Differences in Rehydration of Three Desiccation-tolerant Angiosperm Species
|
|
|
- Darlene Chandler
- 8 years ago
- Views:
Transcription
1 Annals of Botany 78: 73 71, 1996 Differences in Rehydration of Three Desiccation-tolerant Angiosperm Species HEATHER W. SHERWIN* and JILL M. FARRANT Botany Department, Uni ersity of Cape Town, Pri ate Bag, Rondebosch, 77, South Africa Received: 2 February 1996 Accepted: 5 June 1996 The rehydration characteristics of the desiccation-tolerant plants Craterostigma wilmsii and Myrothamnus flabellifolia (homoiochlorophyllous) and Xerophyta iscosa (poikilochlorophyllous) were studied to determine differences among them. A desiccation-sensitive plant (Pisum sati um) was used as a control. Recovery of water content, quantum efficiency (F V ), photosynthetic pigments and chloroplast ultrastructure as well as damage to the plasmamembrane were studied. P. sati um did not recover after desiccation and considerable damage occurred during rehydration. The desiccation-tolerant plants appeared to differ in their responses to dehydration and rehydration. The small herbaceous C. wilmsii generally showed little damage in the dry state and recovered faster than the other tolerant species. M. flabellifolia took longer to recover than C. wilmsii probably due to the presence of a woody stem in which dehydration-induced xylem embolisms slowed the rate of recovery. The poikilochlorophyllous species X. iscosa took the longest to recover because it took longer to reconstitute the chloroplasts and the photosynthetic pigments. Quantum efficiency recovered in all species before water content and chlorophyll content recovered to control levels. The significance of these different responses to desiccation and recovery from desiccation is discussed Annals of Botany Company Key words: Desiccation-tolerant, F V, homoiochlorophyllous, poikilochlorophyllous, chlorophyll, chloroplast, ultrastructure, Craterostigma wilmsii, Myrothamnus flabellifolia, Xerophyta iscosa, Pisum sati um. INTRODUCTION While the vegetative tissues of most angiosperm species cannot survive severe water stress there are a few species which do tolerate desiccation. These plants are commonly known as resurrection plants, due to their unique ability to revive from an air-dried state (Gaff, 1971, 1989). Two types of desiccation-tolerant angiosperms have been recognized: those that lose chlorophyll on drying (poikilochlorophyllous) and those that retain chlorophyll (homoiochlorophyllous) (Gaff, 1977; Bewley, 1979; Tuba et al., 1993). To date there have been no studies reported which have compared the desiccation responses of these two types of plant. It has been suggested that there are several challenges which have to be overcome if a plant tissue is to be truly tolerant of desiccation (Vertucci and Farrant, 1995). These include the ability to: (a) minimize mechanical damage associated with turgor loss; (b) maintain the integrity of macromolecules and membranes by the accumulation of water stress proteins (dehydrins) and compatible solutes; and (c) minimize toxin accumulation and free radical damage generated as metabolism becomes impaired. Upon rehydration, such plant tissues must be able to repair desiccation-induced damage. It has been proposed that different plants will rely on the processes of protection and repair to differing extents (Bewley and Oliver, 1992). Most of the studies on desiccation tolerance have focused on how plants survive in the dry state, while less work has been done * For correspondence $25 on the processes occurring during rehydration. During rehydration the passage to a metabolically active state poses particular problems if metabolic mayhem is to be avoided (Stewart, 199). Too rapid rehydration has been shown to cause damage to dry seeds (Simon, 1974; Hoekstra and van der Wal, 1988). While rehydration causes its own set of challenges to the desiccation-tolerant plant, it is also the period when repair of physical damage and the restoration of metabolic activity must take place. Thus, in studying the phenomenon of desiccation tolerance, the study of the processes occurring on rehydration are important. Studies on the rehydration of desiccation-tolerant mosses, ferns and angiosperms (reviewed in Bewley, 1979; Bewley and Krochko, 1982; Gaff, 1989) have shown that recovery is rapid and that, in general, it is quicker in homoiochlorophyllous than in poikilochlorophyllous plants. Ultrastructural studies on the former have shown that some damage does occur on drying but this is repaired on rehydration (Bewley, 1979; Schneider, et al., 1993; Sherwin, 1995). In Craterostigma nanum, chlorophyll fluorescence parameters were almost fully recovered after 18 h of rehydration (Sherwin, 1995). In the poikilochlorophyllous types examined to date, there were extensive changes to chloroplast structure which can take up to 3 d after rehydration to return to normal (Hetherington, Hallam and Smillie, 1982; Gaff, 1989; Tuba et al., 1993). At least 2 d are required for normal fluorescence characteristics (Hetherington et al., 1982; Tuba et al., 1994). Studies on rehydration of excised leaves of the poikilochlorophyllous Xerophyta scabrida (Tuba et al., 1994) have shown that the leaves take approximately 24 h to rehydrate, after which chlorophyll 1996 Annals of Botany Company
2 74 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species production increases. In all species examined, carbon assimilation reached a maximum after chlorophyll production was complete and chloroplast ultrastructure regained a normal appearance. Studies done to date have used either whole plants or excised leaf parts, making comparisons difficult. Furthermore, the rehydration studies reported have generally only used isolated leaves. Unpublished work from our laboratory has indicated that rehydration of isolated leaves does not result in the full recovery of photosynthetic activity. The aim of this work was to compare the rehydration of whole plants of three different desiccation-tolerant plant species and compare those to the rehydration of a desiccation-sensitive plant, examining specifically the changes occurring to the photosynthetic apparatus. The three tolerant species chosen differed in form and in their physical and physiological response to desiccation. Craterostigma wilmsii Engl. (Scrophulariaceae) is a small herbaceous plant that curls its leaves tightly on drying and is homoiochlorophyllous. Myrothamnus flabellifolia Welw. (Myrothamnaceae) is a woody shrub often reaching heights of 1 m. On dehydration, the leaves fold against the stem and chlorophyll is retained in the adaxial mesophyll layers. The outer, abaxial surfaces become dark brownish-red. Xerophyta iscosa Baker (Velloziaceae), a monocotyledonous plant, is poikilochlorophyllous; the leaves yellow and fold in half along the main rib on drying. The desiccationsensitive plant Pisum sati um L. (Fabaceae) was used as a control to establish differences between the tolerant plants and one sensitive to desiccation. MATERIALS AND METHODS Plants which had been dried to 5% relative water contents (RWC) were allowed to rehydrate and their recovery was monitored. Measurements of electrolyte leakage were performed to assess membrane integrity and thus viability. Changes in pigment content were determined and chloroplast status was assessed in terms of quantum efficiency and subcellular organization. Plant material The desiccation-tolerant plants were collected from the Buffelskloof Nature Reserve near Lydenberg (Mpumalanga Province, South Africa). They were grown in a mixture of peat, river sand and potting soil and were maintained in a greenhouse under 3% shadecloth with no supplementary lighting. To maintain the hardiness of the plants, they were subject to regular cycles of desiccation and rewatering. Seeds of P. sati um (cv. Greenfeast) were sown in trays of potting soil and the material was kept in a greenhouse under the same conditions as the other plants. Plants were dried after 3 weeks of growth. Dehydration and rehydration Whole plants of the four species were dried by withholding water and allowing the plant to dry out naturally. Once no more water was lost from the leaves (RWCs 5%) the plants were left in the dry state exposed to natural sunlight for between 1 and 3 months. Rehydration was carried out by watering the plants using a spray to simulate rainfall. Plants were well watered on the first day and then the soil was kept damp for the remainder of the experiment. P. sati um leaves did not rehydrate upon soil watering. Rehydration was achieved by placing excised leaves directly into water. At regular intervals during the rehydration process, the procedures outlined below were performed on leaf tissues. In the case of C. wilmsii only the middle leaves of the rosette were used and for M. flabellifolia and X. iscosa mature, fully expanded leaves were used. The two youngest fully expanded leaves were used in the case of P. sati um. Several separate rehydration experiments were conducted (four for C. wilmsii; three for M. flabellifolia; two for X. iscosa; and five for P. sati um) and in each case five leaf samples were taken for each of the experimental procedures. Electrolyte leakage Leakage measurements were performed on desiccated and fully rehydrated leaves of each species. As a control, the leakage of leaves which had not been desiccated and rehydrated was also monitored. The leaves (M. flabellifolia) or leaf pieces (C. wilmsii, X. iscosa and P. sati um) were placed directly into pure (distilled, de-ionised and filtered) water and electrolyte leakage was measured every minute for 4 min using a conductivity meter (Jenway 47). The rate of leakage was calculated (the slope of line generated from the time course of leakage) and was corrected by leaf dry mass. Chlorophyll fluorescence A modulated portable fluorometer (OS-5: Opti- Sciences, USA) was used to calculate the quantum efficiency of leaves at various stages of hydration. The initial fluorescence, F O, and maximum fluorescence, F M, using a saturating light intensity of approximately 4 mmol photons m s ) and a duration of 1 s, were measured. F V was obtained by subtracting F O from F M and F V was calculated. Chlorophyll and carotenoid content Photosynthetic pigments were extracted in 1% acetone and the absorbances were measured using a Schimadzu UV- 221 light UV spectrophotometer (Schimadzu Scientific Instruments Inc., USA). Chlorophyll and carotenoid contents were calculated using the adjusted extinction coefficients according to Lichtenthaler (1987). Ultrastructure Leaf segments (approximately 5 mm ) were excised randomly from fully turgid leaves (which had not undergone any dehydration) as well as leaves in the dry and partially rehydrated state (approximately 5% RWC). These were fixed in 2 5% glutaraldehyde in 1 M phosphate buffer
3 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species 75 (ph 7 2) containing 5% caffeine. Post-fixation was in 1% osmium tetroxide in phosphate buffer. Following ethanol dehydration, the material was infiltrated and embedded in epoxy resin (Spurr, 1969). The tissue was sectioned at a gold interference colour (approximately 75 nm thick) using a Reichert Ultracut-S microtome. Sections were stained with uranyl acetate and lead citrate (Reynolds, 1963) and viewed with a Jeol 2CX transmission electron microscope. Observations were made on at least three sections of three different leaf samples. RESULTS Figure 1 shows the time course of rehydration of the four species. The desiccation-sensitive P. sati um did not rehydrate at all while the three desiccation-tolerant plants Water content (% of control) Rehydration time (h) FIG. 1. Increase in water content with time after rehydration. The water content is represented as a percentage of the control value. The plotted data points are means of at least ten measurements and the standard deviations of these means are represented by the vertical lines. ( ) C. wilmsii, ( )M.flabellifolia, ( )X. iscosa and ( ) P. sati um. Electrolyte conductivity (% of control) a a a C. wilmsii a b a M. flabellifolia a c a X. viscosa P. sativum FIG. 2. Changes in electrolyte leakage (as a percentage of the control) in the four species. Control ( ) levels were taken from fully turgid leaves which had not been dehydrated or rehydrated. These were compared with levels of dehydrated ( ) tissue and tissue that had been rehydrated ( ) to full water content. Differences between letters indicate significant differences (Anova and Duncans Multiple Range test, 95% confidence level) in leakage between treatments within and among species. a d e 8 regained full turgor. Leaves of C. wilmsii were fully hydrated by 48 h after rewatering while those of M. flabellifolia took 65 h and X. iscosa 92 h. Both C. wilmsii and X. iscosa rehydrated at relatively constant rates. The more rapid rehydration in C. wilmsii was presumably because it was smaller and is herbaceous. In M. flabellifolia, water uptake during the initial 12 h was slow, but thereafter the rate of rehydration increased and was similar to that of C. wilmsii. M. flabellifolia is a woody plant, which probably accounts for this pattern of water uptake. Once the xylem system became functional (presumably after 12 h) the rate of uptake increased. There was no significant difference in the electrolyte leakage rate among the control, dry and rehydrated leaves of C. wilmsii (Fig. 2), suggesting that the integrity of the plasma membrane was maintained during both drying and rehydration of this species. Leakage in the dry state was greater in X. iscosa than in M. flabellifolia, but in both species leakage returned to control levels on rehydration. Thus drying appeared to cause some change in membrane configuration, but this was reversed and or repaired on rehydration of those species. In contrast to the tolerant species, dry leaves of P. sati um showed a significant increase in electrolyte leakage compared to the control and there was a further substantial increase in leakage upon rehydration of these leaves (Fig. 2). Thus, the desiccationinduced membrane changes were not reversible on rehydration in this species. The changes in quantum efficiency (F V ) with rehydration are shown in Fig. 3A. The desiccation-tolerant species all showed complete recovery of this variable, indicating that chloroplasts became fully functional upon rehydration. C. wilmsii recovered most rapidly, reaching control levels by 32 h. In M. flabellifolia and X. iscosa the rate of recovery was similar, but the latter took longer for the quantum efficiency to return to control levels (76 h in X. iscosa cf. 55 h in M. flabellifolia). In each case, F V recovered to control levels before water content had reached maximum levels. P. sati um did not recover photosynthetic capacity (Fig. 3A). Figure 3B shows the changes in chlorophyll content during rehydration. X. iscosa lost almost all chlorophyll on drying; very little was reconstituted within the first 24 h but thereafter chlorophyll content increased, reaching control levels after 12 h. C. wilmsii and M. flabellifolia lost 5 and 7% of leaf chlorophyll, respectively. The chlorophyll content of M. flabellifolia recovered rapidly and reached control levels after 24 h. The chlorophyll content of C. wilmsii recovered almost to control levels after 45 h. Thus M. flabellifolia recovered chlorophyll content to control levels before F V had recovered fully. On the contrary, C. wilmsii and X. iscosa recovered F V before their chlorophyll contents had fully recovered. As with the other variables P. sati um showed no recovery of the 75% of chlorophyll that it lost on drying. The carotenoid content declined during dehydration of M. flabellifolia, X. iscosa and P. sati um whereas C. wilmsii retained its carotenoid levels (Fig. 3C). Carotenoid levels declined by 6% in X. iscosa and, as with chlorophyll production, there was a 24 h delay before recovery of those
4 76 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species 11 A F v /F m (% of control) Total chlorophyll (% of control) Total carotenoids (% of control) B C Rehydration time (h) FIG. 3. Changes in the quantum efficiency (F V ) (A), chlorophyll (B) and carotenoid contents (C) with time after rehydration. F V, chlorophyll and carotenoid contents are represented as a percentage of the control value. The plotted data points are means of at least ten measurements and the standard deviations of these means are represented by the vertical lines. ( ) C. wilmsii, ( )M.flabellifolia, ( ) X. iscosa and ( ) P. sati um. 8 pigments commenced. Total recovery occurred after 96 h. In M. flabellifolia, the recovery of carotenoid levels was slower (72 h) than the recovery of chlorophyll (24 h). P. sati um showed no recovery of carotenoids on rehydration. Figures 4 7 show the chloroplast organization from control plants and those from dry and partially rehydrated leaves. The use of standard fixation techniques might have allowed some rehydration of the desiccated leaves. However, as all species were fixed in the same manner, comparisons between species are valid. A study using vapour fixation of dried leaves of M. flabellifolia (Goldsworthy and Drennan, 1991) gave very similar results to those observed in the present study. FIG. 4. Micrographs of the chloroplasts of C. wilmsii at full turgor (control) (A) 2, in the dry state (B), 172 and after 12 h of rehydration (C), L, Lipophilic bodies; S, starch. Chloroplasts from control leaves of C. wilmsii were typical of hydrated, photosynthetically active tissues. Thylakoid membranes were clearly defined and some starch was present (Fig. 4A). In the dry state chloroplasts became rounded, the internal membranes were located peripherally and no starch was evident. The boundary membranes were intact and thylakoid stacks were still clearly visible, although
