JOURNAL OF VIROLOGY, Aug. 1999, p Vol. 73, No. 8. Copyright 1999, American Society for Microbiology. All Rights Reserved.
|
|
|
- Brent Owens
- 8 years ago
- Views:
Transcription
1 JOURNAL OF VIROLOGY, Aug. 1999, p Vol. 73, No X/99/$ Copyright 1999, American Society for Microbiology. All Rights Reserved. Association with the Cellular Export Receptor CRM 1 Mediates Function and Intracellular Localization of Epstein-Barr Virus SM Protein, a Regulator of Gene Expression SARAH M. BOYLE, 1 VIVIAN RUVOLO, 1 ASHISH K. GUPTA, 1 AND SANKAR SWAMINATHAN 1,2 * Sealy Center for Oncology and Hematology and Division of Infectious Diseases, Department of Internal Medicine, 1 and Department of Microbiology and Immunology, 2 University of Texas Medical Branch, Galveston, Texas Received 25 February 1999/Accepted 10 May 1999 Splicing and posttranscriptional processing of eukaryotic gene transcripts are linked to their nuclear export and cytoplasmic expression. Unspliced pre-mrnas and intronless transcripts are thus inherently poorly expressed. Nevertheless, human and animal viruses encode essential genes as single open reading frames or in the intervening sequences of other genes. Many retroviruses have evolved mechanisms to facilitate nuclear export of their unspliced mrnas. For example, the human immunodeficiency virus RNA-binding protein Rev associates with the soluble cellular export receptor CRM 1 (exportin 1), which mediates nucleocytoplasmic translocation of Rev-HIV RNA complexes through the nuclear pore. The transforming human herpesvirus Epstein-Barr virus (EBV) expresses a nuclear protein, SM, early in its lytic cycle; SM binds RNA and posttranscriptionally activates expression of certain intronless lytic EBV genes. Here we show that both the trans-activation function and cytoplasmic translocation of SM are dependent on association with CRM 1 in vivo. SM is also shown to be associated in vivo with other components of the CRM 1 export pathway, including the small GTPase Ran and the nucleoporin CAN/Nup214. SM is shown to be present in the cytoplasm, nucleoplasm, and nuclear envelope of transfected cells. Mutation of a leucine-rich region (LRR) of SM inhibited CRM 1-mediated cytoplasmic translocation and SM activity, as did leptomycin B, an inhibitor of CRM 1 complex formation. Surprisingly, however, leptomycin B treatment and mutation of the LRR both led to SM becoming more tightly attached to intranuclear structures. These findings suggest a model in which SM is not merely a soluble carrier protein for RNA but rather is bound directly to intranuclear proteins, possibly including the nuclear pore complex. The Epstein-Barr virus (EBV) protein SM posttranscriptionally activates intronless genes and inhibits expression of intron-containing genes (23, 24, 27, 39). In contrast to the majority of cellular genes, many EBV genes expressed during lytic replication are intronless (2, 26), and SM may therefore be important in enhancing expression of other lytic EBV genes. Activation of intronless genes by SM appears to be exerted both at the level of pre-mrna stability and nucleocytoplasmic mrna export (39). SM binds RNA in vitro and is capable of shuttling from nucleus to cytoplasm in a heterokaryon assay (39, 41). It is therefore probable that SM, like the human immunodeficiency virus (HIV) Rev protein, is an RNA transport protein. It has been shown that several proteins involved in nucleocytoplasmic transport of RNA or protein bind to exportins, such as the recently characterized cellular export receptor CRM 1 (exportin 1) (for a review, see reference 43). Various proteins, including HIV Rev and cyclic AMP-dependent protein kinase inhibitor (PKI), contain leucine-rich regions (LRR) which serve as nuclear export signals (NESs) that are required for nuclear export (12, 45). The mechanism of NES-dependent export has been elucidated by the finding that CRM 1 forms a physical complex with NES-containing peptides and conjugates (14, 15, 33). Complex formation may require the presence of a small GTPase, Ran, associated with GTP (Ran-GTP), resulting in a tripartite complex of CRM 1 with NES-containing * Corresponding author. Mailing address: Sealy Center for Oncology and Hematology, MRB 9.104, University of Texas Medical Branch, Galveston, TX Phone: (409) Fax: (409) sswamina@utmb.edu. proteins and Ran-GTP (14, 38). Current models for NESdependent nuclear export postulate a gradient of Ran-GTP across the nuclear envelope, with more Ran-GDP present in the cytoplasm. Thus, the CRM 1 Ran-GTP NES complex is thought to be formed in the nucleus and translocated to the cytoplasm where Ran-GTP is converted to Ran-GDP, accompanied by release of CRM 1 and the NES protein. CRM 1 also associates with the nuclear pore complex (NPC) via CAN/ Nup214 and other nucleoporins (13). Thus, CRM 1 is postulated to direct movement of its cargo NES protein to the NPC and dock with components of the NPC. The exact details of these interactions and the mechanism of directional translocation of the CRM 1-NES protein complex through the NPC remain to be delineated. SM facilitates the expression of intronless mrnas, as opposed to the unspliced retroviral RNAs transported by Revlike proteins. Nevertheless, it was considered possible that SM-mediated RNA export occurs via a CRM 1-dependent pathway. We therefore performed immunoprecipitation and immunofluorescence experiments to determine whether SM interacts physically with CRM 1 in vivo. The specificity of CRM 1-mediated effects was confirmed with a specific inhibitor of CRM 1 complex formation, leptomycin B (LMB). The role of CRM 1 in SM function was investigated in reporter assays that measure trans activation of gene expression by SM. These studies revealed an in vivo association of SM with CRM 1 that mediates both nuclear export and functional activity of SM. The subcellular distribution of SM was also analyzed, by cellular fractionation studies. These studies revealed an effect of CRM 1 not only on nuclear export of SM but also on the degree to which SM is bound to structural elements of the 6872
2 VOL. 73, 1999 ASSOCIATION OF EBV SM PROTEIN WITH CRM nucleus. Finally, the functional role of a putative leucine-rich SM NES was analyzed by site-directed mutagenesis. These experiments demonstrate that the LRR is important in mediating interaction with CRM 1 and suggest a novel mechanism for SM function. MATERIALS AND METHODS Immunofluorescence assays. Cells were grown on glass coverslips prior to washing in phosphate-buffered saline (PBS) and fixation with ice-cold acetone. Fixed cells were incubated in polyclonal rabbit anti-sm antisera at a 1:500 dilution for 1 h at room temperature, washed three times in PBS, and incubated for 1 h with rhodamine-conjugated affinity-purified F(ab ) 2 goat anti-rabbit antibodies (Rockland, Gilbertsville, Pa.) at a 1:1,000 dilution. Cells were washed and overlaid with glycerol, and immunofluorescent microscopy was performed with a Nikon Optiphot 2 microscope. Deconvoluted fluoromicrographs were acquired with a DeltaVision deconvolution fluorescent microscope system (Applied Precision, Issaquah, Wash.). Individual optical sections of 200-nm thickness were obtained from slides prepared as described above. Nuclei were counterstained with 0.5 g of DAPI (4,6-diamidino-2-phenylindole) per ml, and slides were overlaid with Faramount aqueous mounting medium (DAKO Corporation, Carpinteria, Calif.) prior to microscopy. Cell lines, plasmids, and antibodies. SM, antisense control, and CMV-CAT plasmids have been previously described (39). SM mutants were generated by oligonucleotide-directed site-specific mutagenesis (8). CRM 1 cdna, the influenza virus hemagglutinin (HA)-tagged carboxy-terminal amino acid fragment of CAN/Nup214 (amino acids 1864 to 2090), and polyclonal rabbit anti-crm 1 (13) were kind gifts of G. Grosveld (St. Jude Children s Research Hospital, Memphis, Tenn.). CRM 1 cdna and the HA-CAN/Nup214 fragment were cloned in the pcdna3 expression vector (Invitrogen Corp.). Polyclonal anti-sm antibodies were generated by injecting rabbits with gel-purified SM glutathione S-transferase fusion proteins (39). Polyclonal anti-ran antibodies were purchased from Covance Laboratories (Richmond, Calif.). BJAB, an EBV-negative B lymphoma cell line, and Cos 7 cells have been previously described (16, 31). Anti-FLAG monoclonal antibody was purchased from Sigma (St. Louis, Mo.). Transfections and reporter assays. BJAB cells were electroporated with expression constructs, and chloramphenicol acetyltransferase (CAT) assays were performed exactly as previously described (39). Each data point represents pooled results from at least three independent transfections. LMB (kind gift of B. Wolff; Novartis AG) treatment was begun immediately after transfection at a concentration of 10 nm and continued until harvest 16 h later. Cos 7 cells were transfected with LipofectaminePlus, per the manufacturer s protocol (Gibco Life Sciences). Immunoprecipitation and immunoblotting. Cells were lysed 48 h after transfection in immunoprecipitation buffer (Tris-buffered saline [ph 7.4], 1% Triton X-100, 1 mm dithiothreitol, 100 M GTP- S, and a mixture of protease inhibitors [Sigma protease inhibitor cocktail no. P2714]). One hundred fifty microliters of each lysate was cleared with preimmune serum, incubated with 1 l of undiluted polyclonal antibody and 20 l of protein A-conjugated agarose beads (Sigma) for 90 min at 4 C, and washed four times in immunoprecipitation buffer. Precipitation with anti-ha monoclonal antibodies was performed with 150 l of 12CA5 hybridoma supernatant (10). Precipitates were boiled in protein loading buffer, electrophoresed, and immunoblotted as previously described (44). Immunoblotting was performed with a 1:400 dilution of polyclonal anti-sm antibody and a 1:7,500 dilution of horseradish peroxidase-linked donkey anti-rabbit immunoglobulin or a 1:4,000 dilution of horseradish peroxidase-linked goat anti-mouse immunoglobulin (Amersham), and immunoreactive proteins were detected by enhanced chemiluminescence. Cell fractionation and nuclear extractions. Cos 7 cells were harvested 48 h after transfection by scraping into ice-cold PBS, washed in PBS, and lysed in 0.5% Nonidet P-40, 50 mm Tris 5 mm MgSO 4. Under these conditions the nuclei remain intact, as confirmed by light microscopy. Nuclei were separated by centrifugation at 950 g for 10 min. Nuclei were resuspended in a solution of 250 mm sucrose, 50 mm Tris (ph 7.4), and 5 mm MgSO 4 and treated with DNase (250 g/ml) and RNase A (1 mg/ml) for 2hat4 C, followed by washing and resuspension in 50 mm Tris (ph 7.4) 5 mm MgSO 4. High-salt extractions were performed by dropwise addition of 2 M NaCl 50 mm Tris (ph 7.4) with constant mixing to a final concentration of 1.6 M NaCl and incubation on ice for 30 min. High-salt extractions with -mercaptoethanol were performed identically, with the inclusion of -mercaptoethanol at 1% (vol/vol). The remaining nuclear envelopes were sedimented by centrifugation at 13,000 g for 30 min. The salt-extracted fraction was desalted and concentrated with a Microcon 10 filter apparatus (Amicon, Beverly, Mass.). Protease inhibitors, as described above, were included at all steps of the isolation process. Equal fractions from each step of the fractionation procedure were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted as described above. In experiments with LMB, cells were treated with LMB for 16 h after transfection. Treated and untreated cells were harvested at 16 h posttransfection and fractionated exactly as described above. RESULTS SM activity is dependent on CRM 1 (exportin 1) function. SM-mediated gene activation is correlated with enhanced cytoplasmic accumulation of intronless target gene mrnas (7, 39). SM contains an LRR resembling an NES found in certain proteins which shuttle from nucleus to cytoplasm (45). Such proteins, notably the HIV Rev protein, bind CRM 1 (exportin 1) and the small GTPase Ran in the nucleus (14). Translocation to the cytoplasm is thought to be followed by hydrolysis of Ran-associated GTP and complex dissociation. Thus, it was considered possible that SM chaperones intronless EBV mrnas to the cytoplasm via a CRM 1-dependent pathway. We therefore wished to determine whether SM-mediated gene activation is dependent on an interaction with CRM 1. We first examined the effect of a specific inhibitor of CRM 1 complex formation, LMB (47), on SM function. BJAB cells were transfected with an intronless CAT reporter plasmid (CMV-CAT) and an expression vector encoding either SM or antisense SM as a control. Immediately after transfection, cells were incubated in growth medium containing LMB or in control medium without LMB. Cells were harvested after 16 h, at which time viability was not affected by LMB treatment (data not shown) and CAT activity was measured. As previously reported, SM led to activation of CAT expression (approximately 16-fold). LMB itself did not affect baseline expression of CAT activity from the reporter plasmid. However, LMB treatment led to a marked reduction in activation by SM (to four times that of the control) (Fig. 1A). These experiments indicated that an interaction with CRM 1 was involved in some aspects of gene activation by SM. We therefore attempted to determine whether overexpression of CRM 1 could augment SM-mediated gene activation. A CRM 1 expression plasmid or control vector was cotransfected with CMV-CAT and either SM or antisense SM into BJAB cells, and CAT activity was measured 48 h after transfection. As shown in Fig. 1B, CRM 1 overexpression stimulated SM activation. Activation by SM alone was 17-fold and increased to 40-fold over the control when CRM 1 was cotransfected. CRM 1 overexpression did not increase CAT activity in the absence of SM, demonstrating that CRM 1 does not have a nonspecific stimulatory effect on CAT gene expression. CRM 1 expression affects intracellular localization of SM. The preceding experiments suggested that CRM 1 may be involved in nucleocytoplasmic translocation of SM. While it has been shown that SM can shuttle from nucleus to cytoplasm in a heterokaryon assay (41), immunofluorescence studies have demonstrated exclusively nuclear localization of SM (6, 48). SM-transfected cells typically display a speckled nuclear fluorescence with nucleolar sparing when stained with anti-sm antibodies (Fig. 2). Such apparently exclusive nuclear localization of other known shuttling proteins has been reported, presumably because the concentration of cytoplasmic proteins is below the limits of detection of conventional indirect immunofluorescence microscopy (34). We reasoned that if cytoplasmic transport of SM is CRM 1 dependent, overexpression of CRM 1 might lead to visible cytoplasmic accumulation of SM. We therefore transfected Cos 7 cells, in which nuclei and cytoplasm are easily differentiated, with SM and either CRM 1 expression vector or control plasmid and examined the transfected cells by indirect immunofluorescence microscopy. As shown in Fig. 2, overexpression of CRM 1 led to dramatic intracellular relocalization of SM. The nuclei of CRM 1 cotransfected cells were relatively depleted of SM, and the cytoplasm became diffusely stained by the anti-sm antibodies. To confirm the role of CRM 1 in nucleocytoplasmic export
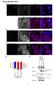
3 6874 BOYLE ET AL. J. VIROL. FIG. 1. Effects of modulating CRM 1 activity on SM function. (A) Effect of LMB on trans-activation by SM. CAT activity was measured in lysates of BJAB cells transfected with SM or antisense control plasmid and CAT reporter plasmid CMV-CAT. Cells were treated with either LMB (10 nm) or control medium immediately after transfection. Results are means of at least three independent transfections. (B) Effect of CRM 1 overexpression on trans-activation by SM. CAT activity was measured in lysates of BJAB cells transfected with SM or antisense control plasmid, CAT reporter plasmid CMV-CAT, and either control or CRM 1 expression vector. of SM, we studied the effect of LMB on cytoplasmic translocation of SM. Cos 7 cells were transfected with SM and CRM 1 expression vectors, as described above, and incubated in growth medium in the presence or absence of LMB. Sixteen hours after transfection, the cells were fixed and stained with anti-sm antibodies and examined by indirect immunofluorescence microscopy. LMB treatment resulted in exclusively nuclear localization of SM despite overexpression of CRM 1 (Fig. 2). These data thus confirm that cytoplasmic translocation of SM is directly or indirectly dependent on CRM 1 complex formation. Mutation of a putative NES impairs SM function. The predicted amino acid sequence of SM contains an LRR which satisfies the consensus requirements (LX 2 3 LX 2 3 LXL) for an NES, as found in other proteins known to interact with CRM 1 (4) (Fig. 3A). It should be noted that although there is not strong selection for a particular amino acid at positions denoted by X, some amino acids at these positions lead to nonfunctional NESs (4). Thus, not all regions meeting these broad criteria are functional NESs. In order to determine if the putative SM NES (amino acids 227 to 236) is required for SM function, we examined the effect of mutating this region on SM-mediated gene activation. Site-directed mutagenesis was used to generate SM mutants altered in the relevant region. The mutant LRR-2 has leucines 234 and 236 replaced by alanine and arginine, respectively, whereas all 11 amino acids are deleted in the LRR- mutant. We then compared these mutants with wild-type SM in the ability to activate gene expression in the CAT reporter assay (Fig. 3B). Both mutants were impaired in activation function, with LRR- being the least active. We have previously demonstrated that in addition to its putative RNA transport function, SM stabilizes and leads to increased accumulation of target gene RNAs in the nucleus as well as the cytoplasm (39). Therefore, the residual activating function of the SM mutants was not unexpected despite a potential defect in the ability to interact with CRM 1 and hence in the ability to translocate to the cytoplasm. Deletion of the LRR abolishes CRM 1-mediated nuclear export of SM. We next wished to determine whether the putative SM NES was important for CRM 1-mediated cytoplasmic localization of SM. Cos cells were transfected with wildtype or LRR mutant plasmids and either CRM 1 plasmid or control vector and examined by immunofluorescence microscopy. In the absence of CRM 1 overexpression, both LRR mutants were detectable only in the nucleus, as expected (Fig. 4). However, both mutants exhibited a more punctate nuclear distribution than wild-type SM. This difference was most obvious with the LRR- mutant, where the fluorescence was most prominent in large nuclear dots. It should be noted that a similar distribution of wild-type SM, albeit not as marked, was observed when SM-transfected cells were treated with LMB (Fig. 2), suggesting that the more diffuse nuclear distri- FIG. 2. Effects of CRM 1 overexpression and LMB treatment on cellular distribution of SM. Cos 7 cells were grown on coverslips prior to transfection, and immunofluorescence microscopy was performed with anti-sm antibodies. Cells were transfected with SM plasmid alone or with SM and CRM 1 expression plasmids. Cells were also transfected with SM and CRM 1 expression plasmid and treated with LMB immediately after transfection.
