Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes
|
|
|
- Alexandra Stevens
- 8 years ago
- Views:
Transcription
1 The new england journal of medicine Original Article, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes Bernard Zinman, M.D., Christoph Wanner, M.D., John M. Lachin, Sc.D., David Fitchett, M.D., Erich Bluhmki, Ph.D., Stefan Hantel, Ph.D., Michaela Mattheus, Dipl. Biomath., Theresa Devins, Dr.P.H., Odd Erik Johansen, M.D., Ph.D., Hans J. Woerle, M.D., Uli C. Broedl, M.D., and Silvio E. Inzucchi, M.D., for the EMPA-REG OUTCOME Investigators ABSTRACT BACKGROUND The effects of empagliflozin, an inhibitor of sodium glucose cotransporter 2, in addition to standard care, on cardiovascular morbidity and mortality in patients with type 2 diabetes at high cardiovascular risk are not known. METHODS We randomly assigned patients to receive 10 mg or 25 mg of empagliflozin or placebo once daily. The primary composite outcome was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, as analyzed in the pooled empagliflozin group versus the placebo group. The key secondary composite outcome was the primary outcome plus hospitalization for unstable angina. RESULTS A total of 7020 patients were treated (median observation time, 3.1 years). The primary outcome occurred in 490 of 4687 patients (10.5%) in the pooled empagliflozin group and in 282 of 2333 patients (12.1%) in the placebo group (hazard ratio in the empagliflozin group, 0.86; 95.02% confidence interval, 0.74 to 0.99; P = 0.04 for superiority). There were no significant between-group differences in the rates of myocardial infarction or stroke, but in the empagliflozin group there were significantly lower rates of death from cardiovascular causes (3.7%, vs. 5.9% in the placebo group; 38% relative risk reduction), hospitalization for heart failure (2.7% and 4.1%, respectively; 35% relative risk reduction), and death from any cause (5.7% and 8.3%, respectively; 32% relative risk reduction). There was no significant between-group difference in the key secondary outcome (P = 0.08 for superiority). Among patients receiving empagliflozin, there was an increased rate of genital infection but no increase in other adverse events. From the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital (B.Z.) and the Divisions of Endocrinology (B.Z.) and Cardiology (D.F.), University of Toronto all in Toronto; the Department of Medicine, Division of Nephrology, Würzburg University Clinic, Würzburg (C.W.), Boehringer Ingelheim Pharma, Biberach (E.B., S.H.), and Boehringer Ingelheim Pharma, Ingelheim (M.M., H.J.W., U.C.B.) all in Germany; the Biostatistics Center, George Washington University, Rockville, MD (J.M.L.); Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT (T.D.); Boehringer Ingelheim Norway, Asker, Norway (O.E.J.); and the Section of Endocrinology, Yale University School of Medicine, New Haven, CT (S.E.I.). Address reprint requests to Dr. Zinman at Mount Sinai Hospital, 60 Murray St., Suite L5-024, Box 17, Toronto, ONT M5T 3L9, Canada, or at zinman@ lunenfeld. ca. This article was published on September 17, 2015, at NEJM.org. N Engl J Med 2015;373: DOI: /NEJMoa Copyright 2015 Massachusetts Medical Society. CONCLUSIONS Patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, as compared with placebo, had a lower rate of the primary composite cardiovascular outcome and of death from any cause when the study drug was added to standard care. (Funded by Boehringer Ingelheim and Eli Lilly; EMPA-REG OUTCOME ClinicalTrials.gov number, NCT ) n engl j med 373;22 nejm.org November 26,
2 The new england journal of medicine Type 2 diabetes is a major risk factor for cardiovascular disease, 1,2 and the presence of both type 2 diabetes and cardiovascular disease increases the risk of death. 3 Evidence that glucose lowering reduces the rates of cardiovascular events and death has not been convincingly shown, 4-6 although a modest cardiovascular benefit may be observed after a prolonged follow-up period. 7 Furthermore, there is concern that intensive glucose lowering or the use of specific glucose-lowering drugs may be associated with adverse cardiovascular outcomes. 8 Therefore, it is necessary to establish the cardiovascular safety benefits of glucose-lowering agents. 9 Inhibitors of sodium glucose cotransporter 2 reduce rates of hyperglycemia in patients with type 2 diabetes by decreasing renal glucose reabsorption, thereby increasing urinary glucose excretion. 10 is a selective inhibitor of sodium glucose cotransporter 2 11 that has been approved for type 2 diabetes. 12 Given as either monotherapy or as an add-on therapy, the drug is reported to reduce glycated hemoglobin levels in patients with type 2 diabetes, including those with stage 2 or 3a chronic kidney disease Furthermore, empagliflozin is associated with weight loss and reductions in blood pressure without increases in heart rate also has favorable effects on markers of arterial stiffness and vascular resistance, 21 visceral adiposity, 22 albuminuria, 20 and plasma urate has been associated with an increase in levels of both low-density lipoprotein (LDL) 14 and high-density lipoprotein (HDL) cholesterol The most common side effects of empagliflozin are urinary tract infection and genital infection. 12 In the EMPA-REG OUTCOME trial, we examined the effects of empagliflozin, as compared with placebo, on cardiovascular morbidity and mortality in patients with type 2 diabetes at high risk for cardiovascular events who were receiving standard care. Methods Study Oversight The trial was designed and overseen by a steering committee that included academic investigators and employees of Boehringer Ingelheim. The role of Eli Lilly was limited to cofunding the trial. Safety data were reviewed by an independent academic data monitoring committee every 90 days or at the discretion of the committee. Cardiovascular outcome events and deaths were prospectively adjudicated by two clinical-events committees (one for cardiac events and the other for neurologic events), as recommended by the Food and Drug Administration (FDA) guidelines. 9 A list of investigators and committee members is provided in Sections A and B, respectively, in the Supplementary Appendix, which is available with the full text of this article at NEJM.org. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the the International Conference on Harmonisation Good Clinical Practice guidelines and was approved by local authorities. An independent ethics committee or institutional review board approved the clinical protocol at each participating center. All the patients provided written informed consent before study entry. All the authors were involved in the study design and had access to the data, which were analyzed by one of the study sponsors, Boehringer Ingelheim. All the authors vouch for the accuracy and completeness of the data analyses and for the fidelity of the study to the protocol, available at NEJM.org. Members of the University of Freiburg conducted an independent statistical analysis of cardiovascular outcomes (Section B in the Supplementary Appendix). The manuscript was drafted by the first and last authors and revised by all the authors. Medical writing assistance, which was paid for by Boehringer Ingelheim, was provided by Fleishman-Hillard Group. Study Design As described previously, 23 this was a randomized, double-blind, placebo-controlled trial to assess the effect of once-daily empagliflozin (at a dose of either 10 mg or 25 mg) versus placebo on cardiovascular events in adults with type 2 diabetes at high cardiovascular risk against a background of standard care. Patients were treated at 590 sites in 42 countries. The trial continued until an adjudicated primary outcome event had occurred in at least 691 patients. Study Patients Eligible patients with type 2 diabetes were adults ( 18 years of age) with a body-mass index (the weight in kilograms divided by the square of the 2118 n engl j med 373;22 nejm.org November 26, 2015
3 in Type 2 Diabetes height in meters) of 45 or less and an estimated glomerular filtration rate (egfr) of at least 30 ml per minute per 1.73 m 2 of body-surface area, according to the Modification of Diet in Renal Disease criteria. All the patients had established cardiovascular disease (as defined in Section C in the Supplementary Appendix) and had received no glucose-lowering agents for at least 12 weeks before randomization and had a glycated hemoglobin level of at least 7.0% and no more than 9.0% or had received stable glucose-lowering therapy for at least 12 weeks before randomization and had a glycated hemoglobin level of at least 7.0% and no more than 10.0%. Other key exclusion criteria are provided in Section D in the Supplementary Appendix. Study Procedures Eligible patients underwent a 2-week, open-label, placebo run-in period in which background glucose-lowering therapy was unchanged. Patients meeting the inclusion criteria were then randomly assigned in a 1:1:1 ratio to receive either 10 mg or 25 mg of empagliflozin or placebo once daily. Randomization was performed with the use of a computer-generated random-sequence and interactive voice- and Web-response system and was stratified according to the glycated hemoglobin level at screening (<8.5% or 8.5%), bodymass index at randomization (<30 or 30), renal function at screening (egfr, 30 to 59 ml, 60 to 89 ml, or 90 ml per minute per 1.73 m 2 ), and geographic region (North America [plus Australia and New Zealand], Latin America, Europe, Africa, or Asia). Background glucose-lowering therapy was to remain unchanged for the first 12 weeks after randomization, although intensification was permitted if the patient had a confirmed fasting glucose level of