International Influenza Surveillance Assessment Tool. National Center for Immunization & Respiratory Diseases Influenza Division
|
|
|
- Reynard Lane
- 8 years ago
- Views:
Transcription
1 International Influenza Surveillance Assessment Tool National Center for Immunization & Respiratory Diseases Influenza Division
2 Introduction: Surveillance Assessment & Review Tool Purpose of Tool The goal of this surveillance review tool is to assist in the systematic, standardized review of influenza sentinel site surveillance systems and to provide a guide for identifying problems and designing solutions to provide support. The specific objectives of this tool include: 1. To provide a guide to CDC epidemiologists and project officers, as well as to Ministry of Health or other national counterparts, for conducting site visits and assessing the functionality, standardization, thoroughness and sensitivity of the national surveillance system(s). 2. To obtain a clear understanding of the structure of the surveillance system as developed, while identifying both strengths and opportunities for improvement. 3. To provide quality technical assistance, feedback, and recommendations for changes in order to achieve system goals. 4. To provide basic recommendations on conducting surveillance data quality assurance and monitoring, and establishing solid laboratory and epidemiologic data integration. Application & Administration This tool is composed of 6 checklists, and a standardized report format. These sections include a broad overview of all influenza-related surveillance systems (e.g. national early notification systems, animal health surveillance, etc), a brief national/central laboratory review, central level ILI and SARI questionnaires, and ILI and SARI sentinel site review guides. All questions in the tool should be answered in the course of a visit, however strict adherence to the format is not necessary. This tool was designed for global use, and not all questions will be applicable in all situations. The tool can be used to evaluate all levels of a surveillance system, from an evaluation of the national surveillance administration and oversight, to an evaluation of site-level functionality. Background Depending on location, capacity, and available resources, both goals of surveillance, and types of surveillance systems in use might vary. Some basic goals and types of surveillance are outlined briefly below. Similarly, depending on resources and capacity, different partners might employ different logistical mechanisms for recording and reporting of data, storage and transport of specimens, and not all solutions will be applicable in all situations. It is important to assess feasibility and consider differing capacities while developing recommendations for improvement. Goals of surveillance/uses of surveillance data: - Monitor trends in influenza activity, describe seasonality, and basic epidemiology of influenza (eg timing, geography, population, type, etc - Detect unusual events, novel viruses - Describe burden of disease - Describe severity of disease - Identify risk factors for severe disease Page 2 Introduction
3 Types of surveillance: ILI sentinel site surveillance meets the following criteria: 1) adherence to a clearly defined case definition, 2) use of a sampling strategy for the collection of viral specimens, 3) reporting of aggregate weekly numbers for patients meeting the case definition and for total clinic visits. Non-sentinel ILI surveillance may be conducted in either a systematic or a non-systematic manner, and may or may not adhere to a standard case definition. SARI sentinel site surveillance meets the following criteria: 1) adherence to a case definition for SARI, 2) collection of viral specimens from all* patients meeting case definition, 3) completion of standard clinical and epidemiologic data forms that can be linked to specimens, and 4) reporting of aggregate weekly totals for SARI patients and for total admissions. * In some locations it may not be possible to sample all SARI admissions. Event-based/outbreak/enhanced surveillance may include supplemental reporting of pandemic H1N1 or increased testing of ILI or SARI cases based on regional outbreak reports, etc. Population-based surveillance Medical records surveillance eg based on discharge or diagnostic codes Mortality surveillance, based on standard codes for causes of mortality Summary Report A standard report format is included with this tool, with a focus on summarizing the system design, system strengths, identification of opportunities for improvement and provision of key recommendations. Making Recommendations Causes of difficulties in the functionality of all surveillance systems will differ. A universal recommendation to be emphasized for all sentinel surveillance systems, especially those in their infancy, is the importance of establishing fewer, highly functional sites prior to the widespread establishment of sites. The clinical, laboratory, data analysis, and reporting capacities reviewed in the administration of the tool should be reviewed when making recommendations, in order to provide the most useful, realistic, and feasible advice and recommendations possible. Useful recommendations might take into consideration total lab testing capacity combined with considerations of realistic storage and transport of specimens from sentinel sites, while also considering the data management and analysis capacities at the central level in order to identify realistic goals for specimen collection and testing on a regular (weekly) basis. Feasible standard goals for specimen and data collection might help in regularizing frequency of data analysis and reporting at the national level. Follow Up Initial surveillance assessments will ideally be followed up annually at the minimum at the national level. Site-level assessments will ideally be conducted on a monthly or quarterly basis by national surveillance staff, in order to ensure that recommendations are being implemented and the quality of data is being monitored. Page 3 Introduction
4 INFLUENZA SURVEILLANCE REVIEW -- NATIONAL OVERVIEW Country Name of Interviewer Date of Site Visit General Information 1 What kind of influenza/respiratory disease surveillance systems are currently operating, for how long, and who is responsible for their operation and oversight? Type of System /DK ILI outpatient sentinel site surveillance Non-sentinel ILI outpatient surveillance SARI/Pneumonia hospital-based sentinel site surveillance Event-based/outbreak surveillance Other (please describe ) 2 Do all of the systems above share their specimens with the national influenza laboratory? () 3 Do all of the systems above share clinical and/or epidemiologic data with national surveillance staff? () 4 Are specimens, testing results, and data from all of the above systems kept track of separately? () (e.g. Are ILI and SARI tracked/able to be analyzed separately) National Data Aggregation & Analysis 5 Are data from different surveillance systems maintained in different databases or otherwise distinguishable from one another? () 6 How frequently are site-level data compiled and analyzed at the national level (eg weekly/monthly, etc)? 6a Who analyzes this data? Length of operation (Y/M) & Ministry/group responsible Page 4 System Overview
5 National Reporting 7 Is a report describing national influenza activity produced at the central office using data received from participating sites? (/DK) 7a If yes, with whom is this report shared? 7b How is this report published? 7c Format Website newsletter listserv Paper reports by post Which of the following analyses/charts are included in this report? / DK Chart/Analysis ILI consultations/total consultations Flu positive ILI specimens/total tested ILI specimens SARI admissions/total admissions Flu positive SARI specimens/total tested SARI specimens Positive flu specimens by type & sub-type Other? Please specify 7d How frequently is this report prepared (eg weekly/monthly, etc)? (If a report is available, please request a copy) National Data Use 8 Does national surveillance staff have a set of indicators used to identify abnormal influenza activity based on data submitted by participating sites? (Y/K) 8a If yes, please describe 8b What is the notification mechanism to senior leadership if abnormal activity is noted? e.g. phone, , fax, other 9 Please list other uses of influenza surveillance data: Page 5 System Overview
6 INFLUENZA SURVEILLANCE REVIEW -- NATIONAL SEVERE ILI/SARI OVERVIEW General Information /DK 1 How many sentinel SARI sites have been established, and in what sorts of facilities? Please list sites by location: # of Type of facility sites Pediatric hospital General hospital Fever hospital Chest clinic Other (please describe ) 1a How were these sites selected? (e.g. what selection criteria were used? ) 1b Do these sites provide a nationally representative sampling of the following: Please describe Age Sex Ethnicity Socio-economic status Risk factors/chronic disease Geography 1c Is participation in the sentinel surveillance program voluntary for each site? () 1d Are any incentives provided to the facility from the national level for undertaking surveillance activities? () 1e If yes, what are those incentives? 2 Does each site have surveillance focal points/staff assigned to oversee surveillance activites? () 2a Who are the staff overseeing surveillance/what are their qualifications? 2b Please describe the duties and responsibilities of site surveillance staff: 2c Are any incentives given to staff to undertake surveillance activities? (eg payment, continuing education credits, etc) () 2d If yes, what are those incentives? Page 6 National -- SARI
7 3 Has a national protocol for SARI (or influenza) surveillance or a set of standard operating procedures (SOPs) been developed? () 3a If yes, who developed this protocol? 3b Does the protocol include clearly defined objectives for the surveillance system? () 3c If yes, what are those objectives? 3d Has a copy of the protocol been distributed to each sentinel surveillance site? () 3e Has the protocol, it's objectives, and the SOPs contained in it been presented to all participating surveillance staff at all sites?/have staff been trained in implementation of the protocol? () 4 How frequently are site-level staff trained in each of the following (eg one time, annually, bi-annually, etc): Training Freque ncy Application of standard case definition & identification of cases Case sampling & enrollment procedures (eg random sampling, etc) Specimen collection, storage, and shipment Completion of specimen collection and clinical/.epidemiologic data forms Recording & reporting of aggregate weekly hospital admissions, SARI admissions, patient enrollment, etc. Standards for SARI Case Detection 5 What is the case definition in use for SARI? 5a Are any exclusion criteria in use, and if yes, what are they? 5b Does the case definition specify a time period of symptom onset? () Page 7 National -- SARI
8 5c If yes, what is that time period? 5d Please describe diagnoses typically used for SARI cases: 6 Does the protocol include a standard method for screening of SARI cases? () 6a If yes, please describe: 6b Would this method mean that only patients who present with SARI upon admission be detected? () 7 Does the protocol indicate if ALL SARI cases should have a specimen collected? () 7a If not all cases are enrolled and sampled, does the protocol include a standard sampling scheme? () 7b If yes, please describe the sampling scheme: 7c 7d Is the sampling scheme random? () If no, please describe how this sampling scheme might bias the data collected: Standards for Epidemiologic Data Collection 8 Does the protocol include a standard SARI case report form to be used at every site? () (if available, please obtain a copy of that form) 8a If yes, are those forms regularly distributed to the sentinel sites for use? () 9 Does the protocol specify that sites should keep a log/record of all SARI cases detected? () 9a Does the protocol specify that sites should keep a log/record of all hospital admissions? () 10 Does the protocol include a standard aggregate SARI 10a reporting form? () If yes, are those forms distributed to the sentinel sites for use? () 11 Does the standard method of recording SARI data include outcome? () Standards for Respiratory Specimen Collection, Storage, and Transport 12 Does the protocol include a standard laboratory specimen collection form to be used at all surveillance sites? () (if available, please obtain a copy of that form ) Page 8 National -- SARI
