LUNG CANCER TREATMENT REGIMENS (Part 1 of 7)
|
|
|
- Gilbert Robbins
- 8 years ago
- Views:
Transcription
1 LUNG CANCER TREATMENT S (Part 1 of 7) Clinical Trials: The NCCN recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced healthcare team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are only provided to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data becomes available. The NCCN Guidelines are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient s care or treatment. The National Comprehensive Cancer Network makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Non-Small Cell Lung Cancer (NSCLC) Note: All recommendations are Category 2A unless otherwise indicated. Chemotherapy Regimens For Neoadjuvant and Adjuvant Therapy 1 Cisplatin + vinorelbine 2 4 Days 1 and 8: Cisplatin 50mg/m 2 IV plus Days 1, 8, 15 and 22: Vinorelbine 25mg/m 2 IV. Day 1: Cisplatin 100mg/m 2 IV plus Days 1, 8, 15 and 22: Vinorelbine 30mg/m 2 IV. Day 1: Cisplatin 75 80mg/m 2 plus Days 1 + 8: Vinorelbine 25 30mg/m 2. Cisplatin + etoposide 3 Day 1: Cisplatin 100mg/m 2 IV plus Cisplatin + vinblastine 3 Days 1, 22, 43, 64: Cisplatin 80mg/m 2 IV. Days 1, 8, 15, 22, 29, and then every 2 weeks after day 43: Vinblastine 4 mg/m 2. Cisplatin + gemcitabine 5 Day 1: Cisplatin 75mg/m 2 IV plus Days 1 and 8: Gemcitabine 1,250mg/m 2 IV. Cisplatin + docetaxel 6 Day 1: Docetaxel 75mg/m 2 IV + cisplatin 75mg/m 2 IV. Cisplatin + pemetrexed 7,8 Day 1: Cisplatin 75mg/m 2 IV + pemetrexed 500mg/m 2 IV. * For patients with comorbidities or patients not able to tolerate cisplatin 1 Paclitaxel + carboplatin 9 Day 1: Paclitaxel 200mg/m 2 IV + carboplatin AUC=6 IV. Repeat cycle every 3 weeks for 4 cycles. Concurrent Chemotherapy/Radiotherapy (RT) 1 Cisplatin + etoposide 10, (preferred regimen) Cisplatin + vinblastine (preferred regimen) 11 Carboplatin + pemetrexed (nonsquamous) 12 Cisplatin + pemetrexed (nonsquamous) 7,8 Days 1, 8, 29 and 36: Cisplatin 50mg/m 2 IV plus Days 1 5 and 29 33: Etoposide 50mg/m 2 IV plus Concurrent thoracic radiotherapy 1.8Gy/day for 5 days/week (total dose, 61Gy). Days 1 and 29: Cisplatin 100mg/m 2 IV plus Days 1, 8, 15, 22 and 29: Vinblastine 5mg/m 2 IV with concurrent thoracic radiotherapy (total dose, 60Gy). Day 1: Carboplatin AUC 5 IV plus Day 1: Pemetrexed 500 mg/m 2 IV with concurrent thoracic radiotherapy. Day 1: Cisplatin 75 mg/m 2 IV. Day 1: Pemetrexed 500 mg/m 2 IV with concurrent thoracic radiotherapy. Repeat every 3 weeks for 3 cycles. Sequential Chemotherapy/ Radiotherapy (RT) 1 Cisplatin + vinblastine 11 Days 1 and 29: Cisplatin 100mg/m 2 IV. Days 1, 8, 15, 22 and 29: Vinblastine 5mg/m 2 IV; followed by thoracic radiotherapy with 60Gy in 30 fractions beginning on Day 50. Paclitaxel + carboplatin 13 Day 1: Paclitaxel 200mg/m 2 IV over 3 hours + carboplatin AUC=6 IV over 1 hour. Repeat every 3 weeks for 2 cycles; followed by thoracic radiotherapy 63Gy beginning on Day 42.
2 LUNG CANCER TREATMENT S (Part 2 of 7) Non-Small Cell Lung Cancer (NSCLC) () Concurrent Chemotherapy/ Radiotherapy (RT) Followed by Chemotherapy 1 Paclitaxel + carboplatin 13 Day 1 (weekly): Paclitaxel 45 50mg/m 2 IV and carboplatin AUC=2 IV. Concurrent thoracic radiotherapy; followed by two additional cycles of paclitaxel 200mg/m 2 IV and carboplatin AUC=6 IV. Cisplatin + etoposide 10 Days 1, 8, 29, and 36: Cisplatin 50mg/m 2 IV. Days 1 5, 29 33: Etoposide 50mg/m 2 IV with concurrent thoracic radiotherapy; followed by two additional cycles of cisplatin 50mg/m 2 IV and etoposide 50mg/m 2 IV. Systemic Therapy for Advanced Disease 1 The drug regimen with the highest likelihood of benefit, with toxicity deemed acceptable to both the physician and the patient, should be given as initial therapy for advanced lung cancer. Stage, weight loss, performance status (PS), and gender predict survival. Platinum-based chemotherapy prolongs survival, improves symptom control, and yields superior quality of life compared to best supportive care. Histology of NSCLC is important in the selection of systemic therapy. New agent/platinum combinations have generated a plateau in overall response rate ( 25% 35%), time to progression (4 6 months), median survival (8 10 months), 1-year survival rate (30% 40%), and 2-year survival rate (10% 15%) in fit patients. Unfit patients of any age (PS 3 4) do not benefit from cytotoxic treatment, except erlotinib for those who are epidermal growth factor receptor (EGFR) mutation-positive. Principals of Maintenance Therapy 1 Continuation maintenance refers to the use of at least one of the agents given in first line, beyond 4 to 6 cycles, in the absence of disease progression. Switch maintenance refers to the initiation of a different agent, not included as part of the first-line regimen, in the absence of disease progression, after 4 to 6 cycles of initial therapy. Continuation Maintenance: Bevacizumab and cetuximab given in combination with chemotherapy should be until evidence of disease progression or unacceptable toxicity, as per the design of the clinical trials supporting their use. Continuation of bevacizumab after 4 6 cycles of platinum-doublet chemotherapy and bevacizumab (category 1). Continuation of cetuximab after 4 6 cycles of cisplatin, vinorelbine, and cetuximab (category 1). Continuation of pemetrexed after 4 6 cycles of cisplatin and pemetrexed chemotherapy, for patients with histologies other than squamous cell carcinoma (category 1). Continuation