Mutation Research/Reviews in Mutation Research
|
|
|
- Lynne Powers
- 8 years ago
- Views:
Transcription
1 Mutation Research 681 (2009) Contents lists available at ScienceDirect Mutation Research/Reviews in Mutation Research journal homepage: Community address: Review Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Nele Hilgert a, Richard J.H. Smith b, Guy Van Camp a, * a Department of Medical Genetics, University of Antwerp (UA), Universiteitsplein 1, B-2610 Antwerp, Belgium b Department of Otolaryngology-Head and Neck Surgery, University of Iowa, Iowa city, IA 52242, USA ARTICLE INFO ABSTRACT Article history: Received 10 June 2008 Received in revised form 4 August 2008 Accepted 22 August 2008 Available online 29 August 2008 Keywords: Hereditary hearing loss ARNSHL ADNSHL GJB2 Gene frequencies Genetic counselling Hearing impairment is the most common sensory disorder, present in 1 of every 500 newborns. With 46 genes implicated in nonsyndromic hearing loss, it is also an extremely heterogeneous trait. Here, we categorize for the first time all mutations reported in nonsyndromic deafness genes, both worldwide and more specifically in Caucasians. The most frequent genes implicated in autosomal recessive nonsyndromic hearing loss are GJB2, which is responsible for more than half of cases, followed by SLC26A4, MYO15A, OTOF, CDH23 and TMC1. None of the genes associated with autosomal dominant nonsyndromic hearing loss accounts for a preponderance of cases, although mutations are somewhat more frequently reported in WFS1, KCNQ4, COCH and GJB2. Only a minority of these genes is currently included in genetic diagnostics, the selection criteria typically reflecting: (1) high frequency as a cause of deafness (i.e. GJB2); (2) association with another recognisable feature (i.e. SLC26A4 and enlarged vestibular aqueduct); or (3) a recognisable audioprofile (i.e. WFS1). New and powerful DNA sequencing technologies have been developed over the past few years, but have not yet found their way into DNA diagnostics. Implementing these technologies is likely to happen within the next 5 years, and will cause a breakthrough in terms of power and cost efficiency. It will become possible to analyze most if not all deafness genes, as opposed to one or a few genes currently. This ability will greatly improve DNA diagnostics, provide epidemiological data on gene-based mutation frequencies, and reveal novel genotype phenotype correlations. ß 2008 Elsevier B.V. All rights reserved. Contents 1. Introduction Frequency of mutations causing monogenic hearing loss Genes as a frequent cause of ARNSHL Genes as a frequent cause of ADNSHL X-linked and mitochondrial hearing loss Clinical diagnostics for hearing impairment Genetic diagnostics for hearing loss Evolution of molecular diagnostics Acknowledgements References Introduction * Corresponding author. Tel.: ; fax: address: guy.vancamp@ua.ac.be (G. Van Camp). Hearing loss (HL) is the most common birth defect in industrialized countries and the most prevalent sensorineural disorder. One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 dbhl. Before the age of 5, the /$ see front matter ß 2008 Elsevier B.V. All rights reserved. doi: /j.mrrev
2 190 N. Hilgert et al. / Mutation Research 681 (2009) prevalence increases to 2.7 per 1000 and to 3.5 per 1000 during adolescence [1]. HL is categorized in several ways: conductive HL typically implies a defect of the outer or middle ear while sensorineural HL refers to a defect of the inner ear. The cooccurrence of both conductive and sensorineural hearing loss is referred to as a mixed hearing loss. Based on the age of onset, HL can also be described as prelingual (before speech development, sometimes referred to as congenital although not all prelingual cases are congenital) or postlingual (after speech development). It is estimated that in developed countries, genetic causes of HL can be found in at least two-thirds of prelingual cases. The remaining one-third of cases can be ascribed to environmental factors and unidentified genetic factors. The most common environmental (non-genetic) cause of congenital hearing loss is congenital cytomegaloviral (CMV) infection. Its overall birth prevalence is 0.64%, with only about 10% of this number having symptomatic CMV. Of asymptomatic cases, 0 4.4% develops unilateral or bilateral HL before the age of 6 years. These figures generally apply to developed countries, although there is wide ethnic variation [2]. In addition, the diagnosis of congenital CMV is often difficult to make, especially in children over 3 weeks of age. As PCR-based techniques using dried bloodspot cards become widely available, reliable screening for in utero CMV exposure will be possible. Other environmental causes of hearing loss in the newborn period include bacterial infection, hyperbilirubinemia, anoxia and the use of ototoxic medications. In most cases, inherited HL is monogenic. In 70% of neonates who fail newborn hearing screens (NBHS) and are presumed to have inherited HL, there are no other distinguishing physical findings and the HL is classified as nonsyndromic. In the remaining 30%, the HL is accompanied by other physical findings and is said to be syndromic [3]. Of the more than 400 syndromes in which HL is a recognized feature, Usher syndrome, Pendred syndrome and Jervell and Lange-Nielsen syndrome are the most frequent [4]. Monogenic hearing loss can be inherited in different ways. Autosomal recessive HL (ARNSHL) occurs in 80% of cases and is typically prelingual, while autosomal dominant HL (ADNSHL) accounts for about 20% of cases and is most often postlingual. In less than 1% of cases, the inheritance occurs through the X- chromosome or the mitochondria [5]. Monogenic hearing loss is an extremely heterogeneous trait, with over 100 mapped loci and 46 causally implicated genes (Hereditary Hearing Loss Homepage; For over half of these loci, specific deafness-causing genes have not yet been found. Their identification will provide further knowledge about the pathways involved in the hearing process and will allow for better genetic diagnostics. The ultimate goal is to provide better and/or new habilitation for hearing impaired persons. Over the past decade, universal newborn hearing screening programs have been developed and implemented in many developed countries. The rationale for this initiative is based on the premise that early detection and intervention for children with hearing loss maximizes opportunities for language and speech development, thereby facilitating the acquisition of normal social, cognitive and motor skills. Stimulation of the auditory cortex before the age of 6 months is crucial for normal development of the auditory tracts. Screening is most often performed by automated auditory brainstem response (ABR) or otoacoustic emission (OAE) testing. 2. Frequency of mutations causing monogenic hearing loss To date, 46 genes have been identified as causally related to nonsyndromic HL. Mutations in these genes do not occur at the same frequencies across ethnicities. In addition, although families with ARNSHL are found around the world, the majority of families reported with ARNSHL originate from the consanguinity belt, a region ranging from North Africa, through the Middle East to India. These consanguineous families have a large power for linkage mapping and allow locus identification on the basis of a single family. Families segregating ADNSHL, in contrast, mainly originate from Europe, North America and Australia. To date, no overview has assessed the number of mutations reported in deafness-causing genes. The lack of this information makes it very hard to estimate the relative contribution of different genes to HL. This information is however of great importance for researchers as well as clinicians. In order to fill this gap, we performed a literature search which allowed counting the number of reported mutations in all 46 nonsyndromic deafness genes. In Table 1, we have collated these mutation numbers, both worldwide and in populations of white European ancestry (whites from Europe, North America, Canada, Australia and New Zealand), which we hereafter call Caucasians. The table also lists the locus name and the presumed function of each gene. Classifying into the worldwide population and Caucasians is an oversimplification as the term Caucasians does not recognize a specific ethnic subgroup. Within the Caucasian population, there is variation in mutation frequencies from country to country. Supplementary Table 1 provides information about the country of origin of the patients in which the mutation was identified, together with references to the reported articles in which the mutation was first described. The number of reported mutations reflects several biases. For example, large genes are less frequently analyzed, numbers for frequently mutated genes like GJB2 and SLC26A4 are likely underestimates, and some genes have distinct audioprofiles to facilitate targeted gene screening. Nevertheless, based on the number of reported mutations, we have estimated the mutation frequencies for all nonsyndromic deafness genes. For most of these genes, the number of mutations identified and the number of reported families (unrelated cases) are identical. In a few specific cases, however, the number of mutations is fewer than the number of reported cases. Examples include GJB2, SLC26A4, OTOF, MT-RNR1 and COCH, all of which have recurrent mutations. It should also be noted that reported mutation numbers for Caucasians are not representative for all European populations and that in some cases, considerable differences in mutations frequencies may be present, even within Europe. These limitations support the need for indepth studies to determine the epidemiology of HL in different populations Genes as a frequent cause of ARNSHL For ARNSHL, the most frequent causative genes in order of frequency are GJB2, SLC26A4, MYO15A, OTOF, CDH23 and TMC1. For each of these genes, at least 20 mutations have been reported. The number of mutations in the other genes is <20 and most of these mutations are reported in consanguineous families and not in Caucasians. GJB2 mutations are the most frequent cause of ARNSHL in most world populations and in some populations account for up to 50% of ARNSHL cases [6]. According to the Connexin-deafness homepage ( 91 different mutations have been identified, some of which are very frequent, others of which are extremely rare. These mutations occur at different frequencies across populations [6]. Because mutations reported after 2003 are not listed, we completed an extensive literature search and estimate that over 220 mutations have been reported.
