Development of a Biomolecular Assay for Postmortem Diagnosis of Taenia saginata Cysticercosis
|
|
|
- Allen Campbell
- 8 years ago
- Views:
Transcription
1 FOODBORNE PATHOGENS AND DISEASE Volume 7, Number 10, 2010 ª Mary Ann Liebert, Inc. DOI: =fpd Original Article Development of a Biomolecular Assay for Postmortem Diagnosis of Taenia saginata Cysticercosis Francesco Chiesa, 1 Alessandra Dalmasso, 1 Alberto Bellio, 1 Manuela Martinetti, 2 Stefano Gili, 2 and Tiziana Civera 1 Abstract Bovine cysticercosis is caused by the larval stage of the human tapeworm Taenia saginata. According to European data on meat inspection, the prevalence ranges from 0.007% to 6.8%, but the real prevalence is considered to be at least 10 times higher. Laboratory confirmation of the etiological agent is based on gross, stereomicroscopic, and histological examination of submitted specimens. False identifications may occur, possibly because of death and degeneration of cysts, or because taeniid larvae and other tissue parasites, such as Sarcocystis spp., may cause similar macroscopic morphological lesions. Therefore, tests that can warrant sure identification of taeniid lesions and calcified cysts in the muscle are needed. The focus of our study was to develop a suitable postmortem test that could be applied on putative lesions by T. saginata cysticerci, as ambiguously diagnosed after routine meat inspection. In particular, we proposed a biomolecular assay targeting the mitochondrial cytochrome c oxidase subunit I gene (COI). For developing the polymerase chain reaction assay, viable cysts of Cysticercus bovis (n ¼ 10) were used as positive reference samples, and those of Echinococcus granulosus (n ¼ 3), Cysticercus tenuicollis (n ¼ 3), and Sarcocystis spp. (n ¼ 4) as reference negative controls. Further, to evaluate the applicability of the proposed assay, 171 samples of bovine muscular tissue, obtained from local slaughterhouses and containing lesions recognized as T. saginata cysticerci by macroscopic examination, were tested. The proposed test confirmed the diagnosis at postmortem inspection in 94.7% (162=171) of samples. In conclusion, the assay developed in this study, amplifying a short fragment from the mitochondrial gene COI, showed to be suitable for samples containing both viable and degenerating T. saginata cysticerci, yielding an unequivocal diagnosis. Introduction Bovine cysticercosis is caused by the larval stage (metacestode) of the human tapeworm Taenia saginata, formerly defined as Cysticercus bovis. Intermediate and final hosts are represented by cattle and humans, respectively. Bovine cysticercosis is caused by the infestation and development of cysticerci of T. saginata in the muscle tissue of cattle (Pawlowski and Schultz, 1972; Murrell and Dorny, 2005). The eggs reach the animals through contact with contaminated materials or proglottids; after ingestion, the oncospheres can penetrate the intestinal wall and travel via blood to striated muscles (Abuseir et al., 2006). Cysts are prevalently found in predilection sites such as the masseter muscles, heart, tongue, and the muscles of the shoulder and diaphragm, although they could also be found in other sites and organs (Minozzo et al., 2002; Wilson and Wilson, 2005). The longevity of the cysts ranges from weeks to years (Urquhart et al., 1998), and after their death they are usually replaced by a caseous, crumby mass, which may become calcified (McGavin et al., 2001). Both living and dead cysts may be found in the same carcass (Gracey et al., 1999). Life cycle is completed with infestation in humans, which may develop taeniosis after consumption of raw or undercooked beef containing viable T. saginata metacestodes (Dorny et al., 2000). As taeniosis is not a notifiable disease, the incidence is usually estimated from the sale of specific drugs, and prevalence rates ranging from 0.01% to 10% have been reported in Europe (Cabaret et al., 2002). Regarding bovine cysticercosis, the prevalence ranges from 0.007% to 6.8% according to European meat inspection data (Pawlowski and Schultz, 1972; Cabaret et al., 2002), but the real prevalence is considered to be at least 10 times higher (Onyango-Abuje et al., 1996; Dorny et al., 2000). According to the Regulation (EC) No. 854=2004, to determine and, if necessary, assess the level of cysticercosis infestation, all bovines of over 6 weeks of age have to be inspected by incision and visual examination of the masseter muscles 1 Department of Animal Pathology, Faculty of Veterinary Medicine, University of Turin, Grugliasco (TO), Italy. 2 Veterinary Service ASL TO1, Turin, Italy. 1
2 2 CHIESA ET AL. and heart. In the case of generalized infestation, the carcass and offal are declared unfit for human consumption but, if the infestation is localized, the parts not infected may be declared fit for human consumption after having undergone a cold treatment (Regulation (EC) No. 854=2004). During the routine meat inspection, the diagnosis of T. saginata cysts is based on the morphological appearance. Any alteration with identifiable cyst and fluid filled, cheesy, or calcified content found in heart or masseter muscles of slaughtered cattle is assumed to be T. saginata (Murrell and Dorny, 2005). Laboratory confirmation of the etiological agent is based on gross, stereomicroscopic, and histological examination of submitted specimens (Ogunremi et al., 2004). False identifications may occur, possibly because of death and degeneration of cysts, or because taeniid larvae and other tissue parasites, such as Sarcocystis spp., may cause similar macroscopic morphological lesions. Therefore, tests that can warrant sure identification of taeniid lesions and calcified cysts in the muscle are needed (Harrison et al., 2005). Different improved postmortem diagnostic methods have been proposed, such as ELISA methods, detecting antigens in meat juice (Abuseir et al., 2007), immunohistochemical techniques, using the avidin biotin complex immunohistochemical method (Ogunremi et al., 2004), and biomolecular assays (Gottstein et al., 1991; Abuseir et al., 2006; Geysen et al., 2007). DNA-sequence-based primers for the diagnosis of T. saginata have been designed mainly to distinguish between taeniosis caused by T. saginata or by Taenia solium, also considering the severity of T. solium infections in humans. The use of polymerase chain reaction (PCR) to confirm or reject the morphological diagnosis of T. saginata metacestodes has been described, but an adequate evaluation of the reliability of these used methods is still lacking (SCVPH, 2000; van der Logt and Gottstein, 2000; Geysen et al., 2007). The focus of our study was to develop an appropriate postmortem test that could be applied on lesions suspected to be T. saginata cysticerci, ambiguously diagnosed after routine meat inspection. In particular, a biomolecular assay targeting the mitochondrial cytochrome c oxidase subunit I gene (COI) was developed. Materials and Methods Samples For the development of the PCR assay, 10 viable cysts of T. saginata metacestodes were used as positive reference samples. Reference negative controls were represented by three samples of Echinococcus granulosus, three of Cysticercus tenuicollis, and four of Sarcocystis spp. To evaluate the applicability of the proposed assay, 171 samples of bovine muscular tissue, containing T. saginata cysticerci diagnosed by macroscopic examination, were tested. In particular, these samples, consisting of portions of miocardic and masseter muscles, were collected from local slaughterhouses during routine meat inspection procedures from March 2008 to July As indicated by Abuseir et al. (2006), cysts were examinated macroscopically and classified as (1) viable when they contained protoscolex, (2) calcified with solid, crumby content, cheesy with soft content, and (3) dull when they appeared neither viable nor degenerating and no obvious cyst structure (wall and subsequent layers) was macroscopically detectable. DNA extraction For each sample, up to 25 (5) mg of material was collected from a single excised cyst; effort was made to collect only the content of the cyst, avoiding the cyst wall to reduce the amount of nontarget bovine DNA. When viable cysts were analyzed, only protoscolex was taken. DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) was used for DNA extraction from all samples. Some minor modification in the protocols consisted of an overnight incubation with Proteinase K and a decrease in the volume of the final elution step (100 ml). Extracted DNA was quantified with a NanoDrop 2000 spectrophotometer (ThermoScientific, Wilmington, NC). Primers For the specific amplification of T. saginata, the forward primer (Tsag) designed by Yamasaki et al. (2004) was used in conjunction with a newly designed reverse primer. For this purpose, the COI gene sequences of several T. saginata, available in the GenBank (AB066495, AB AB107247, AB271695, AB275143, AB AB465248, and AY195858), were aligned using ClustalV software (Higgins et al., 1992). Moreover, the sequences of other Taeniidae species (T. solium, Taenia hydatigena, Taenia crassiceps, and E. granulosus) were included in the alignment to evaluate possible cross reactions. Reverse COI primer was designed as follows: 5 0 -ACGT AAATAAATAAGCCCACAATATT-3 0, matching positions of T. saginata sequence (GenBank accession no. AB465248). Finally, based on the previously produced alignment, a set of internal primers were designed to develop a nested