The University of Texas Southwestern Medical Center at Dallas
|
|
|
- Jesse Manning
- 8 years ago
- Views:
Transcription
1 Page 1 of 6 The University of Texas Southwestern Medical Center at Dallas CONSENT TO PARTICIPATE IN RESEARCH Short Screening Consent Title of Research: Physical and Metabolic Abnormalities in Lipodystrophy and Dyslipidemias Sponsor: NIH Investigators Telephone No(regular office hours) Telephone No.(other times) Abhimanyu Garg, M.D Vinaya Simha, M.D Peter Snell, Ph.D Paul Weatherall, M.D Claudia Quittner, RN David Euhus, M.D Meena Shah, Ph.D Dolores Peterson, M.D., Ph.D Zahid Ahmad, M.D Ron Hoxworth, M.D INVITATION: Note: If you are a parent or guardian of a minor and have been asked to read and sign this form, the you in this document refers to the minor. Instructions: Please read this consent form carefully and take your time making a decision about whether to participate. As the researchers discuss this consent form with you, please ask him/her to explain any words or information that you do not clearly understand. The purpose of the study, risks, inconveniences, discomforts, and other important information about the study are listed below. If you decide to participate, you will be given a copy of this form to keep. You are invited to participate in this research because either you or your child or your family members have abnormalities of fat cells and metabolism, disorders such as lipodystrophy (either congenital-generalized, familial-partial, or acquired lipodystrophy). Or you have another type of body fat disorder involving problems with fat or sugar metabolism including abnormal blood fats (triglycerides and/or cholesterol) or and other syndromes which may be related, such as premature aging syndromes, obesity or lipomatosis. Also, you may qualify if you have lipodystrophy with HIV. You may also participate as a normal volunteer if you do not show signs of lipodystrophy or body fat abnormalities, in order to serve as part of a comparison group. This study does not offer a treatment or intervention for your condition. NUMBER OF PARTICIPANTS: The sponsor plans to include 1800 participants in this research. PURPOSE: You have been asked to undergo a blood test and possibly other tests that will help determine if you have any metabolic disorders such as high blood cholesterol, triglycerides or DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
2 glucose associated with lipodystrophy (a disease of abnormal body fat distribution), or other types of disorders involving problems with fat or sugar metabolism and other syndromes which may be related, such as premature aging syndromes. The overall purpose of this research is to examine the physical and metabolic defects associated with lipodystrophy and related syndromes and learn how these develop. PROCEDURES Screening: You will be asked questions about your health and health history, there will be questionnaires about your body shape changes and quality of life and you may have a physical exam. You will have blood tests, and you may have urine tests and measures of height, weight and body fat (anthropometry). These procedures are being done because you are in this research. Your photographs may also be requested from you or your physician prior to your evaluation here, or we may take photographs while you are here. We use photographs for diagnostic, research and educational purposes. Your photographs may be taken by a professional photographer or a member of Dr. Garg s research team and they may be published in professional scientific journals or medical books but your identity will not be disclosed if any images are published. How long can I expect to be in this study? For this study, you may be seen just once, or yearly or every few years or if your body shape or metabolism changes, or at an interval of your choosing. If you have been seen and then months or years later we develop a new study that might help you, we would contact you to see if you would like to participate. Unless you tell us not to contact you in future, or not to contact you after a certain period of time. You can choose to stop participating for any reason at any time. However, if you decide to stop participating in the study, we encourage you to tell the researchers. Evaluations during the research: INVESTIGATIONAL PROCEDURES: The tests with their known risks are listed below. The investigator obtaining this consent from you will check the boxes for the tests you are being asked to undergo. You should read the description of the requested tests and their associated risks. After you understand the tests and risks fully, you will be asked to check in the boxes yes or no if you agree or not for each of the requested tests. Then place your initials where indicated. And you will be asked to sign the consent on the last page. For children, the blood draw amounts for all the testing will be based on the child's weight and will usually be smaller amounts than those shown for adults. 1. Blood tests, the risks of donating this blood are minimal but include: (1) a lightheaded or dizzy Page 2 of 6 DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
3 feeling while the blood is being drawn, and drawn or very rarely infection. I consent to the following blood tests: a bruise or soreness at the site where the blood was The blood tests are for: YES Cholesterol, triglyceride, lipoprotein, and hormone NO INITIALS (Insulin, leptin, sex hormones, etc.) analysis: 2-4 teaspoons. SMAC-20 (general blood chemistry screen) and/or CBC: (3 to 4 teaspoons) Apolipoprotein (proteins related to blood cholesterol) analysis: (2 tablespoons or 6 teaspoons). Glycosylated hemoglobin A1c (tests for diabetes): (1 teaspoon) Serum protein electrophoresis and/or Thyroid function tests: (2 teaspoons) Anthropometry a. (Height, weight, and percent of body fat measurements): In order to determine how much muscle and fat is in your body, we will obtain detailed measurements of height, weight, and skinfold thickness using a tape measure, scale, and calipers (a fat measuring instrument). You will be weighed and measured while wearing minimal clothing, such as underwear and a patient gown. b. Underwater weighing: This test requires that you put on a bathing suit and get into a tub of lukewarm water. You will be asked to breathe out as much air as you can and go completely under water for a period no longer than seconds. While you are underwater, you will hear the investigator knock on the side of the tub, and that will be a signal for you to come up for air. Since it requires breath holding under water, children less than 6 years of age or unable to hold their breath will not be allowed to do this test. The risks of anthropometry are only the possible discomfort of holding your breath briefly underwater or the slight pinching feeling of the calipers, which measure skinfold thickness ANTHROPOMETRY YES NO INITIALS 3. OGTT, Oral Glucose Tolerance Test: OGTT, Oral glucose tolerance test:: For this test you will have an intravenous (IV) line placed for blood drawing and then receive a sugar drink on an empty stomach in the morning. Blood will be taken from the IV line at 30 minute intervals for 3 hours to check your glucose and insulin response. The total amount of blood drawn for adults will be 45 ml. This is 3 tablespoons. Page 3 of 6 DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
4 The risks of oral glucose tolerance test are the risks of a blood draw or the brief placement of an IV line, bruising, feeling light-headed or dizzy or possible infection, also rarely the sweet drink causes nausea. OGTT YES NO INITIALS 4. Dietary Recall: You will be asked about your food intake with a 1 or 3-day food recall or 3 day food record questionnaire, or by interview or phone interview. You may be called by Dr. Shah or her research assistant to give you additional instructions on completing the food record. This takes up 30 minutes or more of your time. Although careful precautions are taken to protect your privacy, there is an outside chance that an unauthorized person could review your information. DIETARY RECALL YES NO INITIALS POSSIBLE BENEFITS Benefit to you: A benefit you may receive from having these tests is the knowledge that you either do or do not have a metabolic disorder associated with lipodystrophy or a related syndrome. If we discover that you do have a metabolic disorder, proper therapy to control the condition can be instituted, either through our clinic or by your physician. Benefit to other people with lipodystrophy, premature aging or related syndromes: In the future, other people with lipodystrophy, premature aging or other problems related to abnormal fat or glucose metabolism or unusual fat distribution could benefit from the results of this research. Information gained from this research could lead to improved medical care for them. However, your study doctor will not know whether there are benefits to other people with lipodystrophy, premature aging and related syndromes until all of the information obtained from this research has been collected and analyzed. COSTS TO YOU: The sponsor will pay the expenses for the test that is done including laboratory work, underwater weight, and oral glucose tolerance test. Expenses related to standard medical care for your lipodystrophy, HIV, premature aging or other syndromes, diabetes, high triglycerides and other metabolic abnormalities are your responsibility (or the responsibility of your insurance provider or government program). There are no funds available to pay for parking expenses, transportation to and from the research center, lost time away from work and other activities, lost wages, or child care expenses. COMPENSATION FOR INJURY: Compensation for an injury resulting from your participation in this research is not available from the University of Texas Southwestern Medical Center at Dallas or Parkland Health & Hospital System,. You retain your legal rights during your participation in this research. Page 4 of 6 DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