9 13 Does that protocol include standard operating procedures (SOPs) for the following: Task Specimen collection Specimen packaging Specimen storage Specimen transport 13a If yes, how frequently are site level staff trained in these methods? (eg monthly, quarterly, etc) 14 Please describe the specimen collection, storage, and transport SOPs outlined in the national protocol: 15 How often are sites required to send specimens to the national laboratory for testing/verification? (e.g. weekly, bi-weekly, etc) 16 Are sites required to keep a log of total specimens collected? () 17 How frequently are each of the following replaced (eg weekly, monthly, etc), and by whom are they provided? Item Swabs Tongue depressors Gloves Respiratory protection Plastic vials containing viral transport media Specimen collection forms Shipping containers Labels Refrigerants Standards for Data Management, Analysis, and Quality 18 Does the national protocol include standard methods for linking laboratory specimens to data forms? () 19 How are laboratory results merged with case-based data at the national level? (please describe; eg unique ID, last name, etc ) 20 Who is repsonsbile for SARI data management at the national level? Freque ncy Provided by: Page 9 National -- SARI
10 21 How often are SARI data updated at the national level? (eg weekly, monthly, etc) 22 Please indicate in the boxes below how frequently (eg weekly, bi-weekly, monthly, etc) the following are summarized at the national level, and which strata, if any, are used? Item All SARI admissions All sampled/tested SARI admissions All hospital admissions All SARI deaths All hospital deaths Flu positive SARI cases Flu positive SARI cases by risk factor Flu positive SARI cases by symptom Flu positive SARI cases by outcome 22a If age groups are used, please describe the groupings used: 23 Are national SARI trends routinely observed and interpreted? () 23a If yes, with what frequency are trends analyzed/observed? 24 Is there a method for identifying aberrations in SARI data at the: Level Site National 24a If yes, please describe that method: National Data Reporting 25 Is a national report on influenza activity prepared by the national office? () 25a If yes, with what frequency is that report prepared? (eg weekly, monthly, quarterly, intermittently etc) Nation al Site level aggregates Age groups () Page 10 National -- SARI
11 25b With whom is that report shared? Agency/Group Sentinel Sites Ministry of Health leadership WHO Geneva WHO Regional Office WHO Country Office CDC Animal health authorities Other (please list) National Monitoring & Evaluation of SARI Sentinel Sites 26 How frequently do national surveillance staff visit each sentinel site for evaluation, quality control, or assessments? 26a What sorts of activities are performed on these visits? (please describe) 26b Are hospital admission logbooks verified on site visits to verify that all SARI cases are being identified and documented? () 26c Are assessments of the percent of eligible patients that are enrolled done? 26d Are feedback and recommendations from these visits documented? () 26e Are those documents shared with sites? () 27 Does national surveillance staff monitor the quality and completeness of epidemiologic data received from each of the sites? () 27a How is that quality monitored? 27b How frequently are the quality/completeness monitored? 27c How frequently are those quality findings/comments reported back to sites? 27d Is an indicator checklist used to monitor quality and completeness? () (If so, please obtain a copy ) 27e Are feedback and recommendations from these findings given to sites individually? 27f If so, how frequently are such feedback and recommendations provided? Page 11 National -- SARI
12 28 Do national surveillance staff follow up with sites when timely submissions of aggregate data are not received? () 28a What proportion of SARI sites submit their data by the due date on a weekly basis? 29 Do national laboratory staff follow up with sites when specimens are not received on a timely basis? () 29a What proportion of SARI sites submit their respiratory specimens by the due date on a weekly basis? 30 How often is refresher training provided to surveillance staff in: Item SARI case detection Epidemiologic data collection Laboratory specimen packaging, storage, and transportation Freque ncy Training in past year? () Page 12 National -- SARI
13 INFLUENZA SURVEILLANCE REVIEW -- NATIONAL ILI OVERVIEW General Information 1 How many sentinel ILI sites have been established, and in what sorts of facilities? Please list sites by location: 1a 1b Type of facility Pediatric hospital OPD General hospital OPD Outpatient clinic Pediatric outpatient clinic Other (please describe ) How were these sites selected? (eg what selection criteria were used?) Do these sites provide a nationally representative sampling of: Age Sex Ethnicity/minority group Socio-economic status Risk factors/chronic disease Geography 1c Are any incentives provided for undertaking surveillance activities at the site level? () 1d If yes, what are those incentives? 2 Does each site have surveillance focal points/staff to oversee surveillance activites? () 2a If yes, are those staff paid? () 2b If yes, how are they paid? 2c Who are the staff overseeing national surveillance activities/what are their qualifications? 2d Please describe the duties and responsibilities of site surveillance staff: # of sites Please describe Page 13 National -- ILI
14 3 Has a national protocol for ILI surveillance or a set of standard operating procedures (SOPs) been developed? () 3a If yes, who developed this protocol? 3b Does the protocol include clearly defined objectives for the surveillance system? () 3c If yes, what are those objectives? 3d Has a copy of the protocol been distributed to each site? () 3e Has the protocol, it's objectives, and the SOPs contained in it been presented to all participating surveillance staff at all sites/have staff been trained in implementation of that protocol? () 4 How frequently are site-level staff trained in each of the following (eg one time, annually, bi-annually, etc): Training Application of standard case definition & identification of cases Case sampling & enrollment procedures (eg random sampling) Specimen collection, storage, and shipment Completion of specimen collection and clinical/epidemiologic data forms Recording & reporting of aggregate weekly clinic visit, ILI visits, enrollment, etc. Standards for ILI Case Detection 5 What is the case definition in use for ILI? 5a Are any exclusion criteria in use, and if yes, what are they? () 5b Does the case definition specify a period of symptom onset? () 5c If yes, what is that time period? Frequency Page 14 National -- ILI
15 6 Does the protocol define a sampling method for the enrollment of ILI cases and collection of specimens? () 6a If yes, what is that sampling method? 6b 6c Is this sampling method random? () If no, please describe how this sampling scheme might bias the data collected: Standards for Epidemiologic Data Collection 7 Does the protocol include a standard ILI case report form to be used at every site? () (if available, please obtain a copy of that form) 7a If yes, are those forms regularly distributed to the sentinel sites for use? () 8 Does the protocol specify that sites should keep a log/record of all ILI cases detected? () 8a Does the protocol specify that sites should keep a record of total ILI specimens collected? () 8b Does the protocol specify that sites should keep a log/record of all clinic visits? () 9 Does the protocol include a standard aggregate ILI reporting form? () 9a If yes, are those forms distributed to the sentinel sites for use? () Standards for Respiratory Specimen Collection, Storage, and Transport 10 Does the protocol include a standardized swab collection form to be used at all sentinel surveillance sites (if available, please obtain a copy of that form)? 11 Does that protocol include standard operating procedures (SOPs) for the following: Task Specimen collection Specimen packaging Specimen storage Specimen transport 11a If yes, how frequently are staff trained in these methods (eg monthly, quarterly, etc) Page 15 National -- ILI
16 12 Please describe the specimen collection, storage, and transport SOPs outlined in the national protocol: 13 How often are sites required to send specimens to the national laboratory for testing? (e.g. weekly, bi-weekly, etc) 14 How many specimens are sites meant to draw per day/week? 15 How frequently are each of the following replaced (eg weekly, monthly, etc), and by whom are they provided? Item Swabs Tongue depressors Gloves Respiratory protection Plastic vials containing viral transport media Specimen collection forms Shipping containers Labels Refrigerants Standards for Data Management, Analysis, and Quality 16 Does the protocol include standard methods for linking laboratory specimens to data forms? () 17 Does the national protocol include standard methods for linking laboratory specimens to data forms? () 18 Are laboratory results merged with case-based data at the national level? () 18a How are laboratory results merged with case-based data at the national level? (please describe; eg unique ID, last name, etc ) 19 Who is repsonsbile for ILI data management at the national level? Frequ ency Provided by: Page 16 National -- ILI
17 20 How often are ILI data updated at the national level? (eg weekly, monthly, etc) 21 Please indicate in the boxes below how frequently (eg weekly, bi-weekly, monthly, etc) the following are summarized at the national level, and which strata, if any, are used? Item All ILI consultations All sampled/tested ILI consultations All clinic consultations Flu +ve ILI specimens 21a If age groups are used, please describe the groupings used: 22 Are national ILII trends routinely observed and interpreted? () 22a If yes, with what frequency are trends analyzed/observed? 23 Is there a method for identifying aberrations in ILI data at the: Level Site National 23a If yes, please describe that method: National Data Reporting 24 Is a national report on influenza activity prepared by the national office? () 24a If yes, with what frequency is that report prepared? (eg weekly, monthly, quarterly, intermittently etc) Natio nal Site level aggregates Age groups () Page 17 National -- ILI
18 24b With whom is that report shared? Agency/Group Sentinel Sites Ministry of Health leadership WHO Geneva WHO Regional Office WHO Country Office US CDC Animal health authorities Others (please list) National Monitoring & Evaluation of ILI Sentinel Sites 25 How frequently do national surveillance staff visit each sentinel site for evaluation, quality control, or assessments? 25a What sorts of activities are performed on these visits? (please describe) 25b Are clinic logbooks verified on site visits to verify that all ILI cases are being identified and documented? () 25c Are feedback and recommendations from these visits documented? () 25d Are those documents shared with sites? () 26 Does national surveillance staff monitor the quality and completeness of epidemiologic data received from each of the sites? () 26a How is that quality monitored? 26b How frequently are the quality/completeness monitored? 26c How frequently are those quality findings/comments reported back to sites? 26d Is an indicator checklist used to monitor quality and completeness? () (If so, please obtain a copy ) 26e Are feedback and recommendations from these findings given to sites individually? 26f If so, how frequently are such feedback and recommendations provided? Page 18 National -- ILI
