Effect of the Protein Denaturants Urea and Guanidinium on Water Structure: A Structural and Thermodynamic Study
|
|
|
- Rosa Summers
- 8 years ago
- Views:
Transcription
1 10748 J. Am. Chem. Soc. 1998, 120, Effect of the Protein Denaturants Urea and Guanidinium on Water Structure: A Structural and Thermodynamic Study Francesco Vanzi, Bhupinder Madan, and Kim Sharp* Contribution from the Johnson Research Foundation, Department of Biochemistry and Biophysics, UniVersity of PennsylVania, Philadelphia, PennsylVania ReceiVed May 4, 1998 Abstract: The mechanism of the denaturing effects of urea and the guanidinium ion on proteins is still an unsolved and important problem in protein chemistry. Changes in the rogen bond network of water in the first ration shell of urea and guanidinium were analyzed in terms of the random network model using Monte Carlo simulations. Bulk water consists of two populations of rogen bonds: a predominantly linear population and a small but significant population of slightly longer and more bent rogen bonds. In the first shell of urea, rogen bonds between waters solvating the amino groups were shorter and more linear on average than those in bulk water. These changes are caused by a depletion of the more distorted rogen bonds. These changes in ration water structure have previously been seen only around nonpolar solutes of solute groups. Thus urea, being entirely polar, is anomalous in this regard. Hydrogen bonds around guanidinium were longer and more bent than those in bulk water. These distortions are characteristic of a polar solute but are smaller than expected for an ion. The rogen bond structural parameters were combined with a random network model equation of state for heat capacity to calculate the ration heat capacities ( C p ) of urea and guanidinium. The value of C p obtained for urea is positive, characteristic of a nonpolar solute, and in good agreement with the experimental value. Urea and, to a lesser extent, guanidinium are unique among polar molecules in that they are highly soluble yet appear to structure water more like nonpolar solutes. The relevance of this observation to proposed mechanisms of denaturation is discussed. Introduction The mechanism by which urea and the guanidinium ion denature proteins in aqueous solutions is still a mystery, although there exists a large literature on the experimental and theoretical studies of denaturation of proteins by these molecules 1-5 (see reviews by Tanford 6 and Pace 7 ). It is not certain whether these molecules act directly by binding to peptide groups, thereby weakening internal rogen bonds, or indirectly by causing a change in the structure of water s rogen bond network around rophobic groups in proteins, thereby increasing their solubility and weakening the rophobic effect. It is also possible that both mechanisms are operating. Experimental data from an early study on urea s denaturing ability 1 are suggestive of the mechanism that denaturation could be occurring through the changes in the structure of the rogen bond network of water. Wetlaufer et al. studied solubilities of rocarbons with chains longer than two carbon atoms and observed that urea increased the solubility of these rocarbons. They also found that the solubility of these rocarbons depended approximately linearly on the urea concentration, not on the activity of urea, and hence they ruled out solvation of the rocarbons solely by urea. They proposed two possible mechanisms: (i) urea changes the rogen bond network of water and thus helps the ration of rocarbon molecules (1) Wetlaufer, D. B.; Malik, S. K.; Stoller, L.; Coffin, R. I. J. Am. Chem. Soc. 1964, 86, 508. (2) Makhatadze, G.; Privalov, P. J. Mol. Biol. 1992, 226, (3) Schellman, J. A. Biopolymers 1987, 26, (4) Alonso, D. O. V.; Dill, K. A. Biochemistry 1991, 30, (5) Timasheff, S. Biochemistry 1992, 31, (6) Tanford, C. AdV. Protein Chem. 1970, 24, (7) Pace, N. Methods Enzymol. 1986, 131, and (ii) both urea and water molecules solvate the rocarbon molecules. This study concluded that the denaturing effect of urea was partly due to the weakening of rophobic effects. There have been a number of theoretical and experimental studies supporting the more direct mechanism of denaturation, i.e., through better solvation of the protein molecule in the denatured state by urea and guanidinium. 3,5 Schellman proposed a direct binding model for denaturant activity. 3 Since the affinity constant for urea binding to protein required by this model is very low, it has proven difficult to measure directly. Alonso and Dill predicted on theoretical grounds that the denaturants cause unfolding of proteins because the denaturant solutions solvate the rophobic groups in the unfolded states of proteins. 4 However, it is not made clear whether denaturing action occurs through the changes in the rogen bond network of water or through binding to rophobic groups by the denaturant. Myers, Pace, and Scholtz have studied the relation of m values (the rate of change of the unfolding equilibrium with increasing denaturant concentration) and heat capacity changes to the changes in the accessible surface area ( ASA) of protein unfolding by urea and guanidinium ion. 8 They observed that both the m values and the heat capacity changes ( C p ) correlate with the changes in ASA. They also observed that the m values and C p correlate with each other. They concluded that, for the proteins which undergo denaturation by a two-state mechanism, the accessible surface area exposed to solvent during denaturation is a main factor in the determination of the m values for unfolding of proteins by urea and guanidinium. The authors do not give a mechanism by which the (8) Myers, J. K.; Pace, C. N.; Scholtz, J. M. Protein Sci. 1995, 4, /ja981529n CCC: $ American Chemical Society Published on Web 10/03/1998
