 2014 ISSN 2050-4349 Staff satisfaction and organisational performance: evidence from a longitudinal secondary analysis of the NHS staff
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 50 DECEMBER 2014 ISSN 2050-4349 Staff satisfaction and organisational performance: evidence from a longitudinal secondary analysis of the NHS staff
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 37 OCTOBER 2014 ISSN 2050-4349 Improving the effectiveness of multidisciplinary team meetings for patients with chronic diseases: a prospective observational
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 37 OCTOBER 2014 ISSN 2050-4349 Improving the effectiveness of multidisciplinary team meetings for patients with chronic diseases: a prospective observational
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 30 MAY 2014 ISSN 1366-5278 The clinical effectiveness and cost-effectiveness of brief intervention for excessive alcohol consumption among people attending
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 30 MAY 2014 ISSN 1366-5278 The clinical effectiveness and cost-effectiveness of brief intervention for excessive alcohol consumption among people attending
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 42 OCTOBER 2015 ISSN 2050-4349 Measuring prevalence, reliability and variation in high-risk prescribing in general practice using multilevel modelling
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 42 OCTOBER 2015 ISSN 2050-4349 Measuring prevalence, reliability and variation in high-risk prescribing in general practice using multilevel modelling
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 38 SEPTEMBER 2015 ISSN 2050-4349 ReseArch with Patient and Public involvement: a RealisT evaluation the RAPPORT study Patricia Wilson, Elspeth Mathie,
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 38 SEPTEMBER 2015 ISSN 2050-4349 ReseArch with Patient and Public involvement: a RealisT evaluation the RAPPORT study Patricia Wilson, Elspeth Mathie,
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 16 MAY 2014 ISSN 2050-4349 Investigating the contribution of physician assistants to primary care in England: a mixed-methods study Vari M Drennan,
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 16 MAY 2014 ISSN 2050-4349 Investigating the contribution of physician assistants to primary care in England: a mixed-methods study Vari M Drennan,
Psychological therapies for borderline personality disorder. Health Technology Assessment 2006; Vol. 10: No. 35
 Psychological therapies for borderline personality disorder Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic
Psychological therapies for borderline personality disorder Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic
Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): a prospective cohort study
 Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): a prospective cohort study RJ Lilford, 1 * L Bentham, 1 A Girling, 1 I Litchfield, 1 R Lancashire, 1 D Armstrong, 2 R Jones, 2 T Marteau,
Birmingham and Lambeth Liver Evaluation Testing Strategies (BALLETS): a prospective cohort study RJ Lilford, 1 * L Bentham, 1 A Girling, 1 I Litchfield, 1 R Lancashire, 1 D Armstrong, 2 R Jones, 2 T Marteau,
Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review
 Insulin sensitisers in the treatment of non-alcoholic fatty liver disease Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review D Shyangdan, 1 C Clar, 2 N Ghouri,
Insulin sensitisers in the treatment of non-alcoholic fatty liver disease Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review D Shyangdan, 1 C Clar, 2 N Ghouri,
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 70 DECEMBER 2014 ISSN 1366-5278 The use of fenestrated and branched endovascular aneurysm repair for juxtarenal and thoracoabdominal aneurysms: a systematic
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 70 DECEMBER 2014 ISSN 1366-5278 The use of fenestrated and branched endovascular aneurysm repair for juxtarenal and thoracoabdominal aneurysms: a systematic
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 56 JULY 2015 ISSN 1366-5278 Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report Katy Cooper, Robin
HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 56 JULY 2015 ISSN 1366-5278 Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report Katy Cooper, Robin
