Please note: Contact Coppe Laboratories at if archival plasma samples need to be tested.
|
|
|
- Juliana Henderson
- 8 years ago
- Views:
Transcription
1
2 Collecting a Coppe Laboratories Sample The Coppe Laboratories Sample Kit contains: Test Request Form (TRF) Heparin tube Absorbent sheet Biohazard bag Foil pouch Label for blood tube Box Seal Instructions Following the individual specimen instructions is an appendix with additional information about minimum specimen, alternate samples, and reasons for rejection. Please note: Contact Coppe Laboratories at if archival plasma samples need to be tested. Table of Contents Collecting a blood sample 1 Collecting and sending a serum 2 Collecting and sending a bone marrow sample 3 Collecting and sending a cord blood sample 3 Collecting and sending a cerebrospinal fluid sample 4 Collecting and sending a hair follicle sample 5 Collecting and sending a tissue (slide) sample for IHC 6 Testing appendix 7 9 Reasons for sample rejection 10 Contact Us 10
3 Whole Blood (or whole blood for plasma sample) Specimen Type: Whole blood (or whole blood to be processed for plasma) (Plasma tests ordered require heparin whole blood sample which will be processed by Coppe Laboratories. Please call for information about acceptability of other collected plasma samples.) 1. Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature, and test ordered. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 2. Draw blood in a heparin tube and gently invert blood tube 8 10 times. 3. Label the blood tube with patient name, date of birth and date and time of draw. 4. Place blood tube into biohazard bag with absorbent pad and seal. 5. Place the biohazard-labeled bag into the foil pouch and seal. 6. Place the foil pouch and the test request form into the pre-labeled Coppe Laboratories box and seal with enclosed Box Seal. 7. Send the pre-labeled box via FedEx, priority overnight shipping. Place test kit box directly into any FedEx drop box or Call FedEx at for pickup. 1
4 Serum Specimen Type: Serum Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 1. Draw 6 10 ml of blood and gently invert blood tube 8 10 times. 2. Label the SST tube with patient name, date of birth and date and time of draw. 3. Allow blood to clot at room, upright, for minutes 4. Place tube in centrifuge for minutes. 5. Place blood tube into biohazard bag with absorbent pad and seal. 6. Place the biohazard-labeled bag into the foil pouch and seal. 7. Place the foil pouch and the test request form into the prelabeled Coppe Laboratories box and seal with enclosed Box Seal. 8. Send the pre-labeled box via FedEx, priority overnight shipping. Place test kit box directly into any FedEx drop box or Call FedEx at for pickup. 2
5 Bone Marrow/Cord Blood Specimen Type: Bone Marrow or Cord Blood Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature, and test ordered. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 1. Place 2 ml of bone marrow aspirate (or cord blood) in a pediatric (3mL) heparin or ACD tube and gently invert blood tube 8 10 times. 2. Label the tube with patient name, date of birth and date and time of draw. 3. Place blood tube into biohazard bag with absorbent pad and seal. 4. Place the biohazard-labeled bag into the foil pouch and seal. 5. Place the foil pouch and the test request form into the pre-labeled Coppe Laboratories box and seal with enclosed Box Seal. 6. Send the pre-labeled box via FedEx, priority overnight shipping. a. Place test kit box directly into any FedEx drop box or b. Call FedEx at for pickup. 3
6 Cerebrospinal Fluid (CSF) Specimen Type: CSF Since CSF samples will be sent refrigerated, please freeze gel ice packs for 4 hours prior to shipping. Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature, and test ordered. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 1. Place CSF into a sterile, leak-proof plastic tube. 2. Label the CSF specimen with patient name, date of birth and date and time of draw. 3. Place tube into the biohazard bag and seal. 4. Place the frozen gel pack into the foil pouch. 5. Place the biohazard-labeled bag into the foil pouch and seal. 6. Place the foil pouch and the test request form into the pre-labeled Coppe Laboratories box and seal. 7. Send the pre-labeled box via FedEx, priority overnight shipping. a. Call FedEx at for pickup or b. Place test kit box directly into any FedEx drop box. Note: If a sample cannot be immediately shipped, it can be stored for 3 days refrigerated or 1 year frozen. Must be shipped with dry ice if frozen or ice packets if stored refrigerated. 4
7 Hair Folliclle Specimen Type: Hair Follicle Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature, and test ordered. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 1. Explain to the patient that you will need to remove 2 3 hairs from their head. Hair taken from combs or brushes is not an acceptable specimen. 2. Using a tweezer or forceps, grasp the hair near the scalp and pull quickly. 3. Check root to ensure hair follicle is attached (see picture below). Lack of follicle on the hair shaft will cause the sample to be rejected. 4. Repeat steps 2-3 twice. 5. Place hair in small zip lock bag. 6. Label the zip lock specimen bag with the patient first name, last name, date of birth, and specimen removal date. 7. Place zip lock bag in biohazard bag and seal. 8. Place biohazard bag into padded foil bag and seal. 9. Place foil bag and completed test request form in Coppe Laboratories box and seal. 10. Send the pre-labeled box via FedEx. a. Place test kit box directly into any FedEx drop box or b. Call FedEx at for pickup. 5