3 N. Hilgert et al. / Mutation Research 681 (2009) Table 1 Reported number of mutations for all 46 nonsyndromic deafness genes Gene Locus Number of mutations worldwide Number of mutations in Caucasians Function in hearing process OMIM number ARNSHL GJB2 DFNB1 >220 >150 Ion homeostasis SLC26A4 DFNB Ion homeostasis MYO15A DFNB Hair bundle, motor protein OTOF DFNB Exocytosis at auditory ribon synapse CDH23 DFNB Hair bundle, adhesion protein TMC1 DFNB7/ Unknown function TMPRSS3 DFNB8/ Unknown function TECTA DFNB Extracellular matrix protein TRIOBP DFNB Hair bundle, cytoskeletal formation TMIE DFNB6 8 0 Unknown function PJVK DFNB Signalling of hair cells and neurons ESPN DFNB Hair bundle, cytoskeletal formation PCDH15 DFNB Hair bundle, adhesion protein ESRRB DFNB Transcription factor MYO7A DFNB2 5 0 Hair bundle, motor proteins GJB6 DFNB1 4 0 Ion homeostasis TRIC DFNB Ion homeostasis TMHS DFNB Hair bundle, adhesion protein STRC DFNB Extracellular matrix protein CLDN14 DFNB Ion homeostasis RDX DFNB Hair bundle, cytoskeletal formation MYO6 DFNB Hair bundle, motor protein MYO3A DFNB Hair bundle, motor protein SLC26A5 2 2 Molecular motor of OHC WHRN DFNB Hair bundle, scaffolding protein USH1C DFNB Hair bundle, scaffolding protein GJB3 2 0 Ion homeostasis COL11A2 DFNB Extracellular matrix protein OTOA DFNB Extracellular matrix protein ADNSHL WFS1 DFNA6/ Ion homeostasis KCNQ4 DFNA Ion homeostasis COCH DFNA Extracellular matrix protein GJB2 DFNA Ion homeostasis MYO1A DFNA Unknown function TECTA DFNA8/ Extracellular matrix protein ACTG1 DFNA20/ Hair bundle, cytoskeletal formation EYA4 DFNA Transcription factor MYH14 DFNA4 5 5 Unknown function MYO6 DFNA Hair bundle, motor protein MYO7A DFNA Hair bundle, motor protein ESPN 4 4 Hair bundle, cytoskeletal formation DFNA5 DFNA5 4 2 Unknown function GJB3 DFNA2 3 1 Ion homeostasis POU4F3 DFNA Transcription factor TMC1 DFNA Unknown function COL11A2 DFNA Extracellular matrix protein CRYM 2 0 Ion homeostasis TFCP2L3 DFNA Transcription factor GJB6 DFNA3 1 1 Ion homeostasis MYH9 DFNA Hair bundle, motor protein CCDC50 DFNA Hair bundle, cytoskeletal formation DIAPH1 DFNA1 1 0 Hair bundle, cytoskeletal formation X-linked hearing loss POU3F4 DFN Transcription factor Mitochondrial hearing loss MT-RNR1 12S rrna 9 4 RNA translation MT-TS1 trna Ser(UCN) 4 1 RNA translation The number of mutations worldwide and more specifically in Caucasians are listed. The genes are ranked according to the number of mutations worldwide. For each gene, the locus and function of the encoded protein in the hearing process is mentioned, as well as the OMIM number. The table is split up in four parts, depending on the mode of inheritance: ADNSHL, ARNSHL, X-linked HL and mitochondrial HL. The 35delG mutation is most frequent in the majority of Caucasian populations and may account for up to 70% of all GJB2 mutations [7]. The 35delG carrier frequency, however, differs significantly between European populations. A gradient in carrier frequency has been suggested between central-northern and southern Europe, with frequencies in the latter being roughly twice as high [8]. Other frequent mutations in specific populations are 167delT in Ashkenazi Jewish [9] and 235delC in Japanese [10]. The number of deaf persons carrying a single GJB2 mutation is higher than expected and a search for other mutations in or near GJB2 has led to the identification of two large deletions: del(gjb6-d13s1830) and del(gjb6-d13s1854) [11,12]. Both
4 192 N. Hilgert et al. / Mutation Research 681 (2009) deletions not only truncate the neighbouring GJB6-gene but also abolish GJB2 expression, possibly by deleting a currently unidentified GJB2 regulatory element. Therefore, they are considered as GJB2 mutations as well [13]. They are usually found in compound heterozygosity with a GJB2 coding mutation and cause hearing loss that is significantly worse than most other GJB2 mutations, perhaps because expression of both copies of GJB2 and one copy of GJB6 is abolished [7,12]. Del(GJB6- D13S1830) seems to occur worldwide and is much more frequent than del(gjb6-d13s1854), which has been found mainly in Spain and the UK. A significant percentage of GJB2 mutation carriers do not carry either of these deletions indicating that additional unidentified mutations/deletions maybepresentatthedfnb1locus. There is a great deal of variation in the degree of hearing loss caused by GJB2 mutations. A multicenter genotype phenotype correlation study has shown that inactivating mutations cause a more severe phenotype than non-inactivating mutations [7]. In addition, a few common mutations (M34T, V37I, and L90P) are associated with mild-to-moderate hearing impairment. For most genotypes, however, there is considerable variation. As an example, there is striking phenotypic variability among 35delG homozygotes. While the majority does have profound HL, some 35delG homozygotes have only moderate hearing loss and in a few, the loss is only mild [7]. Most likely, this variation is caused by modifier genes, but none has been identified to date. Their identification could substantially contribute to a more accurate diagnosis and more appropriate genetic counselling. Mutations in SLC26A4 are the second most frequent cause of ARNSHL. The associated phenotypic spectrum ranges from Pendred syndrome at one extreme to isolated nonsyndromic HL with enlarged vestibular aqueduct (EVA) at the other (DFNB4 locus). Pendred syndrome is characterized by goiter and congenital, fluctuating, progressive hearing loss associated with Mondini dysplasia (cochlear dysplasia plus EVA) or EVA alone. It can be challenging to distinguish between Pendred syndrome and nonsyndromic HL with EVA because the goiter phenotype is usually not seen until adolescence and in some families, affected siblings can be discordant for thyroid disease [14]. In northern Europe, four mutations are found quite frequently (L236P, T416P, E384G and IVS8 + 1G > A) [15]. Recently, it has been reported that the SLC26A4 promoter contains a key transcriptional regulatory element that binds FOXI1, a transcriptional activator of the gene. Mutations in this regulatory element as well as mutations in FOXI1 cause Pendred syndrome and nonsyndromic hearing loss. One patient has been reported to be double-heterozygous for both an SLC26A4 and a FOXI1 mutation, while all other patients only carry a FOXI1 mutation [16]. In addition, many patients carry single mutations or no mutations in SLC26A4, indicating that the Pendred syndrome EVA phenotypic spectrum is not always inherited in a simple Mendelian way. No clear genotype phenotype correlation has been reported for SLC26A4 mutations [17]. Mutations in MYO15A cause congenital severe-to-profound hearing loss at the DFNB3 locus. All 28 identified mutations have been found by linkage analysis in consanguineous families, most of which originate from Pakistan. They were identified by screening 600 Pakistani consanguineous families for linkage with DFNB3 [18]. It is likely that the number of ARNSHL-causing MYO15A mutations is higher, as the gene is large (66 exons) and mutation analysis is rare if complementary linkage analysis has not been performed. OTOF mutations cause prelingual, profound ARNSHL, which initially may be accompanied by auditory neuropathy in about half of cases with biallelic OTOF mutations [19]. Auditory neuropathy is characterized by the presence of OAE responses in absence of ABR responses. However, as the hearing loss progresses, OHC function is lost, and so is the OAE response. As OTOF mutations have been suggested as the major cause of auditory neuropathy, mutation screening of OTOF should be considered when OAE responses are present in the absence of ABR responses [19]. In the Spanish population, Q829X is the most common OTOF mutation identified and ranks as the third most common cause of ARNSHL in this ethnic group [19,20]. In addition to OTOF, some DFNB59 (PJVK) mutations also cause HL and auditory neuropathy [21]. Mutations in CDH23 cause both Usher syndrome type 1D (USH1D: hearing loss, retinitis pigmentosa and vestibular dysfunction) and moderate-to-profound progressive ARNSHL at the DFNB12 locus. Genotype phenotype studies suggest that missense mutations or in-frame alterations cause ARNSHL, while truncating mutations cause USH1D. This correlation is not absolute, however, as six in-frame mutations have been identified in Usher patients [22]. No single CDH23 mutation predominates as a cause of either USH1D or ARNSHL. TMC1 mutations are one of the more frequent causes of ARNSHL in consanguineous populations. Twenty-one different mutations have been reported in 33 consanguineous families, only one of which was Caucasian. One mutation, c.100c > T seems especially frequent as a cause of ARNSHL, and accounts for over 40% of all TMC1 mutations [23]. All reported cases show a similar phenotype characterized by prelingual severe-to-profound hearing loss Genes as a frequent cause of ADNSHL In contrast to ARNSHL where mutations in two genes are frequently found, none of the genes causing ADNSHL is a frequent cause of hearing loss. Based on our summary, WFS1, KCNQ4, COCH and GJB2 mutations are somewhat more frequent in comparison to the other reported genes. In addition, WFSI, COCH and TECTA mutations cause HL with a recognisable phenotype. Mutations in WFS1 cause both autosomal dominant lowfrequency sensorineural hearing loss (LFSNHL) at the DFNA6/14/38 locus and Wolfram syndrome, characterized by autosomal recessive hearing loss, diabetes mellitus, diabetes insipidus and optic atrophy. In addition, WFS1 may also play a role in the susceptibility to diabetes mellitus and possibly also psychiatric disorders, although its exact role in these disorders needs to be determined [24]. The hearing loss caused by dominant WFS1 mutations is very characteristic, only affecting the low frequencies and rising to normal hearing in the high frequencies. With age, hearing in the high frequencies is lost and the audioprofile flattens. In families segregating LFSNHL, WFS1 mutations are found in 30 80% of pedigrees, depending on whether the families are preselected by linkage analysis prior to WFS1 mutation analysis [25 27]. Interestingly, dominant mutations are nearly always found in the C-terminal domain. WFS1 mutations causing the recessive Wolfram syndrome are numerous and distributed along the entire gene. Two mutations seem to occur recurrently in specific populations: c.424_425ins16 in Spanish people [28] and c.1362_1377del16 in Italians [29]. As a general rule, inactivating mutations cause Wolfram syndrome and missense mutations in the C-terminal domain cause the characteristic low-frequency ADNSHL [24]. KCNQ4 has been identified as a disease-causing gene at the DFNA2 locus. Twelve different mutations have been reported to date (10 missense mutations and 2 deletions), and a genotype phenotype correlation has been proposed [30,31]. Missense mutations are believed to exert a dominant-negative effect by which the mutant protein interferes with the normal channel subunit. These mutations cause hearing loss beginning at a young age and affecting all frequencies. Both deletions, which are proposed to exert a pathogenic effect through haploinsufficiency,
