Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form
|
|
|
- Kathlyn Powell
- 8 years ago
- Views:
Transcription
1 Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form WHO Welke onderneming(en) werd(en) hierbij betrokken? (grootte, bedrijfssector, )? Met welke partner(s) (clusters, O&O-centrum, spin-offs, hubs, )? Three partners are involved in the CERACELL project: Bone Therapeutics, SIRRIS and Image Analysis. Bone Therapeutics (the project coordinator) is a leading international biopharmaceutical SME located in Wallonia (Belgium) that specialises in innovative cell therapy products for the treatment of bone diseases. Bone Therapeutics has developed two top-class products: PREOB, an autologous bone cell therapy product, currently in Phase III clinical trials for the treatment of osteonecrosis and non-union fractures, and ALLOB, an allogeneic bone cell therapy product, now in Phase IIA clinical trials for the treatment of delayed union and spinal fusion. Bone Therapeutics also benefits from the support of university and academic hospitals. SIRRIS is the Belgian research centre for the technological industry. It fosters innovation and supports the transfer of technology to 2,500 companies in the metalworking, plastics, medical, mechanical, electrical and electronic engineering, information and communication technology, and automotive sectors. Image Analysis is a UK-based medical imaging company set up to bridge the gap between the best of academic research and routine clinical practice.
2 WHAT Wat was de doelstelling van de innovatie? Waarin bestaat precies de innovatie (toepassing, soort innovatie product/procedé/businessmodel/support diensten/management, )? Porous bioceramic materials, cements and allografts are already used to treat bone defects, but these treatments are not fully satisfactory and reportedly have limited efficacy. CERACELL is a unique opportunity to manufacture biocompatible 3D pieces tailored to a patient s defect, to be used in conjunction with differentiated bone-forming cells to treat large bone defects. The use of stem cells for cellular therapy offers interesting and important prospects with regard to replacing damaged or lost tissue function. Through the use of mesenchymal stem cells (MSCs), an unlimited supply of fully differentiated cells is within reach, making cellular therapy a reality. In order to directly influence MSCs behaviour, biomaterials are chemically or biologically modified and treated to ultimately manipulate the signal transduction pathways of MSCs towards osteoblasts. Progress/innovation 1 Bone Therapeutics has developed a reproducible manufacturing process to obtain already differentiated bone-forming cells from MSCs. This innovation will allow better control over the cell fate resulting in enhanced efficacy and greater safety for the patient. In the CERACELL project, Bone Therapeutics is in charge of the preclinical (in vitro and in vivo) studies: (i) the behaviour and biocompatibility studies of bone-forming cells with the 3D bioceramic pieces and (ii) the in vivo preliminary studies (biocompatibility and efficacy). Progress/ innovation 2 In the CERACELL project, Image Analysis will develop algorithms and software to provide the necessary input (i.e. printer instructions) to create personalised scaffolds via a 3D printer. This approach is innovative as Image Analysis will attempt to model what is no longer there from the CT scan of bone defects. Progress/ innovation 3 The 3D printing of bioceramics is an innovative development as it combines (i) the conception and manufacturing of a tailored and personalised ceramic piece (for which the company already has background in the field), (ii) the integration of adequate microarchitecture (e.g. porosity) tailored to the inclusion of cells, and (iii) the internal design of the 3D pieces to promote the integration, survival and growth of bone-forming cells. In the CERACELL project, SIRRIS uses the 3D modelling algorithms developed by Image Analysis to manufacture tailor-made 3D bioceramics.
3 IMPACT Voor de business/ de onderneming (verwerving van een nieuwe markt, groei, kostenvermindering, ) Op de markt (eindafnemers, tussenpersonen) Over het geheel genomen, ten aanzien van de maatschappelijke thematiek The number of bone graft procedures performed in EU and the US is increasing year-on-year. Procedures involving bone grafts are predominantly used in the field of spinal surgery and, to a lesser degree, trauma. Fractures characterised by significant bone loss (large defects) and non-unions of long bones are the two main areas in this field. The application under development here primarily targets long bone defects and those involving the spine. Without taking into account the patient population suffering bone loss following a disease, we are looking at an overall population of 160,000 to 240,000 patients per annum in the EU and US. Bone autograft is considered the gold standard for repairing bone defects. However, its usage is limited by donor-site morbidity and supply. Limitations of bone allografts include the host s immunogenic response to the foreign tissue of the graft and the potential for disease transmission. Because of these limitations, the development as well as the availability of new orthobiologic materials to aid the management of bone defects is increasing. CERACELL s bone tissue engineering products are expected to equal the performance of autografts without affecting the patient of the bone graft harvesting and without having to deal with limited supply issues. The developed products should be able to gain a market share in the current allograft sector of the market as well as provide better solutions for patients currently treated with autografts.