2 Water Structure around Urea and Guanidinium J. Am. Chem. Soc., Vol. 120, No. 41, denaturant molecules cause unfolding of proteins. Even though the above-mentioned thermodynamic study suggests that direct interaction with urea and guanidinium ions is a plausible mechanism, this study does not rule out the possibility of the indirect mechanism, in which urea changes the structure of water in the ration shell of proteins. Such an indirect mechanism could still exist because, in this study, the dependence of m values is shown to be quite a coarse parameter for quantitating changes in the accessible surface area during the unfolding phenomenon. A calorimetric study by Zou, Habermann, and Murphy on the energetics of dissolution of cyclic dipeptides in different concentrations of aqueous urea solutions concludes that the urea denaturant effect is twofold: it decreases the rophobic effect and it binds to the peptide groups via rogen bonds. 9 That group further found that the interactions of nonpolar groups with urea are enthalpically unfavorable but entropically favorable, while the reverse is true for urea interactions with polar groups. None of the experimental studies mentioned above can clearly distinguish whether direct interaction with peptides or indirect changes in the rogen bond network of rogen bonds, or both, are operating. Because of this, several theoretical and simulation studies have focused on the changes in the water structure around the urea molecule. Franks and Franks, in their study on aqueous solutions of urea, proposed that the water around urea is less rogen bonded than bulk water. 10 The first simulation study on aqueous solutions of urea, by Kuharski and Rossky, compared the distribution of pair interaction energies of water molecules in the ration shell and in the bulk. 11,12 The authors reached the conclusion that the properties of water in the first shell are very similar to those in bulk. Our unpublished results on the distribution of water-water interaction energies in bulk water and in the ration shells of various nonpolar and polar molecules confirm this observation. However, in a previous study of the random network model (RNM) water structure parameters (the mean and standard deviation of the rogen bond length (d, s) and the root-mean-square rogen bond angle (θ)), we observed significant changes in the ration shell of polar and nonpolar solutes, particularly in θ, with significant but lesser changes in d These results show that the RNM parameters are more sensitive to changes in the rogen bond network than the water-water interaction energy, and hence it is worthwhile to analyze the difference in the RNM parameters for rogen bonds among water molecules in the ration shell of denaturant molecules, urea and guanidinium. A recent simulation study on the effect of urea in aqueous solutions of two methane molecules and two ions of the same radius as methane showed that the first peak in the urea-o/ water-o radial distribution function is at the same position, i.e., 2.8 Å, as the first peak in the water-o/water-o radial distribution peak in pure water. 16 Wallqvist et al. also computed the various O-H radial distribution functions for urea and water oxygens with the urea and water rogens. They found that these O-H radial distribution functions match the corresponding (9) Zou, Q.; Habermann, S.; Murphy, K. P. Proteins 1997, 31, (10) Franks, H. S.; Franks, F. J. Chem. Phys. 1968, 48, (11) Kuharski, R. A.; Rossky, P. J. J. Am. Chem. Soc. 1984, 106, (12) Kuharski, R. A.; Rossky, P. J. J. Am. Chem. Soc. 1984, 106, (13) Madan, B.; Sharp, K. A. J. Phys. Chem. 1996, 100, (14) Madan, B.; Sharp, K. A. J. Phys. Chem. 1997, 101, (15) Sharp, K. A.; Madan, B. J. Phys. Chem. 1997, 101, (16) Wallqvist, A.; Covell, D. G.; Thirumalai, D. J. Am. Chem. Soc. 1998, 120, pure water O-H radial distribution functions. The authors concluded from this observation that urea does not break the water structure in its aqueous solutions. Previous studies by Tsai et. 17 and Astrand et al. 18 also found little change in the water O-O radial distribution function in urea solutions. These results can be rationalized with our previous studies on water around solutes using the random network model, which showed that the mean rogen bond length, d (d is essentially equivalent to the position of the first peak in the water O-O radial distribution function), is a much less sensitive indicator of structural changes than the rogen bond angle between water, θ Wallqvist et al. also observed that the first peak in the charged-methane/urea-c radial distribution function was bigger than the first peak in the uncharged-methane/urea-c radial distribution function, thus concluding that urea gets absorbed selectively on the rophilic groups. The work by Wallqvist et al. is interesting and generates some new questions about the behavior of water in the ration shell of urea. First, if water does not lose its structure because the oxygen atom in urea behaves like the oxygen atom of water, what happens to the water molecules surrounding the amine groups? Second, do the structures of water around oxygen in urea and of water around amine groups in urea look the same? In other words, how do urea and guanidinium change the structure of rogen bond network of water in its ration shell? These are the questions addressed in the present work. We further ask if we can check the consistency of these observations by relating changes in the rogen bond network to measured changes in heat capacity, since we have shown that this quantity is a sensitive indicator of changes in water structure around solutes. In this work, we have set out to accomplish two goals. The first goal is to analyze the changes in the RNM parameters of the rogen bond network in the ration shells of various groups in urea and guanidinium ions. We also discuss the possible role of these changes in the rogen bond network in the denaturation ability of these molecules. The second goal is to compute the heat capacity changes associated with the ration of these molecules using the methodology developed in our earlier papers. We compare the values for the heat capacity of ration of these compounds with the experimental estimates. A good agreement with the experimental values would give us confidence in our analysis of the water structure in the ration shell of these two denaturant molecules. Methods Dilute solutions of urea and guanidinium were simulated by inserting one molecule of the solute into a cubic box of 216 molecules of TIP4P water. 19 The box dimension was 18.6 Å per side. Minimum image periodic boundary conditions were used, with a cutoff of 12.0 Å. The OPLS potential function was used for urea. 20 Parameters for guanidinium were taken from Saigal and Pranata. 21 The partial charge distributions for urea and guanidinium are shown in Figure 1. The configurations of the aqueous solutions of urea and guanidinium ion were sampled using a Metropolis Monte Carlo algorithm implemented in the program BOSS. 22 The simulations were performed at 25 C (17) Tsai, J.; Gerstein, M.; Levitt, M. J. Chem. Phys. 1996, 104, (18) Astrand, P.; Wallqvist, A.; Karlstrom, G. J. Phys. Chem. 1994, 98, 8, (19) Jorgensen, W. L.; Chandrasekhar, J.; Madura, J. D.; Impey, R. W.; Klein, M. L. J. Chem. Phys. 1983, 79, 926. (20) Duffy, E.; Severance, D.; Jorgensen, W. Isr. J. Chem. 1993, 33, (21) Saigal, S.; Pranata, J. Bioorg. Chem. 1997, 25, (22) Jorgensen, W. L. BOSS, Version 3.3; Yale University: New Haven, CT, 1992.