19 27 Do national surveillance staff follow up with sites when timely submissions of aggregate data are not received? () 27a If yes, what is the lag period/when do national staff follow-up? 27b What proportion of ILII sites submit their complete data by the due date on a weekly basis? 28 Do national laboratory staff follow up with sites when specimens are not received on a timely basis? () 28a What proportion of ILII sites submit their respiratory specimens by the due date on a weekly basis? 29 How often is refresher training provided to surveillance staff in: Item ILI case detection Epidemiologic data collection Laboratory specimen packaging, storage, and transportation Frequ ency Training in past year? () Page 19 National -- ILI
20 INFLUENZA SURVEILLANCE REVIEW -- NATIONAL LABORATORY OVERVIEW National Laboratory Name: Date of Interview: Interviewer: Laboratory Staff Interviewed: General Information 1 Does the national laboratory receive all specimens from all ILI/SARI sentinel sites? () 1a If not, how is selection done? 2 Does the national laboratory receive specimens from all other human influenza surveillance systems? () 2a If yes, please explain 3 How frequently does the national laboratory process specimens from participating surveillance sites? (eg daily/weekly/bimonthly, etc) Specimen Processing & Testing 4 What testing platform(s) is/are used in the testing of ILI Platform RT-PCR rt RT-PCR IFA Viral culture Rapid test Other 4a If mulitiple testing platforms are used, is a testing algorithm in use for priority testing by different platforms? () 4b If yes, what is that algorithm? 5 What type of data are recorded for each specimen: Type Subtype Strain Page 20 National - Lab
21 5a If all of the above are not recorded, please indicate the proportion of specimens that are assigned a strain 6 Does this laboratory test animal specimens? () 7 Does the national laboratory routinely test influenza specimens for any other respiratory disease? () 7a If yes, please list pathogen(s) and test(s) 8 What is the weekly maximum sample testing capacity at the national laboratory? 9 How are specimens stored at the national laboratory? 10 What are the typical lag times between receipt of specimens at laboratory and the testing and reporting of results? Data Management & Tracking & Analysis 11 Are specimens received from sites marked with a unique ID that enables a linkage to individual cases and to clinical/epidemiologic data? () 11a If yes, do those IDs differentiate between sentinel ILI or SARI and specimens from other systems (eg is it possible to record test results from different systems separately?) () 12 Are all specimens received accompanied by a corresponding data collection form? () 13 Are laboratory testing results recorded in a national influenza database/table? () 13a Who enters data and manages that database? 13b How frequently is that database updated with testing results? 14 Are ILI and SARI cases identified separately in that database? () 15 Is the total number of specimens received for testing recorded? () 15a Is the total number of specimens tested recorded? 15b Is the total number of positive specimens recorded? () 16 How frequently is this data analyzed to observe patterns in influenza activity? 16a Who does this analysis? Page 21 National - Lab
22 Reporting 17 How frequently are basic testing results shared? 17a With whom is this information shared? 18 How frequently are the analyzed results of testing outcomes shared? 18a With whom are these results shared? 19 How many isolates have been sent from the national laboratory to WHO CC in the past year? In the past three years? 19a With what frequency are samples shared with a WHO CC? 19b Does a protocol exist for sharing specimens with a WHO CC? () If a protocol exists, please ask to see/have a copy 20 Does the national laboratory report results to the WHO regional office? () 20a If yes, how frequently is this information shared? Specimen Quality & Site Monitoring 21 Do national laboratory staff follow up with sentinel sites when specimens are not received by the scheduled date? 21a What proportion of sentinel ILI and SARI sites send their specimens consistenly at the interval specified by the central office? 22 Does the national lab routinely monitor the quality of specimens submitted by sentinel sites? 22a If yes, how is quality monitored? 22b How frequently is quality reported to the sites? 22c Is this feedback documented? 22d Is there a system to track actions taken as a result of feedback/actions to be taken? 22f If yes, please describe that system. Page 22 National - Lab
23 INFLUENZA SURVEILLANCE REVIEW -- DATA MANAGEMENT & ANALYSIS Data Management Please spend some time looking at the surveillance data, how it is stored, recorded, and analyzed. 1 What software is used to store surveillance data? 2 Are ILI and SARI data housed in the same file/database? () 2a Please describe the database structure; how are ILI and SARI data differentiated; can data be analyzed separately, etc. 3 Are laboratory results and epidemiologic data kept in a common database? () 3a If yes, are laboratory results and corresponding epidemiologic data on the same case kept in the same table? () 3b If yes, are laboratory results and corresponding epidemiologic data on the same case kept in the same record in that table? () 3c If no, please describe how laboratory results and clinical/epidemiologic data are linked. 4 Is every laboratory result linked to a case record? () Core Data 5 Are total outpatient visits recorded? () 6 Are total outpatients meeting ILI case defintion recorded? () 6a If no, how are patients recorded? (eg all patients meeting particular ICD-9/10 codes; if codes are used, please specify which codes are counted and summarized for an estimate of ILI visits) 7 Is the total number of ILI cases selected for sampling recorded? () 8 Are total all-cause* hospital admissions recorded? () *all-cause with whichever exceptions have been noted elsewhere Page 23 Data
24 9 Are total SARI admissions recorded? () 9a If no, how are SARI patients recorded? (eg all patients admitted with pneumonia? All patients meeting ICD- 10/ICD-9 codes? Please explain.) 10 Are total SARI cases selected for sampling recorded? () 11 Are total hospital deaths recorded? () 12 Are total SARI deaths recorded? () 12a If no, how are SARI deaths estimated? Please describe 13 Are all SARI cases admitted to ICU recorded? () 13a If no, how are SARI ICU admissions recorded? (eg all pneumonia patients admitted to ICU? Please explain) 14 Is outcome recorded for all SARI cases? () 14a Are patients followed up after discharge or hospital transfer? 15 Is the current year's influenza vaccination history recorded for all sampled ILI cases? () 15a Is the vaccine received (manufacturer, etc) recorded? () 16 Is the current year's influenza vaccination history recorded for all sampled SARI cases? () 16a Is the vaccine received (manufacturer, etc) recorded? () 17 Are total ILI specimens tested recorded? () 18 Are total SARI specimens tested recorded? () 19 Are total influenza positive SARI cases recorded? () 19a For what proportion of influenza positive SARI cases is influenza virus type recorded? 19b For what proportion of A-positive SARI cases is subtype recorded? 19c For what proportion of influenza-positive SARI cases is strain recorded? Page 24 Data
25 20 Are total influenza positive ILI cases recorded? () 20a For what proportion of influenza positive ILI cases is influenza virus type recorded? 20b For what proportion of A-positive ILI cases is subtype recorded? 20c For what proportion of ILI cases is strain recorded? 20d Is a routine tracking system in place to record differential diagnoses? () Basic Analyses Please record whether the data, as recorded and stored, are sufficient to allow for the following analyses. Please have the surveillance staff responsible for data analysis perform these analyses at some point during the site visit. Please also perform some of these analyses on your own and attach both to final surveillance assessment. 21 Total hospital admissions, by week () 21a Total SARI admissions, by week () 21b Proportion of all hospital admissions that met SARI case definition, by week () 21c Number and proportion of all sampled SARI patients that have laboratory confirmed influenza infection by week () 21d Number and proportion of all sampled SARI patients that have laboratory confirmed influenza infection by week, by type/subtype () 21e Number and proportion of all sampled SARI patients that have laboratory confirmed influenza infection by week, by age group () 21f Number and proportion of all sampled SARI patients that have laboratory confirmed influenza infection by week, by geographic region () 22 Total outpatients meeting ILI case definition, by week () 22a Proportion of all outpatient consultations that met ILI case definition, by week () 22b Number and proportion of all sampled ILI patients that have laboratory confirmed influenza infection by week () 22c Number and proportion of all sampled ILI patients that have laboratory confirmed influenza infection by week, by type/subtype () Page 25 Data
26 22d Number and proportion of all sampled ILI patients that have laboratory confirmed influenza infection by week, by age group () 22e Number and proportion of all sampled ILI patients that have laboratory confirmed influenza infection by week, by geographic region () 23 Incidence rate for SARI (in systems where population served/represented is known) () 24 Incidence rate for ILI (in systems where population served/represented is known) () 25 Total influenza positive SARI cases by risk factor () 26 Total influenza positive SARI cases by symptom () 27 Total influenza positive SARI cases by outcome () Completeness of Data Please spend some time analyzing the completeness of surveillance data. It might be helpful to explore completeness by site or by region to compare participation in activities by location. If ILI and SARI data are kept in separate databases and/or separate tables, please complete these questions for each. 28 For what proportion of records/cases is the age field complete? 29 For what proportion of cases is the date of onset field complete? 30 For what proportion of cases is the date of interview field complete? 31 For what proportion of cases is the date of specimen collection field complete? 32 To what proportion of cases is a unique identifier assigned? 33 For what proportion of cases is a temperature recorded? 34 For what proportion of cases is the cough field completed? 35 For what proportion of cases is the sore throat field complete? 36 For what proportion of SARI cases are the underlying conditions recorded/completed? 37 For what proportion of SARI cases are the treatment questions completed? 38 For what proportion of SARI cases are the outcome questions completed? Page 26 Data
27 Completeness & Timeliness of Reporting Please review available reporting documents in order to observe the completeness of reporting 39 When did reporting of surveillance data begin? 40 For how many of the weeks/months (if reporting only on a monthly basis) since reporting began has a report been produced? 41 Upon reviewing the reports, how many of the surveillance sites have submitted up-to-date surveillance data to be included in the weekly report? Page 27 Data
28 INFLUENZA SURVEILLANCE REVIEW -- SARI SITE VISIT Date of Interview Surveillance Staff General Information 1 What is the name of this facility? 2 Where is this facility located? 2a Country: 2b Province: 2c 2d City/Town/Village: How many beds are in this hospital? Non-ICU beds ICU beds 2e For how long has this site been collecting SARI data (years/months)? 3 Who is responsible for coordinating surveillance at this site? 3a What is this person's position? 3b How many surveillance staff are present at this site? 3c Have these staff received training in surveillance data and specimen collection from the national level? () 3d Are these staff given a motivation/incentive to participate? () 3e If yes, what is it? 3f Please describe the duties assigned to designated surveillance staff: 4 Is this a public or a private facility? 4a What type of facility is it? Facility type General hospital Pediatric hospital Infectious disease hospital Specialty/referral hospital Other (please describe) Page 28 SARI Site Visit