Systematic Reviews. knowledge to support evidence-informed health and social care
 Systematic Reviews knowledge to support evidence-informed health and social care By removing uncertainties in science and research, systematic reviews ensure that only the most effective and best-value
Systematic Reviews knowledge to support evidence-informed health and social care By removing uncertainties in science and research, systematic reviews ensure that only the most effective and best-value
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 31 JULY 2015 ISSN 2050-4349 Care and communication between health professionals and patients affected by severe or chronic illness in community care
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 3 ISSUE 31 JULY 2015 ISSN 2050-4349 Care and communication between health professionals and patients affected by severe or chronic illness in community care
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 9 ISSUE 48 JUNE 25 ISSN 366-5278 The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation
HEALTH TECHNOLOGY ASSESSMENT VOLUME 9 ISSUE 48 JUNE 25 ISSN 366-5278 The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 10 APRIL 2014 ISSN 2050-4349 How do managers and leaders in the National Health Service and social care respond to service user involvement in mental
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 2 ISSUE 10 APRIL 2014 ISSN 2050-4349 How do managers and leaders in the National Health Service and social care respond to service user involvement in mental
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 43 JULY 2014 ISSN 1366-5278 The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 43 JULY 2014 ISSN 1366-5278 The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations
Adalimumab for the treatment of psoriasis
 DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 46 JULY 2014 ISSN 1366-5278 Interventions for adult Eustachian tube dysfunction: a systematic review Alexis Llewellyn, Gill Norman, Melissa Harden, Andrew Coatesworth,
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 46 JULY 2014 ISSN 1366-5278 Interventions for adult Eustachian tube dysfunction: a systematic review Alexis Llewellyn, Gill Norman, Melissa Harden, Andrew Coatesworth,
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model
 The effectiveness and cost-effectiveness of storing donated kidneys from deceased donors The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic
The effectiveness and cost-effectiveness of storing donated kidneys from deceased donors The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 47 JUNE 2015 ISSN 1366-5278 Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE
HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 47 JUNE 2015 ISSN 1366-5278 Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence
 Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence Tension-free vaginal tape for treatment of urinary stress
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence Tension-free vaginal tape for treatment of urinary stress
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 23 APRIL 2014 ISSN 1366-5278 The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 23 APRIL 2014 ISSN 1366-5278 The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 17 ISSUE 31 July 2013 ISSN 1366-5278 Clinical effectiveness and cost-effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic
HEALTH TECHNOLOGY ASSESSMENT VOLUME 17 ISSUE 31 July 2013 ISSN 1366-5278 Clinical effectiveness and cost-effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 61 JULY 2015 ISSN 1366-5278 A randomised controlled trial of Outpatient versus inpatient Polyp Treatment (OPT) for abnormal uterine bleeding T Justin Clark,
HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 61 JULY 2015 ISSN 1366-5278 A randomised controlled trial of Outpatient versus inpatient Polyp Treatment (OPT) for abnormal uterine bleeding T Justin Clark,
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 50 JULY 2015 ISSN 1366-5278 A feasibility randomised controlled trial of a motivational interviewing-based intervention for weight loss maintenance in adults
HEALTH TECHNOLOGY ASSESSMENT VOLUME 19 ISSUE 50 JULY 2015 ISSN 1366-5278 A feasibility randomised controlled trial of a motivational interviewing-based intervention for weight loss maintenance in adults
Best supportive care: Do we know what it is?
