Rational Assessment of Drugs and Research. April 2013 TIMELY, INDEPENDENT INFORMATION ABOUT NEW DRUGS. In Brief Pregabalin (Lyrica)...
|
|
|
- Amos Perry
- 8 years ago
- Views:
Transcription
1 Rational Assessment of Drugs and Research April 2013 TIMELY, INDEPENDENT INFORMATION ABOUT NEW DRUGS Pregabalin (Lyrica) for neuropathic pain An alternative adjuvant analgesic for refractory neuropathic pain Imiquimod cream (Aldara)...09 for superficial basal cell carcinoma An alternative topical treatment for low-risk lesions Sitagliptin with simvastatin (Juvicor) fixed-dose combination therapy for type 2 diabetes and hypercholesterolaemia A fixed-dose combination medicine for treating two different conditions In Brief...28 Femme-Tab ED 20/100: low-dose-oestrogen combined oral contraceptive PBS listing for strontium ranelate (Protos) extended to include men with osteoporosis PBS listing for zoledronic acid (Aclasta) changed to include an extended bone mineral density T-score cut-off Durotram (tramadol, extended release) PBS listing deleted Look out for NPS RADAR review online: Rivaroxaban (Xarelto) for deep vein thrombosis Available early June 2013 at npsradar.org.au
2 EDITORIAL Time to revisit the evidence April 2013 Cover image: Synaptic transmission in neuropathic pain GABA analogues for chronic pain M uch attention and publicity attaches to the latest new drugs, especially those that are first in class or offering a new approach to disease treatment and management. In the face of this hype it is easy to lose sight of the fact that for some of the most challenging of human conditions, drugs that have been available for some time remain in common use and have much to offer in achieving management goals. The gamma-aminobutyric acid (GABA) analogue gabapentin and its successor pregabalin are examples. Although the fanfare surrounding their initial release has subsided, it is worth revisiting the evidence for their efficacy, side effects and place in therapy against current alternative options. Approved in Australia in 2002 and 2005, respectively, for adjunctive therapy in epilepsy and for management of neuropathic pain, they were rapidly taken up by medical practitioners seeking therapeutic options for managing chronic pain. Is this place in therapy still justified? Most pain is caused by nociceptor stimulation that resolves once the stimulus is removed and the damage is rectified by the healing process. But chronic neuropathic pain is far more insidious, cure remains elusive and the treatment objective is reduced to how best to manage the pain. Chronic pain is for many people a long-term debilitating condition, and managing chronic pain can be one of the greatest challenges for primary care practitioners. The challenge is made more difficult by the perception of chronic pain being a highly subjective experience. Practitioners know well that the art of pain relief is based on managing a patient s perception of pain rather than attempting to correct its cause. The most common causes of neuropathic pain, such as diabetes and viral neuralgias, are irreversible, intractable and require constant titration and optimisation of treatment strategies. Drug therapy is best used as part of a multifaceted, multidisciplinary, active self-management approach to the physical, psychological, social and vocational impacts of neuropathic pain. It is timely then to be reminded of the options available for the pharmacological management of chronic neuropathic pain. In March 2013 pregabalin was made available on the Pharmaceutical Benefits Scheme as a second-line option, for treatment of neuropathic pain refractory to other treatments. In this issue of NPS RADAR we review the role of pregabalin in managing chronic neuropathic pain, its place in therapy, known harms and benefits, and how it can be used in combination with other pain management options. Independent, not-for-profit and evidence based, NPS enables better decisions about medicines and medical tests. We are funded by the Australian Government Department of Health and Ageing. National Prescribing Service Limited ABN Level 7/418A Elizabeth St Surry Hills NSW 2010 PO Box 1147 Strawberry Hills NSW 2012 P F E. info@nps.org.au NPS RADAR articles may be updated when there is new evidence about safety or efficacy, or in case of regulatory or PBS listing changes. Please refer to for the most recent version as well as any supplementary information. These icons indicate related information is available from NPS Find additional information by going to the link provided Medicine Update articles are available where indicated
3 Pregabalin (Lyrica) 3 FULL REVIEW Pregabalin (Lyrica) for neuropathic pain (pre-gab-a-lin) An alternative adjuvant analgesic for refractory neuropathic pain KEY POINTS Pregabalin appears to have similar efficacy to that of amitriptyline and gabapentin for neuropathic pain Pregabalin is superior in efficacy to placebo and appears to be non-inferior in efficacy and safety to amitriptyline and gabapentin (from indirect comparisons). Consider initial treatment with another agent, such as a tricyclic antidepressant, before pregabalin Pregabalin is only PBS listed for people with refractory neuropathic pain not controlled by other drugs (Authority Required). Dizziness and drowsiness are common dose-dependent adverse events In trials these were the most common adverse effects causing people to stop pregabalin. Dosage reduction is required in people with impaired renal function Assess renal function in people with diabetic neuropathy before starting pregabalin treatment. ADDITIONAL INFORMATION healthpro/explanatorynotes/section1/nursepractitioner PBS listing Authority required (Streamlined) Refractory neuropathic pain not controlled by other drugs. May be prescribed by nurse practitioners Authorised nurse practitioners may prescribe this medicine. See the Pharmaceutical Benefits Scheme (PBS) website for more information on nurse practitioner PBS prescribing. What is it? Pregabalin is a structural analogue of the neurotransmitter GABA. 1 It has analgesic, anticonvulsant, anxiolytic and sleep-modulating activities and is indicated for treatment of epilepsy and neuropathic pain. 2,3 It has a similar pharmacological action to that of gabapentin. 2,4 However, it should be noted that gabapentin is not PBS reimbursed for neuropathic pain. Who is it for? Pregabalin is a treatment option for people with refractory neuropathic pain who have not responded to other drugs. Use with caution in people with renal impairment, as pregabalin is renally excreted. 1 Where does it fit? There is no generally accepted stepwise approach to treating neuropathic pain. There is an array of drugs with limited evidence for differences in efficacy, and often with troublesome adverse effects. Drug therapy is best used as part of a multifaceted, multidisciplinary, active self-management approach to the physical, psychological, social and vocational impacts of neuropathic pain. 5 Assess the nature of the pain experience and inform people of realistic outcomes with treatment. 6,7 The primary goal in most cases is to make the pain tolerable not usually to eliminate the pain. 6 Aim for medium-term drug therapy with a drug holiday after 6 months. Patients who relapse during a drug holiday can resume treatment.