5 VOLUNTARY PARTICIPATION IN RESEARCH: You have the right to agree or refuse to participate in this research. If you decide to participate and later change your mind, you are free to discontinue participation in the research at any time. Refusal to participate will involve no penalty or loss of benefits to which you are otherwise entitled. Refusal to participate will not affect your legal rights or the quality of health care that you receive at this center. Your status as a medical student, fellow, faculty, or staff in the medical center will not be affected in any way. NEW INFORMATION: Any new information which becomes available during your participation in the research and may affect your health, safety, or willingness to continue in the research will be given to you. RECORDS OF YOUR PARTICIPATION IN THIS RESEARCH: You have the right to privacy. Any information about you that is collected for this research will remain confidential as required by law. In addition to this consent form, you will be asked to sign an Authorization for Use and Disclosure of Protected Health Information for Research Purposes, which will contain more specific information about who is authorized to review, use, and/or receive your protected health information for purposes of the study. YOUR QUESTIONS: Your study doctor is available to answer your questions about this research. The Chairman of the IRB is available to answer questions about your rights as a participant in research or to answer your questions about an injury or other complication resulting from your participation in this research. You may telephone the Chairman of the IRB during regular office hours at YOU WILL HAVE A COPY OF THIS CONSENT FORM TO KEEP. Your signature below certifies the following: You have read (or been read) the information provided above. You have received answers to all of your questions. You have freely decided to participate in this research. You understand that you are not giving up any of your legal rights. Participant s Name (printed) Participant s Signature Legally authorized representative s name (printed) Legally authorized representative s Signature Page 5 of 6 DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
6 Name (printed) of person obtaining Consent Signature of person obtaining consent ASSENT OF A MINOR: I have discussed my participation in this research with my mother or father or legal guardian and my study doctor, and I agree to participate in this research. Signature (participants from 10 to 18 years old) Page 6 of 6 DO NOT DISCLOSE Study ID: STU Approved: 10/22/2010 Expiration : 10/3/2011
7 The University of Texas Southwestern Medical Center at Dallas CONSENT TO PARTICIPATE IN RESEARCH TITLE OF RESEARCH: Physical and Metabolic Abnormalities in Lipodystrophy and Dylipidemias SPONSOR: National Institutes of Health INVESTIGATORS: Telephone No. (office hours) Telephone No. (other times) Abhimanyu Garg, M.D Vinaya Simha, M.D Peter Snell, Ph.D Paul Weatherall, M.D Claudia Quittner, RN David Euhus, M.D Meena Shah, Ph.D Dolores Peterson, M.D., Ph.D Zahid Ahmad, M.D Ron Hoxworth, M.D, INVITATION: You are invited to participate in this research because either you or your family members have adipose tissue disorders such as lipodystrophy (either congenital-generalized, familialpartial, or acquired lipodystrophy). Or you have another type of body fat disorder involving problems with fat or sugar metabolism including abnormal blood fats (Triglycerides and/or cholesterol) or and other syndromes which may be related, such as premature aging syndromes, obesity or lipomatosis. Also, you may qualify if you have lipodystrophy with HIV. You may also participate as a normal volunteer if you do not show signs of lipodystrophy or body fat abnormalities, in order to serve as part of a comparison group. NUMBER OF PARTICIPANTS: Dr. Garg plans to include 1800 participants in this research. PURPOSE: Dr. Garg wants to learn more about lipodystrophy and other disorders where there are problems with glucose or fat metabolism, including premature aging syndromes. This research is necessary because there is currently not much information available about these problems. PROCEDURES For this part of the research, you may be asked for a blood sample, a tissue sample, or both. Sample of blood: A doctor, nurse, or licensed technician will collect 4-6 teaspoons ( 20-30ml) of blood by vein one time. We may ask for a second blood sample if the research laboratory cannot process the first sample. The samples will be coded with a unique identifier. This code is based upon the suspected diagnosis and order of presentation to us. This identifier is not kept in your hospital chart. Cells removed during surgery: If you are planning to have surgery for any reason and we are able to coordinate with your surgeon, we may ask to keep some cells already removed during surgery for this research. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
8 Sample of Tissue: For some subjects a tissue biopsy will be requested. This will involve a skin, muscle, or fat biopsy collected for this research. A skilled physician will perform the procedure. Adipose Tissue Biopsy (Fat Biopsy): For this procedure done under sterile conditions, a 2"x 2" area of your skin will be numbed with the anesthetic lidocaine. A small incision ( 1 ½ inches or less) will be made in the skin, and a small piece of fat (about the size of a marble containing about 5-10 grams of fat) will be removed from under your skin. Cautery may be used to decrease the risk of bruising. The incision will be closed using dissolvable sutures and steri-strips or skin adhesive. Biopsies will be taken from one or two sites, most likely sites are the back of the neck, the abdomen and the upper thigh, if you develop fat accumulations at different sites we may request you allow a biopsy from an alternate or additional site. The tissue obtained by this procedure will be used to determine anatomical and biochemical changes in the fat tissue. Children less than 14 years of age will not have this procedure. Muscle Biopsy: If this is a punch biopsy then a small area of skin will be cleaned then numbed with lidocaine, and a tiny incision will be made. A needle will be inserted into your muscle through the incision and a very tiny pea-sized piece of muscle will be withdrawn through the needle (about 200mg). If this is being done as part of a fat biopsy then the procedure is as described for the adipose tissue (fat biopsy) above, the only difference is that the surgeon will also remove a tiny peasized piece of muscle through the incision already created to remove the fat. Cautery will be used to minimize bruising. The site will be closed with dissolvable sutures and steri-strips or skin adhesive. The tissue will be used to determine anatomical and biochemical changes in the muscle tissue. Children less than 16 years of age will not have this procedure. Skin Biopsy: For this procedure one inch by one-inch area of your skin will be cleaned and numbed with the anesthetic lidocaine. A small round piece of skin about 1/16 th inch across will be removed with a punch biopsy instrument. The wound will be closed with steri-strips or skin adhesive and no sutures will be needed. The cells from the skin sample will be grown in culture and anatomical, biochemical and biological changes in these cells will be determined. Children less than 4 years of age will not have this procedure. For more information about the use of your blood or tissue sample in this research, please read More Information about This Research at the end of this consent form. POSSIBLE RISKS Questions: We will ask you personal questions. However, you can skip any question that makes you uncomfortable. Sample of blood: You may experience discomfort, bleeding, and/or bruising. You may feel dizzy or faint. On a rare occasion, an infection could develop at the site where the blood was collected. Sample of Tissue: Adipose Tissue Biopsy: The biopsy site could bleed or form hematoma (collection of blood in tissue), or a bruise. This procedure is done under sterile conditions. There is a minimal risk for infection at the biopsy sites. You could have an allergic reaction to the skin cleanser or to the local anesthetic. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
9 Please tell the study nurse and doctors if you know that you are allergic to betadine, iodine, novocaine or lidocaine. You will have small scars where the incisions are made. You may experience pain during or after the procedure. It is possible that a nerve in your skin might be damaged or cut, causing temporary or permanent numbness in a patch of skin near the incision. Muscle Biopsy: The biopsy site is the adipose tissue biopsy site and the risks are the same as above. There may be pressure or pain during or after the procedure, although a local anesthetic agent is used. You could develop bruising or a hematoma (collection of blood in the tissue), the site could bleed. There is a minimal risk for infection at the site. You will have small scars where the incisions are made. It is possible that a nerve in your skin might be damaged or cut, causing temporary or permanent numbness in a patch of skin near the incision. Skin Biopsy: There is a small risk for bleeding, bruising, pain or infection. You will have a tiny scar at the site. Other risks: The attached document ( More Information about This Research ) describes other possible risks related to this type of research. Unforeseen risks: There could be risks to your participation in this research which Dr. Garg does not know about now. What to do if you have problems: If you have a medical problem after blood or tissue is collected for this research, Dr. Garg can recommend treatment. Please report the problem to Dr. Garg or Claudia Quittner promptly. Call any one of the telephone numbers listed on the first page of this consent form. POSSIBLE BENEFITS To you: Usually there are no personal benefits from participation in this kind of research. To others: The results of this research may help other people in the future. New information may lead to improvements in medical care for lipodystrophy, premature aging or related syndromes. However, research tests using your sample could possibly fail to produce useful information. COMMERCIAL DEVELOPMENTS: Research tests using your sample may possibly result in inventions or procedures that have commercial value and are eligible for protection by a patent. Should future commercial developments occur, there are no plans to provide financial compensation to you from the University of Texas Southwestern Medical Center at Dallas or its researchers, and/or other facilities or researchers whose research may benefit from the use of your sample. By agreeing to the use of your sample in research, you are giving your sample with the understanding that there are no plans of providing you acknowledgment, compensation, interest in any commercial value or patent, or interest of any other type. You retain your legal rights during your participation in this research. PAYMENT TO TAKE PART IN THIS RESEARCH: You will not be paid to participate in this research. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