of bevacizumab + pemetrexed after 4 6 cycles of bevacizumab, pemetrexed, cisplatin/carboplatin, for patients with histologies other than squamous cell carcinoma. Continuation of gemcitabine after 4 6 cycles of platinum-doublet chemotherapy (category 2B). Switch Maintenance: Two studies have shown a benefit in progression-free and overall survival with the initiation of pemetrexed or erlotinib after first-line chemotherapy, in patients without disease progression after 4 6 cycles of therapy. Initiation of pemetrexed after 4 6 cycles of first-line platinum-doublet chemotherapy for patients with histologies other than squamous cell carcinoma (category 2B). Initiation of erlotinib after 4 6 cycles of first-line platinum-doublet chemotherapy (category 2B). Initiation of docetaxel after 4 6 cycles of first-line platinum-doublet chemotherapy in patients with squamous cell carcinoma (category 2B). Close surveillance of patients without therapy is a reasonable alternative to maintenance. Principles of Third-Line Therapy 1 If not already given, options for patients with PS 0 2 include docetaxel, pemetrexed (nonsquamous), erlotinib, or gemcitabine (category 2B for all options). Continuation After Disease Progression 1 With the exception of targeted agents (erlotinib, gefitinib, afatinib, crizotinib, ceritinib) in patients with EGFR-sensitizing mutations or ALK rearrangements who have experienced objective regressions with targeted therapy, no agent should be after disease progression has been documented except in selected situations. (refer to discussion section of NCCN Guidelines for Non-Small Cell Lung Cancer v ) Systemic Treatment Options for Patients with NSCLC 1, Cisplatin Carboplatin 17,18 23 Paclitaxel 14,17,18,20 23 Docetaxel 5,6,19,23,24 Vinorelbine 6,20,21 Gemcitabine 5,16,18 20,24 Etoposide 17 lrinotecan 20 Vinblastine Mitomycin Ifosfamide 23 Pemetrexed 7,8 Erlotinib 25 Bevacizumab 26 Cetuximab 27 Albumin-bound paclitaxel Crizotinib 31 Afatinib 32 Ceritinib 33 Ramucirumab 34 Nivolumab 35
3 LUNG CANCER TREATMENT S (Part 3 of 7) Non-Small Cell Lung Cancer (NSCLC) () First-Line Systemic Therapy for Advanced Disease 1 Bevacizumab carboplatin + paclitaxel 26,36 Cetuximab + cisplatin + vinorelbine 27 Erlotinib 37,38 Cisplatin + paclitaxel 19 Cisplatin + gemcitabine 19 Cisplatin + docetaxel 6 Cisplatin + vinorelbine 6 Carboplatin + paclitaxel 19 Pemetrexed + cisplatin 24,39 Day 1: Paclitaxel 200mg/m 2 IV Day 1: Carboplatin AUC=6 IV. Repeat every 3 weeks for 6 cycles. Day 1: Bevacizumab 15mg/kg IV every 3 weeks until disease progression. Day 1: Cetuximab 400mg/m 2 IV + cisplatin 80mg/m 2 IV, plus Days 1 and 8: Vinorelbine 25mg/m 2 IV, plus Day 8: Cetuximab 250mg/m 2 IV once weekly. Repeat every 3 weeks for 6 cycles. Day 1: Erlotinib 150mg PO once daily; following 4 cycles of platinum-based chemotherapy. Day 1: Paclitaxel 135mg/m 2 IV over 24 hours Day 2: Cisplatin 75mg/m 2 IV. Day 1: Cisplatin 100mg/m 2 IV Days 1, 8 and 15: Gemcitabine 1,000mg/m 2 IV. Repeat cycle every 4 weeks. Day 1: Cisplatin 75mg/m 2 IV + docetaxel 75mg/m 2 IV. Day 1: Cisplatin 100mg/m 2 IV Days 1, 8, 15 and 22: Vinorelbine 25mg/m 2 IV over 10 minutes. Repeat cycle every 4 weeks. Day 1: Carboplatin AUC=5 6 IV Day 1: Paclitaxel 225mg/m 2 IV over 3 hours. Day 1: Pemetrexed 500mg/m 2 IV + cisplatin 75mg/m 2 IV. Crizotinib 40# Crizotinib 250mg PO twice daily. ** Afatinib 32 Afatinib 40mg PO once daily. Principals of First-Line Therapy 1 Bevacizumab + chemotherapy or chemotherapy alone is indicated in patients with PS 0 1 with advanced or recurrent NSCLC. Bevacizumab should be given until disease progression. Cetuximab + vinorelbine/cisplatin is an option for patients with PS 0 1 (category 2B). Erlotinib is recommended as a first-line therapy in patients with sensitizing EGFR mutations and should not be given as first-line therapy to patients negative for these EGFR mutations or with unknown EGFR status. Afatinib is indicated for select patients with sensitizing EGFR mutations. Crizotinib is indicated for select patients with ALK rearrangements. There is superior efficacy and reduced toxicity for cisplatin/pemetrexed in patients with nonsquamous histology compared with cisplatin/gemcitabine. There is superior efficacy for cisplatin/gemcitabine in patients with squamous histology, in comparison to cisplatin/pemetrexed. Two drug regimens are preferred; a third cytotoxic drug increases response rate but not survival. Single-agent therapy or platinum-based combinations are a reasonable alternative in PS 2 patients or the elderly. Cisplatin or carboplatin have been proven effective in combination with any of the following agents: paclitaxel, docetaxel, gemcitabine, etoposide, vinblastine, vinorelbine, pemetrexed, or albumin-bound paclitaxel. New agent/non-platinum combinations are reasonable alternatives if available data show activity and tolerable toxicity (e.g., gemcitabine/docetaxel, gemcitabine/vinorelbine). Response assessment after 1 2 cycles, then every 2 4 cycles. Subsequent Second-Line Systemic Therapy for Advanced Disease 1 Docetaxel 23 Day 1: Docetaxel 75mg/m 2 IV. Pemetrexed 7 Day 1: Pemetrexed 500mg/m 2 IV. Erlotinib 25 Erlotinib 150mg PO once daily. Ceritinib 33# Ceritinib 750mg PO once daily. Ramucirumab + docetaxel 34 Day 1: Ramucirumab 10mg/kg IV + docetaxel 75mg/m 2 IV. Afatinib 32 Afatinib 40mg PO once daily. Nivolumab 35 Nivolumab 3mg/kg IV over 60 minutes every 2 weeks.