5 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species 77 FIG. 5. Micrographs of the chloroplasts of M. flabellifolia at full turgor (A,B), 2 and 59, respectively, in the dry state (C), 165, after 12 h of hydration (D), 165 and after 24 h of rehydration (E) 177. The staircase arrangement of thylakoids is shown in the inset (Fig. 5B). Note the swollen nature of the thylakoids in rehydrating tissue (Fig. 5D). there was a small degree of blistering or separation of the appressed membranes (Fig. 4B). After 12 h of rehydration (6% RWC and 8% recovery of F V ), the chloroplasts resembled those in the dry state, although they were more oval than rounded (Fig. 4C). Upon further hydration the chloroplasts progressively assumed the shape and thylakoid distribution typical of control tissue (not shown). Lipophilic bodies were present at all stages of dehydration and rehydration. Chloroplasts from control plants of M. flabellifolia were typically elongated with well defined thylakoid membranes and starch was present (Fig. 5A). The thylakoid membranes had a unique form of stacking (Fig. 5B) referred to as a staircase arrangement (Wellburn and Wellburn, 1976). They suggested that such an arrangement allows a more effective presentation of photosynthetically active membranes to incoming radiation. This feature is not specific to resurrection species in general, as it has not been found in other resurrection species such as Craterostigma spp. or Xerophyta spp. (Tuba et al., 1993; Sherwin, 1995). In the dry state the chloroplasts appeared ovate, the stroma stained darkly and, while the thylakoids retained their staircase arrangement, they had a blistered appearance. There was no starch present (Fig. 5C). After 12 h of rehydration (2% RWC and 4% recovery of F V ) the appearance of the chloroplasts was very unusual. They had become rounded and the appressed membranes of the thylakoids appeared to have pulled apart (Fig. 5D). This honeycomb appearance of the thylakoids was lost between 18 and 24 h of rehydration. After 24 h (6% RWC and 6% recovery of F V ) the staircase arrangement of the thylakoids was evident, although electron transparent regions were still present within the stroma (Fig. 5E). Like C. wilmsii and M. flabellifolia, chloroplasts from the control plants of X. iscosa were elongated and contained starch. There were few granal layers in this species (Fig. 6A). In the dry state the chloroplasts were rounded and while the outer membranes remained continuous, the internal membranes lost their original conformation and appeared vesiculated (Fig. 6B). An unusual double-membraned structure became evident in virtually all chloroplasts examined (arrowed, Fig. 6B). The origin and function of this structure is unknown, but may have resulted from invagination and vesiculation of the outer boundary membrane. At this stage, the plant had lost more than 9% of its chlorophyll. After 24 h of rehydration (6% RWC) the internal membranes were less vesiculated and the circular membranous structure evident in the dry state was not present (Fig. 6C). At this stage less than 1% of the chlorophyll had been recovered yet there was a 6% recovery of F V. After 48 h of rehydration (8% RWC, 5% chlorophyll recovery and 9% recovery of F V ) the
6 78 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species FIG. 6. Micrographs of the chloroplasts of X. iscosa at full turgor (A), 2; in the dry state (B), 19; after 24 h of rehydration (C), 27; and after 48 h of rehydration (D), 24. Note the circular membranous structure within chloroplasts in the dry state (arrows, Fig. 6B) and RER associated with outer membranes of the chloroplasts in rehydrating tissues (Fig. 6C and D). RER, rough endoplasmic reticulum. thylakoid membranes had reassembled (Fig. 6D). Strands of rough endoplasmic reticulum (RER) and or polysomes were present in close association with the boundary membranes of the chloroplasts (Fig. 6A D). As in C. wilmsii, lipophilic bodies were present at all stages. Figure 7 shows details of chloroplasts of P. sati um in leaves at full turgor (Fig. 7A), in the dry state (Fig. 7B) and after rehydration of individual leaves (Fig. 7C). In the dry state the chloroplasts became rounded and the thylakoids, while clearly visible and still arranged in stacks, were blistered. After rehydration it was difficult to distinguish the chloroplasts. Although in some instances thylakoid stacks were apparent, there was a general disruption of chloroplast membranes. DISCUSSION The results from this study show the unusual ability of three different resurrection plants to survive desiccation. It is also apparent that while these plants presumably undergo similar stresses on dehydration, there are differences in the way in which they cope with these stresses and in the nature of recovery during rehydration. The nature of the damage associated with desiccation is typified in the response of P. sati um to dehydration and rehydration. The pattern of electrolyte leakage suggests that there is irreversible damage to the plasmalemma and, while organelles such as chloroplasts appear to retain some degree of integrity on drying, there is total dissolution on rehydration. Photosynthetic pigments were lost and not recovered. Thus there appears to be no mechanism of subcellular protection operating in this species and, furthermore, there is an inability to repair the damage induced by desiccation (presumably because the repair enzymes are damaged). Thus, on drying, metabolism such as photosynthesis is disrupted (rather than interrupted) by desiccation and this cannot be restored on rehydration. Among the desiccation-tolerant species there were differences in the rate of recovery of water content and in photochemical activity. Recovery was generally more rapid in the homoiochlorophyllous species than in the poikilochlorophyllous X. iscosa, but there was also a difference in the rate of recovery between the homoiochlorophyllous types. The rate of recovery appears to depend on plant morphology and the extent of subcellular repair reconstitution required for the resumption of metabolism. C. wilmsii, being a small herbaceous plant, recovered water content very rapidly and this facilitated a rapid recovery of metabolism. Photochemical activity was restored before the plant was fully hydrated and before chlorophyll content reached control levels, suggesting that maximal
7 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species 79 FIG. 7. Micrographs of the chloroplasts of P. sati um at full turgor (A), 165; in the dry state (B), 18 and after 12 h of rehydration (C), 17. levels of these parameters are not required for full photosynthetic activity. Carotenoid levels were maintained, however, and it is possible that these were sufficient for maximal levels of photosynthesis. The rapid recovery is presumably also facilitated by the apparent ability of this plant to maintain integrity of the plasmalemma and chloroplast organization during dehydration and rehydration. Thus metabolism can resume without the delays caused by reconstituting these organelles. These results suggest that a mechanism of subcellular protection may operate during desiccation. Dehydrin proteins have been reported to be induced by drying in Craterostigma plantagineum (Piatkowski et al., 199) and Craterostigma nanum (Sherwin, 1995). The presence of such proteins in C. wilmsii may contribute towards subcellular stabilization during dehydration. The rehydration of leaves of M. flabellifolia was slower than in C. wilmsii, possibly because of the time to recover hydraulic conductance in the xylem vessels in the former. Embolisms in the xylem vessels could initially have posed a significant resistance to water uptake. Thus, photochemical activity was recovered over a longer period although, like C. wilmsii, this occured before the plant was fully rehydrated. There was a delay in the resumption of photochemical activity during initial rehydration despite recovery of chlorophyll content. This is possibly due to the lack of continuity between adjacent thylakoid membranes and their reconstitution appears to be necessary for full recovery of photosynthetic activity. Increased levels of electrolyte leakage occurred upon desiccation, suggesting that some reconstitution of plasma membranes is required during rehydration. Thus repair reconstitution of subcellular organization appears to play a more significant role in the recovery of this species compared to C. wilmsii. However, considering the degree of subcellular integrity maintained and the relatively rapid recovery of this plant on rehydration, there must also be some mechanism of protection. Drennan et al. (1993) reported an increase in the levels of trehalose and Bianchi et al. (1993) increases in the levels of sucrose, arbutin and glucopyranosyl-β-glycerol on desiccation of this species, which may contribute to subcellular stabilization. There is to date no information on the presence of dehydrins in M. flabellifolia. Recovery of turgor and photochemical activity was slowest in X. iscosa. While slow water uptake must delay metabolic recovery, this species showed the greatest degree of membrane leakage and almost total loss of photosynthetic