 Best supportive care: Do we know what it is? Angela Boland Rumona Dickson Barbara Jack James Stevenson Edge Hill University Faculty of Health www.liv.ac.uk/lrig Collaborative partners Liverpool Reviews
Best supportive care: Do we know what it is? Angela Boland Rumona Dickson Barbara Jack James Stevenson Edge Hill University Faculty of Health www.liv.ac.uk/lrig Collaborative partners Liverpool Reviews
Evaluation of a Primary Care Dermatology Service: final report
 Evaluation of a Primary Care Dermatology Service: final report Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO) December 2005 prepared by Chris Salisbury*
Evaluation of a Primary Care Dermatology Service: final report Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO) December 2005 prepared by Chris Salisbury*
Breast cancer treatment for elderly women: a systematic review
 Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 51 AUGUST 2014 ISSN 1366-5278 Systematic review to identify and appraise outcome measures used to evaluate childhood obesity treatment interventions (CoOR):
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 51 AUGUST 2014 ISSN 1366-5278 Systematic review to identify and appraise outcome measures used to evaluate childhood obesity treatment interventions (CoOR):
HEALTH SERVICES AND DELIVERY RESEARCH
 HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 4 ISSUE 16 MAY 2016 ISSN 2050-4349 Challenges, solutions and future directions in the evaluation of service innovations in health care and public health Rosalind
HEALTH SERVICES AND DELIVERY RESEARCH VOLUME 4 ISSUE 16 MAY 2016 ISSN 2050-4349 Challenges, solutions and future directions in the evaluation of service innovations in health care and public health Rosalind
Evidence Briefing for NHS Bradford and Airedale. Alternatives to inpatient admission for adolescents with eating disorders
 Evidence Briefing for NHS Bradford and Airedale Alternatives to inpatient admission for adolescents with eating disorders NHS Bradford and Airedale currently commissions out of area placements involving
Evidence Briefing for NHS Bradford and Airedale Alternatives to inpatient admission for adolescents with eating disorders NHS Bradford and Airedale currently commissions out of area placements involving
NIHR Research Design Service London Dr Peter Lovell Deputy Director
 NIHR Research Design Service London Dr Peter Lovell Deputy Director Research Design Service (RDS) A national network of support services; Supporting those who are putting together REGIONAL RDS North East
NIHR Research Design Service London Dr Peter Lovell Deputy Director Research Design Service (RDS) A national network of support services; Supporting those who are putting together REGIONAL RDS North East
Chapter 2 What is evidence and evidence-based practice?
 Chapter 2 What is evidence and evidence-based practice? AIMS When you have read this chapter, you should understand: What evidence is Where evidence comes from How we find the evidence and what we do with
Chapter 2 What is evidence and evidence-based practice? AIMS When you have read this chapter, you should understand: What evidence is Where evidence comes from How we find the evidence and what we do with
How To Understand The Cost Effectiveness Of Bortezomib
 DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
How to literature search
 How to literature search Evidence based practice the learning cycle Our ultimate aim is to help you, as a health professional, to make good clinical decisions. This will enable you to give the best possible
How to literature search Evidence based practice the learning cycle Our ultimate aim is to help you, as a health professional, to make good clinical decisions. This will enable you to give the best possible
Systematic reviews and meta-analysis
 Evidence-Based Medicine And Healthcare Singapore Med J 2005 Vol 46(6) : 270 CME Article Systematic reviews and meta-analysis S Green ABSTRACT Systematic reviews form a potential method for overcoming the
Evidence-Based Medicine And Healthcare Singapore Med J 2005 Vol 46(6) : 270 CME Article Systematic reviews and meta-analysis S Green ABSTRACT Systematic reviews form a potential method for overcoming the
Research Design Service London