PCR approach. Nested COI primers were designed as follows: 5 0 -GGGTGCTGGTATAGGGTGGACT-3 0 (forward) and 5 0 -ATTAATAGAACTAAAAATTCTAGACG-3 0 (reverse), corresponding to positions and of T. saginata (GenBank accession no. AB465248), respectively (Fig. 1). For all the samples that showed negative result with the PCR assays described above, further tests were carried out to verify (1) positivity for Sarcocystis spp. with genus-specific primers (Vangeel et al., 2007); (2) misdiagnosis with the larval stage of cestodes other than T. saginata, using specific primer sets for Taeniidae (von Nickisch-Rosenegk et al., 1999); and (3) the presence of detectable DNA and the absence of PCR inhibitors, by means of bovine-specific primers (Bottero et al., 2003). The primers were synthesized by Sigma Aldrich (St. Louis, MO). PCR procedure Both primers for the COI-PCR and the nested PCR were carried out in a final volume of 25 ml containing 20 mm Tris HCl (ph 8.4); 50 mm KCl; 0.5 U of recombinant Taq DNA polymerase (Invitrogen, Paisley, United Kingdom); 0.2 mm each of datp, dctp, dgtp, and dttp (Invitrogen); 2 mm di MgCl 2 ; and 12.5 pmol of primers. DNA was added with a concentration of 100 ng=ml for the COI-PCR, and 2.5 ml of amplification product for the nested PCR. COI-PCR amplification was performed with an initial step of 948C for 5 min, followed by 20 cycles of 948C for 30 sec, with annealing temperatures starting at 658C for 1.30 min
3 PCR FOR DIAGNOSIS OF T. SAGINATA CYSTICERCOSIS 3 FIG. 1. Polymerase chain reaction strategy outline. (decreasing 0.58C=cycle) and 728C for 30 sec for extension. This step was followed by 20 cycles of 948C for 30 sec, 558C for 1.30 min, 728C for 30 sec, and finally 728C for 5 min. Nested PCR conditions consisted of an initial denaturation at 948C for 5 min, followed by 35 cycle of 948C for 30 sec, 1.30 min annealing at 548C, and 30 sec extension at 728C. The final extension was carried out at 728C for 5 min. Bovine, Sarcocystis spp., and taeniid species-specific PCR assays were performed following the author instructions (von Nickisch-Rosenegk et al., 1999; Bottero et al., 2003; Vangeel et al., 2007). Amplimers were resolved by electrophoresis on a 2% agarose gel (Invitrogen), run in Tris acetate ethylenediaminetetraacetic acid buffer for 70 min at 110 V, and stained with ethidium bromide (0.4 ng=ml) for 20 min. Confirmatory sequencing of the COI and nested-pcramplified fragments was carried out for all samples. Amplified products were purified with Exo-Sap treatment according to the manufacturer s recommendations (USB Europe, Staufen, Germany). Forward and reverse sequencing reactions were performed using ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit, version 1.1 (Applied Biosystems, Foster City, CA). Sequenced fragments were purified by DyeEX (Qiagen) and resolved by capillary electrophoresis using an ABI 310 Genetic Analyser (Applied Biosystems). The nucleotide sequences were analyzed using the BLASTN sequence similarity search at the NCBI database (Altschul et al., 1990). Results and Discussion Several different diagnostic tests have been described to support visual diagnosis of T. saginata cysticercosis. Serological methods, such as detecting circulating antigens or antibodies, both in live and slaughtered animals (Dorny et al., 2000; Abuseir et al., 2007) have been studied. However, this technique failed to detect many light infections (Van Kerckhoven et al., 1998), and the possibility of cross reaction with other parasites indicates the need for further investigation (Brandt et al., 1992). Also, an immunohistochemical method has been proposed as a promising alternative by Ogunremi et al. (2004), but results show that cross reaction with other taeniid species may occur. Therefore, histological evaluation of T. saginata cysticercisuspect lesions is still considered the only laboratory diagnostic tool suitable for routine confirmation of undiagnosed lesions during inspection procedures at slaughterhouse. The morphology and cellularity of these putative lesions can be used to distinguish them from other commonly encountered and somewhat similar lesions. Demonstration of a cysticercus-specific host structure is therefore necessary for a definitive diagnosis (Silverman and Hulland, 1961), but an accurate identification cannot always be made, as such features are often not obvious in degenerated specimens and especially if the sample contains calcified tissues (Schandevyl and Vercruysse, 1982). Molecular diagnosis has therefore been considered, considering the high sensitivity, objectivity, and rapidity of most molecular tools. Protocols using multiplex PCR for the molecular differentiation of adult T. saginata, T. saginata asiatica, and T. solium have been developed and successfully applied (Gonzalez et al., 2000; González et al., 2004). However, these same primers yielded unreliable results on both viable and degenerated bovine cysticerci when tested on naturally infected cattle (Abuseir et al., 2006). Geysen et al. (2007) designed a PCR assay, targeting mitochondrial DNA, that was able to amplify the expected fragment (846 bp) in 97% of viable cysts, but only in 60% of calcified specimens. These results have been partially explained by authors with the lacking of amplifiable DNA in positive but degenerated specimens. The onset of degeneration in mature cysts depends on the inflammatory response of the host but generally consists of the breakdown of the cyst wall and cuticle, followed by the disappearance of following layers and eventually in the dissolution of parasite remnants (Silverman and Hulland, 1961). Detectable DNA is therefore likely to be present in a degraded form and in low amounts. Several studies suggested that degraded DNA can still be amplified by PCR if the primers are designed so to recognize and amplify a shorter target sequence, between 100 and 300 bp, preferably of mitochondrial genes (Dalmasso et al., 2004; Hui, 2006). In fact, the circular structure of mtdna has been shown to have a superior preservation to degradation, and because of its presence in multicopy, detection of target DNA is possible even from a small amount of starting material (Fondevila et al., 2008). On the basis of these considerations, a primer set targeting the mitochondrial COI gene was chosen, based on the literature (Yamasaki et al., 2004). The COI gene has been particularly targeted for phylogenetic studies, and thus several sequences are available in nucleotide databases. In particular, in this study a new reverse primer was designed to amplify a shorter fragment. Specificity tests were conducted and all positive reference samples yielded amplicons of the expected size (253 bp). In addition, no cross reaction was observed with samples of phylogenetically correlated species that could affect cattle,
4 4 CHIESA ET AL. FIG. 2. Specificity of polymerase chain reaction amplification of the COI gene of Taenia saginata cysticerci in beef. Lanes 1 5, DNA extracted from viable T. saginata cysticerci; lane 6, Echinococcus granulosus; lane 7, Taenia hydatigena; lane 8, Sarcocystis spp.; lane 9, reagents control; M, 100-bp ladder. Cyst stage Table 1. Results of Applicability Test No. of samples COI- PCR Positive samples Nested PCR Sarcocystis spp. Viable Cheesy Calcified Dull a Total a Two samples negative to all the test performed were positive for the presence of bovine DNA, demonstrating the absence of PCR inhibition. PCR, polymerase chain reaction. such as E. granulosus and T. hydatigena (Euzéby, 1998), and with samples of a coccidian protozoa, such as Sarcocystis spp., that can cause muscular lesions easily misidentified as T. saginata cysticerci (González et al., 2006) (Fig. 2). Further, the specificity of the amplified fragments was confirmed when nucleotide sequences of all reference strains were submitted to BLASTN sequence similarity search (Altschul et al., 1990). As regard to assay applicability, the number of samples that yielded a positive result to COI-PCR was 154 out of 171 (Table 1). When different stages of degeneration were taken in account, differences were observed as resulted positive 11 out of 12 viable cysts, 42 out of 45 cheesy cysts, 93 out of 101 calcified cysts, and 8 out 13 dull cysts, thus confirming greater difficulties in identification when degraded samples were tested (Abuseir et al., 2006; Geysen et al., 2007). To improve the sensitivity of the assay, as suggested by previous studies (Yamasaki et al., 2006; Geysen et al., 2007), we designed two internal primers on the amplified COI and developed a nested approach, which has been used to test negative samples. The nested PCR approach allowed the identification of 8 more samples as positive among the 17 formerly tested as negative. Results have been confirmed by sequencing of specific fragments. For the remaining nine negative samples, seven resulted positives to the specific PCR for the identification of Sarcocystis spp., confirming the possibility of misdiagnosis between degenerating T. saginata cysticerci and lesions caused by Sarcocystis spp. (González et al., 2006). The two remaining negative samples also gave negative results when further analyzed with a specific PCR for taeniid species, and used to determine if the misdiagnosed lesions could be ascribed to the presence of erratic cyst belonging to species other than T. saginata (Euzéby, 1998). These two samples were subsequently tested with a PCR targeting bovine DNA, and gave positive results, demonstrating