4 VOL. 73, 1999 ASSOCIATION OF EBV SM PROTEIN WITH CRM FIG. 3. Effect of mutation of a leucine-rich putative SM NES on SM-mediated activation. (A) Amino acids 227 to 236 of SM, with four leucines separated by three, two, and one amino acid, fits the broad consensus sequence described for an NES (3). LRR-2 and LRR- are SM mutants with two leucines altered or the entire LRR deleted and replaced with an arginine, respectively. Amino acid substitutions are shown in bold. X represents no selection for a particular amino acid at that site. Preferred amino acids at particular sites are shown by their one-letter codes. (B) BJAB cells were transfected with a CAT reporter plasmid and either SM or a mutant SM plasmid, and CAT activity was measured as described in the text. bution normally seen with wild-type SM is dependent on interaction with CRM 1. The effect of CRM 1 overexpression on cytoplasmic translocation of SM in the case of LRR- was also markedly different from wild-type SM or LRR-2. When CRM 1 was overexpressed by cotransfection, the LRR-2 mutant retained the ability to translocate to the cytoplasm (Fig. 4). However, the LRR- mutant displayed a different immunofluorescence pattern, could not be detected in the cytoplasm despite overexpression of CRM 1, and remained confined to large nuclear foci. These data indicate that LRR-2 retains, at least partially, the ability to be exported and interact with CRM 1, whereas deletion of the LRR results in a more severe export defect. These findings thus correlate well with the data on LRR mutant function which revealed that LRR-2 retained more activating capability than LRR-. In order to confirm these findings and further examine the unusual distribution of the LRR- mutant in the nucleus, SMand LRR- -transfected cells were examined by immunofluorescence deconvolution microscopy (1). As shown in Fig. 4B, cotransfection of CRM 1 had the expected effect on wild-type SM, causing cytoplasmic translocation, whereas LRR- remained confined to the nucleus. The LRR- mutant was localized to numerous large circular foci (from 10 to 20 per cell) which appeared to be distributed throughout the nucleus and were not confined to the nuclear rim. These foci were less intensely stained in the center and were approximately 1 min diameter. The size and distribution of these foci were not affected by overexpression of CRM 1. Whether these foci correspond to areas of pre-mrna processing or other intranuclear functions remains to be determined. SM associates with CRM 1 in vivo. In order to determine whether SM could be found complexed to CRM 1 in vivo, we attempted to immunoprecipitate SM from cell lysates with anti-crm 1 antibodies. Since CRM 1-NES complexes exist as ternary complexes with the GTP-bound form of Ran (14, 38), immunoprecipitations were performed in the presence of the nonhydrolyzable GTP derivative GTP- S to maximize the likelihood of detecting SM-CRM 1 complex formation. Proteins immunoprecipitated from SM-transfected Cos 7 cells with anti-crm 1 antibodies were separated by SDS-PAGE and immunoblotted with anti-sm antibodies. Anti-CRM 1 antibodies precipitated a protein of the appropriate molecular weight (detected by anti-sm antibodies) from SM-transfected cells but not from control-transfected cells (Fig. 5A). Comparison with unprecipitated lysate indicates that approximately 30% of the SM in transfected cells is precipitable by anti-crm 1 antibodies under these conditions. However, anti-sm antibodies did not precipitate CRM 1 from SM-transfected cells (data not shown), suggesting that SM antibodies may block complex formation or, less likely, that the CRM 1-bound form of SM is not reactive with our antibody. Similar results were obtained with SM-transfected BJAB cells (Fig. 5B). Since it is known that proteins that are exported by CRM 1 may cooperatively associate with both CRM 1 and the small GTPase Ran (14), we asked whether we could also demonstrate an in vivo association of SM with Ran. Such an association would confirm the functional importance of the CRM 1-SM interaction and suggest that directional export of SM is dependent on the nuclear to cytoplasmic gradient of Ran- GTP. SM-transfected or control-transfected cells were therefore lysed and immunoprecipitated with anti-ran antibodies. As shown in Fig. 5C, anti-ran antibodies also immunoprecipitated SM, suggesting that SM forms a tripartite complex with CRM 1 and Ran GTPase in vivo. A third line of evidence that supports the existence of an SM-CRM 1 interaction was provided by experiments in which the association of SM with a nucleoporin known to interact with CRM 1 was investigated. CRM 1 binds the nucleoporin CAN/Nup214 via a series of FXFG repeats in the carboxyterminal portion of CAN (13). Expression of a carboxy-terminal fragment of CAN (amino acids 1864 to 2090 [ CAN]) containing these repeats has been shown to competitively inhibit CRM 1 function (3, 49). We therefore asked whether a potential indirect association of SM with the carboxy-terminal portion of CAN could be detected by coimmunoprecipitation. Cells were transfected with SM and HA-tagged CAN, lysed, and immunoprecipitated with anti-ha monoclonal antibodies. Immunoprecipitates were analyzed for the presence of SM by SDS-PAGE and immunoblotting. As shown in Fig. 5D, anti-ha antibodies precipitated SM from cells cotransfected with HA- CAN. (Multiple forms of SM, as previously described [7], were visualized in this experiment, as electrophoresis was performed at a higher polyacrylamide concentration). These experiments indicate that SM interacts with at least one component of the NPC, CAN/Nup214, possibly indirectly via CRM 1. Mutation of the LRR alters intranuclear compartmentalization of SM. Based on the above findings, we expected that the
5 6876 BOYLE ET AL. J. VIROL. LRR mutants, particularly LRR-, might not bind to CRM 1 and therefore would not be precipitable with anti-crm 1 antibodies. Parallel immunoprecipitation experiments were therefore performed with lysates from cells transfected with wild-type SM, LRR-2, and LRR-. As expected, little or no mutant protein was precipitated with anti-crm 1 antibodies (Fig. 6A, lanes 2 and 3). Surprisingly, however, the total amount of mutant SM protein in unprecipitated cell lysates was also less than that of wild-type SM (Fig. 6, lanes 4, 5, and 6). This was in spite of there being no obvious difference in the amounts of mutant and wild-type SM protein when assessed by immunofluorescence (Fig. 4) and our previous finding of equal amounts of mutant and wild-type SM in immunoblots of whole-cell lysates of similarly transfected cells (data not shown). We therefore further analyzed the amounts and intracellular distribution of mutant and wild-type SM proteins in transfected cells. Cells transfected with mutant or wild-type SM plasmids were lysed in immunoprecipitation buffer containing 1% Triton X-100, and both the lysate and the remaining nuclear pellet were analyzed by immunoblotting. As shown in Fig. 6B, whereas approximately 50% of wild-type SM was found in the soluble lysate, which is expected to contain soluble cytoplasmic and nucleoplasmic SM, only 10 and 5% of LRR-2 and LRR-, respectively, were present in this fraction. Conversely, correspondingly greater amounts of the mutant SM proteins were found in the nuclear pellet fraction. This effect of LRR mutation on intracellular distribution of SM, resulting in a tighter association with nuclear structures, was thus consistent with the immunofluorescence studies described previously which showed that LRR- did not translocate to the cytoplasm. In addition, it was apparent that a large proportion of even wild-type SM remained associated with the nucleus despite detergent treatment. In order to further analyze the nature of the association of SM with nuclear structures, transfected cells were subjected to a further series of fractionation steps. Cos 7 cells transfected with wild-type SM or LRR- plasmid were washed and rapidly lysed in hypotonic buffer containing 0.5% Nonidet P-40. At this detergent concentration, the nuclei remain physically intact and adherent cytoplasm is minimal, as confirmed by light microscopy (data not shown). The lysates were reserved and the nuclei were nuclease treated and extracted with high-salt buffer with or without -mercaptoethanol. Extraction with -mercaptoethanol reduces and releases proteins which are oxidatively bound to the nuclear matrix, in addition to the soluble matrix-associated fraction extracted with high salt alone (11, 22). Each fraction and the remaining nuclear envelopes were then subjected to SDS-PAGE and immunoblotted with anti-sm antibodies (Fig. 7A). Approximately 20% of wild-type SM was found in the detergent-soluble fraction, compared to 5% of LRR-. Approximately 10% of the remaining nuclear wildtype SM was extracted with high salt, and an additional 30% was extracted with the inclusion of -mercaptoethanol, indicating that the latter fraction was also associated with the nuclear matrix. The remaining 60% was associated with the nuclear envelope fraction, which includes the nuclear pore complexes (35). In contrast, the majority of LRR- remained tightly associated with the nuclear envelope fraction and was resistant to the high-salt extraction steps (Fig. 7A). These data indicate that under normal conditions, wild-type SM is found in both a soluble and a tightly nucleus-bound fraction. Further, while SM is also present in an extractable matrix-bound form, a substantial proportion of SM remains tightly associated with the nuclear envelope. In contrast, the majority of LRR- protein is found in the nuclear fractions and particularly in the nuclear envelope fraction. FIG. 4. Effects of CRM 1 overexpression on cellular distribution of SM and SM LRR mutants. (A) Immunofluorescence microscopy was performed on SMor SM mutant-transfected cells with anti-sm antibodies as for Fig. 2. Cos 7 cells were transfected with either wild-type SM (wt SM), SM mutant plasmid LRR-2 or LRR-, or vector plasmid (C), as indicated. Cells were also cotransfected with either control vector ( CRM 1) or CRM 1 expression plasmid ( CRM 1). (B) Cos 7 cells transfected with wt SM or LRR- plasmid and cotransfected with either CRM 1 or control plasmid were stained with anti-sm antibodies. Nuclei were visualized by staining with DAPI. Immunofluorescence images were acquired with a deconvolution fluorescence microscope system. SM staining appears red, and nuclei are purple. Bar, 15 m. These results were somewhat surprising in the context of the conventional model for CRM 1-mediated export of NES proteins, in which NES proteins are diffusible nucleoplasmic proteins which are bound and transported to the NPC by CRM 1. Further, if CRM 1 mediates docking and interaction with nucleoporins, one might have expected that an inability to interact with CRM 1 would result in less rather than more association with the nuclear envelope. To further test the hypothesis that interaction with CRM 1 modulates the intranuclear attachment of SM, we examined the effect of LMB on the intranuclear compartmentalization of SM. If the increased affinity of LRR- for the nuclear envelope and matrix were
6 VOL. 73, 1999 ASSOCIATION OF EBV SM PROTEIN WITH CRM FIG. 4 Continued. indeed due to decreased CRM 1 binding, LMB treatment should result in similar changes in distribution of wild-type SM. SM-transfected cells were treated with LMB and harvested 16 h posttransfection to minimize toxicity. The cells were fractionated, and the distribution of SM in each fraction was compared to those from non-lmb-treated cells by immunoblotting. SM was present in both the soluble and nuclear fractions of untreated cells, as expected, and could be extracted from the nuclei with high salt plus -mercaptoethanol (Fig. 7B). The proportion of SM that was extractable with high salt and -mercaptoethanol in non-lmb-treated cells was greater than in the previous experiments and may be a reflection of the shorter time interval between transfection and harvest. Nevertheless, as can be seen by the relative amounts in each fraction, LMB treatment led to a decrease in the amount of SM that was extractable with high salt, particularly in the presence of -mercaptoethanol, and to a corresponding increase in the amount that remained attached to the nuclear envelope fraction. These data indicate that LMB, by inhibiting the association of SM with CRM 1, alters its intranuclear compartmentalization in a manner that correlates with the effect of LRR mutation. The LRR therefore appears to be required not only for CRM 1-mediated transport to the cytoplasm but also for the proper association of SM with intranuclear structures.