4 4 Pregabalin (Lyrica) Guidelines recommend starting drug therapy for neuropathic pain with a TCA (amitriptyline or nortriptyline) or an antiepileptic agent (gabapentin or pregabalin) The dose of the drugs can be escalated at weekly intervals if tolerated, but it may take several weeks to achieve clinical efficacy. Consider initial therapy with amitriptyline or nortriptyline TCAs are an established first-line treatment option for neuropathic pain but are not approved by the Therapeutic Goods Administration (TGA) for this indication Amitriptyline is the most studied TCA for neuropathic pain. 13 TCAs exhibit significant adverse effects that limit their clinical use, particularly in elderly people. 13 Anticholinergic adverse events, such as dry mouth, constipation and urinary retention, are common. 13 In addition they may cause serious cardiovascular adverse effects, including postural hypotension, heart block and arrhythmias. 13 Gabapentin is effective for treating neuropathic pain Guidelines recommend gabapentin for the treatment of neuropathic pain However, it is not reimbursed by the PBS for this indication. In a meta-analysis gabapentin was demonstrated to be effective for the treatment of a variety of neuropathic pain conditions. 14 The number needed to treat (NNT) to benefit, as measured by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definition, were 6.8 (95% confidence interval [CI] 5.6 to 8.7) for substantial pain relief (50% over baseline) and 5.8 (95% CI 4.8 to 7.2) for moderate pain relief (35% over baseline). 14 Consider pregabalin for neuropathic pain refractory to other drugs In clinical trials pregabalin provided significant pain relief (see Table 1) and improved quality of sleep in both postherpetic neuralgia and painful diabetic neuropathy. 15 Pregabalin has dose-dependent clinical efficacy and appears not to be effective at 150 mg/day for diabetic neuropathy. 15 Preliminary evidence suggests that pregabalin may be effective in the treatment of trigeminal neuralgia However, further clinical trial data are required before it can be recommended for this indication. The anticonvulsant carbamazepine remains the drug of choice for trigeminal neuralgia. 6,8 Consider combination therapy A significant proportion of people with neuropathic pain will not benefit from treatment with a single medication, even when administered at its maximum tolerated dose. 7,19 Evidence suggests that at least 45% of people with neuropathic pain concurrently receive two or more drugs to treat Table 1. Efficacy outcomes with different doses of pregabalin for the treatment of postherpetic neuralgia and diabetic neuropathy, compared with placebo 15 Pregabalin daily dose Number of studies Participants Relative benefit* (95% CI) At least 50% pain relief NNT (95% CI) Postherpetic neuropathic pain 150 mg (1.6 to 3.4) 6.9 (4.8 to 13) 300 mg (1.9 to 3.4) 5.1 (3.9 to 7.4) 600 mg (2.1 to 3.5) 3.9 (3.1 to 5.1) Diabetic neuropathic pain 150 mg (0.8 to 1.6) NA 300 mg (1.2 to 1.8) 7.5 (5.1 to 14) 600 mg (1.5 to 2.0) 5.0 (4.0 to 6.6) * Relative to placebo NNT were calculated from the combined results of clinical trials with different durations (4 14 weeks) Not effective at 150 mg
5 Pregabalin (Lyrica) 5 EVIDENCE SNAPSHOT WHAT IS KNOWN ABOUT THIS DRUG? Pregabalin is a structural analogue of the neurotransmitter gammaaminobutyric acid (GABA). It appears to have a similar spectrum of benefits and harms to that of other adjuvant analgesics used to treat neuropathic pain. It is clinically superior to placebo, and indirect comparisons suggest it is non-inferior for efficacy and safety to amitriptyline and gabapentin. AREAS OF UNCERTAINTY There are no head-to-head trials comparing the efficacy of pregabalin with that of other drugs for neuropathic pain. Information about its use in people with head and neck pain is lacking. WHAT DOES NPS SAY? Pregabalin is an alternative treatment for people with neuropathic pain that has not responded to other drugs. Consider initial treatment with another adjuvant analgesic, such as a tricyclic antidepressant (TCA), before pregabalin. Be aware that dizziness and drowsiness are common dose-dependent adverse events associated with pregabalin and may persist until treatment is stopped. ADDITIONAL INFORMATION Refer to this review at for more information about TGA approval status and PBS listing restrictions for prescription drugs for adults with neuropathic pain: (December 2012) their pain. 19 Combining two or more different drugs may improve analgesic efficacy and reduce overall adverse events if synergistic interactions allow dose reductions of combined drugs. 19 However, combinations of medicines can be associated with increased adverse events. 19 A specific combination of treatments cannot be recommended due to the limited number of studies for any combination therapy, as well as other study factors, such as the limited trial size and duration. 19 Clinical studies of pregabalin in combination with an antidepressant, a cyclo-oxygenase-2 (COX-2) inhibitor or an opioid have shown positive responses greater than the respective monotherapies in diabetic neuropathy and postherpetic neuralgia (five positive trials and one negative trial). 20 Refractory severe neuropathic pain Assistance from a multidisciplinary pain service may be required for refractory, severe neuropathic pain, as treatment options are complex. 5 The TGA has not approved some of the drugs recommended in guidelines for a neuropathic pain indication and most are not subsidised by the PBS for neuropathic pain Tramadol can be considered as a third-line treatment for refractory neuropathic pain However, other opioids are not recommended without further pain-medicine specialist input due to the problems with tolerance and dependence There is limited evidence for use of selective serotonin reuptake inhibitors or serotoninnoradrenaline reuptake inhibitors in the treatment of neuropathic pain, and further research is required to establish their role. 13 How does it compare? Pregabalin is superior to placebo for the treatment of neuropathic pain A meta-analysis of randomised placebocontrolled trials demonstrated that pregabalin (300 mg, 450 mg and 600 mg daily) significantly reduced subjective pain compared with placebo for neuropathic conditions. 15 Pregabalin administered as 150 mg/day was generally ineffective for diabetic neuropathic pain. 15 After pregabalin treatment a minority of patients had substantial benefit (at least 50% pain relief), and more had moderate benefit (at least 35% pain relief). 15 After treatment with pregabalin ( mg daily) the Patient Global Impressions of Change (PGIC) rating of much or very much improved was achieved in about 35% of patients with postherpetic neuralgia and 50% of those with painful diabetic neuropathy. 15 At all doses a significant proportion of patients had no, or trivial, benefit, or discontinued because of adverse events. 15
6 6 Pregabalin (Lyrica) Limited evidence comparing pregabalin with amitriptyline and gabapentin There are no adequately powered direct headto-head trials comparing pregabalin with other drugs. There are indirect comparisons of pregabalin with amitriptyline and gabapentin using placebo as the common comparator. 21 However, indirect comparisons have numerous limitations, as they compare different patient populations, primary outcomes and pain measurement scales. The PBAC noted that indirect comparisons supported the conclusion that pregabalin was clinically no worse than amitriptyline and gabapentin. 21 The efficacy and safety of pregabalin and amitriptyline were demonstrated to be comparable in two head-to-head clinical trials in patients with diabetic peripheral neuropathy, but these were not powered to detect either superiority or noninferiority of pregabalin versus amitriptyline. 22,23 A 36-day head-to-head dose titration trial in patients with diabetic peripheral neuropathy compared pregabalin (300 mg followed by 600 mg, n = 24), duloxetine (60 mg followed by 120 mg, n = 23) and amitriptyline (50 mg followed by 75 mg, n = 27). 23 All treatments improved subjective pain versus placebo as assessed by the primary outcome measure, the Brief Pain Inventory, and also the secondary outcome measure, the short-form McGill visual analogue scale, with no difference between treatments after 1 week of treatment. 