the presence of detectable DNA and the absence of PCR inhibitors (van der Logt and Gottstein, 2000; Abuseir et al., 2006). These findings suggest that nonspecific lesions can be possibly misdiagnosed as parasitic (Pawlowski and Schultz, 1972; Gracey et al., 1999; van der Logt and Gottstein, 2000). Overall, the proposed test was able to confirm the diagnosis made at postmortem inspection in 94.7% (162=171) of samples, and when considering degenerating specimens, in 94.3% (150=159) of samples. These findings underline the robustness of the developed test and showed its suitability for the identification of degraded cysticerci, which proved to be the main issue to overcome (Abuseir et al., 2006; Geysen et al., 2007). Meat inspection of bovines is the only public health measure performed to prevent T. saginata from entering the food chain (Dorny et al., 2000). The main shortcomings of routine meat inspection may be found in its low sensitivity and that its success heavily depends on the expertise of the inspector. Moreover, the stage of development of the cyst has to be taken into careful consideration because it is difficult to distinguish between old lesions caused by cysticerci and other lesions (Geysen et al., 2007). The assay developed in this study, amplifying a short fragment from the mitochondrial COI gene, yielded an unequivocal diagnosis, showing to be species specific and suitable both for samples containing viable and degenerating T. saginata cysticerci. Rapidity and objectivity in the interpretation of results prove this molecular assay to be a useful, high-throughput laboratory tool for the confirmation of macroscopic diagnosis of ambiguous lesions. Further studies, comparing the proposed method with histological and immunohistochemical techniques, would be of great importance to assess the full performance potential of the developed COI-PCR for the identification of T. saginata cysticerci, assessing the actual sensitivity and specificity of the assay. Acknowledgments Our special thank goes to Dr. M. Tursi for his technical support and advises during the classification of the lesions examined during the study and to Dr. S. Lomonaco for her support and suggestions during the revision process. Disclosure Statement No competing financial interests exist. References Abuseir S, Epe C, Schnieder T, Klein G, and Kühne M. Visual diagnosis of Taenia saginata cysticercosis during meat inspection: is it unequivocal? Parasitol Res 2006;99:
5 PCR FOR DIAGNOSIS OF T. SAGINATA CYSTICERCOSIS 5 Abuseir S, Kühne M, Schnieder T, Klein G, and Epe C. Evaluation of a serological method for the detection of Taenia saginata cysticercosis using serum and meat juice samples. Parasitol Res 2007;101: Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ. Basic local alignment search tool. J Mol Biol 1990;215: Bottero MT, Civera T, Nucera D, Rosati S, Sacchi P, and Turi RM. A multiplex polymerase chain reaction for the identification of cows, goats and sheep s milk in dairy products. Int Dairy J 2003;13: Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibodybased ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 1992;22: Cabaret J, Geerts S, Madeline M, Ballandonne C, and Barbier D. The use of urban sewage sludge on pastures: the cysticercosis threat. Vet Res 2002;33: Dalmasso A, Fontanella E, Piatti P, Civera T, Rosati S, and Bottero MT. A multiplex PCR assay for the identification of animal species in feedstuffs. Mol Cell Probes 2004;18: Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D, and Geerts S. Sero-epidemiological study of Taenia saginata cysticercosis in Belgian cattle. Vet Parasitol 2000;88: Euzéby J. Les parasites des viandes. Lavoisier (In French.) Fondevila M, Phillips C, Naverán N, et al. Challenging DNA: assessment of a range of genotyping approaches for highly degraded forensic samples. Forensic Sci Int Genet Suppl Ser 2008;1: Geysen D, Kanobana K, Victor B, et al. Validation of meat inspection results for Taenia saginata cysticercosis by PCRrestriction fragment length polymorphism. J Food Prot 2007; 70: Gonzalez LM, Montero E, Harrison LJS, Parkhouse RME, and Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol 2000;38: González LM, Montero E, Morakote N, et al. Differential diagnosis of Taenia saginata and Taenia saginata asiatica taeniasis through PCR. Diagn Microbiol Infect Dis 2004;49: González LM, Villalobos N, Montero E, et al. Differential molecular identification of Taeniid spp. and Sarcocystis spp. cysts isolated from infected pigs and cattle. Vet Parasitol 2006;142: Gottstein B, Deplazes P, Tanner I, and Skaggs JS. Diagnostic identification of Taenia saginata with the polymerase chain reaction. Trans R Soc Trop Med Hyg 1991;85: Gracey J, Collins DS, and Huey R. Diseases caused by helminth and arthropod parasites. In: Meat Hygiene. GraceyJ,CollinsDS, and Huey R (eds.). London: Harcourt Brace and Company Limited, 1999, pp Harrison LJS, Garate T, Bryce DM, et al. Ag-ELISA and PCR for monitoring the vaccination of cattle against Taenia saginata cysticercosis using an oncospheral adhesion protein (HP6) with surface and secreted localization. Trop Anim Health Prod 2005;37: Higgins DG, Bleasby AJ, and Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci 1992;8: Hui YH. Handbook of Food Science, Technology, and Engineering, Volume 4. New York: Taylor & Francis Group, McGavin MD, Carlton WW, and Zachary JF. Thomson s Special Veterinary Pathology. St. Louis: Mosby, Minozzo JC, Gusso RLF, Castro EA, Lago O, and Soccol VT. Experimental bovine infection with Taenia saginata eggs: recovery rates and cysticerci location. Braz Arch Biol Technol 2002;45: Murrell KD and Dorny P. WHO=FAO=OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis=Cysticercosis. OIE, Available at ftp:==ftp.fao.org=docrep=fao=011= aj005e=aj005e.pdf, accessed April 22, Ogunremi O, MacDonald G, Geerts S, and Brandt J. Diagnosis of Taenia saginata cysticercosis by immunohistochemical test on formalin-fixed and paraffin-embedded bovine lesions. J Vet Diagn Invest 2004;16: Onyango-Abuje JA, Hughes G, Opicha M, et al. Diagnosis of Taenia saginata cysticercosis in Kenyan cattle by antibody and antigen ELISA. Vet Parasitol 1996;61: Pawlowski Z and Schultz MG. Taeniasis and cysticercosis (Taenia saginata). Adv Parasitol 1972;10: Regulation (EC) No. 854=2004. Regulation of the European Parliament and the Council of 29 April 2004 laying down specific rules for the organization of official controls of products of animal origin intended for human consumption. Available at accessed November 10, (Online.) Schandevyl P and Vercruysse J. Cysticercosis in cattle in Senegal. Vet Parasitol 1982;11: [SCVPH] Scientific Committee on Veterinary Measures relating to Public Health. Opinion on the control of taeniosis= cysticercosis in man and animals, Available at ec.europa.ed=food=fs=sc=scv=out36_en.pdf, accessed April 22, Silverman PH and Hulland TJ. Histological observations on bovine cysticercosis. Res Vet Sci 1961;2: Urquhart GM, Armour J, Duncan JL, Dunn AM, and Jennings FW. Veterinary Parasitology. Oxford: Blackwell Publishing Ltd., van der Logt PB and Gottstein B. Unidentified parasitic cysts in cattle. Vet Rec 2000;146: Vangeel L, Houf K, Chiers K, Vercruysse J, D Herde K, and Ducatelle R. Molecular-based identification of Sarcocystis hominis in Belgian minced beef. J Food Prot 2007;70: Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, and Brandt J. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol 1998; 76: von Nickisch-Rosenegk M, Silva-Gonzalez R, and Lucius R. Modification of universal 12S rdna primers for specific amplification of contaminated Taenia spp.(cestoda) gdna enabling phylogenetic studies. Parasitol Res 1999;85: Wilson A and Wilson W. Wilson s Practical Meat Inspection. Oxford: Blackwell Publishing, Ltd., Yamasaki H, Allan JC, Sato MO, et al. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J Clin Microbiol 2004;42: Yamasaki H, Nagase T, Kiyoshige Y, et al. A case of intramuscular cysticercosis diagnosed definitively by mitochondrial DNA analysis of extremely calcified cysts. Parasitol Int 2006; 55: Address correspondence to: Tiziana Civera, D.V.M., Ph.D. Department of Animal Pathology Faculty of Veterinary Medicine University of Turin da Vinci 44 Grugliasco (TO) Italy tiziana.civera@unito.it
6
Protocols. Internal transcribed spacer region (ITS) region. Niklaus J. Grünwald, Frank N. Martin, and Meg M. Larsen (2013)
 Protocols Internal transcribed spacer region (ITS) region Niklaus J. Grünwald, Frank N. Martin, and Meg M. Larsen (2013) The nuclear ribosomal RNA (rrna) genes (small subunit, large subunit and 5.8S) are
Protocols Internal transcribed spacer region (ITS) region Niklaus J. Grünwald, Frank N. Martin, and Meg M. Larsen (2013) The nuclear ribosomal RNA (rrna) genes (small subunit, large subunit and 5.8S) are
TURIN. Historical capital of Italy. City of Art, Nature, Food and Sport. Turin is crossed by the Po river, the Italy s longest river
 TURIN Historical capital of Italy City of Art, Nature, Food and Sport Turin is crossed by the Po river, the Italy s longest river The Mole Antonelliana (1863-1889), 167.5 Meters tall is the symbol of the
TURIN Historical capital of Italy City of Art, Nature, Food and Sport Turin is crossed by the Po river, the Italy s longest river The Mole Antonelliana (1863-1889), 167.5 Meters tall is the symbol of the
ID kit. imegen Anchovies II. and E. japonicus) DNA detection by. User manual. Anchovies species (E. encrasicolus. sequencing.