8 Slides for Immunohistochemistry Specimen Type: Unstained slides for Immunohistochemistry (4 micron, FFPE slides) Fill in the Test Request Form completely. Be sure to include date and time of specimen collection, physician information and signature, and test ordered. Attach payment information. Incomplete or inaccurate information may cause delays in testing and reporting of results. 1. Label slides with patient first name, last name, and date of birth. 2. Package 6 unstained slides in a slide mailer, transporter or slide holder. 3. Place slides into the biohazard-labeled bag. 4. Place the biohazard-labeled bag into the foil pouch and seal. 5. Place the foil pouch and the test request form into the pre-labeled Coppe Laboratories box and seal. 6. Include a copy of the pathology report in the box. 7. Send the pre-labeled box via FedEx. a. Place test kit box directly into any FedEx drop box or b. Call FedEx at for pickup. 6
9 Appendix Cell-Free and Cellular Samples Real-Time DNA PCR on Cell-Free Samples: Quantitative Detection in Plasma for HHV-6A, HHV-6B, HHV-7 and CMV Qualitative Detection in CSF for HHV-6A, HHV-6B, HHV-7, CMV, EBV Test numbers: 1104, 1105, 1205, 1304, 1305, 1404, 1405 Specimen requirements are as follows: Specimen Volume Minimum Vol. Whole Blood to be processed to plasma CSF 6 10 ml collected in heparin tube. ACD also acceptable 2 ml cryotube Specimen Volume Minimum Vol. Whole Blood Bone Marrow 6 10 ml collected in heparin or ACD tube 2 ml collected in pediatric heparin ro ACD tube Storage/collection to test initiation 3 ml Less than 48 hours from collection to arrival in lab, room 0.5 ml Refrigerated 3 days or frozen up to 1 year Storage/collection to test initiation 3 ml Less than 48 hours from collection to arrival in lab, room 1 ml Shipping Room Dry ice or ice packs Quantitative, Real-Time DNA PCR (qpcr) on Cellular Samples: Whole Blood, Bone Marrow and Cord Blood for HHV-6A, HHV- 6B HHV-7, CMV, EBV Test numbers: 1106, 1206, 1306, 1406 Specimen requirements are as follows: Cord Blood 2 ml collected in pediatric heparin or ACD tube 1 ml Shipping Room 7
10 Appendix Cellular Samples mrna, cihhv-6 Screening Reverse Transcription PCR on Cellular Samples for mrna: Whole Blood, Bone Marrow and Cord Blood for HHV-6A, HHV-6B and CMV Test numbers: 1110, 1303 Specimen Requirements are as follows: Specimen Volume Minimum Vol. Whole Blood Bone Marrow Cord Blood 6 10 ml collected in heparin or ACD tube 2 ml collected in pediatric heparin or ACD tube cihhv-6 Screening Panel: Includes Quantitative, Real-Time DNA PCR (qpcr) on Whole Blood, Reverse Transcription PCR for mrna and Real-Time DNA PCR on Hair Follicle Test number: 1012 Specimen requirements: Specimen Volume Minimum Vol. Whole Blood AND Hair Follicle 6 10 ml collected in heparin or ACD tube 3 4 hairs with follicle Storage/ collection to test initiation 3 ml Less than 48 hours from collection to arrival in lab, 1 ml room Storage /collection to test initiation 3 ml Less than 48 hours from collection to arrival in lab, room At least Room 1 follicle, 1 week 8 Shipping Room temperatur e Shipping Room
11 Appendix Lyme Tests/ Babesia Tests Lyme Tests: Antibodies to Borrelia Test numbers: 3001, 3002, 3003 Specimen Requirements are as follows: Specimen Volume Min. Vol. Whole Blood to be processed to plasma 6 10 ml collected in heparin tube (EDTA also acceptable) Babesia Tests: qpcr, IFA, panel Test numbers: 3004, 3005, 3006 Specimen Requirements are as follows: Specimen Volume Min. Vol. Whole Blood to be processed to plasma by Coppe Laboratories 6 10 ml collected in heparin tube (EDTA also acceptable) Storage/ collection to test initiation 3 ml Room less than 72 hours Storage/ collection to test initiation 3 ml Room less than 72 hours Shipping Room Shipping Room
12 12/16/2015 Specimen Collection and Preparation Laboratory test results are dependent on the quality of the specimen submitted. It is important that all specimens and request forms be properly labeled in the presence of the patient. Specimens should be labeled with patient name and date of birth, the collection date and time. If there is any doubt or question regarding the type of specimen that should be collected, it is imperative that Coppe Laboratories be contacted to clarify the order and specimen requirements. Causes for rejection of sample: Specimens submitted in an improper tube or container Specimens not accompanied by a requisition or incomplete requisition Unlabeled specimens Leaking containers Insufficient quantity of specimen Improper storage Failure to receive samples prior to expiration Box not sealed or seal broken Please contact Coppe Laboratories with any questions: W229N1870 Westwood Drive Waukesha, WI phone fax info@coppelabs.com www. coppelabs.com 10
Guidelines for Collection and Transport of Specimen for Laboratory Diagnosis of Pathogenic Leptospira spp.
 Document: Introduction: Guidelines for Collection and Transport of Specimen for Laboratory Diagnosis of Pathogenic Leptospira spp. Leptospirosis is an acute bacterial infection caused by organism belonging
Document: Introduction: Guidelines for Collection and Transport of Specimen for Laboratory Diagnosis of Pathogenic Leptospira spp. Leptospirosis is an acute bacterial infection caused by organism belonging
WHICH SAMPLES SHOULD BE SUBMITTED WHEN LYMPHOID NEOPLASIA IS SUSPECTED?
 WHICH SAMPLES SHOULD BE SUBMITTED WHEN LYMPHOID NEOPLASIA IS SUSPECTED? Which test should be submitted? The answer to this depends on the clinical signs, and the diagnostic question you are asking. If
WHICH SAMPLES SHOULD BE SUBMITTED WHEN LYMPHOID NEOPLASIA IS SUSPECTED? Which test should be submitted? The answer to this depends on the clinical signs, and the diagnostic question you are asking. If
PEAC CENTRAL LABORATORY SAMPLING MANUAL
 PEAC CENTRAL LABORATORY SAMPLING MANUAL FINAL REVISED VERSION (9.0) PEAC Clinical Study Final 1.0 2 TABLE OF CONTENTS Page No. 1. PEAC CENTRAL LABORATORY - CONTACT NUMBERS... 3 2. IMPORTANT INFORMATION...
PEAC CENTRAL LABORATORY SAMPLING MANUAL FINAL REVISED VERSION (9.0) PEAC Clinical Study Final 1.0 2 TABLE OF CONTENTS Page No. 1. PEAC CENTRAL LABORATORY - CONTACT NUMBERS... 3 2. IMPORTANT INFORMATION...