23 Pregabalin appears to have some distinct pharmacokinetic advantages over gabapentin, with higher bioavailability, more rapid absorption and increased binding affinity. 4 However, headto-head trials are needed to provide evidence supporting the use of pregabalin over gabapentin in the treatment of neuropathic pain. Safety issues Pregabalin is generally well tolerated and is associated with dose-dependent adverse events that are mild to moderate and usually transient. 2 As pregabalin alters neurotransmission, it can cause a variety of neurological adverse events. Common adverse effects reported in at least 3% of all patients treated with pregabalin include: 12 dizziness drowsiness blurred vision fatigue weight gain dry mouth headache impaired balance peripheral oedema. Report suspected adverse reactions to the TGA online ( or by using the Blue Card distributed three times a year with Australian Prescriber. For information about reporting adverse reactions, see the ( Dizziness and drowsiness: the most common reason for stopping pregabalin therapy In clinical trials dizziness and drowsiness were commonly reported (28 36% and 20 24% of patients, respectively, taking pregabalin 300 mg/day). 1 Both adverse effects occurred more often at higher doses and were the most common reasons for stopping pregabalin. 1 About one-third of people reported dizziness and about half reported drowsiness persisting throughout therapy. 1 Weight gain Weight gain occurred more frequently in patients treated with pregabalin compared with placebo. 1 This dose-dependent side effect may be problematic for patients, such as those with diabetes, who may need to adjust hypoglycaemic medications. 1 Occurrence of peripheral oedema In randomised controlled trials, peripheral oedema was seen more frequently in people with neuropathic pain treated with pregabalin than in those in the control group. 1,24 This may be a particular concern with the higher incidence of peripheral oedema in people with diabetes. 24
7 Pregabalin (Lyrica) 7 ADDITIONAL INFORMATION More information available at nsw. gov.au/pain/ health_professionals/ assessment_tools ADDITIONAL INFORMATION Available at medicines/lyrica Congestive heart failure There have been postmarketing reports of congestive heart failure in some people receiving pregabalin. 1 Depression and anxiety Neuropathic pain can be severe and unrelenting; thus it is important to recognise and treat comorbidities such as anxiety and depression. 6 In addition, anticonvulsant drugs, including pregabalin, increase the risk of suicidal thoughts or behaviours in people using them for any indication. 25,26 Monitor people treated with pregabalin for the emergence or worsening of depression, suicidal thoughts or self-harm behaviour and/or any unusual changes in mood or behaviour. 1 Reason for PBS listing The PBAC recommended pregabalin for listing on the basis of an indirect comparison with amitriptyline and gabapentin with placebo as the common comparator. The PBAC accepted that pregabalin was clinically superior to placebo and clinically no worse than amitriptyline or gabapentin. 21 The PBAC recommended listing of pregabalin on the basis of acceptable cost-effectiveness compared with placebo in people with uncontrolled neuropathic pain. It noted the clinical need for an alternative to current treatments for neuropathic pain. 21 A key issue identified by the PBAC was economic uncertainty from the potential for pregabalin use outside the restriction. They considered that it was essential that the drug utilisation subcommittee (DUSC) review usage 12 months after PBS listing. 21 Dosing issues Start with 75 mg twice a day for 3 7 days. If required, increase dose to 150 mg twice a day after 3 7 days, to a maximum of 300 mg twice a day after another 7 days. 1 It may take several weeks to achieve maximal effect with pregabalin. If improvement is satisfactory continue treatment and consider gradually reducing the dose over time if improvement is sustained. 9 Discontinue pregabalin if it does not improve symptoms or is not tolerated by tapering the dose over a minimum of 1 week, or longer depending on the dose and duration of therapy. 1 Abrupt withdrawal may result in adverse events, including insomnia, headache, nausea, anxiety, hyperhidrosis and diarrhoea. 1 Adjust the dose in renal impairment: refer to the product information for details. 1 Conduct early and regular clinical reviews Due to the refractory nature of neuropathic pain, conduct early and regular clinical reviews to monitor the effectiveness of the chosen treatment. 9 The review should assess: pain (utilise multidimensional tools, such as the Brief Pain Inventory, that provide information about pain history, intensity and associated disability) adverse events daily activities (e.g. ability to work or drive) mood (particularly if the person may be depressed and/or anxious) quality of sleep subjective, overall self-reported improvement in pain (e.g. using PGIC). 9 Information for patients Discuss the Lyrica consumer medicine information (CMI) leaflet with the patient and inform about common side effects such as dizziness and drowsiness. Monitor for weight gain and advise that constipation, diarrhoea, nausea, headache, weight gain, dry mouth or blurred vision may occur, and that, if they are affected, not to drive or operate machinery. Advise patients not to stop taking pregabalin suddenly. Stopping suddenly may cause anxiety, insomnia, headache, sweating, nausea and diarrhoea. Advise that it may take several weeks to achieve maximal effect for postherpetic neuralgia.
8 8 Pregabalin (Lyrica) REFERENCES 1. Pfizer Australia. Pregabalin (Lyrica) product information picmi/picmirepository.nsf/pdf?openagent&id=cp PI Gajraj NM. Anesth Analg 2007;105: Australian Government Department of Health and Ageing, Therapeutics Goods Administration. Australian Register of Therapeutic Goods (ARTG). Public Summary LYRICA pregabalin FPublicHTML%2FpdfStore.nsf&docid=B9871D6C ACA2578CD &agid=(PrintDetails Public)&actionid=1 (accessed 5 December 2012). 4. Bockbrader HN, et al. Clin Pharmacokinet 2010;49: Australian Pain Society. Evidence-based recommendations for the pharmacological management of neuropathic pain. Position statement, June owner/files/cz4s7w.pdf (accessed 20 October 2012). 6. Moulin DE, et al. Pain Res Manag 2007;12: Dworkin RH, et al. Mayo Clin Proc 2010;85:S Attal N, et al. Eur J Neurol 2010;17:1113 e National Institute for Health and Clinical Excellence (NICE). Neuropathic pain: The pharmacological management of neuropathic pain in adults in non-specialist settings. Clinical Guideline ; 17 August guidance/cg96 (accessed 20 October 2012). 10. etg complete. Neuropathic pain: treatment (accessed 17 October 2012). 11. Finnerup NB, et al. Pain 2010;150: Australian Medicines Handbook. Analgesics. (accessed 17 October 2012). 13. Saarto T, Wiffen PJ. Cochrane Database Syst Rev 2007:CD Moore RA, et al. Cochrane Database Syst Rev 2011:CD Moore RA, et al. Cochrane Database Syst Rev 2009:CD Perez C, et al. J Clin Pharmacol 2009;49: Perez C, et al. Cephalalgia 2009;29: Obermann M, et al. Cephalalgia 2008;28: Chaparro LE, et al. Cochrane Database Syst Rev 2012;7:CD Vorobeychik Y, et al. CNS Drugs 2011;25: Pharmaceutical Benefits Advisory Committee. Pregabalin, capsules, 25 mg, 75 mg, 150 mg and 300 mg, Lyrica March Public summary document. PBAC, elements/pbac-meetings/psd/ / pregabalin (accessed 22 October 2012). 22. Bansal D, et al. Diabetic Med 2009;26: Boyle J, et al. Diabetes Care Zaccara G, et al. Eur J Clin Pharm 2012;68: Andersohn F, et al. Neurology 2010;75: Mutschler J, et al. Pharmacopsychiatry 2011;44:119. Updated April 2013 to reflect PBS listing effective from 1 March First published: December The information contained in NPS RADAR is derived from a critical analysis of a wide range of authoritative evidence and is current at the time of publication. Any treatment decisions based on the information provided in NPS RADAR should be made in the context of the clinical circumstances of each patient. NPS RADAR articles may be updated when there is new evidence about safety or efficacy, or in case of regulatory or PBS listing changes. Please refer to for the most recent version as well as any supplementary information.