4 KATALYSATOREN & OBSTAKELS Hoe verloopt / verliep de ontwikkeling van het project (duur, algemene indruk)? Wat vergemakkelijkt / vergemakkelijkte het verloop van het project (katalysatoren)? Wat zijn / waren de moeilijkheden en uitdagingen waaraan het hoofd moet /moest worden geboden (hinderpalen)? CERACELL is progressing as planned, having reached the mid-term project period. One obstacle is the high level of innovation required to offer an effective and patienttailored cell therapy treatment for repairing large bone defects. Indeed, according to the Target Product Profile, the product must (i) mimic the natural bone in terms of shape, structure and biomechanical properties and (ii) promote bone formation, remodelling and angiogenesis, (iii) while the scaffold must be progressively bioresorbed and replaced by natural bone tissue. Another identified obstacle is that the current regulatory framework is not suitable for the next generation of combined advanced-therapy medicinal products (ATMP) like Ceracell s. Indeed, in a Reflection paper dated June 2014, the EMA acknowledged that the requirements for the non-clinical and clinical development of ATMPs depended on the specific nature of each product and need to be designed and validated on a flexible case-by-case basis. For cell/scaffold products in particular, the difficulty lies in clarifying whether the scaffold is considered a medical device, an excipient or an active substance. In parallel with the approval granted by the competent authorities based on the product s benefit/risk profile, a solid Health Technology Assessment (HTA) will have to be prepared and thorough discussion will have to be held with the INAMI in order to determine the price, availability, and level of adequate reimbursement by third parties, such as insurance companies, government bodies and other healthcare organisations. These steps may pose an obstacle to commercialisation and the company s financial profit if the maximum price set by ministerial decision is too low to reconcile the company s economic needs. In contrast, the INAMI may decide not to reimburse a pharmaceutical product if its price is considered too high. As a catalyst, the project benefits from the top expertise and complementarity supplied by all three parties, including the highly valuable expertise of Bone Therapeutics in ATMP development from preclinical up to the advanced Phase III clinical stage.
5 LESSONS LEARNT Wat kon er / had er kunnen verbeterd worden om deze innovatie te vergemakkelijken? (enkel invullen indien van toepassing) Organisatie/management van het project Samenwerking/partnerschap Beheer van de intellectuele eigendom Lancering van de innovatie op de markt Financiering van het innovatieproject (fiscaal beleid, beschikbaarheid van kapitaal, investeringssubsidies, enz.) Andere beleidsaspecten /regelgevingsaspecten Market Launch One of the major challenges will be to timely and effectively introduce into medical practice the cell therapy approach as a convincing alternative to standard orthopaedic interventions. To facilitate this process, it will be important to raise physicians awareness of the superior beneficial effects of cell-based products for the treatment and/or prevention of bone diseases and orthopaedic conditions. Bone Therapeutics has already introduced this for its ongoing clinical programmes (e.g. fractures with impaired healing or spinal fusion). Regulatory Pathway To validate the product development and secure future approval from regulatory authorities, it will be necessary to have thorough discussions with the EMA (e.g. through a request for ATMP classification and scientific advice). This dialogue could be initiated during the preparatory phase of the pilot study.
Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form
 Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form WHO Welke onderneming(en) werd(en) hierbij betrokken? (grootte, bedrijfssector, )? Met welke partner(s) (clusters, O&O-centrum,
Innovation Across Borders Forum VBO-FEB Innovation Case Preparation Form WHO Welke onderneming(en) werd(en) hierbij betrokken? (grootte, bedrijfssector, )? Met welke partner(s) (clusters, O&O-centrum,
Technology Breakthrough in Spinal Implants (Technical Insights)
 Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
Technology Breakthrough in Spinal Implants (Technical Insights) Biomaterial innovations is a growth factor for spinal implant market June 2014 Table of Contents Section Page Number Executive Summary 4
The Cell Therapy Catapult
 The Cell Therapy Catapult Keith Thompson CEO January 29 2013 info@ct.catapult.org.uk Catapult is a Technology Strategy Board programme The Launch of Catapults Hauser 2 Hauser Report Creating new manufacturing
The Cell Therapy Catapult Keith Thompson CEO January 29 2013 info@ct.catapult.org.uk Catapult is a Technology Strategy Board programme The Launch of Catapults Hauser 2 Hauser Report Creating new manufacturing
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES
 THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
THE EVOLVING ROLE OF BONE-GRAFT SUBSTITUTES AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 77TH ANNUAL MEETING MARCH 9-13, 2010 NEW ORLEANS, LOUISIANA ORTHOPAEDIC DEVICE FORUM PREPARED BY: A. SETH GREENWALD,
LifeNet Health Presented by James Clagett, PhD., Chief Science Officer LifeNet Health - The Best Keep Secret in Regenerative Medicine
 Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
Presented by James Clagett, PhD., Chief Science Officer - The Best Keep Secret in Regenerative Medicine Corporate Headquarters Virginia Beach, VA Who is? helps save lives and restore health for thousands
Reflection paper on clinical aspects related to tissue engineered products
 1 2 3 19 March 2012 EMA/CAT/CPWP/573420/2009 Committee for Advanced Therapies (CAT) 4 5 6 Reflection paper on clinical aspects related to tissue engineered products Draft Draft agreed by CPWP 7 October
1 2 3 19 March 2012 EMA/CAT/CPWP/573420/2009 Committee for Advanced Therapies (CAT) 4 5 6 Reflection paper on clinical aspects related to tissue engineered products Draft Draft agreed by CPWP 7 October
Reference: NHS England B04/P/a
 Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation (HSCT) (All Ages): Revised Reference: NHS England B04/P/a 1 NHS England Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation
Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation (HSCT) (All Ages): Revised Reference: NHS England B04/P/a 1 NHS England Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation
TERUMO Corporation Business Strategy Conference
 TERUMO Corporation Business Strategy Conference THERAPEUTIC SYSTEMS & CELL PROCESSING MARK FLOWER GLOBAL MARKETING December 5, 2011 Regenerative Medicine, Cell Therapy, & Cell Processing Regenerative Medicine