 User manual imegen Anchovies II ID kit Anchovies species (E. encrasicolus and E. japonicus) DNA detection by sequencing Reference: Made in Spain The information in this guide is subject to change without
User manual imegen Anchovies II ID kit Anchovies species (E. encrasicolus and E. japonicus) DNA detection by sequencing Reference: Made in Spain The information in this guide is subject to change without
VLLM0421c Medical Microbiology I, practical sessions. Protocol to topic J10
 Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
EU Reference Laboratory for E. coli Department of Veterinary Public Health and Food Safety Unit of Foodborne Zoonoses Istituto Superiore di Sanità
 Identification and characterization of Verocytotoxin-producing Escherichia coli (VTEC) by Real Time PCR amplification of the main virulence genes and the genes associated with the serogroups mainly associated
Identification and characterization of Verocytotoxin-producing Escherichia coli (VTEC) by Real Time PCR amplification of the main virulence genes and the genes associated with the serogroups mainly associated
GENOTYPING ASSAYS AT ZIRC
 GENOTYPING ASSAYS AT ZIRC A. READ THIS FIRST - DISCLAIMER Dear ZIRC user, We now provide detailed genotyping protocols for a number of zebrafish lines distributed by ZIRC. These protocols were developed
GENOTYPING ASSAYS AT ZIRC A. READ THIS FIRST - DISCLAIMER Dear ZIRC user, We now provide detailed genotyping protocols for a number of zebrafish lines distributed by ZIRC. These protocols were developed
First Strand cdna Synthesis
 380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
Identification of the VTEC serogroups mainly associated with human infections by conventional PCR amplification of O-associated genes
 Identification of the VTEC serogroups mainly associated with human infections by conventional PCR amplification of O-associated genes 1. Aim and field of application The present method concerns the identification
Identification of the VTEC serogroups mainly associated with human infections by conventional PCR amplification of O-associated genes 1. Aim and field of application The present method concerns the identification
360 Master Mix. , and a supplementary 360 GC Enhancer.
 Product Bulletin AmpliTaq Gold 360 Master Mix and 360 DNA Polymerase AmpliTaq Gold 360 Master Mix AmpliTaq Gold 360 DNA Polymerase 360 Coverage for a Full Range of Targets AmpliTaq Gold 360 Master Mix
Product Bulletin AmpliTaq Gold 360 Master Mix and 360 DNA Polymerase AmpliTaq Gold 360 Master Mix AmpliTaq Gold 360 DNA Polymerase 360 Coverage for a Full Range of Targets AmpliTaq Gold 360 Master Mix
Reverse Transcription System
 TECHNICAL BULLETIN Reverse Transcription System Instruc ons for use of Product A3500 Revised 1/14 TB099 Reverse Transcription System All technical literature is available on the Internet at: www.promega.com/protocols/
TECHNICAL BULLETIN Reverse Transcription System Instruc ons for use of Product A3500 Revised 1/14 TB099 Reverse Transcription System All technical literature is available on the Internet at: www.promega.com/protocols/
Real-Time PCR Vs. Traditional PCR
 Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
Real-Time PCR Vs. Traditional PCR Description This tutorial will discuss the evolution of traditional PCR methods towards the use of Real-Time chemistry and instrumentation for accurate quantitation. Objectives
PyroPhage 3173 DNA Polymerase, Exonuclease Minus (Exo-)
 PyroPhage 3173 DNA Polymerase, Exonuclease Minus (Exo-) FOR RESEARCH USE ONLY. NOT FOR HUMAN OR DIAGNOSTIC USE Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608)
PyroPhage 3173 DNA Polymerase, Exonuclease Minus (Exo-) FOR RESEARCH USE ONLY. NOT FOR HUMAN OR DIAGNOSTIC USE Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608)
NimbleGen DNA Methylation Microarrays and Services
 NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
ABSTRACT. Promega Corporation, Updated September 2008. http://www.promega.com/pubhub. 1 Campbell-Staton, S.
 A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA... A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA from Shed Reptile Skin ABSTRACT
A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA... A Modified Wizard SV Genomic DNA Purification System Protocol to Purify Genomic DNA from Shed Reptile Skin ABSTRACT
IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR)
 IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR) Background Infectious diseases caused by pathogenic organisms such as bacteria, viruses, protozoa,
IIID 14. Biotechnology in Fish Disease Diagnostics: Application of the Polymerase Chain Reaction (PCR) Background Infectious diseases caused by pathogenic organisms such as bacteria, viruses, protozoa,
SYBR Green Realtime PCR Master Mix -Plus-
 Instruction manual SYBR Green Realtime PCR Master Mix -Plus- 0810 F0925K SYBR Green Realtime PCR Master Mix -Plus- Contents QPK-212T 1mLx1 QPK-212 1mLx5 Store at -20 C, protected from light [1] Introduction
Instruction manual SYBR Green Realtime PCR Master Mix -Plus- 0810 F0925K SYBR Green Realtime PCR Master Mix -Plus- Contents QPK-212T 1mLx1 QPK-212 1mLx5 Store at -20 C, protected from light [1] Introduction
The Techniques of Molecular Biology: Forensic DNA Fingerprinting
 Revised Fall 2011 The Techniques of Molecular Biology: Forensic DNA Fingerprinting The techniques of molecular biology are used to manipulate the structure and function of molecules such as DNA and proteins
Revised Fall 2011 The Techniques of Molecular Biology: Forensic DNA Fingerprinting The techniques of molecular biology are used to manipulate the structure and function of molecules such as DNA and proteins
RevertAid Premium First Strand cdna Synthesis Kit
 RevertAid Premium First Strand cdna Synthesis Kit #K1651, #K1652 CERTIFICATE OF ANALYSIS #K1651 Lot QUALITY CONTROL RT-PCR using 100 fg of control GAPDH RNA and GAPDH control primers generated a prominent
RevertAid Premium First Strand cdna Synthesis Kit #K1651, #K1652 CERTIFICATE OF ANALYSIS #K1651 Lot QUALITY CONTROL RT-PCR using 100 fg of control GAPDH RNA and GAPDH control primers generated a prominent
Terra PCR Direct Polymerase Mix User Manual
 Clontech Laboratories, Inc. Terra PCR Direct Polymerase Mix User Manual Cat. Nos. 639269, 639270, 639271 PT5126-1 (031416) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra Bella Avenue, Mountain
Clontech Laboratories, Inc. Terra PCR Direct Polymerase Mix User Manual Cat. Nos. 639269, 639270, 639271 PT5126-1 (031416) Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra Bella Avenue, Mountain
Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.03.2012 to 25.03.2017
Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D-PL-13372-01-00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 26.03.2012 to 25.03.2017
HiPer RT-PCR Teaching Kit
 HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
HiPer RT-PCR Teaching Kit Product Code: HTBM024 Number of experiments that can be performed: 5 Duration of Experiment: Protocol: 4 hours Agarose Gel Electrophoresis: 45 minutes Storage Instructions: The
Genomic DNA Extraction Kit INSTRUCTION MANUAL
 Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Intended Use: The kit is designed to detect the 5 different mutations found in Asian population using seven different primers.
