Publication of Clinical Trials in Scientific Journals: Editorial Issues
|
|
|
- Avis Blake
- 8 years ago
- Views:
Transcription
1 EDITOR S PAGE Publication of Clinical Trials in Scientific Journals: Editorial Issues Fernando Alfonso, a Javier Segovia, b Magda Heras, c and Javier Bermejo b a Editor-in-Chief. b Associate Editor. c Supplements Editor. Since the last update of the International Committee of Medical Journal Editors (ICMJE) 1 recommendations, which we at REVISTA ESPAÑOLA DE CARDIOLOGÍA adopted, 2-5 one of the most important contributions to this field has been the suggestion that that all clinical trials (CT) should be registered prior to definitive publication in biomedical journals The ICMJE believes that from their very inception CTs need to be registered in public databases that are easily accessible both to authors, researchers, and regulatory agencies as well as the general public. The principal objective of this initiative is to improve the trials credibility when finally published and guarantee that the methodology employed, results, and scientific information generated should be freely available for analysis by the international scientific community The very definition of CTs has raised controversy. A CT could be defined broadly as a research study in human volunteers to answer specific health questions. 7 This initial definition would include observational trials and interventional trials without control groups, too. However, from the editorial point of view, and bearing in mind the implications we shall now consider, the definition proposed by the ICMJE is more pragmatic and also more acceptable. 11 Thus, the CT is defined as any research project that prospectively allocates subjects to a particular intervention or comparison group to study the cause-effect relationship between a medical intervention and a health outcome. 11 Such interventions include drugs, surgical procedures, and devices, as well as behavioral treatments or process-of-care changes. 11 In the present article we review: a) principal biases in medical research that affect CTs; b) recommendations to improve the description of CTs in biomedical journals; c) general implications derived from the initiative to register CTs; and d) adapting REVISTA ESPAÑOLA DE CARDIOLOGÍA editorial policy. Correspondence: Revista Española de Cardiología. Nuestra Señora de Guadalupe, Madrid. España. rec@revespcardiol.org CLINICAL TRIALS AND BIAS IN RESEARCH AND PUBLICATION Many publications with false positive results have been a consequence of the pressure experienced by different research groups competing in the same field to publish results that confirm the most attractive physiopathologic hypotheses. Ioannidis 12 analyzed the results of trials that, after publication in medical journals with the highest impact factors, were later the most cited. One third of these articles were questioned by subsequent studies which were better designed or included a greater number of patients that rejected or significantly diminished the effects of the intervention analyzed. Larger trials and those with randomized designs were better able to withstand the passage of time. 12 However, the fact that the bulk of research has been seen to move from academic and university centers to direct contracts between sponsors and private organizations for research by contract 13,14 highlights the gradual loss of the scientific-academic establishment s influence in controlling the research agenda. A recent study 15 has shown that, although the most cited articles continue in the main to be the products of authors with academic affiliations, the number of trials financed exclusively by industry has increased spectacularly. The potential danger of this change is double-edged. On the one hand, scientifically relevant issues are left out in the cold and are increasingly less likely to be investigated. On the other, a plethora of authors have demonstrated that, by comparison with non-sponsored research, sponsored trials are published less frequently and, moreover, have a threefold greater probability of obtaining favorable results than their non-sponsored counterparts Curiously, these differences do not appear to be due to inferior methodology in the trials financed by industry. Specifically, this problem has been analyzed in the context of cardiology, too. In a provocative study that included 324 cardiovascular CTs published between 2000 and 2005 in the 3 medical journals with the 1206 Rev Esp Cardiol. 2006;59(11):
2 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Considerations highest impact factors, Ridker et al 20 analyzed the probability of results being positive according to the source of finance. Trials financed by industry more frequently presented results favorable to the drug or device analyzed than those financed by not for-profit organizations. Moreover, results were more favorable in CTs using surrogate endpoints than in those using clinical endpoints. 20 According to the Declaration of Helsinki, 21 CTs can only be conducted with volunteers and, therefore, medical progress is based on the generosity of people who freely agree to participate in trials. Although these individuals assume risks, their participation in CTs permits them to opt for greater clinical benefits or, at least, means the results obtained can lead to improved treatment for others. 22 As the fundamental objective of research is to extend knowledge, it seems ethically reprehensible to withhold knowledge generated by CTs from the public domain. 22 However, in corporative research, bias can arise when issues of image or, above all, economic motives take precedence over scientific interest. The former frequently underlie the problems of selective publication of results and concealment of data. One recent review showed that 91% of protocols specified limitations in researchers rights of publication. 23,24 In more than half the cases, data were identified as the property of the sponsor who, moreover, had to approve the manuscript prior to its submission for publication. 23,24 Many contracts signed between sponsors and researchers prohibit the latter from disclosing or commenting on results that have been presented to them in private. Not even the powerful US Food and Drug Administration (FDA) can publish all the data it examines 8. These biases limit the information available, condition our knowledge which particularly affects evidence-based medicine and, what is more important, can prejudice the care patients receive. Unfortunately, it has taken revelations of the scandalous concealment of serious adverse events to shake the foundations that underpin the regulation of financing and publishing CTs. 25,26 Although criticism has centered fundamentally on trials sponsored by pharmaceutical companies, substantial problems have also been found in many trials financed by government agencies. Chan et al 27 showed that in trials conducted in the 1990s in Canada there were notable differences between initial protocols and final publications. They found incomplete data on efficacy outcomes and harm outcomes in 31% and 59% of the trials, respectively. More worrying was their demonstration of differences in descriptions of primary outcomes in protocols sent to ethics committees and those in the definitive publications. The same authors conducted a similar study with data from ethics committees in Denmark. 28 More than half of the efficacy or harm outcomes were communicated in an incomplete form so as to favor the results of the intervention and conceal adverse effects. All of the above highlights the problem of publication bias. Some analyses of drug efficacy have found positive results when only evaluating published trials, whereas the inclusion of all trials conducted (published and unpublished) has very often shown that the prejudicial effects can even surpass the benefits. 29,30 Metaanalyses especially suffer the consequences of publication bias. 30 Medical journal editors should favor the publication of correctly designed and conducted trials on topics that are clinically relevant to their readers, whether the trial results are negative or positive. 11 The latest ICMJE recommendations stress aspects of authorship, conflict of interests and control of data that should be analyzed and interpreted directly by the researchers conducting the trial. 11 Moreover, they remind us that failure to submit for publication on the part of authors or non-acceptance on the part of editors simply because the trials concerned present negative results, constitute publication bias. 11 These issues notwithstanding, many negative studies are simply inconclusive and, logically, should be low priorities for publication. 11 Some institutions (Cochrane Library 31 ) have shown themselves to be especially interested in gathering data from all these trials. 11 The policy of registering all CTs seeks to facilitate public access to all scientifically relevant information that, for different motives, may not find its way into conventional publications. RECOMMENDATIONS FOR THE PUBLICATION OF CLINICAL TRIALS (CONSORT RECOMMENDATIONS) In spite of their limitations, randomized trials represent the benchmark approach to learning about the efficacy of a particular treatment. In fact, in the era of evidence-based medicine, the CT has been enthroned at the very highest level of the hierarchy of what has been proven. 19 However, the quality of information in controlled trials has been shown to be frequently deficient. 30,32 When analyzing trials, readers can become frustrated as they realize that information on relevant aspects is missing. Moher et al 30 and Schulz et al 32 showed that inadequate description of randomized trials is associated with a bias in estimating the effects of the interventions being evaluated that tended to overestimate the effects of the treatment. The CONSORT declaration (Consolidated Standards of Reporting Trials) was made by a group of researchers, epidemiologists, statisticians, and biomedical journal editors to improve the presentation of randomized clinical trials. The proposal included following a list of evidence-based variables (Table 1) and a flowchart (Figure) during the process of writing, revising and analyzing CTs. 33 In great detail, the Rev Esp Cardiol. 2006;59(11):