3 10750 J. Am. Chem. Soc., Vol. 120, No. 41, 1998 Vanzi et al. Table 1. RNM Parameters for Hydrogen Bonds Formed in the Hydration Shell of Urea Molecule and Their Contribution to C p H-bond class a d (Å) b s (Å) θ no. of H-bonds C p/water net C p O-O 3.04 ( ( ( ( ( ( 0.3 O-N 2.95 ( ( ( ( ( ( 0.3 O-C 2.92 ( ( ( ( ( ( 0.5 N-C 2.93 ( ( ( ( ( ( 0.3 N-N 2.92 ( ( ( ( ( ( 0.3 C-C 2.92 ( ( ( ( ( ( 0.5 C p 5.0 ( 1 a Hydrogen bonds are classified according to the ration shell to which each water molecule belongs. b For bulk water, d ) 2.94 Å, s ) 0.22 Å, θ ) Figure 1. Charge distributions used for simulating urea and the guanidinium ion. and 1 atm pressure. Flexibility of the solute molecules was not included. The systems were first equilibrated for Monte Carlo steps, following which data were collected for 10 consecutive runs of steps each. Error estimates for various average quantities were determined by computing the standard deviations for each average quantity from the 10 runs. During the data collection runs, an instantaneous configuration of the dilute solution was analyzed every 1000 steps. The values of the various interaction energies, radial distribution functions, g(r), rogen bond distance, and angle distributions of the rogen bond interactions between the water molecules in the solute ration shells were computed from the instantaneous configurations. The ration shell for each group of the solute was determined from the first minimum of the solute-atom/water-oxygen radial distribution function. Two water molecules were defined to be rogen bonded if the instantaneous distance between their oxygen atoms was less than or equal to 3.4 Å, which corresponds to the first minimum in the oxygen-oxygen radial distribution function of water. The rogen bond angle between two such water molecules is defined as the smallest O-O-H angle formed from the four rogens involved. The oxygen-oxygen distances and rogen bond angles for rogen-bonded water molecules were binned together to obtain histograms for the computation of the probability distribution functions of oxygen-oxygen distances and of the rogen bond angles. These probability distribution functions were then used to determine the three random network parameters: average oxygen-oxygen distance between two rogen-bonded water molecules, d, the standard deviation in this distance, s, and the root-meansquare rogen bond angle between two water molecules, θ. Hydrogen bonds among water molecules in the first ration shell were assigned to various classes based on the ration shell of which solute atom each of the water molecule belonged to. Thus, both intragroup and intergroup rogen bonds can occur. The rogen bond interactions were further divided into various classes based on the groups solvated by the two waters. The RNM parameters for these different classes are distinguished by the subscripts O, N, and C for the oxygen, amino, and carbon groups, respectively. For example, a rogen bond between a water molecule in the ration shell of the oxygen atom of urea molecule and another water molecule in the ration shell of the NH2 group of the same urea molecule has parameters X O-N, where X ) d, s, orθ. If a water molecule was at such a position that it could belong to the ration shell of two or more different groups, it was assigned to be in the ration shell of that group which was closest to it. Hydrogen bonds belonging to each of the classes were counted for each snapshot and were averaged over the whole run. The net heat capacity change due to the ration effects ( C p ) for the two solutes was computed from the changes, with Figure 2. Hydrogen bond angle probability distributions for urea. Data are plotted for selected classes of H-bonds: O-O(0), intrashell N-N (- -), intershell N-N(4), and C-C(s). The distribution for bulk water (9) is shown for comparison. respect to bulk water, in the RNM parameters for the rogen bonds in the first ration shell and from the number of rogen bonds in the ration shell. This procedure has been described in detail in previous papers This method provides C p contributions from each class of rogen bonds. These contributions are then summed to obtain the total heat capacity of ration of urea and guanidinium ion from the following equation: C p ) i N i [C p rn (d i,s i,θ i ) - C p rn (d o,s o,θ o )] ) i N i C rn p (d i,s i,θ i ) (1) where C p rn is the contribution to heat capacity arising from a group of N i perturbed H-bonds with average parameters d i, s i, and θ i, where d o, s o, and θ o are the corresponding values for the bulk water. The detailed form of the equation of state for heat capacity C p rn (d i, s iθ i) has been derived in our previous work 13 from the modified version of the random network model of water developed by Henn and Kauzmann. 23 Results Urea. The first ration shell of urea contained, on average, 23 waters, making an average of 32 first shell-first shell water H-bonds. The changes in the characteristics of the rogen bond network between water molecules in the ration shell of urea are shown in the form of the RNM parameters in Table 1 and in the rogen bond angle probability distribution in Figure 2. Table 1 shows the RNM parameters for the rogen bonds between water molecules in the first shell of each of the (23) Henn, A. R.; Kauzmann, W. J. Phys. Chem. 1989, 93,
4 Water Structure around Urea and Guanidinium J. Am. Chem. Soc., Vol. 120, No. 41, three kinds of groups present in the solute molecule. It is observed that the rogen bonds between water molecules present in the ration shell of urea s oxygen atom are similar to those observed previously around polar groups in ethanol and NMA. 15 However, the rogen bonds between water molecules around urea s amine groups are more like the rogen bonds formed between water molecules around nonpolar groups in mixed group molecules such as ethanol and NMA seen in our previous work. This behavior is most evident in θ but is also shown to a lesser extent in d. The average value of θ O-O for urea is 39, while the value θ N-N for urea is 28. For comparison, the value of θ O-O for pure water is Similarly, the value of average oxygen-oxygen distances, d O-O, and d N-N for urea are 3.04 and 2.92 Å, respectively, compared to 2.95 for d O-O of pure water. Similarly, the rogen bonds between waters solvating the carbonyl carbon of urea (class C-C) and between waters solvating both carbon and amino groups (class N-C) are distorted in the direction characteristic of nonpolar groups, i.e., shorter lengths and smaller angles than those for bulk water. The values of θ and d for rogen bonds between water molecules belonging to other classes lie between the two extremes represented by the O-O and N-N/C-C classes. The H-bond geometry is only slightly distorted with respect to that in bulk water, with a barely significant decrease in d and a slight decrease in θ. The changes in length and angle for all classes of groups are highly correlated (R 2 ) 0.8). The changes in RNM parameters with respect to those in bulk water for all classes of rogen bonds in the ration shell are used to compute C p contributions from corresponding groups, as shown in Table 1. The values of ration heat capacity obtained from the different classes of rogen bonds reflect the changes in H-bond length and angle. C p for O-O H-bonds is -1.6 cal mol -1 K -1, while those for the N-N and C-C classes are 4.8 and 0.7 cal mol -1 K -1, respectively; i.e., the changes around these polar groups are more characteristic of nonpolar groups. The sum of these individual contributions gives the total heat capacity of ration for urea, which is 5 ( 1 cal mol -1 K -1. Because of the larger number of H-bonds in the N-N, C-C, and N-C classes, they provide the dominant contribution to the heat capacity of ration, resulting in a net positive C p. Considering the difficulty of calculating heat capacity changes, our calculated value is in good agreement with the experimentally measured value of 