TERUMO Corporation Business Strategy Conference THERAPEUTIC SYSTEMS & CELL PROCESSING MARK FLOWER GLOBAL MARKETING December 5, 2011 Regenerative Medicine, Cell Therapy, & Cell Processing Regenerative Medicine
Support structure for genetic material
 Support structure for genetic material 1 Making proteins in the RER Making copies of humans 2 Making copies of cells Making copies of genetic material 3 Making copies of genetic material Making copies
Support structure for genetic material 1 Making proteins in the RER Making copies of humans 2 Making copies of cells Making copies of genetic material 3 Making copies of genetic material Making copies
Biobanking Pluripotent Stem Cell Lines: UK and EU regulations Glyn Stacey, UK Stem Cell Bank, NIBSC
 Biobanking Pluripotent Stem Cell Lines: UK and EU regulations Glyn Stacey, UK Stem Cell Bank, NIBSC ISCT Conference, New Zealand, April 2013 April 1 st 2013 NIBSC will become part of the Medicines and
Biobanking Pluripotent Stem Cell Lines: UK and EU regulations Glyn Stacey, UK Stem Cell Bank, NIBSC ISCT Conference, New Zealand, April 2013 April 1 st 2013 NIBSC will become part of the Medicines and
A Private Investor Guide to Regenerative Medicine: Unique Opportunities in an Emerging Field
 A Private Investor Guide to Regenerative Medicine: Unique Opportunities in an Emerging Field Introduction The field of regenerative medicine remains at the forefront of personalized medicine and healthcare
A Private Investor Guide to Regenerative Medicine: Unique Opportunities in an Emerging Field Introduction The field of regenerative medicine remains at the forefront of personalized medicine and healthcare
Dr Alexander Henzing
 Horizon 2020 Health, Demographic Change & Wellbeing EU funding, research and collaboration opportunities for 2016/17 Innovate UK funding opportunities in omics, bridging health and life sciences Dr Alexander
Horizon 2020 Health, Demographic Change & Wellbeing EU funding, research and collaboration opportunities for 2016/17 Innovate UK funding opportunities in omics, bridging health and life sciences Dr Alexander
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS
 BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 70th Annual Meeting February 5-9, 2003 New Orleans, Louisiana COMMITTEE ON BIOLOGICAL IMPLANTS Prepared by:
BONE-GRAFT SUBSTITUTES: FACTS, FICTIONS & APPLICATIONS AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 70th Annual Meeting February 5-9, 2003 New Orleans, Louisiana COMMITTEE ON BIOLOGICAL IMPLANTS Prepared by:
STEM CELLS FROM THE UMBLICAL CORD BLOOD AND UMBLICAL CORD TISSUE
 STEM CELLS FROM THE UMBLICAL CORD BLOOD AND UMBLICAL CORD TISSUE What are Stem Cells? Stem cells are the basic building blocks of all the cells, tissues and organs in the human body. The role of the stem
STEM CELLS FROM THE UMBLICAL CORD BLOOD AND UMBLICAL CORD TISSUE What are Stem Cells? Stem cells are the basic building blocks of all the cells, tissues and organs in the human body. The role of the stem
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute
 chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
chronos BOne VOid Filler Beta-Tricalcium Phosphate (b-tcp) bone graft substitute chronos Bone Void Filler Osteoconductive Resorbable Synthetic chronos Granules and Preforms are synthetic, porous, osteoconductive,
Cellular Therapy and Cord Blood Market Report 2013 Page 1. Kathy Gray Worldwide Market Reports & Consulting Coordinator Select Biosciences
 Cellular Therapy and Cord Blood Market Report 2013 Kathy Gray Worldwide Market Reports & Consulting Coordinator Select Biosciences k.gray@selectbio.com Select Biosciences Market Reports Website: SelectBiosciences.com/marketreports/
Cellular Therapy and Cord Blood Market Report 2013 Kathy Gray Worldwide Market Reports & Consulting Coordinator Select Biosciences k.gray@selectbio.com Select Biosciences Market Reports Website: SelectBiosciences.com/marketreports/
4. All cord blood banks should be subject to the same standards, regulations and accreditation requirements.
 WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
Personalised Healthcare Frequently Asked Questions
 Personalised Healthcare Frequently Asked Questions Foreword In one sense, personalised healthcare is nothing new. It is what doctors have aimed to provide for their patients through the exercise of their
Personalised Healthcare Frequently Asked Questions Foreword In one sense, personalised healthcare is nothing new. It is what doctors have aimed to provide for their patients through the exercise of their
The Cell Therapy Catapult UK Clinical Trials Database
 May 2015 The UK Clinical Trials Database covers cell therapy clinical trial activity that the Cell Therapy Catapult believes to be ongoing in the UK as of May 2015. It supersedes the database of April
May 2015 The UK Clinical Trials Database covers cell therapy clinical trial activity that the Cell Therapy Catapult believes to be ongoing in the UK as of May 2015. It supersedes the database of April
well foresight studies opportunities for regenerative medicine in the netherlands royal netherlands academy of arts and sciences
 well underway opportunities for regenerative medicine in the netherlands royal netherlands academy of arts and sciences foresight studies 5. conclusions and recommendations Scientific research in regenerative
well underway opportunities for regenerative medicine in the netherlands royal netherlands academy of arts and sciences foresight studies 5. conclusions and recommendations Scientific research in regenerative
JUMISC JUMISC. For further information visit our website:
 STEM CELL THERAPY COMPANY PROFILE & SCIENTIFIC ACTIVITIES For further information visit our website: WWW.CCMIJESUSUSON.COM 20 14 JUMISC JUMISC Carretera N-521, km. 41,8 10071 CÁCERES (SPAIN) Tel. (+34)
STEM CELL THERAPY COMPANY PROFILE & SCIENTIFIC ACTIVITIES For further information visit our website: WWW.CCMIJESUSUSON.COM 20 14 JUMISC JUMISC Carretera N-521, km. 41,8 10071 CÁCERES (SPAIN) Tel. (+34)
Dental Bone Grafting Options. A review of bone grafting options for patients needing more bone to place dental implants
 Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Dental Bone Grafting Options A review of bone grafting options for patients needing more bone to place dental implants Dental Bone Grafting Options What is bone grafting? Bone grafting options Bone from
Hoofdstuk 2 Samenwerking en afstemming in de zorgketen
 Bijlage 3 Zoekstrategieën Hoofdstuk 2 Samenwerking en afstemming in de zorgketen Database Zoektermen 1 Premature Birth/ (2657) 2 exp infant, low birth weight/ or infant, premature/ (49701) 3 (((preterm
Bijlage 3 Zoekstrategieën Hoofdstuk 2 Samenwerking en afstemming in de zorgketen Database Zoektermen 1 Premature Birth/ (2657) 2 exp infant, low birth weight/ or infant, premature/ (49701) 3 (((preterm