3 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Issves TABLE 1. CONSORT List of Variables Variable (Number) Description Topic and summary (1) Introduction Background (2) Methods Participants (3) Interventions (4) Objectives (5) Results (6) Sample size (7) How participants were allocated to interventions ( random allocation, randomized, or randomly assigned ) Scientific background and explanation of hypothesis Eligibility criteria for participants and settings and locations of data collection Precise details of interventions intended for each group, and how and when they were actually administered Specific objectives and hypotheses Clearly-defined primary and secondary outcome measures and, when applicable, results of methods used to enhance measurement quality (e.g., multiple observations, training of assessors) How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules Randomization Sequence generation (8) Method used to generate random allocation sequence, including details of any restrictions (e.g., blocking, stratification) Allocation concealment (9) Method used to implement random allocation sequence (e.g., numbered containers or central telephone), specifying whether sequence was concealed until interventions were assigned Implementation (10) Who generated the allocation sequence, who enrolled participants, and who assigned participants to their respective groups Blinding (masking) (11) Statistical methods (12) Results Participant flow (13) Recruitment (14) Baseline data (15) Numbers analyzed (16) Outcomes and estimations (17) Ancillary analyses (18) Adverse events (19) Discussion Interpretation (20) Generalizability (21) Overall evidence (22) Specifying whether or not participants, those administering or allocating interventions, and those assessing outcomes were blinded to group allocation and, if so, how the success of masking was evaluated Statistical methods used to compare groups for primary outcome variable(s). Methods used in additional analyses such as subgroup analysis and adjusted analysis Flow of participants through each stage (a diagram is strongly recommended) Specifically, for each group the numbers of participants randomly allocated, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome should be reported. Describe deviations from the initial protocol study, together with reasons Dates defining periods of recruitment and follow-up Baseline demographic and clinical characteristics of each group Number of participants (denominator) in each group included in each analysis, specifying whether analysis was by intention-to-treat. When feasible, stating results in absolute numbers (e.g., 10/20, not 50%) For each primary and secondary outcome, summarize results for each group and the estimated effect size and its precision (e.g., 95% confidence interval) Assess the presence of multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those that were prespecified and those that were exploratory Indicate all important adverse events or side effects in each intervention group Interpretation of results, taking into account study hypotheses, sources of potential bias or imprecision, and the dangers associated with multiplicity of analyses and outcomes Generalizability (external validity) of trial findings General interpretation of results in the context of currently available evidence An additional column (right) should specify the page on which each variable is described. Adapted from Moher et al. 33 flowchart guides the passage of participants through the 4 key phases of a trial: enrolment, allocation to intervention, follow-up, and analysis. The objective was to provide a flexible, regularly-updated working tool that would permit researchers to present CT results systematically and with total transparency, so readers could judge their validity adequately. 33 The initial 1996 declaration was updated in 2001 when, moreover, an additional document was published with examples, explanations and a glossary of terms to guarantee correct application. 34 The authors explained the scientific foundations for their choice of variables and highlighted the coherence of the final list. The most recently updated version of the CONSORT recommendations can be found on their website. 35 Some important issues, which finally could not be incorporated into the list, provoked debate. Interesting variables (ethics committee approval, sources of finance, 1208 Rev Esp Cardiol. 2006;59(11):
4 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Considerations and registry number) were not included. 33 As we will see later, CT registries already facilitate this information. Some researchers noted the lack of a variable that made explicit the sponsor s role in designing, conducting, analyzing, interpreting, and writing up the trial. 36 Others suggested that the process that guarantees a systematic, structured review of the information available should be more detailed as only a minority of CTs analyzed prior information systematically. 37,38 The presentation of a systematic review that justifies the trial guarantees to participants that the design is optimal. 37,38 In some debates, it was suggested that all systematic reviews should be openly accessible It was also proposed that the use of missing data and patients compliance with treatment should be explicitly defined. 40 Finally, it was suggested that the list of variables should include a description of the economic analysis to determine if trials can be conducted on the efficiency of the interventions evaluated. 41 New Proposals Probably, the most interesting advance to occur in this field is that of optimizing the description of harm, risk, and adverse effects in CTs. The CONSORT group has developed a list of 10 variables to improve the description of risk and complications that coincides perfectly with the initial 22 points. 42 It is intended to raise awareness of the importance of informing more widely about the problems of safety detected in CTs. The initial recommendations have also been adapted to cater for trials with specific designs. Thus, the CONSORT group has recently prepared a document 43,44 for trials that randomize groups of patients (cluster trials) as these have been found to present specific problems (design, effective sample size, intragroup correlation coefficient, and selection after randomization) Other adaptations are aimed at equivalence or noninferiority trials, with crossover treatments or multiple intervention groups. Previously, deficiencies in the publication of noninferiority or equivalence trials had been detected. 46 In these trials it is necessary to predefine margins of noninferiority or equivalence and their clinical justification, to mention confidence intervals and analyze results both for intention-to-treat and protocol. 47 Results and Challenges in Their Implementation Implementation of the CONSORT criteria has been shown to improve the quality of published information on randomized trials. Moher et al 48 showed that quality of CT presentation improved notably on following these criteria. In particular, the incorporation of the flowcharts helped improve the quality of publications. 49 Devereaux et al 50 showed that journals that endorsed the CONSORT recommendations improved their quality criteria and increased the number of methodological aspects described. Yank et at 51 compared the quality criteria of 300 CTs published before 1997 in 5 prestigious medical journals with 300 CTs published after Description of informed consent and ethics committee approval significantly improved during the study period. However, although descriptions of randomized trials correlate with methodological quality, substantial differences of quality continue to be found in well-presented trials. 52 Assessed for Eligibility (n=...) [1] Excluded (n=...) Not Meeting Inclusion Criteria (n=...) Refused to Participate (n=...) Other Reasons (n=...) Randomized (n=...) [2] Allocated to Intervention (n=...) Received Allocated Intervention (n=...) Did Not Receive Allocated Intervention (Reasons) (n=...) Allocated to Intervention (n=...) Received Allocated Intervention (n=...) Did Not Receive Allocated Intervention (Reasons) (n=...) [3] Lost to Follow-Up (Reasons) (n=...) Intervention Discontinued (Reasons) (n=...) Lost to Follow-Up (Reasons) (n=...) Intervention Discontinued (Reasons) (n=...) Figure 1. Flowchart of subjects participating in the different phases of randomized trials. [1] Enrolment period; [2] Allocation to intervention; [3] Follow-up; [4] Analysis. [4] Analyzed (n=...) Excluded From Analysis (n=...) Analyzed (n=...) Excluded From Analysis (n=...) Rev Esp Cardiol. 2006;59(11):
5 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Issves Hewitt et al 53 showed that description of concealing group allocation from patients was customary in journals that endorsed CONSORT but infrequent in other journals. This affects result credibility as CTs that omit mentioning allocation concealment more frequently present positive results for their primary outcome. 53 Recently, it has been reported that as many as 70% of randomized trials in cardiovascular disease use subgroup analyses and that these are not always conducted adequately. 54 In spite of the fact that the CONSORT list specifically tackles this issue, few studies clearly state whether or not subgroups are prespecified, if statistical analysis considers multiple comparisons, or if interactions have been evaluated. 54 Finally, Nuovo et al 55 have insisted that, despite all the recommendations, only a limited number of CTs specify the number of patients needed to treat and the absolute reduction in risk. These data confirm that we should continue to strive to improve the presentation of randomized trials in biomedical journals. Altman 56 reviewed the instructions for authors in 167 high-impact factor journals published in Only 23% of these mentioned the CONSORT recommendations and 43% cited the ICMJE recommendations, often with out-of-date references. General medicine journals were more rigorous than specialized journals, and those that adopted the CONSORT proposals also followed ICMJE recommendations. REGISTRY OF CLINICAL TRIALS The idea of registering CTs arose more than 30 years ago in an attempt to avoid publication bias. 8 It has been calculated that only half of the 1 million CTs conducted in the last 50 years has been published and that of these, a considerable number are unavailable via MEDLINE. 8 The net result is a clear bias towards the publication of positive results that systematically favor more innovative and more expensive treatments. Moreover, adverse effects are normally silenced or communicated after some delay. This problem affects clinical practice guideline recommendations 57 and, ultimately, patients health. 57 The question is fundamentally ethical: are CT results the exclusive property of their sponsors or are they also the property of the international scientific community as they have direct repercussions on the health of citizens? From the editorial point of view the reply is clear In fact, the ICMJE proposal has translated into a more effective stimulus to implement CT registries The Declaration of Helsinki 21 requires that trial designs should be available to the public. In the long term, no one wins with the selective dissemination of CT information Consequently, researchers and sponsors hold the weighty responsibility of presenting findings and possible adverse effects within a reasonable period of time Finally, ethics committees should cooperate by insisting on registering CTs prior to giving their definitive agreement. 7 Outstanding advantages of registering CTs 6-11,58 include: a) ethical aspects; b) avoiding harm to patients by allowing access to unpublished information about risks and adverse effects; c) avoiding duplicating research and thus optimizing the efficiency of the investigation and helping improve the design of new trials; d) avoiding publication bias; e) improving transparency, to prevent changes in CT design and favor better interpretation of results; and f) raising the overall quality of the research. 58 At the same time, possible limitations of registering CTs include: a) lack of registers that meet all the required criteria; b) increased bureaucratic red-tape complex enough in itself for research; c) presentation of results prior to their analysis and criticized in the process of peer revision; d) incorrect interpretation of results insufficiently explained by the general public; e) loss of the advantages (and of the resulting economic benefits) for the companies and investment in research and innovation, to the benefit of their competitors; and f) lack of effective measures that penalize nonregistration of CTs. 58 Since 2004, the world s principal pharmaceutical companies have collaborated resolutely to favor registering CTs. In fact, many of them have developed proactive measures aimed at restoring the climate of confidence in research financed by the industry. 8 Some initiatives have centered on developing private but easily accessible registers of all CTs sponsored by each pharmaceutical company. However, attempts by some associations in the industry to unify their registers have yet to produce the desired results. 8 Moreover, due to the inherent conflict of interests, these initiatives do not seem to be the best solution to the problem and we can barely hope for a unification of these registers. The latest ICMJE recommendations specify the obligation to register CTs. 6-11,59 Thus, in 2004 the editors responded to a growing clamor for increased transparency in CTs. This registry would be universal, public, easily accessible (in electronic format) and free. It would remain open prospectively to permit the updating of data and should be managed by a nonprofit making organization. Each CT would have a unique register number appearing at the end of the abstract. Finally, it should be possible to guarantee the validity of the data included. In Table 2, we present the ICMJE recommendations, adapted from the World Health Organization (WHO) proposal, with the minimum data for inclusion in each record. 9 These recommendations are less demanding than the much more ambitious Declaration of Ottawa proposals, with contributions from many organizations, 1210 Rev Esp Cardiol. 2006;59(11):
