Inquiry Labs In The High School Classroom. EDTL 611 Final Project By Misha Stredrick and Laura Rettig
|
|
|
- Ethelbert Owens
- 8 years ago
- Views:
Transcription
1 Inquiry Labs In The High School Classroom EDTL 611 Final Project By Misha Stredrick and Laura Rettig June 2007
2 Table of Contents Research Paper pages 3-7 Introduction to the Lab Activities page 8 State of Ohio Indicators for Scientific Inquiry page 9 State of Ohio Indicators for Physical Science page 10 State of Ohio Indicators for Life Science page 11 Index for Physical Science Laboratory Activities page 12 Lab 1 pages Lab 2 pages Lab 3 pages Lab 4 pages Lab 5 pages Lab 6 pages Index for Biology Laboratory Activities page 29 Lab 1 pages Lab 2 pages Lab 3 pages Lab 4 pages Lab 5 pages 40-42
3 Inquiry Labs in the High School Classroom Research Paper Rationale When we both began this journey to education we assumed that it would be easy to find materials to teach high school science and it was. We were naïve to believe that the lessons found would be inquiry-based and purposeful in light of the Ohio Science Standards. We have been analyzing and observing science lessons throughout our educational experiences, and we have found that most of the lessons were comprised of two components: A lecture and an experiment. The lecture portion often involves extended explanations of the ideas with little student input. This lecture covers the majority of the lesson, leaving little time for the second portion an experiment. The experimental portion of the science lesson is often the application of the idea. It is intended to allow the students to create a first-hand experience of the science concept and to build a deeper understanding of its connectivity to the world. Although we agree with the idea of hands-on science, we acknowledge that some teachers will not have the resources to purchase expensive materials to help their students understand science concepts. We also believe that an effective science lesson should not rely solely on lecture. A productive science lesson should use techniques to reach all of the learning styles (kinesthetic/tactile, auditory, and visual) and help students learn how to work cooperatively and make connections to the real world. Ideally, a single lesson would include multiple state standards.
4 Learning Styles An integral part of a productive science lesson is meeting the needs of students individual learning styles. These learning styles determine how students will interpret and retain the concepts that are taught. They should be considered prior to teaching the lesson. The three learning styles commonly identified are kinesthetic/tactile, auditory, and visual. They can easily be addressed through the following techniques suggested by Marilee Sprenger (2005): Kinesthetic/Tactile: Activities such as role-playing, creating a concept or working with technology. Auditory: Debates, classroom discussions, or use of music to spark memory. Visual: Pictures, video clips, handouts, graphic organizers, overhead transparencies and textbook readings (pp. 26). Another way to assist students is to identify and incorporate their personality types when designing group activities. The six personality types include reactors, workaholics, persisters, dreamers, rebels, and promoters. Identifying these personality types may help teachers to understand how well the students will work together or how they may react to certain assignments. Pauley, Bradley and Pauley (2002) state that if personality types are acknowledged at the beginning of the school year, a teacher will be able to use these personality types to assist students to learn the material and communicate with their peers (pp. 2-6).
5 Cooperative Learning The second piece to an effective science lesson is allowing students to learn cooperatively. In the book entitled, The First Days of School, Wong describes the cooperative learning experience as one in which the teacher presents the lesson and group strategies and allows the students to help one another master the objectives. (2005, pp. 245). We feel that this concept is very important in science because many students may have difficulty with, or disinterest in, the subject matter. Cooperative learning gives students the chance to exhibit leadership abilities and teamwork while exploring new concepts. We tried to incorporate cooperative learning when redesigning science lessons to meet our ideal standard. Real World Connections The next step, application to the real world, is critical for reaching students who do not have a strong science background. This concept is often shown using elements that are of interest to students. Racecar driving, household chemicals, sports and even makeup can all be connected to science. As teachers we must acknowledge that our students need these relationships to help them interpret the phenomena around them. (Brooks & Brooks, 2001, pp. 42). Multiple Standards The final step to having a productive classroom is addressing many of the standards for science. Teachers can do this with the addition of the previous suggestions and by integrating meta-cognitive skills (Bybee, 2002, pp.123). The State of Ohio
6 Science Standards incorporates many indicators that are unrelated to content knowledge. Educators may be overwhelmed by the sheer number of standards and have no idea how to effectively incorporate scientific ways of knowing or scientific inquiry. Teachers who employ meta-cognitive skills can help students by encouraging them to connect ideas and think logically while meeting the scientific inquiry standards. Problem & Potential Solutions We are both science educators who find it unfortunate that many teachers do not have access to materials to create a productive science lesson. We have selected lab activities utilized in Laura s classroom and modified them to include background information, state indicators, safety requirements, materials lists, detailed procedures, and analysis questions. These inexpensive lessons utilize common household supplies and materials readily available to anyone. They will also incorporate the following strategies: Allow students to use simple materials to understand difficult science concepts Create workgroups for students (cooperative learning) Thoroughly explain safety precautions Teach students how to collect and analyze data Utilize different techniques to present data Enable students to describe results, in their own words Access a higher level of thinking Make science connections to the world around them
7 Bibliography Bradley, D. F., Pauley, J. A., & Pauley, J. F. (2002). Here s how to reach me. Baltimore, Maryland: Paul H. Brookes Publishing Co. Brooks, J. G., & Brooks, M. G. (2001). In search of understanding the case for constructivist classrooms. Upper Saddle River, New Jersey: Prentice-Hall, Inc. Bybee, R. W. (2002). Learning science and the science of learning. Arlington, Virginia: National Science Teachers Association. Sprenger, M. (2005). How to teach so students remember. Alexandria, Virginia: Association for Supervision and Curriculum Development. Wong, H. K., & Wong, R. T. (2005). The first days of school. Mountain View, California: Harry K. Wong Publications, Inc.
8 Introduction to the Lab Activities I began my teaching career this year at Fostoria High School through a BGSU grant program. Prior to this I spent eleven years working as a Medical Technologist in a clinical laboratory, so most of my experience in science is laboratory-based. Those of us who entered the teaching profession this year through this program were paid a graduate assistantship, but required to act as the teacher on record and design and implement all lesson plans. This required some flexibility and ingenuity since we were placed in high need districts with many students at or below the Federal Poverty Level and very limited supplies. As I searched for lab activities in textbooks and on the internet, I found that most of the activities were cookbook in nature and guided the students through the lab with explicit directions. Often the provided analysis questions only required students to demonstrate knowledge and understanding, but they did not incorporate more complex thinking skills found in higher levels of Bloom s Taxonomy (application, analysis, synthesis, evaluation). I created the following eleven lab activities for my Physical Science and Biology classes at Fostoria. Through my reading, research, classes, and past experience I have discovered the usefulness of common, inexpensive household materials such as candy, teddy grahams, batteries, Christmas lights, and cleaning products for lab activities. When combined with inquiry methods and complex questioning, the simplest premise for a laboratory activity can be effectively transferred to a high school classroom. For all of these activities I have included the State of Ohio Indicator for content, background information, materials list, safety precautions, the procedure, and the analysis questions. Many of these labs have been modified since I first introduced them, with changes in the procedures or questions to address difficulties my students faced in the learning process. Laura Rettig June 2007
9 State of Ohio Indicators for Scientific Inquiry The eleven lab activities meet one or more of the following State of Ohio Indicators for Scientific Inquiry by grade level: 1. Grade 9 and 10, Research and apply appropriate safety precautions when designing and conducting scientific investigations (e.g., OSHA, Material Safety Data Sheets [MSDS], eyewash, goggles and ventilation. 2. Grade 9, Construct, interpret and apply physical and conceptual models that represent or explain systems, objects, events or concepts. 3. Grade 9, Develop oral and written presentations using clear language, accurate data, appropriate graphs, tables, maps and available technology. 4. Grade 9, Draw logical conclusions based on scientific knowledge and evidence from investigations. 5. Grade 10, Present scientific findings using clear language, accurate data, appropriate graphs, tables, maps and available technology. 6. Grade 10, Use mathematical models to predict and analyze natural phenomena. 7. Grade 10, Draw conclusions from inquiries based on scientific knowledge and principles, the use of logic and evidence (data) from investigations.