INTRODUCTION. Viral shedding is crucial for Gene Therapy Products Safety linked with shedding data
 INTRODUCTION Viral shedding is crucial for Gene Therapy Products Safety linked with shedding data For the patient For families and friends For the environment No treatment possible without good safety
INTRODUCTION Viral shedding is crucial for Gene Therapy Products Safety linked with shedding data For the patient For families and friends For the environment No treatment possible without good safety
(800) 786-7235 Cryo-Cell.com
 HEALTHCARE PROVIDER Instructions Keep these instructions in the kit [ When handling blood products, use personal protective measures, maintain aseptic techniques and adequate room for collection processes.
HEALTHCARE PROVIDER Instructions Keep these instructions in the kit [ When handling blood products, use personal protective measures, maintain aseptic techniques and adequate room for collection processes.
Targeted Variant Test Requisition Form (3/4/2015)
 Targeted Variant Test Requisition Form (3/4/2015) Instructions: Please provide clinical features of family members. All testing must be ordered by a qualified healthcare provider. See Test Selection Box
Targeted Variant Test Requisition Form (3/4/2015) Instructions: Please provide clinical features of family members. All testing must be ordered by a qualified healthcare provider. See Test Selection Box
Blood Collection and Processing SOP
 Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
STEP-BY-STEP INSTRUCTIONS FOR INVESTIGATIONAL USE. Rapid HCV Antibody Test FOR ORAQUICK RAPID HCV ANTIBODY TEST
 Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Content Sheet 5-1: Overview of Sample Management
 Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
Central Line Blood Draw
 Central Line Blood Draw Welcome to the Central Line dressing blood draw refresher. Please use the navigation below to advance to the next page. The Central Line blood draw module is also available as a
Central Line Blood Draw Welcome to the Central Line dressing blood draw refresher. Please use the navigation below to advance to the next page. The Central Line blood draw module is also available as a
HAEMATOLOGY LABORATORY
 HAEMATOLOGY LABORATORY Head of Unit : Dr Raudhawati Osman Phone : +603-26155281 Email : raudhaosman@yahoo.com 1. INTRODUCTION The Haematology Unit, Department of Pathology, HKL provides tertiary diagnostic
HAEMATOLOGY LABORATORY Head of Unit : Dr Raudhawati Osman Phone : +603-26155281 Email : raudhaosman@yahoo.com 1. INTRODUCTION The Haematology Unit, Department of Pathology, HKL provides tertiary diagnostic
Reference Range: 0.5-1.6 mmol/l (arterial) 0.7-2.1 mmol/l (venous) CPT Code: 83605
 LACTIC ACID Fasting, arterial specimen preferred. Please note whether arterial or venous. 0.5 ml heparinized plasma. Green top or PST must be drawn, placed on ice, and spun within 15 minutes. Immediately
LACTIC ACID Fasting, arterial specimen preferred. Please note whether arterial or venous. 0.5 ml heparinized plasma. Green top or PST must be drawn, placed on ice, and spun within 15 minutes. Immediately
14.0 Stem Cell Laboratory Services
 Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
Laboratory Services Contact Information: To inquire about assisting with surgical harvesting of bone marrow, cellular therapy (CT) product processing, cryopreservation, storage, or any other lab services,
Infectious Waste Management Plan
 Infectious Waste Management Plan Infectious Waste Management Plan USC Health & Safety Programs Unit 777-5269 POLICY: A. In keeping with the University of South Carolina's policy of providing protection
Infectious Waste Management Plan Infectious Waste Management Plan USC Health & Safety Programs Unit 777-5269 POLICY: A. In keeping with the University of South Carolina's policy of providing protection
Optimal management of RA patients who require Biologic Therapy. (ORBIT Study) SAMPLING MANUAL
 Optimal management of RA patients who require Biologic Therapy (ORBIT Study) SAMPLING MANUAL VERSION (1.0) 2 TABLE OF CONTENTS Page No. 1. CENTRAL LABORATORY - CONTACT NUMBERS... 3 2. TO RE-ORDER SUPPLIES...
Optimal management of RA patients who require Biologic Therapy (ORBIT Study) SAMPLING MANUAL VERSION (1.0) 2 TABLE OF CONTENTS Page No. 1. CENTRAL LABORATORY - CONTACT NUMBERS... 3 2. TO RE-ORDER SUPPLIES...
Division of Laboratory Medicine Department of Pathology and Laboratory Medicine Hospital of the University of Pennsylvania
 Division of Laboratory Medicine Department of Pathology and Laboratory Medicine Hospital of the University of Pennsylvania General Laboratory Specimen Collection Guidelines The Laboratory will perform
Division of Laboratory Medicine Department of Pathology and Laboratory Medicine Hospital of the University of Pennsylvania General Laboratory Specimen Collection Guidelines The Laboratory will perform
Packaging & Shipping Laboratory Specimens Guide Volume XI
 Packaging & Shipping Laboratory Specimens Guide Volume XI PAML is not certified to provide regulatory training. This guide is to be used in addition to training and certification by a regulated agency.
Packaging & Shipping Laboratory Specimens Guide Volume XI PAML is not certified to provide regulatory training. This guide is to be used in addition to training and certification by a regulated agency.