5. All cord blood banks should be subject to the same standards, regulations and accreditation requirements.
 WMDA Policy Statement for the Utility of Autologous or Family Cord Blood Unit Storage (This policy statement has been approved and adopted by the WMDA board on the 25 th of May 2006) The Cord Blood Registries
WMDA Policy Statement for the Utility of Autologous or Family Cord Blood Unit Storage (This policy statement has been approved and adopted by the WMDA board on the 25 th of May 2006) The Cord Blood Registries
HTA and Post-Launch Studies
 EXPLORING THE FUTURE OF RE FOR DRUGS IN EUROPE ELEMENTS AFFECTING THE FUTURE OF RE IN EUROPE? Baseline factors Payers continue to face austerity pressures Decision making by Payer / HTA bodies remains
EXPLORING THE FUTURE OF RE FOR DRUGS IN EUROPE ELEMENTS AFFECTING THE FUTURE OF RE IN EUROPE? Baseline factors Payers continue to face austerity pressures Decision making by Payer / HTA bodies remains
Pharmacology skills for drug discovery. Why is pharmacology important?
 skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
Stem cells and motor neurone disease
 Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and includes details of the current
Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and includes details of the current
Norbert Weber, Lilly Ostrovsky. Musculoskeletal Transplant Foundation (MTF), Edison, NJ, USA. Disclosures: N. Weber: None. L. Ostrovsky: None.
 Immunogenicity of Biopharmaceuticals in Large Animal Models: Timedependent Formation of Antibodies and Detection of TNF-alpha in Serum and Synovial Fluid Norbert Weber, Lilly Ostrovsky. Musculoskeletal
Immunogenicity of Biopharmaceuticals in Large Animal Models: Timedependent Formation of Antibodies and Detection of TNF-alpha in Serum and Synovial Fluid Norbert Weber, Lilly Ostrovsky. Musculoskeletal
SPINAL FUSION. North American Spine Society Public Education Series
 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
Non-clinical development of biologics
 Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
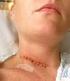 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Titanium Foam Implant
 Titanium Foam Implant The Innovator 2 Move Implants is a Research & Development company which focuses on creating innovating products, not only to improve a product, but also the way of doing business.
Titanium Foam Implant The Innovator 2 Move Implants is a Research & Development company which focuses on creating innovating products, not only to improve a product, but also the way of doing business.
4.1 Objectives of Clinical Trial Assessment
 L1 4.1 Objectives of Clinical Trial Assessment Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office of Clinical
L1 4.1 Objectives of Clinical Trial Assessment Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office of Clinical
Annual General Meeting of Shareholders 19 May 2010
 Annual General Meeting of Shareholders 19 May 2010 2009 results 2010 Q1 highlights Arnoud van Tulder CEO 19 May 2010 www.cryo-savegroup.com Highlights 2009 Strong financial results and cash position year
Annual General Meeting of Shareholders 19 May 2010 2009 results 2010 Q1 highlights Arnoud van Tulder CEO 19 May 2010 www.cryo-savegroup.com Highlights 2009 Strong financial results and cash position year
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
CONSULTATION RESPONSE
 CONSULTATION RESPONSE Department for Business, Innovation and Skills (BIS): Regenerative Medicine Call for Evidence Response by the Wellcome Trust Introduction 1. The Wellcome Trust is pleased to have
CONSULTATION RESPONSE Department for Business, Innovation and Skills (BIS): Regenerative Medicine Call for Evidence Response by the Wellcome Trust Introduction 1. The Wellcome Trust is pleased to have
HTA NETWORK MULTIANNUAL WORK PROGRAMME 2016-2020
 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Healthcare systems, medical products and innovation Medical products: quality, safety, innovation Brussels, 20 May 2016 HTA NETWORK MULTIANNUAL
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Healthcare systems, medical products and innovation Medical products: quality, safety, innovation Brussels, 20 May 2016 HTA NETWORK MULTIANNUAL
TiGenix Business and Financial Update for the First Half of 2015
 REGULATED INFORMATION TiGenix Business and Financial Update for the First Half of 2015 Leuven (BELGIUM) 15 September 2015 TiGenix NV (Euronext Brussels: TIG), an advanced biopharmaceutical company focused
REGULATED INFORMATION TiGenix Business and Financial Update for the First Half of 2015 Leuven (BELGIUM) 15 September 2015 TiGenix NV (Euronext Brussels: TIG), an advanced biopharmaceutical company focused
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Oncology Knowledge Bulletin. Strategies in oncology: Spotlight on clinical pathways
 Strategies in oncology: Spotlight on clinical pathways 2 In response to rising healthcare costs, US payors have increased efforts to control drug costs, including through step edits or discounts for contracts.
Strategies in oncology: Spotlight on clinical pathways 2 In response to rising healthcare costs, US payors have increased efforts to control drug costs, including through step edits or discounts for contracts.