 Research Design Service London Enabling Better Research Peter Lovell Programme Manager 27 th October 2011 Overview of presentation An introduction to the NIHR Research Design Service (RDS) Outline of the
Research Design Service London Enabling Better Research Peter Lovell Programme Manager 27 th October 2011 Overview of presentation An introduction to the NIHR Research Design Service (RDS) Outline of the
Internationale Standards des HTA? Jos Kleijnen Kleijnen Systematic Reviews Ltd
 Internationale Standards des HTA? Jos Kleijnen Kleijnen Systematic Reviews Ltd Conflicts of Interest A Gutachten was commissioned by VFA we had full editorial freedom Kleijnen Systematic Reviews Ltd has
Internationale Standards des HTA? Jos Kleijnen Kleijnen Systematic Reviews Ltd Conflicts of Interest A Gutachten was commissioned by VFA we had full editorial freedom Kleijnen Systematic Reviews Ltd has
Medical Technologies Evaluation Programme Methods guide
 Issue date: April 2011 Medical Technologies Evaluation Programme Methods guide National Institute for Health and Clinical Excellence MidCity Place 71 High Holborn London WC1V 6NA www.nice.org.uk National
Issue date: April 2011 Medical Technologies Evaluation Programme Methods guide National Institute for Health and Clinical Excellence MidCity Place 71 High Holborn London WC1V 6NA www.nice.org.uk National
Evidence-Based Software Engineering. Barbara Kitchenham Tore Dybå (SINTEF) Magne Jørgensen (Simula Laboratory)
 1 Evidence-Based Software Engineering Barbara Kitchenham Tore Dybå (SINTEF) Magne Jørgensen (Simula Laboratory) Agenda The evidence-based paradigm Evidence-Based Software Engineering (EBSE) Goals Procedures
1 Evidence-Based Software Engineering Barbara Kitchenham Tore Dybå (SINTEF) Magne Jørgensen (Simula Laboratory) Agenda The evidence-based paradigm Evidence-Based Software Engineering (EBSE) Goals Procedures
ADOPTION RESEARCH INITIATIVE BRIEFING ENHANCING ADOPTIVE PARENTING: A RANDOMISED CONTROLLED TRIAL OF ADOPTION SUPPORT
 Research Brief DCSF-RBX-19-08 ADOPTION RESEARCH INITIATIVE BRIEFING ENHANCING ADOPTIVE PARENTING: A RANDOMISED CONTROLLED TRIAL OF ADOPTION SUPPORT January 2009 Professor Alan Rushton, Institute of Psychiatry,
Research Brief DCSF-RBX-19-08 ADOPTION RESEARCH INITIATIVE BRIEFING ENHANCING ADOPTIVE PARENTING: A RANDOMISED CONTROLLED TRIAL OF ADOPTION SUPPORT January 2009 Professor Alan Rushton, Institute of Psychiatry,
PUBLIC HEALTH LIMITED OPEN CALL SCHEME (LOCS) PHA202 RESEARCH BRIEFING
 Introduction PUBLIC HEALTH LIMITED OPEN CALL SCHEME (LOCS) PHA202 RESEARCH BRIEFING Following publication of its new national health research strategy Best Research for Best Health (DH 2006a), the Department
Introduction PUBLIC HEALTH LIMITED OPEN CALL SCHEME (LOCS) PHA202 RESEARCH BRIEFING Following publication of its new national health research strategy Best Research for Best Health (DH 2006a), the Department
An Evidence-Based Approach to Reviewing the Science on the Safety of Chemicals in Foods
 An Evidence-Based Approach to Reviewing the Science on the Safety of Chemicals in Foods In considering the safety of chemicals added to foods, or present in foods due to environmental circumstances, we
An Evidence-Based Approach to Reviewing the Science on the Safety of Chemicals in Foods In considering the safety of chemicals added to foods, or present in foods due to environmental circumstances, we
Specification Document (11/1023)
 Responsive funding in the NIHR SDO programme: open call (with special interest in studies with an economic/costing component) Closing date 1.00pm on 15 September 2011 1. Introduction This is the fifth
Responsive funding in the NIHR SDO programme: open call (with special interest in studies with an economic/costing component) Closing date 1.00pm on 15 September 2011 1. Introduction This is the fifth
First Look Summary - Patient level information and costing systems (PLICS): Current practice and future potential for the NHS health economy
 First Look Summary - Patient level information and costing systems (PLICS): Current practice and future potential for the NHS health economy Sue Llewellyn*, Professor of Accountability and Management Control,