apparatus. The greater degree of subcellular reorganization required on rehydration of this species could account for the longer time taken for its recovery compared to the homoiochlorophyllus types. There is no information on the nature of subcellular protection in this species. In the present study, X. iscosa recovered photochemical activity before chlorophyll levels reached their pre-desiccation levels, and before full water content was attained. Tuba et al. (1994) showed that the opposite is true of X. scabrida. Those authors rehydrated isolated leaf tissues in their study, which may account for the discrepancy in results between the studies. We suggest that recovery rate is influenced by plant form (which influences the rate of hydration) and also the extent of reconstitution of subcellular organization that is required. We further suggest that the degree of reconstitution required for the photosynthetic apparatus depends on the strategies adopted to prevent light-related desiccation stresses. Removal of water from plant tissues ultimately results in interruption of metabolic pathways. One consequence of this in chlorophyllous tissue is that the energy generated from light absorbed by a chlorophyll molecule cannot be dissipated via the normal metabolic pathways. The energized electrons (which may in turn generate free radical species) can cause considerable subcellular damage. Poikilochlorophyllous plants like X. iscosa avoid such stress by losing
8 71 Sherwin and Farrant Rehydration of Three Desiccation-tolerant Species chlorophyll and dismantling the thylakoid membranes during desiccation. On rehydration the photosynthetic apparatus has to be reconstituted and this delays recovery of carbon metabolism. Homoiochlorophyllous plants retain most of the photosynthetic apparatus, but prevent lightchlorophyll interactions, for example by leaf curling in C. wilmsii and folding in M. flabellifolia. Recovery of photosynthesis can be more rapid in these species relative to poikilochlorophyllous types. ACKNOWLEDGEMENTS Thanks to John and Sandie Burrows of the Buffelskloof Nature Reserve for help in collecting the plant material. Heather Sherwin acknowledges the Foundation for Research Development (FRD) for a post-doctoral bursary. The project was funded by an FRD grant awarded to Jill Farrant. LITERATURE CITED Bewley JD Physiological aspects of desiccation tolerance. Annual Re iew of Plant Physiology 3: Bewley JD, Krochko JE Desiccation-tolerance. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological plant ecology II. Water and carbon assimilation. Encyclopedia of plant physiology. N.S. Vol. 12B. Berlin: Springer-Verlag, Bewley JD, Oliver MJ Desiccation tolerance in vegetative plant tissues and seeds: protein synthesis in relation to desiccation and a potential role for protection and repair mechanisms. In: Somero GN, Osmond CB, Bolis CL, eds. Water and life: Comparati e analysis of water relationships at the organismic, cellular and molecular le els. Heidelberg: Springer-Verlag, Bianchi G, Gamba A, Limiroli R, Pozzi N, Elster Rl, Salamini F, Bartels D The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiologia Plantarum 87: Drennan PM, Smith MT, Goldsworthy D-A, van Staden J The occurrence of trehalose in leaves of the desiccation tolerant angiosperm Myrothamnus flabellifolia Welw. Journal of Plant Physiology 142: Gaff DF Desiccation tolerant flowering plants in southern Africa. Science 174: Gaff DF Desiccation tolerant vascular plants of southern Africa. Oecologia 31: Gaff DF Responses of desiccation tolerant resurrection plants to water stress. In: Kreeb KH, Richter H, Hinckley TM, eds. Structural and functional responses to en ironmental stresses: water shortages. The Hague, Netherlands: SPB Academic Publishing, Goldsworthy D-A, Drennan PM Anhydrous fixation of desiccated leaves of Myrothamnus flabellifolia Welw. Electron Microscopy Society of South Africa 21: Hetherington SE, Hallam ND, Smillie RM Ultrastructural and compositional changes in chloroplast thylakoids of leaves of Borya nitida during humidity-sensitive degreening. Australian Journal of Plant Physiology 9: Hoekstra FA, van der Wal EW Initial moisture content and temperature of imbibition determine extent of imbibitional injury in pollen. Journal of Plant Physiology 133: Lichtenthaler HK Chlorophylls and carotenoids, the pigments of the photosynthetic biomembranes. Methods in Enzymology 148: Piatkowski D, Schneider K, Salamini F, Bartels D Characterisation of five abscisic acid-responsive cdna clones isolated from the desiccation-tolerant plant Craterostigma plantagineum and their relationship to other water-stress genes. Plant Physiology 94: Reynolds ES The use of lead citrate at high ph as an electron opaque stain for electron microscopy. Journal of Cell Biology 17: Schneider K, Wells B, Schmelzer E, Salamini F, Bartels D Desiccation leads to the rapid accumulation of both cytosolic and chloroplastic proteins in the resurrection plant Craterostigma plantagineum Hochst. Planta 189: Sherwin HW Desiccation tolerance and sensitivity of vegetative plant tissue. PhD thesis, University of Natal, Durban, South Africa. Simon EW Phospholipids and plant membrane permeability. New Phytologist 73: Spurr AR A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: Stewart GR Desiccation injury, anhydrobiosis and survival. In: Jones HG, Flowers TJ, Jones MB, eds. Plants under stress: biochemistry, physiology and ecology and their application to plant impro ement. Cambridge: Cambridge University Press, Tuba Z, Lichtenthaler HK, Maroti I, Csintalan Z Resynthesis of thylakoids and functional chloroplasts in the desiccated leaves of the poikilochlorophyllous plant Xerophyta scabrida upon rehydration. Journal of Plant Physiology 142: Tuba Z, Lichtenthaler HK, Csintalan Z, Nagy Z, Szente K Reconstitution of chlorophylls and photosynthetic CO assimilation upon rehydration of the desiccated poikilochlorophyllous plant Xerophyta scabrida (Pax) Th. Dur. et Schinz. Planta 192: Vertucci CW, Farrant JM Acquisition and loss of desiccation tolerance. In: Kigel J, Galilli G, eds. Seed de elopment and germination. New York: Marcel Dekker, Inc., Wellburn FAM, Wellburn AR Novel chloroplasts and unusual cellular ultrastructure in the resurrection plant Myrothamnus flabellifolia Welw. (Myrothamnaceae). Botanical Journal of the Linnean Society 72:
Which regions of the electromagnetic spectrum do plants use to drive photosynthesis?
 Which regions of the electromagnetic spectrum do plants use to drive photosynthesis? Green Light: The Forgotten Region of the Spectrum. In the past, plant physiologists used green light as a safe light
Which regions of the electromagnetic spectrum do plants use to drive photosynthesis? Green Light: The Forgotten Region of the Spectrum. In the past, plant physiologists used green light as a safe light
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS. II. Ultrastructure of the Beta Granule
 ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
ISOLATION AND PROPERTIES OF SECRETORY GRANULES FROM RAT ISLETS OF LANGERHANS II Ultrastructure of the Beta Granule MARIE H GREIDER, S L HOWELL, and P E LACY From the Department of Pathology, Washington
Photosynthesis and Cellular Respiration. Stored Energy
 Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Photosynthesis and Cellular Respiration Stored Energy What is Photosynthesis? plants convert the energy of sunlight into the energy in the chemical bonds of carbohydrates sugars and starches. SUMMARY EQUATION:
Review Questions Photosynthesis
 Review Questions Photosynthesis 1. Describe a metabolic pathway. In a factory, labor is divided into small individual jobs. A carmaker, for example, will have one worker install the front windshield, another
Review Questions Photosynthesis 1. Describe a metabolic pathway. In a factory, labor is divided into small individual jobs. A carmaker, for example, will have one worker install the front windshield, another
Name Date Period PHOTOSYNTHESIS HW REVIEW ENERGY AND LIFE
 1 Name Date Period PHOTOSYNTHESIS HW REVIEW ENERGY AND LIFE MULTIPLE CHOICE: CIRCLE ALL THE ANSWERS THAT ARE TRUE. THERE MAY BE MORE THAN ONE CORRECT ANSWER! 1. Which molecule stores more than 90 times
1 Name Date Period PHOTOSYNTHESIS HW REVIEW ENERGY AND LIFE MULTIPLE CHOICE: CIRCLE ALL THE ANSWERS THAT ARE TRUE. THERE MAY BE MORE THAN ONE CORRECT ANSWER! 1. Which molecule stores more than 90 times
Question. Which of the following are necessary in order for photosynthesis to occur? A. water B. light energy C. carbon dioxide D.
 Photosynthesis is the process through which plants convert light energy to chemical energy in order to produce food The energy involved in photosynthesis is eventually stored in the chemical bonds of molecules
Photosynthesis is the process through which plants convert light energy to chemical energy in order to produce food The energy involved in photosynthesis is eventually stored in the chemical bonds of molecules
Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis?
 Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis? Photosynthesis: synthesis from light The broad outline: Plants take in CO 2 and release water and O 2 Light is required
Like The Guy From Krypton Photosynthesis: Energy from Sunlight What Is Photosynthesis? Photosynthesis: synthesis from light The broad outline: Plants take in CO 2 and release water and O 2 Light is required
Electron Transport Generates a Proton Gradient Across the Membrane
 Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
Electron Transport Generates a Proton Gradient Across the Membrane Each of respiratory enzyme complexes couples the energy released by electron transfer across it to an uptake of protons from water in
1. f. Students know usable energy is captured from sunlight by chloroplasts and is stored through the synthesis of sugar from carbon dioxide.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Jan Baptisa van Helmont (1648)
 Instructions To help you navigate these slides, you should set your viewer to display thumbnails of these slides. On many viewers, this can be done by pressing the F4 key. The slides should be viewed in
Instructions To help you navigate these slides, you should set your viewer to display thumbnails of these slides. On many viewers, this can be done by pressing the F4 key. The slides should be viewed in
Unit 5 Photosynthesis and Cellular Respiration
 Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
Unit 5 Photosynthesis and Cellular Respiration Advanced Concepts What is the abbreviated name of this molecule? What is its purpose? What are the three parts of this molecule? Label each part with the
- 1 - BISC 367 Plant Physiology Laboratory SFU
 - 1 - BISC 367 Plant Physiology Laboratory SFU CO 2 exchange in plants The effect of light intensity and quality on photosynthetic CO 2 fixation and respiration in C3 and C4 plants Using light energy and
- 1 - BISC 367 Plant Physiology Laboratory SFU CO 2 exchange in plants The effect of light intensity and quality on photosynthetic CO 2 fixation and respiration in C3 and C4 plants Using light energy and
> C 6 H 12 O 6 + 6O 2
 Photosynthesis- is the process that converts light energy into chemical energy. This chemical energy is usually a carbohydrate. Only photoautrotrops can do photosynthesis. Heterotrophs must obtain their
Photosynthesis- is the process that converts light energy into chemical energy. This chemical energy is usually a carbohydrate. Only photoautrotrops can do photosynthesis. Heterotrophs must obtain their
2. PHOTOSYNTHESIS. The general equation describing photosynthesis is light + 6 H 2 O + 6 CO 2 C 6 H 12 O 6 + 6 O 2
 2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
2. PHOTOSYNTHESIS Photosynthesis is the process by which light energy is converted to chemical energy whereby carbon dioxide and water are converted into organic molecules. The process occurs in most algae,
PLANT PHYSIOLOGY. Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP-4.1.2-08/1/A-2009-0010
 PLANT PHYSIOLOGY Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP-4.1.2-08/1/A-2009-0010 The light reactions of the photosynthesis Photosynthesis inhibiting herbicides Overview 1. Photosynthesis, general
PLANT PHYSIOLOGY Az Agrármérnöki MSc szak tananyagfejlesztése TÁMOP-4.1.2-08/1/A-2009-0010 The light reactions of the photosynthesis Photosynthesis inhibiting herbicides Overview 1. Photosynthesis, general
CELLS: PLANT CELLS 20 FEBRUARY 2013
 CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
CELLS: PLANT CELLS 20 FEBRUARY 2013 Lesson Description In this lesson we will discuss the following: The Cell Theory Terminology Parts of Plant Cells: Organelles Difference between plant and animal cells
Photosynthesis: Harvesting Light Energy
 Photosynthesis: Harvesting Light Energy Importance of Photosynthesis A. Ultimate source of energy for all life on Earth 1. All producers are photosynthesizers 2. All consumers and decomposers are dependent
Photosynthesis: Harvesting Light Energy Importance of Photosynthesis A. Ultimate source of energy for all life on Earth 1. All producers are photosynthesizers 2. All consumers and decomposers are dependent
4.1 Chemical Energy and ATP. KEY CONCEPT All cells need chemical energy.
 4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP Molecules in food store chemical energy in their bonds. Starch molecule Glucose molecule The chemical
4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP Molecules in food store chemical energy in their bonds. Starch molecule Glucose molecule The chemical
Lecture 7 Outline (Ch. 10)
 Lecture 7 Outline (Ch. 10) I. Photosynthesis overview A. Purpose B. Location II. The light vs. the dark reaction III. Chloroplasts pigments A. Light absorption B. Types IV. Light reactions A. Photosystems
Lecture 7 Outline (Ch. 10) I. Photosynthesis overview A. Purpose B. Location II. The light vs. the dark reaction III. Chloroplasts pigments A. Light absorption B. Types IV. Light reactions A. Photosystems
A. Incorrect! No, while this statement is correct, it is not the best answer to the question.
 Biochemistry - Problem Drill 18: Photosynthesis No. 1 of 10 1. What is photosynthesis? Select the best answer. (A) Photosynthesis happens in the chloroplasts. (B) Light absorption by chlorophyll induces
Biochemistry - Problem Drill 18: Photosynthesis No. 1 of 10 1. What is photosynthesis? Select the best answer. (A) Photosynthesis happens in the chloroplasts. (B) Light absorption by chlorophyll induces
Photosynthesis Part I: Overview & The Light-Dependent Reactions
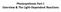 Photosynthesis Part I: Overview & The Light-Dependent Reactions Photosynthesis: The BIG Picture Photosynthesis is the process by which PHOTOAUTOTROPHS convert the energy in SUNLIGHT into the energy stored
Photosynthesis Part I: Overview & The Light-Dependent Reactions Photosynthesis: The BIG Picture Photosynthesis is the process by which PHOTOAUTOTROPHS convert the energy in SUNLIGHT into the energy stored
Biology. Slide 1of 51. End Show. Copyright Pearson Prentice Hall
 Biology 1of 51 8-3 The Reactions of Photosynthesis 2of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3of 51 Inside
Biology 1of 51 8-3 The Reactions of Photosynthesis 2of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3of 51 Inside
3.1 AS Unit: Cells, Exchange and Transport
 3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
3.1 AS Unit: Cells, Exchange and Transport Module 1: Cells 1.1.1 Cell Structure Candidates should be able to: (a) state the resolution and magnification that can be achieved by a light microscope, a transmission
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Photosynthesis 6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2. An anabolic, endergonic, carbon dioxide (CO 2
 PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
Photosynthesis. Photosynthesis: Converting light energy into chemical energy. Photoautotrophs capture sunlight and convert it to chemical energy
 Photosynthesis: Converting light energy into chemical energy Photosynthesis 6 + 12H 2 O + light energy Summary Formula: C 6 H 12 O 6 + 6O 2 + 6H 2 O 6 + 6H 2 O C 6 H 12 O 6 + 6O 2 Photosythesis provides
Photosynthesis: Converting light energy into chemical energy Photosynthesis 6 + 12H 2 O + light energy Summary Formula: C 6 H 12 O 6 + 6O 2 + 6H 2 O 6 + 6H 2 O C 6 H 12 O 6 + 6O 2 Photosythesis provides
Name Date Class. energy phosphate adenine charged ATP chemical bonds work ribose
 Energy in a Cell Reinforcement and Study Guide Section.1 The Need for Energy In your textbook, read about cell energy. Use each of the terms below just once to complete the passage. energy phosphate adenine
Energy in a Cell Reinforcement and Study Guide Section.1 The Need for Energy In your textbook, read about cell energy. Use each of the terms below just once to complete the passage. energy phosphate adenine
Photosynthesis Chapter 8 E N E R G Y T O M A K E F O O D?
 Photosynthesis Chapter 8 H O W D O E S T H E P L A N T U S E T H E S U N S E N E R G Y T O M A K E F O O D? http://www.youtube.com/watch?v=pe82qtkssh4 Autotroph vs. Heterotroph Autotrophs/Producers-organisms
Photosynthesis Chapter 8 H O W D O E S T H E P L A N T U S E T H E S U N S E N E R G Y T O M A K E F O O D? http://www.youtube.com/watch?v=pe82qtkssh4 Autotroph vs. Heterotroph Autotrophs/Producers-organisms
pathway that involves taking in heat from the environment at each step. C.
 Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Study Island Cell Energy Keystone Review 1. Cells obtain energy by either capturing light energy through photosynthesis or by breaking down carbohydrates through cellular respiration. In both photosynthesis
Photosynthesis Practice. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best.
 Photosynthesis Practice Fill in the blanks. Name Date Period 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main
Photosynthesis Practice Fill in the blanks. Name Date Period 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main
Cytology. Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells.
 CYTOLOGY Cytology Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells. A. two major cell types B. distinguished by structural organization See table on handout for differences.
CYTOLOGY Cytology Living organisms are made up of cells. Either PROKARYOTIC or EUKARYOTIC cells. A. two major cell types B. distinguished by structural organization See table on handout for differences.
Localisation of polyphenol oxidase activity in avocado fruit
 South African Avocado Growers Association Yearbook 1987. 10:138-140 Proceedings of the First World Avocado Congress Localisation of polyphenol oxidase activity in avocado fruit AHP ENGELBRECHT Department
South African Avocado Growers Association Yearbook 1987. 10:138-140 Proceedings of the First World Avocado Congress Localisation of polyphenol oxidase activity in avocado fruit AHP ENGELBRECHT Department
Photosynthesis and (Aerobic) Respiration. Photosynthesis
 Photosynthesis and (Aerobic) Respiration These two processes have many things in common. 1. occur in organelles that seem to be descended from bacteria (endosymbiont theory): chloroplasts and mitochondria
Photosynthesis and (Aerobic) Respiration These two processes have many things in common. 1. occur in organelles that seem to be descended from bacteria (endosymbiont theory): chloroplasts and mitochondria
Transpiration. C should equal D.BUT SOMETIMES. 1. Loss in mass is greater than volume of water added.