4 LUNG CANCER TREATMENT S (Part 4 of 7) Non-Small Cell Lung Cancer (NSCLC) () Subsequent Systemic Therapy for Advanced Disease 1 () Principles of Subsequent Therapy 1 In patients who have experienced disease progression either during or after first-line therapy, single-agent docetaxel, pemetrexed, or erlotinib are established second-line agents. Docetaxel is superior to vinorelbine or ifosfamide. Pemetrexed is considered equivalent to docetaxel with less toxicity in patients with adenocarcinoma and large cell carcinoma. Ramucirumab + docetaxel improves survival when compared to docetaxel alone. Erlotinib is superior to best supportive care. Afatinib is indicated for select patients with sensitizing EGFR mutations. Ceritinib is indicated for patients with ALK rearrangements who have disease progression on or are intolerant to crizotinib. If not already given, options for patients with PS 0 2 include docetaxel, pemetrexed (nonsquamous), erlotinib, or gemcitabine (category 2B for all options). Continuation After Disease Progression 1 With the exception of targeted agents (erlotinib, gefitinib, afatinib, crizotinib) in patients with EGFR-sensitizing mutations or ALK rearrangements who have experienced objective regressions with targeted therapy, no agent should be after disease progression has been documented except in selected situations (refer to discussion section of NCCN Guidelines for Non-Small Cell Lung Cancer v ). Small Cell Lung Cancer (SCLC) Chemotherapy as Primary or Adjuvant Therapy Limited Stage (maximum of 4 6 cycles) 1 Cisplatin + etoposide Day 1: Cisplatin 60mg/m 2 IV plus Days 1 3: Etoposide 120mg/m 2 IV. Repeat cycle every 3 weeks for at least 4 cycles. Day 1: Cisplatin 80mg/m 2 IV plus Repeat every 4 weeks for 4 6 cycles. Carboplatin + etoposide 44 Day 1: Carboplatin AUC=5 6 IV plus Repeat every 3 weeks for 4 6 cycles. Extensive Stage (maximum of 4 6 cycles) 1 Cisplatin + etoposide Day 1: Cisplatin 75 80mg/m 2 IV Days 1 3: Etoposide mg/m 2 IV. Repeat every 3 weeks for 4 6 cycles. Days 1 3: Cisplatin 25mg/m 2 IV + etoposide 100mg/m 2 IV. Repeat cycle every 3 weeks for 4-6 cycles. Cisplatin + irinotecan 41,48,49 Day 1: Cisplatin 60mg/m 2 IV Days 1, 8 and 15: Irinotecan 60mg/m 2 IV. Day 1 and 8: Cisplatin 30mg/m 2 IV Day 1 and 8: Irinotecan 65mg/m 2 IV. Repeat every 3 weeks for 4 6 cycles. Carboplatin + irinotecan 50 Day 1: Carboplatin AUC=5 IV plus Days 1, 8 and 15: Irinotecan 50mg/m 2 IV. Repeat cycle every 4 weeks for 4 6 cycles. Carboplatin + etoposide 51 Day 1: Carboplatin AUC=5 6 IV. Repeat every 4 weeks for 4 6 cycles. Subsequent Chemotherapy Relapse <2 3 months, PS Paclitaxel 18,52 Day 1: Paclitaxel 175mg/m 2 IV over 3 hours plus Day 1: Cisplatin 80mg/m 2 IV. Repeat every 3 weeks for at least 2 cycles. Day 1: Paclitaxel 80mg/m 2 IV over 1 hour. Repeat every week for 6 weeks, followed by a 2-week break.
5 LUNG CANCER TREATMENT S (Part 5 of 7) Small Cell Lung Cancer (SCLC) () Subsequent Chemotherapy () Relapse <2 3 months, PS () Docetaxel 53 Day 1: Docetaxel 100mg/m 2 IV over 1 hour. Repeat every 21 days. Topotecan Days 1 5: Topotecan 1.5mg/m 2 IV once daily over 30 minutes. Days 1 5: Topotecan 2.3mg/m 2 PO once daily. Irinotecan 58 Day 1: Irinotecan 100mg/m 2 IV over 90 minutes. Repeat every week. Temozolomide 59 Gemcitabine 60,61 Day 1 21: Temozolomide 75mg/m 2 PO for a 4-week cycle. Days 1, 8, and 15: Gemcitabine 1,000mg/m 2 IV for a 4-week cycle. Ifosfamide 62 Day 1: Ifosfamide/mesna 5,000mg/m 2 IV. Relapse > 2 3 months up to 6 months 1 Topotecan (Category 1) Days 1 5: Topotecan 1.5mg/m 2 IV once daily over 30 minutes. Days 1 5: Topotecan 2.3mg/m 2 PO once daily. Paclitaxel 18,52 Day 1: Paclitaxel 175mg/m 2 IV over 3 hours plus Day 1: Cisplatin 80mg/m 2. Repeat every 3 weeks for at least 2 cycles. Day 1: Paclitaxel 80mg/m 2 IV over 1 hour. Repeat every week for 6 weeks, followed by a 2-week break. Docetaxel 53 Day 1: Docetaxel 100 mg/m 2 IV over 1 hour. Repeat every 21 days. Irinotecan 58 Day 1: Irinotecan 100mg/m 2 IV over 90 minutes. Repeat every week. Gemcitabine 60,61 Vinorelbine 63,64 Etoposide (PO) 65,66 Temozolomide 59 Days 1, 8, and 15: Gemcitabine 1,000mg/m 2 IV for a 4-week cycle. Day 1: Vinorelbine 25 30mg/m 2 IV. Repeat every week Day 1 21: Etoposide 50mg/m 2 PO. Day 1 21: Temozolomide 75mg/m 2 PO for a 4-week cycle. Cyclophosphamide + doxorubicin + vincristine (CAV) 54 Day 1: Cyclophosphamide 1,000 mg/m 2 IV plus Day 1: Doxorubicin 45mg/m 2 IV plus Day 1: Vincristine 2mg IV. Repeat every 21 days. Relapse> 6 months 1 Original regimen 67,68 * For adenocarcinoma, large cell carcinoma, and NSCLC NOS without specific histologic subtype. This regimen can be used as neoadjuvant chemoradiotherapy. Cisplatin and etoposide is the preferred regimen. If weekly carboplatin and paclitaxel is used because the patient is not able to tolerate concurrent full-dose cisplatin and radiotherapy, the treating physician should consider 2 cycles of full-dose platinum therapy after local treatment is completed Most are used in combination, while others are used as monotherapy (e.g., maintenance or second-line therapy). Albumin-bound paclitaxel may be substituted for either paclitaxel or docetaxel in patients who have experienced hypersensitivity reactions after receiving paclitaxel or docetaxel despite premedication, or for patients where the standard premedications (dexamethasone, H2 blockers, H1 blockers) are contraindicated. Indicated in advanced NSCLC. Indicated for EGFR mutation positive patients and may be considered as an option for patients who test positive for an EGFR mutation. # Indicated for ALK-positive patients. ** May reduce to 200mg twice daily not tolerated or toxicity occurs. If further reduction is needed, reduce to 250mg once daily. The regimens included are representative of the more commonly used regimens for small cell lung cancer. Other regimens may be acceptable. The use of rnyeloid growth factors is not recommended during concurrent chemotherapy plus radiotherapy. During chemotherapy + radiotherapy, cisplatin/etoposide is recommended (Category 1). Consider dose reductions versus growth factors in the poor performance status patient.