29 4b What level of care is provided at this hospital? Level of care Primary care (local, non-referral) Secondary (first level of referral) Tertiary (highest level of referral) 4c Which wards participate in SARI surveillance? (e.g. all wards, selected wards; please describe ) 4d Does this site provide a representative sampling of the following? Age Sex Ethnicity Socio-economic status Risk factors/chronic disease Geography 4e How might the answers to questions 4a through 4d bias the surveillance data collected? SARI Case Detection 5 Please describe the procedure used to screen for SARI cases: 6 What is the case definition in use for SARI at this site? 6a Does the case definition include a period of time for symptom onset? () If yes, what is that time period? 6b Is the SARI case definition known and understood by all staff that screen patients for cases? () (If possible, please ask staff about the SARI case definition and their understanding of it) 6c Is the SARI case defintion posted and visible to all staff? () 7 Would patients who develop SARI after hospital admission be detected at this hospital, or would only those patients admitted with SARI be detected/recorded? () Please explain. Page 29 SARI Site Visit
30 8 Does SARI screening happen everyday, at all hours of the day? () 8a If no, when are patients screened? Day Time of Day (Morning, Afternoon, Evening, Night) Monday Tuesday Wednesday Thursday Friday Saturday Sunday 9 How might the screening procedures described above bias or impact the selection of SARI cases? Epidemiologic Data Collection 10 Does the site have standard individual SARI case report forms printed, accessible, and in use? (If possible, please obtain a copy of this form ) () 10a Is this form a standard from provided by the national office, and used at all sentinel sites? () 11 Please indicate which of the following items are included in this form: Item Date of interview Date of admission Date of symptom onset Date of specimen collection Diagnoses at time of admission Age or date of birth (years, months if under 1 year) Sex Patient Unique Identifier Page 30 SARI Site Visit
31 12 Please indicate which of the following signs and symptoms are included in this form: Sign/Symptom Fever >38 measured Temperature recorded Cough Sore throat Shortness of breath or difficulty breathing Requires hospitalization Requires ICU admission Clinical signs of pneumonia Others (please indicate ) 13 Please indicate which of the following underlying/preexisting condiions are included in this form: Condition Heart disease Diabetes Chronic lung disease Asthma Liver disease Immuno-compromised/HIV+/AIDS Neurologic or neuromuscular dysfunction Others (please indicate ) 14 Please indicate which of the following other conditions are included in this form: Condition Pregnancy Obesity reported (BMI>30) Morbid obesity reported (BMI>40) Undernourished Page 31 SARI Site Visit
32 15 Please indicate which of the following items related to treatment are included in this form: Treatment Antiviral use in the previous 14 days (please list those used & date started ) Influenza vaccination for the current season Patient chest x-ray taken 16 Please indicate which of the following items related to outcome are included on this form: Outcome Discharged Died Unknown Transfer to other health care setting Other (please list ) 17 If a SARI logbook/line list is kept, please ask to review a random selection of records from those patients 17a Number of charts reviewed: 17b Number of charts meeting SARI case definition: 17c Number identified as SARI in the logbook/line list: 17d Does a review of charts indicate that all or most cases meeting the SARI case definition are being enrolled? () 17e If yes, what percentage are being enrolled? 17f Please list some of the common admitting diagnoses for SARI cases: 18 Does the site have standard aggregate SARI forms printed, accessible, and in use? () 19 Is a record of total SARI cases maintained, regardless of enrollment/sample collection? ( 19a Is this record maintained by age, or age group? () 19b Who is responsible for maintaining these records? 19c Are records/logbooks of total hospital admissions kept? () Page 32 SARI Site Visit
33 Respiratory Specimen Collection, Packaging, Storage, and Shipment 20 Is a specimen collected from every identified SARI patient? () 20a If all cases are not enrolled/sampled, what is the sampling scheme used/how are cases selected for specimen collection and enrollment? 20b Is this method random? () 20c How might this method be biased? 20d Is there a limit to the number of SARI cases that can be sampled on a weekly basis? () If yes, what is that number? 21 How are laboratory specimens collected in this hospital (please describe ): 21a 21b Which staff are responsible for specimen collection? How frequently are these staff trained in specimen collection and storage methods? 22 How are laboratory specimens packaged in this hospital? (please describe ): 23 How are laboratory specimens transported to the confirmatory laboratory? (please describe ): 24 How frequently are specimens transported to the confirmatory laboratory (e.g. daily/weekly/monthly ): 25 Does the site have standard specimen collection forms printed, available, and in use? () 26 Does the site have standard operating procedures for specimen collection, transportation, and storage written, accessible, and in use? () 26a Is this a standard form provided by the national surveillance office/coordinator? () Page 33 SARI Site Visit
34 27 Please indicate which of the following items are included on the specimen collection form: Item Unique identifier Hospital name Person collecting specimen Age or date of birth Sex Date of symptom onset Date of specimen collection Type of specimen collected: Nasal swab Throat swab Nasopharyngeal swab Nasopharyngeal aspirates or washes Nasal wash Other (please describe ) 27a Is there any protocol in place for multiple collections? (eg Day 1, Day 3, or acute & convalescent sera, etc) () 28 Please indicate which PPE are rountinely used during the collection of specimens: PPE Gloves Gown/Lab coat Safety glasses Respiratory protection type Mask Respirator 28a Is handwashing required before & after specimen collection? Page 34 SARI Site Visit
35 29 Are specimen collection materials readily available? () 29a If yes, for how many specimens are materials routinely available? 29b Please indicate which collection materials are available: Material Tongue depressors Swabs Vials containing VTM at 4 C Alcohol/bleach Packaging materials for transport 30 Is there a lab capable of testing for influenza on-site? () 30a If yes, please indicate which tests are peformed on-site: Test Rapid-test Immunofluorescence assay PCR (typing) PCR (typing & subtyping) Culture Hemmaglutinin inhibition Other (please describe) 30b If specimens are tested in hospital, are total numbers of positive and negative specimens reported to surveillance coordinator on-site? () 30c If yes, how often are they reported? (e.g. daily/weekly/monthly etc) 30d If tested on-site, how often are specimens sent to the national laboratory for confirmatory testing? 30e If no, where are specimens sent for testing? 30f If no, how often are specimens sent for testing (e.g. daily/weekly/monthly/intermittently, etc) Page 35 SARI Site Visit
36 31 How are specimens stored? Storage method Refrigerated Freezer -20 Freezer -70 Liquid nitrogen Cold pack Ambient temperature Other (please describe) 32 For how long are specimens stored before being sent for testing? 33 Is there a system in place for monitoring the temperature of the samples in storage? () 33a If yes, please describe that system: 34 Are total numbers of specimens collected and tested recorded? () 35 Is a unique identifier affixed to the swab to allow for linkage to swab collection form/clinical/epidemiologic data? 36 Are laboratory results reported back to clinicians? 36a If yes, how often are laboratory results received at the site/reported to clinicians? 36b What is the typical lag time between specimen collection & reporting of results to clinicians? 37 Are total numbers of positive and negative specimens reported to a surveillance focal point? 37a If yes, how often are these results reported (eg weekly/monthly, etc)? Data Management, Analysis, and Quality 38 How are surveillance data stored at the site (eg on paper, electronically)? 38a Who enters/records surveillance data? 38b How often is data entered into the storage system? 38c When is data entered? 39 Who is responsible for data management on-site? Page 36 SARI Site Visit
37 40 How is data quality monitored? 40a What data quality indicators are used? 40b How frequently is data quality monitored? 40c Who is notified of data quality issues? 41 Do surveillance staff review admissions logbooks to ensure that all SARI cases have been recorded? 42 How are SARI case-based data stored at the hospital? 43 How are SARI aggregate data stored at the hospital? 44 How often do you summarize total all-cause admissions at this hospital? (e.g. weekly/monthly/intermittently, etc.) 44a Are these summaries divided by age groups? () 44b If yes, please describe age groups. 45 How are laboratory results merged with the case-based reporting system (e.g. to case-based epidemiologic and clinical data forms) 46 How often does the site receive data quality feedback from the national level? 46a How often does the site host site visits/quality assurance visits from the national level? 47 Do site staff receive training updates from the national level? 47a If yes, how often do these trainings take place? 48 Are SARI visits summarized by: Interval Day Week Month Other 49 Is any SARI data analysis performed on site? 49a If yes, what sort of analysis is done? Please describe. 49b Who does on-site data aggregation and analysis? Page 37 SARI Site Visit
38 50 Does the surveillance focal point compile and prepare reports (weekly, monthly, other) at the site level? () 50a If yes, with whom are these reports shared? 50b Is a standard reporting template used? () 50c Do other sites use this template? 51 How is data reported/submitted to the national level? (eg , fax, text message, post, etc) 51a With what frequency is this reporting done? 52 Is there a method in place for identifying changes in activity/abnormal activity at the site level? () 52a If yes, please describe. 52b To whom is this activity reported? 52c Is there a mechanism in place to respond to these changes in activity? If yes, please describe. 52e Is this mechanism used? () 52f If no, why not? Page 38 SARI Site Visit
39 INFLUENZA SURVEILLANCE REVIEW -- ILI SITE VISIT Date of Interview Surveillance Staff General Information 1 What is the name of this facility? 2 Where is this facility located? 2a Country: 2b Province: 2c City/Town/Village: 2d How many patients does this facility typically see on a weekly basis? 2e For how long has this site been collecting ILI data (years/months)? 3 Who is responsible for coordinating surveillance at this site? 3a What is this person's position? 3b How many surveillance staff are present at this site? 3c Have these staff received training in surveillance data and specimen collection from the national level? () 3d If yes, how frequently are staff trained? 3e Are these staff given a motivation/incentive to participate? () 3f If yes, what is it? 3g Please describe the duties assigned to designated surveillance staff: 4 Is this a public or a private clinic? 4a What type of facility is it? Facility type General hospital OPD Pediatric hospital OPD Outpatient clinic Pediatric clinic Other (Please describe) Page 39 ILI Site Visit