 Unzipping Genes MBPCR014 Beta-Thalassemia Detection Kit P r o d u c t I n f o r m a t i o n Description: Thalassemia is a group of genetic disorders characterized by quantitative defects in globin chain
Unzipping Genes MBPCR014 Beta-Thalassemia Detection Kit P r o d u c t I n f o r m a t i o n Description: Thalassemia is a group of genetic disorders characterized by quantitative defects in globin chain
A quick and simple method for the identi cation of meat species and meat products by PCR assay
 Meat Science 51 (1999) 143±148 A quick and simple method for the identi cation of meat species and meat products by PCR assay T. Matsunaga a, K. Chikuni b *, R. Tanabe b, S. Muroya b, K. Shibata a, J.
Meat Science 51 (1999) 143±148 A quick and simple method for the identi cation of meat species and meat products by PCR assay T. Matsunaga a, K. Chikuni b *, R. Tanabe b, S. Muroya b, K. Shibata a, J.
MagExtractor -Genome-
 Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
Instruction manual MagExtractor-Genome-0810 F0981K MagExtractor -Genome- NPK-101 100 preparations Store at 4 C Contents [1] Introduction [2] Components [3] Materials required [4] Protocol 1. Purification
PrimeSTAR HS DNA Polymerase
 Cat. # R010A For Research Use PrimeSTAR HS DNA Polymerase Product Manual Table of Contents I. Description...3 II. III. IV. Components...3 Storage...3 Features...3 V. General Composition of PCR Reaction
Cat. # R010A For Research Use PrimeSTAR HS DNA Polymerase Product Manual Table of Contents I. Description...3 II. III. IV. Components...3 Storage...3 Features...3 V. General Composition of PCR Reaction
RT-PCR: Two-Step Protocol
 RT-PCR: Two-Step Protocol We will provide both one-step and two-step protocols for RT-PCR. We recommend the twostep protocol for this class. In the one-step protocol, the components of RT and PCR are mixed
RT-PCR: Two-Step Protocol We will provide both one-step and two-step protocols for RT-PCR. We recommend the twostep protocol for this class. In the one-step protocol, the components of RT and PCR are mixed
Aurora Forensic Sample Clean-up Protocol
 Aurora Forensic Sample Clean-up Protocol 106-0008-BA-D 2015 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners. http://www.borealgenomics.com support@borealgenomics.com
Aurora Forensic Sample Clean-up Protocol 106-0008-BA-D 2015 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners. http://www.borealgenomics.com support@borealgenomics.com
Taq98 Hot Start 2X Master Mix
 Taq98 Hot Start 2X Master Mix Optimized for 98C Denaturation Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608) 831-9011 FAX: (608) 831-9012 lucigen@lucigen.com
Taq98 Hot Start 2X Master Mix Optimized for 98C Denaturation Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608) 831-9011 FAX: (608) 831-9012 lucigen@lucigen.com
Application Guide... 2
 Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Fungal DNA sequencing from laser capture microdissection samples
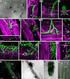 APPLICATION NOTE Laser capture microdissection and Sanger sequencing Fungal DNA sequencing from laser capture microdissection samples Introduction The direct identification of human fungal pathogens by
APPLICATION NOTE Laser capture microdissection and Sanger sequencing Fungal DNA sequencing from laser capture microdissection samples Introduction The direct identification of human fungal pathogens by
ab185916 Hi-Fi cdna Synthesis Kit
 ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
ab185916 Hi-Fi cdna Synthesis Kit Instructions for Use For cdna synthesis from various RNA samples This product is for research use only and is not intended for diagnostic use. Version 1 Last Updated 1
Essentials of Real Time PCR. About Sequence Detection Chemistries
 Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
Essentials of Real Time PCR About Real-Time PCR Assays Real-time Polymerase Chain Reaction (PCR) is the ability to monitor the progress of the PCR as it occurs (i.e., in real time). Data is therefore collected
Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs
 Veterinary Parasitology 79 (1998) 299±313 Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs E. Sciutto a,*, J.J. MartõÂnez b, N.M. Villalobos b,
Veterinary Parasitology 79 (1998) 299±313 Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs E. Sciutto a,*, J.J. MartõÂnez b, N.M. Villalobos b,
Original Article ABSTRACT RESUMO
 Original Article MOLECULAR IDENTIFICATION OF Cysticercus bovis AT DIFFERENT STAGES OF THE HOST-PARASITE INTERACTION PROCESS Eurione Antônio Garcia da Veiga Jardim, 1 Vânia Beatriz Lopes Moura, 2 Marina
Original Article MOLECULAR IDENTIFICATION OF Cysticercus bovis AT DIFFERENT STAGES OF THE HOST-PARASITE INTERACTION PROCESS Eurione Antônio Garcia da Veiga Jardim, 1 Vânia Beatriz Lopes Moura, 2 Marina
CURRICULUM GUIDE. When this Forensics course has been completed successfully, students should be able to:
 CURRICULUM GUIDE NAME OF COURSE: FORENSICS COURSE NUMBER: SCI 40 WRITTEN / REVISED: SEPTEMBER, 2011 LEVEL OF COURSE: REPLACMENT NUMBER OF CREDITS: SIX (6) PREREQUISITES: BIOLOGY GRADE LEVELS OFFERED TO:
CURRICULUM GUIDE NAME OF COURSE: FORENSICS COURSE NUMBER: SCI 40 WRITTEN / REVISED: SEPTEMBER, 2011 LEVEL OF COURSE: REPLACMENT NUMBER OF CREDITS: SIX (6) PREREQUISITES: BIOLOGY GRADE LEVELS OFFERED TO:
Mitochondrial DNA Analysis
 Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
Mitochondrial DNA Analysis Lineage Markers Lineage markers are passed down from generation to generation without changing Except for rare mutation events They can help determine the lineage (family tree)
DNA and Forensic Science
 DNA and Forensic Science Micah A. Luftig * Stephen Richey ** I. INTRODUCTION This paper represents a discussion of the fundamental principles of DNA technology as it applies to forensic testing. A brief
DNA and Forensic Science Micah A. Luftig * Stephen Richey ** I. INTRODUCTION This paper represents a discussion of the fundamental principles of DNA technology as it applies to forensic testing. A brief
Changing Concept of FMD diagnostics: from Central to Local. Aniket Sanyal Project Directorate on FMD Mukteswar, India
 Changing Concept of FMD diagnostics: from Central to Local Aniket Sanyal Project Directorate on FMD Mukteswar, India OBJECTIVES OF DIAGNOSIS IN THE FIELD/LOCAL 1. To arrive at quick diagnosis 2. To implement
Changing Concept of FMD diagnostics: from Central to Local Aniket Sanyal Project Directorate on FMD Mukteswar, India OBJECTIVES OF DIAGNOSIS IN THE FIELD/LOCAL 1. To arrive at quick diagnosis 2. To implement
Rapid Acquisition of Unknown DNA Sequence Adjacent to a Known Segment by Multiplex Restriction Site PCR
 Rapid Acquisition of Unknown DNA Sequence Adjacent to a Known Segment by Multiplex Restriction Site PCR BioTechniques 25:415-419 (September 1998) ABSTRACT The determination of unknown DNA sequences around
Rapid Acquisition of Unknown DNA Sequence Adjacent to a Known Segment by Multiplex Restriction Site PCR BioTechniques 25:415-419 (September 1998) ABSTRACT The determination of unknown DNA sequences around
DNA Isolation Kit for Cells and Tissues
 DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
DNA Isolation Kit for Cells and Tissues for 10 isolations of 00 mg each for tissue or 5 x 10 7 cultured cells Cat. No. 11 81 770 001 Principle Starting material Application Time required Results Benefits
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR. Results Interpretation Guide
 PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
PATHOGEN DETECTION SYSTEMS BY REAL TIME PCR Results Interpretation Guide Pathogen Detection Systems by Real Time PCR Microbial offers real time PCR based systems for the detection of pathogenic bacteria
Animal Pharming: The Industrialization of Transgenic Animals December 1999
 Animal Pharming: The Industrialization of Transgenic Animals December 1999 Animal pharming, the process of using transgenic animals to produce human drugs, is staking its claim in a lucrative world market.