6 LUNG CANCER TREATMENT S (Part 6 of 7) References 1. Referenced with permission from NCCN Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer. v Available at: physician_gls/pdf/nscl.pdf. and Small Cell Lung Cancer. v Available at: physician_gls/pdf/sclc.pdf. Accessed April 10, Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-lung cancer. N Engl J Med. 2005;352: Arriagada R, Bergman B, Dunant A, et al. The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatinbased adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med. 2004; 350: Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7: Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small cell lung cancer. J Clin Oncol. 2000;18: Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003; 21: Hanna NH, Sheperd FA, Fossella FV, et al. Randomized phase III study of pemetrexed versus docetaxel in patients with nonsmall cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22: Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage NSCLC. J Clin Oncol. 2008;26: Strauss GM, Herndon JE III, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study groups. J Clin Oncol. 2008;26: Albain KS, Crowley JJ, Turrisi AT III, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB nonsmall cell lung cancer: a Southwest Oncology Group phase II study, SWOG J Clin Oncol. 2002;20: Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresectable stage III NSCLC: RTOG Proc Am Soc Clin Oncol. 22:621a, 2003 (abstr 2499). 12. Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: Cancer and Leukemia Group B trial J Clin Oncol. 2011;29: Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005; 23: Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18: Wozniak AJ, Crowley JJ, Balcerzak SP, et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small cell lung cancer: A Southwest Oncology Group Study. J Clin Oncol. 1998;16: Cardenal F, Lopez-Cabrerizo MP, Anton A, et al. Randomized phase III study of gemcitabine-cisplatin versus etoposidecisplatin in the treatment of locally advanced or metastatic non-small cell lung cancer. J Clin Oncol. 1999;17: Belani CP, Lee JS, Socinski MA, et al. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005;16: Smit EF, van Meerbeeck JR Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group-ETC J Clin Oncol. 2003; 21: Schiller JH, Harrington D, Belani CR et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N EngI J Med. 2002;346: Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18: Kelly K, Crowley J, Bunn PA, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol. 2001;19: Belani CP, Ramalingam S, Perry MC, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small cell lung cancer. J Clin Oncol. 2008;26: Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small cell lung cancer previously treated with platinum- containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18: Pujol JL, Breton JL, Gervais R, et al. Gemcitabine-docetaxel versus cisplatin-vinorelbine in advanced or metastatic nonsmall cell lung cancer: a phase III study addressing the case for cisplatin. Ann Oncol. 2005;16: Shepherd FA, Pereira JR, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N EngI J Med. 2005;353: Sandler AB, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N EngI J Med. 2006;355: Pirker R, Periera JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small cell lung cancer (FLEX): an open label randomised phase III trial. Lancet. 2009;373: Green M, Manikhas G, Orlov S, et al. Abraxane, a novel Cremophor -free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small cell lung cancer. Ann Oncol. 2006;17: Rizvi N, Riely G, Azzoli, C, et al. Phase I/Il Trial of Weekly Intravenous 130-nm Albumin-Bound Paclitaxel As Initial Chemotherapy in Patients With Stage IV Non-small cell Lung Cancer. J Clin Oncol. 2008;26: Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012:30: Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12: Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27): Shaw AT, Kim D-W, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:
7 LUNG CANCER TREATMENT S (Part 7 of 7) References () 34. Garon EB, Cieleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384: Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-pd-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16: Avastin [prescribing information]. South San Francisco, CA: Genentech, Inc.; Cappuzzo F, Ciuleanu T, Stelmakh L, et al. SATURN investigators. Erlotinib as maintenance treatment in advanced nonsmall-cell lung cancer: a multicentre, randomised, placebocontrolled phase 3 study. Lancet Oncol. 2010;11: Tarceva [prescribing information]. S. San Francisco, CA: Genentech, Inc.; Alimta [prescribing information]. Indianapolis, IN: Eli Lilly & Co.; Xalkori [prescribing information]. New York, NY: Pfizer, Inc.; Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24: Turrisi AT 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Eng J Med. 1999;340: Saito H, Takada Y, Ichinose Y, et al. Phase II study of etoposide and cisplatin with concurrent twice-daily thoracic radiother apy followed by irinotecan and cisplatin in patients with limiteddisease small-cell lung cancer: West Japan Thoracic Oncology Group J Clin Oncol. 2006;24(33): Skarlos DV, Samantas E, Briassoulis E, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HeCOG). Ann Oncol. 2001;12: Ihde DC, Mulshine JL, Kramer BS, et al. Prospective randomized comparison of high-dose and standard-dose etoposide and cisplatin chemotherapy in patients with extensive-stage small-cell lung cancer. J Clin Oncol. 1994;12: Sundstrom S, Bremnes RM, Kaasa S, et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years follow-up. J Clin Oncol. 2002;20: Evans WK, Shepherd FA, Feld R, et al. VP-16 and cisplatin as first line therapy for small-cell lung cancer. J Clin Oncol. 1985;3: Natale RB et al. S0124: a randomized phase III trial comparing irinotecan/cisplatin (IP) with etoposide/cisplatin (EP) in patients (pts) with previously untreated extensive stage small-cell lung cancer (E-SCLC) [Abstract 7512] ASCO annual meeting. 49. Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive smallcell lung cancer. N Engl J Med. 2002;346: Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. For the Arbeitsgemeinschaft Internistische Onkologie thoracic oncology study group. A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposidecarboplatin as first-line therapy for extensive- disease smallcell lung cancer. Ann Oncol. 2011;22(8): Okamoto H, Watanabe K, Nishiwaki Y, et al. Phase II study of area under the plasma-concentration-versus-time curvebased carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J Clin Oncol. 1999;17: Yamamoto, N., Tsurutani, J., Yoshimura, N., et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res,2006;26(1b): Smyth JF, Smith IE, Sessa C, et al. Activity of docetaxel (Taxotere) in small cell lung cancer. Eur J Cancer 1994;30A: von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999; 17(2): Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25: O Brien M, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24: Ardizzoni A, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol. 1997;15: Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol. 1992;10: Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-dna methyltransferase as a potential biomarker. Clin Cancer Res. 2012; 18: Van der Lee I, Smit EF, van Putten JW, et al. Single-agent gemcitabine in patients with resistant small-cell lung cancer. Ann Oncol. 2001;12: Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer. J Clin Oncol. 2003;21: Cantwell BM, Bozzino JM, Corns P, et al. The multidrug resistant phenotype in clinical practice; evaluation of cross resistance to ifosfamide and mesna after VPI6-213, doxorubicin and vincristine (VPAV) for small cell lung cancer. Eur J Cancer Clin Oncol. 1988;24: Jassem J, Karnicka-Mlodkowska H, van Pottelsberghe C, et al. Phase II study of vinorelbine (Navelbine) in previously treated small cell lung cancer patients. Eur J Cancer 1993; 29A: Furuse K, Kuboa K, Kawahara M, et al. Phase II study of vinorelbine in heavily previously treated small cell lung cancer. Oncology. 1996;53: Einhorn LH, Pennington K, McClean J. Phase II trial of daily oral VP-16 in refractory small cell lung cancer. Semin Oncol. 1990;17: Johnson DH, Greco FA, Strupp J, et al. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol. 1990;8: Postmus PE, Berendsen HH, van Zandwijk N, et al. Retreatment with the induction regimen in small cell lung cancer relapsing after an initial response to short term chemotherapy. Eur J Cancer Clin Oncol. 1987;23: Giaccone G, Ferrati P, Donadio M, et al. Reinduction chemotherapy in small cell lung cancer. Eur J Cancer Clin Oncol. 1987;23: (Revised 4/2015) 2015 Haymarket Media, Inc.
