AVS Anchor-C Cervical Cage System Surgical Technique. Large Graft Volume Self-Locking Screws Angled and Flexible Instrumentation
|
|
|
- Alexina Norman
- 8 years ago
- Views:
Transcription
1 AVS Anchor-C Cervical Cage System Large Graft Volume Self-Locking Screws Angled and Flexible Instrumentation
2 Table of Contents Intended Use/Indications... 3 System Overview Implantation Technique Implant Removal Implant Part Numbers Instrument Part Numbers Important Product Information
3 Intended Use/Indications Indications The Stryker Spine AVS Anchor-C Cervical Cage System is indicated for anterior cervical interbody fusion procedures in skeletally mature patients with cervical disc disease at one level from the C2-C3 disc to the C7-T1 disc. Cervical disc disease is defined as intractable radiculopathy and/or myelopathy with herniated disc and/or osteophyte formation on posterior vertebral endplates producing symptomatic nerve root and/or spinal cord compression confirmed by radiographic studies. The AVS Anchor-C Cervical Cage is to be used with autogenous bone graft and implanted via an open, anterior approach. The AVS Anchor-C Cervical Cage must be used with the internal screw fixation provided by AVS Anchor-C Fixation Screws. This cervical device is to be used in patients who have had six weeks of non-operative treatment. Contraindications Contraindications may be relative or absolute. The choice of a particular device must be carefully weighed against the patient s overall evaluation. Circumstances listed below may reduce the chances of a successful outcome: The AVS Anchor-C Cervical Cage should not be implanted in patients with an active infection at the operative site. The AVS Anchor-C Cervical Cage is not intended for use except as indicated. Marked local inflammation. Any abnormality present which affects the normal process of bone remodeling including, but not limited to, severe osteoporosis involving the spine, bone absorption, osteopenia, primary or metastatic tumors involving the spine, active infection at the site or certain metabolic disorders affecting osteogenesis. Any mental or neuromuscular disorder which would create an unacceptable risk of fixation failure or complications in postoperative care. Open wounds. Pregnancy. Inadequate tissue coverage over the operative site. Any neuromuscular deficit which places an unsafe load level on the device during the healing period. Obesity. An overweight or obese patient can produce loads on the spinal system which can lead to failure of the fixation of the device or to failure of the device itself. Obesity is defined according to the W.H.O. standards. A condition of senility, mental illness, or substance abuse. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the implant, leading to failure or other complications. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests must be made prior to material selection or implantation. 3
4 Other medical or surgical condition which would preclude the potential benefit of spinal implant surgery, such as the presence of tumors, congenital abnormalities, elevation of sedimentation rate unexplained by other diseases, elevation of white blood cell count (WBC), or marked left shift in the WBC differential count. Prior fusion at the levels to be treated. These contra-indications can be relative or absolute and must be taken into account by the physician when making his decision. The above list is not exhaustive. Surgeons must discuss the relative contraindications with the patients. Pre-Operative Precautions The surgical indication and the choice of implants must take into account certain important criteria such as: Patients involved in an occupation or activity that applies excessive loading upon the implant (e.g., substantial walking, running, lifting, or muscle strain) may be at increased risk for failure of the fusion and/or the device. Surgeons must instruct patients in detail about the limitations of the implants, including, but not limited to, the impact of excessive loading through patient weight or activity, and be taught to govern their activities accordingly. The procedure will not restore function to the level expected with a normal, healthy spine, and the patient should not have unrealistic functional expectations. A condition of senility, mental illness, chemical dependence or alcoholism. These conditions among others may cause the patients to ignore certain necessary limitations and precautions in the use of the implant, leading to failure and other complications. Foreign body sensitivity. Where material sensitivity is suspected appropriate tests should be made prior to material implantation. Surgeons must advise patients who smoke have been shown to have an increased incidence of non-unions. Such patients must be advised of this fact and warned of the potential consequences. Care must be taken to protect the components from being marred, nicked, or notched as a result of contact with metal or abrasive objects. Intra-Operative Precautions The insertion of the implants must be carried out using instruments designed and provided for this purpose and in accordance with the specific implantation instructions for each implant. Those detailed instructions are provided in the surgical technique brochure supplied by STRYKER Spine. Discard all damaged or mishandled implants. Never reuse an implant, even though it may appear undamaged. 4
5 Caution Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. This device is NOT intended to be used without the AVS Anchor-C Fixation Screws provided. Should removal of the AVS Anchor-C Fixation Screws be necessary during the surgery, the AVS Anchor-C Cage should NOT be implanted alone, without the support of the AVS Anchor-C Fixation Screws. Instruments designed for use with implantation of the AVS Anchor-C Cervical Cage System are provided non-sterile and must be sterilized prior to use. This device is not intended for posterior surgical implantation. The AVS Anchor-C Cervical Cages have not been evaluated for safety and compatibility in the MR environment. AVS Anchor-C Cervical Cages have not been tested for heating or migration in the MR environment. Based on the fatigue testing results, the physician/surgeon must consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact the performance of the intervertebral body fusion device. The implantation of the intervertebral body fusion device must be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient. Potential risks identified with the use of this intervertebral body fusion device, which may require additional surgery, include: device component fracture, loss of fixation, pseudoarthrosis (i.e. non-union), fracture of the vertebrae, neurological injury, and vascular or visceral injury. Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without a previous surgery. The components of the system have been designated to work together. Do not substitute another manufacturer s device for any component of the system. Any such use will negate the responsibility of Stryker Spine for the performance of the resulting mixed component implant. Do not mix metals (e.g. Titanium based devices with stainless steel items). Some corrosion occurs on all implanted metals and alloys. Contact of dissimilar metals, however, may accelerate corrosion. Corrosion may accelerate fatigue fracture of implants, and cause metal compounds to be released into the body. 5
6 System Overview AVS Anchor-C is an interbody fusion device with internal screw fixation and is intended to be used in anterior cervical discectomy and fusion procedures. This system integrates a hollow PEEK cage with a titanium screw locking mechanism and is designed to aid in cervical interbody fusion. A tantalum marker is embedded into the cage to help visually confirm the posterior position under fluoroscopy. The integrated design allows for rigid screw fixation without any added anterior profile. AVS Anchor-C screws feature an outer clip which engages the titanium face plate on the cage. The interbody device is offered in a variety of lengths, heights and lordotic angles. Cages AVS Anchor-C cages are available in 2 footprints, 3 lordosis options and 7 heights from 6mm - 12mm. The cage heights are measured anteriorly. The posterior cage heights are approximately 1mm shorter than the anterior height for every 4 degrees of lordosis. Screw Types and Sizes AVS Anchor-C screws are available in fixed angle and are offered as self-tapping, featuring a cutting flute and a less aggressive screw tip, and self-drilling, designed with a sharp tip for insertion without prior drilling. Self-Tapping 10mm Self-Drilling Diameter: 3.5mm Length: 8-14mm Diameter: 4.0mm Length: 8-14mm 6mm cage with a 10mm screw Inserted 10mm Diameter: 3.5mm Length: 8-14mm Diameter: 4.0mm Length: 8-14mm Note: The screw size indicates the amount of screw purchase in the bone. For example a 10mm screw has 10mm of purchase in bone along the 35 angle. Further, the amount of screw purchase in a given length screw is equivalent across all cage heights. For example, the screw purchase of a 10mm screw in a 6mm cage is equivalent to the screw purchase of a 10mm screw in a 9mm cage. Screw Angulation Cephalad/caudal angulation of 35 Medial/lateral convergence of 10 9mm cage with a 10mm screw Inserted 6