10 COSTS TO YOU: Collecting a sample of blood or tissue and testing it in a research laboratory will not cost you anything (or your insurance company/government program). Expenses for routine health check-ups or care for any medical problem are your responsibility (or the responsibility of your insurance provider or government program). There are no funds available to pay for parking expenses, transportation to and from the research center, lost time away from work and other activities, lost wages, or child care expenses. COMPENSATION FOR INJURY: Compensation for a physical injury or any other complication resulting from participation in this research is not available from the University of Texas Southwestern Medical Center at Dallas or Parkland Health & Hospital System, However, you retain your legal rights during your participation in this research. VOLUNTARY PARTICIPATION IN RESEARCH: You have the right to agree or refuse to participate in this research. If you decide to participate and later change your mind, you are free to stop at any time. Your refusal to participate will not result in any penalty or loss of benefits to which you are otherwise entitled. Your refusal to participate will not affect your legal rights or the quality of health care that you receive at this center. RECORDS OF YOUR PARTICIPATION IN THIS RESEARCH: You have the right to privacy. Any information about you that is collected for this research will remain confidential as required by law. In addition to this consent form, you will be asked to sign an Authorization for Use and Disclosure of Protected Health Information for Research Purposes, which will contain more specific information about who is authorized to review, use, and/or receive your protected health information for the purposes of this study. Certificate of Confidentiality: Dr. Garg has obtained a Certificate of Confidentiality from the Federal government. This Certificate will help researchers protect your privacy. However, the Certificate will not protect your privacy if you consent in writing to the release of information about your participation in this research to anyone else. For more information about a Certificate of Confidentiality, please read More Information about This Research at the end of this consent form. YOUR QUESTIONS: Dr. Garg is available to answer your questions about this research. The Chairman of the IRB is available to answer questions about your rights as a participant in research or to answer your questions about an injury or other complication resulting from your participation. You may telephone the Chairman of the IRB during regular office hours at YOU WILL HAVE A COPY OF THIS CONSENT FORM TO KEEP. Your signature below certifies the following: You have read (or been read) the information provided in this consent form and in the attached document, More Information about This Research. You have received answers to all of your questions. You have freely decided to participate in this research. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
11 You understand that you are not giving up any of your legal rights. Participant s name (printed) Participant s signature and date Legally authorized representative s name (printed) Legally authorized representative s signature and date Name of person obtaining consent (printed) Signature of person obtaining consent and date ASSENT OF A MINOR: I have discussed my participation in this research with my mother or father or legal guardian and [insert name of person obtaining consent], and I agree to participate in this research. Signature (participants from 10 to 18 years of age) and date More Information about This Research What will happen to the samples and information collected for this research? Dr. Garg will compare information about the health of participants with the results of research tests using their DNA, cells or tissue. What is DNA? DNA means deoxyribonucleic acid. DNA is found in almost all of the cells in the body. A gene is that part of DNA which pertains to family traits. How is DNA obtained? Cells from blood or other body materials are processed in a laboratory that has special equipment that can extract DNA and identify genes. How long is the DNA kept? Dr. Garg will keep your sample of DNA, cells or tissue in a research laboratory at this medical center until it is all gone, or until he decides to discard the sample. If there is a power failure in the laboratory, and your frozen sample thaws, the sample could become spoiled and useless for future research. If that happens, the sample would be discarded. If your sample remains stored beyond your lifetime, your sample will be used as described in this document. May other researchers use your DNA? With your permission, Dr. Garg may give some of your DNA, cells or tissue to other medical scientists who are studying lipodystrophy, premature aging or related syndromes. DNA given to other scientists will not be labeled with your name or any other information that could be linked to you in any way. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
12 Who decides which research scientists may receive samples of your DNA? Dr. Garg asks your permission to use your sample of DNA, cells or tissue as he chooses. The sample will be used only for medical research and will not be sold. Dr. Garg will decide which researchers at this medical center and at other medical centers may receive samples of your DNA, cells or tissue. Your samples may be used in other research only if the other research has been reviewed and approved by an Institutional Review Board (IRB). Could your sample be used for other purposes? No one may use your sample or your DNA for purposes other than research without your permission or without the permission of your legally responsible representative and the approval of the IRB at this medical center. Will the results of research tests be reported to you? Dr. Garg will use samples of your DNA, cells or tissue only for research. The samples will not be used to plan your health care. Will you be contacted in the future? You may be contacted later for information about your health. Please keep in touch with Dr. Garg and maintain a current address and telephone number on file. Please notify Dr. Garg if your legal name changes. Dr. Garg or a member of his research team may invite you to participate in other research in the future. Dr. Garg may recommend that you discuss new information about lipodystrophy, premature aging or a related syndrome with your personal physician. Any new information which becomes available during your participation in the research and may affect your willingness to continue in the research will be given to you promptly. Will children be contacted in the future? It is your responsibility to inform a child that samples of his or her DNA, cells or tissue may be kept in a research laboratory at this medical center or possibly other medical centers. The child will not be asked to sign another consent form when he/she reaches age 18. What are some of the risks that could result from participation in this kind of research? Stress: You could experience stress from participating in this kind of research. Knowing that researchers have personal information about you may trouble you. Personal, sensitive information: If you are not the parent of a child in your family, or if you are the parent of a child in another family, that information could be learned from DNA tests. This kind of information will not be reported to you or other family members without your permission. What is a Certificate of Confidentiality? The Department of Health & Human Services issued a Certificate of Confidentiality for this research. This Certificate enables [insert name of investigator] and other researchers associated with this project to withhold information about your participation. The protection afforded by this Certificate lasts forever. However, the Certificate will not provide protection if you consent in writing to the release of information about your participation in the research to anyone else. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