6 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Considerations TABLE 2. Minimum Data That Should Be Registered in Clinical Trials Variable Comment 1. Unique trial number Established by the registry itself 2. Registration date Established by the registry itself 3. Secondary identification Assigned by the sponsor or other interested parties 4. Finance Name of the organization that finances the trial 5. Primary sponsor Primary entity responsible for research 6. Secondary sponsor Secondary entity responsible for research 7. Responsible contact person Public contact person for the trial and for patients 8. Research contact person Person to contact for scientific enquiries about the trial 9. Title of the study Brief title chosen by the research group 10. Official scientific title of the study Must include the type of intervention, the condition being studied and the outcome 11. Research ethics committee review Specify yes/no. It is assumed that all studies have been approved prior to commencing 12. Condition Pathology being analyzed 13. Intervention Description of intervention studied and of that used in the comparison or control group Generic name in the case of registered medication. Must specify the duration of the intervention 14. Principal criteria for inclusion/exclusion Main characteristics of patients that determine eligibility for the study 15. Type of study The database must provide lists of selection specification: randomized or not, type of masking (double blind, etc), type of control (active or placebo), group allocation (parallel, crossover, factorial) 16. Anticipated start date Estimated date of inclusion of first patient 17. Calculation of sample size Total number of patients researchers plan to enroll in the study 18. Recruitment status Specify whether this is available and, if so, provide the information 19. Primary objectives Primary outcome. Should specify exactly when the outcome is analyzed 20. Secondary objectives Secondary outcomes defined in the protocol. Should describe when they are analyzed World Health Organization proposal adopted by the International Committee of Medical Journal Editors (ICMJE). Adapted from de Angelis et al. 9 including the Cochrane Collaboration. 60,61 These proposals suggested that all prospective trials should be registered, including phase I trials, with or without control group. Moreover, any alterations introduced in the trial should be detailed and final results presented, although some delay would be acceptable to allow for their publication. 60,61 Currently Available Registries 1. The US clinicaltrials.gov registry meets all ICMJE requirements. This database, developed by the National Library of Medicine, is available on the internet 62 and, although it depends on the FDA and the National Institute of Health, permits the inclusion of international trials. The registry has been criticized by some European researchers as being overly centered on US CTs and not incorporating information about final results A British private company (Current Controlled Trials 63 ) developed the idea of the standard international registry number. In late 2005, ownership of this database was transferred to a non-profit making organization fulfilling ICMJE requirements. Now, this registry (International Standard Randomised Controlled Trial Number 64 ) is also valid from an editorial point of view The European Community, in a specific harmonization directive (2001/20/CT) introduced legislation that made it obligatory to register clinical studies about medical products for human use and developed the EudraCT database 65 controlled by the European Medicines Agency. Although this database could be very useful for European researchers, at the moment it does not comply with some ICMJE requirements as it is a confidential register, only available to regulatory agencies and funding organizations Many countries, including Spain, have gathered information on all CTs although, again, these data are not available to the public Some medical specialties, such as pediatrics, have been particularly sensitive to the ethical problems in research. In 2004, a registry of drug evaluation in children (DEC-net 66 ) was established, sponsored directly by the European Union, which fully complies with ICMJE requirements Finally, the WHO has developed an international platform to organize CT registries and assume the leadership in this initiative The WHO collaborates with other organizations on projects destined to guarantee consensus over the minimum data contained in the registry, the reliability of the information registered and the implementation of a single international system of numeration Problems and Consequences of Application Some issues have generated considerable discussion. According to the ICMJE, the registry should be public property and non-profit making. 11 However, the editors Rev Esp Cardiol. 2006;59(11):
7 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Issves of the British Medical Journal consider that public ownership of the registry, although desirable, could be an unnecessarily restrictive criterion and, in fact, this apparently minor difference led them to refrain from signing the latest ICMJE declaration. 72 Moreover, although the ICMJE does not demand it, some researchers insist on the usefulness of extending the registries to phase I trials and, above all, on the need to present final results. 58 Also, it is important to see how the WHO initiative and the Declaration of Ottawa 60,61 evolve. Finally, from time to time, we must evaluate the results and implications of this new editorial policy. Zarin et al 73 confirm the important influence of the ICMJE initiative on registering CTs. The number of CTs registered clearly peaked in September A progressive improvement in the quality of the data registered was confirmed. This included the name of the intervention analyzed and the primary outcome. However, the authors observed different concerns at the time of revealing information and many studies were registered simply as drug in research phase instead of specifying the specific name of the intervention. 73 While provision of full information for these fields was practically universal when CTs were financed by academic institutions, CTs sponsored by industry frequently used vague terms to describe these important variables. 22 CONSIDERATIONS OF EDITORIAL POLICY At REVISTA ESPAÑOLA DE CARDIOLOGÍA we have conducted an editorial review of this issue. In contrast with some important American journals, no European publication dedicated to cardiovascular diseases yet insists CTs be registered. However, this issue was debated at the last meeting of national editors of the European Cardiology Society. Moreover, the HEART group (Heart Editors Action Round Table) is actively working to promote agreement among cardiovascular journal editors to facilitate an overall policy of registering CTs. The number of randomized trials submitted to REVISTA ESPAÑOLA DE CARDIOLOGÍA in recent years has been as few as only 2-3 a year. However, during this period we have published a substantial number of sub-trials, analyses of subgroups and follow-ups of different CTs. From now on, the randomized trials published in this journal must comply with the CONSORT recommendations. Moreover, we are committed to adapting our instructions for authors to the advances that are occurring over the registering of CTs, in terms both of editorial requirements and of adapting to current legislation. We hope that the concerns we outline here help present CTs with the greatest methodological clarity possible. In this way, we will improve the quality of the final publication of these trials that are so essential to the advance of scientific knowledge. We trust that these editorial initiatives, together with those previously adopted, contribute to improve the quality and credibility of REVISTA ESPAÑOLA DE CARDIOLOGÍA. REFERENCES 1. Comité Internacional de Editores de Revistas Médicas. Requisitos de uniformidad para los manuscritos enviados a revistas biomédicas: escritura y proceso editorial para la publicación de trabajos biomédicos. Rev Esp Cardiol. 2004;57: Alfonso F, Bermejo J, Segovia J. Nuevas recomendaciones del comité internacional de editores médicos. Cambiando el énfasis: de la uniformidad de los requisitos técnicos a los aspectos bioéticos. Rev Esp Cardiol. 2004;57: Alfonso F, Bermejo J. Revista Española de Cardiología: en camino. Rev Esp Cardiol. 2004;57: Bosch X, Villacastín JP, Alfonso F. Medicina basada en la credibilidad. Rev Esp Cardiol. 2001;54: Alfonso F, Bermejo J, Segovia J. Revista Española de Cardiología. Actividad, difusión internacional e impacto científico. Rev Esp Cardiol. 2004;57: de Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351: Steinbrook R. Public Registration of clinical trials. N Engl J Med. 2004;351: Rennie D. Trial registration. A great idea switches from ignored to irresistible. JAMA. 2004;292: de Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. N Engl J Med. 2005;352: Haug C, Gotzsche PC, Schroeder TV. Registries and registration of clinical trials. N Engl J Med. 2005;353: International Committee of Medical Journal Editors. Uniform requirements for manuscripts submitted to biomedical journals. Update posted on October Available from: Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294: Davidoff F, de Angelis CD, Drazen JM, Hoey J, Hjgaard L, Horton R, et al. Sponsorship, authorship and accountability. Lancet. 2001;358: Johns MM, Barners M, Florencio PS. Restoring balance to industry-academia relationships in an era of institutional financial conflicts of interest: promoting research while maintaining trust. JAMA. 2003;289: Patsopoulos NA, Analatos AA, Ioannidis JP. Origin and funding of the most frequently cited papers in medicine: database analysis. BMJ. 2006;332: Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interes in biomedical research: a systematic review. JAMA. 2003;298: Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: a systematic review. BMJ. 2003;326: Finucane TE, Boult CE. Association of funding and findings of pharmaceutical research at a meeting of a medical professional society. Am J Med. 2004;117: Peiró S. Se puede confiar en los resultados de la investigación clínica? Humanitas. Humanidades Médicas. 2006;5: Rev Esp Cardiol. 2006;59(11):