7.4 cal mol -1 K This agreement provides confidence that the observed structural changes for the ration shell water around various groups of urea are realistic. Mean values of d and θ provide one way to characterize structural changes. However, a more detailed picture of the structural changes is revealed by the H-bond angle probability distribution for different H-bond classes in the ration shell of urea (Figure 2). We have shown in our previous work 15 that this kind of plot for bulk water clearly allows one to distinguish two rogen bond populations: a larger population with quasitetrahedral icelike structure, with θ h 12, and a smaller population in which a fifth molecule, a mismatch water, comes into the coordination shell of the central water molecule, forming a highly distorted H-bond, with θ h 52. We also found in our previous work that nonpolar solutes tend to decrease this second population by competing for the position of the mismatch water molecule. Polar solutes, however, have the opposite effect. The electric field of polar groups tends to align the dipole of water with the intersolute-water axis, producing a large (24) Cabani, S.; Gianni, P.; Mollica, V.; Lepori, L. J. Soln. Chem. 1981, 10, Figure 3. Hydrogen bond angle probability distributions for guanidinium. Data are plotted for selected classes of H-bonds: intrashell N-N (]), intershell N-N (4), and C-C ( ). The distribution for bulk water (b) is shown for comparison. rogen bond angle between two water molecules in the ration shell. Figure 2 shows the dramatic effect of the oxygen atom of urea molecule, acting as a polar group, on the rogen bonds between waters in its ration shell. The probability distribution plot for the rogen bond angles between waters around the oxygen atom shows two peaks, a broader peak at 52 and a much smaller peak at 12. The probability plot for the N-N class shows a small decrease in the 52 peak and an increase in the 12 peak with respect to the bulk water distribution. A similar increase in the 12 peak is seen for the C-C class. This indicates that carbonyl carbon and amine groups, despite having significant partial charge, behave as nonpolar groups. There is no detectable difference in the N-N class of H-bonds between those for waters rating neighboring amino groups and the same amino group. Guanidinium. The first ration shell of guanidinium contained, on average, 23 waters, making an average of 30 first shell-first shell water H-bonds. There are only three kinds of rogen bonds in the ration shell of guanidinium: N-N, C-C, and N-C. The N-N class includes H-bonds between waters rating neighboring amino groups as well as the same amino group. The changes in the rogen bonds in the C-C and N-C classes with respect to those in bulk water are similar to those seen for these classes around the urea molecule: d C-C and d N-C decrease to 2.92 and 2.91 Å, respectively, while the corresponding angles decrease to 28.7 and 28.2, respectively. The corresponding H-bond probability distributions show increases in the 12 peak similar to those seen around urea (Figure 3). These values suggest that the carbon atom of guanidinium acts on water in a manner similar to that for nonpolar groups. For water-water rogen bonds around amine groups (the N-N class), d ) 2.95 Å and θ ) 36.4, both increasing compared to bulk water. The values for these parameters indicate that the amine groups act as rophilic groups, but less strongly than the carbonyl oxygen of urea. This point is emphasized in the H-bond probability distribution, which shows a significant increase in the 52 peak compared to that bulk water, but considerably less than that seen for urea s oxygen (Figures 2 and 3). This behavior is expected because of the uniform delocalization of one positive charge onto three amine groups. Unlike urea, there is a large difference between the
5 10752 J. Am. Chem. Soc., Vol. 120, No. 41, 1998 Vanzi et al. Table 2. RNM Parameters for Hydrogen Bonds Formed in the Hydration Shell of Guanidinium and Their Contribution to C p H-bond class a d (Å) s (Å) θ no. of H-bonds C p/water net C p N-N 2.95 ( ( ( ( ( ( 1 N-C 2.92 ( ( ( ( ( ( 0.5 C-C 2.91 ( ( ( ( ( ( 0.5 C p -3.0 ( 1 a Hydrogen bonds are classified according to the ration shell to which each water molecule belongs. N-N class of H-bonds for waters rating the same amino group (intrashell) and those rating neighboring amino groups (intershell). The figure indicates that the larger distortion effect on H-bond geometry is, in fact, due to intershell water molecules around the amine groups. Hydrogen bonds formed between such water molecules increase the population at 52 at the expense of the population at 12, a behavior which is typical of rogen bonds formed between water molecules around polar groups. Table 2 shows the contributions to C p from rogen bonds formed between different water molecule populations around various groups of guanidinium. The main contribution is from the N-N class because the largest number of H-bonds are formed among water molecules belonging to the ration shell of the amine groups. Our calculated value for the heat capacity of ration of guanidinium is -3.0 ( 1 cal mol -1 K -1. The negative value of heat capacity of ration indicates that guanidinium behaves as a polar compound. We could not find any experimental data on the heat capacity of ration of the guanidinium ion. However, our computed value can be compared to a negative heat capacity of ration (-17 cal mol -1 K -1 ) for the K + ion and a positive heat capacity of ration (36 cal mol -1 K -1 ) for the TMA + ion. Since guanidinium is not as small as K + and neither is it as large as TMA +, nor does it have rophobic groups(methyl groups) in it like TMA + does, we believe a small negative value for the heat capacity of ration for guanidinium is a reasonable value. The guanidinium ion also differs from the TMA + ion in that the polar atom(s) (nitrogens) are on the outside, not on the inside of the ion. It would be expected that a large ion, especially with rophobic groups, would have a positive heat capacity of ration because, first, the charge is distributed over a large volume and, second, the rophobic groups would also contribute to a positive heat capacity change for the ration. Discussion Our results show that the predominantly linear H-bond network of water is maintained, and even enhanced, surrounding the amine groups in urea, while it is distorted surrounding the carbonyl oxygen atom. The rogen bonds around the oxygen atom of urea molecule, however, are of both kinds found in bulk water: the more linear rogen bonds and more strained rogen bonds, but the population of the latter is increased at the expense of the former. Since, on average, there are only 1.6 rogen bonds formed around the oxygen atom, the two peaks suggest that water molecules surrounding the urea oxygen atom sometimes form an almost perfect H-bond (θ h < 12 ) and at other times a bent bond (θ h 52 ). The persistence of the first peak at 12 is in agreement with the results of Wallqvist et al. 16 that urea does not function as structure breaker..., but the peak at 52 suggests that there is an occasional broken rogen bond between water molecules surrounding the oxygen atom of urea. Their conclusion was based on the fact that various permutations of O-H radial distribution functions for urea oxygen and rogen atoms with water rogen and oxygen atoms match the corresponding pure water O-H radial distribution functions. Wallqvist et al. s results as well as our results (data not shown here) show that the urea-o/water-o radial distribution function has its first peak at 2.8 Å, the same position where the water oxygen-oxygen first peak appears. However, we have observed in our previous works that the rogen bond angle is a very sensitive measurement of rogen bond characteristics. We believe that examination of radial distribution functions alone is not sufficient to conclude that the oxygen atom of a