7 6878 BOYLE ET AL. J. VIROL. FIG. 6. Effect of LRR mutation on intracellular compartmentalization of SM. (A) Detergent-solubilized lysates of cells transfected with SM, LRR-2, or LRR- were immunoprecipitated with anti-crm 1 antibodies (anti-crm 1 IP) or electrophoresed directly (non IP lysate) and immunoblotted with anti-sm antibodies. (B) Distribution of SM between detergent-soluble and insoluble fractions. Detergent-soluble (C) and insoluble nuclear pellet (N) fractions of cells transfected with wt SM, LRR-2, or LRR- were analyzed by immunoblotting with anti-sm. FIG. 5. Coimmunoprecipitation of CRM 1 and CRM 1-associated proteins with SM. (A) Lysates of Cos 7 cells transfected with SM or control vector (SM and ) were immunoprecipitated with anti CRM 1 (anti-crm 1 IP) or anti-sm (anti-sm IP) antibodies and immunoblotted to detect SM. A control immunoprecipitation of SM-transfected cell lysate with preimmune rabbit serum (PI) is also shown. (B) Lysates of BJAB cells transfected with SM or control vector were immunoprecipitated with anti-crm 1 antibodies and immunoblotted as in panel A. (C) Lysates of Cos 7 cells transfected with SM and CRM 1 were immunoprecipitated with anti-ran antibodies (anti-ran IP) and immunoblotted to detect SM. Control immunoprecipitations with preimmune rabbit serum (PI) are also shown. (D) Cells were transfected with a plasmid expressing an HA-tagged carboxy-terminal fragment of CAN/Nup214 (HA- CAN) and SM or control plasmid. Lysates were immunoprecipitated with anti-ha monoclonal antibody CA125 (anti-ha IP) and immunoblotted with anti-sm antibodies. A control immunoprecipitation (C) performed with an irrelevant monoclonal antibody (anti-flag) is also shown. In all panels, lanes containing an equivalent amount of unimmunoprecipitated lysate are indicated. in a heterokaryon assay (39, 41). SM also enhances cytoplasmic accumulation of target mrnas (7, 39). Further, SM is homologous to the herpes simplex virus ICP27 protein, which has been shown to shuttle from nucleus to cytoplasm and bind RNA in both locations in vivo (40). Our finding that there is a functionally important association of SM with CRM 1 in vivo indicates that EBV utilizes a cellular DISCUSSION In this study, we demonstrate that the SM protein of EBV binds the export receptor CRM 1 and that CRM 1 binding is important for activity and cytoplasmic localization of SM. The role of CRM 1 binding in both aspects of SM function is shown to be specific by the use of an inhibitor of CRM 1 complex formation, LMB. SM is also shown to associate in vivo with the CRM 1-binding GTPase Ran and the carboxy-terminal portion of a nucleoporin, CAN/Nup214, two components of CRM 1-linked nuclear export pathways. Further, we demonstrate that an LRR of SM is required for function and proper intranuclear distribution of SM. SM is an EBV protein which enhances expression of intronless genes in a gene-dependent manner (23, 30). Although SM clearly has multiple mechanisms of action, including stabilization of RNA and enhancement of posttranscriptional processing (25, 39), it is likely that SM is involved in nucleocytoplasmic export of lytic EBV mrnas. Several lines of evidence support a role for SM as a carrier protein for RNA. SM has been shown to bind RNA in vitro and to shuttle from cytoplasm to nucleus FIG. 7. Effects of LRR mutation and LMB treatment on intranuclear compartmentalization of SM. Cells were lysed and separated into soluble (C) and nuclear (N) fractions. Intact nuclei were nuclease treated and extracted with high salt (HS) or high salt plus 2-mercaptoethanol (HSM). Nuclear envelopes remaining after HS extraction (HS NE) or HSM extraction (HSM NE) were collected by centrifugation. Equivalent amounts of each fraction were analyzed by immunoblotting with anti-sm antibodies. (A) Cells were transfected with SM plasmid or LRR- plasmid, as shown, and harvested for fractionation after 48 h. (B) Cells were transfected with SM plasmid and incubated in growth medium alone (SM) or growth medium with LMB (SM LMB), harvested, and fractionated 16 h posttransfection.
8 VOL. 73, 1999 ASSOCIATION OF EBV SM PROTEIN WITH CRM FIG. 8. Models for intranuclear translocation of SM. (A) Conventional model for CRM 1-mediated export of an NES-containing protein. CRM 1 is shown binding to soluble SM via its NES and transporting it to the NPC. CRM 1 docks at the NPC by binding to an FXFG nucleoporin-binding site (shown in gray). (B) Alternatively, CRM 1 binding detaches SM from its sites on the nuclear matrix or nuclear envelope (diagonal bars). Successive rounds of SM release and binding by CRM 1 constitute a possible mechanism of translocation along the nuclear matrix and through the NPC. export pathway normally utilized for snrna and 5S RNA export (21) to facilitate lytic EBV gene expression. The need for such mechanisms to enhance viral RNA expression may be a reflection of the inherent inefficiency with which intronless RNAs are expressed (19). It has been known for some time that addition of exogenous intron sequences to cdna expression constructs enhances their expression (5). The exact reasons for such a stimulatory effect of intervening sequences on gene expression are poorly understood. The presence of intron sequences facilitates 3 processing and polyadenylation of premrna, and a direct interaction between U1 snrnp protein U1A and polyadenylation factors has been shown (29, 32). Engagement of mrna by the polyadenylation machinery also facilitates nucleocytoplasmic export, possibly due to an interaction of polyadenylation factors with the NPC (9, 20). Lack of assembly of spliceosomes on genes encoded as single open reading frames may thus be a relative barrier to entry of intronless mrnas into a pathway that culminates in nuclear export. SM may allow direct targeting of intronless EBV mrnas for signal-mediated export via CRM 1. Utilization of the CRM 1 pathway also provides another potential advantage for EBV gene expression since, in mammalian cells, the pathways for mrna export and CRM 1-mediated export appear to be independent (3, 14, 47). Thus, inhibition of host cell gene expression during EBV replication could occur without necessarily affecting EBV lytic gene expression. In this context, it is relevant that SM and its homologs in herpes simplex virus and herpesvirus saimiri also inhibit expression of spliced host genes (17, 18, 46). We have shown that the LRR of SM is required for CRM 1-mediated cytoplasmic translocation of SM and for full SM activity. In the case of HIV Rev, several lines of genetic and in vitro evidence indicate that the major function of the NES is CRM 1 binding (14, 15, 33, 42, 47). Mutational analysis of the Rev NES has demonstrated a good correlation between the ability to bind CRM 1 and Rev function (3). It is likely that the SM LRR performs a similar function for the reasons outlined above. Further, LMB treatment produces the same effects as LRR mutation on cytoplasmic localization and SM activity, indicating that the processes are dependent on CRM 1-NES complex formation. However, the additional effects of LMB treatment and LRR mutation on intranuclear distribution of SM suggest that SM may be mechanistically quite different from HIV Rev. The results of LMB treatment and deletion of the LRR suggest that in the absence of CRM 1 binding, SM remains more tightly associated with nuclear structures and particularly with the nuclear envelope. Such a finding is somewhat surprising in the context of current models for CRM 1-NES protein export, in which SM plays the role of a soluble transport substrate for CRM 1 (Fig. 8A). According to such a model, SM might become at least transiently tethered to the NPC via CRM 1. Inhibition of CRM complex formation by mutation of the NES or LMB treatment, while leading to nuclear retention
9 6880 BOYLE ET AL. J. VIROL. of SM, would not be expected to increase the attachment of SM to macromolecular nuclear structures. It is unlikely that the LRR mutations described have merely resulted in an overall decrease in solubility and thus intranuclear aggregation of the mutant proteins for several reasons. First, the SM mutants are properly imported to the nucleus and retain partial transactivating function. Second, a large fraction of wild-type SM is normally associated with the nuclear envelope. Finally, LMB treatment has effects on the intranuclear distribution and attachment of SM that are similar to mutation of the LRR. The finding of SM in the nuclear matrix and nuclear envelope fractions suggests an alternative model for SM transport in which SM interacts directly with nuclear matrix and envelope proteins. In such a scenario, SM with its RNA cargo binds directly to one or more nuclear matrix sites. CRM 1 binding to SM in the presence of Ran-GTP would detach SM from its stationary binding site. Binding of SM to another site on the nuclear matrix accompanied by release from CRM 1 could then occur. Successive rounds of CRM 1 binding, release, and matrix binding could thus result in physical translocation of SM to the cytoplasmic face of the NPC. Such interactions would be expected to increase the efficiency of SM complex movement to the NPC, providing a track along the nuclear matrix. It should be noted that this model is similar to one proposed to explain directional translocation of import substrates into the nucleus (36, 37). In that model, the importin / -Ran-GDP complex dissociates from its cargo nuclear localization signal (NLS) when it binds NPC components. The import receptor is then released from the NPC on binding Ran-GTP, GTP hydrolysis occurs, NLS cargo is bound, and the cycle is repeated, leading to a saltatory movement of the importin-nls complex across the NPC. Our proposed model is similar in that it postulates a successive series of CRM 1-SM binding and release reactions. However, SM is predicted to be also capable of interacting with nucleoporins or other structural nuclear proteins directly and does not invoke GTP hydrolysis, which does not appear to be required for the export process itself (28). Such a model does not preclude CRM 1-nucleoporin interactions and can therefore involve both SM and CRM 1 as active participants in translocation through the NPC. The components and location of the intranuclear foci of SM accumulation, especially those seen with the LRR- mutant, remain to be determined. Based on the known effects of SM on nuclear mrna, it is likely that some of these sites are involved in mrna processing. In summary, our data demonstrate that SM is a transport protein that functionally interacts with CRM 1 but that it may not be merely a soluble carrier of EBV RNA that acts as a link between the RNA, CRM 1, and the NPC. Rather, it is possible that SM exists in a dynamic structural association with the nuclear matrix and other intranuclear structures. Determination of the exact intranuclear sites of SM accumulation and whether SM associates directly with structural components of the NPC is likely to yield further insights into the mechanism of viral and cellular gene regulation at the level of mrna transport. ACKNOWLEDGMENTS This work was supported by a recruitment grant to S.S. from the John Sealy Memorial Endowment Fund for Biomedical Research. We express our appreciation to Barbara Wolff of Novartis AG for providing leptomycin B and to Gerard Grosveld for CRM 1 and CAN/ Nup214 cdna and anti-crm 1 antibodies. We also thank C. Patterson, N. Murray, and A. Fields for many helpful discussions and review of the manuscript. REFERENCES 1. Agard, D. A., Y. Hiraoka, P. Shaw, and J. W. Sedat Fluorescence microscopy in three dimensions. Methods Cell Biol. 30: Baer, R., A. T. Bankier, and M. D. Biggin DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310: Bogerd, H. P., A. Echarri, T. M. Ross, and B. R. Cullen Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J. Virol. 