8 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Considerations 20. Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for profit and not-for-profit organizations: JAMA. 2006;295: World Medical Association. Declaration of Helsinki. Oct [accedido 15 May 2006]. Available from: e/policy/b3.htm 22. Drazen JM, Wood AJJ. Trial registration report card. N Engl J Med. 2005;353: Gotzsche PC, Hrobjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW, et al. Constrains on publication rights in industryinitiated clinical trials. JAMA. 2006;295: Steinbrook R. Gag clauses in clinical-trial agreements. N Engl J Med. 2005;352: Topol EJ. Failng the public health: rofecoxib, Merck and the FDA. N Engl J Med. 2004;351: Kondro W. Drug company experts advised staff to withhold data about SSRI use in children. CMAJ. 2004;170: Chan AW, Krleza-Jerik K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institute of Health Research. CMAJ. 2004;171: Chan AW, Hrobjartsson A, Haarhr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291: Whittington CJ, Kendall T, Fonagly P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363: Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does the quality of reports of randomised trials affects estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352: Cochrane Library. Available from: Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995; 273: Moher D, Schulz K, Altman DG, for the CONSORT group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357: Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. for the CONSORT Group. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134: Available from: Counsell C. Reporting of randomized controlled trials (letter). Lancet. 2001;358: Clarke M, Chalmers I. Discussion sections in reports of controlled trials published in five general medical journals. Islands in search of continents? JAMA. 1998; Clarke M. Doing new research? Don t forget the old. PLoS Medicine. 2004;1:e35: Clarke M, Alderson P, Chambers I. Discussion sections in reports of controlled trials published in general medical journals. JAMA. 2002;287: Shapiro S. The revised CONSORT statement: honing the cutting edge of the randomized controlled trial. CMAJ. 2001;164: Freemantle N, Mason JM, Haines A, Eccles MP. An important step toward evidence-based health care. Ann Intern Med. 1997; 127: Ioannidis JPA, Evans SJW, Gotzsche PC, O Neill RT, Altman DG, Schulz K, et al, for the CONSORT group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141: Campbell MK, Elbourne DR, Altman DG, for the CONSORT Group. CONSORT statement: extension to cluster randomized trials. BMJ. 2004;328: Campbell MJ. Extending CONSORT to include cluster trials. BMJ. 2004;328: Puffer S, Torgerson D, Watson J. Evidence for risk of bias in cluster randomized trials:review of recent trials published in three general medical journals. BMJ. 2003;327: Le Henanff A, Giraudeau B, Baron G, Ravaud P. Quality of reporting of noninferiority or equivalence trials. JAMA. 2006;295: Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW, for the CONSORT group. Reporting of noninferiority and equivalence randomized trials. An extension of the CONSORT statement. JAMA. 2006;295: Moher D, Jones A, Lepage L, for the CONSORT group. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before and after evaluation. JAMA. 2001; 285: Egger M, Jüni P, Bartlett C, for the CONSORT group. Value of flow diagrams in reports of randomised controlled trials. JAMA. 2001;285: Devereaux PJ, Manns BJ, Ghali WA, Quan H, Guyatt GH. The reporting of methodological factors in randomized controlled trials and the association with a journal policy to promote adherence to the consolidated standards of reporting trials (CON- SORT) checklist. Controlled Clinical Trials. 2002;23: Yank V, Rennie D. Reporting of informed consent and ethics committee approval in clinical trials. JAMA. 2002;287: Huwiler-Müntener K, Jüni P, Junker C, Egger M. Quality of reporting of randomized trials as a measure of methodologic quality. JAMA. 2002;287: Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy of reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ. 2005; 330: Hernández AV, Boersma E, Murray GD, Habbema JD. Subgroup analyses in the therapeutic cardiovascular clinical trials. Are most of them misleading? Am Heart J. 2006;151: Nuovo J, Melnikow J, Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA. 2002;287: Altman DG. Endorsement of the CONSORT statement by high impact medical journals: survey of instructions to authors. BMJ. 2005;330: Alfonso F, Bermejo J, Segovia J. Guías Europeas de Práctica Clínica en Revista Española de Cardiología. Hacia una completa «globalización» de la asistencia cardiovascular? Rev Esp Cardiol. 2004;57: Stone JH, Lockshin MD, Katz PP, Yelin EH. New journal policy regarding registration of clinical trials. Arthritis Rheum. 2005;52: Steinbrook R. Registration of clinical trials: voluntary of mandatory? N Engl J Med. 2004;351: Krleza-Jeric K, Chan AW, Dickersin K, Sim I, Grimshaw J, Gluud C, for the Ottawa Group. Principles for international registration of protocol information and results from human trials of health-related interventions: Ottawa statement (part I). BMJ. 2005;330: Erratum in: BMJ 2005;330: Krleza-Jeric K. Clinical trials registration: The differing views of industry, the WHO and the Ottawa group. PloS Medicine. 2005;2:e378( ). 62. National Library of Medicine. Available from: ClinicalTrials.gov 63. Current Controlled Trials. Available from: International Standard Randomised Controlled Trial Number. Available from: EudraCT. Available from: DEC-net. Available from: Bonati M, Pandolfini C. Trial registration, the international committee of medical journals editors statement and pediatric journals. Pediatric Anesthesia. 2006;16:92-9. Rev Esp Cardiol. 2006;59(11):
9 Alfonso F et al. Publication of Clinical Trials in Scientific Journals: Editorial Issves 68. Evans T, Gulmezoglu AM, Pang T. Registering clinical trials: an essential role for WHO. Lancet. 2004;363: Gulmezoglu AN, Pang T, Horton R, Dickersin K. WHO facilitates international collaboration in setting the standards for clinical trials registration. Lancet. 2005;365: Sim I, Chan AW, Gulmezoglu AM, Evans T, Pang T. Clinical trials registration: transparency is the watchword. Lancet. 2006;367: Horton R. Trial registers: protecting patients, advancing trust. Lancet. 2006;367: Abbasi K, Godlee F. Next steps in trial registration. Minimun criteria have been agreed, and intentions restated. BMJ. 2005;330: Zarin DA, Tse T, Ide N. Trial registration at ClinicalTrials.gov between May and October N Engl J Med. 2005;353: Alfonso F, Bermejo J, Segovia J. Publicación duplicada o redundante: podemos permitirnoslo? Rev Esp Cardiol. 2005;58: Alfonso F, Segovia J, Bermejo J. Revista Española de Cardiología 2005: Actividad y Reconocimiento Científico. Rev Esp Cardiol. 2005;58: Alfonso F, Bermejo J, Segovia J. Impactología, impactitis, impactoterapia. Rev Esp Cardiol. 2005;58: Rev Esp Cardiol. 2006;59(11):
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text)
 Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
Biostat Methods STAT 5820/6910 Handout #6: Intro. to Clinical Trials (Matthews text) Key features of RCT (randomized controlled trial) One group (treatment) receives its treatment at the same time another
Guidelines for AJO-DO submissions: Randomized Clinical Trials June 2015
 Guidelines for AJO-DO submissions: Randomized Clinical Trials June 2015 Complete and transparent reporting allows for accurate assessment of the quality of trial and correct interpretation of the trial
Guidelines for AJO-DO submissions: Randomized Clinical Trials June 2015 Complete and transparent reporting allows for accurate assessment of the quality of trial and correct interpretation of the trial
Improving reporting in randomised trials: CONSORT statement and extensions Doug Altman
 Improving reporting in randomised trials: CONSORT statement and extensions Doug Altman Centre for Statistics in Medicine University of Oxford Whatever the outcome of a study, it is really hard for the
Improving reporting in randomised trials: CONSORT statement and extensions Doug Altman Centre for Statistics in Medicine University of Oxford Whatever the outcome of a study, it is really hard for the
Reporting guidelines: past, present and future
 Reporting guidelines: past, present and future Acknowledge: Doug Altman, Isabelle Boutron, John Hoey, Sally Hopewell, Philippe Ravaud, Drummond Rennie, Ken Schulz, and Iveta Simera Health research reporting
Reporting guidelines: past, present and future Acknowledge: Doug Altman, Isabelle Boutron, John Hoey, Sally Hopewell, Philippe Ravaud, Drummond Rennie, Ken Schulz, and Iveta Simera Health research reporting
Current reporting in published research
 Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
Current reporting in published research Doug Altman Centre for Statistics in Medicine, Oxford, UK and EQUATOR Network Research article A published research article is a permanent record that will be used
GUIDE TO RANDOMISED CONTROLLED TRIAL PROTOCOL CONTENT AND FORMAT (FOR NON CTIMP S)
 GUIDE TO RANDOMISED CONTROLLED TRIAL PROTOCOL CONTENT AND FORMAT (FOR NON CTIMP S) The aim of this guide is to help researchers with the content and structure of protocols for randomised controlled trials
GUIDE TO RANDOMISED CONTROLLED TRIAL PROTOCOL CONTENT AND FORMAT (FOR NON CTIMP S) The aim of this guide is to help researchers with the content and structure of protocols for randomised controlled trials
PROTOCOL FILE APPROVALS
 RESEARCH TRAINING ACCREDITATION APPROVALS PRELIMINARY REPORT EQUATOR CHECK UP DATA SHARING IMPLEMENTATION PROTOCOL FILE Evidence METHODOLOGICAL DESIGN Data collection Bias Clinician opinion Patients opinion
RESEARCH TRAINING ACCREDITATION APPROVALS PRELIMINARY REPORT EQUATOR CHECK UP DATA SHARING IMPLEMENTATION PROTOCOL FILE Evidence METHODOLOGICAL DESIGN Data collection Bias Clinician opinion Patients opinion
Singapore Clinical Trials Register. Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY
 Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
Singapore Clinical Trials Register Foo Yang Tong Director Clinical Trials Branch Health Products Regulation Group HEALTH SCIENCES AUTHORITY Clinical Trial Register Global Trend EMA: EU Clinical Trials
Carol Lefebvre Senior Information Specialist UK Cochrane Centre National Institute for Health Research Oxford, UK
 Clinical trials registers and clinical trials results registers: their contribution as health information sources to evidence-based healthcare decision-making Carol Lefebvre Senior Information Specialist
Clinical trials registers and clinical trials results registers: their contribution as health information sources to evidence-based healthcare decision-making Carol Lefebvre Senior Information Specialist
First question to ask yourself Do you really need to do a randomised controlled trial (RCT)?