10 State of Ohio Indicators for Physical Science The eleven lab activities meet one or more of the following State of Ohio Indicators for Physical Science by grade level: 1. Grade 9, Investigate the properties of pure substances and mixtures (e.g., density, conductivity, hardness, properties of alloys, superconductors and semiconductors). 2. Grade 9, Compare the conductivity of different materials and explain the role of electrons in the ability to conduct electricity. 3. Grade 9, Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 4. Grade 9, Show how the properties of a wave depend on the properties of the medium through which it travels. Recognize that electromagnetic waves can be propagated without a medium. 5. Grade 9, Demonstrate that motion is a measurable quantity that depends on the observer s frame of reference and describe the object s motion in terms of position, velocity, acceleration and time. 6. Grade 9, Demonstrate that any object does not accelerate (remains at rest or maintains constant speed and direction of motion) unless an unbalanced (net) force acts on it. 7. Grade 9, Explain the change in motion (acceleration) of an object. Demonstrate that the acceleration is proportional to the mass of the object. (F = ma. Note that weight is the gravitational force on mass). 8. Grade 9, Demonstrate that whenever one object exerts a force on another, an equal amount of force is exerted back on the first object. 9. Grade 9, Demonstrate the ways in which frictional forces constrain the motion of objects (e.g. a car traveling around a curve, a block on an inclined plane, a person running, and airplane in flight).
11 State of Ohio Indicators for Life Science The eleven lab activities meet one or more of the following State of Ohio Indicators for Life Science by grade level: 1. Grade 10, Compare the structure, function and interrelatedness of cell organelles in eukaryotic cells (e.g., nucleus, chromosome, mitochondria, cell membrane, cell wall, chloroplast, cilia, flagella) and prokaryotic cells. 2. Grade 10, Explain the characteristics of life as indicated by cellular processes including a. homeostasis b. energy transfers and transformations c. transportation of molecules d. disposal of wastes e. synthesis of new molecules. 3. Grade 10, Illustrate the relationship of the structure and function of DNA to protein synthesis and the characteristics of an organism. 4. Grade 10, Analyze how evolutionary mechanisms (e.g. genetic drift, immigration, emigration, mutation) and their consequences provide a scientific explanation for the diversity and unity of past life forms, as depicted in the fossil record, and present life forms.
12 Physical Science Laboratory Activities For Grade 9 Index Lab 1: Properties of Substances Lab 2: Identifying Acids and Bases Lab 3: Measuring ph Lab 4: Experimenting with Waves Lab 5: Understanding Motion Lab 6: Understanding Forces
13 Physical Science Properties of Substances State of Ohio Indicators for Physical Science: Grade Investigate the properties of pure substances and mixtures (e.g., density, conductivity, hardness, properties of alloys, superconductors and semiconductors). 2. Compare the conductivity of different materials and explain the role of electrons in the ability to conduct electricity. Background: Substances have specific properties that determine their usage. In this lab activity we will determine characteristics and properties of pure substances and mixtures. Materials: Aluminum nails, steel nails, galvanized steel nails, copper wire, aluminum wire, steel wire, brass hooks, wooden dowels, jute twine, foam cups, salt, calcium carbonate tablets, coated electrical wires, Christmas lights, D batteries, graduated cylinders, rulers, scales, ph paper, five clear liquids. Safety Precautions: Please use care when handling sharp objects such as nail and wires. Do not point toward yourself or others. Goggles and aprons will be worn while handling liquids to prevent splash related injuries. Please try to avoid contact with the copper ends of the stripped wires; they may become warm while in contact with the battery. The battery only provides 1.5 Volts of electricity and will not cause injury. When observing the odor of a substance, do not directly inhale!! Gently wave your hand over the top of the beaker and observe the odor in this manner. Procedure and Analysis: Procedure: Day 1 You are to work in pairs to analyze the properties of the following substances. You will each create a data table that lists the item and all of its measurable properties. 1. Aluminum nail 2. Steel nail 3. Galvanized steel nail 4. Copper wire 5. Aluminum wire 6. Steel wire 7. Brass hook 8. Wooden dowel 9. Jute twine 10. Foam cup Page 1
14 For each of the above items you will be responsible for determining the following properties: 1. Color 2. Shape 3. Size 4. Mass 5. Volume 6. Density 7. Conductivity Note: You may not be able to measure every property for all of these items. If you are unable to adequately measure an item or determine a specific property then mark this on your table!! Create your data table here: In addition to the above items, you will examine two ionic compounds and determine if they are conductors or non-conductors of electricity. 1. Salt (Sodium Chloride); pour a small amount into the foam cup and measure. 2. Calcium tablet (Calcium Carbonate) For these two items, check the conductivity of the substance in dry form as well as dissolved in water. Is there any difference in conductivity? Should there be a difference? Why? Page 2
15 Procedure: Day 2 You may finish any data collection needed for Day 1. Once finished with Day 1 data, you will need to put on goggles and an apron. There are five unknown liquids in beakers on the laboratory counter. You will need a 5 ml aliquot (portion) of each liquid for testing. Please test one liquid at a time and clean your graduated cylinder in between measurements. You will be required to make the following measurements/observations: 1. Color 2. Clarity 3. Odor 4. Volume 5. Mass 6. Density 7. ph 8. Any other observable characteristics Create a data table to organize your results here: Analysis: What do you think each substance is? Why? What other properties, either physical or chemical, would help you to make a determination? Page 3
16 Final Analysis: Day 2/Day 3 1. Describe and differentiate among the following: pure substances and mixtures, elements and compounds, elements and their isotopes, atomic number and atomic mass; protons, electrons, and neutrons; atoms vs. ions. 2. Which of the substances tested were pure substances? Which were mixtures? 3. Compare the conductivity of metals, nonmetals, semi-conductors, and ionic compounds. What role do electrons play in conductivity? 4. What is an alloy? What is their purpose/use? Which of the substances tested were alloys? What are some other common alloys? 5. One of the nails was galvanized steel? What is galvanization and why is it useful for metals? 6. What is the difference between a semi-conductor and a superconductor? Page 4
17 Physical Science Identifying Acids and Bases State of Ohio Indicator for Physical Science: Grade 9, Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral Background: Many common household products are either acids or bases. Today you will be utilizing litmus paper to determine if twelve common substances are acids, bases, or neutral. Materials: Baking powder, baking soda, vinegar, milk, bleach, dish detergent, soda, bubble solution, apple juice, antacids dissolved in water, ketchup, laundry detergent, litmus paper. Safety precaution: Please wear goggles and aprons to prevent any splashing. Although these are common household items, they can be eye irritants. Some items may stain clothing. Procedure: There are two solutions at six different stations around the lab. At each station you will dip one strip of red litmus paper and one strip of blue litmus paper into each solution and record the results. After you have completed your testing, please discard the used litmus papers into the provided containers. 1. Baking powder = 2. Baking soda = 3. Vinegar = 4. Milk = 5. Bleach = 6. Dish Detergent = 7. Soda = 8. Bubble Solution = 9. Apple Juice = 10. Antacids dissolved in water = 11. Ketchup = 12. Laundry detergent = Page 1
18 Analysis: 1. What is the difference between a strong acid or base and a weak acid or base? 2. What is litmus paper? 3. Can we determine if a substance is a strong or weak acid or base using litmus paper? 4. What are some properties of acids and bases? 5. Could any of these substances be conductors of electricity? Why? Page 2