9 Imiquimod cream (Aldara) 9 FULL REVIEW Imiquimod cream (Aldara) for superficial basal cell carcinoma (ih-mih-kwih-mod) An alternative topical treatment for low-risk lesions KEY POINTS Surgical excision is primary treatment for superficial basal cell carcinoma (sbcc) Highest clearance and lowest recurrence rates are seen following surgery. Consider imiquimod only as an alternative when surgery is contraindicated. Imiquimod is indicated for sbcc and not for other forms of BCC Imiquimod is not indicated for recurrent, nodular or morpheaform BCC. Imiquimod is useful treatment for solar keratoses of the face and scalp Do not use imiquimod on solar keratoses on the hands or arms. Local skin reactions are common with imiquimod Interrupt treatment for a few days to allow intolerable reactions to resolve or subside. Imiquimod therapy involves treatment over an extended duration Patients need to continue treatment for up to 6 weeks for sbcc or up to 16 weeks for solar keratoses. PBS listing Authority required Both the sachet and pump presentations (see What is it? at right) are PBS listed for treatment of biopsy-confirmed primary (previously untreated) sbcc in patients with normal immune function for whom surgical excision, cryotherapy, or curettage with diathermy are inappropriate and topical drug therapy is required. The date of the pathology report and name of the Approved Pathology Authority must be provided at the time of application. There is also a Repatriation Pharmaceutical Benefits Scheme (RPBS) listing for the sachet presentation of imiquimod for primary treatment of histopathologically confirmed sbcc (when other standard treatments are inappropriate and topical drug therapy is required) and for treatment of solar keratoses (SK) on the face and scalp (when other standard treatments are inappropriate and topical drug therapy is required as field treatment for clinically visible and subclinical lesions). The maximum quantity is one pack of 12 sachets with one repeat, or two pumps with one repeat. What is it? Imiquimod (Aldara) is an immune response modifier that promotes NF-kappa-B-mediated secretion of pro-inflammatory cytokines, chemokines and other mediators. These immune responses produce cytotoxic effects that are antiproliferative, anti-tumour and with indirect antiviral effects against some viral infections (e.g. human papillomavirus). 1 Imiquimod cream (50 mg/g [5%]) is supplied in a pump that contains 2 g of cream, or as single-use sachets that contain 250 mg. 2 Who is it for? Imiquimod is approved by the Therapeutic Goods Administration (TGA) for treatment of sbcc, SK and external genital and peri-anal warts (condyloma acuminata). Imiquimod is only recommended for people aged over 18 years who will comply with the treatment regimen. The safety and efficacy of imiquimod in patients under 18 have not been established. 2
10 10 Imiquimod cream (Aldara) Where does it fit? The aim for treatment of BCC is to eradicate the tumour in a manner likely to result in a cosmetic outcome that is acceptable to the patient. 3 For most BCC lesions the most effective treatment is excisional surgery, which offers the most prognostically reliable control rates with the advantage of a complete specimen for histological confirmation of tumour clearance, 3,4 and low 5-year recurrence when complete excision is achieved. 5,6 Surgery also delivers good cosmetic results, particularly when excision and wound repair are performed by experienced practitioners. 3 Although surgical excision is often the most effective treatment for BCC there are other alternatives, including: curettage cryosurgery laser treatment surgical excision using various predetermined margins of control excision under frozen section control Mohs micrographic surgery radiotherapy topical treatment. There is a lack of evidence comparing these different treatment modalities. 7 Although not having a PBS-subsidised indication for field treatment for SK lesions on the face and scalp, imiquimod is TGA approved for this use. SK lesions may require treatment for cosmetic reasons or because of irritation or the potential for progression to cancer. 4,8 No data are available assessing imiquimod as a therapy to prevent squamous cell carcinoma (SCC)-related adverse health outcomes. Although SK has the potential to progress to SCC, the risk of progression is low with no way of determining the risk factor for individual lesions. 9 As most lesions do not progress and up to 26% of lesions regress spontaneously, 10 the decision to treat depends on clinical judgement. Where treatment is provided, most SK lesions are cleared with cryotherapy, 5-fluorouracil (5-FU) cream or surgery. 4 For people with multiple SK lesions, field-directed therapies (e.g. topical) are recommended to allow treatment of clinical and sub-clinical lesions (i.e. those not yet visible) within the treatment area. 11 Imiquimod is an alternative when other treatments are not indicated Consider topical treatments when other options are inappropriate or not preferred. In considering the sachet presentation of imiquimod in 2006, the Pharmaceutical Benefits Advisory Committee (PBAC) noted that there are no direct contraindications to surgical excision, cryotherapy and curettage, 12 therefore the choice of treatment will depend on clinical judgement. For any BCC for which non-surgical treatments are considered, treatment should be compared with surgery when discussing with patients the likelihood of complete clearance and the risk of recurrence. 4 Risk of haematoma, infection and wound dehiscence and unacceptable cosmetic outcomes such as deformity, variation in pigmentation, hypertrophic or keloid scarring (especially for lesions of the upper chest and arms) are some of the disadvantages of surgical interventions that may favour other methods. 4 Other patient-related factors such as general fitness, coexisting serious medical conditions or the use of antiplatelet or anticoagulant medication also influence the choice of treatment. 3 Patient choice, local availability of specialised services and the experience of the treating clinician are other factors to be considered. 3,4 Use imiquimod on histologically favourable BCC lesions Imiquimod is an alternative treatment for small primary sbcc. 3,4,7,13 15 The safety and efficacy of imiquimod treatment for BCC of nodular or morpheaform histological subtypes have not been established. 2 Confirm the diagnosis by skin biopsy before starting treatment with imiquimod. 2
11 Imiquimod cream (Aldara) 11 EVIDENCE SNAPSHOT WHAT IS KNOWN ABOUT THIS DRUG? Imiquimod is an option for the treatment of small, low-risk sbcc when surgery, curettage or cryotherapy are inappropriate. Imiquimod is associated with shortterm clearance rates of about 80 87% that are sustained for up to 5-years follow-up. Imiquimod is also useful for the treatment of solar keratoses (SK) but this is not a Pharmaceutical Benefits Scheme (PBS) subsidised indication. Clearance rates for SK are 50%, with recurrences occurring in 25% of people within 16 months. Mild to moderate local skin reactions are a common product of imiquimod s anti-tumour activity and are associated with clinical clearance of lesions in response to imiquimod therapy. AREAS OF UNCERTAINTY It is not known how imiquimod compares with surgical excision, as no head-to-head trials have been reported. There are also few direct comparisons for imiquimod against other treatments and no specific data regarding imiquimod efficacy when other treatments are contraindicated. Imiquimod has utility for multiple sbcc lesions but there are limited safety data, and efficacy is not consistent across multiple lesions. Clearance rates diminish when imiquimod is used on large sbcc lesions, and imiquimod has not been evaluated for sbcc and SK lesions located in high-risk sites such as areas of the face, including eyes, nose, mouth or ears, and SK lesions on the hands or forearms. WHAT DOES NPS SAY? Imiquimod presents an alternative treatment for sbcc and SK. Before considering imiquimod for use on sbccs, assess lesions for prognostic factors that increase the probability of complete clearance. Excisional surgery has a higher clearance and lower recurrence rate for sbcc than topical treatments. Field treatment for solar keratoses must not exceed a treatment area of 25 cm 2. Be aware that local skin reactions are common and reflect the pharmacological effects of imiquimod associated with clearance. Before prescribing, show patients how to apply imiquimod cream and advise on the correct dose required for the prescribed indication. Use imiquimod on clinically favourable sbcc lesions that are low risk Topical treatments are options for treating low-risk lesions, 3 while surgery or radiotherapy should remain the standard for high-risk BCC lesions. 3,7 Identify low risk of recurrence for individual lesions based on prognostic factors such as: smaller size (< 2 cm) 3,4,13 well-defined clinical margins 3,4,13 not fixed to nerve or vascular tissue 3,4,13 not located in sites such as the central face (especially around the eyes, nose, lips and ears) 3,4,13 or lower legs. 4,13 Control rates diminish with increasing size and depth of lesions such that 5-year overall estimated control rates of 95% are seen for lesions 2 cm 2 but only 88% for lesions 2 5 cm 2 in size. 4 Large tumours (> cm 2 ) are usually deeply invasive beyond the subcutaneous tissue and are difficult to treat. 4 There are limited data for large sbcc treated with imiquimod. One small openlabel trial showed lower clearance rates of 65% (95% confidence interval [CI] 38% to 86%) for larger tumours > 7.25 cm 2 compared with 90% (95% CI 78% to 97%) for smaller tumours ranging from 2.0 to 7.25 cm 2. 2,16 Multiple lesions may be problematic and should be considered for specialist referral. 4 In the only trial involving multiple sbccs treated by imiquimod, although the overall tumour response was comparable to those in studies of individual lesions, clearance of one tumour was not predictive of a response in all tumours, and some sites (e.g. lower limb) were associated with a lower clearance rate. 17 Ensure continuous monitoring of all lesions during follow-up of patients with multiple lesions.