7 Implantation Technique Step 1. Patient Positioning and Exposure A direct anterior approach should be selected for the AVS Anchor-C Cervical Cage System. The patient is placed in a supine position with the head turned slightly away from the side of the approach. A transverse or oblique incision parallel to the skin creases of the neck is recommended. Either a left side or right side approach can be used. After blunt dissection through the various layers, the anterior cervical spine is gently exposed. After exposing the vertebral bodies to be fused, prepare the fusion site following the appropriate technique for the given indication. Care should be taken to remove any bony anatomy or osteophytes which might interfere with the instrumentation, as this is required to position the cage flush with the anterior aspect of the cervical spine. Note: Patient positioning should follow the surgeon s standard technique for any anterior cervical discectomy and fusion. Pin Guide Distractor Note: When previous instrumentation is present, pre-operative planning is strongly encouraged to help avoid implant contact. Step 2. Distraction Pin Placement It is recommended to insert the Anchor-C cage under distraction. If distraction pins are used, it is recommended to use either the Pin Guide Distractor or the Guide/ Inserter Tip as a template when placing the distraction pins. Due to the angulation and positioning of the Guide/Inserter Tip, placing the distraction pins centrally into the vertebral bodies may result in the inability to insert the cage using the Guide/ Inserter Tip. Option 1: Use of Pin Guide Distractor To place your distraction pins using the Pin Guide Distractor, a small window is cut into the anterior annulus to allow for insertion of the Pin Guide Distractor. When the Pin Guide Distractor is positioned correctly, distract the instrument such that the top of the paddles is in contact with the endplates. Insert the Straight Awl through each of the guides in the Pin Guide Distractor to create pilot holes for the distraction pins. Remove the Pin Guide Distractor from the disc space and insert distraction pins into the vertebral body using the Pin Inserter from the Reliance C instrumentation set. Note: The pins should be positioned slightly off from center to allow the Guide/ Inserter Tip to be positioned correctly when inserting the cage. 7
8 Option 2: Use of Guide/Inserter Tip To place your distraction pins using the Guide/Inserter Tip, lay the Guide/Inserter Tip on top of the exposed vertebral bodies in the disc space. A cage can be threaded onto the Guide/Inserter Tip during this step to prevent the Guide/Inserter Tip from spinning. When the Guide/Inserter Tip is positioned correctly, the remaining exposed portion of the vertebral body just lateral to the Guide/Inserter Tip should reveal an area where the distraction pins can be placed. Once the pins are inserted into the vertebral body, slide the Parallel Pin Distractor from the Reliance C instrumentation set over the pins and distract the disc space. Step 3. Disc Removal and Endplate Preparation Use preferred surgical approach and technique for the discectomy using curettes, rongeurs, rasps or high speed drills. Step 4. Cage Selection To assist in determining the appropriate cage, several different Cage Trials are available in the set. These trials are designed to approximate the overall height, angulation, and endplate dimensions of the disc space. Each trial option has a matching cage. Trial Options: Height Angulation 0, 4, 8 Footprint 6, 7, 8, 9, 10, 11, 12mm 12 x 14mm, 14 x 16mm Cage Trial See chart on page 19 for Trial part numbers Note: The trials measure line to line (e.g., a 6mm trial measures 6mm), but cage sizes are oversized because they do not include the implant teeth that are.5mm on each side (e.g., a 6mm cage measures 7mm including the teeth). Quick Release Handle Insert the appropriate trial into the Quick Release Handle and carefully insert into the disc space. The trial should pass into the distracted disc space without excessive force. A mallet can be used to aid insertion or removal of the trial. 8
9 Step 5. Cage Preparation and Insertion Once the appropriate cage has been selected, bone graft can be packed into the cage. 6mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip The Guide/Inserter Tip has a size corresponding to each implant height. Select the appropriate Guide/Inserter Tip and insert into the Quick Release Handle. Check both the engraving and the laser-marking on the shaft of the Guide/Inserter Tip to ensure it is the intended size. The cage can now be threaded onto the Guide/Inserter Tip by holding the cage between the thumb and forefinger of one hand and the shaft of the inserter in the other. Continue turning the inserter shaft until it cannot be rotated any further. Tip: When threading the cage onto the Guide/Inserter Tip, hold the instrument at the shaft/tip junction. This will keep the Guide/Inserter Tip from rotating during threading. Note: The cage should be fully threaded to the Guide/Inserter Tip so that it is snug. How to Hold Guide/Inserter Tip How to Attach Anchor-C Cage Note: Applying excessive cantilever force to the Guide/Inserter Tip may cause damage to the instrument. Once the cage is attached to the Guide/Inserter Tip, the assembly should be carefully impacted into the disc space with a mallet. The Guide/Inserter Tip provides a positive stop to place the cage flush with the anterior profile of the vertebral bodies. At this point, distraction should be removed from the disc space and removal of the distractor from the pins is recommended. Before proceeding to the next step, ensure the cage is still tight to the Guide/Inserter Tip. The Quick Release Handle should be removed from the assembly, leaving the Guide/Inserter Tip in place. Note: Excessive pivoting or angulation on the Guide/Inserter Tip while attached to the cage should be avoided as it can damage the instrument. A tantalum marker is embedded into the cage to help visually confirm the posterior position under fluoroscopy. The tantalum marker spans the entire height of the cage to indicate posterior contact with the vertebral endplates and is 1mm from the cage s posterior edge. Tantalum Marker 9
10 Step 6. Screw Hole Preparation and Insertion The Guide/Inserter Tip (without Quick Release Handle) should be left in place when preparing the screw hole. The Guide/Inserter Tip can accommodate awls, drills and screwdrivers. With self-drilling screws: With self-tapping screws: Awl Insert Screw Awl Drill Insert Screw Straight Awl Note: Self-drilling screws do not need prior drilling. However, drilling is required with self-tapping screws. First, create a pilot hole in the vertebral body using the Straight Awl or Angled Awl through the Guide/Inserter Tip for one of the screw holes. Next, select the appropriate sized drill, attach the drill to the Quick Release Handle, and drill a hole into the vertebral body through the Guide/Inserter Tip. Angled Awl Note: There is no angled or flexible drill, so if self-tapping screws are used, a straight drill must also be used. Tip: It is recommend to use self-drilling screws and the angled awl if patient anatomy impedes access to the implant. Drill Bits, which are available in 2.3mm diameter and 3 lengths (8, 10 and 12mm) provide a positive stop (when inserted in a Guide/Inserter Tip) for accurate drilling depth. 8mm Drill Bit mm Drill Bit mm Drill Bit Select the appropriate screw and confirm the length using the screw length gauge in the screw caddy. The screw size indicates the actual amount of screw purchase in the bone (e.g., a 10mm screw protrudes 10mm into the bone, while the head of the screw is contained within the cage). The length of screw used should correspond to the length of drill used to prepare the hole. Note: The 3.5mm screw should be used as the primary screw. The 4.0mm screw is provided as a rescue option and should not be used as the primary screw. 10
11 Due to the position of the screw holes in the cage, the amount of screw purchase in the bone is the same for all heights of implants. However, with different screw lengths and different cage footprints, the screws may protrude beyond the posterior end of the cage. For example, with a 12 x 14mm cage, the 8mm screw will be flush with the posterior end. The picture and tables below detail screw positions with respect to the AVS Anchor-C cage: Amount above Amount protruding Screw Length 12 x 14mm Cage Screw Length Amount of screw past post edge Amount above 0 cage Amount above 4 cage Amount above 8 cage 8 Does not protrude x 16mm Cage Screw Length Amount of screw Amount above Amount above Amount above past post edge 0 cage 4 cage 8 cage 8 Does not protrude Does not protrude measurements in mm 11
12 Screws may be inserted using either the Straight Screwdriver or Flexible Screwdriver. Both screwdrivers feature a square drive with a split tip to hold the screw head securely. Using the screw caddy to load the screws and the Straight Screwdriver or Flexible Screwdriver (in a stab and grab motion), attach the screw to the screwdriver. Straight Screwdriver Flexible Screwdriver Place the screw and screwdriver assembly through the Guide/Inserter Tip and into the hole that has been previously prepared with the awl and/or drill. Note: When using the Flexible Screwdriver, it is recommended that the shaft not be bent past the range indicated in the images below. This will help prevent damage to the shaft. Tip: Do not excessively bend Flexible Screwdriver back and forth; bending the shaft to the full limit repeatedly may result in fracture. Turn until the gold tip and laser marking on the instrument disappear within the Guide/Inserter Tip. This indicates that the clip on the screw is engaged with the implant and the screw is locked. 12
13 Screw is locked when the gold color and the black line are no longer visible. Repeat screw hole preparation and insertion technique for the other screw hole. Note: To ensure proper depth and adequate locking when using the awl, drilling, or locking bone screws, use of a free hand technique is strongly discouraged. Use of the Guide/Inserter Tip is required. Note: Excessive pivoting or angulation on the screwdriver while attached to the screw should be avoided as it can cause damage to the screwdriver and/or screw. Unlocked Locked Remove the Guide/Inserter Tip by fully unthreading the cage. To ensure the screw is locked into the cage, check to make sure the grey clip around the screw is not visible. Tip: After the Guide Inserter/Tip has been removed, screws can be lagged to the bone by slowly turning each screw approximately one quarter turn. Once the cage is in the correct position and the screws are locked, the wound is closed in the normal fashion. 13