 Transpiration Transpiration is the loss of water by evaporation from the leaves through the stomata. The source of water for the plants is soil water. It is taken up by root hair cells by osmosis. Once
Transpiration Transpiration is the loss of water by evaporation from the leaves through the stomata. The source of water for the plants is soil water. It is taken up by root hair cells by osmosis. Once
Chapter 2: Cell Structure and Function pg. 70-107
 UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
UNIT 1: Biochemistry Chapter 2: Cell Structure and Function pg. 70-107 Organelles are internal structures that carry out specialized functions, interacting and complementing each other. Animal and plant
Photosynthesis Reactions. Photosynthesis Reactions
 Photosynthesis Reactions Photosynthesis occurs in two stages linked by ATP and NADPH NADPH is similar to NADH seen in mitochondria; it is an electron/hydrogen carrier The complete process of photosynthesis
Photosynthesis Reactions Photosynthesis occurs in two stages linked by ATP and NADPH NADPH is similar to NADH seen in mitochondria; it is an electron/hydrogen carrier The complete process of photosynthesis
Introduction to the Cell: Plant and Animal Cells
 Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Introduction to the Cell: Plant and Animal Cells Tissues, Organs, and Systems of Living Things Cells, Cell Division, and Animal Systems and Plant Systems Cell Specialization Human Systems All organisms
Chapter 36: Resource Acquisition & Transport in Vascular Plants
 Chapter 36: Resource Acquisition & Transport in Vascular Plants 1. Overview of Transport in Plants 2. Transport of Water & Minerals 3. Transport of Sugars 1. Overview of Transport in Plants H 2 O CO 2
Chapter 36: Resource Acquisition & Transport in Vascular Plants 1. Overview of Transport in Plants 2. Transport of Water & Minerals 3. Transport of Sugars 1. Overview of Transport in Plants H 2 O CO 2
COMPARISON OF PLANT AND ANIMAL CELLS SIMILARITIES IN PLANT & ANIMAL CELLS
 COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
COMPARISON OF PLANT AND ANIMAL CELLS Cells vary widely in structure and function, even within the same organism. The human body, for example, has more than 200 different types of cells, each with a specialized
Photosynthesis-Review. Pigments. Chloroplasts. Chloroplasts 5. Pigments are located in the thylakoid membranes. An Overview of Photosynthesis
 An Overview of Photosynthesis Photosynthesis-Review 1. Photosynthesis uses the energy of sunlight to convert water and carbon dioxide into high-energy sugars and oxygen. 6 CO 2 + 6 H 2 O C 6 H 12 O 6 +
An Overview of Photosynthesis Photosynthesis-Review 1. Photosynthesis uses the energy of sunlight to convert water and carbon dioxide into high-energy sugars and oxygen. 6 CO 2 + 6 H 2 O C 6 H 12 O 6 +
Equation for Photosynthesis
 Photosynthesis Definition The process by which cells harvest light energy to make sugars (glucose). -Sugar is used to power the process of cellular respiration, which produces the ATP that cells utilize
Photosynthesis Definition The process by which cells harvest light energy to make sugars (glucose). -Sugar is used to power the process of cellular respiration, which produces the ATP that cells utilize
Photosynthesis January 23 Feb 1, 2013 WARM-UP JAN 23/24. Mr. Stephens, IB Biology III 1
 WARM-UP JAN 23/24 Mr. Stephens, IB Biology III 1 Photosynthesis & Cellular Respiration What is the connection between Photosynthesis and Cellular Respiration? Energy Production Inorganic Molecules Specialized
WARM-UP JAN 23/24 Mr. Stephens, IB Biology III 1 Photosynthesis & Cellular Respiration What is the connection between Photosynthesis and Cellular Respiration? Energy Production Inorganic Molecules Specialized
Chloroplasts and Mitochondria
 Name: KEY Period: Chloroplasts and Mitochondria Plant cells and some Algae contain an organelle called the chloroplast. The chloroplast allows plants to harvest energy from sunlight to carry on a process
Name: KEY Period: Chloroplasts and Mitochondria Plant cells and some Algae contain an organelle called the chloroplast. The chloroplast allows plants to harvest energy from sunlight to carry on a process
VII. NARRATION FOR PHOTOSYNTHESIS: TRANSFORMING LIGHT TO LIFE
 7. Why do leaves turn color in the fall? 8. How are photosystems I and II different? How are they related? 9. What is the source of energy for dark reactions? 10. Describe the C3 cycle. 11. What is the
7. Why do leaves turn color in the fall? 8. How are photosystems I and II different? How are they related? 9. What is the source of energy for dark reactions? 10. Describe the C3 cycle. 11. What is the
APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips
 APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips Big picture: why are we doing this? A) photosynthesis will explain shortly, b) more generally, interaction of light
APh/BE161: Physical Biology of the Cell Winter 2009 Recap on Photosynthesis Rob Phillips Big picture: why are we doing this? A) photosynthesis will explain shortly, b) more generally, interaction of light
WHAT ARE THE DIFFERENCES BETWEEN VASCULAR AND NON- VASCULAR PLANTS?
 WHAT ARE THE DIFFERENCES BETWEEN VASCULAR AND NON- VASCULAR PLANTS? Let s take a closer look. What makes them different on the outside and inside? Learning Intentions To understand how vascular plant cells
WHAT ARE THE DIFFERENCES BETWEEN VASCULAR AND NON- VASCULAR PLANTS? Let s take a closer look. What makes them different on the outside and inside? Learning Intentions To understand how vascular plant cells
8-3 The Reactions of Photosynthesis Slide 1 of 51
 8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
8-3 The of Photosynthesis 1 of 51 Inside a Chloroplast Inside a Chloroplast In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 2 of 51 Inside a Chloroplast Chloroplasts
Plants, like all other living organisms have basic needs: a source of nutrition (food),
 LEARNING FROM LEAVES: A LOOK AT LEAF SIZE Grades 3 6 I. Introduction Plants, like all other living organisms have basic needs: a source of nutrition (food), water, space in which to live, air, and optimal
LEARNING FROM LEAVES: A LOOK AT LEAF SIZE Grades 3 6 I. Introduction Plants, like all other living organisms have basic needs: a source of nutrition (food), water, space in which to live, air, and optimal
8.3 The Process of Photosynthesis
 8.3 The Process of Photosynthesis Lesson Objectives Describe what happens during the light-dependent reactions. Describe what happens during the light-independent reactions. Identify factors that affect
8.3 The Process of Photosynthesis Lesson Objectives Describe what happens during the light-dependent reactions. Describe what happens during the light-independent reactions. Identify factors that affect
Plant and Animal Cells
 Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
Plant and Animal Cells a. Explain that cells take in nutrients in order to grow, divide and to make needed materials. S7L2a b. Relate cell structures (cell membrane, nucleus, cytoplasm, chloroplasts, and
1. The leaf is the main photosynthetic factory (Fig. 36.1, p. 702)
 TRANSPORT IN PLANTS A. Introduction 1. The leaf is the main photosynthetic factory (Fig. 36.1, p. 702) a. This requires a transport system to move water and minerals from the roots to the leaf. This is
TRANSPORT IN PLANTS A. Introduction 1. The leaf is the main photosynthetic factory (Fig. 36.1, p. 702) a. This requires a transport system to move water and minerals from the roots to the leaf. This is
* Is chemical energy potential or kinetic energy? The position of what is storing energy?
 Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or stored
AP Bio Photosynthesis & Respiration
 AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
AP Bio Photosynthesis & Respiration Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the term used for the metabolic pathway in which
Soybean Physiology: How Well Do You Know Soybeans?
 Soybean Physiology: How Well Do You Know Soybeans? Shaun Casteel, Purdue University Soybean Extension Specialist www.soybeanstation.org 2010-11, Purdue University - 1 Vegetative Growth Stages Reproductive
Soybean Physiology: How Well Do You Know Soybeans? Shaun Casteel, Purdue University Soybean Extension Specialist www.soybeanstation.org 2010-11, Purdue University - 1 Vegetative Growth Stages Reproductive
Cell Structure & Function!
 Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
Cell Structure & Function! Chapter 3! The most exciting phrase to hear in science, the one that heralds new discoveries, is not 'Eureka!' but 'That's funny.! -- Isaac Asimov Animal Cell Plant Cell Cell
While reading these chapters, constantly ask yourself, How is this information helping me to understand how cells get energy from food?
 Biology 160 Reading Guide 07: Photosynthesis NAME: This is DUE: Come prepared to share your findings with your group. ** Fill this reading guide out as you are reading the chapters. This will help you
Biology 160 Reading Guide 07: Photosynthesis NAME: This is DUE: Come prepared to share your findings with your group. ** Fill this reading guide out as you are reading the chapters. This will help you
Water movement in the xylem Water moves from roots to leaves through the xylem. But how? Hypotheses: 1. Capillary action - water will move upward in
 Transport in Plants Two Transport Processes Occur in Plants 1. Carbohydrates carried from leaves (or storage organs) to where they are needed (from sources to sinks) 2. Water transported from roots to
Transport in Plants Two Transport Processes Occur in Plants 1. Carbohydrates carried from leaves (or storage organs) to where they are needed (from sources to sinks) 2. Water transported from roots to
Biology Slide 1 of 51
 Biology 1 of 51 8-3 The Reactions of Photosynthesis 2 of 51 Inside a Chloroplast 1. In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3 of 51 Inside a Chloroplast
Biology 1 of 51 8-3 The Reactions of Photosynthesis 2 of 51 Inside a Chloroplast 1. In plants, photosynthesis takes place inside chloroplasts. Plant Chloroplast Plant cells 3 of 51 Inside a Chloroplast
The Cell: Organelle Diagrams
 The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
The Cell: Organelle Diagrams Fig 7-4. A prokaryotic cell. Lacking a true nucleus and the other membrane-enclosed organelles of the eukaryotic cell, the prokaryotic cell is much simpler in structure. Only
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE. BIOL 101 Introduction to Biology
 STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE BIOL 101 Introduction to Biology Prepared By: W. David Barnes SCHOOL OF SCIENCE, HEALTH & PROFESSIONAL STUDIES SCIENCE
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK COURSE OUTLINE BIOL 101 Introduction to Biology Prepared By: W. David Barnes SCHOOL OF SCIENCE, HEALTH & PROFESSIONAL STUDIES SCIENCE
4.2 Overview of Photosynthesis
 KEY CONCEPT The overall process of photosynthesis produces sugars that store chemical energy. Radiant Energy Chemical Energy A. Organisms are classified according to how they obtain energy. 1. Autotroph/Producers
KEY CONCEPT The overall process of photosynthesis produces sugars that store chemical energy. Radiant Energy Chemical Energy A. Organisms are classified according to how they obtain energy. 1. Autotroph/Producers
Plants, like all living organisms have basic needs: a source of nutrition (food), water,
 WHAT PLANTS NEED IN ORDER TO SURVIVE AND GROW: LIGHT Grades 3 6 I. Introduction Plants, like all living organisms have basic needs: a source of nutrition (food), water, space in which to live, air, and
WHAT PLANTS NEED IN ORDER TO SURVIVE AND GROW: LIGHT Grades 3 6 I. Introduction Plants, like all living organisms have basic needs: a source of nutrition (food), water, space in which to live, air, and
Ch. 4 ATP & Photosynthesis
 Name: Biology G Vocabulary Section 4.1 Ch. 4 ATP & Photosynthesis Period: ADP Adenosine Diphosphate ATP Adenosine Triphosphate Chemosynthesis Vocabulary Section 4.2 Photosynthesis Chlorophyll Thylakoid
Name: Biology G Vocabulary Section 4.1 Ch. 4 ATP & Photosynthesis Period: ADP Adenosine Diphosphate ATP Adenosine Triphosphate Chemosynthesis Vocabulary Section 4.2 Photosynthesis Chlorophyll Thylakoid
Cellular Energy. 1. Photosynthesis is carried out by which of the following?
 Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
Cellular Energy 1. Photosynthesis is carried out by which of the following? A. plants, but not animals B. animals, but not plants C. bacteria, but neither animals nor plants D. all living organisms 2.
UNIT 1 - Living Organisms and the Environment Situations. Cells
 Lesson Summaries HUMAN AND SOCIAL BIOLOGY UNIT 1 - Living Organisms and the Environment Situations Lesson 2 Cells OBJECTIVES At the end of this lesson you will be able to: a) Describe the structure of
Lesson Summaries HUMAN AND SOCIAL BIOLOGY UNIT 1 - Living Organisms and the Environment Situations Lesson 2 Cells OBJECTIVES At the end of this lesson you will be able to: a) Describe the structure of
ecture 16 Oct 7, 2005
 Lecture utline ecture 16 ct 7, 005 hotosynthesis 1 I. Reactions 1. Importance of Photosynthesis to all life on earth - primary producer, generates oxygen, ancient. What needs to be accomplished in photosynthesis
Lecture utline ecture 16 ct 7, 005 hotosynthesis 1 I. Reactions 1. Importance of Photosynthesis to all life on earth - primary producer, generates oxygen, ancient. What needs to be accomplished in photosynthesis
Light in the Greenhouse: How Much is Enough?
 Light in the Greenhouse: How Much is Enough? by: James W. Brown http://www.cropking.com/articlelghe Most of us know that green plants need light for photosynthesis, growth, and development. As important
Light in the Greenhouse: How Much is Enough? by: James W. Brown http://www.cropking.com/articlelghe Most of us know that green plants need light for photosynthesis, growth, and development. As important
Photosynthesis. Chemical Energy (e.g. glucose) - They are the ultimate source of chemical energy for all living organisms: directly or indirectly.
 Photosynthesis Light Energy transduction Chemical Energy (e.g. glucose) - Only photosynthetic organisms can do this (e.g. plants) - They are the ultimate source of chemical energy for all living organisms:
Photosynthesis Light Energy transduction Chemical Energy (e.g. glucose) - Only photosynthetic organisms can do this (e.g. plants) - They are the ultimate source of chemical energy for all living organisms:
Investigating cells. Cells are the basic units of living things (this means that all living things are made up of one or more cells).
 SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
SG Biology Summary notes Investigating cells Sub-topic a: Investigating living cells Cells are the basic units of living things (this means that all living things are made up of one or more cells). Cells
The Cell. Grade 8 Activity Plan
 The Cell Grade 8 Activity Plan Plant Cell Project Objectives: 1. To identify cell organelles and their functions. 2. To demonstrate the difference between plant and animal cells. Keywords/concepts: cells,
The Cell Grade 8 Activity Plan Plant Cell Project Objectives: 1. To identify cell organelles and their functions. 2. To demonstrate the difference between plant and animal cells. Keywords/concepts: cells,
Green pigment that absorbs solar energy and is important in photosynthesis
 PHOTOSYNTHESIS REVIEW SHEET FOR TEST Part A: Match the terms below with the correct description Chlorophyll Chloroplast Electromagnetic spectrum Electron transport chain Grana Light-dependant reactions
PHOTOSYNTHESIS REVIEW SHEET FOR TEST Part A: Match the terms below with the correct description Chlorophyll Chloroplast Electromagnetic spectrum Electron transport chain Grana Light-dependant reactions
Overview of Photosynthesis
 OpenStax-CNX module: m44447 1 Overview of Photosynthesis OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44447 1 Overview of Photosynthesis OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman
 SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
SOME Important Points About Cellular Energetics by Dr. Ty C.M. Hoffman An Introduction to Metabolism Most biochemical processes occur as biochemical pathways, each individual reaction of which is catalyzed
COTTON WATER RELATIONS
 COTTON WATER RELATIONS Dan R. Krieg 1 INTRODUCTION Water is the most abundant substance on the Earth s surface and yet is the most limiting to maximum productivity of nearly all crop plants. Land plants,
COTTON WATER RELATIONS Dan R. Krieg 1 INTRODUCTION Water is the most abundant substance on the Earth s surface and yet is the most limiting to maximum productivity of nearly all crop plants. Land plants,
Carbon Hydrogen Oxygen Nitrogen
 Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Concept 1 - Thinking Practice 1. If the following molecules were to undergo a dehydration synthesis reaction, what molecules would result? Circle the parts of each amino acid that will interact and draw
Name Class Date. Figure 8-1
 Chapter 8 Photosynthesis Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following is an autotroph? a. mushroom
Chapter 8 Photosynthesis Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following is an autotroph? a. mushroom
CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY
 CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY 2 PROCESSES OF PHOTOSYNTHESIS Photosynthesis is actually 2 processes: light reactions - convert solar energy (sunlight) to chemical energy (ATP &
CHAPTER 6: PHOTOSYNTHESIS CAPTURING & CONVERTING ENERGY 2 PROCESSES OF PHOTOSYNTHESIS Photosynthesis is actually 2 processes: light reactions - convert solar energy (sunlight) to chemical energy (ATP &
2. 1. What are the three parts of an ATP molecule? (100 points)
 Photosynthesis Date Created: 12/8/14, 11:22:50 AM Questions: 34 Date Modified: 12/17/14, 8:27:08 AM 1. ATP & Photosynthesis Review Game 30 Multiple Choice Questions Final Question Correct Answers = +$100
Photosynthesis Date Created: 12/8/14, 11:22:50 AM Questions: 34 Date Modified: 12/17/14, 8:27:08 AM 1. ATP & Photosynthesis Review Game 30 Multiple Choice Questions Final Question Correct Answers = +$100
Reactive oxygen species in leaves and how to catch these
 Reactive oxygen species in leaves and how to catch these Éva Hideg Molecular Stress- & Photobiology Group, BRC Szeged EPPN Summer School 2013 Outline How to prove that ROS are involved? The central role
Reactive oxygen species in leaves and how to catch these Éva Hideg Molecular Stress- & Photobiology Group, BRC Szeged EPPN Summer School 2013 Outline How to prove that ROS are involved? The central role
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Plasma Membrane hydrophilic polar heads
 The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
Cell. (1) This is the most basic unit of life inside of our bodies.