 So, you think you want to run a Randomised Controlled Trial? Do you know what is meant by: blinding, stratified randomisation, intention-to-treat analysis, surrogate outcomes, generalisability, confounding
So, you think you want to run a Randomised Controlled Trial? Do you know what is meant by: blinding, stratified randomisation, intention-to-treat analysis, surrogate outcomes, generalisability, confounding
Submission of a clinical trial for access to ECRIN services Notice to the Applicant
 Submission of a clinical trial for access to ECRIN services Notice to the Applicant BEFORE SUBMITTING YOUR PROTOCOL Please, contact the European Correspondent (EuCo) in your country. The list of EuCos
Submission of a clinical trial for access to ECRIN services Notice to the Applicant BEFORE SUBMITTING YOUR PROTOCOL Please, contact the European Correspondent (EuCo) in your country. The list of EuCos
Eliminating Bias in Randomized Controlled Trials: Importance of Allocation Concealment and Masking
 132 February 2007 Family Medicine Research Series Eliminating Bias in Randomized Controlled Trials: Importance of Allocation Concealment and Masking Anthony J. Viera, MD, MPH; Shrikant I. Bangdiwala, PhD
132 February 2007 Family Medicine Research Series Eliminating Bias in Randomized Controlled Trials: Importance of Allocation Concealment and Masking Anthony J. Viera, MD, MPH; Shrikant I. Bangdiwala, PhD
Ethical Principles in Clinical Research. Christine Grady Department of Bioethics NIH Clinical Center
 Ethical Principles in Clinical Research Christine Grady Department of Bioethics NIH Clinical Center 1 Ethical principles Are these studies ethical? How do we know? Ethics of clinical research The goal
Ethical Principles in Clinical Research Christine Grady Department of Bioethics NIH Clinical Center 1 Ethical principles Are these studies ethical? How do we know? Ethics of clinical research The goal
Protocol registration and outcome reporting bias in randomised controlled trials of
 Title: Protocol registration and outcome reporting bias in randomised controlled trials of eczema treatment Rationale: As one of the measures to reduce the likelihood of selective reporting bias in RCTs,
Title: Protocol registration and outcome reporting bias in randomised controlled trials of eczema treatment Rationale: As one of the measures to reduce the likelihood of selective reporting bias in RCTs,
Editor s Note: In order to encourage dissemination of the
 Annals of Internal Medicine Academia and Clinic CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials Kenneth F. Schulz, PhD, MBA; Douglas G. Altman, DSc; and David
Annals of Internal Medicine Academia and Clinic CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials Kenneth F. Schulz, PhD, MBA; Douglas G. Altman, DSc; and David
Comment Form: WHO Statement on Public Disclosure of Clinical Trial Results. Suggested Amendment
 Form: WHO Statement on Public Disclosure of Clinical Trial Results Page 1 of 6 as individual or on behalf of agency or institution?: Institution er's Name in Full, Title, Institution, City, Country, Tel.,
Form: WHO Statement on Public Disclosure of Clinical Trial Results Page 1 of 6 as individual or on behalf of agency or institution?: Institution er's Name in Full, Title, Institution, City, Country, Tel.,
Basic Results Database
 Basic Results Database Deborah A. Zarin, M.D. ClinicalTrials.gov December 2008 1 ClinicalTrials.gov Overview and PL 110-85 Requirements Module 1 2 Levels of Transparency Prospective Clinical Trials Registry
Basic Results Database Deborah A. Zarin, M.D. ClinicalTrials.gov December 2008 1 ClinicalTrials.gov Overview and PL 110-85 Requirements Module 1 2 Levels of Transparency Prospective Clinical Trials Registry
CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials
 Journal of Clinical Epidemiology - (2010) - ORIGINAL ARTICLE CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials David Moher a, *, Sally Hopewell
Journal of Clinical Epidemiology - (2010) - ORIGINAL ARTICLE CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials David Moher a, *, Sally Hopewell
Evaluation of the methodology in publications describing epidemiological design for dental research: a critical analysis
 ISSN: Versão impressa: 1806-7727 Versão eletrônica: 1984-5685 RSBO. 2011 Jan-Mar;8(1):75-80 Original Research Article Evaluation of the methodology in publications describing epidemiological design for
ISSN: Versão impressa: 1806-7727 Versão eletrônica: 1984-5685 RSBO. 2011 Jan-Mar;8(1):75-80 Original Research Article Evaluation of the methodology in publications describing epidemiological design for
Critical appraisal skills are essential to informed decision-making
 Resident s Page Critical appraisal skills are essential to informed decision-making Rahul Mhaskar 1,3, Patricia Emmanuel 3,5, Shobha Mishra 4, Sangita Patel 4, Eknath Naik 5, Ambuj Kumar 1,2,3,5 1 Center
Resident s Page Critical appraisal skills are essential to informed decision-making Rahul Mhaskar 1,3, Patricia Emmanuel 3,5, Shobha Mishra 4, Sangita Patel 4, Eknath Naik 5, Ambuj Kumar 1,2,3,5 1 Center
RESEARCH METHODS & REPORTING
 RESEARCH METHODS & REPORTING CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials David Moher, 1 Sally Hopewell, 2 Kenneth F Schulz, 3 Victor Montori,
RESEARCH METHODS & REPORTING CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials David Moher, 1 Sally Hopewell, 2 Kenneth F Schulz, 3 Victor Montori,
WWW.PUBLICATIONETHICS.ORG
 Note: This document combines the original COPE Guidelines from 1999, the Code of Conduct developed in 2003, and the Best Practice Guidelines developed in 2007. This revision was developed after wide consultation
Note: This document combines the original COPE Guidelines from 1999, the Code of Conduct developed in 2003, and the Best Practice Guidelines developed in 2007. This revision was developed after wide consultation
REPORTING ON PARTICIPANT DISPOSITION IN CLINICAL TRIALS. Dennis C. Turk, Ph.D. 1 University of Washington School of Medicine
 1 REPORTING ON PARTICIPANT DISPOSITION IN CLINICAL TRIALS Dennis C. Turk, Ph.D. 1 University of Washington School of Medicine Running head: Patient Disposition 1 Preparation of this manuscript was supported
1 REPORTING ON PARTICIPANT DISPOSITION IN CLINICAL TRIALS Dennis C. Turk, Ph.D. 1 University of Washington School of Medicine Running head: Patient Disposition 1 Preparation of this manuscript was supported
Principles for Protecting Integrity In the Conduct and Reporting Of Clinical Trials
 Principles for Protecting Integrity In the Conduct and Reporting Of Clinical Trials Susan Ehringhaus David Korn DBHSR January 6, 2006 Association of American Medical Colleges Principles for Protecting
Principles for Protecting Integrity In the Conduct and Reporting Of Clinical Trials Susan Ehringhaus David Korn DBHSR January 6, 2006 Association of American Medical Colleges Principles for Protecting
Priority Program Translational Oncology Applicants' Guidelines
 Stiftung Deutsche Krebshilfe Dr. h.c. Fritz Pleitgen Präsident Spendenkonto Kreissparkasse Köln IBAN DE65 3705 0299 0000 9191 91 BIC COKSDE33XXX Priority Program Translational Oncology Applicants' Guidelines
Stiftung Deutsche Krebshilfe Dr. h.c. Fritz Pleitgen Präsident Spendenkonto Kreissparkasse Köln IBAN DE65 3705 0299 0000 9191 91 BIC COKSDE33XXX Priority Program Translational Oncology Applicants' Guidelines
European Medical Writers Association (EMWA) guidelines on the role of medical writers in developing peer-reviewed publications
 CURRENT MEDICAL RESEARCH AND OPINION VOL. 21, NO. 2, 2005, 317 321 2005 LIBRAPHARM LIMITED 0300-7995 doi:10.1185/030079905x25578 All rights reserved: reproduction in whole or part not permitted COMMENTARY
CURRENT MEDICAL RESEARCH AND OPINION VOL. 21, NO. 2, 2005, 317 321 2005 LIBRAPHARM LIMITED 0300-7995 doi:10.1185/030079905x25578 All rights reserved: reproduction in whole or part not permitted COMMENTARY
Paris, 15 June 2013 Response to a public consultation
 Paris, 15 June 2013 Response to a public consultation Revision of the World Medical Association Helsinki Declaration: - transparency of clinical trial results must be enhanced (articles 23, 24 & 26) -
Paris, 15 June 2013 Response to a public consultation Revision of the World Medical Association Helsinki Declaration: - transparency of clinical trial results must be enhanced (articles 23, 24 & 26) -
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
PEER REVIEW HISTORY ARTICLE DETAILS TITLE (PROVISIONAL)
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
Conduct of clinical Trials Communication of
 PrinciPles on Conduct of clinical Trials Communication of clinical Trial results Table of Contents Preamble...1 Commitment to Protecting Research Participants...5 Conduct of Clinical Trials...7 Ensuring
PrinciPles on Conduct of clinical Trials Communication of clinical Trial results Table of Contents Preamble...1 Commitment to Protecting Research Participants...5 Conduct of Clinical Trials...7 Ensuring
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May 2011
 TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance Session 1: PSI Conference, May 2011 Kerry Gordon, Quintiles 1 E9, and how to locate it 2 ICH E9 Statistical Principles for Clinical Trials (Issued
Adoption by CHMP for release for consultation November 2010. End of consultation (deadline for comments) 31 March 2011
 1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
1 2 3 November 2010 EMA/759784/2010 Committee for Medicinal Products for Human Use 4 5 6 7 Reflection paper on the need for active control in therapeutic areas where use of placebo is deemed ethical and
Clinical trials regulation
 Clinical trials regulation The Proposal for a Regulation of the European Parliament and of the Council on Clinical Trials on Medicinal Products for Human Use and Repealing Directive 2001/20/EC an update
Clinical trials regulation The Proposal for a Regulation of the European Parliament and of the Council on Clinical Trials on Medicinal Products for Human Use and Repealing Directive 2001/20/EC an update
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Guidance for Peer Reviewers. The Journal of the American Osteopathic Association (JAOA)
 Guidance for Peer Reviewers The Journal of the American Osteopathic Association (JAOA) JAOA Editorial Staff This module is available online at http://jaoa.org/documentlibrary/prmodule.pdf Guidance for
Guidance for Peer Reviewers The Journal of the American Osteopathic Association (JAOA) JAOA Editorial Staff This module is available online at http://jaoa.org/documentlibrary/prmodule.pdf Guidance for
What are confidence intervals and p-values?