Opportunities for African Participation in H2020. Research and Innovation Work Programme 2016-2017
 Opportunities for African Participation in H2020 Research and Innovation Work Programme 2016-2017 caast-net-plus.org CAAST-Net Plus is funded by the European Union s Seventh Framework Programme for Research
Opportunities for African Participation in H2020 Research and Innovation Work Programme 2016-2017 caast-net-plus.org CAAST-Net Plus is funded by the European Union s Seventh Framework Programme for Research
QUESTIONS AND ANSWERS ABOUT THE EDQM ACTIVITIES
 QUESTIONS AND ANSWERS ABOUT THE EDQM ACTIVITIES Why are Pharmacopoeias so important in a globalised world? Pharmacopoeias have historically provided collections of medical recipes intended to ensure accurate
QUESTIONS AND ANSWERS ABOUT THE EDQM ACTIVITIES Why are Pharmacopoeias so important in a globalised world? Pharmacopoeias have historically provided collections of medical recipes intended to ensure accurate
End of consultation (deadline for comments) 14 October 2009. Adoption by Committee for advanced therapies 15 October 2010
 15 October 2010 EMA/CAT/418458/2008/corr. Committee for advanced therapies (CAT) Procedural advice on the certification of quality and nonclinical data for small and medium sized enterprises developing
15 October 2010 EMA/CAT/418458/2008/corr. Committee for advanced therapies (CAT) Procedural advice on the certification of quality and nonclinical data for small and medium sized enterprises developing
Cellular Therapy and Cord Blood 2013 Market Report
 Brochure More information from http://www.researchandmarkets.com/reports/2393891/ Cellular Therapy and Cord Blood 2013 Market Report Description: This is the most up-to-date market report focusing on Cellular
Brochure More information from http://www.researchandmarkets.com/reports/2393891/ Cellular Therapy and Cord Blood 2013 Market Report Description: This is the most up-to-date market report focusing on Cellular
EURORDIS Position Paper on Centres of Expertise and European Reference Networks for Rare Diseases
 EURORDIS Position Paper on Centres of Expertise and European Reference Networks for Rare Diseases EURORDIS - the European Organisation for Rare Diseases represents 310 rare disease organisations from 34
EURORDIS Position Paper on Centres of Expertise and European Reference Networks for Rare Diseases EURORDIS - the European Organisation for Rare Diseases represents 310 rare disease organisations from 34
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 Ref. Ares(2011)1044649-03/10/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Pharmaceuticals SANCO/D3/RSR/iv(2011)ddg1.d3. 1137738 Update 1 October 2011 HANDLING
Ref. Ares(2011)1044649-03/10/2011 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Pharmaceuticals SANCO/D3/RSR/iv(2011)ddg1.d3. 1137738 Update 1 October 2011 HANDLING
US Regulations for Import and Export of Cell Therapy Products
 US Regulations for Import and Export of Cell Therapy Products Kurt Gunter, MD U Minnesota UCB Transplants (2001-2007)* Total units: 790 Number US banks: 19 Number non-us banks: 12 % units from non-us banks:
US Regulations for Import and Export of Cell Therapy Products Kurt Gunter, MD U Minnesota UCB Transplants (2001-2007)* Total units: 790 Number US banks: 19 Number non-us banks: 12 % units from non-us banks:
FCT PhD Programme Medicines and Pharmaceutical Innovation (i3du)
 FCT PhD Programme Medicines and Pharmaceutical Innovation (i3du) Course BioPharmaceuticals and Advanced Therapies 28-2 September/October 2015 - Auditorium The FCT PhD Programme in Medicines and Pharmaceutical
FCT PhD Programme Medicines and Pharmaceutical Innovation (i3du) Course BioPharmaceuticals and Advanced Therapies 28-2 September/October 2015 - Auditorium The FCT PhD Programme in Medicines and Pharmaceutical
Adoption by CHMP for release for consultation November 2010. End of consultation (deadline for comments) 31 March 2011
 1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
exactly. The need for efficiency in developing effective new therapeutics has never been greater.
 exactly. The need for efficiency in developing effective new therapeutics has never been greater. As demands on the global healthcare system increase and treating disease becomes more complex, the research,
exactly. The need for efficiency in developing effective new therapeutics has never been greater. As demands on the global healthcare system increase and treating disease becomes more complex, the research,
Overview of Drug Development: the Regulatory Process
 Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
Overview of Drug Development: the Regulatory Process Roger D. Nolan, PhD Director, Project Operations Calvert Research Institute November, 2006 Adapted from course taught by Cato Research Background: Roger
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
THE RESORBABLE MATRIX. Relax. Remaix. The membrane of choice for reliable bone and tissue regeneration
 THE RESORBABLE MATRIX Relax. Remaix. The membrane of choice for reliable bone and tissue regeneration Remaix properties Remaix is a resorbable barrier membrane for use in guided bone regeneration (GBR)
THE RESORBABLE MATRIX Relax. Remaix. The membrane of choice for reliable bone and tissue regeneration Remaix properties Remaix is a resorbable barrier membrane for use in guided bone regeneration (GBR)
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
Biotechnology. Srivatsan Kidambi, Ph.D.
 Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
Stem Stem Cell Cell Engineering-What, Biology and it Application Why, How?? to Biotechnology Srivatsan Kidambi, Ph.D. Assistant Professor Department of Chemical & Biomolecular Engineering University of
The therapies are focused in cardiovascular, orthopedics and neurological indications.