40 4b Does this site provide a representative sampling of the following? Please describe Age Sex Ethnicity Socio-economic status Risk factors/chronic disease Geography 4c How might the answers to questions 4a and 4b bias the surveillance data collected? ILI Case Detection 5 Please describe the procedure used to screen for ILI cases: 6 What is the case definition in use for ILI at this site? 6a Does this case definition include a period of time for symptom onset? 6b If yes, what is that period of time? 6c Are any exclusion criteria in use at this site? 6d If yes, what are those criteria? 7 Is the ILI case definition known and understood by all staff that screen outpatients for ILI? () (Ask a few staff about the case definition for ILI) 7a Is the ILI case defintion posted and visible to all staff? () 8 On which days are patients screened for ILI & at what time of day? Day Time of Day (Morning, Afternoon, Evening, Night) Monday Tuesday Wednesday Thursday Friday Saturday Sunday 8a If patients are not screened daily or only at a particular time of day, how might this bias/impact surveillance? (Please explain) 8b Are any measures used to minimize this bias? (Please explain) Page 40 ILI Site Visit
41 9 What is the sampling scheme in use for the collection of ILI specimens? (Please describe) 9a Is this method random? () 9b If no, how might this impact/bias surveillance? 10 What is the maximum number of ILI specimens collected at this site on a "surveillance day?" 10a What is the maximum number of specimens collected at this site in one week? Epidemiologic Data Collection 11 Does the site have standard individual ILI case report forms printed, accessible, and in use? (If possible, please obtain a copy of this form ) () 11a Is this form a standard from provided by the national office, and used at all sentinel sites? () 11b If no, why are forms not standardized across sites? 12 Please indicate which of the following items are included in this form: Item Date of interview Date of visit Date of symptom onset Date of specimen collection Diagnoses at time of consultation Age or date of birth (years, months if under 1 year) Sex Patient Unique Identifier 13 Please indicate which of the following signs and symptoms are included in this form: Sign/Symptom Fever >38 measured Temperature recorded Cough Sore throat 14 Are records/logbooks kept of total clinic visits/consultations? () 14a If yes, who is responsible for the maintenance of this log? Page 41 ILI Site Visit
Illinois Influenza Surveillance Report
 ILLINOIS DEPARTMENT OF PUBLIC HEALTH Illinois Influenza Surveillance Report Week 8: Week Ending Saturday, February 25, 2012 Division of Infectious Diseases Immunizations Section 3/5/2012 1 Please note
ILLINOIS DEPARTMENT OF PUBLIC HEALTH Illinois Influenza Surveillance Report Week 8: Week Ending Saturday, February 25, 2012 Division of Infectious Diseases Immunizations Section 3/5/2012 1 Please note
Review of Sentinel Hospital Rotavirus Surveillance Systems
 Review of Sentinel Hospital Rotavirus Surveillance Systems Names of assessment team members: Date of assessment: Sentinel Site Information Hospital name: What type of hospital is this? Public Hospital
Review of Sentinel Hospital Rotavirus Surveillance Systems Names of assessment team members: Date of assessment: Sentinel Site Information Hospital name: What type of hospital is this? Public Hospital
Ohio Department of Health Seasonal Influenza Activity Summary MMWR Week 10 March 6 th, March 12 th, 2016
 Ohio Department of Health Seasonal Influenza Activity Summary MMWR Week 10 March 6 th, March 12 th, 2016 Current Influenza Activity: Current Ohio Activity Level (Geographic Spread) - Widespread Definition:
Ohio Department of Health Seasonal Influenza Activity Summary MMWR Week 10 March 6 th, March 12 th, 2016 Current Influenza Activity: Current Ohio Activity Level (Geographic Spread) - Widespread Definition:
ECDC INTERIM GUIDANCE
 ECDC INTERIM GUIDANCE Interim ECDC public health guidance on case and contact management for the new influenza A(H1N1) virus infection Version 3, 19 May 2009 ECDC intends to produce a series of interim
ECDC INTERIM GUIDANCE Interim ECDC public health guidance on case and contact management for the new influenza A(H1N1) virus infection Version 3, 19 May 2009 ECDC intends to produce a series of interim
WHO Regional Office for Europe update on avian influenza A (H7N9) virus
 WHO Regional Office for Europe update on avian influenza A (H7N9) virus Situation update 2: 30 April 2013 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO
WHO Regional Office for Europe update on avian influenza A (H7N9) virus Situation update 2: 30 April 2013 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO
Illinois Long Term Care Facilities and Assisted Living Facilities
 TO: FROM: RE: Illinois Long Term Care Facilities and Assisted Living Facilities Richard Dees, Chief, Bureau of Long Term Care Karen McMahon, Immunization Section Chief Craig Conover, MD, Medical Director,
TO: FROM: RE: Illinois Long Term Care Facilities and Assisted Living Facilities Richard Dees, Chief, Bureau of Long Term Care Karen McMahon, Immunization Section Chief Craig Conover, MD, Medical Director,
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health
 Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
Enhancing Respiratory Infection Surveillance on the Arizona-Sonora Border BIDS Program Sentinel Surveillance Data
 Enhancing Respiratory Infection Surveillance on the Arizona-Sonora Border BIDS Program Sentinel Surveillance Data Catherine Golenko, MPH, Arizona Department of Health Services *Arizona border population:
Enhancing Respiratory Infection Surveillance on the Arizona-Sonora Border BIDS Program Sentinel Surveillance Data Catherine Golenko, MPH, Arizona Department of Health Services *Arizona border population:
Key Facts about Influenza (Flu) & Flu Vaccine
 Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
TUBERCULOSIS (TB) SCREENING GUIDELINES FOR RESIDENTIAL FACILITIES AND DRUG
 TUBERCULOSIS (TB) SCREENING GUIDELINES FOR RESIDENTIAL FACILITIES AND DRUG Tx CENTERS Tuberculosis Control Program Health and Human Services Agency San Diego County INTRODUCTION Reducing TB disease requires
TUBERCULOSIS (TB) SCREENING GUIDELINES FOR RESIDENTIAL FACILITIES AND DRUG Tx CENTERS Tuberculosis Control Program Health and Human Services Agency San Diego County INTRODUCTION Reducing TB disease requires
PREPARING FOR A PANDEMIC. Lessons from the Past Plans for the Present and Future
 PREPARING FOR A PANDEMIC Lessons from the Past Plans for the Present and Future Pandemics Are Inevitable TM And their impact can be devastating 1918 Spanish Flu 20-100 million deaths worldwide 600,000
PREPARING FOR A PANDEMIC Lessons from the Past Plans for the Present and Future Pandemics Are Inevitable TM And their impact can be devastating 1918 Spanish Flu 20-100 million deaths worldwide 600,000
Influenza Surveillance Weekly Report CDC MMWR Week 16: Apr 17 to 23, 2016
 Office of Surveillance & Public Health Preparedness Program of Public Health Informatics Influenza Surveillance Weekly Report CDC MMWR Week 16: Apr 17 to 23, 2016 Influenza Activity by County, State, and
Office of Surveillance & Public Health Preparedness Program of Public Health Informatics Influenza Surveillance Weekly Report CDC MMWR Week 16: Apr 17 to 23, 2016 Influenza Activity by County, State, and
Preparing for the consequences of a swine flu pandemic
 Preparing for the consequences of a swine flu pandemic What CIGNA is Doing To help ensure the health and well-being of the individuals we serve, CIGNA is implementing its action plan to prepare for the
Preparing for the consequences of a swine flu pandemic What CIGNA is Doing To help ensure the health and well-being of the individuals we serve, CIGNA is implementing its action plan to prepare for the
EHR Surveillance for Seasonal and Pandemic Influenza in Primary Care Settings
 EHR Surveillance for Seasonal and Pandemic Influenza in Primary Care Settings Jonathan L. Temte, MD/PhD Chuck Illingworth University of Wisconsin School of Medicine and Public Health Department of Family
EHR Surveillance for Seasonal and Pandemic Influenza in Primary Care Settings Jonathan L. Temte, MD/PhD Chuck Illingworth University of Wisconsin School of Medicine and Public Health Department of Family
WHO checklist for influenza pandemic preparedness planning
 WHO/CDS/CSR/GIP/2005.4 EPIDEMIC ALERT & RESPONSE WHO checklist for influenza pandemic preparedness planning Department of Communicable Disease Surveillance and Response Global Influenza Programme World
WHO/CDS/CSR/GIP/2005.4 EPIDEMIC ALERT & RESPONSE WHO checklist for influenza pandemic preparedness planning Department of Communicable Disease Surveillance and Response Global Influenza Programme World
PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL
 PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL Public Health Action 1. Educate the public, particularly parents of infants, about the dangers of whooping cough and the advantages of initiating immunization
PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL Public Health Action 1. Educate the public, particularly parents of infants, about the dangers of whooping cough and the advantages of initiating immunization
Online Communicable Disease Reporting Handbook For Schools, Child-care Centers & Camps
 Online Communicable Disease Reporting Handbook For Schools, Child-care Centers & Camps More Information On Our Website https://www.accesskent.com/health/commdisease/school_daycare.htm Kent County Health
Online Communicable Disease Reporting Handbook For Schools, Child-care Centers & Camps More Information On Our Website https://www.accesskent.com/health/commdisease/school_daycare.htm Kent County Health
Viral Hepatitis Case Report
 Page 1 of 9 Viral Hepatitis Case Report Perinatal Hepatitis B Virus Infection Michigan Department of Community Health Communicable Disease Division Investigation Information Investigation ID Onset Date
Page 1 of 9 Viral Hepatitis Case Report Perinatal Hepatitis B Virus Infection Michigan Department of Community Health Communicable Disease Division Investigation Information Investigation ID Onset Date
4A. Types of Laboratory Tests Available and Specimens Required. Three main types of laboratory tests are used for diagnosing CHIK: virus
 4. LABORATORY 4A. Types of Laboratory Tests Available and Specimens Required Three main types of laboratory tests are used for diagnosing CHIK: virus isolation, reverse transcriptase-polymerase chain reaction
4. LABORATORY 4A. Types of Laboratory Tests Available and Specimens Required Three main types of laboratory tests are used for diagnosing CHIK: virus isolation, reverse transcriptase-polymerase chain reaction
Chapter 6 Case Ascertainment Methods
 Chapter 6 Case Ascertainment Methods Table of Contents 6.1 Introduction...6-1 6.2 Terminology...6-2 6.3 General Surveillance Development...6-4 6.3.1 Plan and Document... 6-4 6.3.2 Identify Data Sources...
Chapter 6 Case Ascertainment Methods Table of Contents 6.1 Introduction...6-1 6.2 Terminology...6-2 6.3 General Surveillance Development...6-4 6.3.1 Plan and Document... 6-4 6.3.2 Identify Data Sources...
Adapted from a presentation by Sharon Canclini, R.N., MS, FCN Harris College of Nursing and Health Sciences Texas Christian University
 Adapted from a presentation by Sharon Canclini, R.N., MS, FCN Harris College of Nursing and Health Sciences Texas Christian University What is a Pandemic? A pandemic is basically a global epidemic an epidemic
Adapted from a presentation by Sharon Canclini, R.N., MS, FCN Harris College of Nursing and Health Sciences Texas Christian University What is a Pandemic? A pandemic is basically a global epidemic an epidemic
Public Health Measures
 Annex M Public Health Measures Date of Latest Version: October 2006 Note: This is a new annex being released with the 2006 version of the Canadian Pandemic Influenza Plan. Table of Contents 1.0 Introduction.................................................................