5 N. Hilgert et al. / Mutation Research 681 (2009) cause a milder phenotype, have an older age of onset and affect only the high frequencies. The W276S mutation in KCNQ4 may be a mutational hot spot as it has arisen three times independently in two European and one Japanese family [32]. Seven COCH mutations have been reported all of which are missense mutations. Six of these mutations occur in the LCCLdomain of the protein and cause a phenotype characterized by progressive late-onset hearing loss with vestibular impairment [33]. The late onset and the parallel auditory and vestibular decline make this phenotype very recognisable. One mutation is present in the vwfa2 domain and causes earlier onset hearing loss, vestibular dysfunction and abnormal ocular motor responses [34]. The P51S mutation is a common cause of late onset cochleovestibular impairment in the Belgian and Dutch population through a founder effect [35]. Dominant mutations in GJB2 have been reported predominantly in Caucasians and cause both ADNSHL and syndromic hearing loss associated with diverse skin disorders. The skin disorders are very heterogeneous and include diffuse palmoplantar keratodermahyperkeratosis, Vohwinkel syndrome and Keratitis Ichthyosis/ Deafness (KID) syndrome [36,37]. The particular phenotype appears to depend both on the type of mutation and its location. TECTA mutations cause both autosomal dominant midfrequency HL and high-frequency HL. A genotype phenotype correlation has been established that is defined by the protein domain in which the mutation occurs and the nature of the amino acid substitution. Mutations in the zona pellucida domain of a- tectorin cause mid-frequency hearing loss, mutations in the zona adherens domain cause high-frequency hearing loss and cysteinereplacing substitutions cause progressive hearing loss [38] X-linked and mitochondrial hearing loss Hearing loss inherited through the X-chromosome or the mitochondria is not frequent, accounting for less than 1% of all hearing loss cases. However, these genes are often included for mutation analysis because the inheritance pattern is recognisable and there are additional specific HL characteristics. Mutations in POU3F4 cause X-linked HL at locus DFN3. The HL can be mixed or purely sensorineural and may be associated with Mondini dysplasia, cochlear hypoplasia and/or stapes fixation. Identification of POU3F4 as the deafness-causing gene is a contraindication for stapes operations as a perilymphatic gusher may occur during surgery. Twenty different POU3F4 mutations have been identified, 19 of them in Caucasians. These mutations include missense mutations in both DNA-binding domains and deletions in a putative regulatory region about 900 kb upstream of the gene [39]. If HL is found to be X-linked and associated with a defect in the bony labyrinth, mutations in POU3F4 may be suspected. The majority of mutations in mitochondrial genes are the cause of a broad spectrum of maternally inherited multisystem disorders, which is in concordance with the fact that mitochondrial genes are expressed in every cell. However, mutations in a subset of genes, mainly MT-RNR1 and MT-TS1, cause nonsyndromic HL by a currently unknown mechanism. MT-RNR1 encodes for the 12S ribosomal RNA. One mutation in the gene, 1555G > A, is a rather frequent cause of maternally inherited nonsyndromic HL. In some 1555G > A carriers, HL may be induced by the administration of appropriate doses of aminoglycosides. The phenotypic variation caused by this mutation is very high due to the effect of modifier genes [40]. MT-TS1 encodes for the transfer RNA Ser(UCN). The penetrance is however rather low and it has been suggested that MT-TS1 mutations on their own are an insufficient cause of HL. Other modifying factors may be involved, including aminoglycoside-exposure and the 1555G > A mutation in MT-RNR1 [41]. 3. Clinical diagnostics for hearing impairment Diagnosing the cause of HL can be challenging. In many cases, making an accurate diagnosis requires a multidisciplinary team, with involvement of a paediatrician, otolaryngologist, medical geneticist, ophthalmologist, and often additional specialists. Recognizing congenital HL has been facilitated by the implementation of newborn hearing screening in most developed countries. NBHS should be performed just after birth, preferably before hospital discharge and certainly before the age of 1 month. The test is typically either an ABR-based or OAE-based screen, which detects the presence or absence of a response at a specific threshold. If the results are abnormal, a complete ABR or auditory steady state response testing is required. For older children, behavioural or conditioned reflex audiometry can be completed. These tests should be complemented by a thorough physical examination to exclude possibly subtle dysmorphic features that may be indicative of a syndromic form of HL. In young children, an eye examination should also be obtained. Although retinitis pigmentosa associated with USH syndrome is unlikely to be present in early childhood, many children have refractive errors that should be corrected. If the HL progresses, thin-cut computed tomography or magnetic resonance imaging of the temporal bones is required to rule out the presence of an inner ear abnormality like EVA. A thorough family history should also be obtained. Guidelines for early hearing detection and intervention programs can be found in the Year 2007 position statement by The Joint Committee on Infant Hearing [42]. 4. Genetic diagnostics for hearing loss Universal NBHS has lowered the age of detection of HL. In babies who are diagnosed with severe-to-profound HL in the absence of other abnormal findings on physical examination, the single best next diagnostic test is mutation screening for deafness at the DFNB1 locus. This screening should include DNA sequencing of GJB2 and mutation testing for the two large GJB6-containing deletions, as GJB2 mutations cause about half of all genetic hearing loss cases. Table 2 lists all genes that are currently frequently included in genetic diagnostics. All of these genes, with the exception of GJB2, cause a recognisable hearing loss phenotype. According to Table 1, all these genes are also found to be more frequent causes of hearing loss. SLC26A4 should be considered for mutation analysis if there has been evidence of progression of hearing loss or the presence of a goiter, EVA or Mondini dysplasia. In the absence of ABR responses and in the presence of OAE responses, mutation screening of OTOF is warranted. In case of progressive, late-onset HL combined with vestibular abnormalities, COCH should be tested for mutations. In the presence of HL associated with defects of the bony labyrinth, POU3F4 mutations may be causal and GJB2 should be screened for dominant mutations if the HL is associated with skin disorders. Mitochondrial genes may carry mutations in cases of maternal inheritance and in the presence of aminoglycoside-induced HL. TECTA mutations and WFS1 mutations both cause HL with a distinct audioprofile, being moderate mid-frequency HL and lowfrequency HL, respectively. Other genes that have been identified as frequent causes of hearing loss in this review and which are not included in DNA diagnostics cause HL without a recognisable audioprofile. In addition, none of the genes causing ADNSHL is a frequent cause of HL and their screening is therefore not cost-effective. Establishing a genetic diagnosis has been shown to be beneficial to parents as it alleviates parental guilt and anxiety, makes accurate recurrence chance counselling possible, and provides
6 194 N. Hilgert et al. / Mutation Research 681 (2009) Table 2 Overview of genes that are frequently included in genetic diagnostics Gene Frequent mutation(s) Diagnostic criteria GJB2 35delG AR: congenital, mild to profound HL AD: HL associated with skin disorders in half of the cases SLC26A4 L236P, T416P, E384G, IVS8 + 1G > A for Pendred syndrome Pendred syndrome, AR: congenital, fluctuating, progressive HL Often EVA and sometimes euthyroid goiter and Mondini dysplasia ARNSHL associated with EVA WFS1 c.424_425ins16 in Spain and c.1362_1377del16 in Italy for Wolfram syndrome AD, low-frequency hearing loss AR Wolfram syndrome OTOF Q829X ARNSHL, auditory neuropathy: absence of ABR, presence of OAE Prelingual, often severe HL MT-RNR1 1555A > G Mitochondrial, aminoglycoside-induced HL; maternally inherited Varying from severe congenital HL to normal hearing TECTA None AR, moderate-to-severe mid-frequency HL COCH P51S in Belgium and The Netherlands Progressive HL and vestibular impairment with late onset POU3F4 None Mixed HL or pure sensorineural HL Defects in the bony labyrinth, e.g. Mondini dysplasia and stapes fixation Perilymphatic gusher during stapes surgery These genes are a frequent cause of hearing loss and/or they have a recognisable phenotype. Frequent mutations in Caucasians are listed for each gene if present. HL: hearing loss; AD: autosomal dominant; AR: autosomal recessive; EVA: enlarged vestibular aqueduct; ABR: auditory brainstem response; OAE: otoacoustic emission. prognostic information. For example, the parents of a child with GJB2-related deafness have a 25% recurrence chance to have another child with the same GJB2 genotype. Furthermore, if their first child has mild-to-moderate HL and they do have a second hearing-impaired child, there is a 66% chance that the second child will have mild-to-moderate HL and a 34% chance that the HL will be more severe. If their child has severe-to-profound HL and receives a cochlear implant, several studies have shown that the parents can expect their child to have an excellent outcome [43]. It is important to recognize that while genetic testing is crucial, test results must be interpreted correctly and steps must be taken to ensure that parents and family members understand the information presented to them. These responsibilities often fall to paediatricians with experience in genetics and clinical geneticists, as most other health care providers are uncomfortable discussing these issues. 