12 12 Imiquimod cream (Aldara) Imiquimod has not been evaluated for the treatment of sbcc lesions located within 1 cm of the hairline, eyes, nose, mouth or ears. 2 Higher recurrence rates for BCC have been observed for all treatment modalities in the facial region especially in proximity to the nose, eyes and ears compared with non-facial sites. 4 In the US and UK, imiquimod is not approved for lesions located on the face. 15 Only use imiquimod on solar keratoses within a 25 cm 2 field Do not use imiquimod to treat an area of SK in excess of 25 cm 2 due to the potential to cause local skin reactions (LSRs). 2 Use imiquimod on suitable SK lesions of the face and scalp Imiquimod has not been evaluated for treatment on the eyelids, the inside of the nostrils, ears, or the lip area inside the vermillion border and there are insufficient data to support the use of imiquimod on the hands and forearms. Imiquimod is not recommended for the treatment of SK lesions when there is marked hyperkeratosis or hypertrophy. 2 Not PBS listed for genital warts Although not having a PBS-subsidised indication for treatment of external genital and peri-anal warts (condyloma acuminata) in adults, imiquimod is TGA approved for this use. Imiquimod is a common selfapplied treatment for external genital warts and may be used as adjunctive treatment to cryotherapy. 18 Assess treatment success during and after treatment Assess the clinical outcome of imiquimod therapy after treatment following regeneration of the treated area. For sbcc assess the treated skin at least 6 12 weeks after completion of treatment. 2 Longer follow-up periods for some people may be necessary, as persistent residual local effects such as persistent redness can complicate clinical evaluation. 19,20 More than 40% of people will develop a second BCC within 3 years, representing a 10-fold increase in risk compared with the general population. 4 Perform biopsy if there is any doubt about residual tumour. If histological evidence of clearance is not available, follow up initially at 3 months and then 6 12-monthly for up to 3 years. Include a full skin check for new lesions as well as inspection of the site of the original lesion at each examination. 4 For SK, assess clearance after a 4-week treatmentfree period and if any lesions persist repeat treatment for another 4 weeks. 2 Imiquimod is not indicated for use on previously treated sbcc and there is no clinical experience in locally recurrent lesions or incompletely cleared lesions. 2 One study reported definitive outcomes through surgical intervention and suggested practitioners discuss failure rates with patients and recommend surgery for those with persistent lesions. 21 The first opportunity for treatment is the best opportunity to achieve cure. 4 How does it compare? Surgical excision is currently the primary treatment for BCC In a systematic review of randomised controlled trials (RCTs) using indirect comparisons of clinical recurrence at 3 5 years and early treatment failure (measured histologically within 6 months), surgery and radiotherapy were the most effective treatments for BCC, with the lowest failure rates seen for surgery. 7 Another large meta-analysis of treatment of 16,066 lesions reported recurrence rates of < 5% for non-morpheaform BCC (< 2 cm 2 ) after surgical excision with margins ranging from 2 5 mm, and an average recurrence rate of 27% when a positive margin was found. 6 Recurrence rates for radiotherapy were comparable, 4 although a lower 4-year risk of recurrence was reported for surgical excision over radiotherapy in an RCT of treatment of facial lesions. 7,22 Surgery is also associated with more favourable long-term cosmetic outcomes than radiotherapy, 23 though outcomes can be influenced by the skill of the operator. 4
13 Imiquimod cream (Aldara) 13 No head-to-head comparison of imiquimod with surgery There are few studies with sufficient long-term follow-up and subgroup analysis (e.g. lesion type, size and site, patient, operator differences) comparing the range of treatment options available for BCC. 3,7,24 Most studies have been done on low-risk BCCs. Other treatments may have some utility but need to be evaluated against surgery as the gold standard. To date, no head-to-head trials have been reported comparing imiquimod with surgery. 7,14 One RCT is ongoing. 25 Imiquimod is effective for short-term clearance of small primary sbcc In a pooled analysis of early (within 6 months) histologically confirmed failed treatment of BCC lesions, there were significantly fewer treatment failures for lesions treated with imiquimod than for those treated with vehicle-control cream (risk ratio 0.25, 95% CI 0.19 to 0.32). 7 The analysis included five RCTs for imiquimod, 19,26 29 although two of the trials included nodular BCC lesions, which are associated with more modest control rates. 7 For sbcc vehicle-controlled trials alone, the reported recurrence rate for standard once-daily imiquimod treatment 5 or 7 days per week for 6 weeks ranged from 13% to 20%. 19,27,28 Lesions were cm 2 in size and located on the neck, face, forehead, upper extremity, trunk or lower extremity. A more recent meta-analysis considering 15 randomised and non-randomised trials (uncontrolled and vehiclecontrolled) comprising 1088 sbcc lesions showed an overall recurrence rate of 14% (95% CI 10% to 18%); however, significant heterogeneity was reported, likely due to inclusion of a range of treatment and dosing schedules. 24 Imiquimod has a role in treating small primary sbcc although sustained tumour clearance has not been established for imiquimod treatment, with relatively few studies considering clearance rates beyond 1 year. Two open-label, 5-year followup studies reported estimated sustained clearance rates, and overall estimated proportion of patients in whom lesions clinically cleared and remained clear, in the range of 85% to 87% and 78% to 80%, respectively, with more than 70% of recurrences occurring within the first 2 years. 20,30 Imiquimod is effective for short-term clearance of SK Several recent meta-analyses demonstrate that, compared with placebo, imiquimod is an effective therapy for complete clearance of SK lesions located on the face and scalp within treatment areas up to 25 cm 2. 8,31,32 In one pooled analysis of five randomised double-blind trials involving 1293 patients, complete clearance occurred in 50% of patients treated with imiquimod compared with 5% for vehicle-control, with the secondary outcome of partial clearance (> 75%) reported in 65% of patients in the imiquimod group, compared with 11% for vehicle-control. 32 The studies included involved treatment with one sachet of imiquimod two or three times per week for weeks Higher clinical clearance rates have been observed using imiquimod; however, these were in clinical trials in which the size of the treatment area was greater than the recommended 25 cm Subclinical lesions may become apparent in the field of treatment during imiquimod therapy. In clinical studies, higher clearance rates were seen for patients with an increase in lesion count relative to the number of SK lesions present at baseline. 34,35,37 Recurrence rates of 25% within the treatment area have been reported for three doses per week after a median of 16 months. 39,40 There are limited long-term recurrence data beyond 16 months. There are also few data comparing imiquimod with other topical agents or treatment modalities. Imiquimod is reported to be of similar efficacy to cryotherapy and 3% diclofenac (in 2.5% hyaluronic acid) 8 ; however, more studies are needed to confirm its non-inferiority to 5-FU. 8,41 Clearance rates for imiquimod relate to treatment exposure Dose response effects for imiquimod treatment have been investigated in several trials comparing treatments ranging from once or twice daily dosing to 3, 5 or 7 days per week. 26,27,29,42 In a pooled analysis comparing higher- and lower-dose treatment regimens, a 51% (95% CI 35% to 75%) reduction in histologically confirmed risk of BCC recurrence was reported for higher-dose treatments; however, this was also associated with more LSRs. 7