13 Why is a Certificate of Confidentiality needed? Sensitive information about your health and the health of other members of your family may be collected and studied. The Certificate will help DR. Garg avoid having to release identifying information about you which could expose you and your family to unwanted financial, legal, emotional, and social consequences. How does the Certificate of Confidentiality protect your privacy? All persons who are employed by or associated with the University of Texas Southwestern Medical Center at Dallas (and its contractors or cooperating agencies) and who have access to information about your participation in this research may withhold your name and other identifying information from all persons not connected with the conduct of that research. This means that Dr. Garg does not have to identify you as a participant in this research in any Federal, State, or local, civil, criminal, administrative, legislative, or other proceedings. What are the limitations of the Certificate? This Certificate does not stop you or a member of your family from identifying you as a participant in this research. For example, if an insurance provider or employer learns about your participation in this research and obtains your consent to receive research information, Dr. Garg may not use the Certificate of Confidentiality to withhold this information. It is important that you and your family actively protect your own privacy. If Dr. Garg determines that you could be harmful to yourself or to others, he may report such concerns to proper authorities for your safety or the safety of others. If the investigators suspect child, elder or disabled person s abuse, they will report such concerns to the proper authorities as required by law. A Certificate of Confidentiality does not represent an endorsement of this research project by the Department of Health & Human Services or any other Federal government agency. Could there be problems if you or someone else in the family releases information? If you or a member of your family receives private information about you and does not maintain the privacy of that information, there is no way to predict who will have access to that private information. There is no way to predict the risks or damage which could result from unwanted release of that information. How do you stop your participation in the research? If you prefer to stop participation in this research, you may ask Dr. Garg to destroy any record of your participation in this research and to destroy any sample with your name on it. You will not be asked for further information or samples. Your identity will be removed from all research records. However, the resulting data from the research will not be discarded. Copies of DNA and/or growing cells made from your samples will not be destroyed. Samples sent to other scientists cannot be identified and destroyed because your name was removed before the samples were shipped to other medical centers. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
14 The University of Texas Southwestern Medical Center at Dallas Children s Medical Center, Parkland Health & Hospital System Retina Foundation of the Southwest, Texas Scottish Rite Hospital for Children Zale Lipshy University Hospital, St. Paul University Hospital The University of Texas Southwestern Moncrief Cancer Center Authorization for Use and Disclosure of Health Information for Research Purposes NAME OF RESEARCH PARTICIPANT: 1. You agree to let UT Southwestern Medical Center, Parkland Hospital, The Clinical Translational Research Center ( CTRC), share your health information with Dr. Abhimanyu Garg and his or her staff at the University of Texas Southwestern Medical Center at Dallas ( Researchers ) for the purpose of the following research study: Physical and Metabolic Abnormalities in Lipodystrophy, A study to understand different types of fat variations, such as fat loss or fat redistribution and the underlying genetic basis. IRB File # You agree to let the Researchers use your health information for this Research Project. You also agree to let the Researchers share your health information with others who may be working with the Researchers on the Research Project ( Recipients ) as follows. NIH ( National Institutes of Health ) The sponsor includes any people, entities, groups or companies working for or with the sponsor or owned by the sponsor. The sponsor will receive written reports about your participation in the research. The sponsor may look at your health information to assure the quality of the information used in the research. Rogers MRI, CTRC Core Lab, Mineral Metabolism Lab, Quest lab, Aston Radiology. These are other research facilities that are working with UT Southwestern on the Research Project. The UT Southwestern Institutional Review Board (IRB). This is a group of people who are responsible for assuring that the rights of participants in research are respected. Members and staff of the IRB at UT Southwestern may review the records of your participation in this research. A representative of the IRB may contact you for information about your experience with this research. If you do not want to answer their questions, you may refuse to do so. Representatives of the Office of Human Research Protections (OHRP). The OHRP may oversee the Research Project to confirm compliance with laws, regulations and ethical standards. 3. Whenever possible your health information will be kept confidential. Federal privacy laws may not apply to some institutions outside of UT Southwestern. There is a risk that the Recipients could share your information with others without your permission. UT Southwestern cannot guarantee the confidentiality of your health information after it has been shared with the Recipients. 4. You agree to permit the Researchers to use and share your health information as listed below: Medical history, physical exams, DEXA scans, MRI scans, MRS scans, blood tests, urine tests, pregnancy tests, biopsies, HIV status, current and previous medications, questionnaires, and photographs and DNA testing if applicable. 5. The Researchers may use your health information to create research data that does not identify you. Research data that does not identify you may be used and shared by the Researchers (for example, in a publication about the results of the Research Project); it may also be used and shared by the Researchers and Recipients for other research purposes not related to the Research Project. Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
15 6. This authorization is voluntary. Your health care providers must continue to provide you with health care services even if you choose not to sign this authorization. However, if you choose not to sign this authorization, you cannot take part in this Research Project. 7. This Authorization has no expiration date. 8. If you change your mind and do not want us to collect or share your health information, you may cancel this authorization at any time. If you decide to cancel this authorization, you will no longer be able to take part in the Research Project. The Researchers may still use and share the health information that they have already collected before you canceled the authorization. To cancel this authorization, you must make this request in writing to: Claudia Quittner UT Southwestern Medical Center 5323 Harry Hines Blvd. Dallas, Texas Phone A copy of this authorization form will be provided to you. Signature of Research Participant For Legal Representatives of Research Participants (if applicable): Printed Name of Legal Representative: Relationship to Research Participant: I certify that I have the legal authority under applicable law to make this Authorization on behalf of the Research Participant identified above. The basis for this legal authority is:. (e.g. parent, legal guardian, person with legal power of attorney, etc.) Signature of Legal Representative Study ID: STU Approved: 10/11/2010 Expiration : 10/3/2011
16 THE UNIVERSITY OF TEXAS SOUTHWESTERN MEDICAL CENTER AT DALLAS Ambulatory Services Notice of Privacy Practices Acknowledgement of Receipt Form Pt. Name: Address: City State Zip MRN: DOB: SSN: SEX: DOS: Your signature below indicates that you have been offered a copy of UT Southwestern s Notice of Privacy Practices. If you have any questions about the Notice of Privacy Practices, please call The UT Southwestern s Privacy Officer at I have been offered the Notice of Privacy Practices. Patient Signature Print Patient Name Legal Guardian or Patient Representative Signature Print Legal Guardian or Patient Representative Name Relationship to Patient Please describe relationship to patient if other than self. FOR OFFICE USE ONLY: UT Southwestern will make a good faith effort to obtain a written acknowledgement of receipt of the Notice provided to the individual. If the patient is unwilling and or unable to sign this acknowledgment, UT Southwestern must document its good faith efforts to obtain such acknowledgement and record the reason why the acknowledgement was not obtained. Reason: Notice mailed to patient : Staff Signature: Page 1 of 1 COMPLIANCE Form # FMA/NPPARF-001 / 02.03
The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH
 The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH Title of Research: Funding Agency/Sponsor: Study Doctors: Research Personnel:
The University of Texas Southwestern Medical Center at Dallas Retina Foundation of the Southwest CONSENT TO PARTICIPATE IN RESEARCH Title of Research: Funding Agency/Sponsor: Study Doctors: Research Personnel:
If you are signing for a minor child, you refers to your child throughout the consent document.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
Consent Form: Example 2 (DNA Sequencing)
 Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
CONSENT FORM TEMPLATE. Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens
 CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
1. General Information About The Mitochondrial Disease Biobank
 Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Pl"OtocolDirector: Iris Schrijver _ IRB Approval Date: _June 20 2006 IRE Expiration Date: June 19, 2007 _ STANFORD SAMPLE CONSENT FORM
 Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY
 The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
The degree of liver inflammation or damage (grade) Presence and extent of fatty liver or other metabolic liver diseases
 ilearning about your health Liver Biopsy www.cpmc.org/learning What is a Liver Biopsy? A liver biopsy is a procedure where a specially trained doctor (typically a hepatologist, radiologist, or gastroenterologist)
ilearning about your health Liver Biopsy www.cpmc.org/learning What is a Liver Biopsy? A liver biopsy is a procedure where a specially trained doctor (typically a hepatologist, radiologist, or gastroenterologist)
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
RESEARCH SUBJECT INFORMATION AND CONSENT FORM
 1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM
 GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
GONZABA MEDICAL GROUP PATIENT REGISTRATION FORM DATE: CHART#: GUARANTOR INFORMATION LAST NAME: FIRST NAME: MI: ADDRESS: HOME PHONE: ADDRESS: CITY/STATE: ZIP CODE: **************************************************************************************
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