5. Evolution of molecular diagnostics During the last 15 years, major achievements have been made in detecting new deafness genes. These achievements have far outpaced translation of this knowledge to improved clinical care. The gap created reflects the fact that diagnostic tests are still performed using the classical sequencing technology based on the Sanger method. These automatic DNA sequencers are very useful but are limited by slow throughput, which makes extensive sequencing of all known deafness-causing genes very expensive and time consuming. For this reason, only a very small set of genes is routinely screened for mutations with the result that in a large percentage of persons with HL, no genetic cause is identified. An additional problem for extended diagnostic screening is that most genes for ARNSHL lead to congenital severe-to-profound hearing impairment that is indistinguishable between different genes. A single exception is the TECTA-gene, leading to a recognisable milder phenotype, but these cases are rare [44]. The similarity in phenotype makes it impossible to predict the genetic cause based on audioprofiling for almost all cases. This situation highlights the urgent need for new DNA sequencing technologies which allow complete screening of all deafness genes in a quick and cost-effective way. Although a deafness GeneChip using an Affymetrix DNA resequencing microarray platform is being developed [45], we believe that these chips are unlikely to provide a major breakthrough in molecular diagnostic testing for HL because of the inaccuracy of this DNA sequencing system. More promising is the new generation of DNA sequencing technologies that have recently emerged including 454 (Roche), Solexa (Illumina) and SOLiD (Applied Biosystems). These new methods offer much higher throughput with high accuracy and potentially cheaper analyses. However, these machines have not been developed for DNA diagnostics, for which a few specific features are required: (1) high throughput for screening of selected regions; (2) high sensitivity to detect all types of mutations (missense mutations as well as large rearrangements); (3) the need for only small amounts of sample material; (4) high reliability; and (5) costeffectiveness. Recently, the microarray-based genomic selection method has been developed, which allows selecting and enriching large human genomic target regions for resequencing [46]. Combining this technology with the new generation sequencing technologies will allow large-scale resequencing, as required for DNA diagnostics. The benefits will be very large for HL, as this trait is extremely heterogeneous. When such a system is available and affordable, all children who do not pass NBHS could be screened for known causes of nonsyndromic HL. This screen should also include the Usher syndrome-causing genes as recognition of this type of syndromic deafness is difficult in infants and young children, and epidemiological studies indicate that the average prevalence of Usher syndrome among the deaf population is 10% [47]. It is pertinent to note that 5 of the 9 Usher genes also cause nonsyndromic HL. The benefits of a broad-based screening strategy include more accurate estimates of mutation frequency of deafness genes in diverse populations, the identification of novel genotype phenotype correlations, and enhanced clinical care for persons with HL and their families. Acknowledgements This study was supported by grants from EUROHEAR (LSHG-CT ) and the Fund for Scientific Research Flanders (FWO- F, Grant G ). Nele Hilgert is a fellow of the Fund for Scientific Research Flanders (FWO-F). RJHS is the Sterba Hearing Research Professor, University of Iowa College of Medicine, who supported the project with National Institutes of
7 N. Hilgert et al. / Mutation Research 681 (2009) Health (NIH)-National Institute on Deafness and Other Communication Disorders (NIDCD) grants DC02842 and DC Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi: /j.mrrev Conflict of interest None. References [1] C.C. Morton, W.E. Nance, Newborn hearing screening a silent revolution, N. Engl. J. Med. 354 (2006) [2] A. Kenneson, M.J. Cannon, Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection, Rev. Med. Virol. 17 (2007) [3] G. Van Camp, P.J. Willems, R.J. Smith, Nonsyndromic hearing impairment: unparalleled heterogeneity, Am. J. Hum. Genet. 60 (1997) [4] H.V. Toriello, W. Reardon, R.J. Gorlin, Hereditary Hearing Loss and its Syndromes, Oxford University Press Inc., Oxford, [5] K. Cryns, G. Van Camp, Deafness genes and their diagnostic applications, Audiol. Neurootol. 9 (2004) [6] A. Kenneson, K. Van Naarden Braun, C. Boyle, GJB2 (connexin 26) variants and nonsyndromic sensorineural hearing loss: a HuGE review, Genet. Med. 4 (2002) [7] R.L. Snoeckx, P.L. Huygen, D. Feldmann, S. Marlin, F. Denoyelle, J. Waligora, M. Mueller-Malesinska, A. Pollak, R. Ploski, A. Murgia, E. Orzan, P. Castorina, U. Ambrosetti, E. Nowakowska-Szyrwinska, J. Bal, W. Wiszniewski, A.R. Janecke, D. Nekahm-Heis, P. Seeman, O. Bendova, M.A. Kenna, A. Frangulov, H.L. Rehm, M. Tekin, A. Incesulu, H.H. Dahl, D. du Sart, L. Jenkins, D. Lucas, M. Bitner-Glindzicz, K.B. Avraham, Z. Brownstein, I. del Castillo, F. Moreno, N. Blin, M. Pfister, I. Sziklai, T. Toth, P.M. Kelley, E.S. Cohn, L. Van Maldergem, P. Hilbert, A.F. Roux, M. Mondain, L.H. Hoefsloot, C.W. Cremers, T. Lopponen, H. Lopponen, A. Parving, K. Gronskov, I. Schrijver, J. Roberson, F. Gualandi, A. Martini, G. Lina-Granade, N. Pallares-Ruiz, C. Correia, G. Fialho, K. Cryns, N. Hilgert, P. Van de Heyning, C.J. Nishimura, R.J. Smith, G. Van Camp, GJB2 mutations and degree of hearing loss: a multicenter study, Am. J. Hum. Genet. 77 (2005) [8] P. Gasparini, R. Rabionet, G. Barbujani, S. Melchionda, M. Petersen, K. Brondum- Nielsen, A. Metspalu, E. Oitmaa, M. Pisano, P. Fortina, L. Zelante, X. Estivill, High carrier frequency of the 35delG deafness mutation in European populations. Genetic Analysis Consortium of GJB2 35delG, Eur. J. Hum. Genet. 8 (2000) [9] R.J. Morell, H.J. Kim, L.J. Hood, L. Goforth, K. Friderici, R. Fisher, G. Van Camp, C.I. Berlin, C. Oddoux, H. Ostrer, B. Keats, T.B. Friedman, Mutations in the connexin 26 gene (GJB2) among Ashkenazi Jews with nonsyndromic recessive deafness, N. Engl. J. Med. 339 (1998) [10] A. Ohtsuka, I. Yuge, S. Kimura, A. Namba, S. Abe, L. Van Laer, G. Van Camp, S. Usami, GJB2 deafness gene shows a specific spectrum of mutations in Japan, including a frequent founder mutation, Hum. Genet. 112 (2003) [11] I. del Castillo, M. Villamar, M.A. Moreno-Pelayo, F.J. del Castillo, A. Alvarez, D. Telleria, I. Menendez, F. Moreno, A deletion involving the connexin 30 gene in nonsyndromic hearing impairment, N. Engl. J. Med. 346 (2002) [12] F.J. del Castillo, M. Rodriguez-Ballesteros, A. Alvarez, T. Hutchin, E. Leonardi, C.A. de Oliveira, H. Azaiez, Z. Brownstein, M.R. Avenarius, S. Marlin, A. Pandya, H. Shahin, K.R. Siemering, D. Weil, W. Wuyts, L.A. Aguirre, Y. Martin, M.A. Moreno- Pelayo, M. Villamar, K.B. Avraham, H.H. Dahl, M. Kanaan, W.E. Nance, C. Petit, R.J. Smith, G. Van Camp, E.L. Sartorato, A. Murgia, F. Moreno, I. del Castillo, A novel deletion involving the connexin-30 gene, del(gjb6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 nonsyndromic hearing impairment, J. Med. Genet. 42 (2005) [13] J.E. Common, M. Bitner-Glindzicz, E.A. O Toole, M.R. Barnes, L. Jenkins, A. Forge, D.P. Kelsell, Specific loss of connexin 26 expression in ductal sweat gland epithelium associated with the deletion mutation del(gjb6-d13s1830), Clin. Exp. Dermatol. 30 (2005) [14] W. Reardon, O.M. Cf, R. Trembath, H. Jan, P.D. Phelps, Enlarged vestibular aqueduct: a radiological marker of pendred syndrome, and mutation of the PDS gene, QJM 93 (2000) [15] B. Coyle, W. Reardon, J.A. Herbrick, L.C. Tsui, E. Gausden, J. Lee, R. Coffey, A. Grueters, A. Grossman, P.D. Phelps, L. Luxon, P. Kendall-Taylor, S.W. Scherer, R.C. Trembath, Molecular analysis of the PDS gene in Pendred syndrome, Hum. Mol. Genet. 7 (1998) [16] T. Yang, H. Vidarsson, S. Rodrigo-Blomqvist, S.S. Rosengren, S. Enerback, R.J. Smith, Transcriptional control of SLC26A4 is involved in Pendred syndrome and nonsyndromic enlargement of vestibular aqueduct (DFNB4), Am. J. Hum. Genet. 80 (2007) [17] H. Azaiez, T. Yang, S. Prasad, J.L. Sorensen, C.J. Nishimura, W.J. Kimberling, R.J. Smith, Genotype phenotype correlations for SLC26A4-related deafness, Hum. Genet. 122 (2007) [18] N. Nal, Z.M. Ahmed, E. Erkal, O.M. Alper, G. Luleci, O. Dinc, A.M. Waryah, Q. Ain, S. Tasneem, T. Husnain, P. Chattaraj, S. Riazuddin, E. Boger, M. Ghosh, M. Kabra, R.J. Morell, T.B. Friedman, Mutational spectrum of MYO15A: the large N-terminal extension of myosin XVA is required for hearing, Hum. Mutat. 28 (2007) [19] M. Rodriguez-Ballesteros, R. Reynoso, M. Olarte, M. Villamar, C. Morera, R. Santarelli, E. Arslan, C. Meda, C. Curet, C. Volter, M. Sainz-Quevedo, P. Castorina, U. Ambrosetti, S. Berrettini, K. Frei, S. Tedin, J. Smith, M. Cruz Tapia, L. Cavalle, N. Gelvez, P. Primignani, E. Gomez-Rosas, M. Martin, M.A. Moreno-Pelayo, M. Tamayo, J. Moreno-Barral, F. Moreno, I. del Castillo, A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy, Hum. Mutat. 29 (2008) [20] M. Rodriguez-Ballesteros, F.J. del Castillo, Y. Martin, M.A. Moreno-Pelayo, C. Morera, F. Prieto, J. Marco, A. Morant, J. Gallo-Teran, C. Morales-Angulo, C. Navas, G. Trinidad, M.C. Tapia, F. Moreno, I. del Castillo, Auditory neuropathy in patients carrying mutations in the otoferlin gene (OTOF), Hum. Mutat. 22 (2003) [21] S. Delmaghani, F.J. del Castillo, V. Michel, M. Leibovici, A. Aghaie, U. Ron, L. Van Laer, N. Ben-Tal, G. Van Camp, D. Weil, F. Langa, M. Lathrop, P. Avan, C. Petit, Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy, Nat. Genet. 38 (2006) [22] E. Becirovic, I. Ebermann, D. Nagy, E. Zrenner, M.W. Seeliger, H.J. Bolz, Usher syndrome type 1 due to missense mutations on both CDH23 alleles: investigation of mrna splicing, Hum. Mutat. 29 (2008) 452. [23] N. Hilgert, F. Alasti, N. Dieltjens, B. Pawlik, B. Wollnik, O. Uyguner, S. Delmaghani, D. Weil, C. Petit, E. Danis, T. Yang, E. Pandelia, M. Petersen, D. Goossens, J. Favero, M. Sanati, R. Smith, G. Van Camp, Mutation analysis of TMC1 identifies four new mutations and suggests an additional deafness gene at loci DFNA36 and DFNB7/ 11, Clin. Genet. (2008). [24] K. Cryns, T.A. Sivakumaran, J.M. Van den Ouweland, R.J. Pennings, C.W. Cremers, K. Flothmann, T.L. Young, R.J. Smith, M.M. Lesperance, G. Van Camp, Mutational spectrum of the WFS1 gene in Wolfram syndrome, nonsyndromic hearing impairment, diabetes mellitus, and psychiatric disease, Hum. Mutat. 22 (2003) [25] M.M. Lesperance, J.W. Hall III, T.B. San Agustin, S.M. Leal, Mutations in the Wolfram syndrome type 1 gene (WFS1) define a clinical entity of dominant low-frequency sensorineural hearing loss, Arch. Otolaryngol. Head Neck Surg. 129 (2003) [26] H. Fukuoka, Y. Kanda, S. Ohta, S. Usami, Mutations in the WFS1 gene are a frequent cause of autosomal dominant nonsyndromic low-frequency hearing loss in Japanese, J. Hum. Genet. 52 (2007) [27] K. Cryns, M. Pfister, R.J. Pennings, S.J. Bom, K. Flothmann, G. Caethoven, H. Kremer, I. Schatteman, K.A. Koln, T. Toth, S. Kupka, N. Blin, P. Nurnberg, H. Thiele, P.H. van de Heyning, W. Reardon, D. Stephens, C.W. Cremers, R.J. Smith, G. Van Camp, Mutations in the WFS1 gene that cause low-frequency sensorineural hearing loss are small non-inactivating mutations, Hum. Genet. 110 (2002) [28] M. Gomez-Zaera, T.M. Strom, B. Rodriguez, X. Estivill, T. Meitinger, V. Nunes, Presence of a major WFS1 mutation in Spanish Wolfram syndrome pedigrees, Mol. Genet. Metab. 72 (2001) [29] A. Colosimo, V. Guida, L. Rigoli, C. Di Bella, A. De Luca, S. Briuglia, L. Stuppia, D.C. Salpietro, B. Dallapiccola, Molecular detection of novel WFS1 mutations in patients with Wolfram syndrome by a DHPLC-based assay, Hum. Mutat. 