14 14 Imiquimod cream (Aldara) Alternative doses have also been studied for SK involving two or three doses per week in a continuous treatment, cyclical dosing with a treatment-free period, 33,43,44 and lower concentration imiquimod creams. 45 Lower recurrence was observed in an open-label follow-up study in patients treating SK using three applications of imiquimod per week compared with two. 39 Overall, the available data suggest that reducing the dosing frequency does lead to improved tolerability of LSRs but reduces efficacy. Safety issues For all indications, imiquimod causes a strong local inflammatory reaction that leads to mild to moderate application-site reactions in most people, and a rest from treatment may be required. In trials, on rare occasions these reactions were accompanied or preceded by systemic flu-like signs and symptoms or exacerbations of inflammatory skin conditions (e.g. dermatitis or psoriasis). 2 Report suspected adverse reactions to the TGA online ( or by using the Blue Card distributed three times a year with Australian Prescriber. For information about reporting adverse reactions, see the TGA website ( Inflammatory reactions are common and may cause discomfort In clinical studies inflammatory reactions to the cream included oedema, redness, erosion, flaking/scaling, skin thickening and scabbing/ crusting, which occur in more than 50% of patients being treated for sbcc and SK. 2 Other mild skin irritations such as burning, itching and pain were also common for both these indications, and skin infections at the treatment site occurred in about 1% of patients for both sbcc and SK. 2 Although common, LSRs are mostly mild to moderate and tolerated by most people. 7,14 LSR intensity is highest during the treatment period but subsides to levels at or below baseline during the post-treatment period. 19,30,46 With less-frequent dosing, the incidence and severity of LSRs decline. 19,29,42 The intensity of LSRs closely relate to imiquimod s anti-tumour activity such that clearance rate rises with increasing intensity of LSRs. Clinical trials have found significant associations between severity of LSRs and clinical clearance rate for sbcc 19,29,42,46 and SK. 2,34,35,37 Thus an absence of LSRs during imiquimod treatment may be indicative of non-compliance with therapy or a lack of antitumour response mechanism, 46 and could have utility for predicting which patients will not achieve complete clearance of sbcc. 19,46 Advise patients that the LSRs are a normal and expected part of the treatment process and well tolerated by most people. 19 Clinical monitoring of LSRs is advised, as complete clearance may be achieved without the presence of erosion, scabbing or crusting, 19 and there are instances of failed treatment even when LSRs occurred. 21 Advise patients to adhere to the treatment schedule and not to apply excess cream to the treatment area Treatment schedules for imiquimod reflect a balance between achieving therapeutic efficacy with tolerability of LSRs. A treatment regimen of 5 days/week is recommended for sbcc because of the improved safety profile and similar clearance rates with this dose compared with daily treatment, 19 while for SK the recommended treatment schedule is three times per week. 2 Higher than recommended doses may lead to increased LSRs. 2 The contents of one 250 mg sachet or the amount delivered in one full actuation of the pump may be more than is required for a single application. Advise patients against applying excessive cream to the treatment area and to discard unused cream at each application. In the event of a severe LSR, the cream should be removed by washing the treatment area with mild soap and water. Interruptions in treatment are recommended to allow severe inflammatory reactions to resolve. 2 In clinical studies there were few discontinuations due to LSRs, and rest periods were taken around 3 4 weeks after treatment started but occurred as early as the first and as late as the last week of treatment. 19,46
15 Imiquimod cream (Aldara) 15 Imiquimod may cause local skin pigmentation changes following treatment For all indications, cases of localised hypoand hyperpigmentation that may be permanent have been reported after imiquimod use. 2 In one long-term follow-up study of sbccs treated by imiquimod, skin quality changes were most pronounced at 12-week follow-up and 1 year post-treatment and remained stable up to 5 years post treatment. 20 During follow-up compared with baseline, hypopigmentation worsened in 48% 2 and 57% 30 of people, while changes in hyperpigmentation were rare. 2,20,30 Only use imiquimod in people over 18 years who will comply with the treatment regimen The safety and efficacy of imiquimod in patients under age 18 have not been established. 2 Only use imiquimod in people who are able and willing to adhere to the treatment schedule. Use cautiously in immunocompromised patients Imiquimod is not PBS listed for use in immunocompromised patients and there is limited clinical experience with use in these patients. Use with caution in organ transplant patients, those with a pre-existing autoimmune or immune deficiency condition or those receiving immunosuppressive medication because of the potential to exacerbate inflammatory conditions of the skin, including graft-versus-host disease. 2 Use cautiously in people with a high sun exposure Use imiquimod with caution in people with fair skin or those with a high sun exposure. 2 People treated with imiquimod may have heightened sensitivity to sunlight and should avoid exposure to sunlight or artificial solar exposure. Do not use imiquimod on sunburned skin until the area is fully recovered. The potential therapeutic effects of using sunscreens simultaneously with imiquimod have not been examined in clinical trials. Do not use during pregnancy or lactation Due to an absence of clinical studies do not use imiquimod in pregnant or lactating women. 2 Reason for PBS listing The PBAC recommended listing imiquimod 5% cream 2 g multi-use pump on the PBS for the same sbcc indication as the existing single-use sachet. The PBS listing for the sachets was granted on the basis of acceptable cost-effectiveness compared with placebo for patients who cannot have surgical excision, cryotherapy or curettage. The sachets have been PBS-listed since 1 December The pump presentation is considered interchangeable with the sachets and is suitable for substitution at the point of dispensing. Dosing issues Imiquimod multi-use pump offers an alternative to single-use sachets Imiquimod is available as a multi-use pump or single-use sachet presentation. The maximum quantity is one pack of two pumps available with one repeat, or one pack of 12 sachets available with one repeat. Each pump contains 2 g of cream, of which 1.5 g is available due to the operation of an airless pump system that dispenses about 62 mg per actuation. One pump contains a quantity equivalent to six sachets, and four pump actuations are equivalent to one sachet. 2 Ensure patients are provided with the appropriate instructions to match the presentation, as instructions for use differ. The sachets are single use and not intended to be reused after one application. Use the contents of the pump within 4 weeks after opening and discard after this time. 2