University of Pennsylvania RESEARCH Subject Informed Consent Form AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: Evaluating the impact of cocaine use and HIV infection in arterial wall inflammation
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
ANESTHESIA. Anesthesia for Ambulatory Surgery
 ANESTHESIA & YOU Anesthesia for Ambulatory Surgery T oday the majority of patients who undergo surgery or diagnostic tests do not need to stay overnight in the hospital. In most cases, you will be well
ANESTHESIA & YOU Anesthesia for Ambulatory Surgery T oday the majority of patients who undergo surgery or diagnostic tests do not need to stay overnight in the hospital. In most cases, you will be well
RIDGE PHYSICAL THERAPY & WELLNESS CENTER. Intake Form
 Intake Form : Personal Information please print clearly Name: last first middle initial Home Address: Home Telephone: ( ) Cell Phone: E-Mail Address: Social Security #: of Birth: Age: Sex: M F Marital
Intake Form : Personal Information please print clearly Name: last first middle initial Home Address: Home Telephone: ( ) Cell Phone: E-Mail Address: Social Security #: of Birth: Age: Sex: M F Marital
Genes for Good Consent Form
 Genes for Good Consent Form Version 2.1 The next few screens contain information about Genes for Good and the benefits and risks of participating. This is called "informed consent", because we want you
Genes for Good Consent Form Version 2.1 The next few screens contain information about Genes for Good and the benefits and risks of participating. This is called "informed consent", because we want you
RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM
 If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
Presence and extent of fatty liver or other metabolic liver diseases
 UC San Diego Health System Patient Information Sheet: Liver Biopsy What is a Liver Biopsy? A liver biopsy is a procedure where a qualified doctor (typically a hepatologist, radiologist or gastroenterologist)
UC San Diego Health System Patient Information Sheet: Liver Biopsy What is a Liver Biopsy? A liver biopsy is a procedure where a qualified doctor (typically a hepatologist, radiologist or gastroenterologist)
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5320/V-HICS, Version 2.0, 1/29/15: Viral Hepatitis C Infection Long-term Cohort Study (V-HICS) A DIVISION
! 1220 Howell Street Ste. 110, Seattle, WA 98101 (206) 464-9002
 ! 1220 Howell Street Ste. 110, Seattle, WA 98101 (206) 464-9002 PATIENT INFORMATION PATIENT NAME (Last, First, Middle Initial) DATE OF BIRTH AGE ADDRESS SOCIAL SECURITY NUMBER CITY, STATE, ZIP Male GENDER
! 1220 Howell Street Ste. 110, Seattle, WA 98101 (206) 464-9002 PATIENT INFORMATION PATIENT NAME (Last, First, Middle Initial) DATE OF BIRTH AGE ADDRESS SOCIAL SECURITY NUMBER CITY, STATE, ZIP Male GENDER
Inferior Vena Cava filter and removal
 Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
Inferior Vena Cava filter and removal What is Inferior Vena Cava Filter Placement and Removal? An inferior vena cava filter placement procedure involves an interventional radiologist (a specialist doctor)
The Family Library. Understanding Diabetes
 The Family Library Understanding Diabetes What is Diabetes? Diabetes is caused when the body has a problem in making or using insulin. Insulin is a hormone secreted by the pancreas and is needed for the
The Family Library Understanding Diabetes What is Diabetes? Diabetes is caused when the body has a problem in making or using insulin. Insulin is a hormone secreted by the pancreas and is needed for the
SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form
 SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form 1. This Medical Power of Attorney (also known as the Health Care Agent Designation Form) allows you to
SUGGESTIONS & REQUIREMENTS For Medical Power of Attorney & Completing the Texas Will to Live Form 1. This Medical Power of Attorney (also known as the Health Care Agent Designation Form) allows you to
INFORMED CONSENT FOR SLEEVE GASTRECTOMY
 INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
PATIENT INFORMATION FORM
 737 Pearl Street, Suite 108 Phone: 858.456.2114 Fax: 858.456.2103 www.abilityrehabsd.com PATIENT INFORMATION FORM Please print and complete ALL items. If an item doesn t apply, put N/A Patient Name: Age:
737 Pearl Street, Suite 108 Phone: 858.456.2114 Fax: 858.456.2103 www.abilityrehabsd.com PATIENT INFORMATION FORM Please print and complete ALL items. If an item doesn t apply, put N/A Patient Name: Age:
PATIENT INFORMATION INSURANCE INFORMATION
 (mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
(mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
Technical Assistance Document 5
 Technical Assistance Document 5 Information Sharing with Family Members of Adult Behavioral Health Recipients Developed by the Arizona Department of Health Services Division of Behavioral Health Services
Technical Assistance Document 5 Information Sharing with Family Members of Adult Behavioral Health Recipients Developed by the Arizona Department of Health Services Division of Behavioral Health Services
Research Ethics Review Committee (WHO ERC)
 Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Patient Information. Date: Date of Birth: / / Name: Social Security: _- - Address: Street City State Zip
 Personal Insurance Intake Form Patient Information Date of Birth: / / Social Security: _- - Address: Street City State Zip Email Address: Home Phone: Sex: M or F Work Phone:. Cell Phone: Height: Weight:
Personal Insurance Intake Form Patient Information Date of Birth: / / Social Security: _- - Address: Street City State Zip Email Address: Home Phone: Sex: M or F Work Phone:. Cell Phone: Height: Weight:
Personal Injury Intake Form
 Personal Injury Intake Form Patient Information: Name Home Phone Address Work Phone Cell Phone Date of Birth Social Security # Sex Male Female Height Weight lbs Occupation Marital Status Employer No of
Personal Injury Intake Form Patient Information: Name Home Phone Address Work Phone Cell Phone Date of Birth Social Security # Sex Male Female Height Weight lbs Occupation Marital Status Employer No of
INFORMATION LEAFLET. If anything is not clear, or if you would like more information, please
 BIO INFOBK 1492 0410:Layout 1 21/04/2010 15:24 Page 1 INFORMATION LEAFLET You are being invited to take part in a major medical research project called UK Biobank. The purpose of UK Biobank is to set up
BIO INFOBK 1492 0410:Layout 1 21/04/2010 15:24 Page 1 INFORMATION LEAFLET You are being invited to take part in a major medical research project called UK Biobank. The purpose of UK Biobank is to set up
DENVER CHIROPRACTIC CENTER GLENN D. HYMAN, DC, CSCS
 DENVER CHIROPRACTIC CENTER GLENN D. HYMAN, DC, CSCS Are you in the right place? Please read this before proceeding with paperwork: At Denver Chiropractic Center, we specialize in treating muscles with
DENVER CHIROPRACTIC CENTER GLENN D. HYMAN, DC, CSCS Are you in the right place? Please read this before proceeding with paperwork: At Denver Chiropractic Center, we specialize in treating muscles with
Dr. David Y. Liao, D.O. Orthopedic Center, LLC. Release of Information
 Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
CAMARILLO AQUATICS AND REHABILITATION SERVICES
 CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
New Patient Registration Form
 New Patient Registration Form Welcome to Bayside Dental Care! We look forward to giving you the best dental experience possible. Please complete both sides of this form. Let us know if you need any assistance
New Patient Registration Form Welcome to Bayside Dental Care! We look forward to giving you the best dental experience possible. Please complete both sides of this form. Let us know if you need any assistance
PATIENT CONSENT TO PROCEDURE - ROUX-EN-Y GASTRIC BYPASS
 As a patient you must be adequately informed about your condition and the recommended surgical procedure. Please read this document carefully and ask about anything you do not understand. Please initial
As a patient you must be adequately informed about your condition and the recommended surgical procedure. Please read this document carefully and ask about anything you do not understand. Please initial
Frequently Asked Questions
 Frequently Asked Questions 1. What is the purpose of this research study?... 2 2. Who can participate?... 2 3. How is the medication given?... 2 4. Are there any other medications administered as part
Frequently Asked Questions 1. What is the purpose of this research study?... 2 2. Who can participate?... 2 3. How is the medication given?... 2 4. Are there any other medications administered as part
CORD BLOOD TRANSPLANTATION STUDY EXPANDED ACCESS PROTOCOL APPENDIX A SAMPLE CONSENT FORM
 APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
CONSENT TO PARTICIPATE IN A CLINICAL. STUDY NUMBER: 11-E-0072 PRINCIPAL INVESTIGATOR: Frederick Miller, M.D., Ph.D.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient INSTITUTE: National Institute of Environmental Health Sciences STUDY NUMBER: 11-E-0072 PRINCIPAL
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY MEDICAL RECORD Adult Patient or Parent, for Minor Patient INSTITUTE: National Institute of Environmental Health Sciences STUDY NUMBER: 11-E-0072 PRINCIPAL
Ultrasound or Computed Tomography. PATIENT GUIDE and PREPARATION. Liver Biopsy
 Ultrasound or Computed Tomography PATIENT GUIDE and PREPARATION Liver Biopsy What is a Liver Biopsy? A Liver Biopsy is a procedure that involves taking a specimen ( a small amount of tissue) from within
Ultrasound or Computed Tomography PATIENT GUIDE and PREPARATION Liver Biopsy What is a Liver Biopsy? A Liver Biopsy is a procedure that involves taking a specimen ( a small amount of tissue) from within
Copayment Is Due At Time Of Visit. Self-pay (payment due at time of service)
 REGISTRATION FORM Please present your insurance card and photo ID at time of check-in. Settlement of patient financial responsibility is expected at time of service. Copayment Is Due At Time Of Visit.
REGISTRATION FORM Please present your insurance card and photo ID at time of check-in. Settlement of patient financial responsibility is expected at time of service. Copayment Is Due At Time Of Visit.
Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT
 Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT Regulatory changes will occur for investigators studying human DNA The recent acceleration and widening
Human Research Protection Program University of California, San Diego ISSUES ON DNA AND INFORMED CONSENT Regulatory changes will occur for investigators studying human DNA The recent acceleration and widening
Who to call for an emergency: Name: Relationship: Home Phone: ( ) - Work Phone: ( ) - Cell Phone: ( ) -
 4425 Ponce de Leon Blvd., Suite 115 Email:info@ Dr. Mercedes Gonzalez, Pediatric Dermatologist Patient Information: Patient Name: Social Security Number: / / Date of Birth: / / Sex: M / F (Circle one)
4425 Ponce de Leon Blvd., Suite 115 Email:info@ Dr. Mercedes Gonzalez, Pediatric Dermatologist Patient Information: Patient Name: Social Security Number: / / Date of Birth: / / Sex: M / F (Circle one)
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION
 PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
Patient Information Leaflet: Part 1 select-d
 Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM
 INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
INFORMED CONSENT INFORMED CONSENT FOR PARTICIPATION IN A HEALTH AND FITNESS TRAINING PROGRAM NAME: DATE: 1. PURPOSE AND EXPLANATION OF PROCEDURE I hereby consent to voluntarily engage in an acceptable
PATIENT REGISTRATION FORM
 GENERAL INFORMATION PATIENT REGISTRATION FORM All forms must be completed and signed prior to treatment. Account #: Patient Name: Address: Home Phone No: Cell Phone No: First Middle Last Work Phone No:
GENERAL INFORMATION PATIENT REGISTRATION FORM All forms must be completed and signed prior to treatment. Account #: Patient Name: Address: Home Phone No: Cell Phone No: First Middle Last Work Phone No:
Welcome! We look forward to serving YOU. If we can do anything to make your time with us more enjoyable, please let us know.
 Welcome! We want to thank you for allowing us the opportunity to provide you with the highest level of quality rehabilitation services possible. We are committed to providing you with a comfortable, friendly
Welcome! We want to thank you for allowing us the opportunity to provide you with the highest level of quality rehabilitation services possible. We are committed to providing you with a comfortable, friendly
Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes NATIONAL DIABETES INFORMATION CLEARINGHOUSE
 NATIONAL DIABETES INFORMATION CLEARINGHOUSE Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes U.S. Department of Health and Human Services National Institutes of Health
NATIONAL DIABETES INFORMATION CLEARINGHOUSE Am I at Risk for type 2 Diabetes? Taking Steps to Lower the Risk of Getting Diabetes U.S. Department of Health and Human Services National Institutes of Health
INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY
 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL DOCUMENT. BEFORE SIGNING THIS DOCUMENT, YOU SHOULD KNOW THESE IMPORTANT FACTS: Except to the extent you state otherwise,
INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL DOCUMENT. BEFORE SIGNING THIS DOCUMENT, YOU SHOULD KNOW THESE IMPORTANT FACTS: Except to the extent you state otherwise,
Type 2 diabetes Definition
 Type 2 diabetes Definition Type 2 diabetes is a lifelong (chronic) disease in which there are high levels of sugar (glucose) in the blood. Type 2 diabetes is the most common form of diabetes. Causes Diabetes
Type 2 diabetes Definition Type 2 diabetes is a lifelong (chronic) disease in which there are high levels of sugar (glucose) in the blood. Type 2 diabetes is the most common form of diabetes. Causes Diabetes
Taking Part in Research at University Hospitals Birmingham
 University Hospitals Birmingham NHS Foundation Trust The Trust provides free monthly health talks on a variety of medical conditions and treatments. For more information visit www.uhb.nhs.uk or call 0121
University Hospitals Birmingham NHS Foundation Trust The Trust provides free monthly health talks on a variety of medical conditions and treatments. For more information visit www.uhb.nhs.uk or call 0121
Consent for Frozen Donor Oocyte In Vitro Fertilization and Embryo Transfer (Recipient)
 Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
Name of Patient: Name of Partner: We, the Patient and Partner (if applicable) named above, are each over the age of twenty-one (21) years. By our signatures below, I/we request and authorize the performance
Positron Emission Tomography - For Patients
 Positron Emission Tomography - For Patients A physician s written order is required for any PET-CT tests. How should I prepare for my PET-CT? PET-CT is more complicated than most other tests you may be
Positron Emission Tomography - For Patients A physician s written order is required for any PET-CT tests. How should I prepare for my PET-CT? PET-CT is more complicated than most other tests you may be
Blood Transfusion. Red Blood Cells White Blood Cells Platelets
 Blood Transfusion Introduction Blood transfusions are very common. Each year, almost 5 million Americans need a blood transfusion. Blood transfusions are given to replace blood lost during surgery or serious
Blood Transfusion Introduction Blood transfusions are very common. Each year, almost 5 million Americans need a blood transfusion. Blood transfusions are given to replace blood lost during surgery or serious
TEXAS MEDICAL POWER OF ATTORNEY
 TEXAS MEDICAL POWER OF ATTORNEY NAME DATE BHS 80000246 v1 11/13 baptisthealthsystem.com That s Baptist Care. Page 1 of 5 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL
TEXAS MEDICAL POWER OF ATTORNEY NAME DATE BHS 80000246 v1 11/13 baptisthealthsystem.com That s Baptist Care. Page 1 of 5 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL
HOW TO CARE FOR A PATIENT WITH DIABETES
 HOW TO CARE FOR A PATIENT WITH DIABETES INTRODUCTION Diabetes is one of the most common diseases in the United States, and diabetes is a disease that affects the way the body handles blood sugar. Approximately
HOW TO CARE FOR A PATIENT WITH DIABETES INTRODUCTION Diabetes is one of the most common diseases in the United States, and diabetes is a disease that affects the way the body handles blood sugar. Approximately
TREATMENT CONSULTATION FORM
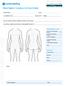 TREATMENT CONSULTATION FORM Consultation led by: Gender: M / F Weight Has your patient had other aesthetic procedures for the body? How did your patient hear about the CoolSculpting procedure? TREATMENT
TREATMENT CONSULTATION FORM Consultation led by: Gender: M / F Weight Has your patient had other aesthetic procedures for the body? How did your patient hear about the CoolSculpting procedure? TREATMENT
MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE
 MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE Complete this form if you are a MEDICAL BENEFITS SETTLEMENT CLASS MEMBER seeking to exercise a BACK END LITIGATION OPTION. In addition to
MEDICAL BENEFITS CLASS ACTION SETTLEMENT NOTICE OF INTENT TO SUE Complete this form if you are a MEDICAL BENEFITS SETTLEMENT CLASS MEMBER seeking to exercise a BACK END LITIGATION OPTION. In addition to
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167
 APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
APPENDIX I-A: INFORMED CONSENT BB IND 11184 Protocol CDC IRB #4167 INFORMED CONSENT FOR USE OF DIPHTHERIA ANTITOXIN (DAT) FOR SUSPECTED DIPHTHERIA CASES Investigational New Drug (IND) BB 11184 Protocol
Patient Registration Form
 Patient Registration Form Name (Last, First, Middle) SSN# Age Marital Status Maiden Name Address Patient Home Phone Patient Business Phone Patient Cell Phone Patient E-mail Patient Occupation Business
Patient Registration Form Name (Last, First, Middle) SSN# Age Marital Status Maiden Name Address Patient Home Phone Patient Business Phone Patient Cell Phone Patient E-mail Patient Occupation Business
BOYER CHIROPRACTIC INC
 Patient Name: Birthdate: Sex: M / F Address: City: State: Zip: Telephone: Social Security #: Driver Lic. #: Occupation: Employer: Work Phone: Address: City: State: Zip: Subscriber Name: Health Plan: Subscriber
Patient Name: Birthdate: Sex: M / F Address: City: State: Zip: Telephone: Social Security #: Driver Lic. #: Occupation: Employer: Work Phone: Address: City: State: Zip: Subscriber Name: Health Plan: Subscriber
How To Get A Medical Insurance Plan From A Doctor
 PATIENT DEMOGRAPHICS SHEET Patient Name: Parent/Legal Guardian Name: Date of Birth: Phone: Address: CITY STATE ZIP Social Security Number: Patient Phone: Sex: M F Home Cell Business Okay to leave detailed
PATIENT DEMOGRAPHICS SHEET Patient Name: Parent/Legal Guardian Name: Date of Birth: Phone: Address: CITY STATE ZIP Social Security Number: Patient Phone: Sex: M F Home Cell Business Okay to leave detailed
Carter Physiotherapy, PLLC. Patient Contact Information
 Carter Physiotherapy, PLLC Patient Contact Information Patient Name Today s Date Address City State Zip Code DOB Gender Marital Status Occupation Home Phone Work Cell Other Fax Email Employer Work Address
Carter Physiotherapy, PLLC Patient Contact Information Patient Name Today s Date Address City State Zip Code DOB Gender Marital Status Occupation Home Phone Work Cell Other Fax Email Employer Work Address
The University of Texas Southwestern Medical Center CONSENT TO PARTICIPATE IN RESEARCH YOU WILL BE GIVEN A COPY OF THIS CONSENT FORM TO KEEP
 The University of Texas Southwestern Medical Center CONSENT TO PARTICIPATE IN RESEARCH YOU WILL BE GIVEN A COPY OF THIS CONSENT FORM TO KEEP Title of Research: Funding Agency/Sponsor: Preventing Perinatal
The University of Texas Southwestern Medical Center CONSENT TO PARTICIPATE IN RESEARCH YOU WILL BE GIVEN A COPY OF THIS CONSENT FORM TO KEEP Title of Research: Funding Agency/Sponsor: Preventing Perinatal
Making Health Care Decisions in North Dakota:
 Making Health Care Decisions in North Dakota: A Summary of North Dakota Law Regarding Health Care Directives Published by: North Dakota Department of Human Services Aging Services Division 1237 W.Divide
Making Health Care Decisions in North Dakota: A Summary of North Dakota Law Regarding Health Care Directives Published by: North Dakota Department of Human Services Aging Services Division 1237 W.Divide
Guardian/Patient Name. Family Dental Care NC. 1701 Country Club Rd---Jacksonville, NC 28546 Telephone: (910) 346-2345 SIGNATURE ON FILE
 Guardian/Patient Name Family Dental Care NC 1701 Country Club Rd---Jacksonville, NC 28546 Telephone: (910) 346-2345 Date/Initial SIGNATURE ON FILE I authorize use of this form on all my insurance submissions.