INMA LABORATORY MANUAL
 INMA LABORATORY MANUAL NUTRITION BIOMARKERS Cohorte-VALENCIA This manual describes the collection, separation, storage and transport of blood samples for the INMA study. Careful collection of samples is
INMA LABORATORY MANUAL NUTRITION BIOMARKERS Cohorte-VALENCIA This manual describes the collection, separation, storage and transport of blood samples for the INMA study. Careful collection of samples is
Human Free Testosterone(F-TESTO) ELISA Kit
 Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
Human Free Testosterone(F-TESTO) ELISA Kit Catalog Number. MBS700040 For the quantitative determination of human free testosterone(f-testo) concentrations in serum, plasma. This package insert must be
RealLine HCV PCR Qualitative - Uni-Format
 Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Instructions for use PCR KIT FOR EXTRACTION OF RNA AND REAL TIME PCR DETECTION KIT FOR HEPATITIS C VIRUS RNA Research Use Only Qualitative Uni Format VBD0798 48 tests valid from: December 2013 Rev11122013
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
Inc. Wuhan. Quantity Pre-coated, ready to use 96-well strip plate 1 Plate sealer for 96 wells 4 Standard (liquid) 2
 Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Uscn Life Science Inc. Wuhan Website: www.uscnk.com Phone: +86 27 84259552 Fax: +86 27 84259551 E-mail: uscnk@uscnk.com ELISA Kit for Human Prostaglandin E1(PG-E1) Instruction manual Cat. No.: E0904Hu
Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens
 Distr.: General Orig.: English Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens WORLD HEALTH ORGANIZATION Division of Emerging and Other Communicable Diseases Surveillance
Distr.: General Orig.: English Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens WORLD HEALTH ORGANIZATION Division of Emerging and Other Communicable Diseases Surveillance
4A. Types of Laboratory Tests Available and Specimens Required. Three main types of laboratory tests are used for diagnosing CHIK: virus
 4. LABORATORY 4A. Types of Laboratory Tests Available and Specimens Required Three main types of laboratory tests are used for diagnosing CHIK: virus isolation, reverse transcriptase-polymerase chain reaction
4. LABORATORY 4A. Types of Laboratory Tests Available and Specimens Required Three main types of laboratory tests are used for diagnosing CHIK: virus isolation, reverse transcriptase-polymerase chain reaction
General Information and Guidelines. Submission of blood tubes
 General Information and Guidelines Submission of blood tubes Note: Do not submit blood samples in syringes (especially with needles attached). Syringes that are submitted to the lab with the needles attached
General Information and Guidelines Submission of blood tubes Note: Do not submit blood samples in syringes (especially with needles attached). Syringes that are submitted to the lab with the needles attached
Guidelines for Handling and Shipping of Blood Samples
 Biofocus Institute for Laboratory Medicine Dr. med. Doris Bachg Dr. med. Uwe Haselhorst Berghäuser Str. 295 45659 Recklinghausen Germany contact: Dr. Doris Bachg (bachg@biofocus.de) phone +49 2361 3000-144
Biofocus Institute for Laboratory Medicine Dr. med. Doris Bachg Dr. med. Uwe Haselhorst Berghäuser Str. 295 45659 Recklinghausen Germany contact: Dr. Doris Bachg (bachg@biofocus.de) phone +49 2361 3000-144
DIRECTED UMBILICAL CORD BLOOD AND TISSUE COLLECTION IN THEATRE (CELLCARE)
 WOMEN AND NEWBORN HEALTH SERVICE King Edward Memorial Hospital CLINICAL GUIDELINES PERIOPERATIVE GUIDELINES DIRECTED CORD CELL COLLECTION IN THEATRE DIRECTED UMBILICAL CORD BLOOD AND TISSUE COLLECTION
WOMEN AND NEWBORN HEALTH SERVICE King Edward Memorial Hospital CLINICAL GUIDELINES PERIOPERATIVE GUIDELINES DIRECTED CORD CELL COLLECTION IN THEATRE DIRECTED UMBILICAL CORD BLOOD AND TISSUE COLLECTION
Streptococcus pneumoniae IgG AB (13 Serotypes), MAID... 7
 Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
Volume 12 December 2011 Table of Contents Summary of Test Changes... 3 Test Changes... 6 Human Anti-Mouse AB (HAMA), ELISA... 6 Hepatitis E Antibody (IgG, IgM)... 6 Hepatitis E Antibody (IgG)... 6 Hepatitis
Family Link Cord Blood Storage Program
 Cord Blood Collection Instructions for the Clinician Dear Collection Staff, Thank you for taking some of your valuable time to read this information. Cord blood harvesting is an elective procedure that
Cord Blood Collection Instructions for the Clinician Dear Collection Staff, Thank you for taking some of your valuable time to read this information. Cord blood harvesting is an elective procedure that
Tests that have had changes to the method/ CPT code, units of measurement, scope of analysis, reference comments, or specimen requirements.
 In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below are the
In our continuing effort to provide you with the highest quality toxicology laboratory services available, we have compiled important changes regarding a number of tests we perform. Listed below are the
Pointers on Shipping: Clinical Samples, Biological Substance Category B(UN 3373) and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B(UN 3373) and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples
Pointers on Shipping: Clinical Samples, Biological Substance Category B(UN 3373) and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit
 Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Rat Creatine Kinase MB isoenzyme,ck-mb ELISA Kit Catalog No: E0479r 96 Tests Operating instructions www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Specimen Collection Guide
 Specimen Collection Guide pathology.mater.org.au Specimen Collection Guide This chart indicates the specimen required for most frequently requested tests. If the desired test is not listed here please
Specimen Collection Guide pathology.mater.org.au Specimen Collection Guide This chart indicates the specimen required for most frequently requested tests. If the desired test is not listed here please
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood
 Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
GTEx Work Instruction for Whole Brain, Brain Stem, and Hair Collection Receipt and Shipping (Green Kit)
 OP-0001-W2 VER. 03.08 Effective Date: mm/dd/yyyy Page 1 of 7 1.0 PURPOSE 1.1 This work instruction provides the specific steps necessary to pack and ship the Comprehensive Biospecimen Resource (CBR)-issued