16 16 Imiquimod cream (Aldara) Information for patients Discuss treatment selection for sbcc with patients and inform patients that: imiquimod may not be as effective as removal by surgical excision there is a one in five chance that the lesion will not clear or will return within 5 10 years surgical removal may be required for lesions not cleared by imiquimod treatment. Imiquimod treatment requires an extended treatment period of 6 weeks for sbcc or up to 16 weeks for solar keratosis. When treating with imiquimod, schedule an appointment to review treatment response after 2 3 weeks and discuss how to manage a rest from treatment if local skin reactions become intolerable. Instruct patients on the proper use of imiquimod cream and refer them to the package insert for further illustrated instructions on how to apply the treatment. For each treatment, patients are advised to: wash hands and the treatment area with soap and water and dry thoroughly dispense a small amount of imiquimod cream onto the fingertips apply the cream to the sbcc and about 1 cm of the surrounding skin (the width of a fingertip) or for solar keratosis apply to an area not larger than 25 cm 2 (about four square inches) rub gently until the cream is no longer visible discard any unused cream remaining from a sachet or pump actuation wash hands with soap and water to remove residual cream wash the cream off with soap and water after 6 10 hours. Refer patients to the Aldara consumer medicine information (CMI) leaflet for more detailed information available at medicines/aldara-cream. Advise patients of common adverse events and safety precautions associated with imiquimod use. Inform patients as follows. Do not use imiquimod near the hairline, eyes, ears, nose or lips and only apply to lesions for which it is prescribed. A local skin reaction at the treatment site is expected and, in severe cases, an interruption from treatment may be needed. Resume treatment after the reaction subsides. When a break in treatment is taken, do not make up missed doses. Stop applying imiquimod as soon as possible if the skin becomes infected or if nausea, fever, tiredness and influenza-like symptoms occur. Wash the treatment area and return to see your doctor. Do not use imiquimod on sunburned skin, wait until the area has healed. Protect skin treated with imiquimod from sunlight or artificial tanning for the duration of the treatment period. Do not cover the treatment area with bandages or other dressings but, if necessary, a cotton gauze may be used to manage skin reactions (e.g. weeping sores). Areas treated with imiquimod may appear lighter or darker than the surrounding skin and for some people these changes will be permanent. The multi-use pump should be primed a number of times until cream appears. Use only enough cream to cover the treatment area, as excessive use can worsen unwanted side effects. The contents of the multi-use pump should be used within 4 weeks of opening and any unused cream should be carefully discarded. Do not use cosmetics or any other skin care products on the treatment area unless advised to do so by a doctor. Do not use imiquimod if you become pregnant. 2
17 Imiquimod cream (Aldara) 17 * In practice most clinicians recommend a treatment regimen of 2 3 times a week for 2 4-week cycles. 4 Treatment schedules for imiquimod vary across indications The treatment area should be washed and dried before applying imiquimod cream to the affected area. A thin layer of the cream should be rubbed into the skin until it is no longer visible. The treatment should be left on for about 8 hours per application (usually overnight) and washed off with mild soap afterwards. Advise patients not to bathe, shower or swim during this time. There is no need to apply an occlusive dressing to the treatment area. Hands should be washed before and after applying the treatment. 2 The recommended treatment schedules are as follows: for superficial BCC apply imiquimod cream to the treatment area including a 1 cm margin around the lesion, once-daily, 5 days a week for 6 weeks for SK* apply imiquimod cream to a treatment area no larger than 25cm 2, three times a week for up to 16 weeks for continuous dosing, or for cycles of 4-week intervals followed by a 4-week treatment-free period for cyclical dosing. Advise patients to apply imiquimod sparingly. Four full actuations of the imiquimod pump, or 250 mg of cream, is reported to be sufficient to cover up to 386 cm 2 of skin area. 47 Therefore, one actuation of the pump (about 62 mg of cream) is in excess of what is likely to be required for a single dose in sbcc and nearly four times the treatable area recommended for SK. Interrupt treatment if excessive inflammation occurs Consider rest periods of several days during therapy to resolve or decrease the intensity of severe or intolerable LSRs. In clinical trials, breaks in treatment were reported for 10 19% of patients who missed a median of five prescribed doses. 19,46 LSRs decrease in intensity or resolve after cessation of treatment; treatment may resume after the reaction subsides. 2 If rest periods are taken, missed doses should not be taken and there is no need to prolong therapy. 2 The maximum PBS quantity of one pack of 12 sachets with one repeat or two pumps with one repeat implies that all patients will take a rest period of at least one week during the 6-week course. If a rest is not needed, stop treatment once cream is all used. REFERENCES 1. Stanley MA. Clin Exp Dermatol 2002;27: inova Pharmaceuticals. Aldara product information. 11 February Telfer NR, et al. Br J Dermatol 2008;159: Cancer Council Australia and Australian Cancer Network. Basal cell carcinoma, squamous cell carcinoma (and related lesions) - a guide to clinical management content/pdf/healthprofessionals/clinicalguidelines/ Basal_cell_carcinoma_Squamous_cell_carcinoma_ Guide_Nov_2008-Final_with_Corrigendums.pdf (accessed 26 November 2012). 5. Walker P, Hill D. Australas J Dermatol 2006;47: Gulleth Y, et al. Plast Reconstr Surg 2010;126: Bath-Hextall FJ, et al. Cochrane Database Syst Rev 2007:CD Gupta AK, et al. Cochrane Database Syst Rev 2012;12:CD Glogau RG. J Am Acad Dermatol 2000;42: Marks R, et al. Br J Dermatol 1986;115: Therapeutic Guidelines Limited. etg Complete. Solar keratoses (accessed 6 December 2012). 12. Pharmaceutical Benefits Advisory Committee. Public Summary Document. Imiquimod, cream, 125 mg per 250 mg single use sachets (5%), 12, Aldara July, internet/main/publishing.nsf/content/pbac-psdimiquimod-july06 (accessed 16 January 2013). 13. National Institute for Health and Clinical Excellence. Improving outcomes for people with skin tumours including melanoma: The management of low-risk basal cell carconomas in the community nicemedia/live/10901/48878/48878.pdf (accessed 29 November 2012). 14. Ormerod A, et al. Clin Evid (Online) 2010:1719.