Guardian/Patient Name Family Dental Care NC 1701 Country Club Rd---Jacksonville, NC 28546 Telephone: (910) 346-2345 Date/Initial SIGNATURE ON FILE I authorize use of this form on all my insurance submissions.
Blood Transfusion. There are three types of blood cells: Red blood cells. White blood cells. Platelets.
 Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
Blood Transfusion Introduction Blood transfusions can save lives. Every second, someone in the world needs a blood transfusion. Blood transfusions can replace the blood lost from a serious injury or surgery.
INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY
 INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL DOCUMENT. BEFORE SIGNING THIS DOCUMENT, YOU SHOULD KNOW THESE IMPORTANT FACTS: Except to the extent you state otherwise,
INFORMATION CONCERNING THE MEDICAL POWER OF ATTORNEY THIS IS AN IMPORTANT LEGAL DOCUMENT. BEFORE SIGNING THIS DOCUMENT, YOU SHOULD KNOW THESE IMPORTANT FACTS: Except to the extent you state otherwise,
INTRODUCTION Thrombophilia deep vein thrombosis DVT pulmonary embolism PE inherited thrombophilia
 INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
Developmental Pediatrics of Central Jersey
 PATIENT INFORMATION: CLIENT INFORMATION Date: SS# Name: (Last) (First) (M.I.) Birthdate: Sex: Race: Address: City: State: Zip: Phone: (Home) (Work) (Cell) Which telephone number is preferred: ( ) Home
PATIENT INFORMATION: CLIENT INFORMATION Date: SS# Name: (Last) (First) (M.I.) Birthdate: Sex: Race: Address: City: State: Zip: Phone: (Home) (Work) (Cell) Which telephone number is preferred: ( ) Home
Electronic Health Records Intake Form
 Dr. Sam Yoder, D.C. 101 Winston Way Ste B Campbellsville, KY 42718 Electronic Health Records Intake Form In compliance with requirements for the government EHR incentive program First Name: Address: Last
Dr. Sam Yoder, D.C. 101 Winston Way Ste B Campbellsville, KY 42718 Electronic Health Records Intake Form In compliance with requirements for the government EHR incentive program First Name: Address: Last
Dr. H. Lokesh M.D Dr. R. Desai M.D Tarah Savino MMS, P.A. C 4804 Rowan Road New Port Richey, FL 34653 (727) 375 5242 (727) 375 5198 Fax
 Practice Policies for Patients It is important to read all the enclosed information carefully. Confirmation and Cancellation of Appointments: Our patients are very important to us. Missed appointments
Practice Policies for Patients It is important to read all the enclosed information carefully. Confirmation and Cancellation of Appointments: Our patients are very important to us. Missed appointments
Client Information for Informed Consent TESTOSTERONE FOR TRANSGENDER PATIENTS
 Client Information for Informed Consent TESTOSTERONE FOR TRANSGENDER PATIENTS You want to take testosterone to masculinize your body. Before taking it, there are several things you need to know about.
Client Information for Informed Consent TESTOSTERONE FOR TRANSGENDER PATIENTS You want to take testosterone to masculinize your body. Before taking it, there are several things you need to know about.
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry
 National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
New Patient Form Please print clearly
 New Patient Form Today s Date: Name: Last First MI Preferred name to be called: Email: Address: Street City State Zip DOB: Age: Sex: SSN#: - - Please check a box for the preferred # to call to confirm
New Patient Form Today s Date: Name: Last First MI Preferred name to be called: Email: Address: Street City State Zip DOB: Age: Sex: SSN#: - - Please check a box for the preferred # to call to confirm
THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS!
 THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS! Please complete and sign all of the enclosed forms. Bring these forms, your physician s referral if required and any other documents required
THANK YOU FOR CHOOSING QPT FOR YOUR PHYSICAL THERAPY NEEDS! Please complete and sign all of the enclosed forms. Bring these forms, your physician s referral if required and any other documents required
For Parents and Students: Minor Donor Permit and Information About Donating Blood
 For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family, and friends! And when blood is needed, blood must be
For Parents and Students: Minor Donor Permit and Information About Donating Blood Every day people like you need blood: students, teachers, family, and friends! And when blood is needed, blood must be
INTRODUCTION Thrombophilia deep vein thrombosis DVT pulmonary embolism PE inherited thrombophilia
 INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
INTRODUCTION Thrombophilia (Hypercoagulability) is a condition in which a person forms blood clots more than normal. Blood clots may occur in the arms or legs (e.g., deep vein thrombosis DVT), the lungs
Liver Transarterial Chemoembolization (TACE) Cancer treatment
 Patient Education Liver Transarterial Chemoembolization (TACE) Cancer treatment This handout explains what liver transarterial chemoembolization (TACE) is and what to expect with this cancer treatment.
Patient Education Liver Transarterial Chemoembolization (TACE) Cancer treatment This handout explains what liver transarterial chemoembolization (TACE) is and what to expect with this cancer treatment.
The Liver and Alpha-1. Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION
 The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION What Is Alpha-1 Antitrypsin Deficiency? Alpha-1 is a condition that may result in serious lung disease in adults and/or liver
The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION What Is Alpha-1 Antitrypsin Deficiency? Alpha-1 is a condition that may result in serious lung disease in adults and/or liver
Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591
 Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591 Andres U. Katz, M.D. Richard S. Anderson, M.D. G. Thomas
Southwest General Surgical Associates General & Vascular Surgery 8230 Walnut Hill Lane Suite 408 Dallas, TX 75231 Phone-214)369-5432 Fax-214)369-5591 Andres U. Katz, M.D. Richard S. Anderson, M.D. G. Thomas
PATIENT INFORMATION PATIENT NAME (LAST, FIRST, MIDDLE) SEX DOB MAILING ADDRESS CITY STATE ZIP SSN
 PATIENT INFORMATION PATIENT NAME (LAST, FIRST, MIDDLE) SEX DOB MAILING ADDRESS CITY STATE ZIP SSN STREET ADDRESS (IF DIFFERENT FROM ABOVE) CITY STATE ZIP HOME PHONE NUMBER EMPLOYER CELL PHONE NUMBER WORK
PATIENT INFORMATION PATIENT NAME (LAST, FIRST, MIDDLE) SEX DOB MAILING ADDRESS CITY STATE ZIP SSN STREET ADDRESS (IF DIFFERENT FROM ABOVE) CITY STATE ZIP HOME PHONE NUMBER EMPLOYER CELL PHONE NUMBER WORK
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004
 Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
The Dermatology & Laser Group of Irvine, A.M.C. 16300 Sand Canyon Avenue, Suite 612 Irvine, CA 92618-3706 Phone# 949-753-1001 Fax# 949-753-1115
 16300 Sand Canyon Avenue, Suite 612 Irvine, CA 92618-3706 Phone# 949-753-1001 Fax# 949-753-1115 (Please Print) Today s Date / / PATIENT INFORMATION Name Last First M.I. Maiden Name: SS# Email: Mr. Ms.