14 Implant Removal To remove the implant, first remove each of the bone screws using the Screw Extractor, and then use the Guide/Inserter Tip to remove the cage. Bone Screw Removal: Screw Extractor The Screw Extractor allows for removal of a bone screw from the cage after the screw has been locked within the cage. To begin removal of the screw, the outer sleeve of the Screw Extractor should be threaded up to the handle, to keep the sleeve from impeding visibility when seating the instrument within the screw. The Screw Extractor should then be fully seated within the screw. Ensure the instrument is aligned at the same 35 angle as the screw. Insert the Draw Rod through the instrument and thread into the screw until the knob will no longer turn. To remove the screw, unthread the outer sleeve towards the cage, ensuring that the angle on the tip mates with the face of the cage. Hold onto the outer sleeve and unthread the bone screw from the cage. It is inadvisable to attempt to remove the screw from the cage using only the Draw Rod. Note: Excessive pivoting or angulation on the Screw Extractor while attached to the screw should be avoided as it can cause damage to the Screw Extractor and/or screw. Note: Once a screw is removed from the cage after it has been locked, the screw cannot be reused. For cleaning, the outer sleeve should be completely unthreaded from the handle and the Draw Rod removed from within the handle. After the components have been cleaned the threaded handle should be dried prior to re-assembly to the outer threaded sleeve. 14
15 Cage Removal: To remove the cage, thread the cage with the Guide/Inserter Tip, and gently remove the cage from the disc space. Note: It is recommended that the Parallel Pin Distractor and Pins from the Reliance C instrumentation set be used to distract the disc space before removing the cage. If necessary, the entire construct (cage and screws) may be removed by using the Straight Screwdriver. If both screws are in the cage, alternate backing the screws out with the Straight Screwdriver and distracting the disc space until the construct is removed from the vertebral body. If only one screw remains in the cage, distract the disc space and use the Straight Screwdriver to backout the screw with the cage attached. 15
16 Implant Part Numbers AVS Anchor-C Cages 12 x 14mm Footprint Part # Description x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x x 12 x 14 x 8 14 x 16mm Footprint Part # Description x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x x 14 x 16 x 8 16
17 Bone Screws Screw Type Part # Size (Diameter x Length) Ø3.5mm x 8mm 3.5mm Self-Tapping Ø3.5mm x 10mm Ø3.5mm x 12mm Ø3.5mm x 14mm Ø3.5mm x 8mm 3.5mm Self-Drilling Ø3.5mm x 10mm Ø3.5mm x 12mm Ø3.5mm x 14mm Ø4.0mm x 8mm 4.0mm Self-Tapping Ø4.0mm x 10mm Ø4.0mm x 12mm Ø4.0mm x 14mm Ø4.0mm x 8mm 4.0mm Self-Drilling Ø4.0mm x 10mm Ø4.0mm x 12mm Ø4.0mm x 14mm 17
18 Instrument Part Numbers AVS Anchor-C General Instruments Part # Description Quick Release Handle Straight Awl Angled Awl mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Guide/Inserter Tip mm Drill Bit mm Drill Bit mm Drill Bit Straight Screwdriver Flexible Screwdriver Screw Extractor Pin Guide Distractor Screw Caddy Implant Caddy Container 18
19 AVS Anchor-C Trials 12 x 14mm Footprint (depth x width) 14 x 16mm Footprint (depth x width) Height Parallel (0 ) Lordotic (4 ) Lordotic (8 ) Parallel (0 ) Lordotic (4 ) Lordotic (8 ) 6mm mm mm mm mm mm mm
20 IMPORTANT PRODUCT INFORMATION FOR STRYKER SPINE AVS ANCHOR-C CERVICAL CAGE SYSTEM NON STERILE PRODUCT Description The AVS Anchor-C Cervical Cage is a hollow, rectangular-shaped PEEK Optima LT1 (per ASTM F2026) cage assembled to a titanium alloy (per ASTM F136 and ISO ) plate and has one tantalum marker (per ASTM F560). It is intended for use as an interbody fusion device and is offered in a variety of heights, footprints, and lordotic angles to adapt to varying patient anatomies. The PEEK Optima LT1 cage portion consists of one closed pocket for graft containment and has serrations on the superior and inferior surfaces of the cage. The implant is designed to be used exclusively with the internal supplemental fixation provided (AVS Anchor-C Fixation Screws). The AVS Anchor-C Fixation Screws are constructed from titanium alloy and possess clips that mate with internal features located within the AVS Anchor-C Cervical Cage. Once fully seated into the holes, the screws are designed to lock into the titanium plate. Materials All components of the system are manufactured out of the following materials: Cage: Polyetheretherketone (PEEK Optima LT1) (ASTM F2026), Titanium Alloy Ti6Al4V (ISO , ASTM F136) with a tantalum marker (ASTM F560) Fixation Screws: Titanium Alloy Ti6Al4V (ISO , ASTM F136) Indications The Stryker Spine AVS Anchor-C Cervical Cage System is indicated for anterior cervical interbody fusion procedures in skeletally mature patients with cervical disc disease at one level from the C2-C3 disc to the C7-T1 disc. Cervical disc disease is defined as intractable radiculopathy and/or myelopathy with herniated disc and/or osteophyte formation on posterior vertebral endplates producing symptomatic nerve root and/or spinal cord compression confirmed by radiographic studies. The AVS Anchor-C Cervical Cage is to be used with autogenous bone graft and implanted via an open, anterior approach. The AVS Anchor-C Cervical Cage must be used with the internal screw fixation provided by AVS Anchor-C Fixation Screws. This cervical device is to be used in patients who have had six weeks of non-operative treatment. General Conditions Of Use The implantation of intervertebral body fusion devices must be performed only by experienced spinal surgeons having 20 undergone the necessary specific training in the use of such systems because this is a technically demanding procedure presenting a risk of serious injury to the patient. The information contained in the Package Insert is necessary but not sufficient for the use of this device. This information is in no sense intended as a substitute for the professional judgment, skill and experience of the surgeon in careful patient selection, preoperative planning and device selection, knowledge of the anatomy and biomechanics of the spine, understanding of the materials and the mechanical characteristics of the implants used, training and skill in spinal surgery and the use of associated instruments for implantation, securing the patient s cooperation in following an appropriately defined post-operative management program and conducting scheduled post-operative follow-up examinations. Caution Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. This device is NOT intended to be used without the AVS Anchor-C fixation screws provided. Should removal of the AVS Anchor-C Fixation Screws be necessary during the surgery, the AVS Anchor-C cage should NOT be implanted alone, without the support of the AVS Anchor-C fixation screws. Instruments designed for use with implantation of the AVS Anchor-C Cervical Cage System are provided non-sterile and must be sterilized prior to use. This device is not intended for posterior surgical implantation. The AVS Anchor-C Cervical Cages have not been evaluated for safety and compatibility in the MR environment. AVS Anchor-C Cervical Cages have not been tested for heating or migration in the MR environment. Based on the fatigue testing results, the physician/surgeon must consider the levels of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact the performance of the intervertebral body fusion device. The implantation of the intervertebral body fusion device must be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient. Potential risks identified with the use of this intervertebral body fusion device, which may require additional surgery, include: device component fracture, loss of fixation, pseudoarthrosis (i.e. non-union), fracture of the vertebrae, neurological injury, and vascular or visceral injury. Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without a previous surgery. The components of the system should not be used with components of any other system or manufacturer. Any such use will negate the responsibility of Stryker Spine for the performance of the resulting mixed component implant. Do not mix metals (e.g. Titanium based devices with stainless steel items). Some corrosion occurs on all implanted metals and alloys. Contact of dissimilar metals, however, may accelerate corrosion. Corrosion may accelerate fatigue fracture of implants, and cause metal compounds to be released into the body. Infection Transient bacteremia can occur in daily life. Dental manipulation, endoscopic examination and other minor surgical procedures have been associated with transient bacteremia. To help prevent infection at the implant site, it may be advisable to use antibiotic prophylaxis before and after such procedures. Instruments Instruments are provided by STRYKER Spine and must be used to assure accurate implantation of the device. While rare, intraoperative fracture or breakage of instruments can occur. Instruments which have experienced extensive use or extensive force are more susceptible to fracture depending on the operative precaution, number of procedures, and disposal attention. Instruments must be examined for wear or damage prior to surgery. Reuse Never reuse or reimplant spinal surgical implants. These could become contaminated resulting in infection. In addition, even though the device appears undamaged, it may have small defects which could compromise structural integrity reducing its service life and/or leading to patient injury. Surgeons must verify that the instruments are in good condition and operating order prior to use during surgery.