19 Physical Science Measuring ph State of Ohio Indicator for Physical Science: Grade 9, Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral Background: Common household products that are acids or bases can be further tested to determine their exact ph. Today you will be utilizing ph paper to measure the ph of twelve common substances. Materials: Baking powder, baking soda, vinegar, milk, pineapple juice, dish detergent, soda, bubble solution, apple juice, antacids dissolved in water, ketchup, and laundry detergent. Safety precaution: Please wear goggles and aprons to prevent any splashing. Although these are common household items, they can be eye irritants. Some items may stain clothing. Procedure: There are two solutions at six different stations around the lab. At each station you will dip one strip of ph paper into each solution and record the results. After you have completed your testing, please discard the used ph papers into the provided containers. For each of the following substances, please write down the measured ph as well as the Molar Concentration for acids and neutral substances. For example, a ph of 7 would have a Molar Concentration of 1x10-7 M or mol/l. 1. Baking powder = 2. Baking soda = 3. Vinegar = 4. Milk = 5. Pineapple juice = 6. Dish detergent = 7. Soda = 8. Bubble solution = 9. Apple juice = 10. Antacids dissolved in water = 11. Ketchup = 12. Laundry detergent = Page 1
20 Analysis: 1. What is the ph scale? How are acids, bases and neutral substances numerically classified on this scale? 2. Would ph testing be a qualitative or quantitative test? Would litmus testing be a qualitative or quantitative test? Why? 3. When would a solution be neutral? How would the ion concentration be different in an acid or base? 4. If one acid has a ph of 1 and a second acid has a ph of 3, how much more acidic is the first substance? 5. If one base has a ph of 11 and a second base has a ph of 14, how much more alkaline is the second substance? 6. On a separate piece of paper draw a replica of the ph scale. Mark all twelve substances on this ph scale and label appropriately. Page 2
21 Physical Science Experimenting with Waves State of Ohio Indicator for Physical Science: Grade 9, Show how the properties of a wave depend on the properties of the medium through which it travels. Recognize that electromagnetic waves can be propagated without a medium. Background: You will be working in pairs to discover the characteristics and properties of waves. There are four different stations in this activity: One area utilizes rope to create waves, a second area uses Slinky toys, a third area involves dropping tennis balls into water, and the final station allows you to experiment with tuning forks. Carefully read the directions for each activity and record all of your observations on a separate piece of paper. You will only submit one set of observations and one set of answers to the analysis questions per pair. Materials: Ropes, ribbon, Slinkys, tennis balls, tuning forks, and a plastic tub filled with water. Safety Precautions: None Procedure: Station 1: Ropes Tie a small piece of ribbon in the center of the rope. Each partner will grab one end of the rope and create a wave pattern. Which direction does the ribbon move in relation to the wave? If you move the rope more quickly, do the waves get smaller, larger, or stay the same size? If you move the rope more slowly is there any change in appearance? What other observations can you make? Station 2: Slinky Tie a small piece of ribbon in the center of the Slinky. Each partner will grab one end of the Slinky and create compressions and rarefactions. Do not stretch the Slinky out of shape!! Which direction does the ribbon move in relation to the wave? What other observations can you make? Page 1
22 Station 3: Tennis Balls Drop a tennis ball into the water and observe the waves. In which direction do the waves travel? If you drop the ball from a higher point or a lower point does it change the waves in any way? Try placing tiny pieces of paper in the water before the ball is dropped. Is it easier to visualize the direction of the wave? What other observations can you make? Station 4: Tuning Forks Strike the tuning fork and observe the vibrations. What type of sound is produced when striking the fork more forcefully? By striking the fork more gently? What happens to the sound as you move the fork away from your body? What happens to the sound if your partner strikes the fork and moves toward or away from you? Observe what happens if you strike the fork and gently touch the top of the water. Try placing tiny pieces of paper in the water and then strike the fork and touch the water. What other observations can you make? Analysis: 1. What types of waves require a medium? 2. What is a medium? 3. What types of waves do not require a medium? What are some examples of this? 4. What type of wave is created by the rope transverse, longitudinal, or surface waves? Why? Page 2
23 5. What type of wave is created by the slinky transverse, longitudinal, or surface waves? Why? 6. What type of wave is created by the tennis ball transverse, longitudinal, or surface waves? Why? 7. What type of wave is created by the tuning fork on water transverse, longitudinal, or surface waves? Why? 8. How are vibrations and waves related? Page 3
24 Physical Science Understanding Motion State of Ohio Indicator for Physical Science: Grade 9, Demonstrate that motion is a measurable quantity that depends on the observer s frame of reference and describe the object s motion in terms of position, velocity, acceleration, and time. Background: We will utilize toy cars and ramps to demonstrate that motion is a measurable quantity and calculate the velocity and acceleration of each car. Materials: Matchbox or Hot Wheels toy cars, ramps (use wood, plywood, metal or cardboard to create), meter stick, stopwatch. Make sure each ramp is equal in length and is placed at the same height. Safety Precaution: None Procedure and Analysis: At each lab station are two different models of toy cars. One ramp has been placed between each lab station, for a total of six ramps. Each one of you will examine the cars at your station and determine which of the two cars you believe will have the fastest overall velocity. You will measure the time it takes for each of the cars to travel down the ramp and from this calculate the velocity and acceleration. You will perform all testing in groups of 4, but you will submit lab reports as pairs. 1. State your hypothesis (which car will be the fastest) and explain why. 2. What is the independent variable in this experiment (what is manipulated)? 3. What is the dependent variable in this experiment (what is measured)? 4. What is a reference point? What is the reference point for the motion you are measuring? Page 1
25 5. Each car at your station (total of 2) will have three time trials to determine its velocity. Create a data table to organize this information here: 6. What is velocity? How is it different than speed? Calculate the average velocity for both of the cars at your station. Hint: Distance should be calculated in meters. Include the correct units! 7. What is acceleration? Assuming the velocity you calculated for each car in step 6 is the final velocity, what would the average acceleration be in.5 seconds? Remember to use the correct units! Page 2
26 Physical Science Understanding Forces State of Ohio Indicators for Physical Science: Grade Demonstrate that any object does not accelerate (remains at rest or maintains a constant speed and direction of motion) unless an unbalanced (net) force acts on it. 2. Explain the change in motion (acceleration) of an object. Demonstrate that the acceleration is proportional to the net force acting on the object and inversely proportional to the mass of the object. (F = ma. Note that weight is the gravitational force on a mass). 3. Demonstrate that whenever one object exerts a force on another, an equal amount of force is exerted back on the first object. 4. Demonstrate the ways in which frictional forces constrain the motion of objects (e.g. a car traveling around a curve, a block on an inclined plane, a person running, and airplane in flight). Background: Newton s Three Laws of Motion explain the relationship between forces and motion. In this lab activity we will observe the affect force has on the motion of an object (a toy car). Materials: Matchbox or Hot Wheels Toy Cars, a section of vinyl flooring, a section of carpet, stopwatch, and a meter stick. Safety Precautions: None. Procedure and Analysis: 1. What is Newton s First Law of Motion? In the previous lab experiment involving cars and ramps, what force(s) initially acted on the cars to create movement? 2. If an outside force did not act on the cars at the top of the ramp, what would happen? If additional force were applied to the cars at the top of the ramp, what would happen? Page 1