Animal Pharming: The Industrialization of Transgenic Animals December 1999 Animal pharming, the process of using transgenic animals to produce human drugs, is staking its claim in a lucrative world market.
Cestodes. Tapeworms Complex Life Cycles Definitive host. 1 or 2 Intermediate Hosts. Adult worms Sexual reproduction
 Cestodes Tapeworms Complex Life Cycles Definitive host Adult worms Sexual reproduction 1 or 2 Intermediate Hosts Larval Tapeworms (Metacestodes) Some show Asexual reproduction Morphologic Characteristics
Cestodes Tapeworms Complex Life Cycles Definitive host Adult worms Sexual reproduction 1 or 2 Intermediate Hosts Larval Tapeworms (Metacestodes) Some show Asexual reproduction Morphologic Characteristics
DNA Fingerprinting. Unless they are identical twins, individuals have unique DNA
 DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
GenScript BloodReady TM Multiplex PCR System
 GenScript BloodReady TM Multiplex PCR System Technical Manual No. 0174 Version 20040915 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI
GenScript BloodReady TM Multiplex PCR System Technical Manual No. 0174 Version 20040915 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI
DNA Sequence Analysis
 DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
DNA Sequence Analysis Two general kinds of analysis Screen for one of a set of known sequences Determine the sequence even if it is novel Screening for a known sequence usually involves an oligonucleotide
Introduction To Real Time Quantitative PCR (qpcr)
 Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Introduction To Real Time Quantitative PCR (qpcr) SABiosciences, A QIAGEN Company www.sabiosciences.com The Seminar Topics The advantages of qpcr versus conventional PCR Work flow & applications Factors
Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit
 Product Bulletin Human Identification Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit The Quantifiler kits produce reliable and reproducible results, helping to
Product Bulletin Human Identification Quantifiler Human DNA Quantification Kit Quantifiler Y Human Male DNA Quantification Kit The Quantifiler kits produce reliable and reproducible results, helping to
1/12 Dideoxy DNA Sequencing
 1/12 Dideoxy DNA Sequencing Dideoxy DNA sequencing utilizes two steps: PCR (polymerase chain reaction) amplification of DNA using dideoxy nucleoside triphosphates (Figures 1 and 2)and denaturing polyacrylamide
1/12 Dideoxy DNA Sequencing Dideoxy DNA sequencing utilizes two steps: PCR (polymerase chain reaction) amplification of DNA using dideoxy nucleoside triphosphates (Figures 1 and 2)and denaturing polyacrylamide
BacReady TM Multiplex PCR System
 BacReady TM Multiplex PCR System Technical Manual No. 0191 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI Detailed Experimental
BacReady TM Multiplex PCR System Technical Manual No. 0191 Version 10112010 I Description.. 1 II Applications 2 III Key Features.. 2 IV Shipping and Storage. 2 V Simplified Procedures. 2 VI Detailed Experimental
PicoMaxx High Fidelity PCR System
 PicoMaxx High Fidelity PCR System Instruction Manual Catalog #600420 (100 U), #600422 (500 U), and #600424 (1000 U) Revision C Research Use Only. Not for Use in Diagnostic Procedures. 600420-12 LIMITED
PicoMaxx High Fidelity PCR System Instruction Manual Catalog #600420 (100 U), #600422 (500 U), and #600424 (1000 U) Revision C Research Use Only. Not for Use in Diagnostic Procedures. 600420-12 LIMITED
Sequencing Guidelines Adapted from ABI BigDye Terminator v3.1 Cycle Sequencing Kit and Roswell Park Cancer Institute Core Laboratory website
 Biomolecular Core Facility AI Dupont Hospital for Children, Rockland Center One, Room 214 Core: (302) 651-6712, Office: (302) 651-6707, mbcore@nemours.org Katia Sol-Church, Ph.D., Director Jennifer Frenck
Biomolecular Core Facility AI Dupont Hospital for Children, Rockland Center One, Room 214 Core: (302) 651-6712, Office: (302) 651-6707, mbcore@nemours.org Katia Sol-Church, Ph.D., Director Jennifer Frenck
Veterinary Testing. Classes of Test
 Veterinary Testing Classes of Test July 2014 Copyright National Association of Testing Authorities, Australia 2014 This publication is protected by copyright under the Commonwealth of Australia Copyright
Veterinary Testing Classes of Test July 2014 Copyright National Association of Testing Authorities, Australia 2014 This publication is protected by copyright under the Commonwealth of Australia Copyright
mircute mirna qpcr Detection Kit (SYBR Green)
 mircute mirna qpcr Detection Kit (SYBR Green) For detection of mirna using real-time RT-PCR (SYBR Green I) www.tiangen.com QP110302 mircute mirna qpcr Detection Kit (SYBR Green) Kit Contents Cat. no. FP401
mircute mirna qpcr Detection Kit (SYBR Green) For detection of mirna using real-time RT-PCR (SYBR Green I) www.tiangen.com QP110302 mircute mirna qpcr Detection Kit (SYBR Green) Kit Contents Cat. no. FP401
HBV Quantitative Real Time PCR Kit
 Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Revision No.: ZJ0002 Issue Date: Aug 7 th, 2008 HBV Quantitative Real Time PCR Kit Cat. No.: HD-0002-01 For Use with LightCycler 1.0/LightCycler2.0/LightCycler480 (Roche) Real Time PCR Systems (Pls ignore
Troubleshooting Sequencing Data
 Troubleshooting Sequencing Data Troubleshooting Sequencing Data No recognizable sequence (see page 7-10) Insufficient Quantitate the DNA. Increase the amount of DNA in the sequencing reactions. See page
Troubleshooting Sequencing Data Troubleshooting Sequencing Data No recognizable sequence (see page 7-10) Insufficient Quantitate the DNA. Increase the amount of DNA in the sequencing reactions. See page
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
Introduction. Preparation of Template DNA
 Procedures and Recommendations for DNA Sequencing at the Plant-Microbe Genomics Facility Ohio State University Biological Sciences Building Room 420, 484 W. 12th Ave., Columbus OH 43210 Telephone: 614/247-6204;
Procedures and Recommendations for DNA Sequencing at the Plant-Microbe Genomics Facility Ohio State University Biological Sciences Building Room 420, 484 W. 12th Ave., Columbus OH 43210 Telephone: 614/247-6204;
IDENTIFICATION OF Echinococcus granulosus COMPLEX AT GENOTYPE/SPECIES LEVEL FROM HYDATID CYSTS BY PCR AND SEQUENCING
 IDENTIFICATION OF Echinococcus granulosus COMPLEX AT GENOTYPE/SPECIES LEVEL FROM HYDATID CYSTS BY PCR AND SEQUENCING INDEX 1. Aim and field of application 2 2. Principle of the method 2 3. References 3
IDENTIFICATION OF Echinococcus granulosus COMPLEX AT GENOTYPE/SPECIES LEVEL FROM HYDATID CYSTS BY PCR AND SEQUENCING INDEX 1. Aim and field of application 2 2. Principle of the method 2 3. References 3
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
Pure-IP Western Blot Detection Kit
 Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Custom Antibody Services
 prosci-inc.com Custom Antibody Services High Performance Antibodies and More Broad Antibody Catalog Extensive Antibody Services CUSTOM ANTIBODY SERVICES Established in 1998, ProSci Incorporated is a leading
prosci-inc.com Custom Antibody Services High Performance Antibodies and More Broad Antibody Catalog Extensive Antibody Services CUSTOM ANTIBODY SERVICES Established in 1998, ProSci Incorporated is a leading
PNA BRAF Mutation Detection Kit
 - PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
- PNA BRAF Mutation Detection Kit Catalog Number KA2102 50 tests/kit Version: 01 Intended for research use only www.abnova.com Introduction and Background Intended use The PNA BRAF Mutation Detection Kit
Diagnostic Testing and Strategies for BVDV
 Diagnostic Testing and Strategies for BVDV Dan Grooms Dept. of Large Animal Clinical Sciences College of Veterinary Medicine Introduction Clinical diseases in cattle resulting from infection with bovine
Diagnostic Testing and Strategies for BVDV Dan Grooms Dept. of Large Animal Clinical Sciences College of Veterinary Medicine Introduction Clinical diseases in cattle resulting from infection with bovine
PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI,
 Supplemental Text/Tables PCR Amplification and Sequencing PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI, Foster City, CA). Each PCR reaction contained 20 ng genomic
Supplemental Text/Tables PCR Amplification and Sequencing PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI, Foster City, CA). Each PCR reaction contained 20 ng genomic
Detection of HBV DNA in Serum Using a PCR-Based Assay
 2 Detection of HBV DNA in Serum Using a PCR-Based Assay Hau Tim Chung 1. Introduction Detection of minute amounts of hepatitis B virus (HBV) DNA in the serum using polymerase chain reaction (PCR) based
2 Detection of HBV DNA in Serum Using a PCR-Based Assay Hau Tim Chung 1. Introduction Detection of minute amounts of hepatitis B virus (HBV) DNA in the serum using polymerase chain reaction (PCR) based
Taenia solium and Taenia saginata
 Taenia solium and Taenia saginata Taenia solium The common name for Taenia solium is pork tapeworm i.e the armed tapeworm of man. The restellum resembles the conventional figures of the sun, hence the
Taenia solium and Taenia saginata Taenia solium The common name for Taenia solium is pork tapeworm i.e the armed tapeworm of man. The restellum resembles the conventional figures of the sun, hence the
Table of Contents. I. Description... 2. II. Kit Components... 2. III. Storage... 2. IV. 1st Strand cdna Synthesis Reaction... 3
 Table of Contents I. Description... 2 II. Kit Components... 2 III. Storage... 2 IV. 1st Strand cdna Synthesis Reaction... 3 V. RT-PCR, Real-time RT-PCR... 4 VI. Application... 5 VII. Preparation of RNA
Table of Contents I. Description... 2 II. Kit Components... 2 III. Storage... 2 IV. 1st Strand cdna Synthesis Reaction... 3 V. RT-PCR, Real-time RT-PCR... 4 VI. Application... 5 VII. Preparation of RNA
Data Analysis for Ion Torrent Sequencing
 IFU022 v140202 Research Use Only Instructions For Use Part III Data Analysis for Ion Torrent Sequencing MANUFACTURER: Multiplicom N.V. Galileilaan 18 2845 Niel Belgium Revision date: August 21, 2014 Page
IFU022 v140202 Research Use Only Instructions For Use Part III Data Analysis for Ion Torrent Sequencing MANUFACTURER: Multiplicom N.V. Galileilaan 18 2845 Niel Belgium Revision date: August 21, 2014 Page
UltraClean Forensic DNA Isolation Kit (Single Prep Format)
 UltraClean Forensic DNA Isolation Kit (Single Prep Format) Catalog No. Quantity 14000-10 10 preps 14000-S 1 prep Instruction Manual Please recycle Version: 10302012 1 Table of Contents Introduction...