21 Handling Correct handling of the implant is extremely important. The operating surgeon must avoid notching or scratching the device. Allergy and Hypersensitivity to Foreign Bodies When hypersensitivity is suspected or proven, it is highly recommended that the tolerance of the skin to the materials that make up the implants be checked before they are implanted. Contraindications Contraindications may be relative or absolute. The choice of a particular device must be carefully weighed against the patient s overall evaluation. Circumstances listed below may reduce the chances of a successful outcome: The AVS Anchor-C Cervical Cage should not be implanted in patients with an active infection at the operative site. The AVS Anchor-C Cervical Cage is not intended for use except as indicated. Marked local inflammation. Any abnormality present which affects the normal process of bone remodeling including, but not limited to, severe osteoporosis involving the spine, bone absorption, osteopenia, primary or metastatic tumors involving the spine, active infection at the site or certain metabolic disorders affecting osteogenesis. Any mental or neuromuscular disorder which would create an unacceptable risk of fixation failure or complications in postoperative care. Open wounds. Pregnancy. Inadequate tissue coverage over the operative site. Any neuromuscular deficit which places an unsafe load level on the device during the healing period. Obesity. An overweight or obese patient can produce loads on the spinal system which can lead to failure of the fixation of the device or to failure of the device itself. Obesity is defined according to the W.H.O. standards. A condition of senility, mental illness, or substance abuse. These conditions, among others, may cause the patient to ignore certain necessary limitations and precautions in the use of the implant, leading to failure or other complications. Foreign body sensitivity. Where material sensitivity is suspected, appropriate tests must be made prior to material selection or implantation. Other medical or surgical condition which would preclude the potential benefit of spinal implant surgery, such as the presence of tumors, congenital abnormalities, elevation of sedimentation rate unexplained by other diseases, elevation of white blood cell count (WBC), or marked left shift in the WBC differential count. Prior fusion at the levels to be treated These contra-indications can be relative or absolute and must be taken into account by the physician when making his decision. The above list is not exhaustive. Surgeons must discuss the relative contraindications with the patients. Information for Patients The surgeon must discuss all physical and psychological limitations inherent to the use of the device with the patient. This includes the rehabilitation regimen, physical therapy, and wearing an appropriate orthosis as prescribed by the physician. Particular discussion should be directed to the issues of premature weightbearing, activity levels, and the necessity for periodic medical follow-up. The surgeon must warn the patient of the surgical risks and make them aware of possible adverse effects. The surgeon must warn the patient that the device cannot and does not replicate the flexibility, strength, reliability or durability of normal healthy bone, that the implant can break or become damaged as a result of strenuous activity or trauma, and that the device may need to be replaced in the future. If the patient is involved in an occupation or activity which applies inordinate stress upon the implant (e.g., substantial walking, running, lifting, or muscle strain) the surgeon must advise the patient that resultant forces can cause failure of the device. Patients who smoke have been shown to have an increased incidence of non-unions. Such patients should be advised of this fact and warned of the potential consequences. For patients with degenerative disease, the progression of degenerative disease may be so advanced at the time of implantation that it may substantially decrease the expected useful life of the appliance. In such cases, orthopaedic devices may be considered only as a delaying technique or to provide temporary relief. Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without a previous surgery. Pre-Operative Precautions The surgical indication and the choice of implants must take into account certain important criteria such as: Patients involved in an occupation or activity that applies excessive loading upon the implant (e.g., substantial walking, running, lifting, or muscle strain) may be at increased risk for failure of the fusion and/or the device. Surgeons must instruct patients in detail about the limitations of the implants, including, but not limited to, the impact of excessive loading through patient weight or activity, and be taught to govern their activities accordingly. The procedure will not restore function to the level expected with a normal, healthy spine, and the patient should not have unrealistic functional expectations. A condition of senility, mental illness, chemical dependence or alcoholism. These conditions among others may cause the patients to ignore certain necessary limitations and precautions in the use of the implant, leading to failure and other complications. Foreign body sensitivity. Where material sensitivity is suspected appropriate tests should be made prior to material implantation. Surgeons must advise patients who smoke have been shown to have an increased incidence of non-unions. Such patients must be advised of this fact and warned of the potential consequences. Care must be taken to protect the components from being marred, nicked, or notched as a result of contact with metal or abrasive objects. The Choice of Implants The choice of proper shape, size and design of the implant for each patient is crucial to the success of the surgery. The surgeon is responsible for this choice, which depends on each patient. Patients who are overweight may be responsible for additional stresses and strains on the device which can speed up implant fatigue and/or lead to deformation or failure of the implants. The size and shape of the bone structures determine the size, shape and type of the implants. Once implanted, the implants are subjected to stresses and strains. These repeated stresses on the implants must be taken into consideration by the surgeon at the time of the choice of the implant, during implantation as well as in the post-operative follow-up period. Indeed, the stresses and strains on the implants may cause fatigue, fracture or deformation of the implants, before the bone graft has become completely consolidated. This may result in further side effects or necessitate the early removal of the osteosynthesis device. Intra-Operative Precautions The insertion of the implants must be carried out using instruments designed and provided for this purpose and in accordance with the specific implantation instructions for each implant. Those detailed instructions are provided in the surgical technique brochure supplied by STRYKER Spine. 21
22 Discard all damaged or mishandled implants. Never reuse an implant, even though it may appear undamaged. Patient Care Following Treatment Prior to adequate maturation of the fusion mass, implanted spinal instrumentation may need additional help to accommodate full load bearing. External support may be recommended by the physician from two to four months postoperatively or until x-rays or other procedures confirm adequate maturation of the fusion mass; external immobilization by bracing or casting may be employed. Surgeons must instruct patients regarding appropriate and restricted activities during consolidation and maturation for the fusion mass in order to prevent placing excessive stress on the implants which may lead to fixation or implant failure and accompanying clinical problems. Surgeons must instruct patients to report any unusual changes of the operative site to his/her physician. The physician must closely monitor the patient if a change at the site has been detected. Adverse Effects Include but are not limited to: Late bone fusion or no visible fusion mass and pseudarthrosis; While the expected life of spinal implant components is difficult to estimate, it is finite. These components are made of foreign materials which are placed within the body for the potential fusion of the spine and reduction of pain. However, due to the many biological, mechanical and physicochemical factors which affect these devices but cannot be evaluated in vivo, the components cannot be expected to indefinitely withstand the activity level and loads of normal healthy bone; Superficial or deep-set infection and inflammatory phenomena; Allergic reactions to the implanted materials, although uncommon, can occur; Decrease in bone density due to stress shielding; Dural leak requiring surgical repair; Peripheral neuropathies, nerve damage, heterotopic bone formation and neurovascular compromise, including paralysis, loss of bowel or bladder function, or foot-drop may occur. Cessation of growth of the fused portion of the spine; Loss of proper spinal curvature, correction, height and/or reduction; Delayed Union or Nonunion: Internal fixation appliances are load sharing devices which are used to obtain alignment until normal healing occurs. In the event that healing is delayed, does not occur, or failure to immobilize the delayed/nonunion 22 results, the implant will be subject to excessive and repeated stresses which can eventually cause loosening, bending or fatigue fracture. The degree or success of union, loads produced by weight bearing, and activity levels will, among other conditions, dictate the longevity of the implant. If a nonunion develops or if the implants loosen, bend or break, the device(s) must be revised or removed immediately before serious injury occurs; Neurological and spinal dura mater lesions from surgical trauma; Early loosening may result from inadequate initial fixation, latent infection, premature loading of the device or trauma. Late loosening may result from trauma, infection, biological complications or mechanical problems, with the subsequent possibility of bone erosion, or pain. Serious complications may occur with any spinal surgery. These complications include, but are not limited to, genitourinary disorders; gastrointestinal disorders; vascular disorders, including thrombus; bronchopulmonary disorders, including emboli; bursitis, hemorrhage, myocardial infarction, infection, paralysis or death. Inappropriate or improper surgical placement of this device may cause distraction or stress shielding of the graft or fusion mass. This may contribute to failure of an adequate fusion mass to form. Intraoperative fissure, fracture, or perforation of the spine can occur due to implantation of the components. Postoperative fracture of bone graft or the intervertebral body above or below the level of surgery can occur due to trauma, the presence of defects, or poor bone stock. Adverse effects may necessitate reoperation or