 What is...? series Second edition Statistics Supported by sanofi-aventis What are confidence intervals and p-values? Huw TO Davies PhD Professor of Health Care Policy and Management, University of St Andrews
What is...? series Second edition Statistics Supported by sanofi-aventis What are confidence intervals and p-values? Huw TO Davies PhD Professor of Health Care Policy and Management, University of St Andrews
PCORI Methodology Standards: Academic Curriculum. 2016 Patient-Centered Outcomes Research Institute. All Rights Reserved.
 PCORI Methodology Standards: Academic Curriculum 2016 Patient-Centered Outcomes Research Institute. All Rights Reserved. Module 5: Step 3 Search the Literature Category 11: Systematic Reviews Prepared
PCORI Methodology Standards: Academic Curriculum 2016 Patient-Centered Outcomes Research Institute. All Rights Reserved. Module 5: Step 3 Search the Literature Category 11: Systematic Reviews Prepared
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It
 nature publishing group The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It RT O Neill 1 and R Temple 2 At the request of the Food and
nature publishing group The Prevention and Treatment of Missing Data in Clinical Trials: An FDA Perspective on the Importance of Dealing With It RT O Neill 1 and R Temple 2 At the request of the Food and
Intent-to-treat Analysis of Randomized Clinical Trials
 Intent-to-treat Analysis of Randomized Clinical Trials Michael P. LaValley Boston University http://people.bu.edu/mlava/ ACR/ARHP Annual Scientific Meeting Orlando 10/27/2003 Outline Origin of Randomization
Intent-to-treat Analysis of Randomized Clinical Trials Michael P. LaValley Boston University http://people.bu.edu/mlava/ ACR/ARHP Annual Scientific Meeting Orlando 10/27/2003 Outline Origin of Randomization
Pharmaceutical Companies and Medical Publishers Working together in a world of increasing regulation
 Pharmaceutical Companies and Medical Publishers Working together in a world of increasing regulation Sarah L Feeny Head of Scientific Direction, Complete Medical Communications Board of Trustees, International
Pharmaceutical Companies and Medical Publishers Working together in a world of increasing regulation Sarah L Feeny Head of Scientific Direction, Complete Medical Communications Board of Trustees, International
Movember Clinical Trial Award (CTA)
 Movember Clinical Trial Awards Part 1: Overview Participating Organisation (s) Funding Category Description The Movember Foundation and Prostate Cancer Foundation of Australia Movember Clinical Trial Award
Movember Clinical Trial Awards Part 1: Overview Participating Organisation (s) Funding Category Description The Movember Foundation and Prostate Cancer Foundation of Australia Movember Clinical Trial Award
Missing trial data briefing notes.
 Missing trial data briefing notes. Doctors need the results of clinical trials to make decisions about which treatment is best. Currently, drug companies and researchers are allowed to withhold the results
Missing trial data briefing notes. Doctors need the results of clinical trials to make decisions about which treatment is best. Currently, drug companies and researchers are allowed to withhold the results
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009
 Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 1 Updated November 10, 2009 The innovative pharmaceutical industry 2 is committed to the transparency
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance
 Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Clinical Trials Reporting Program (CTRP) and Clinicaltrials.gov (CT.gov) Helpful tips and guidance Objectives NCI s Clinical Trials Reporting Program (CTRP) Overview CTRP What is it? CTRP is an NCI mandated
Publication Bias in medical research: issues and communities
 Publication Bias in medical research: issues and communities Edgar Schiebel and Maria Elisabeth Züger edgar.schiebel@ait.ac.at, maria-elisabeth.zueger@ait.ac.at AIT Austrian Institute of Technology GmbH,
Publication Bias in medical research: issues and communities Edgar Schiebel and Maria Elisabeth Züger edgar.schiebel@ait.ac.at, maria-elisabeth.zueger@ait.ac.at AIT Austrian Institute of Technology GmbH,
Submission of comments on 'Policy 0070 on publication and access to clinical-trial data'
 EMA/240810/2013 Submission of comments on 'Policy 0070 on publication and access to clinical-trial data' s from: Name and affiliation For the Austrian Medicines & Medical Devices Agency (AGES), Austria,
EMA/240810/2013 Submission of comments on 'Policy 0070 on publication and access to clinical-trial data' s from: Name and affiliation For the Austrian Medicines & Medical Devices Agency (AGES), Austria,
Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data)
 Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data) 3rd EUDAT Conference 25 September 2014 Amsterdam, Netherlands Prof. Dr. C. Ohmann ECRIN-ERIC Major
Specifics of the clinical trials data life cycle (momentum for providing access to patient-level data) 3rd EUDAT Conference 25 September 2014 Amsterdam, Netherlands Prof. Dr. C. Ohmann ECRIN-ERIC Major
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects
 WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by
WORLD MEDICAL ASSOCIATION DECLARATION OF HELSINKI Ethical Principles for Medical Research Involving Human Subjects Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by
How to Write a Systematic Review
 CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 455, pp. 23 29 2007 Lippincott Williams & Wilkins How to Write a Systematic Review Rick W. Wright, MD * ; Richard A. Brand, MD ; Warren Dunn, MD, ; and
CLINICAL ORTHOPAEDICS AND RELATED RESEARCH Number 455, pp. 23 29 2007 Lippincott Williams & Wilkins How to Write a Systematic Review Rick W. Wright, MD * ; Richard A. Brand, MD ; Warren Dunn, MD, ; and
PEER REVIEW HISTORY ARTICLE DETAILS
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (http://bmjopen.bmj.com/site/about/resources/checklist.pdf)
Protocol: Consort extension to stepped wedge cluster randomised controlled trial
 Protocol: Consort extension to stepped wedge cluster randomised controlled trial Version 1 Investigators Karla Hemming 1 * Alan Girling 1 Terry Haines 2 Richard Lilford 3 1 School of Health and Population
Protocol: Consort extension to stepped wedge cluster randomised controlled trial Version 1 Investigators Karla Hemming 1 * Alan Girling 1 Terry Haines 2 Richard Lilford 3 1 School of Health and Population
Yale University Open Data Access (YODA) Project Procedures to Guide External Investigator Access to Clinical Trial Data Last Updated August 2015
 OVERVIEW Yale University Open Data Access (YODA) Project These procedures support the YODA Project Data Release Policy and more fully describe the process by which clinical trial data held by a third party,
OVERVIEW Yale University Open Data Access (YODA) Project These procedures support the YODA Project Data Release Policy and more fully describe the process by which clinical trial data held by a third party,
LEADING RESEARCH MEASURES THAT COUNT
 Considerations for Implementing Surveys Evaluating Effectiveness: Sample Recruitment, Ethics, and Privacy Laurie Zografos Senior Director, Surveys and Observational Studies RTI Health Solutions zografos@rti.org
Considerations for Implementing Surveys Evaluating Effectiveness: Sample Recruitment, Ethics, and Privacy Laurie Zografos Senior Director, Surveys and Observational Studies RTI Health Solutions zografos@rti.org
PEER REVIEW HISTORY ARTICLE DETAILS TITLE (PROVISIONAL)
 PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
PEER REVIEW HISTORY BMJ Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form (see an example) and are provided with free text boxes to
Random Allocation Software for Parallel Group Randomized Trials
 Random Allocation Software for Parallel Group Randomized Trials Mahmood Saghaei 1 1 Department of Anaesthesia, Isfahan University of Medical Sciences, Isfahan, Iran Corresponding author Email addresses:
Random Allocation Software for Parallel Group Randomized Trials Mahmood Saghaei 1 1 Department of Anaesthesia, Isfahan University of Medical Sciences, Isfahan, Iran Corresponding author Email addresses:
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON DATA MONITORING COMMITTEES
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 27 July 2005 Doc. Ref. EMEA/CHMP/EWP/5872/03 Corr COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE
Critical appraisal. Gary Collins. EQUATOR Network, Centre for Statistics in Medicine NDORMS, University of Oxford
 Critical appraisal Gary Collins EQUATOR Network, Centre for Statistics in Medicine NDORMS, University of Oxford EQUATOR Network OUCAGS training course 25 October 2014 Objectives of this session To understand
Critical appraisal Gary Collins EQUATOR Network, Centre for Statistics in Medicine NDORMS, University of Oxford EQUATOR Network OUCAGS training course 25 October 2014 Objectives of this session To understand
MISSING DATA: THE POINT OF VIEW OF ETHICAL COMMITTEES