 1 2 CescaTherapeutics is (to our knowledge) the first company to integrate all of the essential: 1. Cell friendly devices, 2. Proprietary cell formulations, and Strict clinical steps to ensure the autologous
1 2 CescaTherapeutics is (to our knowledge) the first company to integrate all of the essential: 1. Cell friendly devices, 2. Proprietary cell formulations, and Strict clinical steps to ensure the autologous
Life Science Sector Opportunities Northern Ireland. Clinical Trials. investni.com
 Life Science Sector Opportunities Northern Ireland investni.com Introduction Clinical trials commonly refer to testing the effectiveness of experimental drugs and are typically categorised into four phases;
Life Science Sector Opportunities Northern Ireland investni.com Introduction Clinical trials commonly refer to testing the effectiveness of experimental drugs and are typically categorised into four phases;
EU Clinical Trials Register. www.clinicaltrialsregister.eu. An agency of the European Union
 EU Clinical Trials Register www.clinicaltrialsregister.eu An agency of the European Union The EU Clinical Trials Register provides public access to information from the European Union (EU) clinical trial
EU Clinical Trials Register www.clinicaltrialsregister.eu An agency of the European Union The EU Clinical Trials Register provides public access to information from the European Union (EU) clinical trial
Evaluation reports from the Commission on Reimbursement of Medicines (CRM) and the Medical Pharmaceutical Committee (MFC) as specific target group
 Evaluation reports from the Commission on Reimbursement of Medicines (CRM) and the Medical Pharmaceutical Committee (MFC) as specific target group Philippe Van Wilder RIZIV-INAMI Belgian National Health
Evaluation reports from the Commission on Reimbursement of Medicines (CRM) and the Medical Pharmaceutical Committee (MFC) as specific target group Philippe Van Wilder RIZIV-INAMI Belgian National Health
Master of Science in Biomedical Engineering. Faculty of Engineering Science
 Master of Science in Biomedical Engineering Faculty of Engineering Science Master of Science in Biomedical Engineering The role of technology in contemporary medicine has evolved considerably over the
Master of Science in Biomedical Engineering Faculty of Engineering Science Master of Science in Biomedical Engineering The role of technology in contemporary medicine has evolved considerably over the
What Lies Ahead? Trends to Watch: Health Care Product Development in North America
 What Lies Ahead? Trends to Watch: Health Care Product Development in North America What Lies Ahead? for 2015 DIA has released its third annual What Lies Ahead? report, providing experts insights into the
What Lies Ahead? Trends to Watch: Health Care Product Development in North America What Lies Ahead? for 2015 DIA has released its third annual What Lies Ahead? report, providing experts insights into the
DZIF-Product Development Unit (PDU)
 November 29, 2013 7 th International VPM Days DZIF-Product Development Unit (PDU) - DZIF-TPMO at HZI - DZIF-OSRA at PEI Thomas Hesterkamp, Helmholtz Zentrum für Infektionsforschung Support from TPMO &
November 29, 2013 7 th International VPM Days DZIF-Product Development Unit (PDU) - DZIF-TPMO at HZI - DZIF-OSRA at PEI Thomas Hesterkamp, Helmholtz Zentrum für Infektionsforschung Support from TPMO &
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Alexander Smolyaninov,
 «German-Russian Forum Biotechnology and Life Sciences» September, 2006 St. Petersburg Center of Cell and Gene Therapy «CRYOCENTER SAINT PETERSBURG» Stem Cell Bank «POKROVSKI» Alexander Smolyaninov, M.D.,Ph.D.,
«German-Russian Forum Biotechnology and Life Sciences» September, 2006 St. Petersburg Center of Cell and Gene Therapy «CRYOCENTER SAINT PETERSBURG» Stem Cell Bank «POKROVSKI» Alexander Smolyaninov, M.D.,Ph.D.,
博 士 論 文 ( 要 約 ) A study on enzymatic synthesis of. stable cyclized peptides which. inhibit protein-protein interactions
 博 士 論 文 ( 要 約 ) 論 文 題 目 A study on enzymatic synthesis of stable cyclized peptides which inhibit protein-protein interactions ( 蛋 白 質 間 相 互 作 用 を 阻 害 する 安 定 な 環 状 化 ペプチドの 酵 素 合 成 に 関 する 研 究 ) 氏 名 張 静 1
博 士 論 文 ( 要 約 ) 論 文 題 目 A study on enzymatic synthesis of stable cyclized peptides which inhibit protein-protein interactions ( 蛋 白 質 間 相 互 作 用 を 阻 害 する 安 定 な 環 状 化 ペプチドの 酵 素 合 成 に 関 する 研 究 ) 氏 名 張 静 1
The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings. Prof. Tony Pagliuca
 The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings Prof. Tony Pagliuca UK STEM CELL STRATEGIC FORUM The future of unrelated donor SCT in the UK Antonio Pagliuca, Transplant
The future of unrelated Stem Cell Transplant in the UK: DOH Working Party Findings Prof. Tony Pagliuca UK STEM CELL STRATEGIC FORUM The future of unrelated donor SCT in the UK Antonio Pagliuca, Transplant
Kansen in KP7 NMP. Aansluitend op de HTSM Roadmap Nanotechnologie. 11 juni 2012. Melvin A. Kasanrokijat
 Kansen in KP7 NMP Aansluitend op de HTSM Roadmap Nanotechnologie 11 juni 2012 Melvin A. Kasanrokijat Mogelijkheden in KP7 - Cooperation Groot programma met 10 verschillende thema s NMP, ICT, Health, Energy,
Kansen in KP7 NMP Aansluitend op de HTSM Roadmap Nanotechnologie 11 juni 2012 Melvin A. Kasanrokijat Mogelijkheden in KP7 - Cooperation Groot programma met 10 verschillende thema s NMP, ICT, Health, Energy,
Singapore Clinical Trials Register. Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY
 Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
Stem cells and motor neurone disease
 Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and how they may be used to create
Stem cells and motor neurone disease F Stem cell research has fuelled hope of a treatment for a variety of conditions. This information sheet explains what these cells are and how they may be used to create
Expanded Access Programs. Richard Klein Office of Special Health issues Food and Drug Administration