 Cytology Overview Cell (1) This is the most basic unit of life inside of our bodies. ATP (2) Each of our cell s requires energy in order to carry out its day to day func>ons. This is the energy all cells
Cytology Overview Cell (1) This is the most basic unit of life inside of our bodies. ATP (2) Each of our cell s requires energy in order to carry out its day to day func>ons. This is the energy all cells
Cells. Cell Theory. plant cell. Cytoplasm and Organelles. animal cell
 Cells Have you ever seen a cell? Cells are the smallest unit of life. They are called the building blocks of life. We cannot see single cells with just our eyes. We must use a microscope to see them. Cell
Cells Have you ever seen a cell? Cells are the smallest unit of life. They are called the building blocks of life. We cannot see single cells with just our eyes. We must use a microscope to see them. Cell
Chapter 9 Review Worksheet Cellular Respiration
 1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
1 of 5 11/9/2011 8:11 PM Name: Hour: Chapter 9 Review Worksheet Cellular Respiration Energy in General 1. Differentiate an autotroph from a hetertroph as it relates to obtaining energy and the processes
Membrane Structure and Function
 Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
Membrane Structure and Function Part A Multiple Choice 1. The fluid mosaic model describes membranes as having A. a set of protein channels separated by phospholipids. B. a bilayer of phospholipids in
A B C D. Name Class Date
 Chapter 8 Photosynthesis Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following is an autotroph? a. mushroom
Chapter 8 Photosynthesis Chapter Test A Multiple Choice Write the letter that best answers the question or completes the statement on the line provided. 1. Which of the following is an autotroph? a. mushroom
Bioenergetics Module A Anchor 3
 Bioenergetics Module A Anchor 3 Key Concepts: - ATP can easily release and store energy by breaking and re-forming the bonds between its phosphate groups. This characteristic of ATP makes it exceptionally
Bioenergetics Module A Anchor 3 Key Concepts: - ATP can easily release and store energy by breaking and re-forming the bonds between its phosphate groups. This characteristic of ATP makes it exceptionally
The Cell Teaching Notes and Answer Keys
 The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
The Cell Teaching Notes and Answer Keys Subject area: Science / Biology Topic focus: The Cell: components, types of cells, organelles, levels of organization Learning Aims: describe similarities and differences
2. Which type of macromolecule contains high-energy bonds and is used for long-term energy storage?
 Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Energy Transport Study Island 1. During the process of photosynthesis, plants use energy from the Sun to convert carbon dioxide and water into glucose and oxygen. These products are, in turn, used by the
Adaptations of higher plant cell walls to water loss: drought vs desiccation
 Physiologia Plantarum 134: 237 245. 2008 Copyright ª Physiologia Plantarum 2008, ISSN 0031-9317 MINIREVIEW Adaptations of higher plant cell walls to water loss: drought vs desiccation John P. Moore a,
Physiologia Plantarum 134: 237 245. 2008 Copyright ª Physiologia Plantarum 2008, ISSN 0031-9317 MINIREVIEW Adaptations of higher plant cell walls to water loss: drought vs desiccation John P. Moore a,
protocol handbook 3D cell culture mimsys G hydrogel
 handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
handbook 3D cell culture mimsys G hydrogel supporting real discovery handbook Index 01 Cell encapsulation in hydrogels 02 Cell viability by MTS assay 03 Live/Dead assay to assess cell viability 04 Fluorescent
Effect of Light Colors on Bean Plant Growth
 Effect of Light Colors on Bean Plant Growth Teacher Edition Grade: Grades 6-8 Delaware State Science Standard: Science Standard 6 - Life Processes Strand: Structure/Function Relationship Strand: Matter
Effect of Light Colors on Bean Plant Growth Teacher Edition Grade: Grades 6-8 Delaware State Science Standard: Science Standard 6 - Life Processes Strand: Structure/Function Relationship Strand: Matter
Comparing Plant and Animal Cells
 1.2 Comparing Plant and Animal Cells Here is a summary of what you will learn in this section: Plant and animal cell structures are called organelles. Plant and animal cells perform some similar functions,
1.2 Comparing Plant and Animal Cells Here is a summary of what you will learn in this section: Plant and animal cell structures are called organelles. Plant and animal cells perform some similar functions,
Introduction: Growth analysis and crop dry matter accumulation
 PBIO*3110 Crop Physiology Lecture #2 Fall Semester 2008 Lecture Notes for Tuesday 9 September How is plant productivity measured? Introduction: Growth analysis and crop dry matter accumulation Learning
PBIO*3110 Crop Physiology Lecture #2 Fall Semester 2008 Lecture Notes for Tuesday 9 September How is plant productivity measured? Introduction: Growth analysis and crop dry matter accumulation Learning
Water Relations, Root Growth Potential and Plant Survival of Cold Stored Pinus radiata D. Don Seedlings
 Phyton (Austria) Special issue: "Root-soil interactions" Vol. 40 Fasc. 4 (143)-(148) 25.7.2000 Water Relations, Root Growth Potential and Plant Survival of Cold Stored Pinus radiata D. Don Seedlings By
Phyton (Austria) Special issue: "Root-soil interactions" Vol. 40 Fasc. 4 (143)-(148) 25.7.2000 Water Relations, Root Growth Potential and Plant Survival of Cold Stored Pinus radiata D. Don Seedlings By
OBJECTIVES PROCEDURE. Lab 2- Bio 160. Name:
 Lab 2- Bio 160 Name: Prokaryotic and Eukaryotic Cells OBJECTIVES To explore cell structure and morphology in prokaryotes and eukaryotes. To gain more experience using the microscope. To obtain a better
Lab 2- Bio 160 Name: Prokaryotic and Eukaryotic Cells OBJECTIVES To explore cell structure and morphology in prokaryotes and eukaryotes. To gain more experience using the microscope. To obtain a better
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
3) Transpiration creates a force that pulls water upward in. xylem. 2) Water and minerals transported upward form roots to shoots in.
 3) Transpiration creates a force that pulls water upward in xylem Figure 36.1 An overview of transport in whole plants (Layer 1) Transport in plants 2) Water and minerals transported upward form roots
3) Transpiration creates a force that pulls water upward in xylem Figure 36.1 An overview of transport in whole plants (Layer 1) Transport in plants 2) Water and minerals transported upward form roots
Plant Structure, Growth, and Development. Chapter 35
 Plant Structure, Growth, and Development Chapter 35 PLANTS developmental plasticity = ability of plant to alter form to respond to environment Biological heirarchy Cell basic unit of life Tissue group
Plant Structure, Growth, and Development Chapter 35 PLANTS developmental plasticity = ability of plant to alter form to respond to environment Biological heirarchy Cell basic unit of life Tissue group