OP-0001-W2 VER. 03.08 Effective Date: mm/dd/yyyy Page 1 of 7 1.0 PURPOSE 1.1 This work instruction provides the specific steps necessary to pack and ship the Comprehensive Biospecimen Resource (CBR)-issued
Protocols for Collecting and Storing DNA Samples. Created: March 28, 1998 Revised December 12, 2006
 Protocols for Collecting and Storing DNA Samples Michael K. Schwartz Research Ecologist Rocky Mountain Research Station Missoula Montana Kristy Pilgrim Laboratory Supervisor Rocky Mountain Research Station
Protocols for Collecting and Storing DNA Samples Michael K. Schwartz Research Ecologist Rocky Mountain Research Station Missoula Montana Kristy Pilgrim Laboratory Supervisor Rocky Mountain Research Station
2. TRansport ARRANGEMENTS... 5 2.1. Flight arrangements... 5 2.2. Ground transport arrangements... 5
 World Marrow Donor Association (WMDA) Guidelines for couriers and the transportation of haematopoietic progenitor cells (HPC-BM, apheresis and therapeutic cells- T Cells) 1. COURIERS... 3 1.1. Courier
World Marrow Donor Association (WMDA) Guidelines for couriers and the transportation of haematopoietic progenitor cells (HPC-BM, apheresis and therapeutic cells- T Cells) 1. COURIERS... 3 1.1. Courier
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES
 UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES November 2003 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES November 2003 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
Standard Operating Procedure (SOP) Work Package 8. Sample Collection and Storage
 Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
IgM ELISA. For the quantitative determination of IgM in human serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
Medical Necessity and Advanced Beneficiary Notice (ABN) Policy and Form
 Medical Necessity and Advanced Beneficiary Notice (ABN) Policy and Form Billings Clinic Laboratory believes all health-care providers should order only appropriate tests for the diagnosis and treatment
Medical Necessity and Advanced Beneficiary Notice (ABN) Policy and Form Billings Clinic Laboratory believes all health-care providers should order only appropriate tests for the diagnosis and treatment
VS Guidance 6702.1 Date 04/26/13
 The IDEXX Antibody (Ab) Serological Test for Diagnosing Bovine Tuberculosis (TB) in TB-Affected Cattle Herds 1. Purpose and Background The caudal fold tuberculin skin test (CFT), the gamma interferon test,
The IDEXX Antibody (Ab) Serological Test for Diagnosing Bovine Tuberculosis (TB) in TB-Affected Cattle Herds 1. Purpose and Background The caudal fold tuberculin skin test (CFT), the gamma interferon test,
CHAPTER 3 COLLECTION PROCEDURES
 CHAPTER 3 COLLECTION PROCEDURES Chapter 3 COLLECTION PROCEDURES 3.1 COLLECTION KIT Principle The cord blood collection kit will be carefully assembled by the Cord Blood Bank (CBB) supervisor or his/her
CHAPTER 3 COLLECTION PROCEDURES Chapter 3 COLLECTION PROCEDURES 3.1 COLLECTION KIT Principle The cord blood collection kit will be carefully assembled by the Cord Blood Bank (CBB) supervisor or his/her
PROCUREMENT OF BONE MARROW SPECIMENS
 Page 1 of 8 SITES AFFECTED _X Enterprise Chandler Good Samaritan PROCUREMENT OF BONE MARROW SPECIMENS A technologist at Chandler CORE and Samaritan are assigned to Bone Marrow duties Monday through Friday
Page 1 of 8 SITES AFFECTED _X Enterprise Chandler Good Samaritan PROCUREMENT OF BONE MARROW SPECIMENS A technologist at Chandler CORE and Samaritan are assigned to Bone Marrow duties Monday through Friday
VENOUS BLOOD COLLECTION
 Venous Blood Collection p. 1 of 5 Johns Hopkins Medical Laboratories Department of Pathology Core Lab Clinical Services Zd7: Word: JHH Venous Blood Collection Procedure Signature Date Signature Date Written
Venous Blood Collection p. 1 of 5 Johns Hopkins Medical Laboratories Department of Pathology Core Lab Clinical Services Zd7: Word: JHH Venous Blood Collection Procedure Signature Date Signature Date Written
User Manual. CelluLyser Lysis and cdna Synthesis Kit. Version 1.4 Oct 2012 From cells to cdna in one tube
 User Manual CelluLyser Lysis and cdna Synthesis Kit Version 1.4 Oct 2012 From cells to cdna in one tube CelluLyser Lysis and cdna Synthesis Kit Table of contents Introduction 4 Contents 5 Storage 5 Additionally
User Manual CelluLyser Lysis and cdna Synthesis Kit Version 1.4 Oct 2012 From cells to cdna in one tube CelluLyser Lysis and cdna Synthesis Kit Table of contents Introduction 4 Contents 5 Storage 5 Additionally
mircute mirna qpcr Detection Kit (SYBR Green)
 mircute mirna qpcr Detection Kit (SYBR Green) For detection of mirna using real-time RT-PCR (SYBR Green I) www.tiangen.com QP110302 mircute mirna qpcr Detection Kit (SYBR Green) Kit Contents Cat. no. FP401
mircute mirna qpcr Detection Kit (SYBR Green) For detection of mirna using real-time RT-PCR (SYBR Green I) www.tiangen.com QP110302 mircute mirna qpcr Detection Kit (SYBR Green) Kit Contents Cat. no. FP401
PROCEDURES SURROUNDING CORD BLOOD COLLECTION TABLE OF CONTENTS 1. PURPOSE... 2 2. SCOPE AND RESPONSIBILITIES... 2 3. REQUIRED DOCUMENTS...
 Pour copie papier seulement : Le document original est approuvé avec signature numérique sous la responsabilité de CFA. TABLE OF CONTENTS 1. PURPOSE... 2 2. SCOPE AND RESPONSIBILITIES... 2 3. REQUIRED
Pour copie papier seulement : Le document original est approuvé avec signature numérique sous la responsabilité de CFA. TABLE OF CONTENTS 1. PURPOSE... 2 2. SCOPE AND RESPONSIBILITIES... 2 3. REQUIRED
Mouse krebs von den lungen 6 (KL-6) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
KAMIYA BIOMEDICAL COMPANY Mouse krebs von den lungen 6 (KL-6) ELISA For the quantitative determination of mouse KL-6 in serum, plasma, cell culture supernatants, body fluid and tissue homogenate Cat. No.
CHAPTER 5 SHIPPING PROCEDURES
 CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
REQUEST FOR TESTING TRANSFUSION SERVICES
 REQUEST FOR TESTING TRANSFUSION SERVICES 921 Terry Avenue Seattle, WA 98104-1256 TO REORDER FORMS CALL 425-656-3019 PSBC Tech PSBC ID / CL # Time Received See the back of this order form for sample requirements.
REQUEST FOR TESTING TRANSFUSION SERVICES 921 Terry Avenue Seattle, WA 98104-1256 TO REORDER FORMS CALL 425-656-3019 PSBC Tech PSBC ID / CL # Time Received See the back of this order form for sample requirements.