Annex M Public Health Measures Date of Latest Version: October 2006 Note: This is a new annex being released with the 2006 version of the Canadian Pandemic Influenza Plan. Table of Contents 1.0 Introduction.................................................................
ECDC SURVEILLANCE REPORT
 ECDC SURVEILLANCE REPORT Pandemic (H1N1) 2009 Weekly report: Individual case reports EU/EEA countries 31 July 2009 Summary The pandemic A(H1N1) 2009 is still spreading despite the fact that the regular
ECDC SURVEILLANCE REPORT Pandemic (H1N1) 2009 Weekly report: Individual case reports EU/EEA countries 31 July 2009 Summary The pandemic A(H1N1) 2009 is still spreading despite the fact that the regular
SPECIAL REPORTING ISSUE 2007
 We want your opinion! see page 2 Volume 7 Number 1 January 2007 SPECIAL REPORTING ISSUE 2007 UPCOMING CHANGES FOR 2007 COMING SOON The list of reportable diseases and conditions is currently being updated
We want your opinion! see page 2 Volume 7 Number 1 January 2007 SPECIAL REPORTING ISSUE 2007 UPCOMING CHANGES FOR 2007 COMING SOON The list of reportable diseases and conditions is currently being updated
Laboratory confirmation requires isolation of Bordetella pertussis or detection of B. pertussis nucleic acid, preferably from a nasopharyngeal swab.
 Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
Overview. Why this policy? Influenza. Vaccine or mask policies. Other approaches Conclusion. epidemiology transmission vaccine
 Overview Why this policy? Influenza epidemiology transmission vaccine Vaccine or mask policies development and implementation Other approaches Conclusion Influenza or mask policy Receive the influenza
Overview Why this policy? Influenza epidemiology transmission vaccine Vaccine or mask policies development and implementation Other approaches Conclusion Influenza or mask policy Receive the influenza
State HAI Template Utah. 1. Develop or Enhance HAI program infrastructure
 State HAI Template Utah 1. Develop or Enhance HAI program infrastructure Successful HAI prevention requires close integration and collaboration with state and local infection prevention activities and
State HAI Template Utah 1. Develop or Enhance HAI program infrastructure Successful HAI prevention requires close integration and collaboration with state and local infection prevention activities and
Surveillance of Influenza 2009/2010
 Surveillance of Influenza 2009/2010 Reporting protocol for Influenza data submission to TESSy (2009-08-28) Surveillance of Influenza 2009/2010... 1 1. Background information... 1 2. Overview of the data
Surveillance of Influenza 2009/2010 Reporting protocol for Influenza data submission to TESSy (2009-08-28) Surveillance of Influenza 2009/2010... 1 1. Background information... 1 2. Overview of the data
Why you and your Family Should Get the Flu Shot
 Why you and your Family Should Get the Flu Shot Why Get VaCCinated against influenza? Influenza (flu) is a virus that can lead to serious complications, hospitalization, or even death. Even healthy children
Why you and your Family Should Get the Flu Shot Why Get VaCCinated against influenza? Influenza (flu) is a virus that can lead to serious complications, hospitalization, or even death. Even healthy children
PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County
 PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County DATE: 04/02/2015 TO: Health Alert Network FROM: Karen M. Murphy, PhD, RN, Acting Secretary of Health SUBJECT: DISTRIBUTION:
PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County DATE: 04/02/2015 TO: Health Alert Network FROM: Karen M. Murphy, PhD, RN, Acting Secretary of Health SUBJECT: DISTRIBUTION:
Referral Guidelines for TB/HIV co-management. (First Edition)
 Referral Guidelines for TB/HIV co-management (First Edition) Government of Lesotho April 2011 1 REFERRAL GUIDELINES FOR TB/HIV CO-MANAGEMENT INTRODUCTION Many TB patients are infected with HIV. Many people
Referral Guidelines for TB/HIV co-management (First Edition) Government of Lesotho April 2011 1 REFERRAL GUIDELINES FOR TB/HIV CO-MANAGEMENT INTRODUCTION Many TB patients are infected with HIV. Many people
FOR INFORMATION CONTACT:
 NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
Guidance for School Responses to Influenza - 2009 2010
 Guidance for School Responses to Influenza - 2009 2010 West Virginia Department of Education and West Virginia Department of Health and Human Resources August 19, 2009 8/19/2009 1 Purpose To provide local
Guidance for School Responses to Influenza - 2009 2010 West Virginia Department of Education and West Virginia Department of Health and Human Resources August 19, 2009 8/19/2009 1 Purpose To provide local
Version 1.0. HEAL NY Phase 5 Health IT & Public Health Team. Version Released 1.0. HEAL NY Phase 5 Health
 Statewide Health Information Network for New York (SHIN-NY) Health Information Exchange (HIE) for Public Health Use Case (Patient Visit, Hospitalization, Lab Result and Hospital Resources Data) Version
Statewide Health Information Network for New York (SHIN-NY) Health Information Exchange (HIE) for Public Health Use Case (Patient Visit, Hospitalization, Lab Result and Hospital Resources Data) Version
Maria Dalbey RN. BSN, MA, MBA March 17 th, 2015
 Maria Dalbey RN. BSN, MA, MBA March 17 th, 2015 2 Objectives Participants will be able to : Understand the Pathogenesis of Tuberculosis (TB) Identify the Goals of Public Health for TB Identify Hierarchy
Maria Dalbey RN. BSN, MA, MBA March 17 th, 2015 2 Objectives Participants will be able to : Understand the Pathogenesis of Tuberculosis (TB) Identify the Goals of Public Health for TB Identify Hierarchy
AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY
 AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY 1.0 PURPOSE To help ensure that those at greatest risk of complications and death from influenza are optimally protected through the appropriate
AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY 1.0 PURPOSE To help ensure that those at greatest risk of complications and death from influenza are optimally protected through the appropriate
Patient Progress Note & Dictation Standard
 Objective: The patient progress note serves as a basis for planning patient care, documenting communication between the health care provider and any other health professional contributing to the patient's
Objective: The patient progress note serves as a basis for planning patient care, documenting communication between the health care provider and any other health professional contributing to the patient's
Competency 1 Describe the role of epidemiology in public health
 The Northwest Center for Public Health Practice (NWCPHP) has developed competency-based epidemiology training materials for public health professionals in practice. Epidemiology is broadly accepted as
The Northwest Center for Public Health Practice (NWCPHP) has developed competency-based epidemiology training materials for public health professionals in practice. Epidemiology is broadly accepted as
COMMONWEALTH of VIRGINIA Department of Health
 COMMONWEALTH of VIRGINIA Department of Health MARISSA J. LEVINE, MD, MPH, FAAFP PO BOX 2448 TTY 7-1-1 OR STATE HEALTH COMMISSIONER RICHMOND, VA 23218 1-800-828-1120 Dear Colleague: Emerging Infections
COMMONWEALTH of VIRGINIA Department of Health MARISSA J. LEVINE, MD, MPH, FAAFP PO BOX 2448 TTY 7-1-1 OR STATE HEALTH COMMISSIONER RICHMOND, VA 23218 1-800-828-1120 Dear Colleague: Emerging Infections
Solid Organ Transplantation
 Solid Organ Transplantation Infection Prevention And Control Transplant Atlantic 2011 October 13/2011 Kathy Hart Introduction In the past several years, the drugs that we use, the surgeries themselves,
Solid Organ Transplantation Infection Prevention And Control Transplant Atlantic 2011 October 13/2011 Kathy Hart Introduction In the past several years, the drugs that we use, the surgeries themselves,
Pandemic Risk Assessment
 Research Note Pandemic Risk Assessment By: Katherine Hagan Copyright 2013, ASA Institute for Risk & Innovation Keywords: pandemic, Influenza A, novel virus, emergency response, monitoring, risk mitigation
Research Note Pandemic Risk Assessment By: Katherine Hagan Copyright 2013, ASA Institute for Risk & Innovation Keywords: pandemic, Influenza A, novel virus, emergency response, monitoring, risk mitigation
CONNECTICUT TECHNICAL HIGH SCHOOL SYSTEM PANDEMIC INFLUENZA & HIGHLY COMMUNICABLE DISEASE PLAN
 CONNECTICUT TECHNICAL HIGH SCHOOL SYSTEM PANDEMIC INFLUENZA & HIGHLY COMMUNICABLE DISEASE PLAN August 2009 Revised February 2015 Contents Introduction... Error! Bookmark not defined. Plan... 3 Immunization...
CONNECTICUT TECHNICAL HIGH SCHOOL SYSTEM PANDEMIC INFLUENZA & HIGHLY COMMUNICABLE DISEASE PLAN August 2009 Revised February 2015 Contents Introduction... Error! Bookmark not defined. Plan... 3 Immunization...
Swine Influenza Special Edition Newsletter
 Swine Influenza Newsletter surrounding swine flu, so that you ll have the right facts to make smart decisions for yourself and your family. While the World Health Organization (WHO) and the Centers for
Swine Influenza Newsletter surrounding swine flu, so that you ll have the right facts to make smart decisions for yourself and your family. While the World Health Organization (WHO) and the Centers for
Norovirus Outbreak Among Residents of an Assisted Living Facility, Houston County, Alabama 2010 (AL1003NRV 35a)
 Norovirus Outbreak Among Residents of an Assisted Living Facility, Houston County, Alabama 2010 (AL1003NRV 35a) Introduction On March 9, 2010, Public Health Area (PHA) 10 surveillance nurse contacted the
Norovirus Outbreak Among Residents of an Assisted Living Facility, Houston County, Alabama 2010 (AL1003NRV 35a) Introduction On March 9, 2010, Public Health Area (PHA) 10 surveillance nurse contacted the
Pandemic Influenza Preparedness Plan for Maryland Version 5
 Pandemic Influenza Preparedness Plan for Maryland Version 5 Epidemiology and Disease Control Program Community Health Administration Maryland Department of Health and Mental Hygiene Table of Contents Acknowledgments..............................................................
Pandemic Influenza Preparedness Plan for Maryland Version 5 Epidemiology and Disease Control Program Community Health Administration Maryland Department of Health and Mental Hygiene Table of Contents Acknowledgments..............................................................