72: Bogerd, H. P., R. A. Fridell, R. E. Benson, J. Hua, and B. R. Cullen Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell. Biol. 16: Callis, J., M. Fromm, and V. Walbot Introns increase gene expression in cultured maize cells. Genes Dev. 1: Cho, M. S., K. T. Jeang, and S. D. Hayward Localization of the coding region for an Epstein-Barr virus early antigen and inducible expression of this 60-kilodalton nuclear protein in transfected fibroblast cell lines. J. Virol. 56: Cook, I. D., F. Shanahan, and P. J. Farrell Epstein-Barr virus SM protein. Virology 205: Deng, W. P., and J. A. Nickoloff Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal. Biochem. 200: Eckner, R., W. Ellmeier, and M. L. Birnstiel Mature mrna 3 end formation stimulates RNA export from the nucleus. EMBO J. 10: Field, J., J. Nikawa, D. Broek, B. MacDonald, L. Rodgers, I. A. Wilson, R. A. Lerner, and M. Wigler Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol. Cell. Biol. 8: Fields, A. P., S. H. Kaufmann, and J. H. Shaper Analysis of the internal nuclear matrix. Oligomers of a 38 kd nucleolar polypeptide stabilized by disulfide bonds. Exp. Cell Res. 164: Fischer, U., J. Huber, W. C. Boelens, I. W. Mattaj, and R. Luhrmann The HIV-1 rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82: Fornerod, M., J. van Deursen, S. van Baal, A. Reynolds, D. Davis, K. G. Murti, J. Fransen, and G. Grosveld The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16: Fornerod, M., M. Ohno, and M. Yoshida CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90: Fukuda, M., S. Asano, T. Nakamura, M. Adachi, M. Yoshida, M. Yanagida, and E. Nishida CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390: Gluzman, Y SV40-transformed simian cells support the replication of early SV40 mutants. Cell 23: Hardwicke, M. A., and R. M. Sandri-Goldin The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mrna levels during infection. J. Virol. 68: Hardy, W. R., and R. M. Sandri-Goldin Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68: Huang, M. T. F., and C. M. Gorman Intervening sequences increase efficiency of RNA 3 processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 18: Huang, Y., and G. G. Carmichael Role of polyadenylation in nucleocytoplasmic transport of mrna. Mol. Cell. Biol. 16: Jarmolowski, A., W. C. Boelens, E. Izaurralde, and I. W. Mattaj Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol. 124: Kaufmann, S. H., W. Gibson, and J. H. Shaper Characterization of the major polypeptides of the rat liver nuclear envelope. J. Biol. Chem. 258: Kenney, S., J. Kamine, E. Holley-Guthrie, E. C. Mar, J. C. Lin, D. Markovitz, and J. Pagano The Epstein-Barr virus immediate-early gene product, BMLF1, acts in trans by a posttranscriptional mechanism which is reporter gene dependent. J. Virol. 63: Kenney, S., J. Kamine, D. Markovitz, R. Fenrick, and J. Pagano An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc. Natl. Acad. Sci. USA 85: Key, S. C., T. Yoshizaki, and J. S. Pagano The Epstein-Barr virus (EBV) SM protein enhances pre-mrna processing of the EBV DNA polymerase transcript. J. Virol. 72: Kieff, E Epstein-Barr virus and its replication, p In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa. 27. Lieberman, P. M., P. O Hare, G. S. Hayward, and S. D. Hayward Promiscuous trans activation of gene expression by an Epstein-Barr virusencoded early nuclear protein. J. Virol. 60: Love, D. C., T. D. Sweitzer, and J. A. Hanover Reconstitution of HIV-1
10 VOL. 73, 1999 ASSOCIATION OF EBV SM PROTEIN WITH CRM rev nuclear export: independent requirements for nuclear import and export. Proc. Natl. Acad. Sci. USA 95: Lutz, C. S., K. G. K. Murthy, N. Schek, J. P. O Conner, J. L. Manley, and J. C. Alwine Interaction between the U1 snrnp-a protein and the 160-kD subunit of cleavage-polyadenylation specificity factor increases polyadenylation efficiency in vitro. Genes Dev. 10: Markovitz, D. M., S. Kenney, J. Kamine, M. S. Smith, M. Davis, and E.-S. Huang Disparate effects of two herpesvirus immediate-early gene trans-activators on the HIV-1 LTR. Virology 173: Menezes, J., W. Liebold, G. Klein, and G. Clements Establishment and characterization of an Epstein-Barr virus (EBV)-negative lymphoblastoid B cell line (BJA-B) from an exceptional EBV-genome-negative African Burkitt s lymphoma. Biomedicine 22: Niwa, M., S. D. Rose, and S. M. Berget In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 4: Ossareh-Nazari, B., F. Bachelerie, and C. Dargemont Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278: Pinol-Roma, S., and G. Dreyfuss Shuttling of pre-mrna binding proteins between nucleus and cytoplasm. Nature 355: Radu, A., G. Blobel, and R. W. Wozniak Nup155 is a novel nuclear pore complex protein that contains neither repetitive sequence motifs nor reacts with WGA. J. Cell Biol. 121: Radu, A., M. S. Moore, and G. Blobel The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell 81: Rexach, M., and G. Blobel Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 83: Richards, S. A., K. L. Carey, and I. G. Macara Requirement of guanosine triphosphate-bound ran for signal-mediated nuclear protein export. Science 276: Ruvolo, V., E. Wang, S. Boyle, and S. Swaminathan The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc. Natl. Acad. Sci. USA 95: Sandri-Goldin, R. M ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12: Semmes, O. J., L. Chen, R. T. Sarisky, Z. Gao, L. Zhong, and S. D. Hayward Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mrna. J. Virol. 72: Stade, K., C. S. Ford, C. Guthrie, and K. Weis Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90: Stutz, F., and M. Rosbash Nuclear RNA export. Genes Dev. 12: Swaminathan, S., B. Tomkinson, and E. Kieff Recombinant Epstein- Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc. Natl. Acad. Sci. USA 88: Wen, W., J. L. Meinkoth, R. Y. Tsien, and S. S. Taylor Identification of a signal for rapid export of proteins from the nucleus. Cell 82: Whitehouse, A., M. Cooper, and D. M. Meredith The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level. J. Virol. 72: Wolff, B., J. J. Sanglier, and Y. Wang Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mrna. Chem. Biol. 4: Wong, K.-M., and A. J. Levine Identification and mapping of Epstein- Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J. Virol. 60: Zolotukhin, A. S., and B. K. Felber Nucleoporins Nup98 and Nup214 participate in nuclear export of human immunodeficiency virus type 1 Rev. J. Virol. 73:
Anti-ATF6 α antibody, mouse monoclonal (1-7)
 Anti-ATF6 α antibody, mouse monoclonal (1-7) 73-500 50 ug ATF6 (activating transcription factor 6) is an endoplasmic reticulum (ER) membrane-bound transcription factor activated in response to ER stress.
Anti-ATF6 α antibody, mouse monoclonal (1-7) 73-500 50 ug ATF6 (activating transcription factor 6) is an endoplasmic reticulum (ER) membrane-bound transcription factor activated in response to ER stress.
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
RNA Viruses. A Practical Approac h. Alan J. Cann
 RNA Viruses A Practical Approac h Alan J. Cann List of protocols page xiii Abbreviations xvii Investigation of RNA virus genome structure 1 A j. Easton, A.C. Marriott and C.R. Pringl e 1 Introduction-the
RNA Viruses A Practical Approac h Alan J. Cann List of protocols page xiii Abbreviations xvii Investigation of RNA virus genome structure 1 A j. Easton, A.C. Marriott and C.R. Pringl e 1 Introduction-the
Chapter 2 Antibodies. Contents. Introduction
 Chapter 2 Antibodies Keywords Immunohistochemistry Antibody labeling Fluorescence microscopy Fluorescent immunocytochemistry Fluorescent immunohistochemistry Indirect immunocytochemistry Immunostaining
Chapter 2 Antibodies Keywords Immunohistochemistry Antibody labeling Fluorescence microscopy Fluorescent immunocytochemistry Fluorescent immunohistochemistry Indirect immunocytochemistry Immunostaining
Chromatin Immunoprecipitation
 Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro, Monica Venere, Thanos Halazonetis, Nicholas E. Sherman, and Michele Pagano
 Molecular Cell, Volume 23 Supplemental Data SCF βtrcp -Mediated Degradation of Claspin Regulates Recovery from the DNA Replication Checkpoint Response Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro,
Molecular Cell, Volume 23 Supplemental Data SCF βtrcp -Mediated Degradation of Claspin Regulates Recovery from the DNA Replication Checkpoint Response Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro,
Cross-reactivity of the anti-pml antibody PG-M3 with the herpes simplex virus type 1 immediate early protein ICP4
 Journal of General Virology (2000), 81, 1773 1777. Printed in Great Britain... SHORT COMMUNICATION Cross-reactivity of the anti-pml antibody PG-M3 with the herpes simplex virus type 1 immediate early protein
Journal of General Virology (2000), 81, 1773 1777. Printed in Great Britain... SHORT COMMUNICATION Cross-reactivity of the anti-pml antibody PG-M3 with the herpes simplex virus type 1 immediate early protein
SUPPLEMENTARY DATA 1
 SUPPLEMENTARY DATA 1 Supplementary Figure S1. Overexpression of untagged Ago2 inhibits the nuclear transport of the dotted foci of GFP-signal of myc-gfp-tnrc6a-nes-mut. (A C) HeLa cells expressing myc-gfp-tnrc6a-nes-mut
SUPPLEMENTARY DATA 1 Supplementary Figure S1. Overexpression of untagged Ago2 inhibits the nuclear transport of the dotted foci of GFP-signal of myc-gfp-tnrc6a-nes-mut. (A C) HeLa cells expressing myc-gfp-tnrc6a-nes-mut
Rapid GST Inclusion Body Solubilization and Renaturation Kit
 Product Manual Rapid GST Inclusion Body Solubilization and Renaturation Kit Catalog Number AKR-110 FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Bacteria are widely used for His
Product Manual Rapid GST Inclusion Body Solubilization and Renaturation Kit Catalog Number AKR-110 FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Bacteria are widely used for His
PROTOCOL. Immunocytochemistry (ICC) MATERIALS AND EQUIPMENT REQUIRED
 PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
PROTOCOL Immunocytochemistry (ICC) 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 11-07 MATERIALS AND EQUIPMENT REQUIRED Materials: MitoSciences primary monoclonal antibody/antibodies Fluorophore-conjugated
Chapter 8. Summary and Perspectives
 Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Chapter 8 Summary and Perspectives 131 Chapter 8 Summary Overexpression of the multidrug resistance protein MRP1 confer multidrug resistance (MDR) to cancer cells. The contents of this thesis describe
Visualization of Myc/Max/Mad Family Dimers and the Competition for Dimerization in Living Cells
 MOLECULAR AND CELLULAR BIOLOGY, May 2004, p. 4294 4308 Vol. 24, No. 10 0270-7306/04/$08.00 0 DOI: 10.1128/MCB.24.10.4294 4308.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved.