more than 240 mg per deciliter (>13.3 mmol per liter). In cases of medical necessity, dose reduction or discontinuation of background medication could occur. After week 12, investigators were encouraged to adjust glucoselowering therapy at their discretion to achieve glycemic control according to local guidelines. Throughout the trial, investigators were encouraged to treat other cardiovascular risk factors (including dyslipidemia and hypertension) to achieve the best available standard of care according to local guidelines. Patients were instructed to attend the clinic at prespecified times, which included a follow-up visit 30 days after the end of treatment. Patients who prematurely discontinued a study drug were to be followed for ascertainment of cardiovascular outcomes, and attempts were made to collect vital-status information for any patient who was lost to follow-up, as allowed by local guidelines. Study Outcomes The primary outcome was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent myocardial infarction), or nonfatal stroke. The key secondary outcome was a composite of the primary outcome plus hospitalization for unstable angina. Definitions of the major clinical outcomes are provided in Section E in the Supplementary Appendix. Safety was assessed on the basis of adverse events that occurred during treatment or within 7 days after the last dose of a study drug and were coded with the use of the Medical Dictionary for Regulatory Activities, version Adverse events of special interest included confirmed hypoglycemic adverse events (plasma glucose level, 70 mg per deciliter [3.9 mmol per liter] or an event requiring assistance), and adverse events reflecting urinary tract infection, genital infection, volume depletion, acute renal failure, bone fracture, diabetic ketoacidosis, and thromboembolic events. Statistical Analysis The primary hypothesis was noninferiority for the primary outcome with empagliflozin (pooled doses of 10 mg and 25 mg) versus placebo with a margin of 1.3 for the hazard ratio. 9 We used a four-step hierarchical-testing strategy for the pooled empagliflozin group versus the placebo group in the following order: noninferiority for the primary outcome, noninferiority for the key secondary outcome, superiority for the primary outcome, and superiority for the key secondary outcome. Since interim data from the trial were included in a new-drug application submitted to the FDA, under the Haybittle Peto rule, a two-sided P value of or less was considered to indicate statistical significance in the final analyses. 23 For the test of noninferiority for the primary outcome with a margin of 1.3 at a one-sided level of , at least 691 events were required to provide a power of at least 90% on the assumption of a true hazard ratio of 1.0. Noninferiority for n engl j med 373;22 nejm.org November 26,
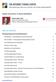
4 The new england journal of medicine the primary outcome was determined if the upper boundary of the two-sided 95.02% confidence interval was less than 1.3. Analyses were based on a Cox proportional-hazards model, with study group, age, sex, baseline body-mass index, baseline glycated hemoglobin level, baseline egfr, and geographic region as factors. Estimates of cumulative-incidence function were corrected for death as a competing risk, 24 except for death from any cause, for which Kaplan Meier estimates are presented. Because of the declining numbers of patients at risk, cumulative-incidence plots have been truncated at 48 months. We calculated the number of patients who would need to be treated to prevent one death on the basis of the exponential distribution. We performed the primary analysis using a modified intention-to-treat approach among patients who had received at least one dose of a study drug. Data for patients who did not have an event were censored on the last day they were known to be free of the outcome. Secondary analyses included comparisons of the 10-mg dose of empagliflozin versus placebo and the 25-mg dose versus placebo. Sensitivity analyses are described in the Section F in the Supplementary Appendix. We analyzed the changes from baseline in glycated hemoglobin level, weight, waist circumference, systolic and diastolic blood pressure, heart rate, LDL and HDL cholesterol, and uric acid using a repeated-measures analysis as a mixed model. Subgroup analyses are described in Section F in the Supplementary Appendix. Results Study Patients A total of 7028 patients underwent randomization from September 2010 through April Of these patients, 7020 were treated and included in the primary analysis (Fig. S1 in Section G in the Supplementary Appendix). Reasons for premature discontinuation are provided in Table S1 in Section H in the Supplementary Appendix. Overall, 97.0% of patients completed the study, with 25.4% of patients prematurely discontinuing a study drug. Final vital status was available for 99.2% of patients. At baseline, demographic and clinical characteristics were well balanced between the placebo group and the empagliflozin group (Table S2 in Section I in the Supplementary Appendix). According to the inclusion criteria, more than 99% of patients had established cardiovascular disease, and patients were well treated with respect to the use of lipid-lowering therapy and antihypertensive medications at baseline. The median duration of treatment was 2.6 years, and the median observation time was 3.1 years; both durations were similar in the pooled empagliflozin group and the placebo group (Table S3 in Section J in the Supplementary Appendix). Cardiovascular Outcomes The primary outcome occurred in a significantly lower percentage of patients in the empagliflozin group (490 of 4687 [10.5%]) than in the placebo group (282 of 2333 [12.1%]) (hazard ratio in the empagliflozin group, 0.86; 95.02% confidence interval [CI], 0.74 to 0.99; P<0.001 for noninferiority and P = 0.04 for superiority) (Fig. 1A). The key secondary outcome occurred in 599 of 4687 patients (12.8%) in the empagliflozin group and 333 of 2333 patients (14.3%) in the placebo group (hazard ratio, 0.89; 95% CI, 0.78 to 1.01; P<0.001 for noninferiority and P = 0.08 for superiority). As compared with placebo, empagliflozin resulted in a significantly lower risk of death from cardiovascular causes (hazard ratio, 0.62; 95% CI, 0.49 to 0.77; P<0.001) (Fig. 1B), death from any cause (hazard ratio, 0.68; 95% CI, 0.57 to 0.82, P<0.001; Fig. 1C), and hospitalization for heart failure (hazard ratio, 0.65; 95% CI, 0.50 to 0.85; P = 0.002) (Fig. 1D). Hazard ratios for cardiovascular outcomes with empagliflozin versus placebo are shown in Table 1. Absolute reductions in incidence rates for cardiovascular outcomes are provided in Table S4 in Section K in the Supplementary Appendix. All categories of death from cardiovascular causes contributed to the reduction in cardiovascular death in the empagliflozin group (Table S5 in Section L in the Supplementary Appendix). There were no significant between-group differences in the occurrence of myocardial infarction or stroke (Table 1). Myocardial infarction was reported in 4.8% of patients in the empagliflozin group and 5.4% of those in the placebo group, and stroke in 3.5% and 3.0% of patients, respectively. For the primary and key secondary outcomes, hazard ratios for the comparison between the 10-mg dose of empagliflozin versus placebo and the 25-mg dose versus placebo were virtually 2120 n engl j med 373;22 nejm.org November 26, 2015
5 in Type 2 Diabetes A Primary Outcome Patients with Event (%) 0 Hazard ratio, 0.86 (95.02% CI, ) P=0.04 for superiority Month B Death from Cardiovascular Causes Patients with Event (%) Hazard ratio, 0.62 (95% CI, ) P< Month No. at Risk No. at Risk C Death from Any Cause Hazard ratio, 0.68 (95% CI, ) P<0.001 D Hospitalization for Heart Failure Patients with Event (%) Patients with Event (%) 1 Hazard ratio, 0.65 (95% CI, ) P= Month Month No. at Risk No. at Risk Figure 1. Cardiovascular Outcomes and Death from Any Cause. Shown are the cumulative incidence of the primary outcome (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) (Panel A), cumulative incidence of death from cardiovascular causes (Panel B), the Kaplan Meier estimate for death from any cause (Panel C), and the cumulative incidence of hospitalization for heart failure (Panel D) in the pooled empagliflozin group and the placebo group among patients who received at least one dose of a study drug. Hazard ratios are based on Cox regression analyses. n engl j med 373;22 nejm.org November 26,