21 (2003) [30] V. Topsakal, R.J. Pennings, H. te Brinke, B. Hamel, P.L. Huygen, H. Kremer, C.W. Cremers, Phenotype determination guides swift genotyping of a DFNA2/KCNQ4 family with a hot spot mutation (W276S), Otol. Neurotol. 26 (2005) [31] F. Kamada, S. Kure, T. Kudo, Y. Suzuki, T. Oshima, A. Ichinohe, K. Kojima, T. Niihori, J. Kanno, Y. Narumi, A. Narisawa, K. Kato, Y. Aoki, K. Ikeda, T. Kobayashi, Y. Matsubara, A novel KCNQ4 one-base deletion in a large pedigree with hearing loss: implication for the genotype-phenotype correlation, J. Hum. Genet. 51 (2006) [32] G. Van Camp, P.J. Coucke, J. Akita, E. Fransen, S. Abe, E.M. De Leenheer, P.L. Huygen, C.W. Cremers, S. Usami, A mutational hot spot in the KCNQ4 gene responsible for autosomal dominant hearing impairment, Hum. Mutat. 20 (2002) [33] M.H. Kemperman, E.M. De Leenheer, P.L. Huygen, G. van Duijnhoven, C.C. Morton, N.G. Robertson, F.P. Cremers, H. Kremer, C.W. Cremers, Audiometric, vestibular, and genetic aspects of a DFNA9 family with a G88E COCH mutation, Otol. Neurotol. 26 (2005) [34] V.A. Street, J.C. Kallman, N.G. Robertson, S.F. Kuo, C.C. Morton, J.O. Phillips, A novel DFNA9 mutation in the vwfa2 domain of COCH alters a conserved cysteine residue and intrachain disulfide bond formation resulting in progressive hearing loss and site-specific vestibular and central oculomotor dysfunction, Am. J. Med. Genet. A 139 (2005) [35] E. Fransen, M. Verstreken, S.J. Bom, F. Lemaire, M.H. Kemperman, Y.J. De Kok, F.L. Wuyts, W.I. Verhagen, P.L. Huygen, W.T. McGuirt, R.J. Smith, L.V. Van Maldergem, F. Declau, C.W. Cremers, P.H. Van De Heyning, F.P. Cremers, G. Van Camp, A common ancestor for COCH related cochleovestibular (DFNA9) patients in Belgium and The Netherlands bearing the P51S mutation, J. Med. Genet. 38 (2001) [36] E.A. de Zwart-Storm, H. Hamm, J. Stoevesandt, P.M. Steijlen, P.E. Martin, M. van Geel, M.A. van Steensel, A novel missense mutation in GJB2 disturbs gap junction protein transport and causes focal palmoplantar keratoderma with deafness, J. Med. Genet. 45 (2008)
8 196 N. Hilgert et al. / Mutation Research 681 (2009) [37] G. Richard, F. Rouan, C.E. Willoughby, N. Brown, P. Chung, M. Ryynanen, E.W. Jabs, S.J. Bale, J.J. DiGiovanna, J. Uitto, L. Russell, Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis ichthyosis deafness syndrome, Am. J. Hum. Genet. 70 (2002) [38] M. Pfister, H. Thiele, G. Van Camp, E. Fransen, F. Apaydin, O. Aydin, P. Leistenschneider, M. Devoto, H.P. Zenner, N. Blin, P. Nurnberg, H. Ozkarakas, S. Kupka, A genotype phenotype correlation with gender-effect for hearing impairment caused by TECTA mutations, Cell. Physiol. Biochem. 14 (2004) [39] A.P. Vore, E.H. Chang, J.E. Hoppe, M.G. Butler, S. Forrester, M.C. Schneider, L.L. Smith, D.W. Burke, C.A. Campbell, R.J. Smith, Deletion of and novel missense mutation in POU3F4 in 2 families segregating X-linked nonsyndromic deafness, Arch. Otolaryngol. Head Neck Surg. 131 (2005) [40] H. Kokotas, M.B. Petersen, P.J. Willems, Mitochondrial deafness, Clin. Genet. 71 (2007) [41] L. Jin, A. Yang, Y. Zhu, J. Zhao, X. Wang, L. Yang, D. Sun, Z. Tao, A. Tsushima, G. Wu, L. Xu, C. Chen, B. Yi, J. Cai, X. Tang, J. Wang, D. Li, Q. Yuan, Z. Liao, J. Chen, Z. Li, J. Lu, M.X. Guan, Mitochondrial trnaser(ucn) gene is the hot spot for mutations associated with aminoglycoside-induced and non-syndromic hearing loss, Biochem. Biophys. Res. Commun. 361 (2007) [42] J.C.o.I. Hearing, Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs, Pediatrics 120 (2007) [43] S.S. Connell, S.I. Angeli, H. Suarez, A.V. Hodges, T.J. Balkany, X.Z. Liu, Performance after cochlear implantation in DFNB1 patients, Otolaryngol. Head Neck Surg. 137 (2007) [44] F. Alasti, M.H. Sanati, A.H. Behrouzifard, A. Sadeghi, A.P. de Brouwer, H. Kremer, R.J. Smith, G. Van Camp, A novel TECTA mutation confirms the recognizable phenotype among autosomal recessive hearing impairment families, Int. J. Pediatr. Otorhinolaryngol. 72 (2008) [45] S. Cox, P. Kothiyal, S.R. DePalma, J. Ebert, F. Thompson, M.A. Kenna, J.G. Seidman, B. Aronow, J.H. Greinwald, H. Rehm, An integrated approach to molecular diagnostic testing for hearing loss and related syndromes, (abstract P80), in: 6th Molecular Biology of Hearing & Deafness Conference, Hinxton, UK, [46] D.T. Okou, K.M. Steinberg, C. Middle, D.J. Cutler, T.J. Albert, M.E. Zwick, Microarray-based genomic selection for high-throughput resequencing, Nature Methods 4 (2007) [47] M. Cohen, M. Bitner-Glindzicz, L. Luxon, The changing face of Usher syndrome: clinical implications, Int. J. Audiol. 46 (2007)
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics
 G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
G. Shashidhar Pai, MD MUSC Children s Hospital Department of Pediatrics Division of Genetics One of every 500 newborns has bilateral permanent sensorineural hearing loss 40 db which makes it the most common
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Genetic Testing for Hereditary Hearing Loss
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDIX HISTORY Genetic Testing for Hereditary Hearing Loss Number 12.04.87 Effective
Genetic Testing for Hereditary Hearing Loss. Populations Interventions Comparators Outcomes Interventions of interest are: Genetic testing
 Protocol Genetic Testing for Hereditary Hearing Loss (20487) Medical Benefit Effective Date: 01/01/15 Next Review Date: 11/16 Preauthorization Yes Review Dates: 01/14, 11/14, 11/15 Preauthorization is
Protocol Genetic Testing for Hereditary Hearing Loss (20487) Medical Benefit Effective Date: 01/01/15 Next Review Date: 11/16 Preauthorization Yes Review Dates: 01/14, 11/14, 11/15 Preauthorization is
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
The Genetics of Hearing Loss. Heidi L. Rehm, Ph.D. Harvard Medical School Boston, MA
 The Genetics of Hearing Loss Heidi L. Rehm, Ph.D. Harvard Medical School Boston, MA Faculty Disclosure Information In the past 12 months, I have not had a significant financial interest or other relationship
The Genetics of Hearing Loss Heidi L. Rehm, Ph.D. Harvard Medical School Boston, MA Faculty Disclosure Information In the past 12 months, I have not had a significant financial interest or other relationship
Jennifer A. Defant, M.S., C.G.C. Certified Genetic Counselor Division of Genetics and Metabolism University of Florida
 Jennifer A. Defant, M.S., C.G.C. Certified Genetic Counselor Division of Genetics and Metabolism University of Florida 60% of childhood hearing loss is genetic Syndromic Nonsyndromic 40% of childhood hearing
Jennifer A. Defant, M.S., C.G.C. Certified Genetic Counselor Division of Genetics and Metabolism University of Florida 60% of childhood hearing loss is genetic Syndromic Nonsyndromic 40% of childhood hearing
Objectives Role of Medical Genetics in Hearing Loss Evaluation. 5 y.o. boy with severe SNHL
 Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
Objectives Role of Medical Genetics in Hearing Loss Evaluation Millan Patel, MD UBC Dept. of Medical Genetics October 22, 2010 Case presentation to illustrate importance of defining syndromic hearing loss
BEST PRACTICES Pediatric Bilateral Sensorineural Hearing Loss
 BEST PRACTICES Pediatric Bilateral Sensorineural Hearing Loss Christina L. Runge, PhD, CCC-A Associate Professor Chief, Division of Communication Sciences Director, Koss Cochlear Implant Program Resources
BEST PRACTICES Pediatric Bilateral Sensorineural Hearing Loss Christina L. Runge, PhD, CCC-A Associate Professor Chief, Division of Communication Sciences Director, Koss Cochlear Implant Program Resources
Medical Policy Manual. Date of Origin: October 2014. Topic: Genetic Testing for Hereditary Hearing Loss. Last Reviewed Date: December 2015
 Medical Policy Manual Topic: Genetic Testing for Hereditary Hearing Loss Section: Genetic Testing Policy No: 36 Date of Origin: October 2014 Last Reviewed Date: December 2015 Effective Date: February 1,
Medical Policy Manual Topic: Genetic Testing for Hereditary Hearing Loss Section: Genetic Testing Policy No: 36 Date of Origin: October 2014 Last Reviewed Date: December 2015 Effective Date: February 1,
Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics
 Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Genetic Testing for Hearing Loss
 Genetic Testing for Hearing Loss S H O B A N A K U B E N D R A N, M B B S, M S, C G C G E N E T I C C O U N S E L O R A S S T P R O F E S S O R D E P T O F P E D I A T R I C S K U S M - W Objectives Indications
Genetic Testing for Hearing Loss S H O B A N A K U B E N D R A N, M B B S, M S, C G C G E N E T I C C O U N S E L O R A S S T P R O F E S S O R D E P T O F P E D I A T R I C S K U S M - W Objectives Indications
Goals for Today. Clinical and Molecular Aspects of Genetic Hearing Loss. Case presentation. Case presentation continued. Case presentation continued
 Clinical and Molecular Aspects of Genetic Hearing Loss Kathleen Arnos, PhD Department of Science, Technology, & Mathematics Gallaudet University Washington, DC 20002 kathleen.arnos@gallaudet.edu Goals
Clinical and Molecular Aspects of Genetic Hearing Loss Kathleen Arnos, PhD Department of Science, Technology, & Mathematics Gallaudet University Washington, DC 20002 kathleen.arnos@gallaudet.edu Goals
About The Causes of Hearing Loss
 About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
About 1 in 500 infants is born with or develops hearing loss during early childhood. Hearing loss has many causes: some are genetic (that is, caused by a baby s genes) or non-genetic (such as certain infections
The future role of genetic screening to detect newborns at risk of childhood-onset hearing loss
 International Journal of Audiology 2013; 52: 124 133 Discussion Article The future role of genetic screening to detect newborns at risk of childhood-onset hearing loss Luan Linden Phillips *, Maria Bitner-Glindzicz,
International Journal of Audiology 2013; 52: 124 133 Discussion Article The future role of genetic screening to detect newborns at risk of childhood-onset hearing loss Luan Linden Phillips *, Maria Bitner-Glindzicz,
commentary Genetics IN Medicine 521 November/December 2004 Vol. 6 No. 6
 November/December 2004 Vol. 6 No. 6 commentary Genetic testing as part of the Early Hearing Detection and Intervention (EHDI) process Lisa A. Schimmenti, MD 1, Ariadna Martinez, MS, MS 2, Michelle Fox,
November/December 2004 Vol. 6 No. 6 commentary Genetic testing as part of the Early Hearing Detection and Intervention (EHDI) process Lisa A. Schimmenti, MD 1, Ariadna Martinez, MS, MS 2, Michelle Fox,
Medical Policy Genetic Testing for Hereditary Hearing Loss
 Medical Policy Genetic Testing for Hereditary Hearing Loss Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization
Medical Policy Genetic Testing for Hereditary Hearing Loss Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization
Cochlear Implantation for Children With GJB2-Related Deafness
 The Laryngoscope Lippincott Williams & Wilkins, Inc. 2004 The American Laryngological, Rhinological and Otological Society, Inc. Cochlear Implantation for Children With GJB2-Related Deafness Robert D.