 I CONGRESSO NAZIONALE BIAS 2009 29/30 APRILE 2009 ELI LILLY SESTO FIORENTINO (FI) MISSING DATA: THE POINT OF VIEW OF ETHICAL COMMITTEES Anna Chiara Frigo Department of Environmental Medicine and Public
I CONGRESSO NAZIONALE BIAS 2009 29/30 APRILE 2009 ELI LILLY SESTO FIORENTINO (FI) MISSING DATA: THE POINT OF VIEW OF ETHICAL COMMITTEES Anna Chiara Frigo Department of Environmental Medicine and Public
Advancing research: a physician s guide to clinical trials
 Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
Advancing research: a physician s guide to clinical trials Recruiting and retaining trial participants is one of the greatest obstacles to developing the next generation of Alzheimer s treatments Alzheimer
Professional Standards and Guidelines
 College of Physicians and Surgeons of British Columbia Professional Standards and Guidelines Conflict of Interest Arising from Clinical Research Preamble This document is a guideline of the Board of the
College of Physicians and Surgeons of British Columbia Professional Standards and Guidelines Conflict of Interest Arising from Clinical Research Preamble This document is a guideline of the Board of the
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device
 Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device List of Contents Page 1. Introduction 1.1 The Medical Device Administrative Control System and the 3 proposed legislation
Clinical research: where are we with the new (Paediatric) RC trial Regulation
 where are we with the new (Paediatric) RC trial Regulation, MD, PhD Ethical Committee DEEP Former member of the PDCO EMA With the aid of Fabio D'Atri European commission and Anabela Marcal of EMA The new
where are we with the new (Paediatric) RC trial Regulation, MD, PhD Ethical Committee DEEP Former member of the PDCO EMA With the aid of Fabio D'Atri European commission and Anabela Marcal of EMA The new
Response of the German Medical Association
 Response of the German Medical Association to the European Commission proposal for a regulation of the European Parliament and of the Council on clinical trials on medicinal products for human use, and
Response of the German Medical Association to the European Commission proposal for a regulation of the European Parliament and of the Council on clinical trials on medicinal products for human use, and
Submission of comments on 'Policy 0070 on publication and access to clinical-trial data'
 EMA/240810/2013 Submission of comments on 'Policy 0070 on publication and access to clinical-trial data' s from: Name and affiliation PHARMIG - Association of the Austrian pharmaceutical industry Please
EMA/240810/2013 Submission of comments on 'Policy 0070 on publication and access to clinical-trial data' s from: Name and affiliation PHARMIG - Association of the Austrian pharmaceutical industry Please
The Product Review Life Cycle A Brief Overview
 Stat & Quant Mthds Pharm Reg (Spring 2, 2014) Lecture 2,Week 1 1 The review process developed over a 40 year period and has been influenced by 5 Prescription User Fee Act renewals Time frames for review
Stat & Quant Mthds Pharm Reg (Spring 2, 2014) Lecture 2,Week 1 1 The review process developed over a 40 year period and has been influenced by 5 Prescription User Fee Act renewals Time frames for review
Template for essential information to be provided for proposals including clinical trials / studies / investigations
 Template for essential information to be provided for proposals including clinical trials / studies / investigations Document history Version 2016callsV1 September 2015 Modifications (compared to previous
Template for essential information to be provided for proposals including clinical trials / studies / investigations Document history Version 2016callsV1 September 2015 Modifications (compared to previous
Precision and Negative Predictive Value of Links between ClinicalTrials.gov and PubMed
 Precision and Negative Predictive Value of Links between ClinicalTrials.gov and PubMed Abstract Vojtech Huser, MD, PhD, James J. Cimino, MD Laboratory for Informatics Development, NIH Clinical Center,
Precision and Negative Predictive Value of Links between ClinicalTrials.gov and PubMed Abstract Vojtech Huser, MD, PhD, James J. Cimino, MD Laboratory for Informatics Development, NIH Clinical Center,
GUEST EDITORIAL. Twenty Statistical Errors Even YOU Can Find in Biomedical Research Articles. Tom Lang. Tom Lang Communications, Murphys, Ca, USA
 5():-7, GUEST EDITORIAL Twenty Statistical Errors Even YOU Can Find in Biomedical Research Articles Tom Lang Tom Lang Communications, Murphys, Ca, USA Critical reviewers of the biomedical literature have
5():-7, GUEST EDITORIAL Twenty Statistical Errors Even YOU Can Find in Biomedical Research Articles Tom Lang Tom Lang Communications, Murphys, Ca, USA Critical reviewers of the biomedical literature have
Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations
 Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations Clinical Trials Registry S. 1082 (Title II, Subtitle C) H.R.
Comparison of Clinical Trials Registry Legislation in the Senate and House Prepared by Tannaz Rasouli, AAMC Office of Governmental Relations Clinical Trials Registry S. 1082 (Title II, Subtitle C) H.R.
Analyzing Research Articles: A Guide for Readers and Writers 1. Sam Mathews, Ph.D. Department of Psychology The University of West Florida
 Analyzing Research Articles: A Guide for Readers and Writers 1 Sam Mathews, Ph.D. Department of Psychology The University of West Florida The critical reader of a research report expects the writer to
Analyzing Research Articles: A Guide for Readers and Writers 1 Sam Mathews, Ph.D. Department of Psychology The University of West Florida The critical reader of a research report expects the writer to
(2) The neurological surgeon shall not participate in any activity, which is not in the best interest of the patient.
 AANS Code of Ethics a) General Statement of Purpose The American Association of Neurological Surgeons has established a Code of Ethics for neurological surgeons as guidelines in medical, social, and professional
AANS Code of Ethics a) General Statement of Purpose The American Association of Neurological Surgeons has established a Code of Ethics for neurological surgeons as guidelines in medical, social, and professional
CLINICAL TRIALS WITH MEDICINES IN EUROPE
 CLINICAL TRIALS WITH MEDICINES IN EUROPE REGULATORY FRAMEWORK FOR CLINICAL TRIALS WITH MEDICINES IN EUROPE The pharmaceutical industry is the most highly regulated sector in Europe. The Commission has
CLINICAL TRIALS WITH MEDICINES IN EUROPE REGULATORY FRAMEWORK FOR CLINICAL TRIALS WITH MEDICINES IN EUROPE The pharmaceutical industry is the most highly regulated sector in Europe. The Commission has
GUIDELINES ON MEDICAL DEVICES EVALUATION OF CLINICAL DATA : A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology MEDDEV. 2.7.1 April 2003 GUIDELINES
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single Market : regulatory environment, standardisation and New Approach Pressure equipment, medical devices, metrology MEDDEV. 2.7.1 April 2003 GUIDELINES
Authors Checklist for Manuscript Submission to JPP
 Authors Checklist for Manuscript Submission to JPP Prospective authors should use the following checklist guide in order to maximize the chance for their manuscript to be published and to ease our reviewers
Authors Checklist for Manuscript Submission to JPP Prospective authors should use the following checklist guide in order to maximize the chance for their manuscript to be published and to ease our reviewers
Guide to Chronic Disease Management and Prevention
 Family Health Teams Advancing Primary Health Care Guide to Chronic Disease Management and Prevention September 27, 2005 Table of Contents 3 Introduction 3 Purpose 4 What is Chronic Disease Management
Family Health Teams Advancing Primary Health Care Guide to Chronic Disease Management and Prevention September 27, 2005 Table of Contents 3 Introduction 3 Purpose 4 What is Chronic Disease Management
Issues Regarding Use of Placebo in MS Drug Trials. Peter Scott Chin, MD Novartis Pharmaceuticals Corporation
 Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Operational aspects of a clinical trial
 Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
Operational aspects of a clinical trial Carlo Tomino Pharm.D. Coordinator Pre-authorization Department Head of Research and Clinical Trial Italian Medicines Agency Mwanza (Tanzania), June 11, 2012 1 Declaration
The PCORI Methodology Report. Appendix A: Methodology Standards
 The Appendix A: Methodology Standards November 2013 4 INTRODUCTION This page intentionally left blank. APPENDIX A A-1 APPENDIX A: PCORI METHODOLOGY STANDARDS Cross-Cutting Standards for PCOR 1: Standards
The Appendix A: Methodology Standards November 2013 4 INTRODUCTION This page intentionally left blank. APPENDIX A A-1 APPENDIX A: PCORI METHODOLOGY STANDARDS Cross-Cutting Standards for PCOR 1: Standards
ChangingPractice. Appraising Systematic Reviews. Evidence Based Practice Information Sheets for Health Professionals. What Are Systematic Reviews?