6 The new england journal of medicine Table 1. Primary and Secondary Cardiovascular Outcomes. Outcome Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke: primary outcome* (N = 2333) (N = 4687) Hazard Ratio (95% CI) P Value rate/1000 rate/1000 no. (%) patient-yr no. (%) patient-yr 282 (12.1) (10.5) ( ) Noninferiority <0.001 Superiority 0.04 Death from cardiovascular causes, nonfatal myocardial 333 (14.3) (12.8) ( ) infarction, nonfatal stroke, or hospi- talization for unstable angina: key secondary outcome* Noninferiority <0.001 Superiority 0.08 Death From any cause 194 (8.3) (5.7) ( ) <0.001 From cardiovascular causes 137 (5.9) (3.7) ( ) <0.001 Fatal or nonfatal myocardial infarction excluding silent myocardial infarction 126 (5.4) (4.8) ( ) 0.23 Nonfatal myocardial infarction excluding silent myocardial infarction 121 (5.2) (4.5) ( ) 0.22 Silent myocardial infarction 15 (1.2) (1.6) ( ) 0.42 Hospitalization for unstable angina 66 (2.8) (2.8) ( ) 0.97 Coronary revascularization procedure 186 (8.0) (7.0) ( ) 0.11 Fatal or nonfatal stroke 69 (3.0) (3.5) ( ) 0.26 Nonfatal stroke 60 (2.6) (3.2) ( ) 0.16 Transient ischemic attack 23 (1.0) (0.8) ( ) 0.54 Hospitalization for heart failure 95 (4.1) (2.7) ( ) Hospitalization for heart failure or death from cardiovascular causes excluding fatal stroke 198 (8.5) (5.7) ( ) <0.001 * Data were analyzed with the use of a four-step hierarchical-testing strategy for the pooled empagliflozin group versus the placebo group in the following order: noninferiority for the primary outcome, noninferiority for the key secondary outcome, superiority for the primary outcome, and superiority for the key secondary outcome. Each successive hypothesis could be tested, provided that those preceding it met the designated level of significance. Data are based on Cox regression analyses in patients who received at least one dose of a study drug. One-sided P values are shown for tests of noninferiority, and two-sided P values are shown for tests of superiority. Silent myocardial infarction was analyzed in 2378 patients in the empagliflozin group and 1211 patients in the placebo group. identical to those in the pooled analysis, but the individual dose effects were not significant, owing to the smaller numbers of outcome events in the individual groups (Table S6 and Fig. S2 in Section M in the Supplementary Appendix). The hazard ratios for the primary outcome were 0.85 (95% CI, 0.72 to 1.01; P = 0.07) for the 10-mg dose of empagliflozin versus placebo and 0.86 (95% CI, 0.73 to 1.02; P = 0.09) for the 25-mg dose versus placebo. In subgroup analyses, there was some heterogeneity for the primary outcome. In contrast, there was a consistent benefit of empagliflozin versus placebo on death from cardiovascular causes across all subgroups (Fig. 2, and Tables S7 and S8 in Section N in the Supplementary Appendix). In prespecified sensitivity analyses based on events that occurred within 30 days after last dose of a study drug, results for the primary outcome, cardiovascular death, myocardial infarction, and stroke were consistent with the 2122 n engl j med 373;22 nejm.org November 26, 2015
7 in Type 2 Diabetes primary analyses, and the point estimate for the hazard ratio for stroke was closer to 1.00 (Tables S9 and S10 in Section O in the Supplementary Appendix). A sensitivity analysis of death from any cause in which it was assumed that all patients who were lost to follow-up in the empagliflozin group died and all patients who were lost to follow-up in the placebo group were alive showed a significant benefit of empagliflozin versus placebo (hazard ratio, 0.77; 95% CI, 0.65 to 0.93; P = 0.005). Glycemic Control After 12 weeks, during which glucose-lowering therapy was to remain unchanged, the adjusted mean differences in the glycated hemoglobin level between patients receiving empagliflozin and those receiving placebo were 0.54 percentage points (95% CI, 0.58 to 0.49) in the 10-mg group and 0.60 percentage points (95% CI, 0.64 to 0.55) in the 25-mg group (Fig. 3). At week 94, the adjusted mean differences in the glycated hemoglobin level between patients receiving empagliflozin and those receiving placebo were 0.42 percentage points (95% CI, 0.48 to 0.36) and 0.47 percentage points (95% CI, 0.54 to 0.41), respectively; at week 206, the differences were 0.24 percentage points (95% CI, 0.40 to 0.08) and 0.36 percentage points (95% CI, 0.51 to 0.20). Cardiovascular Risk Factors Over the course of the study, empagliflozin, as compared with placebo, was associated with small reductions in weight, waist circumference, uric acid level, and systolic and diastolic blood pressure with no increase in heart rate and small increases in both LDL and HDL cholesterol (Fig. S3 in Section P in the Supplementary Appendix). A higher percentage of patients in the placebo group received additional glucose-lowering medications (including sulfonylurea and insulin), antihypertensive medications (including diuretics), and anticoagulants during the trial, with no between-group difference in the receipt of lipidlowering drugs (Tables S11 and S12 in Section Q in the Supplementary Appendix). Safety and Adverse Events The proportions of patients who had adverse events, serious adverse events, and adverse events leading to the discontinuation of a study drug were similar in the empagliflozin group and the placebo group (Table 2). Genital infection was reported in a higher percentage of patients in the pooled empagliflozin group. The proportions of patients with confirmed hypoglycemic adverse events, acute renal failure, diabetic ketoacidosis, thromboembolic events, bone fracture, and events consistent with volume depletion were similar in the two study groups. Urosepsis was reported in 0.4% of patients in the empagliflozin group and 0.1% of those in the placebo group, but there was no imbalance in overall rates of urinary tract infection, complicated urinary tract infection, or pyelonephritis (Table S13 in Section R in the Supplementary Appendix). Clinical laboratory data are provided in Table S14 in Section S in the Supplementary Appendix. There were no relevant changes in electrolytes in the two study groups. Hematocrit values were higher in the empagliflozin groups than in the placebo group (mean [±SD] changes from baseline, 4.8±5.5% in the group receiving 10 mg of empagliflozin, 5.0±5.3% in the group receiving 25 mg of empagliflozin, and 0.9±4.7% in the placebo group). Discussion Among patients with type 2 diabetes at high risk for cardiovascular events, those receiving empagliflozin had a lower rate of the primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke than did patients receiving placebo. The difference between empagliflozin and placebo was driven by a significant reduction in death from cardiovascular causes, with no significant between-group difference in the risk of myocardial infarction or stroke. Since the two groups had similar rates of hospitalization for unstable angina, there was no significant difference in the key secondary outcome, which included the risk of hospitalization for unstable angina. Patients in the empagliflozin group had significantly lower risks of death from any cause and for hospitalization for heart failure than did those in the placebo group. Although a small dose response effect for the 10-mg dose of empagliflozin versus placebo and the 25-mg dose versus placebo has been documented for metabolic responses, in our study the two dose groups had similar hazard n engl j med 373;22 nejm.org November 26,
8 The new england journal of medicine Subgroup All patients Age <65 yr 65 yr Sex Male Female Race White Asian Black Glycated hemoglobin <8.5% 8.5% Body-mass index <30 30 Blood pressure control SBP 140 mm Hg or DBP 90 mm Hg SBP <140 mm Hg and DBP <90 mm Hg Estimated glomerular filtration rate 90 ml/min/1.73 m 2 60 to <90 ml/min/1.73 m 2 <60 ml/min/1.73 m 2 Urine albumin-to-creatinine ratio <30 mg/g 30 to 300 mg/g >300 mg/g Cardiovascular risk Only cerebrovascular disease Only coronary artery disease Only peripheral artery disease 2 or 3 high-risk categories Insulin No Yes Statins or ezetimibe No Yes Antihypertensive therapy No Yes ACE inhibitor or ARB No Yes Beta-blocker No Yes Diuretic No Yes no. in subgroup Hazard Ratio (95% CI) Primary Outcome P Value for Interaction Death from Cardiovascular Causes Hazard Ratio (95% CI) P Value for Interaction Favors Favors Favors Favors 2124 n engl j med 373;22 nejm.org November 26, 2015
9 in Type 2 Diabetes Figure 2 (facing page). Subgroup Analyses for the Primary Outcome and Death from Cardiovascular Causes. Shown are the results of a prespecified Cox regression analysis of data for subgroups of patients with respect to the primary outcome. Subgroup analyses of death from cardiovascular causes were conducted post hoc. P values are for tests of homogeneity of between-group differences among subgroups with no adjustment for multiple testing. The size of the ovals is proportional to the number of patients in the subgroup. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, DBP diastolic blood pressure, and SBP systolic blood pressure. ratios for cardiovascular outcomes. Thus, in clinical practice, the choice of the empagliflozin dose will probably depend primarily on the achievement of metabolic targets and the occurrence of adverse events. These benefits were observed in a population with established cardiovascular disease in whom cardiovascular risk factors, including blood pressure and dyslipidemia, were well treated with the use of renin angiotensin aldosterone system inhibitors, statins, and acetylsalicylic acid. The reductions in the risk of cardiovascular death in the empagliflozin group were consistent across subgroups according to baseline characteristics. Notably, reductions in the risks of death from cardiovascular causes and from any cause occurred early in the trial, and these benefits continued throughout the study. The relative reduction of 32% in the risk of death from any cause in the pooled empagliflozin group means that 39 patients (41 in the 10-mg group and 38 in the 25-mg group) would need to be treated during a 3-year period to prevent one death, but these numbers cannot be extrapolated to patient populations with other clinical characteristics. Even though investigators were encouraged to adjust glucose-lowering therapy according to local guidelines, many patients did not reach their glycemic targets, with an adjusted mean glycated hemoglobin level at week 206 of 7.81% in the pooled empagliflozin group and 8.16% in the mg 25 mg Adjusted Mean Glycated Hemoglobin (%) No. at Risk 10 mg 25 mg Week Figure 3. Glycated Hemoglobin Levels. Shown are mean (±SE) glycated hemoglobin levels in the three study groups, as calculated with the use of a repeated-measures analysis as a mixed model of all data for patients who received at least one dose of a study drug and had a baseline measurement. The model included baseline glycated hemoglobin as a linear covariate, with baseline estimated glomerular filtration rate, geographic region, body-mass index, the last week a patient could have had a glycated hemoglobin measurement, study group, visit, visit according to treatment interaction, and baseline glycated hemoglobin according to visit interaction as fixed effects. n engl j med 373;22 nejm.org November 26,