The Laryngoscope Lippincott Williams & Wilkins, Inc. 2004 The American Laryngological, Rhinological and Otological Society, Inc. Cochlear Implantation for Children With GJB2-Related Deafness Robert D.
Deafness and Hereditary Hearing Loss Overview
 Page 1 of 25 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews [Internet]. Seattle (WA): University
Page 1 of 25 NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Pagon RA, Bird TD, Dolan CR, et al., editors. GeneReviews [Internet]. Seattle (WA): University
Single Gene and NextGen Panels
 Molecular Testing for Hearing Loss: Single Gene and NextGen Panels Honey V Reddi, PhD, FACMG Clinical Molecular Geneticist Prevention Genetics, Marshfield, WI www.preventiongenetics.com Outline of Presentation
Molecular Testing for Hearing Loss: Single Gene and NextGen Panels Honey V Reddi, PhD, FACMG Clinical Molecular Geneticist Prevention Genetics, Marshfield, WI www.preventiongenetics.com Outline of Presentation
CONGENITAL HEARING LOSS
 CONGENITAL HEARING LOSS Congenital hearing loss, for the purposes of this fact sheet, is defined as permanent and is bilateral or unilateral, is sensory or conductive, and averages 30 db or more in the
CONGENITAL HEARING LOSS Congenital hearing loss, for the purposes of this fact sheet, is defined as permanent and is bilateral or unilateral, is sensory or conductive, and averages 30 db or more in the
Supervisors Prof.Dr.Ahmad Sameh Farid Professor of ENT and Audiology Faculty of Medicine Cairo University
 Connexin 26 gene in non-syndromic hearing loss Thesis Submitted for partial fulfillment of MD degree in Audiology by Mona Ahmed El-Akkad M.B, B.CH., MSc. Supervisors Prof.Dr.Ahmad Sameh Farid Professor
Connexin 26 gene in non-syndromic hearing loss Thesis Submitted for partial fulfillment of MD degree in Audiology by Mona Ahmed El-Akkad M.B, B.CH., MSc. Supervisors Prof.Dr.Ahmad Sameh Farid Professor
DURATION OF HEARING LOSS
 When your child is diagnosed with a hearing loss, it may be very overwhelming. This may be a difficult time for you and your family. However, gaining a greater knowledge in this area is crucial in helping
When your child is diagnosed with a hearing loss, it may be very overwhelming. This may be a difficult time for you and your family. However, gaining a greater knowledge in this area is crucial in helping
Hearing loss is an etiologically heterogeneous trait with
 The new england journal of medicine review article Current Concepts Newborn Hearing Screening A Silent Revolution Cynthia C. Morton, Ph.D., and Walter E. Nance, M.D., Ph.D. Hearing loss is an etiologically
The new england journal of medicine review article Current Concepts Newborn Hearing Screening A Silent Revolution Cynthia C. Morton, Ph.D., and Walter E. Nance, M.D., Ph.D. Hearing loss is an etiologically
Usher Syndrome Genetics
 Usher Syndrome Genetics October 2012 Page 1 of 20 Introduction Usher syndrome is a genetic or inherited condition that affects hearing, vision and balance The sight loss is caused by an eye condition known
Usher Syndrome Genetics October 2012 Page 1 of 20 Introduction Usher syndrome is a genetic or inherited condition that affects hearing, vision and balance The sight loss is caused by an eye condition known
Congenital Hearing Loss
 Congenital Hearing Loss A resident asks... Why would a primary care physician want to know about the genetics of hearing loss? Key Points: Newborn screening for hearing loss is being implemented in many
Congenital Hearing Loss A resident asks... Why would a primary care physician want to know about the genetics of hearing loss? Key Points: Newborn screening for hearing loss is being implemented in many
Congenital Deafness and Goiter: Pendred Syndrome
 This text is a translation from the original German which should be used for referencing. The German version is authoritative. REVIEW ARTICLE Congenital Deafness and Goiter: Pendred Syndrome Guntram Borck,
This text is a translation from the original German which should be used for referencing. The German version is authoritative. REVIEW ARTICLE Congenital Deafness and Goiter: Pendred Syndrome Guntram Borck,
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT. Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy
 COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
Genetic Mutations Cause Many Birth Defects:
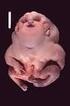 Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Bases of Hearing Loss: Future Treatment Implications
 Genetic Bases of Hearing Loss: Future Treatment Implications Luis F. Escobar, MD Medical Director Medical Genetics & Neurodevelopmental Pediatrics of Indiana Peyton Manning Children s Hospital St. Vincent
Genetic Bases of Hearing Loss: Future Treatment Implications Luis F. Escobar, MD Medical Director Medical Genetics & Neurodevelopmental Pediatrics of Indiana Peyton Manning Children s Hospital St. Vincent
SENSORINEURAL HEARING LOSS INVESTIGATION & REFERRAL
 Epidemiology NZ Hearing Screening Referral Pathway Important Services When to Investigate Initial Assessment Referrals Investigations Indications for Cochlear Transplant Acknowledgements References Epidemiology
Epidemiology NZ Hearing Screening Referral Pathway Important Services When to Investigate Initial Assessment Referrals Investigations Indications for Cochlear Transplant Acknowledgements References Epidemiology
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Victims Compensation Claim Status of All Pending Claims and Claims Decided Within the Last Three Years
 Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD. Department of Otolaryngology Hacettepe University ANKARA, TURKEY
 GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
Understanding the Genetics of Deafness
 Understanding the Genetics of Deafness A Guide for Patients and Families Harvard Medical School Center For Hereditary Deafness Understanding the Genetics of Deafness Understanding the Genetics of Deafness
Understanding the Genetics of Deafness A Guide for Patients and Families Harvard Medical School Center For Hereditary Deafness Understanding the Genetics of Deafness Understanding the Genetics of Deafness
Breast cancer and the role of low penetrance alleles: a focus on ATM gene
 Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
Modena 18-19 novembre 2010 Breast cancer and the role of low penetrance alleles: a focus on ATM gene Dr. Laura La Paglia Breast Cancer genetic Other BC susceptibility genes TP53 PTEN STK11 CHEK2 BRCA1
Why does my child have a hearing loss?