 Supplement 1, 2000 ChangingPractice Evidence Based Practice Information Sheets for Health Professionals Appraising Systematic Reviews The series Changing Practice has been designed to support health professionals
Supplement 1, 2000 ChangingPractice Evidence Based Practice Information Sheets for Health Professionals Appraising Systematic Reviews The series Changing Practice has been designed to support health professionals
Participating in Alzheimer s Disease Clinical Trials and Studies
 Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
Sheffield Kidney Institute. Planning a Clinical Trial
 Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Planning a Clinical Trial Clinical Trials Testing a new drug Ethical Issues Liability and Indemnity Trial Design Trial Protocol Statistical analysis Clinical Trials Phase I: Phase II: Phase III: Phase
Improving the Reporting Quality of Nonrandomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement
 Improving the Reporting Quality of Nonrandomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement Developing an evidence base for making public health decisions will require
Improving the Reporting Quality of Nonrandomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement Developing an evidence base for making public health decisions will require
Peer / Scientific review of research and the role of NRES Research Ethics Committees (RECs)
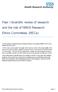 Peer / Scientific review of research and the role of NRES Research Ethics Committees (RECs) The Governance Arrangements for Research Ethics Committees (GAfREC 2011) now defines the RECs role; A REC need
Peer / Scientific review of research and the role of NRES Research Ethics Committees (RECs) The Governance Arrangements for Research Ethics Committees (GAfREC 2011) now defines the RECs role; A REC need
International Ethical Principles for Scholarly Publication
 International Ethical Principles for Scholarly Publication 1. Aims and Scope Publication (usually in a peer reviewed learned journal) is an important component of the overall research process, and provides
International Ethical Principles for Scholarly Publication 1. Aims and Scope Publication (usually in a peer reviewed learned journal) is an important component of the overall research process, and provides
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Prepared by:jane Healey (Email: janie_healey@yahoo.com) 4 th year undergraduate occupational therapy student, University of Western Sydney
 1 There is fair (2b) level evidence that living skills training is effective at improving independence in food preparation, money management, personal possessions, and efficacy, in adults with persistent
1 There is fair (2b) level evidence that living skills training is effective at improving independence in food preparation, money management, personal possessions, and efficacy, in adults with persistent
A Guide to Clinical Trials
 A Guide to Clinical Trials For young people with cancer and their parents Children s Cancer and Leukaemia Group www.cclg.org.uk Original booklet produced in conjunction with the CCLG Patient Advocacy Committee.
A Guide to Clinical Trials For young people with cancer and their parents Children s Cancer and Leukaemia Group www.cclg.org.uk Original booklet produced in conjunction with the CCLG Patient Advocacy Committee.
Clinical trials for medical devices: FDA and the IDE process
 Clinical trials for medical devices: FDA and the IDE process Owen Faris, Ph.D. Deputy Director Division of Cardiovascular Devices Office of Device Evaluation Center for Devices and Radiological Health,
Clinical trials for medical devices: FDA and the IDE process Owen Faris, Ph.D. Deputy Director Division of Cardiovascular Devices Office of Device Evaluation Center for Devices and Radiological Health,
Outline. Publication and other reporting biases; funnel plots and asymmetry tests. The dissemination of evidence...
 Cochrane Methodology Annual Training Assessing Risk Of Bias In Cochrane Systematic Reviews Loughborough UK, March 0 Publication and other reporting biases; funnel plots and asymmetry tests Outline Sources
Cochrane Methodology Annual Training Assessing Risk Of Bias In Cochrane Systematic Reviews Loughborough UK, March 0 Publication and other reporting biases; funnel plots and asymmetry tests Outline Sources
2015 Workshop Report. Key words: systematic reviews; project management; data management; librarians, authorship.
 2015 Workshop Report An overview of the role of librarians in systematic reviews: from expert search to project manager Margaret J. Foster Medical Sciences Library, Texas A&M University, College Station,
2015 Workshop Report An overview of the role of librarians in systematic reviews: from expert search to project manager Margaret J. Foster Medical Sciences Library, Texas A&M University, College Station,
April 2013 THE PROCESS OF INFORMED CONSENT
 April 2013 THE PROCESS OF INFORMED CONSENT Introduction Every investigator and clinical research coordinator (CRC) should recognize the importance of obtaining valid and appropriate informed consent as
April 2013 THE PROCESS OF INFORMED CONSENT Introduction Every investigator and clinical research coordinator (CRC) should recognize the importance of obtaining valid and appropriate informed consent as
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Health Care Job Information Sheet #20. Clinical Research
 Health Care Job Information Sheet #20 Clinical Research A. Background B. Occupations 1) Clinical Research Associate (Study Monitor) 2) Clinical Research Coordinator 3) Other positions in the field C. Labour
Health Care Job Information Sheet #20 Clinical Research A. Background B. Occupations 1) Clinical Research Associate (Study Monitor) 2) Clinical Research Coordinator 3) Other positions in the field C. Labour
History and Principles of Good Clinical Practice
 History and Principles of Good Clinical Practice Cristina E. Torres, Ph.D. Social Science Professor, UPM-NIH FERCAP Coordinator ICH: International Conference on Harmonization GCP: Good Clinical Practices
History and Principles of Good Clinical Practice Cristina E. Torres, Ph.D. Social Science Professor, UPM-NIH FERCAP Coordinator ICH: International Conference on Harmonization GCP: Good Clinical Practices
Competency Statements for Dental Public Health*
 Competency Statements for Dental Public Health* Preamble Competency statements for dental public health, and the performance indicators by which they can be measured, were developed at a workshop in San
Competency Statements for Dental Public Health* Preamble Competency statements for dental public health, and the performance indicators by which they can be measured, were developed at a workshop in San
Early Phase Clinical Trials: Public Access to the EU Database Repository
 European CRO Federation Via Lucrezio Caro, 63 00193 Roma, Italy Tel.: +39 06 807 60 72 Fax: +39 06 807 60 85 Email: info@eucrof.eu Internet: www.eucrof.eu Early Phase Clinical Trials: Public Access to
European CRO Federation Via Lucrezio Caro, 63 00193 Roma, Italy Tel.: +39 06 807 60 72 Fax: +39 06 807 60 85 Email: info@eucrof.eu Internet: www.eucrof.eu Early Phase Clinical Trials: Public Access to
Please find below some views on the issues raised by the EMA Senior Medical Officer's Reflections on the legal basis for EMA/CHMP opinions.
 Jacques Ropers Afssaps Pierre Demolis Afssaps (French CHMP Member) Philippe Lechat Afssaps (French Alternate CHMP Member) Dear Colleagues, Please find below some views on the issues raised by the EMA Senior
Jacques Ropers Afssaps Pierre Demolis Afssaps (French CHMP Member) Philippe Lechat Afssaps (French Alternate CHMP Member) Dear Colleagues, Please find below some views on the issues raised by the EMA Senior
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
CLINICAL RESEARCH PROTOCOL CHECKLIST
 CLINICAL RESEARCH PROTOCOL CHECKLIST [taken from ICH GCP : Guidance for Industry, Good Clinical Practice: Consolidated Guidance, Revision 1 (R1) June 1996] ICH GCP, Section 6. CLINICAL TRIAL PROTOCOL AND
CLINICAL RESEARCH PROTOCOL CHECKLIST [taken from ICH GCP : Guidance for Industry, Good Clinical Practice: Consolidated Guidance, Revision 1 (R1) June 1996] ICH GCP, Section 6. CLINICAL TRIAL PROTOCOL AND