MOLECULAR AND CELLULAR BIOLOGY, May 2004, p. 4294 4308 Vol. 24, No. 10 0270-7306/04/$08.00 0 DOI: 10.1128/MCB.24.10.4294 4308.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved.
Chapter 6: Antigen-Antibody Interactions
 Chapter 6: Antigen-Antibody Interactions I. Strength of Ag-Ab interactions A. Antibody Affinity - strength of total noncovalent interactions between single Ag-binding site on an Ab and a single epitope
Chapter 6: Antigen-Antibody Interactions I. Strength of Ag-Ab interactions A. Antibody Affinity - strength of total noncovalent interactions between single Ag-binding site on an Ab and a single epitope
CD3/TCR stimulation and surface detection Determination of specificity of intracellular detection of IL-7Rα by flow cytometry
 CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
THE His Tag Antibody, mab, Mouse
 THE His Tag Antibody, mab, Mouse Cat. No. A00186 Technical Manual No. TM0243 Update date 01052011 I Description.... 1 II Key Features. 2 III Storage 2 IV Applications.... 2 V Examples - ELISA..... 2 VI
THE His Tag Antibody, mab, Mouse Cat. No. A00186 Technical Manual No. TM0243 Update date 01052011 I Description.... 1 II Key Features. 2 III Storage 2 IV Applications.... 2 V Examples - ELISA..... 2 VI
Protein Expression. A Practical Approach J. HIGGIN S
 Protein Expression A Practical Approach S. J. HIGGIN S B. D. HAMES List of contributors Abbreviations xv Xvi i 1. Protein expression in mammalian cell s Marlies Otter-Nilsson and Tommy Nilsso n 1. Introduction
Protein Expression A Practical Approach S. J. HIGGIN S B. D. HAMES List of contributors Abbreviations xv Xvi i 1. Protein expression in mammalian cell s Marlies Otter-Nilsson and Tommy Nilsso n 1. Introduction
Protein immunoblotting
 Protein immunoblotting (Western blotting) Dr. Serageldeen A. A. Sultan Lecturer of virology Dept. of Microbiology SVU, Qena, Egypt seaas@lycos.com Western blotting -It is an analytical technique used to
Protein immunoblotting (Western blotting) Dr. Serageldeen A. A. Sultan Lecturer of virology Dept. of Microbiology SVU, Qena, Egypt seaas@lycos.com Western blotting -It is an analytical technique used to
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
In vitro analysis of pri-mirna processing. by Drosha-DGCR8 complex. (Narry Kim s lab)
 In vitro analysis of pri-mirna processing by Drosha-DGCR8 complex (Narry Kim s lab) 1-1. Preparation of radiolabeled pri-mirna transcript The RNA substrate for a cropping reaction can be prepared by in
In vitro analysis of pri-mirna processing by Drosha-DGCR8 complex (Narry Kim s lab) 1-1. Preparation of radiolabeled pri-mirna transcript The RNA substrate for a cropping reaction can be prepared by in
+ - bcma_03. Materiale per uso didattico 1. Kinesin light chain Kinesin heavy chain Kinesin heavy chain Kinesin light chain. tetratricopeptide repeat
 p.959-970 Kinesin light chain Kinesin heavy chain Kinesin heavy chain Kinesin light chain + - tetratricopeptide repeat Zona di possibile interazione con cargo Materiale per uso didattico 1 JIP-1 COOH-term
p.959-970 Kinesin light chain Kinesin heavy chain Kinesin heavy chain Kinesin light chain + - tetratricopeptide repeat Zona di possibile interazione con cargo Materiale per uso didattico 1 JIP-1 COOH-term
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus
 Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
How To Understand How Gene Expression Is Regulated
 What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
Chapter 11. Rapid Screening of Gli2/3 Mutants Using the Flp-In System. Pawel Niewiadomski and Rajat Rohatgi. Abstract.
 Chapter 11 Rapid Screening of Gli2/3 Mutants Using the Flp-In System Abstract Gli2 and Gli3 respond to the Hedgehog (Hh) signal in mammals by undergoing posttranslational modifications and moving to the
Chapter 11 Rapid Screening of Gli2/3 Mutants Using the Flp-In System Abstract Gli2 and Gli3 respond to the Hedgehog (Hh) signal in mammals by undergoing posttranslational modifications and moving to the
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
Pure-IP Western Blot Detection Kit
 Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Chapter 6. Antigen-Antibody Properties 10/3/2012. Antigen-Antibody Interactions: Principles and Applications. Precipitin reactions
 Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
Chapter 6 Antigen-Antibody Interactions: Principles and Applications Antigen-Antibody Properties You must remember antibody affinity (single) VS avidity (multiple) High affinity: bound tightly and longer!
Transfection-Transfer of non-viral genetic material into eukaryotic cells. Infection/ Transduction- Transfer of viral genetic material into cells.
 Transfection Key words: Transient transfection, Stable transfection, transfection methods, vector, plasmid, origin of replication, reporter gene/ protein, cloning site, promoter and enhancer, signal peptide,
Transfection Key words: Transient transfection, Stable transfection, transfection methods, vector, plasmid, origin of replication, reporter gene/ protein, cloning site, promoter and enhancer, signal peptide,
The world of non-coding RNA. Espen Enerly
 The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
The world of non-coding RNA Espen Enerly ncrna in general Different groups Small RNAs Outline mirnas and sirnas Speculations Common for all ncrna Per def.: never translated Not spurious transcripts Always/often
Notch 1 -dependent regulation of cell fate in colorectal cancer
 Notch 1 -dependent regulation of cell fate in colorectal cancer Referees: PD Dr. Tobias Dick Prof. Dr. Wilfried Roth http://d-nb.info/1057851272 CONTENTS Summary 1 Zusammenfassung 2 1 INTRODUCTION 3 1.1
Notch 1 -dependent regulation of cell fate in colorectal cancer Referees: PD Dr. Tobias Dick Prof. Dr. Wilfried Roth http://d-nb.info/1057851272 CONTENTS Summary 1 Zusammenfassung 2 1 INTRODUCTION 3 1.1
Antibody Production Price List
 Antibody Production Price List Presenting Insight Biotechnology s price list for custom polyclonal and monoclonal antibody production services. We are happy to tailor individual packages towards the specific
Antibody Production Price List Presenting Insight Biotechnology s price list for custom polyclonal and monoclonal antibody production services. We are happy to tailor individual packages towards the specific
WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TROUBLESHOOTING GUIDE
 WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TIPS FOR SUCCESSFUL WESTERB BLOTS TROUBLESHOOTING GUIDE 1. Suboptimal protein transfer. This is the most common complaint with western blotting and could
WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TIPS FOR SUCCESSFUL WESTERB BLOTS TROUBLESHOOTING GUIDE 1. Suboptimal protein transfer. This is the most common complaint with western blotting and could
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
Chromatin Immunoprecipitation (ChIP)
 Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Methods for Protein Analysis
 Methods for Protein Analysis 1. Protein Separation Methods The following is a quick review of some common methods used for protein separation: SDS-PAGE (SDS-polyacrylamide gel electrophoresis) separates
Methods for Protein Analysis 1. Protein Separation Methods The following is a quick review of some common methods used for protein separation: SDS-PAGE (SDS-polyacrylamide gel electrophoresis) separates
Custom Antibody Services
 Custom Antibody Services Custom service offerings DNA sequence Plasmid Peptide Structure Protein Peptide Small molecule Cells Spleen Lymphocytes Antigen Preparation Immunization Fusion & Subcloning Expansion
Custom Antibody Services Custom service offerings DNA sequence Plasmid Peptide Structure Protein Peptide Small molecule Cells Spleen Lymphocytes Antigen Preparation Immunization Fusion & Subcloning Expansion
Structure and Function of DNA
 Structure and Function of DNA DNA and RNA Structure DNA and RNA are nucleic acids. They consist of chemical units called nucleotides. The nucleotides are joined by a sugar-phosphate backbone. The four
Structure and Function of DNA DNA and RNA Structure DNA and RNA are nucleic acids. They consist of chemical units called nucleotides. The nucleotides are joined by a sugar-phosphate backbone. The four
Custom Antibody Services
 prosci-inc.com Custom Antibody Services High Performance Antibodies and More Broad Antibody Catalog Extensive Antibody Services CUSTOM ANTIBODY SERVICES Established in 1998, ProSci Incorporated is a leading
prosci-inc.com Custom Antibody Services High Performance Antibodies and More Broad Antibody Catalog Extensive Antibody Services CUSTOM ANTIBODY SERVICES Established in 1998, ProSci Incorporated is a leading
Applications of Ab Molecules. Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128)
 Applications of Ab Molecules Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128) Monoclonal Antibodies Clonal Selection of B Lymphocytes Hybridoma Köhler and Milsten (1975) - continuous
Applications of Ab Molecules Chapter 4 Monoclonal Ab (p.99) Chapter 5 Ab genes and Ab Engineering (p.128) Monoclonal Antibodies Clonal Selection of B Lymphocytes Hybridoma Köhler and Milsten (1975) - continuous
Investigating the role of a Cryptosporidium parum apyrase in infection
 Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
TABLE OF CONTENT. Page ACKNOWLEDGEMENTS. iii ENGLISH ABSTRACT THAI ABSTRACT. vii LIST OF TABLES LIST OF FIGURES. xvi ABBREVIATIONS.