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Medication Policy Manual. Topic: Alimta, pemetrexed Date of Origin: May 12, 2010
 Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Medication Policy Manual Policy No: dru213 Topic: Alimta, pemetrexed Date of Origin: May 12, 2010 Committee Approval Date: February 17, 2015 Next Review Date: February 2016 Effective Date: March 1, 2015
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Chapter 7: Lung Cancer
 Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chapter 7: Lung Cancer Contents Chapter 7: Lung Cancer... 1 Small Cell... 2 Good PS + Limited stage... 2 Cisplatin/etoposide... 2 Concurrent chemotherapy + XRT... 2 Good / Intermediate PS... 2 Carboplatin
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status
 Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Chemotherapy in Advanced Non-Small Cell Lung Cancer: Optimal Treatment Approach for Elderly and Patients With Poor Performance Status Tracey L. Evans, MD Abstract In spite of advances in molecular profiling
Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer
 J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
J Hong Kong Col Radiol. 2010;13(Suppl):S16-21 ORIGINAL ARTICLE Shifting the Paradigm for Maintenance Therapy for Non small-cell Lung Cancer VHF Lee Department of Clinical Oncology, Queen Mary Hospital,
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
SYSTEMIC THERAPY FOR STAGE IV NON-SMALL CELL LUNG CANCER: AMERICAN SOCIETY OF CLINICAL ONCOLOGY CLINICAL PRACTICE GUIDELINE UPDATE
 Which patients with stage IV NSCLC should be treated with chemotherapy? NSCLC with nonsquamous cell carcinoma, negative or unknown EGFR-sensitizing mutation and ALK gene rearrangement status, and PS 0-1
Which patients with stage IV NSCLC should be treated with chemotherapy? NSCLC with nonsquamous cell carcinoma, negative or unknown EGFR-sensitizing mutation and ALK gene rearrangement status, and PS 0-1
Emerging Drug List GEFITINIB
 Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Generic (Trade Name): Manufacturer: Gefitinib (Iressa ) formerly referred to as ZD1839 AstraZeneca NO. 52 JANUARY 2004 Indication: Current Regulatory Status: Description: Current Treatment: Cost: Evidence:
Non-small cell lung cancer (NSCLC) is the most
 Vol 3 April 2011 Clinical Pharmacist 109 First-line treatment for non-small cell lung cancer is surgery; but many patients are not suitable and, for these patients, management may involve radiotherapy,
Vol 3 April 2011 Clinical Pharmacist 109 First-line treatment for non-small cell lung cancer is surgery; but many patients are not suitable and, for these patients, management may involve radiotherapy,
More than half of all lung cancer patients present with. New Combinations in the Treatment of Lung Cancer* A Time for Optimism
 New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
New Combinations in the Treatment of Lung Cancer* A Time for Optimism Paul A. Bunn, Jr., MD; and Karen Kelly, MD Strides have been made in the treatment of lung cancer in the last decade that warrant a
Systemic Therapy for Stage IV Non-Small Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update
 Systemic Therapy for Stage IV Non-Small Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Introduction The purpose of this guideline update is to revise the 2011
Systemic Therapy for Stage IV Non-Small Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Introduction The purpose of this guideline update is to revise the 2011
Non-Small Cell Lung Cancer Treatment Protocols
 Medscape Reference Reference News Reference Education MEDLINE Non-Small Cell Lung Cancer Treatment Protocols Author: Marvaretta M Stevenson, MD; Chief Editor: Jules E Harris, MD more... Updated: Oct 16,
Medscape Reference Reference News Reference Education MEDLINE Non-Small Cell Lung Cancer Treatment Protocols Author: Marvaretta M Stevenson, MD; Chief Editor: Jules E Harris, MD more... Updated: Oct 16,
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Systemic Chemotherapy for Advanced Non-Small Cell Lung Cancer: Recent Advances and Future Directions
 Systemic Chemotherapy for Advanced Non-Small Cell Lung Cancer: Recent Advances and Future Directions Suresh Ramalingam, a Chandra Belani b a Lung & Thoracic Malignancies Program, University of Pittsburgh
Systemic Chemotherapy for Advanced Non-Small Cell Lung Cancer: Recent Advances and Future Directions Suresh Ramalingam, a Chandra Belani b a Lung & Thoracic Malignancies Program, University of Pittsburgh
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with relatively good performance status
 Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Available online at www.sciencedirect.com Journal of the Chinese Medical Association 74 (2011) 209e214 Original Article Third-line or fourth-line chemotherapy in non-small-cell lung cancer patients with
Cetuximab (Erbitux) MM.04.005 05/10/2005. HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s) of Service: Office: Outpatient
 Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Cetuximab (Erbitux) Policy Number: Original Effective Date: MM.04.005 05/10/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 01/01/2015 Section: Prescription Drugs Place(s)
Lung cancer is the leading cause of cancer-related death in the United
 Evolving Treatment Paradigms in Non-Small Cell Lung Cancer MARGARET E. M. VAN METER, MD Medical Oncology Fellow Division of Cancer Medicine The University of Texas M.D. Anderson Cancer Center Houston,
Evolving Treatment Paradigms in Non-Small Cell Lung Cancer MARGARET E. M. VAN METER, MD Medical Oncology Fellow Division of Cancer Medicine The University of Texas M.D. Anderson Cancer Center Houston,
Advancing Personalized Therapy for Advanced Non-Small Cell Lung Cancer
 GUIDING THE WAY White Paper Advancing Personalized Therapy for Advanced Non-Small Cell Lung Cancer is a serum proteomic test for patients with advanced non-small cell lung cancer that helps healthcare
GUIDING THE WAY White Paper Advancing Personalized Therapy for Advanced Non-Small Cell Lung Cancer is a serum proteomic test for patients with advanced non-small cell lung cancer that helps healthcare
Summary ID# 13095. Clinical Study Summary: Study H3E-EW-B012
 Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
Page 1 Summary ID# 13095 Clinical Study Summary: Study H3E-EW-B012 First-line Treatment of Non-Small Cell Lung Cancer under Routine Conditions: Observational Study on Overall Survival Date summary electronically
SAKK Lung Cancer Group. Current activities and future projects
 SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
SAKK Lung Cancer Group Current activities and future projects SAKK Lung Cancer Group Open group of physicians interested in lung cancer Mostly Medical Oncologists, but also Thoracic Surgeons Radiation
Small-Cell Lung Cancer: Is There a Standard Therapy?
 Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
 Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Evolving Treatment Paradigms in Non-Small Cell Lung Cancer
 PRINTER-FRIENDLY VERSION AT PHARMACYPRACTICE NEWS.COM Evolving Treatment Paradigms in Non-Small Cell Lung Cancer CALEB T. CHU, MD, MPH Postdoctoral Fellow Department of Thoracic/Head and Neck Medical Oncology
PRINTER-FRIENDLY VERSION AT PHARMACYPRACTICE NEWS.COM Evolving Treatment Paradigms in Non-Small Cell Lung Cancer CALEB T. CHU, MD, MPH Postdoctoral Fellow Department of Thoracic/Head and Neck Medical Oncology
Schedule: Drug Dose iv/infusion/oral q Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8
 Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Carboplatin/Gemcitabine Lung Cancer (non-small cell) - Advanced Carboplatin AUC 5 500mls 5% dex/1hr Day 1 Gemcitabine 1200mg/m 2 200mls N. Saline/30mins Days 1 & 8 Cycle frequency: Every three weeks Total
Treatment Paradigm in NSCLC Treatment
 Treatment Paradigm in NSCLC Treatment Era of Targeted Therapy Aumkhae Sookprasert, MD Medicine Department, KKU Which factors taken to be account in NSCLC treatment? 1. Staging 2. ECOG performance status
Treatment Paradigm in NSCLC Treatment Era of Targeted Therapy Aumkhae Sookprasert, MD Medicine Department, KKU Which factors taken to be account in NSCLC treatment? 1. Staging 2. ECOG performance status
REPORT ASCO 2011 CHICAG0 : RESPIRATORY ONCOLOGY Johan Vansteenkiste / Christophe Dooms, Univ. Hospital Leuven and Leuven Lung Cancer Group
 1 REPORT ASCO 2011 CHICAG0 : RESPIRATORY ONCOLOGY Johan Vansteenkiste / Christophe Dooms, Univ. Hospital Leuven and Leuven Lung Cancer Group 10 MESSAGE HIGHLIGHTS Early stage non-small cell lung cancer
1 REPORT ASCO 2011 CHICAG0 : RESPIRATORY ONCOLOGY Johan Vansteenkiste / Christophe Dooms, Univ. Hospital Leuven and Leuven Lung Cancer Group 10 MESSAGE HIGHLIGHTS Early stage non-small cell lung cancer
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue)
 Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Sur les nouveaux médicaments et les perspectives qu ils offrent (traitement à la carte et survie longue) Professeur Jean Trédaniel Unité de cancérologie thoracique Hôpital Saint-Louis Comparison of Four
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Non-Small Cell Lung Cancer
 Non-Small Cell Lung Cancer in East tasia Chia-Chi (Josh) Lin, MD, PhD 林 家 齊 Director of Phase I Center, e Department of Oncology, National Taiwan University Hospital Clinical Associate Professor, Department
Non-Small Cell Lung Cancer in East tasia Chia-Chi (Josh) Lin, MD, PhD 林 家 齊 Director of Phase I Center, e Department of Oncology, National Taiwan University Hospital Clinical Associate Professor, Department
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Stage IIIB disease includes patients with T4 tumors,
 Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
Guidelines on Treatment of Stage IIIB Non-small Cell Lung Cancer* James R. Jett, MD, FCCP; Walter J. Scott, MD, FCCP; M. Patricia Rivera MD, FCCP; and William T. Sause, MD, FACR Stage IIIB includes patients
NON-SMALL CELL LUNG CANCER STAGE IV
 NON-SMALL CELL LUNG CANCER STAGE IV Effective Date: November, 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently
NON-SMALL CELL LUNG CANCER STAGE IV Effective Date: November, 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently
Adjuvant Chemotherapy of Non-Small Cell Lung Cancer
 Review Article 2012 NRITLD, National Research Institute of Tuberculosis and Lung Disease, Iran ISSN: 1735-0344 Tanaffos 2012; 11 (1): 12-17 TANAFFOS Adjuvant Chemotherapy of Non-Small Cell Lung Cancer
Review Article 2012 NRITLD, National Research Institute of Tuberculosis and Lung Disease, Iran ISSN: 1735-0344 Tanaffos 2012; 11 (1): 12-17 TANAFFOS Adjuvant Chemotherapy of Non-Small Cell Lung Cancer
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline An ASCO Endorsement of Treatment of Small Cell Lung Cancer:
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART
 Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Harmesh Naik, MD. Hope Cancer Clinic HOW DO I MANAGE STAGE 4 NSCLC IN 2012: STATE OF THE ART Goals Discuss treatment options for stage 4 lung cancer: New and old Discuss new developments in personalized
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Small Cell Lung Cancer* State-of-the-Art Therapy 1994
 Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Small Cell Lung Cancer* State-of-the-Art Therapy 1994 Daniel C. Ihde, MD In the United States, small cell lung cancer (SCLC) accounts for about 20% of all cases of lung cancer. Without treatment, tumor
Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens
 VOLUME 32 NUMBER 18 JUNE 20 2014 JOURNAL OF CLINICAL ONCOLOGY ONCOLOGY GRAND ROUNDS Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens Alona Zer and Natasha
VOLUME 32 NUMBER 18 JUNE 20 2014 JOURNAL OF CLINICAL ONCOLOGY ONCOLOGY GRAND ROUNDS Second-Line Therapy in Non Small-Cell Lung Cancer: The DELTA Between Different Genotypes Widens Alona Zer and Natasha
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
New Trends & Current Research in the Treatment of Lung Cancer, Pt. II
 New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board
 Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
Management of Stage III, N2 NSCLC: A Virtual Thoracic Oncology Tumor Board Abstract Introduction Management of stage III non small-cell lung cancer (NSCLC) is complex and requires careful work-up, staging,
NON-SMALL CELL LUNG CANCER
 NON-SMALL CELL LUNG CANCER Executive Summary In 2012, an estimated 1.8 million people were diagnosed with lung cancer resulting in 1.6 million deaths (http://globocan.iarc.fr/pages/fact_sheets_cancer.aspx).
NON-SMALL CELL LUNG CANCER Executive Summary In 2012, an estimated 1.8 million people were diagnosed with lung cancer resulting in 1.6 million deaths (http://globocan.iarc.fr/pages/fact_sheets_cancer.aspx).
( targeted therapy ) ( oncogenesis ) ( epidermal. growth factor receptor tyrosine kinase inhibitor EGFR-TKI ) ( epidermal growth
 2008 19 8-13 growth factor receptor tyrosine kinase inhibitor EGFR-TKI ) ( targeted therapy ) ( oncogenesis ) ( epidermal factor receptor monoclonal antibody EGFR-moAb ) growth factor monoclonal antibody
2008 19 8-13 growth factor receptor tyrosine kinase inhibitor EGFR-TKI ) ( targeted therapy ) ( oncogenesis ) ( epidermal factor receptor monoclonal antibody EGFR-moAb ) growth factor monoclonal antibody
SECOND-LINE CHEMOTHERAPY in advanced non
 Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
Gemcitabine as Second-Line Treatment for Advanced Non Small-Cell Lung Cancer: A Phase II Trial By Lucio Crinò, Anna Maria Mosconi, Giorgio Scagliotti, Giovanni Selvaggi, Silvia Novello, Massimo Rinaldi,
NON-SMALL CELL LUNG CANCER STAGE III
 NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
NON-SMALL CELL LUNG CANCER STAGE III Effective Date: April, 2012 The recommendations contained in this guideline are a consensus of the Alberta Provincial Thoracic Tumour Team synthesis of currently accepted
Targeted therapies and brain metastases in lung cancer patients. Benjamin Besse, MD, PhD. Medical Oncologist. 19 septembre 2014
 Targeted therapies and brain metastases in lung cancer patients Benjamin Besse, MD, PhD Medical Oncologist 19 septembre 2014 Targeted therapies and brain mets! Brain mets in NSCLC! Specific targeted therapies!
Targeted therapies and brain metastases in lung cancer patients Benjamin Besse, MD, PhD Medical Oncologist 19 septembre 2014 Targeted therapies and brain mets! Brain mets in NSCLC! Specific targeted therapies!