27 3. What is inertia? Why did the cars stop moving after leaving the ramp? What might happen if the cars masses were significantly greater? 4. What is Newton s Second Law of Motion? 5. Select one car from your lab station. Propel the car across a one-meter section of vinyl flooring, recording the time it takes to travel this distance. Using the equation, Force = Mass x Acceleration, calculate the force necessary to propel the car across the floor. (Hints: Assume the calculated velocity is your final velocity. Find the acceleration in 0.5 seconds. Mass should be in kg). Report the correct units! 6. What is friction? Propel the car across a one-meter section of the carpeted flooring in the same manner as you did in step #5. Record the time it takes to travel this distance, and calculate the force necessary to propel the car across the floor. How do the calculated forces compare between vinyl and carpet? Would you expect to see a difference in the force? Why? 7. How much more force will an object exert if its mass is doubled and its acceleration stays the same? If the force remains constant, what will happen to the acceleration if the mass doubles? 8. What is Newton s Third Law of Motion? Page 2
28 9. If an object exerts 200 N of force on the floor, how much force does the floor exert on the object? Why? 10. All moving objects have momentum. What is momentum? How does mass affect momentum? 11. What is the Law of Conservation of Momentum? Page 3
29 Biology Lab Activities For Grade 10 Index Lab 1: Creating A Cell Model Lab 2: Osmosis and Diffusion Lab 3: Creating A DNA Model Lab 4: Modeling RNA Transcription Lab 5: Hardy-Weinberg Equilibrium and Evolutionary Mechanisms
30 Biology Creating a Cell Model State of Ohio Indicator for Life Science: Grade 10, Compare the structure, function and interrelatedness of cell organelles in eukaryotic cells (e.g., nucleus, chromosome, mitochondria, cell membrane, cell wall, chloroplast, cilia, flagella) and prokaryotic cells. Background: Eukaryotic cells contain a nucleus and membrane-bound organelles that are necessary for the maintenance and survival of the cell. Prokaryotic cells do not contain a nucleus or membrane-bound organelles. In today s lab activity you will create a model and sketch of a eukaryotic cell and label the appropriate parts. Materials: Plastic bags, vegetable oil, beans, assorted pasta shapes (mini-lasagna noodles, elbow macaroni, corkscrew pasta), assorted candy (Mike n Ikes, jelly beans, caramel nips, licorice, etc.). Safety Precautions: None. Procedure: Create a cell model using the supplies available. Keep in mind the shape and purpose of each organelle as you select from the materials. Each of the following organelles must be represented in your model: 1. Nucleus 2. Mitochondria 3. Ribosomes 4. Golgi Apparatus 5. Endoplasmic Reticulum 6. Cytoskeleton components (microtubules) 7. Plasma Membrane 8. Cytoplasm After creating the cell model, draw a sketch on the provided 11x14 sheet of paper. Identify each of the organelles in your model (as well as the food item that represents it) and explain their purpose and function in the cell. Page 1
31 Analysis: 1. How could you differentiate between a eukaryotic cell and a prokaryotic cell with an electron microscope? 2. What are some examples of eukaryotic cells? What is an example of a prokaryotic cell? 3. How are organelles interrelated in prokaryotic and eukaryotic cells? 4. What three structures may be present in a plant cell but not in animal cells? Why? 5. What is the purpose of cilia and flagella in cells? Page 2
32 Biology Osmosis and Diffusion State of Ohio Indicator for Life Science: Grade 10, Explain the characteristics of life as indicated by cellular processes including a. homeostasis b. energy transfers and transformations c. transportation of molecules d. disposal of wastes e. synthesis of new molecules Background: Osmosis is the movement of water from an area of higher concentration to an area of lower concentration through a selectively permeable membrane. Diffusion is the movement of a substance from an area of higher concentration to an area of lower concentration (down a concentration gradient). Both processes occur in living cells to help maintain equilibrium. Materials: Potatoes, eggs, vinegar, corn syrup, sugar, salt, water. Safety: Students must wear goggles and aprons to guard against splashing. Procedure: In Groups of 4 Day 1 1. Prepare a 50% sucrose solution and a 50% salt solution using water as your solvent in two separate beakers. Also fill a third beaker with water only. 2. Obtain slices of potato from Mrs. Rettig. Immediately measure the mass of each potato slice and note the texture of the potato. Is it firm or soft? Place each potato slice in one of the solutions. Allow it to sit for 30 minutes undisturbed. 3. Meanwhile, place a raw egg in a beaker and cover completely with vinegar. Observe what happens. What do you think will occur in the next 24 hours? Leave the egg, undisturbed, in the vinegar solution. Page 1
33 4. After 30 minutes, record any changes to your potato slices. Measure the masses and note any changes to texture. Empty the solutions and clean up the lab area. Day 2 1. Check the egg. Note if any shell remains. If shell is still present, keep in vinegar for another 24 hours. Skip to Day 3 instructions. 2. If the shell has dissolved, remove the egg and empty the vinegar solution. What does the egg look like? Why does it have this appearance? 3. Each of the lab groups will be assigned a solution. Some will place their eggs in water and others will place their eggs in corn syrup. Leave them, undisturbed, for 24 hours. What do you think will happen? Day 3 1. If the shell has now dissolved, follow the directions for Day 2, steps 2 and If the egg was already immersed in water or corn syrup, remove the egg and note any changes to its appearance. What do you think happened? Why? Analysis: 1. Are osmosis and diffusion active or passive transport? Why? 2. At what point will equilibrium be achieved? 3. Will the molecules stop moving once equilibrium is achieved? Page 2
34 4. Why use eggs and potatoes to demonstrate this process? 5. If we used human red blood cells and placed them in a 50% sucrose solution, a 50% salt solution, and pure water what would happen in each instance? Page 3
35 Biology Creating a DNA Model State of Ohio Indicators for Life Science: Grade 10, Illustrate the relationship of the structure and function of DNA to protein synthesis and the characteristics of an organism. Background: DNA contains the information needed to synthesize proteins and create living things. In today s lab activity you will create a 3-Dimensional model of DNA that will be used to demonstrate Transcription in a future lab activity. Materials: Wire, gumdrops, toothpicks, tape. Safety Precautions: Wires and toothpicks are sharp. Please point away from your body. Procedure: 1. Select four different gumdrop colors to represent the four nitrogenous bases of DNA. Write down your color choices with the name of each corresponding nitrogenous base on a separate piece of paper. 2. Using the following DNA sequence, assemble your template DNA strand using wire and threading the gumdrops in the appropriate order. Label this strand as the template with a piece of tape. GGTAATTGACTT 3. Using the second piece of wire, assemble the complementary DNA sequence using the appropriate base pairing. 4. Connect the strands by placing toothpicks between each gumdrop base pair. Keep in mind that Adenine and Thymine are connected by a double hydrogen bond and Cytosine and Guanine are connected by a triple hydrogen bond. 5. Twist the two strands into the appropriate shape to mimic a DNA molecule. 6. Draw a sketch of this structure on the same piece of paper that you listed gumdrop colors. 7. The DNA model will remain in the lab and be utilized for a future lab involving RNA Transcription. Page 1
36 Analysis: 1. What are the four nitrogenous bases of DNA? 2. What are the complementary base pairs for these nitrogenous bases? 3. What does the wire represent in relation to the actual DNA molecule? 4. What do the gumdrops and toothpicks represent? 5. Which of the nitrogenous bases are purines and which are pyramidines? 6. What scientists discovered the structure of DNA? What is the structure? 7. What is a nucleotide? Page 2