 Expanded Access Programs Richard Klein Office of Special Health issues Food and Drug Administration Expanded Access Programs (EAPs) What is expanded access? History Legislative background General principles
Expanded Access Programs Richard Klein Office of Special Health issues Food and Drug Administration Expanded Access Programs (EAPs) What is expanded access? History Legislative background General principles
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: quality issues (revision 1)
 22 May 2014 EMA/CHMP/BWP/247713/2012 Committee for Medicinal Products for Human Use (CHMP) Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance:
22 May 2014 EMA/CHMP/BWP/247713/2012 Committee for Medicinal Products for Human Use (CHMP) Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance:
THE MEDICAL TREATMENT GUIDELINES
 THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
THE MEDICAL TREATMENT GUIDELINES I. INTRODUCTION A. About the Medical Treatment Guidelines. On December 1, 2010, the NYS Workers' Compensation Board is implementing new regulations and Medical Treatment
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
The National Centre for Biomedical Engineering Science
 The How this Integrated Technology Platform has impacted the development of R&D Clusters in Galway Jacinta Thornton, PhD Executive Manager, NCBES National Centre for Biomedical Engineering g Science Mission
The How this Integrated Technology Platform has impacted the development of R&D Clusters in Galway Jacinta Thornton, PhD Executive Manager, NCBES National Centre for Biomedical Engineering g Science Mission
PL 17871/0208 UKPAR TABLE OF CONTENTS
 Fultium-D 3 3,200 IU Capsules PL 17871/0208 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 11 Summary of Product Characteristics Page 12 Patient
Fultium-D 3 3,200 IU Capsules PL 17871/0208 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 4 Steps taken for assessment Page 11 Summary of Product Characteristics Page 12 Patient
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA Brussels, 27.03.2014 ENTR/6283/00 Rev 4 orphan\guidelines\format content
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, EMA Brussels, 27.03.2014 ENTR/6283/00 Rev 4 orphan\guidelines\format content
The NHS as a Driver for Growth. A report by The Prime Minister s Council for Science and Technology
 The NHS as a Driver for Growth A report by The Prime Minister s Council for Science and Technology September 2011 The NHS as a Driver for Growth The 21 st Century is bringing a revolution in the life sciences,
The NHS as a Driver for Growth A report by The Prime Minister s Council for Science and Technology September 2011 The NHS as a Driver for Growth The 21 st Century is bringing a revolution in the life sciences,
THE LINCOLN INSTITUTE OF HEALTH
 THE LINCOLN INSTITUTE OF HEALTH Background The Chair in the Care of the Older Person will be part of the new Lincoln Institute of Health, a cross disciplinary research collaboration linking schools, colleges
THE LINCOLN INSTITUTE OF HEALTH Background The Chair in the Care of the Older Person will be part of the new Lincoln Institute of Health, a cross disciplinary research collaboration linking schools, colleges
. Soft Tissue Regeneration... Consultant. RTI Surgical...Consultant. OSSUR...Consultant. Arthrex...Consultant
 sl23lls 1 E ACL Graft Choice in the Elite Athlete and When Do I Let Them Return to Sport: Graft Ghoice: Allografts : @ Peter A. lndelicato MD Emeritus Professor Sports Medicine Department of OrthoPaedic
sl23lls 1 E ACL Graft Choice in the Elite Athlete and When Do I Let Them Return to Sport: Graft Ghoice: Allografts : @ Peter A. lndelicato MD Emeritus Professor Sports Medicine Department of OrthoPaedic
Eisen en Verplichtingen
 Registratie ti van Biosimilars: i il Eisen en Verplichtingen Dr. Thijs J. Giezen Ziekenhuisapotheker, Stichting Apotheek Haarlemse Ziekenhuizen Lid, CHMP Biosimilar Working Party (BMWP), European Medicines
Registratie ti van Biosimilars: i il Eisen en Verplichtingen Dr. Thijs J. Giezen Ziekenhuisapotheker, Stichting Apotheek Haarlemse Ziekenhuizen Lid, CHMP Biosimilar Working Party (BMWP), European Medicines
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: adoptive_immunotherapy 11/1993 3/2016 3/2017 3/2016 Description of Procedure or Service The spontaneous regression
San Diego Stem Cell Treatment Center Frequently Asked Questions
 San Diego Stem Cell Treatment Center Frequently Asked Questions What is a Stem Cell? A stem cell is basically any cell that can replicate and differentiate. This means the cell can not only multiply, but
San Diego Stem Cell Treatment Center Frequently Asked Questions What is a Stem Cell? A stem cell is basically any cell that can replicate and differentiate. This means the cell can not only multiply, but
Overview of Phase 1 Oncology Trials of Biologic Therapeutics
 Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Overview of Phase 1 Oncology Trials of Biologic Therapeutics Susan Jerian, MD ONCORD, Inc. February 28, 2008 February 28, 2008 Phase 1 1 Assumptions and Ground Rules The goal is regulatory approval of
Summary of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Status
 of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Shelley Grant Branch Chief, Blood Stem Cell Transplantation Program September 11, 2015 Overview December 20, 2005 - HRSA began
of Advisory Council on Blood Stem Cell Transplantation: Recommendations and Shelley Grant Branch Chief, Blood Stem Cell Transplantation Program September 11, 2015 Overview December 20, 2005 - HRSA began
Market Access for Medical Technology & Pharmaceutical Companies An Organizational Priority in Times of Economic Austerity and Reform
 Market Access for Medical Technology & Pharmaceutical Companies An Organizational Priority in Times of Economic Austerity and Reform By Joakim Steen Mikkelsen, Managing Healthcare Counselor, Embassy of