SEARCH METHODOLOGY SEARCH STRATEGY #1 (SYSTEMATIC REVIEWS): PubMed 1966-3/16/2012 Cochrane Database of Systematic Reviews All years
 Appendix A. Search Strategy for Systematic Reviews and Cost-Effectiveness Analyses (Search #1) TREATMENT OF METASTATIC NON-SMALL-CELL LUNG CANCER SEARCH METHODOLOGY SEARCH STRATEGY #1 (SYSTEMATIC REVIEWS):
Appendix A. Search Strategy for Systematic Reviews and Cost-Effectiveness Analyses (Search #1) TREATMENT OF METASTATIC NON-SMALL-CELL LUNG CANCER SEARCH METHODOLOGY SEARCH STRATEGY #1 (SYSTEMATIC REVIEWS):
A Review of Treatment in Non-small-cell Lung Cancer. Athanasios G Pallis
 A Review of Treatment in Non-small-cell Lung Cancer Athanasios G Pallis Medical Oncologist, Department of Medical Oncology, University General Hospital of Heraklion Abstract Non-small-cell lung cancer
A Review of Treatment in Non-small-cell Lung Cancer Athanasios G Pallis Medical Oncologist, Department of Medical Oncology, University General Hospital of Heraklion Abstract Non-small-cell lung cancer
Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small Cell Lung Cancer
 EBS 7-1-2 EDUCATION AND INFORMATION 2013 Evidence-based Series 7-1-2: EDUCATION AND INFORMATION- 2013 Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small
EBS 7-1-2 EDUCATION AND INFORMATION 2013 Evidence-based Series 7-1-2: EDUCATION AND INFORMATION- 2013 Postoperative Adjuvant Chemotherapy, with or without Radiotherapy, in Completely Resected Non-Small
REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) GSK1572932A (NON-SMALL CELL LUNG CANCER) - Executive Summary GSK1572932A (MAGE-A3): Key Metrics in NSCLC Markets
REFERENCE CODE GDHC212DFR PUBLICAT ION DATE JUNE 2013 GSK1572932A (NON-SMALL CELL LUNG CANCER) GSK1572932A (NON-SMALL CELL LUNG CANCER) - Executive Summary GSK1572932A (MAGE-A3): Key Metrics in NSCLC Markets
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
 Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Treatment Paradigms in Advanced Non Small-Cell Lung Cancer
 Treatment Paradigms in Advanced Non Small-Cell Lung Cancer Caroline E. McCoach, MD, PhD, and Karen Kelly, MD Dr McCoach is a clinical research fellow and Dr Kelly is a professor of medicine, an associate
Treatment Paradigms in Advanced Non Small-Cell Lung Cancer Caroline E. McCoach, MD, PhD, and Karen Kelly, MD Dr McCoach is a clinical research fellow and Dr Kelly is a professor of medicine, an associate
Italian clinical research in non-small-cell lung cancer
 Annals of Oncology 16 (Supplement 4): iv110 iv115, 2005 doi:10.1093/annonc/mdi919 Italian clinical research in non-small-cell lung cancer C. Gridelli 1, A. Rossi 1, D. Galetta 2, P. Maione 1, C. Ferrara
Annals of Oncology 16 (Supplement 4): iv110 iv115, 2005 doi:10.1093/annonc/mdi919 Italian clinical research in non-small-cell lung cancer C. Gridelli 1, A. Rossi 1, D. Galetta 2, P. Maione 1, C. Ferrara
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
 Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Department of Veterans Affairs Health Services Research & Development Service Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost-Effectiveness
Network Chemotherapy Regimens
 Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Network Chemotherapy Regimens Lung Cancer Notes from the editor Thames Valley Cancer Network These protocols are available on the Network website www.tvcn.org.uk. Any correspondence about the protocols
Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment?
 4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
4 The Open Lung Cancer Journal, 2011, 4, 4-9 Open Access Elderly Patients with Advanced Non-Small Cell Lung Cancer: What Treatment? Antonio Rossi * Division of Medical Oncology, S.G. Moscati Hospital,
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
Chemotherapy for advanced lung cancer: A 5-year experience
 Original Article Chemotherapy for advanced lung cancer: A 5-year experience Rajappa S, Gundeti S, Talluri MR 1, Digumarti R Departments of Medical Oncology and 1 Internal Medicine, Nizam s Institute of
Original Article Chemotherapy for advanced lung cancer: A 5-year experience Rajappa S, Gundeti S, Talluri MR 1, Digumarti R Departments of Medical Oncology and 1 Internal Medicine, Nizam s Institute of
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
4.8 Lung cancer. 4.8.5 In the UK, three fractionation regimens are most commonly used:
 4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
4.8 Lung cancer 4.8.1 In 2005, both NICE (National Institute for Health and Clinical Excellence) and SIGN (Scottish Intercollegiate Guidelines Network) published guidelines on the management of lung cancer.
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)?
 Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
Is an evidence-based approach realistic in non-small cell lung cancer (NSCLC)? Authors Key words P.A. Coucke, N. Barthelemy, L. Bosquee, J.P. van Meerbeeck NSCLC, sequential and concomitant chemo-radiotherapy,
KRAS Mutation Analysis in Non-Small Cell Lung Cancer (NSCLC) Original Policy Date
 MP 2.04.43 KRAS Mutation Analysis in Non-Small Cell Lung Cancer (NSCLC) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
MP 2.04.43 KRAS Mutation Analysis in Non-Small Cell Lung Cancer (NSCLC) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin
 COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
COMPENDIA TRANSPARENCY TRACKING FORM DRUG: Docetaxel INDICATION: Non-small cell lung cancer, advanced or metastatic, switch-therapy after gemcitabine/carboplatin COMPENDIA TRANSPARENCY REQUIREMENTS 1 Provide
Lung Cancer: New Biological Insights and Recent Therapeutic Advances
 CA CANCER J CLIN 2011;61:91 112 Lung Cancer: New Biological Insights and Recent Therapeutic Advances Suresh S. Ramalingam, MD 1 ; Taofeek K. Owonikoko, MD, PhD 2 ; Fadlo R. Khuri, MD 3 Abstract Approximately
CA CANCER J CLIN 2011;61:91 112 Lung Cancer: New Biological Insights and Recent Therapeutic Advances Suresh S. Ramalingam, MD 1 ; Taofeek K. Owonikoko, MD, PhD 2 ; Fadlo R. Khuri, MD 3 Abstract Approximately
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Thames Valley Chemotherapy Regimens
 Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
Chemotherapy Regimens Lung Cancer Notes from the editor These regimens are available on the Network website www.tvcn.org.uk. Any correspondence about the regimens should be addressed to: Sally Coutts,
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
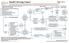 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
Thorakale Onkologie. ASCO 2013 Aktuelle Studien TZ Berlin Buch 12.6.2013
 Triple negativ Thorakale Onkologie ASCO 2013 Aktuelle Studien TZ Berlin Buch 12.6.2013 Driver Oncogene Mutations in non sq-nsclc Genome Res. 2012 November; 22(11): 2109 2119 8021: Detection of EGFR-activating
Triple negativ Thorakale Onkologie ASCO 2013 Aktuelle Studien TZ Berlin Buch 12.6.2013 Driver Oncogene Mutations in non sq-nsclc Genome Res. 2012 November; 22(11): 2109 2119 8021: Detection of EGFR-activating
non-small-cell lung cancer Quimioterapia adjuvante para câncer de pulmão não pequenas
 Adjuvant chemotherapy for non-small-cell lung cancer Quimioterapia adjuvante para câncer de pulmão não pequenas células Daniel B. Costa, MD, PhD, MMSc Division i i of Hematology/Oncology l Beth Israel
Adjuvant chemotherapy for non-small-cell lung cancer Quimioterapia adjuvante para câncer de pulmão não pequenas células Daniel B. Costa, MD, PhD, MMSc Division i i of Hematology/Oncology l Beth Israel
Future Directions in Clinical Research. Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center
 Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Future Directions in Clinical Research Karen Kelly, MD Associate Director for Clinical Research UC Davis Cancer Center Outline 1. Status of Cancer Treatment 2. Overview of Clinical Research at UCDCC 3.