37 Biology Modeling RNA Transcription State of Ohio Indicators for Life Science: Grade 10, Illustrate the relationship of the structure and function of DNA to protein synthesis and the characteristics of an organism. Background: DNA contains the information needed to synthesize proteins and create living things. RNA Transcription is necessary to take the information from DNA out of the nucleus to the ribosomes for protein synthesis. Materials: Your DNA model from previous lab, gumdrops, toothpicks, wire. Safety Precaution: Wires and toothpicks are sharp. Point away from your body. Procedure: 1. Using your original DNA model, unzip the strands. 2. Create the corresponding RNA strand using your original DNA template strand. Write down your color choices for each nitrogenous base. 3. Connect the nitrogenous base pairs using toothpicks. Hydrogen bonds will remain the same. 4. Label the RNA strand with a piece of tape. Analysis: 1. What are the four nitrogenous bases of RNA? 2. What are the complementary base pairs for these nitrogenous bases? 3. What enzyme bonds to the DNA molecule to allow transcription to begin? 4. What is the name of the sequence to which this enzyme binds? Page 1
38 5. What will happen to the DNA and RNA molecules at the end of transcription? 6. What are the three types of RNA? What purpose does each serve? 7. What will happen during the process of Translation? 8. Utilizing the Amino Acid/ RNA Codon chart given, what amino acids would this strand of RNA code for? 9. What are the structural differences between DNA and RNA? Page 2
39 AMINO ACID ALANINE ARGININE ASPARAGINE ASPARTIC ACID CYSTEINE GLUTAMIC ACID GLUTAMINE GLYCINE HISTIDINE ISOLEUCINE LEUCINE LYCINE METHIONINE (INITIATION) PHENYLALANINE PROLINE SERINE THREONINE TRYPTOPHAN TYROSINE VALINE STOP RNA CODON GCC, GCA, GCG, GCU AGA, AGG, CGU, CGA, CGC, CGG AAC, AAU GAC, GAU UGC, UGU GAA, GAG CAA, CAG GGA, GGC, GGG, GGU CAC, CAU AUA, AUC, AUU UUA, UUG, CUA, CUC, CUG, CUU AAA, AAG AUG UUC, UUU CCA, CCC, CCG, CCU UCA, UCC, UCG, UCU, AGC, AGU ACA, ACC, ACG, ACU UGG UAC, UAU GUA, GUC, GUG, GUU UAA, UAG, UGA
40 Biology Hardy-Weinberg Equilibrium and Evolutionary Mechanisms State of Ohio Indicators for Life Science: Grade 10, Analyze how evolutionary mechanisms (e.g. genetic drift, immigration, emigration, mutation) and their consequences provide a scientific explanation for the diversity and unity of past life forms, as depicted in the fossil record, and present life forms. Background: You will be utilizing Teddy Grahams to determine gene frequencies within a population for two specific circumstances: First, if Hardy-Weinberg Genetic Equilibrium is observed; and second, if evolutionary mechanisms such as emigration, immigration, mutation and genetic drift had taken place. You may work in pairs for this activity. Two types of bears are present in the Teddy Graham population. The first type is Happy Bear, who holds his hands up in the air. The second type is Sad Bear, who keeps his hands by his side. Happy Bear is homozygous dominant for the happy gene, Sad Bear is homozygous recessive for the happy gene Materials: Teddy Grahams Safety Precautions: None. Procedure: Activity One: Record all results on a separate piece of paper. You receive a randomly selected population of twenty Teddy Grahams. Calculate what percentage of your population is composed of Happy Bears. Calculate what percentage is composed of Sad Bears. Assuming this population follows the rules of Hardy- Weinberg Genetic Equilibrium, how might you determine the percentage of Happy Bears and Sad Bears found in generation two? What would the gene frequencies be for these generation two offspring? Activity Two: Record all results on a separate piece of paper. Using the same randomly selected population of twenty Teddy Grahams, once again record the percentage of Happy Bears and Sad Bears. This population of bears is now subjected to a variety of evolutionary mechanisms: Page 1
41 1. Happy Bears taste sweet and are easy to catch. Sad Bears taste bitter and are sneaky and difficult to catch. Half of your Happy Bears are consumed by a hungry predator. 2. Happy Bears are very threatened by this new predator, so half of the remaining Happy Bears emigrate to a new community. 3. Sad Bears love to mate with other Sad Bears, so they immigrate to your population and take the place of all the Happy Bears. 4. Now, record the percentage of Happy Bears and Sad Bears within your population. Assuming that these bears practice non-random mating, so that Happy Bears only mate with Happy Bears and Sad Bears mate with other Sad Bears, what would the percentage of each type be in generation two? What would the gene frequencies be for these generation two offspring? Analysis: 1. Define the following terms: Immigration, emigration, natural selection, genetic drift, and mutation. 2. What affect do the above mechanisms have on gene frequencies in a population? 3. How did immigration, emigration, natural selection, and non-random mating affect the Happy Bear population? 4. If all of these circumstances continued, what might happen to the Happy Bear population in this geographical area? 5. Would the population of Sad Bears remain consistent throughout time? Why? Page 2
42 6. In order for Hardy-Weinberg Genetic Equilibrium to occur what must happen? Does this occur often? 7. How does natural selection and evolutionary mechanisms such as immigration and mutation provide an explanation for the diversity of life? Page 3
43
Gene Finding CMSC 423
 Gene Finding CMSC 423 Finding Signals in DNA We just have a long string of A, C, G, Ts. How can we find the signals encoded in it? Suppose you encountered a language you didn t know. How would you decipher
Gene Finding CMSC 423 Finding Signals in DNA We just have a long string of A, C, G, Ts. How can we find the signals encoded in it? Suppose you encountered a language you didn t know. How would you decipher
Molecular Facts and Figures
 Nucleic Acids Molecular Facts and Figures DNA/RNA bases: DNA and RNA are composed of four bases each. In DNA the four are Adenine (A), Thymidine (T), Cytosine (C), and Guanine (G). In RNA the four are
Nucleic Acids Molecular Facts and Figures DNA/RNA bases: DNA and RNA are composed of four bases each. In DNA the four are Adenine (A), Thymidine (T), Cytosine (C), and Guanine (G). In RNA the four are
Hands on Simulation of Mutation
 Hands on Simulation of Mutation Charlotte K. Omoto P.O. Box 644236 Washington State University Pullman, WA 99164-4236 omoto@wsu.edu ABSTRACT This exercise is a hands-on simulation of mutations and their
Hands on Simulation of Mutation Charlotte K. Omoto P.O. Box 644236 Washington State University Pullman, WA 99164-4236 omoto@wsu.edu ABSTRACT This exercise is a hands-on simulation of mutations and their
Mutation. Mutation provides raw material to evolution. Different kinds of mutations have different effects
 Mutation Mutation provides raw material to evolution Different kinds of mutations have different effects Mutational Processes Point mutation single nucleotide changes coding changes (missense mutations)
Mutation Mutation provides raw material to evolution Different kinds of mutations have different effects Mutational Processes Point mutation single nucleotide changes coding changes (missense mutations)
(http://genomes.urv.es/caical) TUTORIAL. (July 2006)
 (http://genomes.urv.es/caical) TUTORIAL (July 2006) CAIcal manual 2 Table of contents Introduction... 3 Required inputs... 5 SECTION A Calculation of parameters... 8 SECTION B CAI calculation for FASTA
(http://genomes.urv.es/caical) TUTORIAL (July 2006) CAIcal manual 2 Table of contents Introduction... 3 Required inputs... 5 SECTION A Calculation of parameters... 8 SECTION B CAI calculation for FASTA
Protein Synthesis Simulation
 Protein Synthesis Simulation Name(s) Date Period Benchmark: SC.912.L.16.5 as AA: Explain the basic processes of transcription and translation, and how they result in the expression of genes. (Assessed
Protein Synthesis Simulation Name(s) Date Period Benchmark: SC.912.L.16.5 as AA: Explain the basic processes of transcription and translation, and how they result in the expression of genes. (Assessed
PRACTICE TEST QUESTIONS
 PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
Coding sequence the sequence of nucleotide bases on the DNA that are transcribed into RNA which are in turn translated into protein
 Assignment 3 Michele Owens Vocabulary Gene: A sequence of DNA that instructs a cell to produce a particular protein Promoter a control sequence near the start of a gene Coding sequence the sequence of
Assignment 3 Michele Owens Vocabulary Gene: A sequence of DNA that instructs a cell to produce a particular protein Promoter a control sequence near the start of a gene Coding sequence the sequence of
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
Provincial Exam Questions. 9. Give one role of each of the following nucleic acids in the production of an enzyme.
 Provincial Exam Questions Unit: Cell Biology: Protein Synthesis (B7 & B8) 2010 Jan 3. Describe the process of translation. (4 marks) 2009 Sample 8. What is the role of ribosomes in protein synthesis? A.