revision. The surgeon must warn the patient of these adverse effects as deemed necessary. Removal If fusion / bone graft growth occurs, the device will be deeply integrated into the bony tissues. As a result, the AVS Anchor-C is not intended to be removed unless the management of a complication or adverse event requires the removal. Any decision by a physician to remove the device should take into consideration such factors as: The risk to the patient of the additional surgical procedure as well as the difficulty of removal. Migration of the implant, with subsequent pain and/or neurological, articular or soft tissue lesions Pain or abnormal sensations due to the presence of the implants Infection or inflammatory reactions Reduction in bone density due to the different distribution of mechanical and physiological stresses and strains. Packaging The implants are single use devices, provided non-sterile, and delivered in individual packages. The typical packaging used is clear plastic tubes and polyethylene bags. The packages must be intact at the time of receipt. Implants must be removed entirely from their packaging prior to sterilization. The implants may also be supplied as a complete set: implants and instruments are arranged on trays and placed in specially designed storage boxes. Complaints Any health professional having a complaint or grounds for dissatisfaction relating to the quality of the product, its identity, its durability, its reliability, safety, effectiveness and / or its performance, should notify STRYKER Spine or its representative. Moreover, if a device has malfunctioned, or is suspected of having malfunctioned, STRYKER Spine or its representative must be advised immediately. If a STRYKER Spine product has ever worked improperly and could have caused or contributed to the death of or serious injury to a patient, the distributor or STRYKER Spine must be informed as soon as possible by telephone, fax or in writing. For all complaints, please give the name and reference along with the batch number of the component(s), your name and address and a complete description of the event to help STRYKER Spine understand the causes of the complaint. For further information or complaints, please contact: STRYKER Spine ZI de Marticot, CESTAS France Tel. (33) (0) Fax. (33) (0) (Customer Service) Fax. (33) (0) (Quality Assurance) STRYKER Spine 2 Pearl Court Allendale, NJ USA Tel Fax (Customer Service)
23 Notes 23
24 US Operations 2 Pearl Court Allendale, New Jersey Phone: Fax: Web: EU Operations ZI Marticot Cestas France t: +33 (0) f: +33 (0) A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery. The information presented is intended to demonstrate the breadth of Stryker product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area. Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: AVS, Stryker. All other trademarks are trademarks of their respective owners or holders. Literature Number: CVANCST11042 SC/GS 11/11 Copyright 2011 Stryker Printed in USA
(English) NEXUS SPINE SPACER SYSTEM
 (English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
(English) NEXUS SPINE SPACER SYSTEM INDICATIONS FOR USE NEXUS Spine Spacer System, a GEO Structure is indicated for use in the thoraco-lumbar spine (i.e., T1 to L5) to replace a diseased vertebral body
4052 Slimplicity Tech final_layout 1 6/29/15 3:29 PM Page 2 Surgical Technique
 Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
Surgical Technique TABLE OF CONTENTS Slimplicity Anterior Cervical Plate System Overview 2 Indications 2 Implants 3 Instruments 4 Surgical Technique 6 1. Patient Positioning and Approach 6 2. Plate Selection
TABLE OF CONTENTS. Surgical Technique 2. Indications 4. Product Information 5. 1. Patient Positioning and Approach 2
 Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Surgical Technique TABLE OF CONTENTS Surgical Technique 2 1. Patient Positioning and Approach 2 2. Intervertebral Device Implanted 2 3. Buttress Plate Selection 2 4. Awl Insertion 2 5. Screw Insertion
Information for the Patient About Surgical
 Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
Information for the Patient About Surgical Decompression and Stabilization of the Spine Aging and the Spine Daily wear and tear, along with disc degeneration due to aging and injury, are common causes
ANTERIOR LUMBAR INTERBODY FUSION (ALIF) Basic Anatomical Landmarks: Anterior Lumbar Spine
 (ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
(ALIF) Anterior In human anatomy, referring to the front surface of the body or the position of one structure relative to another Lumbar Relating to the loins or the section of the back and sides between
ANTERIOR CERVICAL DISCECTOMY AND FUSION. Basic Anatomical Landmarks: Anterior Cervical Spine
 Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
Anterior In the human anatomy, referring to the front surface of the body or position of one structure relative to another Cervical Relating to the neck, in the spine relating to the first seven vertebrae
Degenerative Spine Solutions
 Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
Degenerative Spine Solutions The Backbone for Your Surgical Needs Aesculap Spine Backbone for Your Degenerative Spine Needs Comprehensive operative solutions, unique product technology and world-class
BRYAN. Cervical Disc System. Patient Information
 BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
BRYAN Cervical Disc System Patient Information 3 BRYAN Cervical Disc System PATIENT INFORMATION BRYAN Cervical Disc System PATIENT INFORMATION 1 BRYAN Cervical Disc System This patient information brochure
Orthopaedic Spine Center. Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs
 Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
Orthopaedic Spine Center Graham Calvert MD James Woodall MD PhD Anterior Cervical Discectomy and Fusion (ACDF) Normal Discs The cervical spine consists of the bony vertebrae, discs, nerves and other structures.
Anterior Lumbar Interbody Fusion (ALIF). Instrument set supports placement of ALIF spacers using anterior or anterolateral approach.
 Anterior Lumbar Interbody Fusion (ALIF). Instrument set supports placement of ALIF spacers using anterior or anterolateral approach. Technique Guide Instruments and implants approved by the AO Foundation
Anterior Lumbar Interbody Fusion (ALIF). Instrument set supports placement of ALIF spacers using anterior or anterolateral approach. Technique Guide Instruments and implants approved by the AO Foundation
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF).
 Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
Patient Information. Lateral Lumbar Interbody Fusion Surgery (LLIF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra, allows
SPINAL FUSION. North American Spine Society Public Education Series
 SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
SPINAL FUSION North American Spine Society Public Education Series WHAT IS SPINAL FUSION? The spine is made up of a series of bones called vertebrae ; between each vertebra are strong connective tissues
FUSEFORCE. Hand Fixation System SURGIC A L T ECHNIQUE
 FUSEFORCE Hand Fixation System SURGIC A L T ECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
FUSEFORCE Hand Fixation System SURGIC A L T ECHNIQUE Contents Chapter 1 3 Chapter 2 4 Appendix A 7 Intended Use Surgical Technique Ordering Information Indication Sizing Reference Proper surgical procedures
CeSpace Titanium / PEEK
 Aesculap Anterior Cervical Interbody Fusion System CeSpace Titanium / PEEK Aesculap Spine CeSpace Content A Foreword 3 B Implant Material 4 C Implant Features 6 D Surgical Technique 8 E Ordering Information
Aesculap Anterior Cervical Interbody Fusion System CeSpace Titanium / PEEK Aesculap Spine CeSpace Content A Foreword 3 B Implant Material 4 C Implant Features 6 D Surgical Technique 8 E Ordering Information
Patient Guide to Neck Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
The following is a sampling of products offered by Zimmer Spine for use in Anterior Cervical Fusion procedures. Patient Guide to Neck Surgery Anterior Cervical Fusion Trinica Select With the Trinica and
Zimmer Small Fragment Universal Locking System. Surgical Technique
 Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
ZERO-P VA. Variable angle zero-profile anterior cervical interbody fusion (ACIF) device SURGICAL TECHNIQUE
 ZERO-P VA Variable angle zero-profile anterior cervical interbody fusion (ACIF) device SURGICAL TECHNIQUE Table of Contents Introduction Zero-P VA Instruments and Implants 2 AO Principles 4 Indications
ZERO-P VA Variable angle zero-profile anterior cervical interbody fusion (ACIF) device SURGICAL TECHNIQUE Table of Contents Introduction Zero-P VA Instruments and Implants 2 AO Principles 4 Indications
.org. Fractures of the Thoracic and Lumbar Spine. Cause. Description
 Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Fractures of the Thoracic and Lumbar Spine Page ( 1 ) Spinal fractures can vary widely in severity. While some fractures are very serious injuries that require emergency treatment, other fractures can
Patient Guide to Lower Back Surgery
 The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
The following is a sampling of products offered by Zimmer Spine for use in Open Lumbar Fusion procedures. Patient Guide to Lower Back Surgery Open Lumbar Fusion Dynesys The Dynesys Dynamic Stabilization
Anterior Cervical Discectomy and Fusion
 A Patient s Guide to Anterior Cervical Discectomy and Fusion 651 Old Country Road Plainview, NY 11803 Phone: 5166818822 Fax: 5166813332 p.lettieri@aol.com DISCLAIMER: The information in this booklet is
A Patient s Guide to Anterior Cervical Discectomy and Fusion 651 Old Country Road Plainview, NY 11803 Phone: 5166818822 Fax: 5166813332 p.lettieri@aol.com DISCLAIMER: The information in this booklet is
Lentur Cable System. Surgical Technique
 Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
Lentur Cable System Surgical Technique Contents Introduction... Page 1 System Design Features And Benefits... Page 2 Implants... Page 3 Instrumentation... Page 4 Surgical Technique... Page 5 Single Cable...
A Patient s Guide to Artificial Cervical Disc Replacement
 A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
A Patient s Guide to Artificial Cervical Disc Replacement Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness
Minimally Invasive Lumbar Fusion
 Minimally Invasive Lumbar Fusion Biomechanical Evaluation (1) coflex-f screw Biomechanical Evaluation (1) coflex-f intact Primary Stability intact Primary Stability Extension Neutral Position Flexion Coflex
Minimally Invasive Lumbar Fusion Biomechanical Evaluation (1) coflex-f screw Biomechanical Evaluation (1) coflex-f intact Primary Stability intact Primary Stability Extension Neutral Position Flexion Coflex
Y O U R S U R G E O N S. choice of. implants F O R Y O U R S U R G E R Y
 Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Y O U R S U R G E O N S choice of implants F O R Y O U R S U R G E R Y Your Surgeon Has Chosen the C 2 a-taper Acetabular System The
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF).
 Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
Patient Information. Anterior Cervical Discectomy and Fusion Surgery (ACDF). Understanding your spine Disc Between each pair of vertebrae there is a disc that acts as a cushion to protect the vertebra,
PRESTIGE LP. Cervical Disc System. Patient Information
 PRESTIGE LP Cervical Disc System Patient Information 3 PRESTIGE LP Cervical Disc System Patient Information This patient information brochure is designed to help you understand one treatment option for
PRESTIGE LP Cervical Disc System Patient Information 3 PRESTIGE LP Cervical Disc System Patient Information This patient information brochure is designed to help you understand one treatment option for
Adult Forearm Fractures
 Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
Adult Forearm Fractures Your forearm is made up of two bones, the radius and ulna. In most cases of adult forearm fractures, both bones are broken. Fractures of the forearm can occur near the wrist at
The information contained in this document is intended for healthcare professionals only.
 The information contained in this document is intended for healthcare professionals only. Dall-Miles Cabling System Dall-Miles Recon and Trauma Cable System Trochanteric Reattachment Using the Trochanteric
The information contained in this document is intended for healthcare professionals only. Dall-Miles Cabling System Dall-Miles Recon and Trauma Cable System Trochanteric Reattachment Using the Trochanteric
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study
 Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
Options for Cervical Disc Degeneration A Guide to the Fusion Arm of the M6 -C Artificial Disc Study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE:
 James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
James A. Sanfilippo, M.D. CONSENT FOR SPINAL SURGERY PATIENT: DATE: 1. I have been strongly advised to carefully read and consider this operative permit. I realize that it is important that I understand
Exeter. Surgical Technique. V40 Stem Cement-in-Cement. Orthopaedics
 Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Exeter Orthopaedics V40 Stem Cement-in-Cement Surgical Technique Exeter V40 Stem Cement-in-Cement Surgical Technique Table of Contents Indications and Contraindications...2 Warnings and Precautions...2
Options for Cervical Disc Degeneration A Guide to the M6-C. clinical study
 Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Options for Cervical Disc Degeneration A Guide to the M6-C clinical study Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause
Technique Guide. Screw Removal Set. Instruments for removing Synthes screws.
 Technique Guide Screw Removal Set. Instruments for removing Synthes screws. Table of Contents Introduction Screw Removal Set 2 Surgical Technique Preoperative Planning and Preparation 6 Removal of Intact
Technique Guide Screw Removal Set. Instruments for removing Synthes screws. Table of Contents Introduction Screw Removal Set 2 Surgical Technique Preoperative Planning and Preparation 6 Removal of Intact
Knotilus TM. Anchor Instability Repair. Technique Guide
 Knotilus TM Anchor Instability Repair Technique Guide Instability Repair Using the Knotilus TM Anchor Introduction While the shoulder has more mobility than any other joint in the body, it is also the
Knotilus TM Anchor Instability Repair Technique Guide Instability Repair Using the Knotilus TM Anchor Introduction While the shoulder has more mobility than any other joint in the body, it is also the
Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion
 Surgical Technique Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion Prepared in consultation with: Phinit Phisitkul, MD Department of Orthopedics and Rehabilitation University of Iowa
Surgical Technique Foot and Ankle Technique Guide Proximal Inter-Phalangeal (PIP) Fusion Prepared in consultation with: Phinit Phisitkul, MD Department of Orthopedics and Rehabilitation University of Iowa
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine
 A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
A Patient s Guide to the Disorders of the Cervical and Upper Thoracic Spine General Anatomy of the Spine The spine can be divided into four regions based on vertebral shape and sagittal plane curve.» CERVICAL:
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery.
 Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
Orthopedic Foot Instruments. Dedicated instruments for reconstructive foot surgery. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by
Zimmer Periarticular Proximal Tibial Locking Plate
 Zimmer Periarticular Proximal Tibial Locking Plate Surgical Technique The Science of the Landscape Zimmer Periarticular Proximal Tibial Locking Plate 1 Table of Contents Introduction 2 Locking Screw Technology
Zimmer Periarticular Proximal Tibial Locking Plate Surgical Technique The Science of the Landscape Zimmer Periarticular Proximal Tibial Locking Plate 1 Table of Contents Introduction 2 Locking Screw Technology
Posterior Cervical Decompression
 Posterior Cervical Decompression Spinal Unit Tel: 01473 702032 or 702097 Issue 2: January 2009 Following your recent MRI scan and consultation with your spinal surgeon, you have been diagnosed with a
Posterior Cervical Decompression Spinal Unit Tel: 01473 702032 or 702097 Issue 2: January 2009 Following your recent MRI scan and consultation with your spinal surgeon, you have been diagnosed with a
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary?
 White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
White Paper: Cervical Disc Replacement: When is the Mobi-C Cervical Disc Medically Necessary? For Health Plans, Medical Management Organizations and TPAs Cervical Disc Disease: An Overview The cervical
Wrist and Hand. Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Fractures of the Wrist and Hand: Carpal bones
 Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
Patient Information Guide to Bone Fracture, Bone Reconstruction and Bone Fusion: Wrist and Hand Fractures of the Wrist and Hand: Fractures of the wrist The wrist joint is made up of the two bones in your
TABLE OF CONTENTS. Indications and Contraindications 3. Features and Benefits 4. Ease of connection 4 Stable fixation concept 5
 Surgical Technique TABLE OF CONTENTS Indications and Contraindications 3 Features and Benefits 4 Ease of connection 4 Stable fixation concept 5 Surgical Technique Steps 6 Identification and preparation
Surgical Technique TABLE OF CONTENTS Indications and Contraindications 3 Features and Benefits 4 Ease of connection 4 Stable fixation concept 5 Surgical Technique Steps 6 Identification and preparation
The Total Ankle Replacement
 The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
The Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please consult your physician
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS?
 SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
SPINAL STENOSIS Information for Patients WHAT IS SPINAL STENOSIS? The spinal canal is best imagined as a bony tube through which nerve fibres pass. The tube is interrupted between each pair of adjacent
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, The Cervical Spine. What is the Cervical Spine?
 Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
Each year, hundreds of thousands of adults are diagnosed with Cervical Disc Degeneration, an upper spine condition that can cause pain and numbness in the neck, shoulders, arms, and even hands. This patient
visualized. The correct level is then identified again. With the use of a microscope and
 SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
SURGERY FOR SPINAL STENOSIS Laminectomy A one inch (or longer for extensive stenosis) incision is made in the middle of the back over the effected region of the spine. The muscles over the bone are moved
How To Use A Phoenix Retrograde Femoral Nail
 Phoenix Retrograde Femoral Nail System Featuring CoreLock Technology Surgical Technique Contents Introduction... Page 1 Indications... Page 2 Design Features... Page 3 Surgical Technique... Page 6 Product
Phoenix Retrograde Femoral Nail System Featuring CoreLock Technology Surgical Technique Contents Introduction... Page 1 Indications... Page 2 Design Features... Page 3 Surgical Technique... Page 6 Product
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances?
 Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
Does the pain radiating down your legs, buttocks or lower back prevent you from walking long distances? Do you experience weakness, tingling, numbness, stiffness, or cramping in your legs, buttocks or
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS?
 IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
IMPLANT CONSENT FORM WHAT ARE DENTAL IMPLANTS? Dental implants are a very successful and accepted treatment option to replace lost or missing teeth. A dental implant is essentially an artificial tooth
Patient Labeling Information System Description
 Patient Labeling Information System Description The Trident Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The
Patient Labeling Information System Description The Trident Ceramic Acetabular System is an artificial hip replacement device that features a new, state-of-the-art ceramic-on-ceramic bearing couple. The
SAMBA Screw System INSTRUCTIONS FOR USE TABLE OF CONTENTS. Description. Indications. Contraindications. Warnings. Potential Adverse Events
 SAMBA Screw System INSTRUCTIONS FOR USE SAMBA Screw System TABLE OF CONTENTS Description Indications Contraindications Warnings Potential Adverse Events How Supplied and Handled Inspection Cleaning Sterilization
SAMBA Screw System INSTRUCTIONS FOR USE SAMBA Screw System TABLE OF CONTENTS Description Indications Contraindications Warnings Potential Adverse Events How Supplied and Handled Inspection Cleaning Sterilization
ZEPHIR Anterior Cervical Plate System
 SOFAMOR DANEK ZEPHIR Anterior Cervical Plate System Surgical Technique as described by: Richard Assaker, M.D. Lille University Hospital Lille, France Paul McCormick, M.D. Columbia Presbyterian Hospital
SOFAMOR DANEK ZEPHIR Anterior Cervical Plate System Surgical Technique as described by: Richard Assaker, M.D. Lille University Hospital Lille, France Paul McCormick, M.D. Columbia Presbyterian Hospital
V-TEK IVP System 2.7 System 4.0
 V-TEK IVP System Ankle 2.7 Fix System 4.0 Surgical Technique Surgical Technique Titanium osteosynthesis system for tibio-talar and tibio-talo-calcaneal fusion SECTION 1 Ankle Fix System 4.0 Titanium osteosynthesis
V-TEK IVP System Ankle 2.7 Fix System 4.0 Surgical Technique Surgical Technique Titanium osteosynthesis system for tibio-talar and tibio-talo-calcaneal fusion SECTION 1 Ankle Fix System 4.0 Titanium osteosynthesis
.org. Herniated Disk in the Lower Back. Anatomy. Description
 Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
BONE PRESERVATION STEM
 TRI-LOCK BONE PRESERVATION STEM Featuring GRIPTION Technology SURGICAL TECHNIQUE IMPLANT GEOMETRY Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK BONE PRESERVATION STEM Featuring GRIPTION Technology SURGICAL TECHNIQUE IMPLANT GEOMETRY Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery.
 Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Scout Vessel Guard. A cover for vessels during anterior lumbar spine surgery. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Scout Vessel Guard 2
Hip Replacement Surgery Understanding the Risks
 Hip Replacement Surgery Understanding the Risks Understanding the Risks of Hip Replacement Surgery Introduction This booklet is designed to help your doctor talk to you about the most common risks you
Hip Replacement Surgery Understanding the Risks Understanding the Risks of Hip Replacement Surgery Introduction This booklet is designed to help your doctor talk to you about the most common risks you
THE LUMBAR SPINE (BACK)
 THE LUMBAR SPINE (BACK) At a glance Chronic back pain, especially in the area of the lumbar spine (lower back), is a widespread condition. It can be assumed that 75 % of all people have it sometimes or
THE LUMBAR SPINE (BACK) At a glance Chronic back pain, especially in the area of the lumbar spine (lower back), is a widespread condition. It can be assumed that 75 % of all people have it sometimes or
Minimally Invasive Spine Surgery For Your Patients
 Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
Minimally Invasive Spine Surgery For Your Patients Lukas P. Zebala, M.D. Assistant Professor Orthopaedic and Neurological Spine Surgery Department of Orthopaedic Surgery Washington University School of
NCB Distal Femur System. Surgical Technique
 NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
Advances In Spine Care. James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery
 Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Advances In Spine Care James D. Bruffey M.D. Scripps Clinic Division of Orthopaedic Surgery Section of Spinal Surgery Introduction The Spine - A common source of problems Back pain is the #2 presenting
Temple Physical Therapy
 Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery
 Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Marc A. Cohen, MD, FAAOS, FACS Diplomate American Board of Spinal Surgery Fellow American College of Spinal Surgery 221 Madison Ave Morristown, New Jersey 07960 (973) 538 4444 Fax (973) 538 0420 Patient
Technique Guide. VersiTomic. Michael A. Rauh, MD. Anterior Cruciate Ligament Reconstruction
 Technique Guide VersiTomic Anterior Cruciate Ligament Reconstruction Michael A. Rauh, MD The opinions expressed are those of Dr. Rauh and are not necessarily those of Stryker VersiTomic Anterior Cruciate
Technique Guide VersiTomic Anterior Cruciate Ligament Reconstruction Michael A. Rauh, MD The opinions expressed are those of Dr. Rauh and are not necessarily those of Stryker VersiTomic Anterior Cruciate
Lumbar Laminectomy and Interspinous Process Fusion
 Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Lumbar Laminectomy and Interspinous Process Fusion Introduction Low back and leg pain caused by pinched nerves in the back is a common condition that limits your ability to move, walk, and work. This condition
Patient Information. Posterior Cervical Surgery. Here to help. Respond Deliver & Enable
 Here to help Our Health Information Centre (HIC) provides advice and information on a wide range of health-related topics. We also offer: Services for people with disabilities. Information in large print,
Here to help Our Health Information Centre (HIC) provides advice and information on a wide range of health-related topics. We also offer: Services for people with disabilities. Information in large print,
Minimally Invasive Spine Surgery
 Chapter 1 Minimally Invasive Spine Surgery 1 H.M. Mayer Primum non nocere First do no harm In the long history of surgery it always has been a basic principle to restrict the iatrogenic trauma done to
Chapter 1 Minimally Invasive Spine Surgery 1 H.M. Mayer Primum non nocere First do no harm In the long history of surgery it always has been a basic principle to restrict the iatrogenic trauma done to
Integra. Subtalar MBA Implant
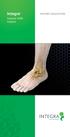 Integra Subtalar MBA Implant Patient EDUCATION Overview: What is Flatfoot? Flatfoot is a physical deformity where there is an absence of the arch that runs from the heel of the foot to the toes. A common
Integra Subtalar MBA Implant Patient EDUCATION Overview: What is Flatfoot? Flatfoot is a physical deformity where there is an absence of the arch that runs from the heel of the foot to the toes. A common
Patient information for cervical spinal fusion.
 Patient information for cervical spinal fusion. Introduction This booklet has been compiled to help you understand spinal cervical fusion surgery and postoperative rehabilitation. Anatomy The cervical
Patient information for cervical spinal fusion. Introduction This booklet has been compiled to help you understand spinal cervical fusion surgery and postoperative rehabilitation. Anatomy The cervical
Minimally Invasive Spine Surgery What is it and how will it benefit patients?
 Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Minimally Invasive Spine Surgery What is it and how will it benefit patients? Dr Raoul Pope MBChB, FRACS, Neurosurgeon and Minimally Invasive Spine Surgeon Concord Hospital and Mater Private Hospital Sydney
Anterior Hip Replacement
 Disclaimer This movie is an educational resource only and should not be used to manage Orthopaedic health. All decisions about the management of hip replacement and arthritis management must be made in
Disclaimer This movie is an educational resource only and should not be used to manage Orthopaedic health. All decisions about the management of hip replacement and arthritis management must be made in
SALVATION. Fusion Bolts and Beams SURGICAL TECHNIQUE
 SALVATION Fusion Bolts and Beams SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 4 Intended Use Chapter 3 4 Device Description 4 Fusion Beams 5 Fusion Bolts Chapter 4 5 Preoperative Planning
SALVATION Fusion Bolts and Beams SURGICAL TECHNIQUE Contents Chapter 1 4 Introduction Chapter 2 4 Intended Use Chapter 3 4 Device Description 4 Fusion Beams 5 Fusion Bolts Chapter 4 5 Preoperative Planning
Achilles Tendon Repair, Operative Technique
 *smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
*smith&nephew ANKLE TECHNIQUE GUIDE Achilles Tendon Repair, Operative Technique Prepared in Consultation with: C. Niek van Dijk, MD, PhD KNEE HIP SHOULDER EXTREMITIES Achilles Tendon Repair, Operative
VENTURE. Anterior Cervical Plate System Surgical Technique
 VENTURE Anterior Cervical Plate System Surgical Technique as described by: Regis Haid, MD Atlanta Brain and Spine Care Atlanta, Georgia Iain Kalfas, MD Cleveland Clinic Foundation Cleveland, Ohio Steve
VENTURE Anterior Cervical Plate System Surgical Technique as described by: Regis Haid, MD Atlanta Brain and Spine Care Atlanta, Georgia Iain Kalfas, MD Cleveland Clinic Foundation Cleveland, Ohio Steve
Do you have Back Pain? Associated with:
 Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
Do you have Back Pain? Associated with: Herniated Discs? Protruding Discs? Degenerative Disk Disease? Posterior Facet Syndrome? Sciatica? You may be a candidate for Decompression Therapy The Dynatronics
If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time.