urea molecule always fits into the network of water molecules, since the probability distribution function of rogen bond angles for water molecules around the urea oxygen atom differs markedly from that in bulk water. Hydrogen bond angles also appear to be a more sensitive indicator of structural perturbations in the ration shell of urea than the distribution of water pair interaction energies studied by Kuharski and Rossky, 11,12 since there are significant differences in the former, but not in the latter. Hydrogen bonds around amine groups of urea are much like those of the bulk water. Similar kinds of changes take place for the rogen bonds between water molecules in the ration shell of guanidinium. One mechanism postulated for the denaturing activity of urea and guanidinium involves binding to protein groups exposed upon unfolding. Another possible mechanism is through their effect on water structure, and thus on the strength of the rophobic effect. Demonstrating that this mechanism, rather than direct binding to protein groups, is sufficient to denature proteins has proved elusive. A necessary condition for the indirect mechanism is that these denaturants affect the structural and thermodynamic properties of water in a unique way (unique in the sense that distinguishes them from the effect on water of solutes that are nondenaturing). While our work does not directly address the mechanism of denaturation, the results presented here and in previous work show that analyzing the random network model parameters, particularly the rogen bond angle between waters in the first ration shell, is a powerful way to analyze solute-induced perturbations of water for two reasons: (1) It is sensitive to structural perturbations. Indeed, it may reveal perturbations that do not show up in changes in the radial distribution functions traditionally used to analyze liquid structure (Madan and Sharp, communicated to Biophysical Chemistry). This is merely a reflection of the crucial importance of orientational structuring in water, most notably in the tetrahedral nature of the coordination shell. (2) The structural changes may be directly and quantitatively related to a key thermodynamic property, the heat capacity. Ample experimental data have shown that ration heat capacity change is the most revealing of the common thermodynamic functions (the others being free energy, enthalpy, and entropy) in terms of the differences between ration of polar and nonpolar (rophobic) solutes. Our previous analysis of 12 solutes of widely differing characteristics, comprising more than 17 different functional
6 Water Structure around Urea and Guanidinium J. Am. Chem. Soc., Vol. 120, No. 41, groups, has established an overall pattern of ration: bulk water contains two populations of H bonds, about 80% that are approximately linear (with an angle of θ h 12 ), the rest being more bent (θ h 52 ) and slightly longer. Both polar and nonpolar solutes perturb water, producing concerted changes in mean H-bond angle and length. Nonpolar groups decrease the mean angle and length by displacing the more bent population of H-bonds. This results in an increase in water s heat capacity. Polar and ionic groups, through the orienting effect of their electrostatic fields, increase the mean angle and length by increasing the population of more bent H-bonds. This results in a decrease in water s heat capacity. In this regard, urea is unique. Although it is an entirely polar molecule (judged by the significant partial charge on all its atoms, Figure 1), the amino groups perturb the water in a way characteristic of nonpolar groups, and the net C p is positive. No other solute we have examined shows this behavior. Guanidinium is isosteric to urea but differs by the replacement of the oxygen with an amino group, which enables it to support more positive charge, indeed, a formal charge of 1 (Figure 1). Guanidinium shares some of the nonpolar-like effect on water structure with urea, notable in the structuring effect on water rating the carbon and carbon/amino groups, although, because of its formal charge, it has a net negative but very small C p. We may thus characterize urea, and more tentatively, guanidinium, as sharing both polar and nonpolar characteristics that could be important in their denaturing action. First, they are both highly soluble. This is a requirement since rather large concentrations are required for denaturation, whatever the mechanism. Second, they could, because of their dual nature, interact with both polar and nonpolar groups. The picture of urea that emerges here is consistent with recent work by Zou et al., 9 which implies that urea binds to both polar and nonpolar groups of peptides, driven by enthalpy and entropy, respectively. Acknowledgment. Financial support is acknowledged from NIH (GM54105). JA981529N
Hydrophobic Effect, Water Structure, and Heat Capacity Changes
 J. Phys. Chem. B 1997, 101, 4343-4348 4343 Hydrophobic Effect, Water Structure, and Heat Capacity Changes Kim A. Sharp* and Bhupinder Madan Department of Biochemistry & Biophysics, UniVersity of PennsylVania,
J. Phys. Chem. B 1997, 101, 4343-4348 4343 Hydrophobic Effect, Water Structure, and Heat Capacity Changes Kim A. Sharp* and Bhupinder Madan Department of Biochemistry & Biophysics, UniVersity of PennsylVania,
Hydrogen Bonds The electrostatic nature of hydrogen bonds
 Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
2054-2. Structure and Dynamics of Hydrogen-Bonded Systems. 26-27 October 2009. Hydrogen Bonds and Liquid Water
 2054-2 Structure and Dynamics of Hydrogen-Bonded Systems 26-27 October 2009 Hydrogen Bonds and Liquid Water Ruth LYNDEN-BELL University of Cambridge, Department of Chemistry Lensfield Road Cambridge CB2
2054-2 Structure and Dynamics of Hydrogen-Bonded Systems 26-27 October 2009 Hydrogen Bonds and Liquid Water Ruth LYNDEN-BELL University of Cambridge, Department of Chemistry Lensfield Road Cambridge CB2
Water structure changes induced by hydrophobic and polar solutes revealed by simulations and infrared spectroscopy
 JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 4 22 JANUARY 2001 Water structure changes induced by hydrophobic and polar solutes revealed by simulations and infrared spectroscopy Kim A. Sharp, a) Bhupinder
JOURNAL OF CHEMICAL PHYSICS VOLUME 114, NUMBER 4 22 JANUARY 2001 Water structure changes induced by hydrophobic and polar solutes revealed by simulations and infrared spectroscopy Kim A. Sharp, a) Bhupinder
Section Activity #1: Fill out the following table for biology s most common elements assuming that each atom is neutrally charged.
 LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
Water s Hydrogen Bonds in the Hydrophobic Effect: A Simple Model
 J. Phys. Chem. B 2005, 109, 23611-23617 23611 Water s Hydrogen Bonds in the Hydrophobic Effect: A Simple Model Huafeng Xu and Ken A. Dill* Department of Pharmaceutical Chemistry and Graduate Group of Biophysics,
J. Phys. Chem. B 2005, 109, 23611-23617 23611 Water s Hydrogen Bonds in the Hydrophobic Effect: A Simple Model Huafeng Xu and Ken A. Dill* Department of Pharmaceutical Chemistry and Graduate Group of Biophysics,
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
Type of Chemical Bonds
 Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
Type of Chemical Bonds Covalent bond Polar Covalent bond Ionic bond Hydrogen bond Metallic bond Van der Waals bonds. Covalent Bonds Covalent bond: bond in which one or more pairs of electrons are shared
EXPERIMENT 9 Dot Structures and Geometries of Molecules
 EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
Why? Intermolecular Forces. Intermolecular Forces. Chapter 12 IM Forces and Liquids. Covalent Bonding Forces for Comparison of Magnitude
 1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
1 Why? Chapter 1 Intermolecular Forces and Liquids Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature for such a small molecule? Why does ice float on water?