Provincial Exam Questions Unit: Cell Biology: Protein Synthesis (B7 & B8) 2010 Jan 3. Describe the process of translation. (4 marks) 2009 Sample 8. What is the role of ribosomes in protein synthesis? A.
http://www.life.umd.edu/grad/mlfsc/ DNA Bracelets
 http://www.life.umd.edu/grad/mlfsc/ DNA Bracelets by Louise Brown Jasko John Anthony Campbell Jack Dennis Cassidy Michael Nickelsburg Stephen Prentis Rohm Objectives: 1) Using plastic beads, construct
http://www.life.umd.edu/grad/mlfsc/ DNA Bracelets by Louise Brown Jasko John Anthony Campbell Jack Dennis Cassidy Michael Nickelsburg Stephen Prentis Rohm Objectives: 1) Using plastic beads, construct
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
a. Ribosomal RNA rrna a type ofrna that combines with proteins to form Ribosomes on which polypeptide chains of proteins are assembled
 Biology 101 Chapter 14 Name: Fill-in-the-Blanks Which base follows the next in a strand of DNA is referred to. as the base (1) Sequence. The region of DNA that calls for the assembly of specific amino
Biology 101 Chapter 14 Name: Fill-in-the-Blanks Which base follows the next in a strand of DNA is referred to. as the base (1) Sequence. The region of DNA that calls for the assembly of specific amino
Animal & Plant Cell Slides
 Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
Animal & Plant Cell Slides Category: Biology Type: Class Experiment, 60 min class Materials: 2 Glass Slides 2 Cover Slips 1 Bottle of methylene blue (optional) 1 Plastic tray 1 Bottle of iodine 1 Plastic
ISTEP+: Biology I End-of-Course Assessment Released Items and Scoring Notes
 ISTEP+: Biology I End-of-Course Assessment Released Items and Scoring Notes Page 1 of 22 Introduction Indiana students enrolled in Biology I participated in the ISTEP+: Biology I Graduation Examination
ISTEP+: Biology I End-of-Course Assessment Released Items and Scoring Notes Page 1 of 22 Introduction Indiana students enrolled in Biology I participated in the ISTEP+: Biology I Graduation Examination
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Physical and Chemical Changes
 Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 jbarrow@dsdmail.net Eighth Grade Integrated Science Standard I: Students will understand the
Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 jbarrow@dsdmail.net Eighth Grade Integrated Science Standard I: Students will understand the
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Protein Synthesis. Page 41 Page 44 Page 47 Page 42 Page 45 Page 48 Page 43 Page 46 Page 49. Page 41. DNA RNA Protein. Vocabulary
 Protein Synthesis Vocabulary Transcription Translation Translocation Chromosomal mutation Deoxyribonucleic acid Frame shift mutation Gene expression Mutation Point mutation Page 41 Page 41 Page 44 Page
Protein Synthesis Vocabulary Transcription Translation Translocation Chromosomal mutation Deoxyribonucleic acid Frame shift mutation Gene expression Mutation Point mutation Page 41 Page 41 Page 44 Page
Transcription and Translation of DNA
 Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz
 OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
OSMOSIS AND DIALYSIS 2003 BY Wendy Weeks-Galindo with modifications by David A. Katz OSMOSIS Osmosis is the reason that a fresh water fish placed in the ocean desiccates and dies. Osmosis is the reason
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
From DNA to Protein. Proteins. Chapter 13. Prokaryotes and Eukaryotes. The Path From Genes to Proteins. All proteins consist of polypeptide chains
 Proteins From DNA to Protein Chapter 13 All proteins consist of polypeptide chains A linear sequence of amino acids Each chain corresponds to the nucleotide base sequence of a gene The Path From Genes
Proteins From DNA to Protein Chapter 13 All proteins consist of polypeptide chains A linear sequence of amino acids Each chain corresponds to the nucleotide base sequence of a gene The Path From Genes
Insulin mrna to Protein Kit
 Insulin mrna to Protein Kit A 3DMD Paper BioInformatics and Mini-Toober Folding Activity Teacher Key and Teacher Notes www. Insulin mrna to Protein Kit Contents Becoming Familiar with the Data... 3 Identifying
Insulin mrna to Protein Kit A 3DMD Paper BioInformatics and Mini-Toober Folding Activity Teacher Key and Teacher Notes www. Insulin mrna to Protein Kit Contents Becoming Familiar with the Data... 3 Identifying
Cell and Membrane Practice. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
Name: ate: 1. Which structure is outside the nucleus of a cell and contains N?. chromosome. gene. mitochondrion. vacuole 2. potato core was placed in a beaker of water as shown in the figure below. Which
13.2 Ribosomes & Protein Synthesis
 13.2 Ribosomes & Protein Synthesis Introduction: *A specific sequence of bases in DNA carries the directions for forming a polypeptide, a chain of amino acids (there are 20 different types of amino acid).
13.2 Ribosomes & Protein Synthesis Introduction: *A specific sequence of bases in DNA carries the directions for forming a polypeptide, a chain of amino acids (there are 20 different types of amino acid).
Letter to the Student... 5 Test-Taking Checklist... 6 Next Generation Sunshine State Standards Correlation Chart... 7
 Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism )
 Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Hiding Data in DNA. 1 Introduction
 Hiding Data in DNA Boris Shimanovsky *, Jessica Feng +, and Miodrag Potkonjak + * XAP Corporation + Dept. Computer Science, Univ. of California, Los Angeles Abstract. Just like disk or RAM, DNA and RNA
Hiding Data in DNA Boris Shimanovsky *, Jessica Feng +, and Miodrag Potkonjak + * XAP Corporation + Dept. Computer Science, Univ. of California, Los Angeles Abstract. Just like disk or RAM, DNA and RNA
Cellular Structure and Function
 Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
Chapter Test A CHAPTER 7 Cellular Structure and Function Part A: Multiple Choice In the space at the left, write the letter of the term or phrase that best answers each question. 1. Which defines a cell?
Concluding lesson. Student manual. What kind of protein are you? (Basic)
 Concluding lesson Student manual What kind of protein are you? (Basic) Part 1 The hereditary material of an organism is stored in a coded way on the DNA. This code consists of four different nucleotides:
Concluding lesson Student manual What kind of protein are you? (Basic) Part 1 The hereditary material of an organism is stored in a coded way on the DNA. This code consists of four different nucleotides:
RNA and Protein Synthesis
 Name lass Date RN and Protein Synthesis Information and Heredity Q: How does information fl ow from DN to RN to direct the synthesis of proteins? 13.1 What is RN? WHT I KNOW SMPLE NSWER: RN is a nucleic
Name lass Date RN and Protein Synthesis Information and Heredity Q: How does information fl ow from DN to RN to direct the synthesis of proteins? 13.1 What is RN? WHT I KNOW SMPLE NSWER: RN is a nucleic
Energy and Energy Transformations Test Review
 Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
UNIT (12) MOLECULES OF LIFE: NUCLEIC ACIDS
 UIT (12) MLECULE F LIFE: UCLEIC ACID ucleic acids are extremely large molecules that were first isolated from the nuclei of cells. Two kinds of nucleic acids are found in cells: RA (ribonucleic acid) is
UIT (12) MLECULE F LIFE: UCLEIC ACID ucleic acids are extremely large molecules that were first isolated from the nuclei of cells. Two kinds of nucleic acids are found in cells: RA (ribonucleic acid) is
Thymine = orange Adenine = dark green Guanine = purple Cytosine = yellow Uracil = brown
 1 DNA Coloring - Transcription & Translation Transcription RNA, Ribonucleic Acid is very similar to DNA. RNA normally exists as a single strand (and not the double stranded double helix of DNA). It contains
1 DNA Coloring - Transcription & Translation Transcription RNA, Ribonucleic Acid is very similar to DNA. RNA normally exists as a single strand (and not the double stranded double helix of DNA). It contains
DNA, RNA, Protein synthesis, and Mutations. Chapters 12-13.3
 DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
Acids, Bases, and ph
 CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
One Stop Shop For Teachers
 Physical Science Curriculum The Georgia Performance Standards are designed to provide students with the knowledge and skills for proficiency in science. The Project 2061 s Benchmarks for Science Literacy
Physical Science Curriculum The Georgia Performance Standards are designed to provide students with the knowledge and skills for proficiency in science. The Project 2061 s Benchmarks for Science Literacy
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
Molecular Genetics. RNA, Transcription, & Protein Synthesis
 Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
called a cell wall. The cell wall protects against mechanical stress and keeps the cell from becoming over-filled with water.