 x TABLE OF CONTENT ACKNOWLEDGEMENTS ENGLISH ABSTRACT THAI ABSTRACT LIST OF TABLES LIST OF FIGURES ABBREVIATIONS iii iv vii xv xvi xviii CHAPTER I: INTRODUCTION 1.1 Statement of problems 1 1.2 Literature
x TABLE OF CONTENT ACKNOWLEDGEMENTS ENGLISH ABSTRACT THAI ABSTRACT LIST OF TABLES LIST OF FIGURES ABBREVIATIONS iii iv vii xv xvi xviii CHAPTER I: INTRODUCTION 1.1 Statement of problems 1 1.2 Literature
Understanding the immune response to bacterial infections
 Understanding the immune response to bacterial infections A Ph.D. (SCIENCE) DISSERTATION SUBMITTED TO JADAVPUR UNIVERSITY SUSHIL KUMAR PATHAK DEPARTMENT OF CHEMISTRY BOSE INSTITUTE 2008 CONTENTS Page SUMMARY
Understanding the immune response to bacterial infections A Ph.D. (SCIENCE) DISSERTATION SUBMITTED TO JADAVPUR UNIVERSITY SUSHIL KUMAR PATHAK DEPARTMENT OF CHEMISTRY BOSE INSTITUTE 2008 CONTENTS Page SUMMARY
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College
 Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
2.1.2 Characterization of antiviral effect of cytokine expression on HBV replication in transduced mouse hepatocytes line
 i 1 INTRODUCTION 1.1 Human Hepatitis B virus (HBV) 1 1.1.1 Pathogenesis of Hepatitis B 1 1.1.2 Genome organization of HBV 3 1.1.3 Structure of HBV virion 5 1.1.4 HBV life cycle 5 1.1.5 Experimental models
i 1 INTRODUCTION 1.1 Human Hepatitis B virus (HBV) 1 1.1.1 Pathogenesis of Hepatitis B 1 1.1.2 Genome organization of HBV 3 1.1.3 Structure of HBV virion 5 1.1.4 HBV life cycle 5 1.1.5 Experimental models
Proteomics Research with BIOCHAIN
 Proteomics Research with IOCHIN Protein Extraction CNMCS Compartmental Protein Extraction Kit Cytoplasmic, Nuclear, Membrane, and Cytoskeleton Protein One kit isolates four different proteins sequentially
Proteomics Research with IOCHIN Protein Extraction CNMCS Compartmental Protein Extraction Kit Cytoplasmic, Nuclear, Membrane, and Cytoskeleton Protein One kit isolates four different proteins sequentially
Fluorescein Isothiocyanate (FITC)- conjugated Antibodies
 USER GUIDE Fluorescein Isothiocyanate (FITC)- conjugated Antibodies Catalog Numbers R933-25, R953-25, R963-25 Document Part Number 25-0376 Publication Number MAN0000194 Revision 2.0 For Research Use Only.
USER GUIDE Fluorescein Isothiocyanate (FITC)- conjugated Antibodies Catalog Numbers R933-25, R953-25, R963-25 Document Part Number 25-0376 Publication Number MAN0000194 Revision 2.0 For Research Use Only.
Supplemental Information. McBrayer et al. Supplemental Data
 1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
HuCAL Custom Monoclonal Antibodies
 HuCAL Custom Monoclonal HuCAL Custom Monoclonal Antibodies Highly Specific, Recombinant Antibodies in 8 Weeks Highly Specific Monoclonal Antibodies in Just 8 Weeks HuCAL PLATINUM (Human Combinatorial Antibody
HuCAL Custom Monoclonal HuCAL Custom Monoclonal Antibodies Highly Specific, Recombinant Antibodies in 8 Weeks Highly Specific Monoclonal Antibodies in Just 8 Weeks HuCAL PLATINUM (Human Combinatorial Antibody
Custom Antibodies Services. GeneCust Europe. GeneCust Europe
 GeneCust Europe Laboratoire de Biotechnologie du Luxembourg S.A. 6 rue Dominique Lang L-3505 Dudelange Luxembourg Tél. : +352 27620411 Fax : +352 27620412 Email : info@genecust.com Web : www.genecust.com
GeneCust Europe Laboratoire de Biotechnologie du Luxembourg S.A. 6 rue Dominique Lang L-3505 Dudelange Luxembourg Tél. : +352 27620411 Fax : +352 27620412 Email : info@genecust.com Web : www.genecust.com
Protein Expression and Analysis. Vijay Yajnik, MD, PhD GI Unit MGH
 Protein Expression and Analysis Vijay Yajnik, MD, PhD GI Unit MGH Identify your needs Antigen production Biochemical studies Cell Biology Protein interaction studies including proteomics Structural studies
Protein Expression and Analysis Vijay Yajnik, MD, PhD GI Unit MGH Identify your needs Antigen production Biochemical studies Cell Biology Protein interaction studies including proteomics Structural studies
Lecture 8. Protein Trafficking/Targeting. Protein targeting is necessary for proteins that are destined to work outside the cytoplasm.
 Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Protocol for Western Blotting
 Protocol for Western Blotting Materials Materials used on Day 3 Protease inhibitor stock: 1 μg/μl pepstatin A in DMSO 200 μm leupeptin in OG Buffer 200 mm PMSF: Freshly made. Ex) 34.8 mg PMSF in 1 ml isopropanol
Protocol for Western Blotting Materials Materials used on Day 3 Protease inhibitor stock: 1 μg/μl pepstatin A in DMSO 200 μm leupeptin in OG Buffer 200 mm PMSF: Freshly made. Ex) 34.8 mg PMSF in 1 ml isopropanol
TOOLS sirna and mirna. User guide
 TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
High Resolution Epitope Mapping of Human Autoimmune Sera against Antigens CENPA and KDM6B. PEPperPRINT GmbH Heidelberg, 06/2014
 High Resolution Epitope Mapping of Human Autoimmune Sera against Antigens CENPA and KDM6B PEPperPRINT GmbH Heidelberg, 06/2014 Introduction This report describes the epitope mapping of autoimmune sera
High Resolution Epitope Mapping of Human Autoimmune Sera against Antigens CENPA and KDM6B PEPperPRINT GmbH Heidelberg, 06/2014 Introduction This report describes the epitope mapping of autoimmune sera
Outline. 1. Experiment. 2. Sample analysis and storage. 3. Image analysis and presenting data. 4. Probemaker
 Tips and tricks Note: this is just an informative document with general recommendations. Please contact support@olink.com should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
Tips and tricks Note: this is just an informative document with general recommendations. Please contact support@olink.com should you have any queries. Document last reviewed 2011-11-17 Outline 1. Experiment
Aviva Systems Biology
 Aviva Custom Antibody Service and Price Mouse Monoclonal Antibody Service Package Number Description Package Contents Time Price Customer provides antigen protein $6,174 Monoclonal package1 (From protein
Aviva Custom Antibody Service and Price Mouse Monoclonal Antibody Service Package Number Description Package Contents Time Price Customer provides antigen protein $6,174 Monoclonal package1 (From protein
Sickle cell anemia: Altered beta chain Single AA change (#6 Glu to Val) Consequence: Protein polymerizes Change in RBC shape ---> phenotypes
 Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Protein Structure Polypeptide: Protein: Therefore: Example: Single chain of amino acids 1 or more polypeptide chains All polypeptides are proteins Some proteins contain >1 polypeptide Hemoglobin (O 2 binding
Protein Synthesis How Genes Become Constituent Molecules
 Protein Synthesis Protein Synthesis How Genes Become Constituent Molecules Mendel and The Idea of Gene What is a Chromosome? A chromosome is a molecule of DNA 50% 50% 1. True 2. False True False Protein
Protein Synthesis Protein Synthesis How Genes Become Constituent Molecules Mendel and The Idea of Gene What is a Chromosome? A chromosome is a molecule of DNA 50% 50% 1. True 2. False True False Protein
CUSTOM ANTIBODIES. Fully customised services: rat and murine monoclonals, rat and rabbit polyclonals, antibody characterisation, antigen preparation
 CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
ArC Amine Reactive Compensation Bead Kit
 ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
ArC Amine Reactive Compensation Bead Kit Catalog no. A1346 Table 1. Contents and storage information. Material Amount Composition Storage Stability ArC reactive beads (Component A) ArC negative beads (Component
TECHNICAL BULLETIN. FluoroTag FITC Conjugation Kit. Product Number FITC1 Storage Temperature 2 8 C
 FluoroTag FITC Conjugation Kit Product Number FITC1 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The FluoroTag FITC Conjugation Kit is suitable for the conjugation of polyclonal and
FluoroTag FITC Conjugation Kit Product Number FITC1 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The FluoroTag FITC Conjugation Kit is suitable for the conjugation of polyclonal and
Custom Antibodies & Recombinant Proteins
 Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
Custom Antibodies & Recombinant Proteins INTRODUCTION Custom services to meet your research and development requirements Improvements in health, medicine and diagnostics over the past century can be largely
First Strand cdna Synthesis
 380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
4.1 3T12 and 312 are immortalized cell lines with transforming potential:
 DISCUSSION CHAPTER 4 4.1 3T12 and 312 are immortalized cell lines with transforming potential: Transformation of a normal cell with finite number of divisions into a tumorigenic cell of potentially infinite
DISCUSSION CHAPTER 4 4.1 3T12 and 312 are immortalized cell lines with transforming potential: Transformation of a normal cell with finite number of divisions into a tumorigenic cell of potentially infinite
Approaches that can be used to study expression of specific proteins
 Approaches that can be used to study expression of specific proteins Receptors and transporters Homogenate binding studies Receptor autoradiography Radiochemical Western blotting Immunohistochemistry/cytochemistry
Approaches that can be used to study expression of specific proteins Receptors and transporters Homogenate binding studies Receptor autoradiography Radiochemical Western blotting Immunohistochemistry/cytochemistry
How To Make A Tri Reagent
 TRI Reagent For processing tissues, cells cultured in monolayer or cell pellets Catalog Number T9424 Store at room temperature. TECHNICAL BULLETIN Product Description TRI Reagent is a quick and convenient
TRI Reagent For processing tissues, cells cultured in monolayer or cell pellets Catalog Number T9424 Store at room temperature. TECHNICAL BULLETIN Product Description TRI Reagent is a quick and convenient
Chapter 18 Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression 18.1. Gene Regulation Is Necessary By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection
Chapter 18 Regulation of Gene Expression 18.1. Gene Regulation Is Necessary By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE Q5B
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
pcas-guide System Validation in Genome Editing
 pcas-guide System Validation in Genome Editing Tagging HSP60 with HA tag genome editing The latest tool in genome editing CRISPR/Cas9 allows for specific genome disruption and replacement in a flexible
pcas-guide System Validation in Genome Editing Tagging HSP60 with HA tag genome editing The latest tool in genome editing CRISPR/Cas9 allows for specific genome disruption and replacement in a flexible
Lecture Series 7. From DNA to Protein. Genotype to Phenotype. Reading Assignments. A. Genes and the Synthesis of Polypeptides
 Lecture Series 7 From DNA to Protein: Genotype to Phenotype Reading Assignments Read Chapter 7 From DNA to Protein A. Genes and the Synthesis of Polypeptides Genes are made up of DNA and are expressed
Lecture Series 7 From DNA to Protein: Genotype to Phenotype Reading Assignments Read Chapter 7 From DNA to Protein A. Genes and the Synthesis of Polypeptides Genes are made up of DNA and are expressed
ab185915 Protein Sumoylation Assay Ultra Kit
 ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
INSTRUCTION Probemaker
 INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
Virological Methods. Flint et al. Principles of Virology (ASM), Chapter 2
 Virological Methods Flint et al. Principles of Virology (ASM), Chapter 2 Overview The most commonly used laboratory methods for the detection of viruses and virus components in biological samples can be
Virological Methods Flint et al. Principles of Virology (ASM), Chapter 2 Overview The most commonly used laboratory methods for the detection of viruses and virus components in biological samples can be
QUANTITATIVE RT-PCR. A = B (1+e) n. A=amplified products, B=input templates, n=cycle number, and e=amplification efficiency.