First Look Summary - Patient level information and costing systems (PLICS): Current practice and future potential for the NHS health economy Sue Llewellyn*, Professor of Accountability and Management Control,
Health Technology Assessment
 Health Technology Assessment VOLUME 17 ISSUE 23 June 2013 ISSN 1366-5278 Risk Adjustment In Neurocritical care (RAIN) prospective validation of risk prediction models for adult patients with acute traumatic
Health Technology Assessment VOLUME 17 ISSUE 23 June 2013 ISSN 1366-5278 Risk Adjustment In Neurocritical care (RAIN) prospective validation of risk prediction models for adult patients with acute traumatic
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study
 Health Technology Assessment 2008; Vol. 12: No. 10 Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study J Raftery, J Bryant, J Powell, C Kerr and
Health Technology Assessment 2008; Vol. 12: No. 10 Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study J Raftery, J Bryant, J Powell, C Kerr and
Policy Research Programme Summary Final Report Form
 Policy Research Programme Summary Final Report Form 1. Project Details Project Title: Project Duration: (months) Evaluation of the costs and benefits of computerised on-scene decision support for ambulance
Policy Research Programme Summary Final Report Form 1. Project Details Project Title: Project Duration: (months) Evaluation of the costs and benefits of computerised on-scene decision support for ambulance
. 1 : N Glos ry a d l t o f a 6 Systematic review of economic evaluations utive summary er 7 Computerised decision support ssion and conclusions
 Health Technology Assessment 2010; Vol. 14: No. 48 Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: systematic reviews of the effects
Health Technology Assessment 2010; Vol. 14: No. 48 Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: systematic reviews of the effects
ChangingPractice. Appraising Systematic Reviews. Evidence Based Practice Information Sheets for Health Professionals. What Are Systematic Reviews?
 Supplement 1, 2000 ChangingPractice Evidence Based Practice Information Sheets for Health Professionals Appraising Systematic Reviews The series Changing Practice has been designed to support health professionals
Supplement 1, 2000 ChangingPractice Evidence Based Practice Information Sheets for Health Professionals Appraising Systematic Reviews The series Changing Practice has been designed to support health professionals
HEALTH TECHNOLOGY ASSESSMENT
 HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 45 JULY 2014 ISSN 1366-5278 Vitamin D supplementation in pregnancy: a systematic review Nicholas C Harvey, Christopher Holroyd, Georgia Ntani, Kassim Javaid,
HEALTH TECHNOLOGY ASSESSMENT VOLUME 18 ISSUE 45 JULY 2014 ISSN 1366-5278 Vitamin D supplementation in pregnancy: a systematic review Nicholas C Harvey, Christopher Holroyd, Georgia Ntani, Kassim Javaid,
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial
 Health Technology Assessment 2009; Vol. 13: No. 39 Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial SG Parker, P Oliver, M Pennington,
Health Technology Assessment 2009; Vol. 13: No. 39 Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial SG Parker, P Oliver, M Pennington,
To achieve this aim the specific objectives of this PhD will include:
 PhD Project Proposal - Living well with dementia: a PhD programme to develop a complex intervention that integrates healthcare for people with dementia 1. Background to the study There are 800,000 people
PhD Project Proposal - Living well with dementia: a PhD programme to develop a complex intervention that integrates healthcare for people with dementia 1. Background to the study There are 800,000 people
33 % of whiplash patients develop. headaches originating from the upper. cervical spine
 33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
33 % of whiplash patients develop headaches originating from the upper cervical spine - Dr Nikolai Bogduk Spine, 1995 1 Physical Treatments for Headache: A Structured Review Headache: The Journal of Head
Funding opportunities for research and career development
 Funding opportunities for research and career development funding leading edge research and supporting research professionals Contents Introduction 3 Research programmes Efficacy and Mechanism Evaluation