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
Treatment of Advanced Non Small-Cell Lung Cancer: A Review of Current Randomized Clinical Trials and an Examination of Emerging Therapies
 Progress in the treatment of advanced non small-cell lung cancer allows patients to live longer and with better quality of life. Milton Rochman. Mother s Comfort. Acrylic on canvas, 24 30. Courtesy of
Progress in the treatment of advanced non small-cell lung cancer allows patients to live longer and with better quality of life. Milton Rochman. Mother s Comfort. Acrylic on canvas, 24 30. Courtesy of
REPORT PERSPECTIVES IN LUNG CANCER 2010 AMSTERDAM
 REPORT PERSPECTIVES IN LUNG CANCER 2010 AMSTERDAM Valerie Van Damme, Isabelle Wauters, Johan Vansteenkiste Univ. Hospital Leuven and Leuven Lung Cancer Group Introduction Perspectives in Lung Cancer (PILC)
REPORT PERSPECTIVES IN LUNG CANCER 2010 AMSTERDAM Valerie Van Damme, Isabelle Wauters, Johan Vansteenkiste Univ. Hospital Leuven and Leuven Lung Cancer Group Introduction Perspectives in Lung Cancer (PILC)
The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy
 17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
17 (Supplement 10): x108 x112, 2006 doi:10.1093/annonc/mdl247 The expanding role of systemic treatment in non-small cell lung cancer neo-adjuvant therapy E. Felip & E. Vilar Oncology Department, Vall d
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
Non Small Cell Lung Cancer: Scientific Discoveries and the Pursuit of Progress
 Non Small Cell Lung Cancer: Scientific Discoveries and the Pursuit of Progress Lung Cancer Accounts for 14% of All New Cancer Diagnoses in the United States 1 Lung cancer is the second most common malignancy
Non Small Cell Lung Cancer: Scientific Discoveries and the Pursuit of Progress Lung Cancer Accounts for 14% of All New Cancer Diagnoses in the United States 1 Lung cancer is the second most common malignancy
Highlights in NSCLC From the 15th World Conference on Lung Cancer
 January 214 Volume 12, Issue 1, Supplement 1 A SPECIAL MEETING REVIEW EDITION Highlights in CLC From the 15th World Conference on Lung Cancer A Review of Selected Presentations From the 15th World Conference
January 214 Volume 12, Issue 1, Supplement 1 A SPECIAL MEETING REVIEW EDITION Highlights in CLC From the 15th World Conference on Lung Cancer A Review of Selected Presentations From the 15th World Conference
National Clinical Trials Network Groups Update Fall 2014
 National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
National Clinical Trials Network Groups Update Fall 2014 Walter J Curran, Jr, MD An NRG Oncology Group Chair Executive Director Winship Cancer Institute of Emory University Atlanta, GA NCTN Groups Update
Lung cancer remains the leading cause of cancer-related death in the. Evolving Treatment Paradigms in Non-Small Cell Lung Cancer
 PRINTER-FRIENDLY VERSION AT CLINICALONCOLOGY.COM Evolving Treatment Paradigms in Non-Small Cell Lung Cancer KATHRYN F. MILEHAM, MD Staff Oncologist HEATHER D. BROOKS, MD Staff Oncologist EDWARD S. KIM,
PRINTER-FRIENDLY VERSION AT CLINICALONCOLOGY.COM Evolving Treatment Paradigms in Non-Small Cell Lung Cancer KATHRYN F. MILEHAM, MD Staff Oncologist HEATHER D. BROOKS, MD Staff Oncologist EDWARD S. KIM,
Treatment for Lung Cancer: Drug Therapy
 Treatment for Lung Cancer: Drug Therapy Living in the Molecular Age: Advances Drug Therapy of Lung Cancer John C. Morris, M.D. Division of Hematology-Oncology November 1, 2014 Non-small cell lung cancer:
Treatment for Lung Cancer: Drug Therapy Living in the Molecular Age: Advances Drug Therapy of Lung Cancer John C. Morris, M.D. Division of Hematology-Oncology November 1, 2014 Non-small cell lung cancer:
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology. Endorsement of the American College of Chest Physicians (ACCP) Guideline
 Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Treatment of Small Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians (ACCP) Guideline Table of Contents Data Supplement 1: Summary of included
Special Article. Received 5 December 2014; revised 18 February 2015; accepted 25 February 2015. Practical Radiation Oncology (2015) 5, 141-148
 Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Practical Radiation Oncology (2015) 5, 141-148 www.practicalradonc.org Special Article Definitive radiation therapy in locally advanced non-small cell lung cancer: Executive summary of an American Society
Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer
 REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
REVIEW ARTICLE Adjuvant Chemotherapy After Complete Resection of Non-Small Cell Lung Cancer Eckart Laack, Carsten Bokemeyer, Dieter Kurt Hossfeld SUMMARY Introduction: In non-small cell lung cancer (NSCLC)
Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies
 Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies Filippo de Marinis, a Stefano De Santis, a and Luigi De Petris b Current options for
Second-Line Treatment Options in Non Small Cell Lung Cancer: A Comparison of Cytotoxic Agents and Targeted Therapies Filippo de Marinis, a Stefano De Santis, a and Luigi De Petris b Current options for
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Targeting angiogenesis in NSCLC: Clinical trial update Martin Reck Lung Clinic Grosshansdorf Grosshansdorf, Germany
 Targeting angiogenesis in NSCLC: Clinical trial update Martin Reck Lung Clinic Grosshansdorf Grosshansdorf, Germany This presentation was selected by the 15 th World Conference on Lung Cancer Program Committee
Targeting angiogenesis in NSCLC: Clinical trial update Martin Reck Lung Clinic Grosshansdorf Grosshansdorf, Germany This presentation was selected by the 15 th World Conference on Lung Cancer Program Committee
Treatment of Small Cell Lung Cancer
 Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Introduction. Acta Medica Mediterranea, 2015, 31: 883 BOZKURT DUMAN 5, SEMRA PAYDAŞ 4, VEHBI ERCOLAK 6 1
 Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
Acta Medica Mediterranea, 2015, 31: 883 THE EFFECT OF CHEMORADIOTHERAPY ON SURVIVAL IN LOCALLY ADVANCED UNRESECTABLE NON-SMALL CELL LUNG CANCER PATIENTS: EXPERIENCE FROM THE SOUTHEAST REGION OF TURKEY