 QUANTITATIVE RT-PCR Application: Quantitative RT-PCR is used to quantify mrna in both relative and absolute terms. It can be applied for the quantification of mrna expressed from endogenous genes, and
QUANTITATIVE RT-PCR Application: Quantitative RT-PCR is used to quantify mrna in both relative and absolute terms. It can be applied for the quantification of mrna expressed from endogenous genes, and
Application Guide... 2
 Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
Protocol for GenomePlex Whole Genome Amplification from Formalin-Fixed Parrafin-Embedded (FFPE) tissue Application Guide... 2 I. Description... 2 II. Product Components... 2 III. Materials to be Supplied
TECHNICAL BULLETIN. HIS-Select Nickel Affinity Gel. Catalog Number P6611 Storage Temperature 2 8 C
 HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
Basics of Immunology
 Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Basics of Immunology 2 Basics of Immunology What is the immune system? Biological mechanism for identifying and destroying pathogens within a larger organism. Pathogens: agents that cause disease Bacteria,
Predict Reactivity Note Chicken (100%), Sheep (100%), Rhesus Monkey (100%), Chimpanzee (100%), Bovine (100%), Guinea pig (100%)
 Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
HighPure Maxi Plasmid Kit
 HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a
 Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method)
 Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
Immune Tolerance Network RPCI 004 v.002 Staining Procedure For all Directly Conjugated Reagents (Whole Blood Method) Author: Paul Wallace, Director, RPCI Laboratory of Flow Cytometry Approved by: Paul
The role of IBV proteins in protection: cellular immune responses. COST meeting WG2 + WG3 Budapest, Hungary, 2015
 The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
The role of IBV proteins in protection: cellular immune responses COST meeting WG2 + WG3 Budapest, Hungary, 2015 1 Presentation include: Laboratory results Literature summary Role of T cells in response
GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps)
 1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
1 GRS Plasmid Purification Kit Transfection Grade GK73.0002 (2 MaxiPreps) (FOR RESEARCH ONLY) Sample : Expected Yield : Endotoxin: Format : Operation Time : Elution Volume : 50-400 ml of cultured bacterial
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small
 The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
Luísa Romão. Instituto Nacional de Saúde Dr. Ricardo Jorge Av. Padre Cruz, 1649-016 Lisboa, Portugal. Cooper et al (2009) Cell 136: 777
 Luísa Romão Instituto Nacional de Saúde Dr. Ricardo Jorge Av. Padre Cruz, 1649-016 Lisboa, Portugal Cooper et al (2009) Cell 136: 777 PTC = nonsense or stop codon = UAA, UAG, UGA PTCs can arise in a variety
Luísa Romão Instituto Nacional de Saúde Dr. Ricardo Jorge Av. Padre Cruz, 1649-016 Lisboa, Portugal Cooper et al (2009) Cell 136: 777 PTC = nonsense or stop codon = UAA, UAG, UGA PTCs can arise in a variety
T Cell Maturation,Activation and Differentiation
 T Cell Maturation,Activation and Differentiation Positive Selection- In thymus, permits survival of only those T cells whose TCRs recognize self- MHC molecules (self-mhc restriction) Negative Selection-
T Cell Maturation,Activation and Differentiation Positive Selection- In thymus, permits survival of only those T cells whose TCRs recognize self- MHC molecules (self-mhc restriction) Negative Selection-
MicroRNA formation. 4th International Symposium on Non-Surgical Contraceptive Methods of Pet Population Control
 MicroRNA formation mirna s are processed from several precursor stages Mammalian genomes seem to have 100 s of mirna s Nucleotides in positions 2-8 of an mirna are considered the mirna seed 5 Methyl-G
MicroRNA formation mirna s are processed from several precursor stages Mammalian genomes seem to have 100 s of mirna s Nucleotides in positions 2-8 of an mirna are considered the mirna seed 5 Methyl-G
Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study.
 1 Supplemental data Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study. Supplemental Fig. S2. Ingenuity Pathway Analysis (IPA) of the 56 putative caspase substrates
1 Supplemental data Supplemental Fig. S1. The schematic diagrams of the expression constructs used in this study. Supplemental Fig. S2. Ingenuity Pathway Analysis (IPA) of the 56 putative caspase substrates
About Our Products. Blood Products. Purified Infectious/Inactivated Agents. Native & Recombinant Viral Proteins. DNA Controls and Primers for PCR
 About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
About Our Products Purified Infectious/Inactivated Agents ABI produces a variety of specialized reagents, allowing researchers to choose the best preparations for their studies. Available reagents include
biomapping FLAG Epitope System Original & Proven System for Demanding Applications
 biomapping FLAG Epitope System Original & Proven System for Demanding Applications biomapping FLAG Epitope System Original & Proven System for Demanding Applications Overview A Proven System for Detection
biomapping FLAG Epitope System Original & Proven System for Demanding Applications biomapping FLAG Epitope System Original & Proven System for Demanding Applications Overview A Proven System for Detection
Viruses. Viral components: Capsid. Chapter 10: Viruses. Viral components: Nucleic Acid. Viral components: Envelope
 Viruses Chapter 10: Viruses Lecture Exam #3 Wednesday, November 22 nd (This lecture WILL be on Exam #3) Dr. Amy Rogers Office Hours: MW 9-10 AM Too small to see with a light microscope Visible with electron
Viruses Chapter 10: Viruses Lecture Exam #3 Wednesday, November 22 nd (This lecture WILL be on Exam #3) Dr. Amy Rogers Office Hours: MW 9-10 AM Too small to see with a light microscope Visible with electron
Aviva Systems Biology
 Aviva Custom Antibody Services and Prices Rabbit Polyclonal Antibody Service Package Number Description Package Contents Time Price Polyclonal package 1 (From protein to antiserum) Polyclonal package 2
Aviva Custom Antibody Services and Prices Rabbit Polyclonal Antibody Service Package Number Description Package Contents Time Price Polyclonal package 1 (From protein to antiserum) Polyclonal package 2
Antigens & Antibodies II. Polyclonal antibodies vs Monoclonal antibodies
 A Brief Review of Antibody Structure A Brief Review of Antibody Structure The basic antibody is a dimer of dimer (2 heavy chain-light chain pairs) composed of repeats of a single structural unit known
A Brief Review of Antibody Structure A Brief Review of Antibody Structure The basic antibody is a dimer of dimer (2 heavy chain-light chain pairs) composed of repeats of a single structural unit known
Aurora Forensic Sample Clean-up Protocol
 Aurora Forensic Sample Clean-up Protocol 106-0008-BA-D 2015 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners. http://www.borealgenomics.com support@borealgenomics.com
Aurora Forensic Sample Clean-up Protocol 106-0008-BA-D 2015 Boreal Genomics, Inc. All rights reserved. All trademarks are property of their owners. http://www.borealgenomics.com support@borealgenomics.com
Chapter 3.2» Custom Monoclonal
 198 3 3.2 Custom Monoclonal 199 Mouse monoclonal antibody development Chapter 3.2» Custom Monoclonal 200 In vitro monoclonals expression service 201 Mouse monoclonal antibody additional services 202 Magnetic
198 3 3.2 Custom Monoclonal 199 Mouse monoclonal antibody development Chapter 3.2» Custom Monoclonal 200 In vitro monoclonals expression service 201 Mouse monoclonal antibody additional services 202 Magnetic
mirnaselect pep-mir Cloning and Expression Vector
 Product Data Sheet mirnaselect pep-mir Cloning and Expression Vector CATALOG NUMBER: MIR-EXP-C STORAGE: -80ºC QUANTITY: 2 vectors; each contains 100 µl of bacterial glycerol stock Components 1. mirnaselect
Product Data Sheet mirnaselect pep-mir Cloning and Expression Vector CATALOG NUMBER: MIR-EXP-C STORAGE: -80ºC QUANTITY: 2 vectors; each contains 100 µl of bacterial glycerol stock Components 1. mirnaselect
APPLICATION FOCUS. Application Solutions for Western Blotting
 APPLICATION FOCUS Application Solutions for Western Blotting WESTERN BLOTTING Companion Products Solutions for consistently better blotting. Companion products from Cell Signaling Technology (CST) are
APPLICATION FOCUS Application Solutions for Western Blotting WESTERN BLOTTING Companion Products Solutions for consistently better blotting. Companion products from Cell Signaling Technology (CST) are
MAB Solut. MABSolys Génopole Campus 1 5 rue Henri Desbruères 91030 Evry Cedex. www.mabsolut.com. is involved at each stage of your project
 Mabsolus-2015-UK:Mise en page 1 03/07/15 14:13 Page1 Services provider Department of MABSolys from conception to validation MAB Solut is involved at each stage of your project Creation of antibodies Production
Mabsolus-2015-UK:Mise en page 1 03/07/15 14:13 Page1 Services provider Department of MABSolys from conception to validation MAB Solut is involved at each stage of your project Creation of antibodies Production
6 Characterization of Casein and Bovine Serum Albumin
 6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