10 The new england journal of medicine (N = 2333), 10 mg (N = 2345), 25 mg (N = 2342) Pooled (N = 4687) Event number of patients (percent) Any adverse event 2139 (91.7) 2112 (90.1) 2118 (90.4) 4230 (90.2) Severe adverse event 592 (25.4) 536 (22.9) 564 (24.1) 1100 (23.5) Serious adverse event Any 988 (42.3) 876 (37.4) 913 (39.0) 1789 (38.2) Death 119 (5.1) 97 (4.1) 79 (3.4) 176 (3.8) Adverse event leading to discontinuation of a 453 (19.4) 416 (17.7) 397 (17.0) 813 (17.3) study drug Confirmed hypoglycemic adverse event Any 650 (27.9) 656 (28.0) 647 (27.6) 1303 (27.8) Requiring assistance 36 (1.5) 33 (1.4) 30 (1.3) 63 (1.3) Event consistent with urinary tract infection 423 (18.1) 426 (18.2) 416 (17.8) 842 (18.0) Male patients 158 (9.4) 180 (10.9) 170 (10.1) 350 (10.5) Female patients 265 (40.6) 246 (35.5) 246 (37.3) 492 (36.4) Complicated urinary tract infection** 41 (1.8) 34 (1.4) 48 (2.0) 82 (1.7) Event consistent with genital infection 42 (1.8) 153 (6.5) 148 (6.3) 301 (6.4) Male patients 25 (1.5) 89 (5.4) 77 (4.6) 166 (5.0) Female patients 17 (2.6) 64 (9.2) 71 (10.8) 135 (10.0) Event consistent with volume depletion 115 (4.9) 115 (4.9) 124 (5.3) 239 (5.1) Acute renal failure 155 (6.6) 121 (5.2) 125 (5.3) 246 (5.2) Acute kidney injury 37 (1.6) 26 (1.1) 19 (0.8) 45 (1.0) Diabetic ketoacidosis 1 (<0.1) 3 (0.1) 1 (<0.1) 4 (0.1) Thromboembolic event 20 (0.9) 9 (0.4) 21 (0.9) 30 (0.6) Bone fracture 91 (3.9) 92 (3.9) 87 (3.7) 179 (3.8) * Data are for patients who had one or more event and who had received at least one dose of a study drug. All events occurred within 7 days after the last receipt of the study drug. P<0.001 for the comparison with placebo. P<0.05 for the comparison with placebo. P<0.01 for the comparison with placebo. A confirmed hypoglycemic adverse event was a plasma glucose level of less than 70 mg per deciliter (3.9 mmol per liter) or an event requiring assistance. Table 2. Adverse Events.* The definition of urinary tract infection was based on 79 preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA). Percentages were calculated as the proportions of all men and all women with the event. ** Complicated urinary tract infection was defined as pyelonephritis, urosepsis, or a serious adverse event consistent with urinary tract infection. A breakdown of such events according to MedDRA preferred terms is provided in Table S13 in Section R in the Supplementary Appendix. The definition of genital infection was based on 88 MedDRA preferred terms. Percentages were calculated as the proportions of all men and all women with the event. The definition of volume depletion was based on 8 MedDRA preferred terms. The definitions of acute renal failure and thromboembolic event were based on 1 standardized MedDRA query for each. The definition of ketoacidosis was based on 4 MedDRA preferred terms. The definition of bone fracture was based on 62 MedDRA preferred terms. placebo group. Our trial was designed to assess the specific effects of empagliflozin on clinical outcomes, and the mechanisms behind the observed benefits are speculative. As such, we infer that the mechanisms behind the cardiovascular benefits of empagliflozin are multidimensional 25 and possibly involve changes in arterial stiffness, 26,27 cardiac function, and cardiac oxygen 2126 n engl j med 373;22 nejm.org November 26, 2015
11 in Type 2 Diabetes demand (in the absence of sympathetic-nerve activation), 26 as well as cardiorenal effects, 21,26,28,29 reduction in albuminuria, 20,30 reduction in uric acid, and established effects on hyperglycemia, weight, visceral adiposity, and blood pressure Our trial provides data to support the longterm use of empagliflozin, as well as strong evidence for a reduction in cardiovascular risk. As observed in previous trials, genital infection was more common in patients treated with empagliflozin. Urosepsis was infrequent but reported in more patients treated with empagliflozin, although there was no increase in the overall rate of urinary tract infection, complicated urinary tract infection, or pyelonephritis. The proportions of patients with diabetic ketoacidosis, volume depletion, thromboembolic events, and bone fracture were low (ranging from <1% for ketoacidosis and thromboembolic events to 5% for volume depletion) and similar in the empagliflozin groups and the placebo group. Concern has been expressed about the renal safety of inhibitors of sodium glucose cotransporter 2 over time. However, the percentage of patients with acute renal failure (including acute kidney injury) was lower in the empagliflozin groups than in the placebo group, and renal function was maintained with empagliflozin. In conclusion, patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin had significantly lower rates of the primary composite cardiovascular outcome and of death from any cause than did those in the placebo group when the study drugs were added to standard care. Supported by Boehringer Ingelheim and Eli Lilly. Dr. Zinman reports receiving consulting fees from Merck, Novo Nordisk, Sanofi Aventis, Eli Lilly, Takeda, AstraZeneca, and Janssen and grant support through his institution from Merck and Novo Nordisk; Dr. Wanner, receiving grant support from the European Foundation of Studies in Diabetes Boehringer Ingelheim European Diabetes Research Programme; Dr. Lachin, receiving fees for serving on a steering committee from Merck, fees for serving on data monitoring committees from Gilead, Janssen, and Novartis, and consulting fees from AstraZeneca; Dr. Fitchett, receiving fees for serving on a data and safety monitoring board from Novo Nordisk and consulting fees from AstraZeneca, Sanofi, and Merck; Drs. Bluhmki, Hantel, Johansen, Woerle, and Broedel and Ms. Mattheus, being employees of Boehringer Ingelheim; and Dr. Inzucchi, receiving fees for serving on advisory boards from Merck, Janssen, Sanofi/Regeneron, Poxel, Boehringer Ingelheim, and Eli Lilly, fees for serving on data monitoring committees from Novo Nordisk and Intarcia, fees for serving on a steering committee from Lexicon, serving as an expert witness on behalf of Takeda in a patent suit, and receiving clinical-study drugs from Takeda, and receiving funding through his institution from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Abbott, Merck, and Sanofi. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. We thank the patients who participated in this trial and Elizabeth Ng and Wendy Morris of Fleishman-Hillard Group for their assistance in medical writing. References 1. Sarwar N, Gao P, Seshasai SR, et al. of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 Chatterjee S, Farkouh ME, Scirica BM. 8. Udell JA, Cavender MA, Bhatt DL, prospective studies. Lancet 2010; 375: Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with 2. Beckman JA, Creager MA, Libby P. or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lan- Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. cet Diabetes Endocrinol 2015; 3: JAMA 2002; 287: Department of Health and Human 3. Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometa- Guidance for industry: diabetes mellitus Services, Food and Drug Administration. bolic multimorbidity with mortality. JAMA evaluating cardiovascular risk in new 2015; 314: antidiabetic therapies to treat type 2 diabetes ( downloads/ drugs/ 4. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J guidances/ ucm pdf). guidancecomplianceregulatoryinformation/ Med 2015; 373: Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for dia- 5. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome betes: basic physiology and consequences. in patients with type 2 diabetes. N Engl J Diab Vasc Dis Res 2015; 12: Med 2013; 369: Grempler R, Thomas L, Eckhardt M, 6. Scirica BM, Bhatt DL, Braunwald E, et et al., a novel selective al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes inhibitor: characterisation and compari- sodium glucose cotransporter-2 (SGLT-2) mellitus. N Engl J Med 2013; 369: son with other SGLT-2 inhibitors. Diabetes Obes Metab 2012; 14: Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up 12. Boehringer Ingelheim Pharmaceuticals. Jardiance (empagliflozin) tablets; prescribing information ( BIWebAccess/ ViewServlet.ser?docBase=renetnt&folder Path=/ Prescribing+Information/ PIs/ Jardiance/ jardiance.pdf). 13. Häring HU, Merker L, Seewaldt-Becker E, et al. as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013; 36: Häring HU, Merker L, Seewaldt-Becker E, et al. as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014; 37: Kovacs CS, Seshiah V, Swallow R, et al. improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014; 16: Roden M, Weng J, Eilbracht J, et al. monotherapy with sitagliptin as an active comparator in pa- n engl j med 373;22 nejm.org November 26,