Chemical Bonds. Chemical Bonds. The Nature of Molecules. Energy and Metabolism < < Covalent bonds form when atoms share 2 or more valence electrons.
 The Nature of Molecules Chapter 2 Energy and Metabolism Chapter 6 Chemical Bonds Molecules are groups of atoms held together in a stable association. Compounds are molecules containing more than one type
The Nature of Molecules Chapter 2 Energy and Metabolism Chapter 6 Chemical Bonds Molecules are groups of atoms held together in a stable association. Compounds are molecules containing more than one type
Bonds. Bond Length. Forces that hold groups of atoms together and make them function as a unit. Bond Energy. Chapter 8. Bonding: General Concepts
 Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
Bonds hapter 8 Bonding: General oncepts Forces that hold groups of atoms together and make them function as a unit. Bond Energy Bond Length It is the energy required to break a bond. The distance where
5s Solubility & Conductivity
 5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
Water: cavity size distribution and hydrogen bonds
 Chemical Physics Letters 396 (2004) 226 231 www.elsevier.com/locate/cplett Water: cavity size distribution and hydrogen bonds Giuseppe Graziano * Department of Biological and Environmental Sciences, University
Chemical Physics Letters 396 (2004) 226 231 www.elsevier.com/locate/cplett Water: cavity size distribution and hydrogen bonds Giuseppe Graziano * Department of Biological and Environmental Sciences, University
1 The water molecule and hydrogen bonds in water
 The Physics and Chemistry of Water 1 The water molecule and hydrogen bonds in water Stoichiometric composition H 2 O the average lifetime of a molecule is 1 ms due to proton exchange (catalysed by acids
The Physics and Chemistry of Water 1 The water molecule and hydrogen bonds in water Stoichiometric composition H 2 O the average lifetime of a molecule is 1 ms due to proton exchange (catalysed by acids
CHEMISTRY BONDING REVIEW
 Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Chemical Bonds and Groups - Part 1
 hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
1 Peptide bond rotation
 1 Peptide bond rotation We now consider an application of data mining that has yielded a result that links the quantum scale with the continnum level electrostatic field. In other cases, we have considered
1 Peptide bond rotation We now consider an application of data mining that has yielded a result that links the quantum scale with the continnum level electrostatic field. In other cases, we have considered
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.
 CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
Role of Hydrogen Bonding on Protein Secondary Structure Introduction
 Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
H 2O gas: molecules are very far apart
 Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
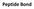 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1. Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57, 61, 63, 67, 69, 71(a), 73, 75, 79
 Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
Sample Exercise 8.1 Magnitudes of Lattice Energies
 Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the ionic compounds NaF, CsI, and CaO in order of increasing lattice energy. Analyze From the formulas for three
Hydrophobic Tendencies of Polar Groups as a Major Force in Molecular Recognition
 Tigran V. Chalikian Department of Pharmaceutical Sciences, Leslie Dan Faculty of Pharmacy, University of Toronto, 19 Russell St., Toronto, Ontario M5S 2S2, Canada Hydrophobic Tendencies of Polar Groups
Tigran V. Chalikian Department of Pharmaceutical Sciences, Leslie Dan Faculty of Pharmacy, University of Toronto, 19 Russell St., Toronto, Ontario M5S 2S2, Canada Hydrophobic Tendencies of Polar Groups
5. Structure, Geometry, and Polarity of Molecules
 5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
The strength of the interaction
 The strength of the interaction Host Guest Supramolecule (host-guest complex) When is the host capable to recognize the guest? How do we define selectivity Which element will we use to design the host
The strength of the interaction Host Guest Supramolecule (host-guest complex) When is the host capable to recognize the guest? How do we define selectivity Which element will we use to design the host
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
C has 4 valence electrons, O has six electrons. The total number of electrons is 4 + 2(6) = 16.
 129 Lewis Structures G. N. Lewis hypothesized that electron pair bonds between unlike elements in the second (and sometimes the third) row occurred in a way that electrons were shared such that each element
129 Lewis Structures G. N. Lewis hypothesized that electron pair bonds between unlike elements in the second (and sometimes the third) row occurred in a way that electrons were shared such that each element
4.5 Physical Properties: Solubility
 4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
Sample Exercise 8.1 Magnitudes of Lattice Energies
 Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the following ionic compounds in order of increasing lattice energy: NaF, CsI, and CaO. Analyze: From the formulas
Sample Exercise 8.1 Magnitudes of Lattice Energies Without consulting Table 8.2, arrange the following ionic compounds in order of increasing lattice energy: NaF, CsI, and CaO. Analyze: From the formulas
Health Science Chemistry I CHEM-1180 Experiment No. 15 Molecular Models (Revised 05/22/2015)
 (Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
(Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
Getting the most from this book...4 About this book...5
 Contents Getting the most from this book...4 About this book....5 Content Guidance Topic 1 Atomic structure and the periodic table...8 Topic 2 Bonding and structure...14 Topic 2A Bonding....14 Topic 2B
Contents Getting the most from this book...4 About this book....5 Content Guidance Topic 1 Atomic structure and the periodic table...8 Topic 2 Bonding and structure...14 Topic 2A Bonding....14 Topic 2B
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Chapter 2 Polar Covalent Bonds: Acids and Bases
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
Symmetric Stretch: allows molecule to move through space
 BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
BACKGROUND INFORMATION Infrared Spectroscopy Before introducing the subject of IR spectroscopy, we must first review some aspects of the electromagnetic spectrum. The electromagnetic spectrum is composed
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
Question 4.2: Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
 Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents (atoms, ions etc.) together in a chemical species. Various theories
Question 4.1: Explain the formation of a chemical bond. A chemical bond is defined as an attractive force that holds the constituents (atoms, ions etc.) together in a chemical species. Various theories
Chemistry 105, Chapter 7 Exercises
 hemistry 15, hapter 7 Exercises Types of Bonds 1. Using the periodic table classify the bonds in the following compounds as ionic or covalent. If covalent, classify the bond as polar or not. Mg2 4 i2 a(3)2
hemistry 15, hapter 7 Exercises Types of Bonds 1. Using the periodic table classify the bonds in the following compounds as ionic or covalent. If covalent, classify the bond as polar or not. Mg2 4 i2 a(3)2
Introduction, Noncovalent Bonds, and Properties of Water
 Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Non-Covalent Bonds (Weak Bond)
 Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
Chapter 6 An Overview of Organic Reactions
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras
 Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Modern Construction Materials Prof. Ravindra Gettu Department of Civil Engineering Indian Institute of Technology, Madras Module - 2 Lecture - 2 Part 2 of 2 Review of Atomic Bonding II We will continue
Thermodynamics of Mixing
 Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Thermodynamics of Mixing Dependence of Gibbs energy on mixture composition is G = n A µ A + n B µ B and at constant T and p, systems tend towards a lower Gibbs energy The simplest example of mixing: What
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
CHAPTER 6 REVIEW. Chemical Bonding. Answer the following questions in the space provided.
 Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Name Date lass APTER 6 REVIEW hemical Bonding SETIN 1 SRT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence
Communicated March 31, 1951 CHR CHR CHR. *H* zz '" *H _ 0.-...H / k C,.. CHR CNR CHR CHR CHR *HN/' N 'H_N/' H_./ - H-(H.
 VOL. 37, 1951 CHEMISTR Y: PA ULING AND COREY 251 THE PLEATED SHEET, A NEW LAYER CONFIGURATION OF POL YPEPTIDE CHAINS BY LINUS PAULING AND ROBERT B. COREY GATES AND CRELLIN LABORATORIES OF CHEMISTRY,* CALIFORNIA
VOL. 37, 1951 CHEMISTR Y: PA ULING AND COREY 251 THE PLEATED SHEET, A NEW LAYER CONFIGURATION OF POL YPEPTIDE CHAINS BY LINUS PAULING AND ROBERT B. COREY GATES AND CRELLIN LABORATORIES OF CHEMISTRY,* CALIFORNIA
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory
 EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
Use the Force! Noncovalent Molecular Forces
 Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
Name: Class: Date: 3) The bond angles marked a, b, and c in the molecule below are about,, and, respectively.
 Name: Class: Date: Unit 9 Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1) The basis of the VSEPR model of molecular bonding is. A) regions of
Name: Class: Date: Unit 9 Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1) The basis of the VSEPR model of molecular bonding is. A) regions of
Quantum Calculations on Hydrogen Bonds in Certain Water Clusters Show Cooperative Effects
 J. Chem. Theory Comput. 2007, 3, 103-114 103 Quantum Calculations on Hydrogen Bonds in Certain Water Clusters Show Cooperative Effects Vasiliy S. Znamenskiy and Michael E. Green* Department of Chemistry,
J. Chem. Theory Comput. 2007, 3, 103-114 103 Quantum Calculations on Hydrogen Bonds in Certain Water Clusters Show Cooperative Effects Vasiliy S. Znamenskiy and Michael E. Green* Department of Chemistry,
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
The Strength of Hydrogen Bonds in Liquid Water and Around Nonpolar Solutes
 J. Am. Chem. Soc. 2000, 122, 8037-8041 8037 The Strength of Hydrogen Bonds in Liquid Water and Around Nonpolar Solutes Kevin A. T. Silverstein, A. D. J. Haymet,*, and Ken A. Dill*, Contribution from the
J. Am. Chem. Soc. 2000, 122, 8037-8041 8037 The Strength of Hydrogen Bonds in Liquid Water and Around Nonpolar Solutes Kevin A. T. Silverstein, A. D. J. Haymet,*, and Ken A. Dill*, Contribution from the
INTERMOLECULAR FORCES
 INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Determination of Equilibrium Constants using NMR Spectrscopy
 CHEM 331L Physical Chemistry Laboratory Revision 1.0 Determination of Equilibrium Constants using NMR Spectrscopy In this laboratory exercise we will measure a chemical equilibrium constant using key proton
CHEM 331L Physical Chemistry Laboratory Revision 1.0 Determination of Equilibrium Constants using NMR Spectrscopy In this laboratory exercise we will measure a chemical equilibrium constant using key proton
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 4 ENZYMATIC CATALYSIS
 ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 4 ENZYMATIC CATALYSIS We will continue today our discussion on enzymatic catalysis
ENZYME SCIENCE AND ENGINEERING PROF. SUBHASH CHAND DEPARTMENT OF BIOCHEMICAL ENGINEERING AND BIOTECHNOLOGY IIT DELHI LECTURE 4 ENZYMATIC CATALYSIS We will continue today our discussion on enzymatic catalysis
Lecture 15: Enzymes & Kinetics Mechanisms
 ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7. 4. Which of the following weak acids would make the best buffer at ph = 5.0?
 Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Chapter10 Tro. 4. Based on the Lewis structure, the number of electron domains in the valence shell of the CO molecule is A) 1 B) 2 C) 3 D) 4 E) 5
 Chapter10 Tro 1. All of the geometries listed below are examples of the five basic geometries for molecules with more than 3 atoms except A) planar triangular B) octahedral C) tetrahedral D) trihedral
Chapter10 Tro 1. All of the geometries listed below are examples of the five basic geometries for molecules with more than 3 atoms except A) planar triangular B) octahedral C) tetrahedral D) trihedral
Since we will be dealing with aqueous acid and base solution, first we must examine the behavior of water.