SWINE FLU: FROM CONTAINMENT TO TREATMENT
 SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
Pneumonia Education and Discharge Instructions
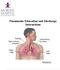 Pneumonia Education and Discharge Instructions Pneumonia Education and Discharge Instructions Definition: Pneumonia is an infection of the lungs. Many different organisms can cause it, including bacteria,
Pneumonia Education and Discharge Instructions Pneumonia Education and Discharge Instructions Definition: Pneumonia is an infection of the lungs. Many different organisms can cause it, including bacteria,
See page 331 of HEDIS 2013 Tech Specs Vol 2. HEDIS specs apply to plans. RARE applies to hospitals. Plan All-Cause Readmissions (PCR) *++
 Hospitalizations Inpatient Utilization General Hospital/Acute Care (IPU) * This measure summarizes utilization of acute inpatient care and services in the following categories: Total inpatient. Medicine.
Hospitalizations Inpatient Utilization General Hospital/Acute Care (IPU) * This measure summarizes utilization of acute inpatient care and services in the following categories: Total inpatient. Medicine.
Exelon Pandemic Planning. Utility Interface With the NRC. Ralph Bassett
 Exelon Pandemic Planning Utility Interface With the NRC Ralph Bassett Meeting Goals Provide the NRC with a high level overview of the Exelon Nuclear Pandemic Response Plan Review interface with the Regional
Exelon Pandemic Planning Utility Interface With the NRC Ralph Bassett Meeting Goals Provide the NRC with a high level overview of the Exelon Nuclear Pandemic Response Plan Review interface with the Regional
http://www.who.int/csr/disease/avian_influenza/phase/en 4 http://new.paho.org/hq/index.php?option=com_content&task=view&id=1283&itemid=569
 Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
CHAPTER 13. Quality Control/Quality Assurance
 CHAPTER 13 Quality Control/Quality Assurance Quality Control/Quality Assurance (QC/QA) can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements
CHAPTER 13 Quality Control/Quality Assurance Quality Control/Quality Assurance (QC/QA) can be defined as the set of planned and systematic activities focused on providing confidence that quality requirements
Requirements for Prevention and Detection of Influenza Outbreaks Aide Memoire 2015-2016 Season
 Requirements for Prevention and Detection of Influenza Outbreaks Aide Memoire 2015-2016 Season Liz Forde, Cork Community Services Patricia Coughlan, Cork & Kerry Disability Services Outline Guidelines
Requirements for Prevention and Detection of Influenza Outbreaks Aide Memoire 2015-2016 Season Liz Forde, Cork Community Services Patricia Coughlan, Cork & Kerry Disability Services Outline Guidelines
Planning for 2009 H1N1 Influenza. A Preparedness Guide for Small Business
 09 Planning for 2009 H1N1 Influenza A Preparedness Guide for Small Business Table of Contents 02 Foreword 03 Introduction 04 How to Write Your Plan 05 Keeping Healthy: 10 Tips for Businesses 06 Keeping
09 Planning for 2009 H1N1 Influenza A Preparedness Guide for Small Business Table of Contents 02 Foreword 03 Introduction 04 How to Write Your Plan 05 Keeping Healthy: 10 Tips for Businesses 06 Keeping
Kim Cervantes, MPH, Coordinator / Regional Epidemiology Program Barbara Carothers, LPN, Public Health Representative 1
 Kim Cervantes, MPH, Coordinator / Regional Epidemiology Program Barbara Carothers, LPN, Public Health Representative 1 Overview NJDOH Outbreak Training 2013, Jan 2014 NJLMN Practice Exchange: NJACCHO Outbreak
Kim Cervantes, MPH, Coordinator / Regional Epidemiology Program Barbara Carothers, LPN, Public Health Representative 1 Overview NJDOH Outbreak Training 2013, Jan 2014 NJLMN Practice Exchange: NJACCHO Outbreak
READ THIS LEAFLET VERY CAREFULLY, AND KEEP IT IN A SAFE PLACE. FLU IS SPREADING IN IRELAND, AND THIS INFORMATION IS IMPORTANT FOR YOU AND YOUR FAMILY.
 READ THIS LEAFLET VERY CAREFULLY, AND KEEP IT IN A SAFE PLACE. FLU IS SPREADING IN IRELAND, AND THIS INFORMATION IS IMPORTANT FOR YOU AND YOUR FAMILY. Information and medical advice for the public on Pandemic
READ THIS LEAFLET VERY CAREFULLY, AND KEEP IT IN A SAFE PLACE. FLU IS SPREADING IN IRELAND, AND THIS INFORMATION IS IMPORTANT FOR YOU AND YOUR FAMILY. Information and medical advice for the public on Pandemic
A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned
 A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned Presenter: Kristen George MPH Candidate Johns Hopkins University Bloomberg School of Public Health Preceptor:
A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned Presenter: Kristen George MPH Candidate Johns Hopkins University Bloomberg School of Public Health Preceptor:
WHEREAS, Ebola Virus Disease (EVD) is a rare and potentially deadly disease caused
 STATE OF NEW YORK : DEPARTMENT OF HEALTH --------------------------------------------------------------------------X IN THE MATTER OF THE PREVENTION AND CONTROL OF EBOLA VIRUS DISEASE ORDER FOR SUMMARY
STATE OF NEW YORK : DEPARTMENT OF HEALTH --------------------------------------------------------------------------X IN THE MATTER OF THE PREVENTION AND CONTROL OF EBOLA VIRUS DISEASE ORDER FOR SUMMARY
Major Public Health Threat
 GAO United States General Accounting Office Testimony Before the Committee on Government Reform, House of Representatives For Release on Delivery Expected at 10:00 a.m. EST Thursday, February 12, 2004
GAO United States General Accounting Office Testimony Before the Committee on Government Reform, House of Representatives For Release on Delivery Expected at 10:00 a.m. EST Thursday, February 12, 2004
Ontario Pandemic Influenza Plan for Continuity of Electricity Operations
 Planning Guideline GDE-162 Ontario Pandemic Influenza Plan for Continuity of Electricity Operations Planning Guideline Issue 4.0 October 13, 2015 Emergency Preparedness Task Force This planning guide provides
Planning Guideline GDE-162 Ontario Pandemic Influenza Plan for Continuity of Electricity Operations Planning Guideline Issue 4.0 October 13, 2015 Emergency Preparedness Task Force This planning guide provides
Hospital Discussion Guide
 Hospital Discussion Guide For Pandemic Influenza Planning Prepared for Healthcare Preparedness Activity Centers for Disease Control and Prevention By Oak Ridge Institute for Science and Education January
Hospital Discussion Guide For Pandemic Influenza Planning Prepared for Healthcare Preparedness Activity Centers for Disease Control and Prevention By Oak Ridge Institute for Science and Education January
Internet Search Activity & Crowdsourcing
 Novel Data Sources for Disease Surveillance and Epidemiology: Internet Search Activity & Crowdsourcing Sumiko Mekaru, DVM, MPVM, MLIS HealthMap, Boston Children s Hospital American College of Epidemiology
Novel Data Sources for Disease Surveillance and Epidemiology: Internet Search Activity & Crowdsourcing Sumiko Mekaru, DVM, MPVM, MLIS HealthMap, Boston Children s Hospital American College of Epidemiology
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Centers for Disease Control and Prevention
 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Centers for Disease Control and Prevention National Center for HIV, STD, and TB Prevention Division of Tuberculosis Elimination Public
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Centers for Disease Control and Prevention National Center for HIV, STD, and TB Prevention Division of Tuberculosis Elimination Public
Tuberculosis. Subject. Goal/Objective. Instructions. Rationale. Operations Directorate, Health Branch Immigration Medical Examination Instructions
 Subject Instructions for the screening of clients to detect tuberculosis (TB) in the context of the Canadian immigration medical examination (IME). Goal/Objective These instructions are provided to ensure
Subject Instructions for the screening of clients to detect tuberculosis (TB) in the context of the Canadian immigration medical examination (IME). Goal/Objective These instructions are provided to ensure
FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH)
 FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH) What is pertussis? General Questions About Pertussis Pertussis, or whooping cough, is a contagious illness that is spread when an infected person
FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH) What is pertussis? General Questions About Pertussis Pertussis, or whooping cough, is a contagious illness that is spread when an infected person
KANSAS PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN Version 2.2.4 January 2015
 KANSAS PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN Version 2.2.4 January 2015 Kansas Response Plan Biological Incident Annex Attachment 1 Table of Contents Introduction... 4 Situation... 4 Influenza
KANSAS PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN Version 2.2.4 January 2015 Kansas Response Plan Biological Incident Annex Attachment 1 Table of Contents Introduction... 4 Situation... 4 Influenza
Quality Assurance and Data Management Processes
 Quality Assurance and Data Management Processes Version 2.0 9 th January 2013 Table of Contents 1.0 Preamble... 3 2.0 Roles and responsibilities of AuSCR staff... 3 3.0 Standard quality assurance data
Quality Assurance and Data Management Processes Version 2.0 9 th January 2013 Table of Contents 1.0 Preamble... 3 2.0 Roles and responsibilities of AuSCR staff... 3 3.0 Standard quality assurance data
PTE Pediatric Asthma Metrics Reporting Updated January 2015
 PTE Pediatric Asthma Metrics Reporting Updated January 20 Introduction: The Maine Health Management Coalition s (MHMC) Pathways to Excellence (PTE) Program is preparing for its next round of PTE Pediatric
PTE Pediatric Asthma Metrics Reporting Updated January 20 Introduction: The Maine Health Management Coalition s (MHMC) Pathways to Excellence (PTE) Program is preparing for its next round of PTE Pediatric
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
Family Care Clinic Guidelines: Virtual Telephone Visits
 Family Care Clinic Guidelines: Virtual Telephone Visits Health System Family Health Center Guidelines December 2013 Family Care Clinic Guidelines: Virtual Telephone Visits Table of Contents Background...3
Family Care Clinic Guidelines: Virtual Telephone Visits Health System Family Health Center Guidelines December 2013 Family Care Clinic Guidelines: Virtual Telephone Visits Table of Contents Background...3
North Texas School Health Surveillance: First-Year Progress and Next Steps
 Dean Lampman, MBA, Regional Surveillance Coordinator, Southwest Center for Advanced Public Health Practice* (Fort Worth, TX), dflampman@tarrantcounty.com Tabatha Powell, MPH, Doctoral student, University
Dean Lampman, MBA, Regional Surveillance Coordinator, Southwest Center for Advanced Public Health Practice* (Fort Worth, TX), dflampman@tarrantcounty.com Tabatha Powell, MPH, Doctoral student, University