12 in Type 2 Diabetes tients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014; 37: Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, doubleblind, placebo-controlled trial. Diabetes Obes Metab 2015 June 4 (Epub ahead of print). 19. Tikkanen I, Narko K, Zeller C, et al. reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38: Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, doubleblind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: Chilton RC, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2014 September 7 (Epub ahead of print). 22. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014; 2: Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol 2014; 13: Beyersmann J, Allignol A, Schumacher M. Competing risks and multistate models with R. New York: Springer, Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015; 12: Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13: Cardoso CR, Ferreira MT, Leite NC, Salles GF. Prognostic impact of aortic stiffness in high-risk type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes Care 2013; 36: Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J 2010; 31: Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014; 37: Copyright 2015 Massachusetts Medical Society n engl j med 373;22 nejm.org November 26, 2015
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results
 Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Type 2 Diabetes. Tabinda Dugal GP Day 4/05/16
 Type 2 Diabetes Tabinda Dugal GP Day 4/05/16 Diabetes Diabetes.a growing health crisis in Britain 869m per year 10% of NHS budget Projections.. 5 million by 2025 Youngest patient? T2DM Type 2 diabetes
Type 2 Diabetes Tabinda Dugal GP Day 4/05/16 Diabetes Diabetes.a growing health crisis in Britain 869m per year 10% of NHS budget Projections.. 5 million by 2025 Youngest patient? T2DM Type 2 diabetes
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes
 ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
ADVANCE: a factorial randomised trial of blood pressure lowering and intensive glucose control in 11,140 patients with type 2 diabetes Effects of a fixed combination of the ACE inhibitor, perindopril,
Second- and Third-Line Approaches for Type 2 Diabetes Workgroup: Topic Brief
 Second- and Third-Line Approaches for Type 2 Diabetes Workgroup: Topic Brief March 7, 2016 Session Objective: The objective of this workshop is to assess the value of undertaking comparative effectiveness
Second- and Third-Line Approaches for Type 2 Diabetes Workgroup: Topic Brief March 7, 2016 Session Objective: The objective of this workshop is to assess the value of undertaking comparative effectiveness
Cardiovascular Effects of Drugs to Treat Diabetes
 Cardiovascular Effects of Drugs to Treat Diabetes Steven E. Nissen MD Chairman, Department of Cardiovascular Medicine Cleveland Clinic Disclosure Consulting: Many pharmaceutical companies Clinical Trials:
Cardiovascular Effects of Drugs to Treat Diabetes Steven E. Nissen MD Chairman, Department of Cardiovascular Medicine Cleveland Clinic Disclosure Consulting: Many pharmaceutical companies Clinical Trials:
DISCLOSURES RISK ASSESSMENT. Stroke and Heart Disease -Is there a Link Beyond Risk Factors? Daniel Lackland, MD
 STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
STROKE AND HEART DISEASE IS THERE A LINK BEYOND RISK FACTORS? D AN IE L T. L AC K L AN D DISCLOSURES Member of NHLBI Risk Assessment Workgroup RISK ASSESSMENT Count major risk factors For patients with
How To Get Better Health Care
 Kardiovaskulär säkerhet vid behandling av typ 2-diabetes Vad säger senaste data? Michael Alvarsson Kliniken för Endokrinologi, Metabolism och Diabetes Karolinska Universitetssjukhuset Solna Near-normal
Kardiovaskulär säkerhet vid behandling av typ 2-diabetes Vad säger senaste data? Michael Alvarsson Kliniken för Endokrinologi, Metabolism och Diabetes Karolinska Universitetssjukhuset Solna Near-normal
Harmony Clinical Trial Medical Media Factsheet
 Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Overview Harmony is the global Phase III clinical trial program for Tanzeum (albiglutide), a product developed by GSK for the treatment of type 2 diabetes. The comprehensive program comprised eight individual
Cardiovascular Disease in Diabetes
 Cardiovascular Disease in Diabetes Where Do We Stand in 2012? David M. Kendall, MD Distinguished Medical Fellow Lilly Diabetes Associate Professor of Medicine University of MInnesota Disclosure - Duality
Cardiovascular Disease in Diabetes Where Do We Stand in 2012? David M. Kendall, MD Distinguished Medical Fellow Lilly Diabetes Associate Professor of Medicine University of MInnesota Disclosure - Duality
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial
 Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
Main Effect of Screening for Coronary Artery Disease Using CT
 Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety
 Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety Anders Svensson MD, PhD Head of Global Clinical Development Metabolism, F Hoffmann LaRoche Ltd. Basel, Switzerland Overview of
Novel Trial Designs in T2D to Satisfy Regulatory Requirements for CV Safety Anders Svensson MD, PhD Head of Global Clinical Development Metabolism, F Hoffmann LaRoche Ltd. Basel, Switzerland Overview of
Use of Glycated Hemoglobin and Microalbuminuria in the Monitoring of Diabetes Mellitus
 Agency for Healthcare Research and Quality Evidence Report/Technology Assessment Number 84 Use of Glycated Hemoglobin and Microalbuminuria in the Monitoring of Diabetes Mellitus Summary Overview Clinical
Agency for Healthcare Research and Quality Evidence Report/Technology Assessment Number 84 Use of Glycated Hemoglobin and Microalbuminuria in the Monitoring of Diabetes Mellitus Summary Overview Clinical
Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence
 Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Guidelines Secondary Stroke Prevention Luke Bradbury, MD 10/4/14 Fall WAPA Conferfence Stroke/TIA Nearly 700,000 ischemic strokes and 240,000 TIAs every year in the United States Currently, the risk for
Rivaroxaban for acute coronary syndromes
 Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Northern Treatment Advisory Group Rivaroxaban for acute coronary syndromes Lead author: Nancy Kane Regional Drug & Therapeutics Centre (Newcastle) May 2014 2014 Summary Current long-term management following
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Prescription Pattern of Anti Hypertensive Drugs used in Hypertensive Patients with Associated Type2 Diabetes Mellitus in A Tertiary Care Hospital
 Research Article Prescription Pattern of Anti Hypertensive Drugs used in Hypertensive Patients with Associated Type2 Diabetes Mellitus in A Tertiary Care Hospital *T. JANAGAN 1, R. KAVITHA 1, S. A. SRIDEVI
Research Article Prescription Pattern of Anti Hypertensive Drugs used in Hypertensive Patients with Associated Type2 Diabetes Mellitus in A Tertiary Care Hospital *T. JANAGAN 1, R. KAVITHA 1, S. A. SRIDEVI
Metabolic Syndrome Overview: Easy Living, Bitter Harvest. Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007
 Metabolic Syndrome Overview: Easy Living, Bitter Harvest Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007 Evolution of Metabolic Syndrome 1923: Kylin describes clustering
Metabolic Syndrome Overview: Easy Living, Bitter Harvest Sabrina Gill MD MPH FRCPC Caroline Stigant MD FRCPC BC Nephrology Days, October 2007 Evolution of Metabolic Syndrome 1923: Kylin describes clustering
SUPPLEMENTARY MATERIALS. Rivaroxaban for Stroke Prevention in East Asian Patients from the ROCKET AF Trial
 1 SUPPLEMENTARY MATERIALS Rivaroxaban for Stroke Prevention in East Asian Patients from the ROCKET AF Trial Professor Ka Sing Lawrence Wong on behalf of The Executive Steering Committee and the ROCKET
1 SUPPLEMENTARY MATERIALS Rivaroxaban for Stroke Prevention in East Asian Patients from the ROCKET AF Trial Professor Ka Sing Lawrence Wong on behalf of The Executive Steering Committee and the ROCKET
CASE A1 Hypoglycemia in an Elderly T2DM Patient with Heart Failure
 Hypoglycemia in an Elderly T2DM Patient with Heart Failure 1 I would like to introduce you to Sophie, an elderly patient with long-standing type 2 diabetes, who has a history of heart failure, a common
Hypoglycemia in an Elderly T2DM Patient with Heart Failure 1 I would like to introduce you to Sophie, an elderly patient with long-standing type 2 diabetes, who has a history of heart failure, a common
Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease
 Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
Diabetic nephropathy is detected clinically by the presence of persistent microalbuminuria or proteinuria.
 Kidney Complications Diabetic Nephropathy Diabetic nephropathy is detected clinically by the presence of persistent microalbuminuria or proteinuria. The peak incidence of nephropathy is usually 15-25 years
Kidney Complications Diabetic Nephropathy Diabetic nephropathy is detected clinically by the presence of persistent microalbuminuria or proteinuria. The peak incidence of nephropathy is usually 15-25 years
Prognostic impact of uric acid in patients with stable coronary artery disease
 Prognostic impact of uric acid in patients with stable coronary artery disease Gjin Ndrepepa, Siegmund Braun, Martin Hadamitzky, Massimiliano Fusaro, Hans-Ullrich Haase, Kathrin A. Birkmeier, Albert Schomig,
Prognostic impact of uric acid in patients with stable coronary artery disease Gjin Ndrepepa, Siegmund Braun, Martin Hadamitzky, Massimiliano Fusaro, Hans-Ullrich Haase, Kathrin A. Birkmeier, Albert Schomig,
嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯
 The Clinical Efficacy and Safety of Sodium Glucose Cotransporter-2 (SGLT2) Inhibitors in Adults with Type 2 Diabetes Mellitus 嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯 Diabetes Mellitus : A group of diseases characterized
The Clinical Efficacy and Safety of Sodium Glucose Cotransporter-2 (SGLT2) Inhibitors in Adults with Type 2 Diabetes Mellitus 嘉 義 長 庚 醫 院 藥 劑 科 Speaker : 翁 玟 雯 Diabetes Mellitus : A group of diseases characterized
Addendum to Clinical Review for NDA 22-512
 Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
FULL COVERAGE FOR PREVENTIVE MEDICATIONS AFTER MYOCARDIAL INFARCTION NEW ENGLAND JOURNAL OF MEDICINE 2011; DOI: 10.