 Acids and Bases Know the definition of Arrhenius, Bronsted-Lowry, and Lewis acid and base. Autoionization of Water Since we will be dealing with aqueous acid and base solution, first we must examine the
Acids and Bases Know the definition of Arrhenius, Bronsted-Lowry, and Lewis acid and base. Autoionization of Water Since we will be dealing with aqueous acid and base solution, first we must examine the
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Topic 2: Energy in Biological Systems
 Topic 2: Energy in Biological Systems Outline: Types of energy inside cells Heat & Free Energy Energy and Equilibrium An Introduction to Entropy Types of energy in cells and the cost to build the parts
Topic 2: Energy in Biological Systems Outline: Types of energy inside cells Heat & Free Energy Energy and Equilibrium An Introduction to Entropy Types of energy in cells and the cost to build the parts
Chemistry 151 Final Exam
 Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Chemistry B11 Chapter 4 Chemical reactions
 Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
Chemistry B11 Chapter 4 Chemical reactions Chemical reactions are classified into five groups: A + B AB Synthesis reactions (Combination) H + O H O AB A + B Decomposition reactions (Analysis) NaCl Na +Cl
Peptide Bonds: Structure
 Peptide Bonds: Structure Peptide primary structure The amino acid sequence, from - to C-terminus, determines the primary structure of a peptide or protein. The amino acids are linked through amide or peptide
Peptide Bonds: Structure Peptide primary structure The amino acid sequence, from - to C-terminus, determines the primary structure of a peptide or protein. The amino acids are linked through amide or peptide
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Ionic and Covalent Bonds
 Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
Ionic and Covalent Bonds Ionic Bonds Transfer of Electrons When metals bond with nonmetals, electrons are from the metal to the nonmetal The becomes a cation and the becomes an anion. The between the cation
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
CHEM 36 General Chemistry EXAM #1 February 13, 2002
 CHEM 36 General Chemistry EXAM #1 February 13, 2002 Name: Serkey, Anne INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show
CHEM 36 General Chemistry EXAM #1 February 13, 2002 Name: Serkey, Anne INSTRUCTIONS: Read through the entire exam before you begin. Answer all of the questions. For questions involving calculations, show
Name Lab #3: Solubility of Organic Compounds Objectives: Introduction: soluble insoluble partially soluble miscible immiscible
 Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
7.14 Linear triatomic: A-----B-----C. Bond angles = 180 degrees. Trigonal planar: Bond angles = 120 degrees. B < B A B = 120
 APTER SEVEN Molecular Geometry 7.13 Molecular geometry may be defined as the three-dimensional arrangement of atoms in a molecule. The study of molecular geometry is important in that a molecule s geometry
APTER SEVEN Molecular Geometry 7.13 Molecular geometry may be defined as the three-dimensional arrangement of atoms in a molecule. The study of molecular geometry is important in that a molecule s geometry
Chapter 1 Structure and Bonding. Modified by Dr. Daniela Radu
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
2/10/2011. Stability of Cycloalkanes: Ring Strain. Stability of Cycloalkanes: Ring Strain. 4.3 Stability of Cycloalkanes: Ring Strain
 4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
INTRODUCTION TO PROTEIN STRUCTURE
 Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
CHAPTER 10 THE SHAPES OF MOLECULES
 ATER 10 TE AE MLEULE 10.1 To be the central atom in a compound, the atom must be able to simultaneously bond to at least two other atoms. e,, and cannot serve as central atoms in a Lewis structure. elium
ATER 10 TE AE MLEULE 10.1 To be the central atom in a compound, the atom must be able to simultaneously bond to at least two other atoms. e,, and cannot serve as central atoms in a Lewis structure. elium
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
VSEPR Model. The Valence-Shell Electron Pair Repulsion Model. Predicting Molecular Geometry
 VSEPR Model The structure around a given atom is determined principally by minimizing electron pair repulsions. The Valence-Shell Electron Pair Repulsion Model The valence-shell electron pair repulsion
VSEPR Model The structure around a given atom is determined principally by minimizing electron pair repulsions. The Valence-Shell Electron Pair Repulsion Model The valence-shell electron pair repulsion
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK
 Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
CHEMISTRY 113 EXAM 4(A)
 Summer 2003 1. The molecular geometry of PF 4 + ion is: A. bent B. trigonal planar C. tetrahedral D. octahedral CHEMISTRY 113 EXAM 4(A) 2. The Cl-C-Cl bond angle in CCl 2 O molecule (C is the central atom)
Summer 2003 1. The molecular geometry of PF 4 + ion is: A. bent B. trigonal planar C. tetrahedral D. octahedral CHEMISTRY 113 EXAM 4(A) 2. The Cl-C-Cl bond angle in CCl 2 O molecule (C is the central atom)
Order of Filling Subshells
 Bonding: General Concepts Ionic Bonds Sections 13.2-13.6 Covalent Bonds Section 13.7 Covalent Bond Energy & Chemical Reactions Section 13.8-13.9 Lewis Structures Sections 13.10-13.12 VSEPR Theory Section
Bonding: General Concepts Ionic Bonds Sections 13.2-13.6 Covalent Bonds Section 13.7 Covalent Bond Energy & Chemical Reactions Section 13.8-13.9 Lewis Structures Sections 13.10-13.12 VSEPR Theory Section
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Studying an Organic Reaction. How do we know if a reaction can occur? And if a reaction can occur what do we know about the reaction?
 Studying an Organic Reaction How do we know if a reaction can occur? And if a reaction can occur what do we know about the reaction? Information we want to know: How much heat is generated? How fast is
Studying an Organic Reaction How do we know if a reaction can occur? And if a reaction can occur what do we know about the reaction? Information we want to know: How much heat is generated? How fast is
The peptide bond Peptides and proteins are linear polymers of amino acids. The amino acids are
 Introduction to Protein Structure Proteins are large heteropolymers usually comprised of 50 2500 monomer units, although larger proteins are observed 7. The monomer units of proteins are amino acids. The
Introduction to Protein Structure Proteins are large heteropolymers usually comprised of 50 2500 monomer units, although larger proteins are observed 7. The monomer units of proteins are amino acids. The
CHAPTER 10 THE SHAPES OF MOLECULES
 ATER 10 TE AE MLEULE EMIAL ETI BED READIG RBLEM B10.1 lan: Examine the Lewis structure, noting the number of regions of electron density around the carbon and nitrogen atoms in the two resonance structures.
ATER 10 TE AE MLEULE EMIAL ETI BED READIG RBLEM B10.1 lan: Examine the Lewis structure, noting the number of regions of electron density around the carbon and nitrogen atoms in the two resonance structures.
Chemistry. The student will be able to identify and apply basic safety procedures and identify basic equipment.
 Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Acids and Bases: Molecular Structure and Acidity
 Acids and Bases: Molecular Structure and Acidity Review the Acids and Bases Vocabulary List as needed. Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive
Acids and Bases: Molecular Structure and Acidity Review the Acids and Bases Vocabulary List as needed. Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive
SHAPES OF MOLECULES (VSEPR MODEL)
 1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
PV (0.775 atm)(0.0854 L) n = = = 0.00264 mol RT -1-1
 catalyst 2 5 g ¾¾¾¾ 2 4 g 2 g DH298 = rxn DS298 C H OH( ) C H ( ) + H O( ) 45.5 kj/mol ; = 126 J/(K mol ) ethanol ethene water rxn 1 atm 760 torr PV (0.775 atm)(0.0854 L) n = = = 0.00264 mol RT -1-1 (0.08206
catalyst 2 5 g ¾¾¾¾ 2 4 g 2 g DH298 = rxn DS298 C H OH( ) C H ( ) + H O( ) 45.5 kj/mol ; = 126 J/(K mol ) ethanol ethene water rxn 1 atm 760 torr PV (0.775 atm)(0.0854 L) n = = = 0.00264 mol RT -1-1 (0.08206
Chapter 2 Polar Covalent Bonds; Acids and Bases
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