 Introduction This factsheet will tell you about the range of tests that can be carried out to try to find the cause of your child s hearing loss. The process to find out why a child is deaf is sometimes
Introduction This factsheet will tell you about the range of tests that can be carried out to try to find the cause of your child s hearing loss. The process to find out why a child is deaf is sometimes
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Screening of genetic alterations related to non-syndromic hearing loss using MassARRAY iplex technology
 Svidnicki et al. BMC Medical Genetics (2015) 16:85 DOI 10.1186/s12881-015-0232-8 RESEARCH ARTICLE Open Access Screening of genetic alterations related to non-syndromic hearing loss using MassARRAY iplex
Svidnicki et al. BMC Medical Genetics (2015) 16:85 DOI 10.1186/s12881-015-0232-8 RESEARCH ARTICLE Open Access Screening of genetic alterations related to non-syndromic hearing loss using MassARRAY iplex
A CASE FOR ETIOLOGIC FOCUS IN AUDIOLOGY GENETIC TESTING. Thesis
 A CASE FOR ETIOLOGIC FOCUS IN AUDIOLOGY GENETIC TESTING Thesis Presented in Partial Fulfillment of the Requirements for The Masters in Speech and Hearing Science in the Graduate School of The Ohio State
A CASE FOR ETIOLOGIC FOCUS IN AUDIOLOGY GENETIC TESTING Thesis Presented in Partial Fulfillment of the Requirements for The Masters in Speech and Hearing Science in the Graduate School of The Ohio State
EXECUTIVE SUMMARY OF JOINT COMMITTEE ON INFANT HEARING YEAR 2007 POSITION STATEMENT. Intervention Programs
 EXECUTIVE SUMMARY OF JOINT COMMITTEE ON INFANT HEARING YEAR 2007 POSITION STATEMENT Principles and Guidelines for Early Hearing Detection and Intervention Programs The Joint Committee on Infant Hearing
EXECUTIVE SUMMARY OF JOINT COMMITTEE ON INFANT HEARING YEAR 2007 POSITION STATEMENT Principles and Guidelines for Early Hearing Detection and Intervention Programs The Joint Committee on Infant Hearing
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
So, how do we hear? outer middle ear inner ear
 The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Cordblood-Based High-Throughput Screening for Deafness Gene of 646 Newborns in Jinan Area of China
 Original Article Clinical and Experimental Otorhinolaryngology Vol. 8, No. 3: 211-217, September 2015 http://dx.doi.org/10.3342/ceo.2015.8.3.211 pissn 1976-8710 eissn 2005-0720 Cordblood-Based High-Throughput
Original Article Clinical and Experimental Otorhinolaryngology Vol. 8, No. 3: 211-217, September 2015 http://dx.doi.org/10.3342/ceo.2015.8.3.211 pissn 1976-8710 eissn 2005-0720 Cordblood-Based High-Throughput
Genetic diagnostics the gateway to personalized medicine
 Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
Micronova 20.11.2012 Genetic diagnostics the gateway to personalized medicine Kristiina Assoc. professor, Director of Genetic Department HUSLAB, Helsinki University Central Hospital The Human Genome Packed
A Common Genetic Disorder Deafness
 A Common Genetic Disorder Deafness Shahid Hussain Department of Bioinformatics, Mohammad Ali Jinnah University Islamabad, Pakistan Abstract: Deafness is a complete or partial loss of hearing, caused due
A Common Genetic Disorder Deafness Shahid Hussain Department of Bioinformatics, Mohammad Ali Jinnah University Islamabad, Pakistan Abstract: Deafness is a complete or partial loss of hearing, caused due
Questions and Answers for Parents
 Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
HEARING & HEARING LOSS. Dr I Butler 2015
 HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
A pproximately one child in 500 650 is born deaf,
 147 ORIGINAL ARTICLE A genotype-phenotype correlation for GJB2 (connexin 26) deafness K Cryns*, E Orzan*, A Murgia*, P L M Huygen, F Moreno, I del Castillo, G Parker Chamberlin, H Azaiez, S Prasad, R A
147 ORIGINAL ARTICLE A genotype-phenotype correlation for GJB2 (connexin 26) deafness K Cryns*, E Orzan*, A Murgia*, P L M Huygen, F Moreno, I del Castillo, G Parker Chamberlin, H Azaiez, S Prasad, R A
Paediatric Hearing Assessment
 Information for parents Paediatric Hearing Assessment Hearing assessment of infants is limited by their ability to respond to sounds. This is determined by both the development of the hearing system and
Information for parents Paediatric Hearing Assessment Hearing assessment of infants is limited by their ability to respond to sounds. This is determined by both the development of the hearing system and
Newborn Genetic Screening for Hearing Impairment: A Preliminary Study at a Tertiary Center
 Newborn Genetic Screening for Hearing Impairment: A Preliminary Study at a Tertiary Center Chen-Chi Wu 1,2, Chia-Cheng Hung 2,5, Shin-Yu Lin 2, Wu-Shiun Hsieh 3, Po-Nien Tsao 3, Chien-Nan Lee 4, Yi-Ning
Newborn Genetic Screening for Hearing Impairment: A Preliminary Study at a Tertiary Center Chen-Chi Wu 1,2, Chia-Cheng Hung 2,5, Shin-Yu Lin 2, Wu-Shiun Hsieh 3, Po-Nien Tsao 3, Chien-Nan Lee 4, Yi-Ning
UNIT 13 (OPTION) Genetic Abnormalities
 Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
Unit 13 Genetic Abnormailities 1 UNIT 13 (OPTION) Genetic Abnormalities Originally developed by: Hildur Helgedottir RN, MN Revised (2000) by: Marlene Reimer RN, PhD, CCN (C) Associate Professor Faculty
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES
 DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
Infant hearing screening will not hurt your baby, and will only take between 5 and 20 minutes. Ideally it is done whilst baby is asleep or settled.
 Early diagnosis of hearing loss will make a difference to your baby s life. Significant hearing loss is the most common condition present at birth. In the private sector, 3 in every 1000 babies are born
Early diagnosis of hearing loss will make a difference to your baby s life. Significant hearing loss is the most common condition present at birth. In the private sector, 3 in every 1000 babies are born
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians
 Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
Heritability: Twin Studies. Twin studies are often used to assess genetic effects on variation in a trait
 TWINS AND GENETICS TWINS Heritability: Twin Studies Twin studies are often used to assess genetic effects on variation in a trait Comparing MZ/DZ twins can give evidence for genetic and/or environmental
TWINS AND GENETICS TWINS Heritability: Twin Studies Twin studies are often used to assess genetic effects on variation in a trait Comparing MZ/DZ twins can give evidence for genetic and/or environmental
Coding Fact Sheet for Primary Care Pediatricians
 1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
Lecture 3: Mutations
 Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
Lecture 3: Mutations Recall that the flow of information within a cell involves the transcription of DNA to mrna and the translation of mrna to protein. Recall also, that the flow of information between
GJB2 Gene Testing, Etiologic Diagnosis and Genetic Counseling in Romanian Persons With Prelingual Hearing Loss
 Elmer ress Original Article Int J Clin Pediatr. 2015;4(1):121-126 GJB2 Gene Testing, Etiologic Diagnosis and Genetic Counseling in Romanian Persons With Prelingual Hearing Loss Cristina Dragomir a, Adriana
Elmer ress Original Article Int J Clin Pediatr. 2015;4(1):121-126 GJB2 Gene Testing, Etiologic Diagnosis and Genetic Counseling in Romanian Persons With Prelingual Hearing Loss Cristina Dragomir a, Adriana
Understanding Hearing Loss 404.591.1884. www.childrensent.com
 Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Genetic Aspects of Mental Retardation and Developmental Disabilities
 Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP Kozmac@georgetown.edu cck2@gunet.georgetown.edu Genetic Aspects of Mental Retardation and Developmental Disabilities
Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP Kozmac@georgetown.edu cck2@gunet.georgetown.edu Genetic Aspects of Mental Retardation and Developmental Disabilities
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
CONNEXINS IN HEARING LOSS: A COMPREHENSIVE OVERVIEW
 CONNEXINS IN HEARING LOSS: A COMPREHENSIVE OVERVIEW Adi D. Sabag, Orit Dagan and Karen B. Avraham Department of Human Genetics and Molecular Medicine, Sackler School of Medicine, Tel Aviv University, Tel
CONNEXINS IN HEARING LOSS: A COMPREHENSIVE OVERVIEW Adi D. Sabag, Orit Dagan and Karen B. Avraham Department of Human Genetics and Molecular Medicine, Sackler School of Medicine, Tel Aviv University, Tel
Roberto Ciccone, Orsetta Zuffardi Università di Pavia
 Roberto Ciccone, Orsetta Zuffardi Università di Pavia XIII Corso di Formazione Malformazioni Congenite dalla Diagnosi Prenatale alla Terapia Postnatale unipv.eu Carrara, 24 ottobre 2014 Legend:Bluebars
Roberto Ciccone, Orsetta Zuffardi Università di Pavia XIII Corso di Formazione Malformazioni Congenite dalla Diagnosi Prenatale alla Terapia Postnatale unipv.eu Carrara, 24 ottobre 2014 Legend:Bluebars
Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan
 Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan Definition Deaf A deaf person is one whose hearing disability precludes successful processing of linguistic information
Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan Definition Deaf A deaf person is one whose hearing disability precludes successful processing of linguistic information
COCHLEAR IMPLANTATION IN A PATIENT WITH LARGE VESTIBULAR AQUEDUCT SYNDROME: A CASE REPORT GENİŞ VESTİBÜLER SENDROMLU HASTADA KOKLEAR İMPLANTASYON
 CASE REPORT COCHLEAR IMPLANTATION IN A PATIENT WITH LARGE VESTIBULAR AQUEDUCT SYNDROME: A CASE REPORT Ufuk Derinsu, Ayça Çiprut, Sezer Külekçi, Ferda Akdaş Sub-department of Audiology, Department of Otorhinolaryngology,
CASE REPORT COCHLEAR IMPLANTATION IN A PATIENT WITH LARGE VESTIBULAR AQUEDUCT SYNDROME: A CASE REPORT Ufuk Derinsu, Ayça Çiprut, Sezer Külekçi, Ferda Akdaş Sub-department of Audiology, Department of Otorhinolaryngology,
AGE Iowa Midwest Nation N % N % N % Total students 256 100.0 9272 100.0 37500 100.0 Information NOT reported 7 2.7 229 2.5 841 2.2
 202-651-5575 * 1-800-451-8834 ext 5575 Page 1 of 9 AGE Iowa Midwest Nation Information NOT reported 7 2.7 229 2.5 841 2.2 Total known information 249 100.0 9043 100.0 36659 100.0 Under 3 years 16 6.4 201
202-651-5575 * 1-800-451-8834 ext 5575 Page 1 of 9 AGE Iowa Midwest Nation Information NOT reported 7 2.7 229 2.5 841 2.2 Total known information 249 100.0 9043 100.0 36659 100.0 Under 3 years 16 6.4 201
How To Know If A Cochlear Implant Is Right For You
 MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
The Genomics of Hearing Loss
 The Genomics of Hearing Loss Ian Krantz, M.D. The Children s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania Objectives 1. Understand the clinical and genetic
The Genomics of Hearing Loss Ian Krantz, M.D. The Children s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania Objectives 1. Understand the clinical and genetic
Attitudes toward genetic testing
 DOI 10.1515/jbcpp-2013-0063 J Basic Clin Physiol Pharmacol 2013; 24(3): 165 170 Mini Review Natali Idan, Zippora Brownstein, Shaked Shivatzki and Karen B. Avraham* Advances in genetic diagnostics for hereditary
DOI 10.1515/jbcpp-2013-0063 J Basic Clin Physiol Pharmacol 2013; 24(3): 165 170 Mini Review Natali Idan, Zippora Brownstein, Shaked Shivatzki and Karen B. Avraham* Advances in genetic diagnostics for hereditary
Genetics of Hearing Loss
 2 Genetics of Hearing Loss Ella Shalit and Karen B. Avraham 1. Introduction The revolution in genetics in the past decades has enabled identification of many of the genes associated with human hereditary
2 Genetics of Hearing Loss Ella Shalit and Karen B. Avraham 1. Introduction The revolution in genetics in the past decades has enabled identification of many of the genes associated with human hereditary
Genetic Mutations. Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes.
 Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Genetic Mutations Indicator 4.8: Compare the consequences of mutations in body cells with those in gametes. Agenda Warm UP: What is a mutation? Body cell? Gamete? Notes on Mutations Karyotype Web Activity
Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal
 www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
Hearing impairment in Estonia: An algorithm to investigate genetic causes in pediatric patients
 Advances in Medical Sciences Vol. 58(2) 2013 pp 419-428 DOI: 10.2478/ams-2013-0001 Medical University of Bialystok, Poland Hearing impairment in Estonia: An algorithm to investigate genetic causes in pediatric
Advances in Medical Sciences Vol. 58(2) 2013 pp 419-428 DOI: 10.2478/ams-2013-0001 Medical University of Bialystok, Poland Hearing impairment in Estonia: An algorithm to investigate genetic causes in pediatric
1/26/2011. 50% of deafness and hearing impairment is avoidable through prevention, early diagnosis, and management.
 Hearing Impairment Roseann Mulligan, DDS, MS Herman Ostrow School of Dentistry of the University of Southern California 1 JAMA, July 4, 2007 Vol 298, No. 1 2 278 million - moderate to profound bilateral
Hearing Impairment Roseann Mulligan, DDS, MS Herman Ostrow School of Dentistry of the University of Southern California 1 JAMA, July 4, 2007 Vol 298, No. 1 2 278 million - moderate to profound bilateral
Genetic Testing for Hereditary Breast and Ovarian Cancer - BRCA1/2 ANALYSIS -
 Genetic Testing for Hereditary Breast and Ovarian Cancer - BRCA1/2 ANALYSIS - January 2005 SCIENTIFIC BACKGROUND Breast cancer is considered to be one of the most prevalent cancer in women. The overall
Genetic Testing for Hereditary Breast and Ovarian Cancer - BRCA1/2 ANALYSIS - January 2005 SCIENTIFIC BACKGROUND Breast cancer is considered to be one of the most prevalent cancer in women. The overall
The Work-Up of Childhood Sensorineural Hearing Loss Jonathan M. Sherman, Eliot Shearer, Richard J.H. Smith, and Dana Suskind
 2 The Work-Up of Childhood Sensorineural Hearing Loss Jonathan M. Sherman, Eliot Shearer, Richard J.H. Smith, and Dana Suskind The Problem Hearing loss in children is common in the United States with an
2 The Work-Up of Childhood Sensorineural Hearing Loss Jonathan M. Sherman, Eliot Shearer, Richard J.H. Smith, and Dana Suskind The Problem Hearing loss in children is common in the United States with an
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance
 Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
Genetic Hearing Loss
 Genetic Hearing Loss Stephanie Cordes, MD Faculty Advisor: Norman Friedman, MD The University of Texas Medical Branch Department of Otolaryngology Grand Rounds Presentation April 2000 Introduction Deafness
Genetic Hearing Loss Stephanie Cordes, MD Faculty Advisor: Norman Friedman, MD The University of Texas Medical Branch Department of Otolaryngology Grand Rounds Presentation April 2000 Introduction Deafness
A bout 1 in 1000 British children are born deaf.
 1of5 ELECTRONIC LETTER Genetic information but not termination: pregnant women s attitudes and willingness to pay for carrier screening for deafness genes M Ryan, Z Miedzybrodzka, L Fraser, M Hall... J
1of5 ELECTRONIC LETTER Genetic information but not termination: pregnant women s attitudes and willingness to pay for carrier screening for deafness genes M Ryan, Z Miedzybrodzka, L Fraser, M Hall... J
PROVIDER POLICIES & PROCEDURES
 PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
PROVIDER POLICIES & PROCEDURES BRCA GENETIC TESTING The purpose of this document is to provide guidance to providers enrolled in the Connecticut Medical Assistance Program (CMAP) on the requirements for
MCB41: Second Midterm Spring 2009
 MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
MCB41: Second Midterm Spring 2009 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 7 pages including this page. You will have 50 minutes for
Exome sequencing e targeted sequencing in diagnostica e ricerca applicata: pro e contro
 Exome sequencing e targeted sequencing in diagnostica e ricerca applicata: pro e contro P. Gasparini University of Trieste IRCCS-Burlo Garofolo Children Hospital SIGU Sorrento 22/11/2012 Our NGS facility
Exome sequencing e targeted sequencing in diagnostica e ricerca applicata: pro e contro P. Gasparini University of Trieste IRCCS-Burlo Garofolo Children Hospital SIGU Sorrento 22/11/2012 Our NGS facility
Prevalence of otological disorders in diabetic patients with hearing loss
 Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
Spinal Muscular Atrophy
 Maryam Oskoui, MD, MSc, FRCPC Pediatric Neurologist Spinal Muscular Atrophy Elise Historical Timeline In vitro and animal studies Werdnig and Hoffmann describe SMA 1 (Arch Psych Nervenkrankheiten) Disease
Maryam Oskoui, MD, MSc, FRCPC Pediatric Neurologist Spinal Muscular Atrophy Elise Historical Timeline In vitro and animal studies Werdnig and Hoffmann describe SMA 1 (Arch Psych Nervenkrankheiten) Disease
Nouvelles méthodes de diagnostic génétique des maladies mitochondriales
 Nouvelles méthodes de diagnostic génétique des maladies mitochondriales Mitochondrial disorders 1/8000 live birth Primary mitochondrial disorders Mutations in 80 nuclear genes Mutations in 13 mitochondrial
Nouvelles méthodes de diagnostic génétique des maladies mitochondriales Mitochondrial disorders 1/8000 live birth Primary mitochondrial disorders Mutations in 80 nuclear genes Mutations in 13 mitochondrial
Accuracy of OAE and BERA to Detect the Incidence of Hearing Loss in Newborn
 P IHS P P P 2 International Journal of Scientific and Research Publications, Volume 5, Issue 6, June 2015 1 Accuracy of OAE and BERA to Detect the Incidence of Hearing Loss in Newborn Dr Jaideep Bhatt,
P IHS P P P 2 International Journal of Scientific and Research Publications, Volume 5, Issue 6, June 2015 1 Accuracy of OAE and BERA to Detect the Incidence of Hearing Loss in Newborn Dr Jaideep Bhatt,
Section 4. Hearing loss and hearing tests
 Section 4 Hearing loss and hearing tests How we hear Outer Ear Middle Ear Inner Ear 4. 7. 8. 1. 3. 6. 2. 5. 1. 2. 3. 4. 5. 6. 7. 8. Ear canal Ear drum Middle ear cavity Middle ear bones the malleus, incus,
Section 4 Hearing loss and hearing tests How we hear Outer Ear Middle Ear Inner Ear 4. 7. 8. 1. 3. 6. 2. 5. 1. 2. 3. 4. 5. 6. 7. 8. Ear canal Ear drum Middle ear cavity Middle ear bones the malleus, incus,
RETRIEVING SEQUENCE INFORMATION. Nucleotide sequence databases. Database search. Sequence alignment and comparison
 RETRIEVING SEQUENCE INFORMATION Nucleotide sequence databases Database search Sequence alignment and comparison Biological sequence databases Originally just a storage place for sequences. Currently the
RETRIEVING SEQUENCE INFORMATION Nucleotide sequence databases Database search Sequence alignment and comparison Biological sequence databases Originally just a storage place for sequences. Currently the
Genetics Review for USMLE (Part 2)
 Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Single Gene Disorders Genetics Review for USMLE (Part 2) Some Definitions Alleles variants of a given DNA sequence at a particular location (locus) in the genome. Often used more narrowly to describe alternative
Chromosomes, Mapping, and the Meiosis Inheritance Connection
 Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Original Article. Abstract. Introduction: Nazia M 1, Sadaf N 2, Saima T 3
 Original Article Trends For Investigations Carried Out For Detection Of Severe To Profound Hearing Impairment For Children In Pakistan Nazia M 1, Sadaf N 2, Saima T 3 Abstract Background: Hearing impairment
Original Article Trends For Investigations Carried Out For Detection Of Severe To Profound Hearing Impairment For Children In Pakistan Nazia M 1, Sadaf N 2, Saima T 3 Abstract Background: Hearing impairment
New Estimates of the Economic Benefits of Newborn Screening for Congenital Hypothyroidism in the US
 The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
Sickle cell anemia: Altered beta chain Single AA change (#6 Glu to Val) Consequence: Protein polymerizes Change in RBC shape ---> phenotypes
 Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Algorithms in Computational Biology (236522) spring 2007 Lecture #1
 Algorithms in Computational Biology (236522) spring 2007 Lecture #1 Lecturer: Shlomo Moran, Taub 639, tel 4363 Office hours: Tuesday 11:00-12:00/by appointment TA: Ilan Gronau, Taub 700, tel 4894 Office
Algorithms in Computational Biology (236522) spring 2007 Lecture #1 Lecturer: Shlomo Moran, Taub 639, tel 4363 Office hours: Tuesday 11:00-12:00/by appointment TA: Ilan Gronau, Taub 700, tel 4894 Office
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
Information leaflet. Centrum voor Medische Genetica. Version 1/20150504 Design by Ben Caljon, UZ Brussel. Universitair Ziekenhuis Brussel
 Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Information on genome-wide genetic testing Array Comparative Genomic Hybridization (array CGH) Single Nucleotide Polymorphism array (SNP array) Massive Parallel Sequencing (MPS) Version 120150504 Design
Acute Profound Deafness
 Sensory Organ Disorders Acute Profound Deafness How much do we now know about the clinical condition? JMAJ 46(7): 285 290, 2003 Jin KANZAKI Director, International University of Health and Welfare Atami
Sensory Organ Disorders Acute Profound Deafness How much do we now know about the clinical condition? JMAJ 46(7): 285 290, 2003 Jin KANZAKI Director, International University of Health and Welfare Atami
Delivering the power of the world s most successful genomics platform
 Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