 FULL COVERAGE FOR PREVENTIVE MEDICATIONS AFTER MYOCARDIAL INFARCTION NEW ENGLAND JOURNAL OF MEDICINE 2011; DOI: 10.1056/NEJMSA1107913 Niteesh K. Choudhry, MD, PhD, 1 Jerry Avorn, MD, 1 Robert J. Glynn,
FULL COVERAGE FOR PREVENTIVE MEDICATIONS AFTER MYOCARDIAL INFARCTION NEW ENGLAND JOURNAL OF MEDICINE 2011; DOI: 10.1056/NEJMSA1107913 Niteesh K. Choudhry, MD, PhD, 1 Jerry Avorn, MD, 1 Robert J. Glynn,
MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES
 MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest EXPLAINING
MANAGEMENT OF LIPID DISORDERS: IMPLICATIONS OF THE NEW GUIDELINES Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Declaration of full disclosure: No conflict of interest EXPLAINING
Statins and Risk for Diabetes Mellitus. Background
 Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
Statins and Risk for Diabetes Mellitus Kevin C. Maki, PhD, FNLA Midwest Center for Metabolic & Cardiovascular Research and DePaul University, Chicago, IL 1 Background In 2012 the US Food and Drug Administration
SHORT CLINICAL GUIDELINE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SHORT CLINICAL GUIDELINE SCOPE 1 Guideline title Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes 1.1 Short title Type 2
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SHORT CLINICAL GUIDELINE SCOPE 1 Guideline title Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes 1.1 Short title Type 2
Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus
 original article Effects of Blood-Pressure Control in Type 2 Diabetes Mellitus The ACCORD Study Group* ABSTRACT Background There is no evidence from randomized trials to support a strategy of lowering
original article Effects of Blood-Pressure Control in Type 2 Diabetes Mellitus The ACCORD Study Group* ABSTRACT Background There is no evidence from randomized trials to support a strategy of lowering
Aggressive Lowering of Blood Pressure in type 2 Diabetes Mellitus: The Diastolic Cost
 Aggressive Lowering of Blood Pressure in type 2 Diabetes Mellitus: The Diastolic Cost Naftali Stern Institute of Endocrinology, Metabolism and Hypertension Tel Aviv -Sourasky Medical Center and Sackler
Aggressive Lowering of Blood Pressure in type 2 Diabetes Mellitus: The Diastolic Cost Naftali Stern Institute of Endocrinology, Metabolism and Hypertension Tel Aviv -Sourasky Medical Center and Sackler
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013
 Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
Kevin Saunders MD CCFP Rivergrove Medical Clinic Wellness Institute @ SOGH April 17 2013 Family physician with Rivergrove Medical Clinic Practice in the north end since 1985 Medical Director of the Wellness
CASE B1. Newly Diagnosed T2DM in Patient with Prior MI
 Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
Newly Diagnosed T2DM in Patient with Prior MI 1 Our case involves a gentleman with acute myocardial infarction who is newly discovered to have type 2 diabetes. 2 One question is whether anti-hyperglycemic
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Primary prevention of chronic kidney disease: managing diabetes mellitus to reduce the risk of progression to CKD
 Primary prevention of chronic kidney disease: managing diabetes mellitus to reduce the risk of progression to CKD Date written: July 2012 Author: Kate Wiggins, Graeme Turner, David Johnson GUIDELINES We
Primary prevention of chronic kidney disease: managing diabetes mellitus to reduce the risk of progression to CKD Date written: July 2012 Author: Kate Wiggins, Graeme Turner, David Johnson GUIDELINES We
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial
 The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
Individual Study Table Referring to Part of Dossier: Volume: Page:
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Trilipix (ABT-335) Name of Active Ingredient: choline salt of fenofibric acid Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
2.0 Synopsis AbbVie Inc. Name of Study Drug: Trilipix (ABT-335) Name of Active Ingredient: choline salt of fenofibric acid Individual Study Table Referring to Part of Dossier: Volume: Page: (For National
Hypertension and Diabetes
 Hypertension and Diabetes C.W. Spellman, D.O., Ph.D., FACOI Professor & Associate Dean Research Dir. Center Diabetes & Metabolic Disorders Texas Tech University Health Science Center Midland-Odessa, Texas
Hypertension and Diabetes C.W. Spellman, D.O., Ph.D., FACOI Professor & Associate Dean Research Dir. Center Diabetes & Metabolic Disorders Texas Tech University Health Science Center Midland-Odessa, Texas
Intensive Diabetes Therapy and Glomerular Filtration Rate in Type 1 Diabetes
 original article Diabetes and Glomerular Filtration Rate in Type 1 Diabetes The DCCT/EDIC Research Group* A BS TR AC T Background An impaired glomerular filtration rate (GFR) leads to end-stage renal disease
original article Diabetes and Glomerular Filtration Rate in Type 1 Diabetes The DCCT/EDIC Research Group* A BS TR AC T Background An impaired glomerular filtration rate (GFR) leads to end-stage renal disease
The largest clinical study of Bayer's Xarelto (rivaroxaban) Wednesday, 14 November 2012 07:38
 Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study
 DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study National Diabetes Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What
DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study National Diabetes Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What
linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd
 linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd 09 December 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of
linagliptin, 5mg film-coated tablet (Trajenta ) SMC No. (746/11) Boehringer Ingelheim / Eli Lilly and Company Ltd 09 December 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of
How To Know If You Have Microalbuminuria
 3 PREVALENCE AND PREDICTORS OF MICROALBUMINURIA IN PATIENTS WITH TYPE 2 DIABETES MELLITUS: A CROSS-SECTIONAL OBSERVATIONAL STUDY Dr Ashok S Goswami *, Dr Janardan V Bhatt**; Dr Hitesh Patel *** *Associate
3 PREVALENCE AND PREDICTORS OF MICROALBUMINURIA IN PATIENTS WITH TYPE 2 DIABETES MELLITUS: A CROSS-SECTIONAL OBSERVATIONAL STUDY Dr Ashok S Goswami *, Dr Janardan V Bhatt**; Dr Hitesh Patel *** *Associate
Improving drug prescription in elderly diabetic patients. FRANCESC FORMIGA Hospital Universitari de Bellvitge
 Improving drug prescription in elderly diabetic patients FRANCESC FORMIGA Hospital Universitari de Bellvitge High prevalence, but also increases the incidence. The older the patients, the higher the percentages
Improving drug prescription in elderly diabetic patients FRANCESC FORMIGA Hospital Universitari de Bellvitge High prevalence, but also increases the incidence. The older the patients, the higher the percentages
PEER REVIEW HISTORY ARTICLE DETAILS
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE
 ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE I- BACKGROUND: Coronary artery disease and stoke are the major killers in the United States.
ROLE OF LDL CHOLESTEROL, HDL CHOLESTEROL AND TRIGLYCERIDES IN THE PREVENTION OF CORONARY HEART DISEASE AND STROKE I- BACKGROUND: Coronary artery disease and stoke are the major killers in the United States.
Guidelines for the management of hypertension in patients with diabetes mellitus
 Guidelines for the management of hypertension in patients with diabetes mellitus Quick reference guide In the Eastern Mediterranean Region, there has been a rapid increase in the incidence of diabetes
Guidelines for the management of hypertension in patients with diabetes mellitus Quick reference guide In the Eastern Mediterranean Region, there has been a rapid increase in the incidence of diabetes
Can Common Blood Pressure Medications Cause Diabetes?