3. Blood and blood products such as serum, plasma, and other blood components.
 Mississippi Downloaded 01/2011 101.11 Infectious Medical Waste. The term "infectious medical waste" includes solid or liquid wastes which may contain pathogens with sufficient virulence and quantity such
Mississippi Downloaded 01/2011 101.11 Infectious Medical Waste. The term "infectious medical waste" includes solid or liquid wastes which may contain pathogens with sufficient virulence and quantity such
The H1N1 Flu in Ontario. A Report by Ontario s Chief Medical Officer of Health
 The H1N1 Flu in Ontario A Report by Ontario s Chief Medical Officer of Health September 2009 Ministry of Health and Long-Term Care Ministère de la Santé et des Soins de longue durée Chief Medical Officer
The H1N1 Flu in Ontario A Report by Ontario s Chief Medical Officer of Health September 2009 Ministry of Health and Long-Term Care Ministère de la Santé et des Soins de longue durée Chief Medical Officer
Ebola: Teaching Points for Nurse Educators
 Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION
 PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health
 Recommendations f the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk f influenza-related complications
Recommendations f the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk f influenza-related complications
Algorithm for detecting Zika virus (ZIKV) 1
 Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
Algorithm for detecting Zika virus (ZIKV) 1 This algorithm is addressed to laboratories with established capacity (molecular, antigenic and/or serological) to detect dengue (DENV), Zika (ZIKV) 2, and chikungunya
Roger Williams University. Bloodborne Pathogens Exposure Control Plan
 Roger Williams University Bloodborne Pathogens Exposure Control Plan Revised 12/2010 ROGER WILLIAMS UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Roger
Roger Williams University Bloodborne Pathogens Exposure Control Plan Revised 12/2010 ROGER WILLIAMS UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Roger
Viral Hepatitis. 2009 APHL survey report
 Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
Issues in Brief: viral hepatitis testing Association of Public Health Laboratories May Viral Hepatitis Testing 9 APHL survey report In order to characterize the role that the nation s public health laboratories
ATTACHMENT A PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN
 APPENDIX 3 TO ANNEX H - EPIDEMIOLOGY AND SURVEILLANCE ATTACHMENT A PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN City of Houston Department of Health and Human Services February 17, 2006 RECORD OF
APPENDIX 3 TO ANNEX H - EPIDEMIOLOGY AND SURVEILLANCE ATTACHMENT A PANDEMIC INFLUENZA PREPAREDNESS AND RESPONSE PLAN City of Houston Department of Health and Human Services February 17, 2006 RECORD OF
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION
 LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
Adult Vaccination Frequently Asked Questions: The Basics
 The Basics Why should I get vaccinated? Vaccination is the best way to protect against infections that can make you sick and be passed on to those around you. 1 What kinds of side effects will I get from
The Basics Why should I get vaccinated? Vaccination is the best way to protect against infections that can make you sick and be passed on to those around you. 1 What kinds of side effects will I get from
KUMC. Medical Surveillance Program
 KUMC Medical Surveillance Program INTRODUCTION Employees at KUMC could potentially be exposed to safety hazards such as chemical, biological, and physical hazards. These hazards are most likely to occur
KUMC Medical Surveillance Program INTRODUCTION Employees at KUMC could potentially be exposed to safety hazards such as chemical, biological, and physical hazards. These hazards are most likely to occur
Pandemic Influenza Response Plan. Licking County Health Department 675 Price Road Newark, OH 43055
 Pandemic Influenza Response Plan Licking County Health Department 675 Price Road Newark, OH 43055 I. EXECUTIVE SUMMARY The Licking County Health Department Pandemic Influenza Response describes the policies
Pandemic Influenza Response Plan Licking County Health Department 675 Price Road Newark, OH 43055 I. EXECUTIVE SUMMARY The Licking County Health Department Pandemic Influenza Response describes the policies
CONFERENCE CALL. Flu School Closing Guidance U.S. Department of Education Moderator: Bill Modzeleski May 5, 2009 3 pm ET
 Page 1 CONFERENCE CALL Flu School Closing Guidance U.S. Department of Education May 5, 2009 3 pm ET Welcome and thank you for standing by. At this time all participants are in a listen-only mode. After
Page 1 CONFERENCE CALL Flu School Closing Guidance U.S. Department of Education May 5, 2009 3 pm ET Welcome and thank you for standing by. At this time all participants are in a listen-only mode. After
Assessment of the 2009 Influenza A (H1N1) Pandemic on Selected Countries in the Southern Hemisphere: Argentina, Australia, Chile, New Zealand and
 Assessment of the 2009 Influenza A (H1N1) Pandemic on Selected Countries in the Southern Hemisphere: Argentina, Australia, Chile, New Zealand and Uruguay Developed by the Department of Health and Human
Assessment of the 2009 Influenza A (H1N1) Pandemic on Selected Countries in the Southern Hemisphere: Argentina, Australia, Chile, New Zealand and Uruguay Developed by the Department of Health and Human
GUIDELINES FOR VIRAL HEPATITIS SURVEILLANCE AND CASE MANAGEMENT
 GUIDELINES FOR VIRAL HEPATITIS SURVEILLANCE AND CASE MANAGEMENT January 2005 Guidelines for Viral Hepatitis Surveillance and Case Management Ordering information To order a copy of this manual, write to:
GUIDELINES FOR VIRAL HEPATITIS SURVEILLANCE AND CASE MANAGEMENT January 2005 Guidelines for Viral Hepatitis Surveillance and Case Management Ordering information To order a copy of this manual, write to:
H1N1 Flu Vaccine Available to All Virginia Beach City Public Schools Students
 V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
4.06. Infection Prevention and Control at Long-term-care Homes. Chapter 4 Section. Background. Follow-up on VFM Section 3.06, 2009 Annual Report
 Chapter 4 Section 4.06 Infection Prevention and Control at Long-term-care Homes Follow-up on VFM Section 3.06, 2009 Annual Report Background Long-term-care nursing homes and homes for the aged (now collectively
Chapter 4 Section 4.06 Infection Prevention and Control at Long-term-care Homes Follow-up on VFM Section 3.06, 2009 Annual Report Background Long-term-care nursing homes and homes for the aged (now collectively
Original Research Paper
 Original Research Paper Sore throat diagnosis and management in a general practice after-hours service Marjan Kljakovic is a senior lecturer, department of general practice, Wellington School of Medicine
Original Research Paper Sore throat diagnosis and management in a general practice after-hours service Marjan Kljakovic is a senior lecturer, department of general practice, Wellington School of Medicine
FLU VACCINATION - FREQUENTLY ASKED QUESTIONS
 FLU VACCINATION - FREQUENTLY ASKED QUESTIONS SEASONAL FLU VACCINATION 2015 2016 As a health care worker, am I required to be vaccinated against influenza (the flu)? It is not mandatory to be vaccinated
FLU VACCINATION - FREQUENTLY ASKED QUESTIONS SEASONAL FLU VACCINATION 2015 2016 As a health care worker, am I required to be vaccinated against influenza (the flu)? It is not mandatory to be vaccinated
Global Influenza Surveillance Network (GISN) Activities in the Eastern Mediterranean Region
 Global Influenza Surveillance Network (GISN) Activities in the Eastern Mediterranean Region Dr Hassan El Bushra Regional Adviser, Emerging Diseases, Communicable Diseases Surveillance, Forecasting and
Global Influenza Surveillance Network (GISN) Activities in the Eastern Mediterranean Region Dr Hassan El Bushra Regional Adviser, Emerging Diseases, Communicable Diseases Surveillance, Forecasting and
ADDENDUM 1 MEDICAL HOME TO SOONERCARE PHYSICIAN AGREEMENT FOR CHOICE PRIMARY CARE PROVIDERS
 ADDENDUM 1 MEDICAL HOME TO SOONERCARE PHYSICIAN AGREEMENT FOR CHOICE PRIMARY CARE PROVIDERS 1.0 PURPOSE The purpose of this Addendum is for OHCA and PROVIDER to contract for PCP services in OHCA s SoonerCare
ADDENDUM 1 MEDICAL HOME TO SOONERCARE PHYSICIAN AGREEMENT FOR CHOICE PRIMARY CARE PROVIDERS 1.0 PURPOSE The purpose of this Addendum is for OHCA and PROVIDER to contract for PCP services in OHCA s SoonerCare
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Pertussis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 December 2005 Case Definition
August 2011 Pertussis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 December 2005 Case Definition
Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.
 Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.org/ Fifth disease (erythema infectiosum) is a common, mild, childhood
Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.org/ Fifth disease (erythema infectiosum) is a common, mild, childhood
University of Dubuque Master in Physician Assistant Studies Student Clinical Rotation Guide
 University of Dubuque Master in Physician Assistant Studies Student Clinical Rotation Guide 2017 2018 Contents General Policies and Procedures... 3 Clinical Rotation Requirements... 3 Immunizations...
University of Dubuque Master in Physician Assistant Studies Student Clinical Rotation Guide 2017 2018 Contents General Policies and Procedures... 3 Clinical Rotation Requirements... 3 Immunizations...
SA Health Hazard Leader for Human Disease
 SA Health Hazard Leader for Human Disease (including Pandemic Influenza) Val Smyth Director Emergency Management Unit SA Health 2013 SA Health > Hazard Leader for Human Disease Includes Pandemic Influenza
SA Health Hazard Leader for Human Disease (including Pandemic Influenza) Val Smyth Director Emergency Management Unit SA Health 2013 SA Health > Hazard Leader for Human Disease Includes Pandemic Influenza
MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams
 MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams Document control Title Type Author/s Management of tuberculosis in prisons: Guidance for prison healthcare teams Operational
MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams Document control Title Type Author/s Management of tuberculosis in prisons: Guidance for prison healthcare teams Operational
swine flu vaccination:
 swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
AAP Meaningful Use: Certified EHR Technology Criteria
 AAP Meaningful Use: Certified EHR Technology Criteria On July 13, 2010, the US Centers for Medicare and Medicaid Services (CMS) released a Final Rule establishing the criteria with which eligible pediatricians,
AAP Meaningful Use: Certified EHR Technology Criteria On July 13, 2010, the US Centers for Medicare and Medicaid Services (CMS) released a Final Rule establishing the criteria with which eligible pediatricians,