 If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time. You may be worried about your future, both in respect of finances and
If you or a loved one have suffered because of a negligent error during spinal surgery, you will be going through a difficult time. You may be worried about your future, both in respect of finances and
INFUSE Bone Graft. Patient Information Brochure
 INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
INFUSE Bone Graft Patient Information Brochure This Patient Guide is designed to help you decide whether or not to have surgery using INFUSE Bone Graft to treat your broken tibia (lower leg). There are
Herniated Lumbar Disc
 Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
SPINE AND NECK SURGERY: MAKING A DECISION THAT S RIGHT FOR YOU
 1. GET THE FACTS: Back and neck pain affects 8 out of 10 people at some point in their life. Acute back and neck pain comes on suddenly and usually lasts from a few days to a few weeks. Chronic back and
1. GET THE FACTS: Back and neck pain affects 8 out of 10 people at some point in their life. Acute back and neck pain comes on suddenly and usually lasts from a few days to a few weeks. Chronic back and
Surgery for cervical disc prolapse or cervical osteophyte
 Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: paul@pauldurso.com
Mr Paul S. D Urso MBBS(Hons), PhD, FRACS Neurosurgeon Provider Nº: 081161DY Epworth Centre Suite 6.1 32 Erin Street Richmond 3121 Tel: 03 9421 5844 Fax: 03 9421 4186 AH: 03 9483 4040 email: paul@pauldurso.com
Magnetic Resonance Imaging
 Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Get the Facts, Be Informed, Make YOUR Best Decision. Pelvic Organ Prolapse
 Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
Pelvic Organ Prolapse ETHICON Women s Health & Urology, a division of ETHICON, INC., a Johnson & Johnson company, is dedicated to providing innovative solutions for common women s health problems and to
How To Fix A Radial Head Plate
 Mayo Clinic CoNGRUENT RADIAL HEAD PLATE Since 1988 Acumed has been designing solutions to the demanding situations facing orthopedic surgeons, hospitals and their patients. Our strategy has been to know
Mayo Clinic CoNGRUENT RADIAL HEAD PLATE Since 1988 Acumed has been designing solutions to the demanding situations facing orthopedic surgeons, hospitals and their patients. Our strategy has been to know
Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization
 APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
APPROVED IRN10389-1.1-04 Direct Lateral Interbody Fusion A Minimally Invasive Approach to Spinal Stabilization Because it involves accessing the spine through the patient s side, the Direct Lateral approach
Introduction to the Bertram Hip Spacer
 Introduction to the Bertram Hip Spacer Approximately 200,000 hip replacement surgeries are done every year in United States. Fortunately the incidence of infection is routinely 0.5 percent-1 percent. However
Introduction to the Bertram Hip Spacer Approximately 200,000 hip replacement surgeries are done every year in United States. Fortunately the incidence of infection is routinely 0.5 percent-1 percent. However
.org. Distal Radius Fracture (Broken Wrist) Description. Cause
 Distal Radius Fracture (Broken Wrist) Page ( 1 ) The radius is the larger of the two bones of the forearm. The end toward the wrist is called the distal end. A fracture of the distal radius occurs when
Distal Radius Fracture (Broken Wrist) Page ( 1 ) The radius is the larger of the two bones of the forearm. The end toward the wrist is called the distal end. A fracture of the distal radius occurs when
SPINE ANATOMY AND PROCEDURES. Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132
 SPINE ANATOMY AND PROCEDURES Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132 SPINE ANATOMY The spine consists of 33 bones called vertebrae. The top 7 are cervical, or neck
SPINE ANATOMY AND PROCEDURES Tulsa Spine & Specialty Hospital 6901 S. Olympia Avenue Tulsa, Oklahoma 74132 SPINE ANATOMY The spine consists of 33 bones called vertebrae. The top 7 are cervical, or neck
Integra. Subtalar MBA and bioblock Implant SURGICAL TECHNIQUE
 Integra Subtalar MBA and bioblock Implant SURGICAL TECHNIQUE Table of contents Introduction Description... 2 Indications... 2 Contraindications... 2 Surgical Technique Step 1: Incision and Dissection...3
Integra Subtalar MBA and bioblock Implant SURGICAL TECHNIQUE Table of contents Introduction Description... 2 Indications... 2 Contraindications... 2 Surgical Technique Step 1: Incision and Dissection...3
Consent for Anterior Cervical Discectomy With Fusion and a Metal Plate at
 STEPHEN MARANO, M.D. JAMES COOK PA-C Consent for Anterior Cervical Discectomy With Fusion and a Metal Plate at Patient Name: Patient Diagnosis: Cervical Degenerative Disc Disease (wear and tear on the
STEPHEN MARANO, M.D. JAMES COOK PA-C Consent for Anterior Cervical Discectomy With Fusion and a Metal Plate at Patient Name: Patient Diagnosis: Cervical Degenerative Disc Disease (wear and tear on the
MINI FRAGMENT SYSTEM. Instruments and implants for 1.5 mm, 2.0 mm, and 2.4 mm plate fixation PRODUCT OVERVIEW
 MINI FRAGMENT SYSTEM Instruments and implants for 1.5 mm, 2.0 mm, and 2.4 mm plate fixation PRODUCT OVERVIEW TABLE OF CONTENTS INTRODUCTION Mini Fragment System 2 PRODUCT INFORMATION Plates 4 Screws 6
MINI FRAGMENT SYSTEM Instruments and implants for 1.5 mm, 2.0 mm, and 2.4 mm plate fixation PRODUCT OVERVIEW TABLE OF CONTENTS INTRODUCTION Mini Fragment System 2 PRODUCT INFORMATION Plates 4 Screws 6
.org. Arthritis of the Hand. Description
 Arthritis of the Hand Page ( 1 ) The hand and wrist have multiple small joints that work together to produce motion, including the fine motion needed to thread a needle or tie a shoelace. When the joints
Arthritis of the Hand Page ( 1 ) The hand and wrist have multiple small joints that work together to produce motion, including the fine motion needed to thread a needle or tie a shoelace. When the joints
1 REVISOR 5223.0070. (4) Pain associated with rigidity (loss of motion or postural abnormality) or
 1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
1 REVISOR 5223.0070 5223.0070 MUSCULOSKELETAL SCHEDULE; BACK. Subpart 1. Lumbar spine. The spine rating is inclusive of leg symptoms except for gross motor weakness, bladder or bowel dysfunction, or sexual
Calcaneus (Heel Bone) Fractures
 Copyright 2010 American Academy of Orthopaedic Surgeons Calcaneus (Heel Bone) Fractures Fractures of the heel bone, or calcaneus, can be disabling injuries. They most often occur during high-energy collisions
Copyright 2010 American Academy of Orthopaedic Surgeons Calcaneus (Heel Bone) Fractures Fractures of the heel bone, or calcaneus, can be disabling injuries. They most often occur during high-energy collisions
Robotic-Arm Assisted Surgery
 Mako TM Robotic-Arm Assisted Surgery for Total Hip Replacement A Patient s Guide Causes of Your Hip Pain Your joints are involved in almost every activity you do. Movements such as walking, bending and
Mako TM Robotic-Arm Assisted Surgery for Total Hip Replacement A Patient s Guide Causes of Your Hip Pain Your joints are involved in almost every activity you do. Movements such as walking, bending and
Technique Guide. Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting.
 Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
Technique Guide Reamer/Irrigator/Aspirator (RIA). For intramedullary reaming and bone harvesting. Table of Contents Introduction Reamer/ Irrigator/ Aspirator (RIA) 2 Indications 4 Surgical Technique Preparation
coligne treatment technology
 coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
coligne treatment technology coligne a strategy in spine Leadership begins before the patient enters the surgical theater, among the spine care professionals forming the coalliance. This may be a small
Titanium Wire with Barb and Needle. For canthal tendon procedures.
 Titanium Wire with Barb and Needle. For canthal tendon procedures. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Titanium Wire with Barb and Needle
Titanium Wire with Barb and Needle. For canthal tendon procedures. Technique Guide Instruments and implants approved by the AO Foundation Table of Contents Introduction Titanium Wire with Barb and Needle
Minimally Invasive Hip Replacement through the Direct Lateral Approach
 Surgical Technique INNOVATIONS IN MINIMALLY INVASIVE JOINT SURGERY Minimally Invasive Hip Replacement through the Direct Lateral Approach *smith&nephew Introduction Prosthetic replacement of the hip joint
Surgical Technique INNOVATIONS IN MINIMALLY INVASIVE JOINT SURGERY Minimally Invasive Hip Replacement through the Direct Lateral Approach *smith&nephew Introduction Prosthetic replacement of the hip joint
ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015
 Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
Presented by: Teri Romano, RN, MBA, CPC, CMDP ICD 10 CM IMPLEMENTATION DATE OCT 1, 2015 Source: http://journal.ahima.org/2015/02/04/us house committee to hold hearing on icd 10 implementation/ 2 2015 Web_Non
KnifeLight. Carpal Tunnel Ligament Release. Operative Technique
 KnifeLight Carpal Tunnel Ligament Release Operative Technique Contents Page 1. Features & Benefits 3 Intended Use and Indications 3 Contraindications 3 Features & Benefits 3 2. Operative Technique 4 Antegrade
KnifeLight Carpal Tunnel Ligament Release Operative Technique Contents Page 1. Features & Benefits 3 Intended Use and Indications 3 Contraindications 3 Features & Benefits 3 2. Operative Technique 4 Antegrade