Market Access for Medical Technology & Pharmaceutical Companies An Organizational Priority in Times of Economic Austerity and Reform By Joakim Steen Mikkelsen, Managing Healthcare Counselor, Embassy of
Nanomedicine in Horizon 2020
 Nanomedicine in Horizon 2020 Heico FRIMA Programme Officer European Commission Directorate-General for Research & Directorate D: Key Enabling Technologies heico.frima@ec.europa.eu ETPN Annual Event 2014
Nanomedicine in Horizon 2020 Heico FRIMA Programme Officer European Commission Directorate-General for Research & Directorate D: Key Enabling Technologies heico.frima@ec.europa.eu ETPN Annual Event 2014
EURORDIS Position Paper on Specialised Services for People Living with Rare Diseases
 EURORDIS Position Paper on Specialised Services for People Living with Rare Diseases EURORDIS - the European Organisation for Rare Diseases represents 310 rare disease organisations from 34 different countries,
EURORDIS Position Paper on Specialised Services for People Living with Rare Diseases EURORDIS - the European Organisation for Rare Diseases represents 310 rare disease organisations from 34 different countries,
Clinical Data for Medical Devices
 White Paper Clinical Data for Medical Devices Preparing for increased requirements in the EU Table of Contents 1. Regulation of medical devices in the EU: on the cusp of change... 3 2. Traditional differences
White Paper Clinical Data for Medical Devices Preparing for increased requirements in the EU Table of Contents 1. Regulation of medical devices in the EU: on the cusp of change... 3 2. Traditional differences
Cord Blood Bank Business Plan
 Cord Blood Bank Business Plan A sample of how to create a new program Produced by the AABB subsection: Cellular Therapy Business Management with major contributions by Nicole Omer TABLE OF CONTENTS Page
Cord Blood Bank Business Plan A sample of how to create a new program Produced by the AABB subsection: Cellular Therapy Business Management with major contributions by Nicole Omer TABLE OF CONTENTS Page
Drug Safety of Stem Cells and other Novel Therapeutics
 Course 1.2 Drug Safety of Stem Cells and other Novel Therapeutics 13 17 October 2014 MRC Centre for Drug Safety Science Institute of Translational Medicine University of Liverpool, Liverpool, UK European
Course 1.2 Drug Safety of Stem Cells and other Novel Therapeutics 13 17 October 2014 MRC Centre for Drug Safety Science Institute of Translational Medicine University of Liverpool, Liverpool, UK European
Do you have anything to add? If so, I d love to hear from you! Jessica Robinson Conference Manager Life Sciences @jessbiopharma
 1 Who is the most influential figure in cord blood around the world? What is the biggest challenge to overcome in the use of cord blood as a source of stem cells? We asked 10 leading experts in the cord
1 Who is the most influential figure in cord blood around the world? What is the biggest challenge to overcome in the use of cord blood as a source of stem cells? We asked 10 leading experts in the cord
Mitchell S. Fourman M.Phil Eugene Borst BS Eric Bogner, MD S. Robert Rozbruch MD Austin T. Fragomen, MD
 Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Post-Operative CT Measurements Accurately Predict the Need for Clinical Intervention Retrospective Findings to Support Prospective Clinical Trial Protocol Mitchell S. Fourman M.Phil Eugene Borst BS Eric
Call 2014: High throughput screening of therapeutic molecules and rare diseases
 Call 2014: High throughput screening of therapeutic molecules and rare diseases The second call High throughput screening of therapeutic molecules and rare diseases launched by the French Foundation for
Call 2014: High throughput screening of therapeutic molecules and rare diseases The second call High throughput screening of therapeutic molecules and rare diseases launched by the French Foundation for
GMP-Z Annex 15: Kwalificatie en validatie
 -Z Annex 15: Kwalificatie en validatie item Gewijzigd richtsnoer -Z Toelichting Principle 1. This Annex describes the principles of qualification and validation which are applicable to the manufacture
-Z Annex 15: Kwalificatie en validatie item Gewijzigd richtsnoer -Z Toelichting Principle 1. This Annex describes the principles of qualification and validation which are applicable to the manufacture
S YN T H E T I C C A N C E L LO U S B O N E
 S YN T H E T I C C A N C E L LO U S B O N E CELLPLEX TCP Graft is a synthetic cancellous scaffold that provides an optimum environment for cellular infiltration. Composed of tricalcium phosphate, the scaffold
S YN T H E T I C C A N C E L LO U S B O N E CELLPLEX TCP Graft is a synthetic cancellous scaffold that provides an optimum environment for cellular infiltration. Composed of tricalcium phosphate, the scaffold
Program of Study: Bachelor of Science in Athletic Training
 Program of Study: Bachelor of Science Training Program Description Athletic training, as defined by the National Athletic Trainer s Association, is practiced by athletic trainers, health care professionals
Program of Study: Bachelor of Science Training Program Description Athletic training, as defined by the National Athletic Trainer s Association, is practiced by athletic trainers, health care professionals
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood
 Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Guidance For Research Involving Human Embryonic Stem Cells, Germ Cells, And Cells Obtained From Cord Blood Supreme Council of Health Department of Research Guidance Regarding Research Involving Human Embryonic
Mobile: +31 6 48 26 19 29 1
 , Roadmap 1 Social-economic developments.over de weg naar duurzame en goede zorg een beschouwing vanuit internationaal maatschappelijk-economisch perspectief NEVI zorgcongres 05-02-2015 2 Healthcare paradigm
, Roadmap 1 Social-economic developments.over de weg naar duurzame en goede zorg een beschouwing vanuit internationaal maatschappelijk-economisch perspectief NEVI zorgcongres 05-02-2015 2 Healthcare paradigm