 Can Common Blood Pressure Medications Cause Diabetes? By Nieske Zabriskie, ND High blood pressure, or hypertension, is a major risk factor for cardiovascular disease. In the United States, approximately
Can Common Blood Pressure Medications Cause Diabetes? By Nieske Zabriskie, ND High blood pressure, or hypertension, is a major risk factor for cardiovascular disease. In the United States, approximately
Epidemiology of Hypertension 陈 奕 希 3120000591 李 禾 园 3120000050 王 卓 3120000613
 Epidemiology of Hypertension 陈 奕 希 3120000591 李 禾 园 3120000050 王 卓 3120000613 1 Definition Hypertension is a chronic medical condition in which the blood pressure in the arteries is elevated. 2 Primary
Epidemiology of Hypertension 陈 奕 希 3120000591 李 禾 园 3120000050 王 卓 3120000613 1 Definition Hypertension is a chronic medical condition in which the blood pressure in the arteries is elevated. 2 Primary
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 25 September 2008 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
Committee Approval Date: September 12, 2014 Next Review Date: September 2015
 Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
Medication Policy Manual Policy No: dru361 Topic: Pradaxa, dabigatran Date of Origin: September 12, 2014 Committee Approval Date: September 12, 2014 Next Review Date: September 2015 Effective Date: November
New and Future Treatments for Diabetes. Mary Charlton Specialty Doctor in Diabetes University Hospital Birmingham BARS Oct 2014
 New and Future Treatments for Diabetes Mary Charlton Specialty Doctor in Diabetes University Hospital Birmingham BARS Oct 2014 Conflicts of interest Investigator Carmelina study of Linagliptin (Boehringer
New and Future Treatments for Diabetes Mary Charlton Specialty Doctor in Diabetes University Hospital Birmingham BARS Oct 2014 Conflicts of interest Investigator Carmelina study of Linagliptin (Boehringer
Antiplatelet and Antithrombotics From clinical trials to guidelines
 Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
trends in the treatment of Diabetes type 2 - New classes of antidiabetic drugs. IAIM, 2015; 2(4): 223-
 Review Article Pharmacological trends in the treatment of Diabetes type 2 - New classes of antidiabetic Silvia Mihailova 1*, Antoaneta Tsvetkova 1, Anna Todorova 2 1 Assistant Pharmacist, Education and
Review Article Pharmacological trends in the treatment of Diabetes type 2 - New classes of antidiabetic Silvia Mihailova 1*, Antoaneta Tsvetkova 1, Anna Todorova 2 1 Assistant Pharmacist, Education and
Cardiovascular Risk in Diabetes
 Cardiovascular Risk in Diabetes Lipids Hypercholesterolaemia is an important reversible risk factor for cardiovascular disease and should be tackled aggressively in all diabetic patients. In Type 1 patients,
Cardiovascular Risk in Diabetes Lipids Hypercholesterolaemia is an important reversible risk factor for cardiovascular disease and should be tackled aggressively in all diabetic patients. In Type 1 patients,
Xarelto (Rivaroxaban)
 Xarelto (Rivaroxaban) Hightly selective, reversible, direct oral FXa inhibitor Maxium concentratiion after 2 to 4 hrs High bioavailability(66%),increase with food ( suggest with food) 1/3 from renal excretion,
Xarelto (Rivaroxaban) Hightly selective, reversible, direct oral FXa inhibitor Maxium concentratiion after 2 to 4 hrs High bioavailability(66%),increase with food ( suggest with food) 1/3 from renal excretion,
Prediction of Kidney Disease Progression in Patients with Diabetes
 Prediction of Kidney Disease Progression in Patients with Diabetes John Arthur, MD, PhD Medical University of South Carolina SEKDC Meeting September 8, 2012 Objectives Understand the importance of predicting
Prediction of Kidney Disease Progression in Patients with Diabetes John Arthur, MD, PhD Medical University of South Carolina SEKDC Meeting September 8, 2012 Objectives Understand the importance of predicting
NCT00272090. sanofi-aventis HOE901_3507. insulin glargine
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Generic drug name:
The WHI 12 Years Later: What Have We Learned about Postmenopausal HRT?
 AACE 23 rd Annual Scientific and Clinical Congress (2014) Syllabus Materials: The WHI 12 Years Later: What Have We Learned about Postmenopausal HRT? JoAnn E. Manson, MD, DrPH, FACP, FACE Chief, Division
AACE 23 rd Annual Scientific and Clinical Congress (2014) Syllabus Materials: The WHI 12 Years Later: What Have We Learned about Postmenopausal HRT? JoAnn E. Manson, MD, DrPH, FACP, FACE Chief, Division
Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D.
 Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D. Associate Investigator Palo Alto Medical Foundation Research Institute Consulting Assistant
Clinical Research on Lifestyle Interventions to Treat Obesity and Asthma in Primary Care Jun Ma, M.D., Ph.D. Associate Investigator Palo Alto Medical Foundation Research Institute Consulting Assistant
A list of FDA-approved testosterone products can be found by searching for testosterone at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/.
 FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke
FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke
CHAPTER V DISCUSSION. normal life provided they keep their diabetes under control. Life style modifications
 CHAPTER V DISCUSSION Background Diabetes mellitus is a chronic condition but people with diabetes can lead a normal life provided they keep their diabetes under control. Life style modifications (LSM)
CHAPTER V DISCUSSION Background Diabetes mellitus is a chronic condition but people with diabetes can lead a normal life provided they keep their diabetes under control. Life style modifications (LSM)
Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes
 Original Article and Cardiovascular Outcomes in Type 2 Diabetes Steven P. Marso, M.D., Gilbert H. Daniels, M.D., Kirstine Brown Frandsen, M.D., Peter Kristensen, M.D., E.M.B.A., Johannes F.E. Mann, M.D.,
Original Article and Cardiovascular Outcomes in Type 2 Diabetes Steven P. Marso, M.D., Gilbert H. Daniels, M.D., Kirstine Brown Frandsen, M.D., Peter Kristensen, M.D., E.M.B.A., Johannes F.E. Mann, M.D.,
New Cholesterol Guidelines: Carte Blanche for Statin Overuse Rita F. Redberg, MD, MSc Professor of Medicine
 New Cholesterol Guidelines: Carte Blanche for Statin Overuse Rita F. Redberg, MD, MSc Professor of Medicine Disclosures & Relevant Relationships I have nothing to disclose No financial conflicts Editor,
New Cholesterol Guidelines: Carte Blanche for Statin Overuse Rita F. Redberg, MD, MSc Professor of Medicine Disclosures & Relevant Relationships I have nothing to disclose No financial conflicts Editor,
Comparing Medications for Adults With Type 2 Diabetes Focus of Research for Clinicians
 Clinician Research Summary Diabetes Type 2 Diabetes Comparing Medications for Adults With Type 2 Diabetes Focus of Research for Clinicians A systematic review of 166 clinical studies published between
Clinician Research Summary Diabetes Type 2 Diabetes Comparing Medications for Adults With Type 2 Diabetes Focus of Research for Clinicians A systematic review of 166 clinical studies published between
ABOUT XARELTO CLINICAL STUDIES
 ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR DISEASE PREVENTION
 European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
European Medicines Agency London, 19 July 2007 Doc. Ref. EMEA/CHMP/EWP/311890/2007 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS (CHMP) DRAFT GUIDELINE ON THE EVALUATION OF MEDICINAL PRODUCTS FOR CARDIOVASCULAR
COST ANALYSIS OF ANTIDIABETIC DRUGS FOR DIABETES MELLITUS OUTPATIENT IN KODYA YOGYAKARTA HOSPITAL
 Malaysian Journal of Pharmaceutical Sciences, Vol. 5, No. 1, 19 23 (2007) COST ANALYSIS OF ANTIDIABETIC DRUGS FOR DIABETES MELLITUS OUTPATIENT IN KODYA YOGYAKARTA HOSPITAL TRI MURTI ANDAYANI* AND IKE IMANINGSIH
Malaysian Journal of Pharmaceutical Sciences, Vol. 5, No. 1, 19 23 (2007) COST ANALYSIS OF ANTIDIABETIC DRUGS FOR DIABETES MELLITUS OUTPATIENT IN KODYA YOGYAKARTA HOSPITAL TRI MURTI ANDAYANI* AND IKE IMANINGSIH
Atrial Fibrillation: A Different Perspective. Michael Heffernan MD PhD FRCPC FACC Staff Cardiologist Oakville Hospital
 Atrial Fibrillation: A Different Perspective Michael Heffernan MD PhD FRCPC FACC Staff Cardiologist Oakville Hospital Faculty/Presenter Disclosure Faculty: Dr. Michael Heffernan Relationships with commercial
Atrial Fibrillation: A Different Perspective Michael Heffernan MD PhD FRCPC FACC Staff Cardiologist Oakville Hospital Faculty/Presenter Disclosure Faculty: Dr. Michael Heffernan Relationships with commercial
DM Management in Elderly- What are the glucose targets?
 DM Management in Elderly- What are the glucose targets? AFSHAN ZAHEDI, BASC, MD, FRCP(C) ENDOCRINOLOGY WOMEN S COLLEGE HOSPITAL ASSISTANT PROFESSOR OF MEDICINE UNIVERSITY OF TORONTO NOVEMBER 2, 2011 Disclosures
DM Management in Elderly- What are the glucose targets? AFSHAN ZAHEDI, BASC, MD, FRCP(C) ENDOCRINOLOGY WOMEN S COLLEGE HOSPITAL ASSISTANT PROFESSOR OF MEDICINE UNIVERSITY OF TORONTO NOVEMBER 2, 2011 Disclosures
New Treatments for Stroke Prevention in Atrial Fibrillation. John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013
 New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
New Treatments for Stroke Prevention in Atrial Fibrillation John C. Andrefsky, MD, FAHA NEOMED Internal Medicine Review course May 5 th, 2013 Classification Paroxysmal atrial fibrillation (AF) Last < 7
Evidence-Based Secondary Stroke Prevention and Adherence to Guidelines
 Evidence-Based Secondary Stroke Prevention and Adherence to Guidelines Mitchell S.V. Elkind, MD, MS Associate Professor of Neurology Columbia University New York, NY Presenter Disclosure Information Mitchell
Evidence-Based Secondary Stroke Prevention and Adherence to Guidelines Mitchell S.V. Elkind, MD, MS Associate Professor of Neurology Columbia University New York, NY Presenter Disclosure Information Mitchell
4/4/2013. Mike Rizo, Pharm D, MBA, ABAAHP THE PHARMACIST OF THE FUTURE? METABOLIC SYNDROME AN INTEGRATIVE APPROACH
 METABOLIC SYNDROME AN INTEGRATIVE APPROACH AN OPPORTUNITY FOR PHARMACISTS TO MAKE A DIFFERENCE Mike Rizo, Pharm D, MBA, ABAAHP THE EVOLUTION OF THE PHARMACIST 1920s 1960s 2000s THE PHARMACIST OF THE FUTURE?