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Human Umbilical Cord Blood CD34 + Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.03 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+ Progenitor Cells, cryopreserved Cryopreserved human umbilical
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit
 Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma and tissue
SAMPLE COLLECTION MANUAL
 SAMPLE COLLECTION MANUAL Revised and Updated v.2 February 2005 Table of Contents I. INTRODUCTION...1 NEW YORK STATE COLLECTIONS...1 II. COMPANY OVERVIEW...2 III. SAFETY...3 Universal Precautions (UP)...3
SAMPLE COLLECTION MANUAL Revised and Updated v.2 February 2005 Table of Contents I. INTRODUCTION...1 NEW YORK STATE COLLECTIONS...1 II. COMPANY OVERVIEW...2 III. SAFETY...3 Universal Precautions (UP)...3
GENOME RUSSIA PROJECT BLOOD SAMPLES COLLECTION, DNA EXTRACTION AND DNA QUALITY CONTROL PROTOCOLS
 ST-PETERSBURG STATE UNIVERSITY THEODOSIUS DOBZHANSKY CENTER FOR GENOME BIOINFORMATICS GENOME RUSSIA PROJECT BLOOD SAMPLES COLLECTION, DNA EXTRACTION AND DNA QUALITY CONTROL PROTOCOLS 2015 The object of
ST-PETERSBURG STATE UNIVERSITY THEODOSIUS DOBZHANSKY CENTER FOR GENOME BIOINFORMATICS GENOME RUSSIA PROJECT BLOOD SAMPLES COLLECTION, DNA EXTRACTION AND DNA QUALITY CONTROL PROTOCOLS 2015 The object of
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit
 Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma, tissue homogenates.
Rat creatine kinase MM isoenzyme (CK-MM) ELISA Kit Catalog Number. CSB-E14405r For the quantitative determination of rat creatine kinase MM isoenzyme (CK-MM) concentrations in serum, plasma, tissue homogenates.
Transport and Mailing Systems
 Transport and Mailing Systems For transportation of diagnostic samples and blood products The complete product range for mailing and transport requirements Safe and compliant with transportation guidelines
Transport and Mailing Systems For transportation of diagnostic samples and blood products The complete product range for mailing and transport requirements Safe and compliant with transportation guidelines
Appendix H IBC Managing Biohazardous Waste SOP
 Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Chromatin Immunoprecipitation (ChIP)
 Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method)
 Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
APPENDIX D INFECTIOUS WASTE MANAGEMENT GUIDE
 APPENDIX D INFECTIOUS WASTE MANAGEMENT GUIDE TABLE OF CONTENTS INTRODUCTION... PAGE 1 SUMMARY OF REGULATIONS... PAGE 1 WRIGHT STATE UNIVERSITY INFECTIOUS WASTE STRATEGY... PAGE 5 PROCEDURES FOR WSU INFECTIOUS
APPENDIX D INFECTIOUS WASTE MANAGEMENT GUIDE TABLE OF CONTENTS INTRODUCTION... PAGE 1 SUMMARY OF REGULATIONS... PAGE 1 WRIGHT STATE UNIVERSITY INFECTIOUS WASTE STRATEGY... PAGE 5 PROCEDURES FOR WSU INFECTIOUS
Instructions for Drawing Blood Samples
 Instructions for Drawing Blood Samples For Blood Collection Supplies please contact us. *Whatever means of restraint is used it should cause minimal distress and should be quick and safe for both the animal
Instructions for Drawing Blood Samples For Blood Collection Supplies please contact us. *Whatever means of restraint is used it should cause minimal distress and should be quick and safe for both the animal
Instructions for Abroad Clinical Rotation
 Revised 7/12 Instructions for Abroad Clinical Rotation While travelling abroad you may have limited access to medical care. These instructions help prepare you for a blood/ body fluid exposure. Timely
Revised 7/12 Instructions for Abroad Clinical Rotation While travelling abroad you may have limited access to medical care. These instructions help prepare you for a blood/ body fluid exposure. Timely
Policy for the Disposal of Biological Waste
 Policy for the Disposal of Biological Waste I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 24 Street 1603 Building 4127, Livingston Campus Piscataway,
Policy for the Disposal of Biological Waste I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 24 Street 1603 Building 4127, Livingston Campus Piscataway,
Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit
 Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit Catalog No. CSB-E15852c (96T) This immunoassay kit allows for the in vitro quantitative determination of canine CK-MB concentrations in serum and plasma.
Canine creatine kinase MB isoenzyme (CK-MB)ELISA Kit Catalog No. CSB-E15852c (96T) This immunoassay kit allows for the in vitro quantitative determination of canine CK-MB concentrations in serum and plasma.
6 Body Fluid Stains and Standards
 6 Body Fluid Stains and Standards Laboratory examination of body fluids (i.e., blood, semen, saliva, etc.) may produce significant information in certain investigations. This chapter considers the recognition,
6 Body Fluid Stains and Standards Laboratory examination of body fluids (i.e., blood, semen, saliva, etc.) may produce significant information in certain investigations. This chapter considers the recognition,
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA. kit
 BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
BlueGene Biotech. Tel: 0086-21-61471242 Fax: 0086-21-61471242 ext 806 E-mail: sales@bluegene.cc tech@bluegene.cc www.elisakit.cc www.bluegene.cc Canine Creatine Kinase MM isoenzyme(ck-mm) ELISA kit 96
UMBILICAL CORD BLOOD COLLECTION
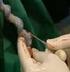 UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
UMBILICAL CORD BLOOD COLLECTION by Frances Verter, PhD Founder & Director, Parent's Guide to Cord Blood Foundation info@parentsguidecordblood.org and Kim Petrella, RN Department of Obstetrics and Gynecology
RealStar HBV PCR Kit 1.0 11/2012
 RealStar HBV PCR Kit 1.0 11/2012 RealStar HBV PCR Kit 1.0 For research use only! (RUO) Product No.: 201003 96 rxns INS-201000-GB-02 Store at -25 C... -15 C November 2012 altona Diagnostics GmbH Mörkenstraße
RealStar HBV PCR Kit 1.0 11/2012 RealStar HBV PCR Kit 1.0 For research use only! (RUO) Product No.: 201003 96 rxns INS-201000-GB-02 Store at -25 C... -15 C November 2012 altona Diagnostics GmbH Mörkenstraße
Archived. Gloves should be changed frequently during the analysis.