 What are Cells? By: Byron Norelius About Cells A cell is the basic unit of life. All living organisms are composed of one (unicellular) or more (multicellular) cells. In unicellular organisms, like many
What are Cells? By: Byron Norelius About Cells A cell is the basic unit of life. All living organisms are composed of one (unicellular) or more (multicellular) cells. In unicellular organisms, like many
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
The Acid Test Grade Nine
 Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
tissues are made of cells that work together, organs are )
 Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
Study Guide Cells Unit Test Matching. Write the letter of the correct response on the line. You may use the responses more than once. A. proteins B. simple carbohydrates C. complex carbohydrates D. lipids
PLANT AND ANIMAL CELL ORGANELLES
 reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
reflect The heart is an example of an organ. Think for a minute about your body. It s organized into parts that perform specific functions. For example, your heart functions to help transport materials
Unit I: Introduction To Scientific Processes
 Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Unit I: Introduction To Scientific Processes This unit is an introduction to the scientific process. This unit consists of a laboratory exercise where students go through the QPOE2 process step by step
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
The Cell Grade Ten. Estimated Duration: Three hours
 Ohio Standards Connection: Life Sciences Benchmark A Explain that cells are the basic unit of structure and function of living organisms, that once life originated all cells come from pre-existing cells,
Ohio Standards Connection: Life Sciences Benchmark A Explain that cells are the basic unit of structure and function of living organisms, that once life originated all cells come from pre-existing cells,
5s Solubility & Conductivity
 5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS
 DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS Have you ever wondered why your fingers have wrinkles after soaking in a bath tub? Your students have probably wondered the
DIFFUSION (HYPERTONIC, HYPOTONIC, & ISOTONIC SOLUTIONS) THE GUMMY BEAR LAB PASS Have you ever wondered why your fingers have wrinkles after soaking in a bath tub? Your students have probably wondered the
Complete tests for CO 2 and H 2 Link observations of acid reactions to species
 Acids and Bases 1. Name common acids and bases found at home and at school 2. Use formulae for common acids and bases 3. Give examples of the uses of acids and bases 4. State that all solutions are acidic,
Acids and Bases 1. Name common acids and bases found at home and at school 2. Use formulae for common acids and bases 3. Give examples of the uses of acids and bases 4. State that all solutions are acidic,
Cell Structure & Function!
 Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
From DNA to Protein
 Nucleus Control center of the cell contains the genetic library encoded in the sequences of nucleotides in molecules of DNA code for the amino acid sequences of all proteins determines which specific proteins
Nucleus Control center of the cell contains the genetic library encoded in the sequences of nucleotides in molecules of DNA code for the amino acid sequences of all proteins determines which specific proteins
Given these characteristics of life, which of the following objects is considered a living organism? W. X. Y. Z.
 Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
Cell Structure and Organization 1. All living things must possess certain characteristics. They are all composed of one or more cells. They can grow, reproduce, and pass their genes on to their offspring.
Sugar or Salt? Ionic and Covalent Bonds
 Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Household Acids and Bases
 Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Biological One-way Functions
 Biological One-way Functions Qinghai Gao, Xiaowen Zhang 2, Michael Anshel 3 gaoj@farmingdale.edu zhangx@mail.csi.cuny.edu csmma@cs.ccny.cuny.edu Dept. Security System, Farmingdale State College / SUNY,
Biological One-way Functions Qinghai Gao, Xiaowen Zhang 2, Michael Anshel 3 gaoj@farmingdale.edu zhangx@mail.csi.cuny.edu csmma@cs.ccny.cuny.edu Dept. Security System, Farmingdale State College / SUNY,
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students
 Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students Activity Title: Quick Hit Goal of Activity: To perform formative and summative assessments
Quick Hit Activity Using UIL Science Contests For Formative and Summative Assessments of Pre-AP and AP Biology Students Activity Title: Quick Hit Goal of Activity: To perform formative and summative assessments
Date: Student Name: Teacher Name: Jared George. Score: 1) A cell with 1% solute concentration is placed in a beaker with a 5% solute concentration.
 Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Biology Keystone (PA Core) Quiz Homeostasis and Transport - (BIO.A.4.1.1 ) Plasma Membrane, (BIO.A.4.1.2 ) Transport Mechanisms, (BIO.A.4.1.3 ) Transport Facilitation Student Name: Teacher Name: Jared
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
The Steps. 1. Transcription. 2. Transferal. 3. Translation
 Protein Synthesis Protein synthesis is simply the "making of proteins." Although the term itself is easy to understand, the multiple steps that a cell in a plant or animal must go through are not. In order
Protein Synthesis Protein synthesis is simply the "making of proteins." Although the term itself is easy to understand, the multiple steps that a cell in a plant or animal must go through are not. In order
Plant and Animal Cells
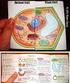 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Properties of Acids and Bases
 Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Module 3: Strawberry DNA Extraction
 Module 3: Strawberry DNA Extraction Teacher/Leader Target Audience: 7-12 Life Science, Biology, Ag Science Overview: In this lab, students will extract DNA from a strawberry using everyday materials and
Module 3: Strawberry DNA Extraction Teacher/Leader Target Audience: 7-12 Life Science, Biology, Ag Science Overview: In this lab, students will extract DNA from a strawberry using everyday materials and
Plant and Animal Cells
 Plant and Animal Cells Strand Topic Life Systems Investigating organelles and their functions in cells of living things Primary SOL LS.2 The student will investigate and understand that all living things
Plant and Animal Cells Strand Topic Life Systems Investigating organelles and their functions in cells of living things Primary SOL LS.2 The student will investigate and understand that all living things
Pipe Cleaner Proteins. Essential question: How does the structure of proteins relate to their function in the cell?
 Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
MCAS Biology. Review Packet
 MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
MCAS Biology Review Packet 1 Name Class Date 1. Define organic. THE CHEMISTRY OF LIFE 2. All living things are made up of 6 essential elements: SPONCH. Name the six elements of life. S N P C O H 3. Elements
Lab # 12: DNA and RNA
 115 116 Concepts to be explored: Structure of DNA Nucleotides Amino Acids Proteins Genetic Code Mutation RNA Transcription to RNA Translation to a Protein Figure 12. 1: DNA double helix Introduction Long
115 116 Concepts to be explored: Structure of DNA Nucleotides Amino Acids Proteins Genetic Code Mutation RNA Transcription to RNA Translation to a Protein Figure 12. 1: DNA double helix Introduction Long
Film Canister ROCKETS. An activity of reaction rates and the scientific method
 Film Canister ROCKETS An activity of reaction rates and the scientific method Developed by: Elisabeth Mills, UCLA NSF GK-12 Fellow Title of Lesson: Film Canister Rockets Grade level: 8 th Grade Subject(s):
Film Canister ROCKETS An activity of reaction rates and the scientific method Developed by: Elisabeth Mills, UCLA NSF GK-12 Fellow Title of Lesson: Film Canister Rockets Grade level: 8 th Grade Subject(s):
7.2 Cell Structure. Lesson Objectives. Lesson Summary. Cell Organization Eukaryotic cells contain a nucleus and many specialized structures.