UltraClean Forensic DNA Isolation Kit (Single Prep Format) Catalog No. Quantity 14000-10 10 preps 14000-S 1 prep Instruction Manual Please recycle Version: 10302012 1 Table of Contents Introduction...
Use of the Agilent 2100 Bioanalyzer and the DNA 500 LabChip in the Analysis of PCR Amplified Mitochondrial DNA Application
 Use of the Agilent 2100 Bioanalyzer and the DNA LabChip in the Analysis of PCR Amplified Mitochondrial DNA Application Homeland Security/Forensics Author Mark Jensen Agilent Technologies, Inc. 2850 Centerville
Use of the Agilent 2100 Bioanalyzer and the DNA LabChip in the Analysis of PCR Amplified Mitochondrial DNA Application Homeland Security/Forensics Author Mark Jensen Agilent Technologies, Inc. 2850 Centerville
http://www.who.int/csr/disease/avian_influenza/phase/en 4 http://new.paho.org/hq/index.php?option=com_content&task=view&id=1283&itemid=569
 Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
Forensic DNA Testing Terminology
 Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Forensic DNA Testing Terminology ABI 310 Genetic Analyzer a capillary electrophoresis instrument used by forensic DNA laboratories to separate short tandem repeat (STR) loci on the basis of their size.
Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated. Screening
 Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated in situ TREC qpcr Assay for SCID Screening J Bai, T Henry, J Benfer, S Berberich, T Kreman, and L DesJardin State
Molecular Assessment of Dried Blood Spot Quality during Development of a Novel Automated in situ TREC qpcr Assay for SCID Screening J Bai, T Henry, J Benfer, S Berberich, T Kreman, and L DesJardin State
Genolution Pharmaceuticals, Inc. Life Science and Molecular Diagnostic Products
 Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
DNA Integrity Number (DIN) For the Assessment of Genomic DNA Samples in Real-Time Quantitative PCR (qpcr) Experiments
 DNA Integrity Number () For the Assessment of Genomic DNA Samples in Real-Time Quantitative PCR (qpcr) Experiments Application Note Nucleic Acid Analysis Author Arunkumar Padmanaban Agilent Technologies,
DNA Integrity Number () For the Assessment of Genomic DNA Samples in Real-Time Quantitative PCR (qpcr) Experiments Application Note Nucleic Acid Analysis Author Arunkumar Padmanaban Agilent Technologies,
MICB ABI PRISM 310 SEQUENCING GUIDE SEQUENCING OF PLASMID DNA
 Plasmid DNA Preparation MICB ABI PRISM 310 SEQUENCING GUIDE SEQUENCING OF PLASMID DNA Introduction: I have always used the classic Alkaline Lysis miniprep method to isolate plasmid DNA. (See below) If
Plasmid DNA Preparation MICB ABI PRISM 310 SEQUENCING GUIDE SEQUENCING OF PLASMID DNA Introduction: I have always used the classic Alkaline Lysis miniprep method to isolate plasmid DNA. (See below) If
IKDT Laboratory. IKDT as Service Lab (CRO) for Molecular Diagnostics
 Page 1 IKDT Laboratory IKDT as Service Lab (CRO) for Molecular Diagnostics IKDT lab offer is complete diagnostic service to all external customers. We could perform as well single procedures or complex
Page 1 IKDT Laboratory IKDT as Service Lab (CRO) for Molecular Diagnostics IKDT lab offer is complete diagnostic service to all external customers. We could perform as well single procedures or complex
Supplementary Information - PCR amplification PCR amplification reactions for the partial mitochondrial cytochrome oxidase subunit I (COI), the
 Supplementary Information - PCR amplification PCR amplification reactions for the partial mitochondrial cytochrome oxidase subunit I (COI), the ribosomal 16S rdna gene and a fragment of the nuclear single
Supplementary Information - PCR amplification PCR amplification reactions for the partial mitochondrial cytochrome oxidase subunit I (COI), the ribosomal 16S rdna gene and a fragment of the nuclear single
CompleteⅡ 1st strand cdna Synthesis Kit
 Instruction Manual CompleteⅡ 1st strand cdna Synthesis Kit Catalog # GM30401, GM30402 Green Mountain Biosystems. LLC Web: www.greenmountainbio.com Tel: 800-942-1160 Sales: Sales@ greenmountainbio.com Support:
Instruction Manual CompleteⅡ 1st strand cdna Synthesis Kit Catalog # GM30401, GM30402 Green Mountain Biosystems. LLC Web: www.greenmountainbio.com Tel: 800-942-1160 Sales: Sales@ greenmountainbio.com Support:
Hepatitis B Virus Genemer Mix
 Product Manual Hepatitis B Virus Genemer Mix Primer Pair for amplification of HBV Specific DNA Fragment Includes Internal Negative Control Primers and Template Catalog No.: 60-2007-12 Store at 20 o C For
Product Manual Hepatitis B Virus Genemer Mix Primer Pair for amplification of HBV Specific DNA Fragment Includes Internal Negative Control Primers and Template Catalog No.: 60-2007-12 Store at 20 o C For
The RNAi Consortium (TRC) Broad Institute
 TRC Laboratory Protocols Protocol Title: One Step PCR Preparation of Samples for Illumina Sequencing Current Revision Date: 11/10/2012 RNAi Platform,, trc_info@broadinstitute.org Brief Description: This
TRC Laboratory Protocols Protocol Title: One Step PCR Preparation of Samples for Illumina Sequencing Current Revision Date: 11/10/2012 RNAi Platform,, trc_info@broadinstitute.org Brief Description: This
DNA Core Facility: DNA Sequencing Guide
 DNA Core Facility: DNA Sequencing Guide University of Missouri-Columbia 216 Life Sciences Center Columbia, MO 65211 http://biotech.missouri.edu/dnacore/ Table of Contents 1. Evaluating Sequencing Data..
DNA Core Facility: DNA Sequencing Guide University of Missouri-Columbia 216 Life Sciences Center Columbia, MO 65211 http://biotech.missouri.edu/dnacore/ Table of Contents 1. Evaluating Sequencing Data..