Funding opportunities for research and career development funding leading edge research and supporting research professionals Contents Introduction 3 Research programmes Efficacy and Mechanism Evaluation
Current reporting in published research
 Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
Healthcare Associate Infections Priority Setting Partnership. PROTOCOL 1 st July 2014
 Healthcare Associate Infections Priority Setting Partnership PROTOCOL 1 st July 2014 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the Healthcare Associated
Healthcare Associate Infections Priority Setting Partnership PROTOCOL 1 st July 2014 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the Healthcare Associated
Department of Veterans Affairs Health Services Research and Development - A Systematic Review
 Department of Veterans Affairs Health Services Research & Development Service Effects of Health Plan-Sponsored Fitness Center Benefits on Physical Activity, Health Outcomes, and Health Care Costs and Utilization:
Department of Veterans Affairs Health Services Research & Development Service Effects of Health Plan-Sponsored Fitness Center Benefits on Physical Activity, Health Outcomes, and Health Care Costs and Utilization:
Evidence-based guideline development. Dr. Jako Burgers/dr. H.P.Muller Dutch Institute for Healthcare Improvement CBO, Utrecht, The Netherlands
 Evidence-based guideline development Dr. Jako Burgers/dr. H.P.Muller Dutch Institute for Healthcare Improvement CBO, Utrecht, The Netherlands Outline lecture/workshop 1. Aims and objectives of guidelines
Evidence-based guideline development Dr. Jako Burgers/dr. H.P.Muller Dutch Institute for Healthcare Improvement CBO, Utrecht, The Netherlands Outline lecture/workshop 1. Aims and objectives of guidelines
Article Four Different Types of Evidence / Literature Reviews
 Article Four Different Types of Evidence / Literature Reviews The rapid growth in the number of reviews undertaken can partly be explained by the current emphasis on evidence-based practice. Healthcare
Article Four Different Types of Evidence / Literature Reviews The rapid growth in the number of reviews undertaken can partly be explained by the current emphasis on evidence-based practice. Healthcare
Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries
 Health Technology Assessment 2012; Vol. 16: No. 49 ISSN 1366-5278 Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries SE Lamb, MA Williams, EM
Health Technology Assessment 2012; Vol. 16: No. 49 ISSN 1366-5278 Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries SE Lamb, MA Williams, EM
Critical Appraisal of a Research Paper
 Critical Appraisal of a Research Paper Andrew MacInnes, BDS (Hons.) MFDS, RCPS (Glasgow), Senior House Officer 1 Thomas Lamont, BDS, MFDS, RCPS (Glasgow), Clinical Research Fellow/Honorary Restorative
Critical Appraisal of a Research Paper Andrew MacInnes, BDS (Hons.) MFDS, RCPS (Glasgow), Senior House Officer 1 Thomas Lamont, BDS, MFDS, RCPS (Glasgow), Clinical Research Fellow/Honorary Restorative
BriefingPaper. Towards faster treatment: reducing attendance and waits at emergency departments ACCESS TO HEALTH CARE OCTOBER 2005
 ACCESS TO HEALTH CARE OCTOBER 2005 BriefingPaper Towards faster treatment: reducing attendance and waits at emergency departments Key messages based on a literature review which investigated the organisational
ACCESS TO HEALTH CARE OCTOBER 2005 BriefingPaper Towards faster treatment: reducing attendance and waits at emergency departments Key messages based on a literature review which investigated the organisational
Impact of cochlear implants on the functional health status of older adults Francis H W, Chee N, Yeagle J, Cheng A, Niparko J K
 Impact of cochlear implants on the functional health status of older adults Francis H W, Chee N, Yeagle J, Cheng A, Niparko J K Record Status This is a critical abstract of an economic evaluation that
Impact of cochlear implants on the functional health status of older adults Francis H W, Chee N, Yeagle J, Cheng A, Niparko J K Record Status This is a critical abstract of an economic evaluation that
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion therapy in diabetes.