METABOLIC SYNDROME AN INTEGRATIVE APPROACH AN OPPORTUNITY FOR PHARMACISTS TO MAKE A DIFFERENCE Mike Rizo, Pharm D, MBA, ABAAHP THE EVOLUTION OF THE PHARMACIST 1920s 1960s 2000s THE PHARMACIST OF THE FUTURE?
Dual Antiplatelet Therapy. Stephen Monroe, MD FACC Chattanooga Heart Institute
 Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION
 Hull & East Riding Prescribing Committee PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION For guidance on Primary Prevention please see NICE guidance http://www.nice.org.uk/guidance/cg181
Hull & East Riding Prescribing Committee PRESCRIBING GUIDELINES FOR LIPID LOWERING TREATMENTS for SECONDARY PREVENTION For guidance on Primary Prevention please see NICE guidance http://www.nice.org.uk/guidance/cg181
Isabella Sudano & Franco Muggli
 Swiss Hypertension Guidelines Isabella Sudano & Franco Muggli CoLaus, Swisshype ESC 2005 Dokumentenname Datum Seite 1 European Journal of Cardiovascular Prevention and Rehabilitation 2009 Guideline...
Swiss Hypertension Guidelines Isabella Sudano & Franco Muggli CoLaus, Swisshype ESC 2005 Dokumentenname Datum Seite 1 European Journal of Cardiovascular Prevention and Rehabilitation 2009 Guideline...
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes
 Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Trends in Prescribing of Drugs for Type 2 Diabetes in General Practice in England (Chart 1) Other intermediate and long-acting insulins
 Type 2 Diabetes Type 2 diabetes is the most common form of diabetes, accounting for 90 95% of cases. 1 Charts 1 and 2 reflect the effect of increasing prevalence on prescribing and costs of products used
Type 2 Diabetes Type 2 diabetes is the most common form of diabetes, accounting for 90 95% of cases. 1 Charts 1 and 2 reflect the effect of increasing prevalence on prescribing and costs of products used
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Making Sense of the New Statin guidelines. They are more than just lowering your cholesterol!
 Making Sense of the New Statin guidelines They are more than just lowering your cholesterol! No Disclosures Margaret (Peg) O Donnell DNPs, FNP, ANP B-C, FAANP Senior Nurse Practitioner South Nassau Communities
Making Sense of the New Statin guidelines They are more than just lowering your cholesterol! No Disclosures Margaret (Peg) O Donnell DNPs, FNP, ANP B-C, FAANP Senior Nurse Practitioner South Nassau Communities
PROCEEDINGS DIABETIC NEPHROPATHY: DETECTION AND TREATMENT OF RENAL DISEASE IN PATIENTS WITH DIABETES* Jiten Vora, MA, MD, FRCP ABSTRACT
 DIABETIC NEPHROPATHY: DETECTION AND TREATMENT OF RENAL DISEASE IN PATIENTS WITH DIABETES* Jiten Vora, MA, MD, FRCP ABSTRACT Diabetic nephropathy affects people with either type 1 or type 2 diabetes mellitus.
DIABETIC NEPHROPATHY: DETECTION AND TREATMENT OF RENAL DISEASE IN PATIENTS WITH DIABETES* Jiten Vora, MA, MD, FRCP ABSTRACT Diabetic nephropathy affects people with either type 1 or type 2 diabetes mellitus.
Absolute cardiovascular disease risk assessment
 Quick reference guide for health professionals Absolute cardiovascular disease risk assessment This quick reference guide is a summary of the key steps involved in assessing absolute cardiovascular risk
Quick reference guide for health professionals Absolute cardiovascular disease risk assessment This quick reference guide is a summary of the key steps involved in assessing absolute cardiovascular risk
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
David Shu, MD, FRCPC Endocrinology, Royal Columbian Hospital October 8 th, 2010
 David Shu, MD, FRCPC Endocrinology, Royal Columbian Hospital October 8 th, 2010 Objectives At the end of the talk, the participants will be able to: 1. Identify the increasing prevalence of type 2 diabetes
David Shu, MD, FRCPC Endocrinology, Royal Columbian Hospital October 8 th, 2010 Objectives At the end of the talk, the participants will be able to: 1. Identify the increasing prevalence of type 2 diabetes
Adult Diabetes Clinician Guide
 Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Adult Diabetes Clinician Guide Introduction JANUARY 2016 This evidence-based guideline summary is based on the 2016 National Diabetes Guideline.
Kaiser Permanente National CLINICAL PRACTICE GUIDELINES Adult Diabetes Clinician Guide Introduction JANUARY 2016 This evidence-based guideline summary is based on the 2016 National Diabetes Guideline.
ASN DIALYSIS ADVISORY GROUP ASN DIALYSIS CURRICULUM
 ASN DIALYSIS ADVISORY GROUP ASN DIALYSIS CURRICULUM Management of Traditional Cardiovascular Risk Factors in ESRD (Hypertension, Dyslipidemia, Glycemic Control) ROBERT D. TOTO, M.D. PROFESSOR OF MEDICINE
ASN DIALYSIS ADVISORY GROUP ASN DIALYSIS CURRICULUM Management of Traditional Cardiovascular Risk Factors in ESRD (Hypertension, Dyslipidemia, Glycemic Control) ROBERT D. TOTO, M.D. PROFESSOR OF MEDICINE
How To Treat Dyslipidemia
 An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
An International Atherosclerosis Society Position Paper: Global Recommendations for the Management of Dyslipidemia Introduction Executive Summary The International Atherosclerosis Society (IAS) here updates
Olmesartan for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes
 original article for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes Hermann Haller, M.D., Sadayoshi Ito, M.D., Ph.D., Joseph L. Izzo, Jr., M.D., Andrzej Januszewicz, M.D., Shigehiro Katayama,
original article for the Delay or Prevention of Microalbuminuria in Type 2 Diabetes Hermann Haller, M.D., Sadayoshi Ito, M.D., Ph.D., Joseph L. Izzo, Jr., M.D., Andrzej Januszewicz, M.D., Shigehiro Katayama,
Follow-up of Blood-Pressure Lowering and Glucose Control in Type 2 Diabetes
 The new england journal of medicine original article Follow-up of Blood-Pressure Lowering and Glucose in Type Diabetes S. Zoungas, J. Chalmers, B. Neal, L. Billot, Q. Li, Y. Hirakawa, H. Arima, H. Monaghan,
The new england journal of medicine original article Follow-up of Blood-Pressure Lowering and Glucose in Type Diabetes S. Zoungas, J. Chalmers, B. Neal, L. Billot, Q. Li, Y. Hirakawa, H. Arima, H. Monaghan,
Bios 6648: Design & conduct of clinical research
 Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Perspectives on the Selection and Duration of Dual Antiplatelet Therapy
 Perspectives on the Selection and Duration of Dual Antiplatelet Therapy Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University
Perspectives on the Selection and Duration of Dual Antiplatelet Therapy Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI Director of Cardiovascular Research Associate Professor of Medicine University
CME Test for AMDA Clinical Practice Guideline. Diabetes Mellitus
 CME Test for AMDA Clinical Practice Guideline Diabetes Mellitus Part I: 1. Which one of the following statements about type 2 diabetes is not accurate? a. Diabetics are at increased risk of experiencing
CME Test for AMDA Clinical Practice Guideline Diabetes Mellitus Part I: 1. Which one of the following statements about type 2 diabetes is not accurate? a. Diabetics are at increased risk of experiencing
The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery
 The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery Michael E. Farkouh, MD, MSc Peter Munk Chair in Multinational Clinical Trials Director, Heart and Stroke
The Link Between Obesity and Diabetes The Rapid Evolution and Positive Results of Bariatric Surgery Michael E. Farkouh, MD, MSc Peter Munk Chair in Multinational Clinical Trials Director, Heart and Stroke
HYPERCHOLESTEROLAEMIA STATIN AND BEYOND
 HYPERCHOLESTEROLAEMIA STATIN AND BEYOND Andrea Luk Division of Endocrinology Department of Medicine & Therapeutics The Chinese University of Hong Kong HA Convention 4 May 2016 Statins reduce CVD and all-cause
HYPERCHOLESTEROLAEMIA STATIN AND BEYOND Andrea Luk Division of Endocrinology Department of Medicine & Therapeutics The Chinese University of Hong Kong HA Convention 4 May 2016 Statins reduce CVD and all-cause