Cloning Blunt-End Pfu DNA Polymerase- Generated PCR Fragments into pgem -T Vector Systems
 Promega Notes Number 71, 1999, p. 10 Blunt-End Pfu DNA Polymerase- Generated PCR Fragments into pgem -T Vector Systems By Kimberly Knoche, Ph.D., and Dan Kephart, Ph.D. Promega Corporation Corresponding
Promega Notes Number 71, 1999, p. 10 Blunt-End Pfu DNA Polymerase- Generated PCR Fragments into pgem -T Vector Systems By Kimberly Knoche, Ph.D., and Dan Kephart, Ph.D. Promega Corporation Corresponding
SOP Title: Multiplex-PCR check of genomic DNA isolated from FFPE tissue for its usability in array CGH analysis
 SOP Title: Multiplex-PCR check of genomic DNA isolated from FFPE tissue for its usability in array CGH analysis The STORE processing methods were shown to be fit-for purpose for DNA, RNA and protein extraction
SOP Title: Multiplex-PCR check of genomic DNA isolated from FFPE tissue for its usability in array CGH analysis The STORE processing methods were shown to be fit-for purpose for DNA, RNA and protein extraction
Speed Matters - Fast ways from template to result
 qpcr Symposium 2007 - Weihenstephan Speed Matters - Fast ways from template to result March 28, 2007 Dr. Thorsten Traeger Senior Scientist, Research and Development - 1 - Overview Ạgenda Fast PCR The Challenges
qpcr Symposium 2007 - Weihenstephan Speed Matters - Fast ways from template to result March 28, 2007 Dr. Thorsten Traeger Senior Scientist, Research and Development - 1 - Overview Ạgenda Fast PCR The Challenges
A Fast, Accurate, and Automated Workflow for Multi Locus Sequence Typing of Bacterial Isolates
 Application Note MLST A Fast, Accurate, and Automated Workflow for Multi Locus Sequence Typing of Bacterial Isolates Using Applied Biosystems 3130 and 3730 Series Capillary Electrophoresis Systems and
Application Note MLST A Fast, Accurate, and Automated Workflow for Multi Locus Sequence Typing of Bacterial Isolates Using Applied Biosystems 3130 and 3730 Series Capillary Electrophoresis Systems and
Genomic DNA Clean & Concentrator Catalog Nos. D4010 & D4011
 Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
HuCAL Custom Monoclonal Antibodies
 HuCAL Custom Monoclonal Antibodies Highly Specific Monoclonal Antibodies in just 8 Weeks PROVEN, HIGHLY SPECIFIC, HIGH AFFINITY ANTIBODIES IN 8 WEEKS WITHOUT HuCAL PLATINUM IMMUNIZATION (Human Combinatorial
HuCAL Custom Monoclonal Antibodies Highly Specific Monoclonal Antibodies in just 8 Weeks PROVEN, HIGHLY SPECIFIC, HIGH AFFINITY ANTIBODIES IN 8 WEEKS WITHOUT HuCAL PLATINUM IMMUNIZATION (Human Combinatorial
Molecular Diagnosis of Hepatitis B and Hepatitis D infections
 Molecular Diagnosis of Hepatitis B and Hepatitis D infections Acute infection Detection of HBsAg in serum is a fundamental diagnostic marker of HBV infection HBsAg shows a strong correlation with HBV replication
Molecular Diagnosis of Hepatitis B and Hepatitis D infections Acute infection Detection of HBsAg in serum is a fundamental diagnostic marker of HBV infection HBsAg shows a strong correlation with HBV replication
GUIDELINES FOR THE VALIDATION OF FOOD SAFETY CONTROL MEASURES CAC/GL 69-2008
 CAC/GL 69-2008 Page 1 of 16 GUIDELINES FOR THE VALIDATION OF FOOD SAFETY CONTROL MEASURES I. INTRODUCTION CAC/GL 69-2008 The control of hazards potentially associated with foods typically involves the
CAC/GL 69-2008 Page 1 of 16 GUIDELINES FOR THE VALIDATION OF FOOD SAFETY CONTROL MEASURES I. INTRODUCTION CAC/GL 69-2008 The control of hazards potentially associated with foods typically involves the
Real-time quantitative RT -PCR (Taqman)
 Real-time quantitative RT -PCR (Taqman) Author: SC, Patti Lab, 3/03 This is performed as a 2-step reaction: 1. cdna synthesis from DNase 1-treated total RNA 2. PCR 1. cdna synthesis (Advantage RT-for-PCR
Real-time quantitative RT -PCR (Taqman) Author: SC, Patti Lab, 3/03 This is performed as a 2-step reaction: 1. cdna synthesis from DNase 1-treated total RNA 2. PCR 1. cdna synthesis (Advantage RT-for-PCR
Effects of Herceptin on circulating tumor cells in HER2 positive early breast cancer
 Effects of Herceptin on circulating tumor cells in HER2 positive early breast cancer J.-L. Zhang, Q. Yao, J.-H. Chen,Y. Wang, H. Wang, Q. Fan, R. Ling, J. Yi and L. Wang Xijing Hospital Vascular Endocrine
Effects of Herceptin on circulating tumor cells in HER2 positive early breast cancer J.-L. Zhang, Q. Yao, J.-H. Chen,Y. Wang, H. Wang, Q. Fan, R. Ling, J. Yi and L. Wang Xijing Hospital Vascular Endocrine
About Our Products. Blood Products. Purified Infectious/Inactivated Agents. Native & Recombinant Viral Proteins. DNA Controls and Primers for PCR
 About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
REFERENCE LABORATORY TESTING
 Diagnostic Services Price List Tel: +44 (0) 1483 232441 Fax: +44 (0) 1483 232621 Reference Laboratories Incoming.Samples@pirbright.ac.uk October 2014 1 REFERENCE LABORATORY TESTING The Pirbright Institute
Diagnostic Services Price List Tel: +44 (0) 1483 232441 Fax: +44 (0) 1483 232621 Reference Laboratories Incoming.Samples@pirbright.ac.uk October 2014 1 REFERENCE LABORATORY TESTING The Pirbright Institute
HuCAL Custom Monoclonal Antibodies
 HuCAL Custom Monoclonal HuCAL Custom Monoclonal Antibodies Highly Specific, Recombinant Antibodies in 8 Weeks Highly Specific Monoclonal Antibodies in Just 8 Weeks HuCAL PLATINUM (Human Combinatorial Antibody
HuCAL Custom Monoclonal HuCAL Custom Monoclonal Antibodies Highly Specific, Recombinant Antibodies in 8 Weeks Highly Specific Monoclonal Antibodies in Just 8 Weeks HuCAL PLATINUM (Human Combinatorial Antibody
Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual
 Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual F- 470S 20 cdna synthesis reactions (20 µl each) F- 470L 100 cdna synthesis reactions (20 µl each) Table of contents 1. Description...
Thermo Scientific DyNAmo cdna Synthesis Kit for qrt-pcr Technical Manual F- 470S 20 cdna synthesis reactions (20 µl each) F- 470L 100 cdna synthesis reactions (20 µl each) Table of contents 1. Description...
WHO/FAO/OIE Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis
 WHO/FAO/OIE Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis Editor: K.D. Murrell Associate Editors: P. Dorny A. Flisser S. Geerts N.C. Kyvsgaard D. McManus T. Nash Z.
WHO/FAO/OIE Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis Editor: K.D. Murrell Associate Editors: P. Dorny A. Flisser S. Geerts N.C. Kyvsgaard D. McManus T. Nash Z.
STA DARD OPERATI G PROCEDURE FOR THE DETECTIO OF AFRICA SWI E FEVER VIRUS (ASFV) BY CO VE TIO AL POLYMERASE CHAI REACTIO (PCR)
 STA DARD OPERATI G PROCEDURE FOR THE DETECTIO OF AFRICA SWI E FEVER VIRUS (ASFV) BY CO VE TIO AL POLYMERASE CHAI REACTIO (PCR) jmvizcaino@vet.ucm.es Av/ Puerta de Hierro s/n. 28040 Madrid. Tel: (34) 913944082
STA DARD OPERATI G PROCEDURE FOR THE DETECTIO OF AFRICA SWI E FEVER VIRUS (ASFV) BY CO VE TIO AL POLYMERASE CHAI REACTIO (PCR) jmvizcaino@vet.ucm.es Av/ Puerta de Hierro s/n. 28040 Madrid. Tel: (34) 913944082