 PROTOCOL Clinical and cost-effectiveness of continuous subcutaneous insulin infusion therapy in diabetes. A. This the revised protocol (April 2002) B. Review team Contact for correspondence: Dr Jill Colquitt
PROTOCOL Clinical and cost-effectiveness of continuous subcutaneous insulin infusion therapy in diabetes. A. This the revised protocol (April 2002) B. Review team Contact for correspondence: Dr Jill Colquitt
Evidence briefing on integrated care pathways in mental health settings
 Evidence briefing on integrated care pathways in mental health settings Leeds Partnerships Foundation NHS Trust (LPFT) is undertaking a project to restructure many of its services based around the use
Evidence briefing on integrated care pathways in mental health settings Leeds Partnerships Foundation NHS Trust (LPFT) is undertaking a project to restructure many of its services based around the use
The Development of the Clinical Healthcare Support Worker Role: A Review of the Evidence. Executive Summary
 The Development of the Clinical Healthcare Support Worker Role: A Review of the Evidence Executive Summary The Development of the Clinical Healthcare Support Worker Role: A Review of the Evidence Executive
The Development of the Clinical Healthcare Support Worker Role: A Review of the Evidence Executive Summary The Development of the Clinical Healthcare Support Worker Role: A Review of the Evidence Executive
Using Qualitative Information For Impact Assessment: Introducing The Qualitative Impact Assessment Protocol (QUIP)
 Using Qualitative Information For Impact Assessment: Introducing The Qualitative Impact Assessment Protocol (QUIP) By Katie Wright-Revolledo, INTRAC Introduction Qualitative methods can provide insight
Using Qualitative Information For Impact Assessment: Introducing The Qualitative Impact Assessment Protocol (QUIP) By Katie Wright-Revolledo, INTRAC Introduction Qualitative methods can provide insight
PEER REVIEW HISTORY ARTICLE DETAILS TITLE (PROVISIONAL)
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
Does contracting out services improve access to care in low and middle-income countries?
 August 2008 SUPPORT Summary of a systematic review Does contracting out services improve access to care in low and middle-income countries? Contracting out of health services is a formal contractual relationship
August 2008 SUPPORT Summary of a systematic review Does contracting out services improve access to care in low and middle-income countries? Contracting out of health services is a formal contractual relationship
Careers Destinations Project Phase 1 Report
 Careers Destinations Project Phase 1 Report Dr Lisa Morrison Coulthard Lead Policy Advisor Background: In August 2011, the Society launched a longitudinal study to determine the career destinations of
Careers Destinations Project Phase 1 Report Dr Lisa Morrison Coulthard Lead Policy Advisor Background: In August 2011, the Society launched a longitudinal study to determine the career destinations of
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation
 Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Recruiting Patients to Cancer Trials. Paper Prepared for the Prostate Cancer Clinical Studies Group
 Recruiting Patients to Cancer Trials Paper Prepared for the Prostate Cancer Clinical Studies Group 1. The Problem 1.1.There is general agreement that the recruitment of patients to clinical trials is a
Recruiting Patients to Cancer Trials Paper Prepared for the Prostate Cancer Clinical Studies Group 1. The Problem 1.1.There is general agreement that the recruitment of patients to clinical trials is a
Do nurse practitioners working in primary care provide equivalent care to doctors?
 August 2008 SUPPORT Summary of a systematic review Do nurse practitioners working in primary care provide equivalent care to doctors? Nurse practitioners are nurses who have undergone further training,
August 2008 SUPPORT Summary of a systematic review Do nurse practitioners working in primary care provide equivalent care to doctors? Nurse practitioners are nurses who have undergone further training,
Technology Assessment Report commissioned by the NHS R&D HTA Programme on behalf of the National Institute for Health and Clinical Excellence
 Technology Assessment Report commissioned by the NHS R&D HTA Programme on behalf of the National Institute for Health and Clinical Excellence Protocol 26 October 2007 PROJECT TITLE Bevacizumab, sorafenib
Technology Assessment Report commissioned by the NHS R&D HTA Programme on behalf of the National Institute for Health and Clinical Excellence Protocol 26 October 2007 PROJECT TITLE Bevacizumab, sorafenib
What factors determine poor functional outcome following Total Knee Replacement (TKR)?