 7.2 Cell Structure Lesson Objectives Describe the structure and function of the cell nucleus. Describe the role of vacuoles, lysosomes, and the cytoskeleton. Identify the role of ribosomes, endoplasmic
7.2 Cell Structure Lesson Objectives Describe the structure and function of the cell nucleus. Describe the role of vacuoles, lysosomes, and the cytoskeleton. Identify the role of ribosomes, endoplasmic
DNA Paper Model Activity Level: Grade 6-8
 Karen Mayes DNA Paper Model Activity Level: Grade 6-8 Students will be able to: 1. Identify the component molecules of DNA. 2. Construct a model of the DNA double-helix. 3. Identify which bases are found
Karen Mayes DNA Paper Model Activity Level: Grade 6-8 Students will be able to: 1. Identify the component molecules of DNA. 2. Construct a model of the DNA double-helix. 3. Identify which bases are found
Teacher Guide: Have Your DNA and Eat It Too ACTIVITY OVERVIEW. http://gslc.genetics.utah.edu
 ACTIVITY OVERVIEW Abstract: Students build an edible model of DNA while learning basic DNA structure and the rules of base pairing. Module: The Basics and Beyond Prior Knowledge Needed: DNA contains heritable
ACTIVITY OVERVIEW Abstract: Students build an edible model of DNA while learning basic DNA structure and the rules of base pairing. Module: The Basics and Beyond Prior Knowledge Needed: DNA contains heritable
Modeling DNA Replication and Protein Synthesis
 Skills Practice Lab Modeling DNA Replication and Protein Synthesis OBJECTIVES Construct and analyze a model of DNA. Use a model to simulate the process of replication. Use a model to simulate the process
Skills Practice Lab Modeling DNA Replication and Protein Synthesis OBJECTIVES Construct and analyze a model of DNA. Use a model to simulate the process of replication. Use a model to simulate the process
High School Science Course Correlations between Ohio s 2010 Course Syllabi and the First Draft of the High School NGSS
 High School Science Course Correlations between Ohio s 2010 Course Syllabi and the First Draft of the High School NGSS This document correlates the content in Ohio s course syllabi with the performance
High School Science Course Correlations between Ohio s 2010 Course Syllabi and the First Draft of the High School NGSS This document correlates the content in Ohio s course syllabi with the performance
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
DNA Replication & Protein Synthesis. This isn t a baaaaaaaddd chapter!!!
 DNA Replication & Protein Synthesis This isn t a baaaaaaaddd chapter!!! The Discovery of DNA s Structure Watson and Crick s discovery of DNA s structure was based on almost fifty years of research by other
DNA Replication & Protein Synthesis This isn t a baaaaaaaddd chapter!!! The Discovery of DNA s Structure Watson and Crick s discovery of DNA s structure was based on almost fifty years of research by other
Lab 4: Osmosis and Diffusion
 Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment. It is not an impermeable barrier, but like all biological membranes,
Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment. It is not an impermeable barrier, but like all biological membranes,
Review of the Cell and Its Organelles
 Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Biology Learning Centre Review of the Cell and Its Organelles Tips for most effective learning of this material: Memorize the names and structures over several days. This will help you retain what you
Lesson Plan: How Do We Know What is Healthy Water?
 Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
How Much Water Fits on a Penny? 6
 6 Students conduct an experiment to determine how many drops of water will fit on a penny and apply their knowledge of the properties of water and chemical bonds to explain the phenomenon. Suggested Grade
6 Students conduct an experiment to determine how many drops of water will fit on a penny and apply their knowledge of the properties of water and chemical bonds to explain the phenomenon. Suggested Grade
Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE
 Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE Course Title: Applied Chemistry Course Number: G 2614 Department: Science Grade(s): 11-12 Level(s): General Credit: 1 Course Description This is
Wallingford Public Schools - HIGH SCHOOL COURSE OUTLINE Course Title: Applied Chemistry Course Number: G 2614 Department: Science Grade(s): 11-12 Level(s): General Credit: 1 Course Description This is
A Correlation of Miller & Levine Biology 2014
 A Correlation of Miller & Levine Biology To Ohio s New Learning Standards for Science, 2011 Biology, High School Science Inquiry and Application Course Content A Correlation of, to Introduction This document
A Correlation of Miller & Levine Biology To Ohio s New Learning Standards for Science, 2011 Biology, High School Science Inquiry and Application Course Content A Correlation of, to Introduction This document
Target Mole Lab. Mole Relationships and the Balanced Equation. For each student group Hydrochloric acid solution, HCl, 3 M, 30 ml
 elearning 2009 Introduction Target Mole Lab Mole Relationships and the Balanced Equation Publication No. A common chemical reaction used in chemistry class is zinc and hydrochloric In this lab, students
elearning 2009 Introduction Target Mole Lab Mole Relationships and the Balanced Equation Publication No. A common chemical reaction used in chemistry class is zinc and hydrochloric In this lab, students
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
IIn our high tech world, one of the hottest areas of development
 Topic 1.1 Why are cells important? Key Concepts Studying cells helps us understand how organisms function. Cellular organelles work together to carry out life functions. Cellular processes enable organisms
Topic 1.1 Why are cells important? Key Concepts Studying cells helps us understand how organisms function. Cellular organelles work together to carry out life functions. Cellular processes enable organisms
Week 1 EOC Review Cell Theory, Cell Structure, Cell Transport
 Week 1 EOC Review Cell Theory, Cell Structure, Cell Transport Benchmarks: SC.912.L.14.1 Describe the scientific theory of cells (cell theory) and relate the history of its discovery to the processes of
Week 1 EOC Review Cell Theory, Cell Structure, Cell Transport Benchmarks: SC.912.L.14.1 Describe the scientific theory of cells (cell theory) and relate the history of its discovery to the processes of
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5
 First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
5th Grade Lesson Plan: The Cell: The building blocks of life
 5th Grade Lesson Plan: The Cell: The building blocks of life Overview This series of lessons was designed to meet the needs of gifted children for extension beyond the standard curriculum with the greatest
5th Grade Lesson Plan: The Cell: The building blocks of life Overview This series of lessons was designed to meet the needs of gifted children for extension beyond the standard curriculum with the greatest
Chapter 3. Cellular Structure and Function Worksheets. 39 www.ck12.org
 Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Chapter 3 Cellular Structure and Function Worksheets (Opening image copyright by Sebastian Kaulitzki, 2010. Used under license from Shutterstock.com.) Lesson 3.1: Introduction to Cells Lesson 3.2: Cell
Cell Unit Practice Test #1
 ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ell Unit Practice Test #1 Name: ate: 1. Which organelle is primarily concerned with the conversion of potential energy of organic compounds into suitable form for immediate use by the cell?. mitochondria.
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
Ms. Campbell Protein Synthesis Practice Questions Regents L.E.
 Name Student # Ms. Campbell Protein Synthesis Practice Questions Regents L.E. 1. A sequence of three nitrogenous bases in a messenger-rna molecule is known as a 1) codon 2) gene 3) polypeptide 4) nucleotide
Name Student # Ms. Campbell Protein Synthesis Practice Questions Regents L.E. 1. A sequence of three nitrogenous bases in a messenger-rna molecule is known as a 1) codon 2) gene 3) polypeptide 4) nucleotide
EXPERIMENT 2 EGG OBSERVATIONS. Contents: Pages 1-4: Teachers Guide Page 5: Student Worksheet. An Osmosis Eggsperiment ACKNOWLEDGEMENTS
 EXPERIMENT 2 EGG OBSERVATIONS An Osmosis Eggsperiment Contents: Pages 1-4: Teachers Guide Page 5: Student Worksheet ACKNOWLEDGEMENTS The creation of this experiment and its support materials would not
EXPERIMENT 2 EGG OBSERVATIONS An Osmosis Eggsperiment Contents: Pages 1-4: Teachers Guide Page 5: Student Worksheet ACKNOWLEDGEMENTS The creation of this experiment and its support materials would not
Biology. STANDARD II: Objective 3. Osmosis Inquiry Labs
 Biology STANDARD II: Objective 3 Osmosis Inquiry Labs Background Knowledge: Students should have used a microscope before and be familiar with the parts. They should also know how to make a wet mount slide.
Biology STANDARD II: Objective 3 Osmosis Inquiry Labs Background Knowledge: Students should have used a microscope before and be familiar with the parts. They should also know how to make a wet mount slide.