 Introduction Gloves and laboratory coats Small tools Specific clean-up and housekeeping procedures are used to help protect evidence samples from conditions and agents that might serve to destroy, deteriorate,
Introduction Gloves and laboratory coats Small tools Specific clean-up and housekeeping procedures are used to help protect evidence samples from conditions and agents that might serve to destroy, deteriorate,
KGI MEDICAL WASTE MANAGEMENT PLAN
 1revised 2012 I. Facility Information KGI MEDICAL WASTE MANAGEMENT PLAN a. Contact Person: Barbara Erwin Director Research Operations/Chemical and Biological Laboratory Safety/Chair. 909-607-0160 i. Facility:
1revised 2012 I. Facility Information KGI MEDICAL WASTE MANAGEMENT PLAN a. Contact Person: Barbara Erwin Director Research Operations/Chemical and Biological Laboratory Safety/Chair. 909-607-0160 i. Facility:
URINE COLLECTION, PREPARATION AND HANDLING
 URINE III-38 URINE COLLECTION, PREPARATION AND HANDLING I. SPECIMEN COLLECTION A. Introduction Laboratory tests requiring urine specimens involve a wide variety of procedures. A basic urinalysis is almost
URINE III-38 URINE COLLECTION, PREPARATION AND HANDLING I. SPECIMEN COLLECTION A. Introduction Laboratory tests requiring urine specimens involve a wide variety of procedures. A basic urinalysis is almost
Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit
 Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit Catalog No. MBS702810 (96 tests) This immunoassay kit allows for the in vitro semi-quantitative determination of mouse KLH(IgM)antibody concentrations
Mouse Keyhole Limpet Hemocyanin antibody(igm) ELISA Kit Catalog No. MBS702810 (96 tests) This immunoassay kit allows for the in vitro semi-quantitative determination of mouse KLH(IgM)antibody concentrations
Safe Blood Sampling Training Package
 Better Blood Transfusion - Education Programme Safe Blood Sampling Training Package SBS Training Package version 2010 to SNBTS www.learnbloodtransfusion.org.uk Learning Outcomes Following this session
Better Blood Transfusion - Education Programme Safe Blood Sampling Training Package SBS Training Package version 2010 to SNBTS www.learnbloodtransfusion.org.uk Learning Outcomes Following this session
Cord Blood Collections for the Texas Cord Blood Bank. Obstetrical Providers Training Module
 Cord Blood Collections for the Texas Cord Blood Bank Obstetrical Providers Training Module The Texas Cord Blood Bank The Texas Cord Blood Bank is a network of maternity hospitals and a central laboratory
Cord Blood Collections for the Texas Cord Blood Bank Obstetrical Providers Training Module The Texas Cord Blood Bank The Texas Cord Blood Bank is a network of maternity hospitals and a central laboratory
REGULATED MEDICAL WASTE MANAGEMENT. Title 15, Article 27, Environmental Conservation Law (DEC)
 REGULATED MEDICAL WASTE MANAGEMENT Medical Waste Laws/Regulations: The definitions of medical waste, requirements for handling, disposal and tracking are primarily determined by: Title XIII, Article 13,
REGULATED MEDICAL WASTE MANAGEMENT Medical Waste Laws/Regulations: The definitions of medical waste, requirements for handling, disposal and tracking are primarily determined by: Title XIII, Article 13,
STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH. Research Centre for Women s and Infants Health (RCWIH) BioBank
 STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH Research Centre for Women s and Infants Health (RCWIH) BioBank Mount Sinai Hospital Samuel Lunenfeld Research Institute
STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH Research Centre for Women s and Infants Health (RCWIH) BioBank Mount Sinai Hospital Samuel Lunenfeld Research Institute
Appendix H Managing Biohazardous Waste SOP
 Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management. Part A. Biological Waste Management Safety Plan
 The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management Part A Biological Waste Management Safety Plan i. SIGNATURE PAGE This Biological Waste Management Safety
The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management Part A Biological Waste Management Safety Plan i. SIGNATURE PAGE This Biological Waste Management Safety
Percentage of the Medical Waste Stream That Is Regulated Medical Waste Microbiological Waste Pathological Waste Blood and Body Fluids
 Percentage of the Medical Waste Stream That Is Regulated Medical Waste Most medical waste may be handled as general solid waste and does not require treatment. Regulated medical waste makes up only a very
Percentage of the Medical Waste Stream That Is Regulated Medical Waste Most medical waste may be handled as general solid waste and does not require treatment. Regulated medical waste makes up only a very
Annual Biomedical Waste Code Training
 Annual Biomedical Waste Code Training Provided by: Barbara D. Will, MPH Biomedical Waste Program Supervisor To protect, promote and improve the health of all people in Florida through integrated state,
Annual Biomedical Waste Code Training Provided by: Barbara D. Will, MPH Biomedical Waste Program Supervisor To protect, promote and improve the health of all people in Florida through integrated state,
VialSafe. DHL VialSafe. UN3373 Packaging Solutions
 VialSafe DHL VialSafe UN3373 Packaging Solutions The DHL VialSafe pack is a complete packaging system certified to meet the strict requirements of ICAO/IATA packing instructions PI650 for the safe transportation
VialSafe DHL VialSafe UN3373 Packaging Solutions The DHL VialSafe pack is a complete packaging system certified to meet the strict requirements of ICAO/IATA packing instructions PI650 for the safe transportation
Gravity Bag Cord Blood Collection Method Training Manual
 Gravity Bag Cord Blood Collection Method Training Manual CordBank Ltd. Training Manual Page 1 of 13 Table of Contents Chapter Page Introduction 3 Requirements for Collection of Cord Blood 3 Collector s
Gravity Bag Cord Blood Collection Method Training Manual CordBank Ltd. Training Manual Page 1 of 13 Table of Contents Chapter Page Introduction 3 Requirements for Collection of Cord Blood 3 Collector s