16300 Sand Canyon Avenue, Suite 612 Irvine, CA 92618-3706 Phone# 949-753-1001 Fax# 949-753-1115 (Please Print) Today s Date / / PATIENT INFORMATION Name Last First M.I. Maiden Name: SS# Email: Mr. Ms.
MILLENNIUM PHYSICAL THERAPY & SPORTS MEDICINE
 A) PATIENT INTAKE/TREATMENT FORM 1) Patient Name: 2) Social Security #: 3) Home Phone number: ( ), Cell: ( ), Work: ( ) 4) Address: City, State, Zip Code 5) Gender: M F 6) Date of Birth (DOB): / / 7) Marital
A) PATIENT INTAKE/TREATMENT FORM 1) Patient Name: 2) Social Security #: 3) Home Phone number: ( ), Cell: ( ), Work: ( ) 4) Address: City, State, Zip Code 5) Gender: M F 6) Date of Birth (DOB): / / 7) Marital
State University of New York at Canton Institutional Review Board. Sample Informed Consent Document
 State University of New York at Canton Institutional Review Board Sample Informed Consent Document The following sample informed consent document includes instructions to the person writing the document,
State University of New York at Canton Institutional Review Board Sample Informed Consent Document The following sample informed consent document includes instructions to the person writing the document,
Integrative Therapies
 Hello! And welcome to Integrative Therapies! Integrative Therapies 7E Oak Branch Drive Greensboro, NC 27407 www.integrativetherapies.net 336-294- 0910 We are very happy that you have chosen to partner
Hello! And welcome to Integrative Therapies! Integrative Therapies 7E Oak Branch Drive Greensboro, NC 27407 www.integrativetherapies.net 336-294- 0910 We are very happy that you have chosen to partner
7% - 1 /% % 1.14 0 "1,( (1,( 14 - "!#% #"!A(" "4:2 4!(!2"= B"!2 #!B! !("! B!!2"!!"!" -2!
 7% -!"!#$$ %&" '()* +,- *+$./- *+$#-*+$ 0 & - 1,-1./-1#-10!1121 1(1.31-2!21021(14 1 /% % 1.14 0 "1,( (1,( 14,35!,%#!61#1,(01141-1-"&-" 1-%11( -" 171.!153-2 -- "-8 -#1#&(19!1&&:1-! &(";!"./
7% -!"!#$$ %&" '()* +,- *+$./- *+$#-*+$ 0 & - 1,-1./-1#-10!1121 1(1.31-2!21021(14 1 /% % 1.14 0 "1,( (1,( 14,35!,%#!61#1,(01141-1-"&-" 1-%11( -" 171.!153-2 -- "-8 -#1#&(19!1&&:1-! &(";!"./
Electrodiagnostic Testing
 Electrodiagnostic Testing Electromyogram and Nerve Conduction Study North American Spine Society Public Education Series What Is Electrodiagnostic Testing? The term electrodiagnostic testing covers a
Electrodiagnostic Testing Electromyogram and Nerve Conduction Study North American Spine Society Public Education Series What Is Electrodiagnostic Testing? The term electrodiagnostic testing covers a
Please fill out forms, sign where needed and bring with you to your first visit. If you have any questions please call the office at 212-751-8300.
 Welcome to Manhattan Sports Medicine and the office of Dr. Kyle Worell. Before we get started please see all forms below: Personal History (Intake) Informed Consent Payments HIPPA Please fill out forms,
Welcome to Manhattan Sports Medicine and the office of Dr. Kyle Worell. Before we get started please see all forms below: Personal History (Intake) Informed Consent Payments HIPPA Please fill out forms,
Signature: Date: Witness:
 : Patient Relationship to Guarantor: of Birth: Sex: M F Social Security Number: Home Address: City: State: Zip Code: Home Telephone:( ) Referred By: Pharmacy of Choice: Pharmacy Address: Pharmacy Phone
: Patient Relationship to Guarantor: of Birth: Sex: M F Social Security Number: Home Address: City: State: Zip Code: Home Telephone:( ) Referred By: Pharmacy of Choice: Pharmacy Address: Pharmacy Phone
Advanced Rheumatology of Houston Offices of Dr. Tamar F Brionez
 Advanced Rheumatology of Houston Offices of Dr. Tamar F Brionez New patient history form Patient name DOB Allergies to Medicines: Current Medications Name Dose Times/day taken Social History Married/single/widowed/divorced
Advanced Rheumatology of Houston Offices of Dr. Tamar F Brionez New patient history form Patient name DOB Allergies to Medicines: Current Medications Name Dose Times/day taken Social History Married/single/widowed/divorced
Borgess Diabetes Center PATIENT REGISTRATION/DEMOGRAPHICS
 Borgess Diabetes Center PATIENT REGISTRATION/DEMOGRAPHICS Please complete the following form by filling in the blanks or by circling the answer provided. Last Name: First Name M.I. Address: City, State,
Borgess Diabetes Center PATIENT REGISTRATION/DEMOGRAPHICS Please complete the following form by filling in the blanks or by circling the answer provided. Last Name: First Name M.I. Address: City, State,
Policy & Procedure AUTUMN RIDGE RESIDENTIAL CARE. March, 2013
 AUTUMN RIDGE RESIDENTIAL CARE Policy & Procedure HIPAA / PRIVACY NOTICE OF PRIVACY PRACTICES FUNCTION NUMBER PRIOR ISSUE EFFECTIVE DATE March, 2013 PURPOSE To ensure that a Notice of Privacy Practices
AUTUMN RIDGE RESIDENTIAL CARE Policy & Procedure HIPAA / PRIVACY NOTICE OF PRIVACY PRACTICES FUNCTION NUMBER PRIOR ISSUE EFFECTIVE DATE March, 2013 PURPOSE To ensure that a Notice of Privacy Practices
HI *Home Phone: Alternate Phone: Driver License No.: Email Address: INSURANCE COVERAGE & SUBSCRIBER INFORMATION (person that has the insurance policy)
 HAWAII PHYSICAL THERAPY INC. -- PATIENT REGISTRATION FORM Please fill out this form to register as a patient of Hawaii Physical Therapy Inc. All fields with an asterisk (*) are REQUIRED. We cannot register
HAWAII PHYSICAL THERAPY INC. -- PATIENT REGISTRATION FORM Please fill out this form to register as a patient of Hawaii Physical Therapy Inc. All fields with an asterisk (*) are REQUIRED. We cannot register
Legal Issues for People with HIV
 Legal Issues for People with HIV Duke Legal Project Box 90360 Durham, NC 27708-0360 (919) 613-7169 (888) 600-7274 Duke Legal Project is a clinical legal education program of Duke Law School. Legal Representation
Legal Issues for People with HIV Duke Legal Project Box 90360 Durham, NC 27708-0360 (919) 613-7169 (888) 600-7274 Duke Legal Project is a clinical legal education program of Duke Law School. Legal Representation
Stanislaw Facial Plastic Surgery Center LLC Paul Stanislaw Jr., M.D.
 Patient Information Stanislaw Facial Plastic Surgery Center LLC Paul Stanislaw Jr., M.D. Patient Name Date of Birth Age Address Marital Status Sex Address Home ( ) City State Zip Cell ( ) Employer Work
Patient Information Stanislaw Facial Plastic Surgery Center LLC Paul Stanislaw Jr., M.D. Patient Name Date of Birth Age Address Marital Status Sex Address Home ( ) City State Zip Cell ( ) Employer Work