 Specific Question: What factors determine poor functional outcome following Total Knee Replacement ()? Clinical bottom line All groups derived benefit from undergoing a, reviews suggests that the decision
Specific Question: What factors determine poor functional outcome following Total Knee Replacement ()? Clinical bottom line All groups derived benefit from undergoing a, reviews suggests that the decision
The QuEST for a decent place to live.
 The QuEST for a decent place to live Helen Killaspy Professor and Honoury Consultant in Rehabilitation Psychiatry, University College London and Camden & Islington NHS Foundation Trust #ImROC @ImROC_comms
The QuEST for a decent place to live Helen Killaspy Professor and Honoury Consultant in Rehabilitation Psychiatry, University College London and Camden & Islington NHS Foundation Trust #ImROC @ImROC_comms
Developing a network for clinical research: The UK Dermatology Clinical Trials Network
 Developing a network for clinical research: The UK Dermatology Clinical Trials Network (UK DCTN) experience Carron Layfield*, Tessa Clarke, Kim Thomas and Hywel Williams Centre of Evidence Based Dermatology,
Developing a network for clinical research: The UK Dermatology Clinical Trials Network (UK DCTN) experience Carron Layfield*, Tessa Clarke, Kim Thomas and Hywel Williams Centre of Evidence Based Dermatology,
Guidance on NHS patients who wish to pay for additional private care
 Guidance on NHS patients who wish to pay for additional private care DH INFORMATION READER BOX Policy HR / Workforce Management Planning / Clinical Document Purpose Gateway Reference Title Author Publication
Guidance on NHS patients who wish to pay for additional private care DH INFORMATION READER BOX Policy HR / Workforce Management Planning / Clinical Document Purpose Gateway Reference Title Author Publication
A Guide to Efficient Trial Management. Trials Managers Network
 A Guide to Efficient Trial Management Trials Managers Network The Fourth Edition (2014) of the Guide to Efficient Trial Management was produced by an appointed Editorial Board and a dedicated group of
A Guide to Efficient Trial Management Trials Managers Network The Fourth Edition (2014) of the Guide to Efficient Trial Management was produced by an appointed Editorial Board and a dedicated group of
Royal College of Obstetricians and Gynaecologists. Faculty of Sexual and Reproductive Healthcare
 Royal College of Obstetricians and Gynaecologists Faculty of Sexual and Reproductive Healthcare Supporting Information for Appraisal and Revalidation: Guidance for Obstetrics and Gynaecology and / or Sexual
Royal College of Obstetricians and Gynaecologists Faculty of Sexual and Reproductive Healthcare Supporting Information for Appraisal and Revalidation: Guidance for Obstetrics and Gynaecology and / or Sexual
HEE/NIHR Integrated Clinical Academic (ICA) Programme for Non-Medical/Dental Healthcare Professionals
 HEE/NIHR Integrated Clinical Academic (ICA) Programme for Non-Medical/Dental Healthcare Professionals Invitation to Tender for Masters in Clinical Research Studentships Introduction Health Education England
HEE/NIHR Integrated Clinical Academic (ICA) Programme for Non-Medical/Dental Healthcare Professionals Invitation to Tender for Masters in Clinical Research Studentships Introduction Health Education England
Map 1 Statutory specialist services and organisations in England
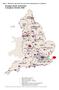 Map 1 Statutory specialist services and organisations in England Isle of Man 31 Neuro Rehab Units (YDU) Spinal Injury Centres Brain Injury Services - NHS Brain Injury Service non NHS Stroke Units Regional
Map 1 Statutory specialist services and organisations in England Isle of Man 31 Neuro Rehab Units (YDU) Spinal Injury Centres Brain Injury Services - NHS Brain Injury Service non NHS Stroke Units Regional