BLOOD BANK Department
 BLOOD BANK Department INTRODUCTION Blood products used at Saint Michael s Hospital are obtained from the BloodCenter of Wisconsin. There is no charge for the blood, but there is a fee for testing and processing
BLOOD BANK Department INTRODUCTION Blood products used at Saint Michael s Hospital are obtained from the BloodCenter of Wisconsin. There is no charge for the blood, but there is a fee for testing and processing
Bloodborne Pathogens Program Revised July, 5 2012
 Bloodborne Pathogens Program Revised July, 5 2012 Page 1 of 16 Table of Contents 1.0 INTRODUCTION...3 1.1 Purpose...3 1.2 Policy.3 2.0 EXPOSURE CONTROL METHODS 4 2.1 Universal Precautions.4 2.2 Engineering
Bloodborne Pathogens Program Revised July, 5 2012 Page 1 of 16 Table of Contents 1.0 INTRODUCTION...3 1.1 Purpose...3 1.2 Policy.3 2.0 EXPOSURE CONTROL METHODS 4 2.1 Universal Precautions.4 2.2 Engineering
General Information. Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations
 General Information Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations Our Background Foundation Laboratory is an advanced, accredited and certified clinical diagnostic
General Information Our Background Automated Phone Services Holiday Coverage Licenses & Accreditations Our Background Foundation Laboratory is an advanced, accredited and certified clinical diagnostic
Animal Health Diagnostic Center. Test & Fee Schedule
 Animal Health Diagnostic Center Test & Fee Schedule and Submission Guidelines Documents included within this manual are the most up to date versions available at the time of printing. For the most current
Animal Health Diagnostic Center Test & Fee Schedule and Submission Guidelines Documents included within this manual are the most up to date versions available at the time of printing. For the most current
2.3. The management in each HCF shall be responsible for ensuring good waste management practices in their premises.
 1. PURPOSE Health-care activities lead to production of medical waste that may lead to adverse health effects. Most of this waste is not more dangerous than regular household waste. However, some types
1. PURPOSE Health-care activities lead to production of medical waste that may lead to adverse health effects. Most of this waste is not more dangerous than regular household waste. However, some types
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs)
 HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
HARVESTING AND CRYOPRESERVATION OF HUMAN EMBRYONIC STEM CELLS (hescs) OBJECTIVE: can be cryopreserved in a liquid nitrogen (LN 2 ) freezer for long-term storage. This Standard Operating Procedure (SOP)
STANDARD OPERATING PROCEDURE
 TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
History Note:Statutory Authority G.S. 130A-309.26; Eff. Oct. 1, 1990; Amended Eff. April 1, 1993.
 15A NCAC 13B.1200 SECTION.1200 - MEDICAL WASTE MANAGEMENT 1201 DEFINITIONS For the purpose of the Section, the following definitions apply: (1) "Blood and body fluids" means liquid blood, serum, plasma,
15A NCAC 13B.1200 SECTION.1200 - MEDICAL WASTE MANAGEMENT 1201 DEFINITIONS For the purpose of the Section, the following definitions apply: (1) "Blood and body fluids" means liquid blood, serum, plasma,
Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA
 Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA For the quantitative determination of rat CKMB in serum, plasma, tissue homogenates and other biological fluids. Cat. No. KT-12247 For Research Use Only. Not
Rat Creatine Kinase MB Isoenzyme (CKMB) ELISA For the quantitative determination of rat CKMB in serum, plasma, tissue homogenates and other biological fluids. Cat. No. KT-12247 For Research Use Only. Not
STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH
 STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH Research Centre for Women s and Infants Health (RCWIH) BioBank Mount Sinai Hospital Abbreviated Version 4.0 January
STANDARD OPERATING PROCEDURES FOR THE COLLECTION OF PERINATAL SPECIMENS FOR RESEARCH Research Centre for Women s and Infants Health (RCWIH) BioBank Mount Sinai Hospital Abbreviated Version 4.0 January
How To Store An Acam2000 Vaccine
 ACAM2000 Smallpox (Vaccinia) Vaccine, Live Deployment Guidance - Acambis Inc. Revision: October 2008 Information contained in this guidance will also be available through Acambis, in the event that questions,
ACAM2000 Smallpox (Vaccinia) Vaccine, Live Deployment Guidance - Acambis Inc. Revision: October 2008 Information contained in this guidance will also be available through Acambis, in the event that questions,
DRAFT Standard Operating Procedure for Long-Term Archiving of PM 2.5 Filters and Extracts
 Page 1 of 6 DRAFT Standard Operating Procedure for Long-Term Archiving of PM 2.5 Filters and Extracts Environmental and Industrial Sciences Division Research Triangle Institute Research Triangle Park,
Page 1 of 6 DRAFT Standard Operating Procedure for Long-Term Archiving of PM 2.5 Filters and Extracts Environmental and Industrial Sciences Division Research Triangle Institute Research Triangle Park,
Malondialdehyde (MDA) ELISA
 Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
Malondialdehyde (MDA) ELISA For the quantitative determination of MDA in serum, plasma and other biological fluids Cat. No. KT-21493 For Research Use Only. Not for use in diagnostic procedures. Page 1
MANITOBA PATIENT SERVICE CENTRE STANDARDS
 MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
HBV Quantitative Real Time PCR Kit
 Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Reagents Quantity Reagents Quantity. Pre-coated, ready to use 96-well strip plate 1 Plate sealer for 96 wells 4. Standard 2 Standard Diluent 1 20mL
 FOR IN VITRO AND RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES SEA479Ra 96 Tests Enzyme-linked Immunosorbent Assay Kit For Creatine Kinase MB Isoenzyme (CKMB) Organism Species: Rattus
FOR IN VITRO AND RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES SEA479Ra 96 Tests Enzyme-linked Immunosorbent Assay Kit For Creatine Kinase MB Isoenzyme (CKMB) Organism Species: Rattus