18 18 Imiquimod cream (Aldara) REFERENCES Continued 15. Raasch B. Clin Cosmet Investig Dermatol 2009;2: Shumack S, et al. Arch Dermatol 2004;140: Marks R, et al. Arch Dermatol 2004;140: Professional advisory board of the Australia and New Zealand HPV Project. Guidelines for the management of genital HPV in Australia and New Zealand preparation%20ck%20khong/sexual%20health/ HPV%20guidelines.pdf (accessed 6 December 2012). 19. Geisse J, et al. J Am Acad Dermatol 2004;50: Gollnick H, et al. Eur J Dermatol 2008;18: Murphy ME, et al. Dermatol Surg 2008;34: Avril MF, et al. Br J Cancer 1997;76: Petit JY, et al. Plast Reconstr Surg 2000;105: Roozeboom MH, et al. Br J Dermatol 2012;167: Ozolins M, et al. Trials 2010;11: Beutner KR, et al. J Am Acad Dermatol 1999;41: Geisse JK, et al. J Am Acad Dermatol 2002;47: Schulze HJ, et al. Br J Dermatol 2005;152: Shumack S, et al. Arch Dermatol 2002;138: Quirk C, et al. Cutis 2010;85: Falagas ME, et al. J Am Acad Dermatol 2006;55: Hadley G, et al. J Invest Dermatol 2006;126: Chen K, et al. Australas J Dermatol 2003;44: Korman N, et al. Arch Dermatol 2005;141: Lebwohl M, et al. J Am Acad Dermatol 2004;50: Stockfleth E, et al. Arch Dermatol 2002;138: Szeimies RM, et al. J Am Acad Dermatol 2004;51: Stockfleth E, et al. Br J Dermatol 2007;157 Suppl 2: Lee PK, et al. Dermatol Surg 2005;31: Stockfleth E, et al. Arch Dermatol 2004;140: Gupta AK, et al. J Cutan Med Surg 2005;9: Marks R, et al. J Am Acad Dermatol 2001;44: Alomar A, et al. Br J Dermatol 2007;157: Jorizzo J, et al. J Am Acad Dermatol 2007;57: Hanke CW, et al. J Am Acad Dermatol 2010;62: Gollnick H, et al. Eur J Dermatol 2005;15: Berman B, et al. Dermatol Surg 2004;30: Date published: April 2013 The information contained in NPS RADAR is derived from a critical analysis of a wide range of authoritative evidence and is current at the time of publication. Any treatment decisions based on the information provided in NPS RADAR should be made in the context of the clinical circumstances of each patient. NPS RADAR articles may be updated when there is new evidence about safety or efficacy, or in case of regulatory or PBS listing changes. Please refer to for the most recent version as well as any supplementary information.
19 Sitagliptin with simvastatin (Juvicor) 19 FULL REVIEW Sitagliptin with simvastatin (Juvicor) Fixed-dose combination therapy for type 2 diabetes and hypercholesterolaemia A fixed-dose combination medicine for treating two different conditions KEY POINTS There are no clinical data comparing fixed-dose combination sitagliptin and simvastatin with each taken separately In patients with type 2 diabetes and hyperlipidaemia there is no evidence of additional clinical benefit using the fixed-dose combination formulation over concomitant use of sitagliptin and simvastatin taken separately. Fixed-dose combination products have benefits but may cause confusion for some Benefits include reducing pill burden and out-of-pocket cost to the patient. Advise patients starting Juvicor to return their previous supply of sitagliptin and simvastatin to their pharmacy. Statins treat hyperlipidaemia and reduce overall cardiovascular risk Statin monotherapy is effective as first-line therapy for reducing the risk of cardiovascular events in people with type 2 diabetes and hyperlipidaemia. Sitagliptin improves glycaemic control in type 2 diabetes There are no data for an effect of sitagliptin on macrovascular disease or diabetes-related complications or mortality. ADDITIONAL INFORMATION healthpro/explanatorynotes/gs-lipid-loweringdrugs ADDITIONAL INFORMATION healthpro/explanatorynotes/section1/nursepractitioner PBS listing Authority required (Streamlined) For use in patients with type 2 diabetes who satisfy the criteria for prescribing dipeptidyl peptidase-4 (DPP-4) inhibitors and who meet the criteria set out in the General Statement for Lipid-Lowering Drugs 1 on the PBS website. May be prescribed by nurse practitioners Authorised nurse practitioners may prescribe this medicine. See the PBS website for more information on nurse practitioner PBS prescribing. What is it? Juvicor is the first fixed-dose combination (FDC) medicine approved by the Therapeutic Goods Administration (TGA) for treating conditions within two different body systems, in this case cardiovascular and endocrine. Sitagliptin is a DPP-4 inhibitor. DPP-4 inhibitors ( gliptins ) are a recently introduced class of oral drugs for type 2 diabetes. They act to block metabolism by the DPP-4 enzyme of incretin hormones, including glucagon-like peptide and glucose-dependent insulinotropic polypeptide, which are secreted by the intestine in response to food. 2 This increases the levels of active incretins, prolonging their effect in stimulating insulin release and decreasing glucagon secretion. 2 Simvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme that catalyses an early and rate-limiting step in the biosynthesis of cholesterol. This results in reduced levels of total plasma cholesterol, low-density lipoprotein cholesterol (LDL-c) and very-low-density lipoprotein cholesterol. In addition, simvastatin increases high-density lipoprotein cholesterol (HDL-c) and reduces plasma triglyceride levels. 3
20 20 Sitagliptin with simvastatin (Juvicor) ADDITIONAL INFORMATION For more information see NPS News August 2012: Type 2 diabetes ( news_80). Who is it for? Juvicor is for patients with type 2 diabetes who satisfy the criteria for prescription of dipeptidyl peptidase-4 (DPP-4) inhibitors and who meet the criteria set out in the PBS General Statement for Lipid-Lowering Drugs (see PBS listing on previous page). While Juvicor has the potential to reduce a patient s pill burden and overall medicine costs, there is no clinical reason to switch patients to Juvicor if they are responding well to other type 2 diabetes and lipid-modifying medicines. 4 Choose type 2 diabetes and lipidmodifying therapy according to the clinical needs of the individual Do not choose therapy solely based on the possibility of improved adherence, convenience or cost advantages of the FDC preparation. While FDC medicines are associated with a significant improvement in adherence, 5 7 there is no evidence that improved adherence alone translates to improved health outcomes. 4,8,9 Use of once-daily formulations and combination products does not by itself ensure improved adherence in every individual. A large number of factors can contribute to non-adherence, such as the lack of a support network, a language barrier limiting communication or a fear of adverse effects. 4,8,9 Where does it fit? Primary prevention of cardiovascular disease in people with type 2 diabetes Having type 2 diabetes more than doubles the risk of mortality due to cardiovascular causes. 10,11 In the short term (3 5 years) tight glycaemic control has a limited effect on cardiovascular outcomes compared with lowering lipids 18 or blood pressure. 12,19 Lifestyle changes such as smoking cessation, consuming a diet rich in vegetables and fruit and doing at least 30 minutes of physical activity on most, preferably every, day of the week is recommended in all people to reduce cardiovascular risk. 20 People at greatest absolute cardiovascular risk derive the most benefit from lipid-lowering treatment. 20 Dual therapy for glycaemic control Glycaemic control with monotherapy tends to deteriorate with time, as diabetes is a progressive disease. 21,22 Metformin (or, when this is contraindicated, a sulfonylurea) is the drug of first choice for people with type 2 diabetes who have inadequate glycaemic control after 3 months of making lifestyle changes. 23,24 Adding a sulfonylurea to metformin is the preferred dual therapy for glycaemic control when monotherapy is no longer effective Sitagliptin with metformin or a sulfonylurea is an option for people who require dual therapy but who are intolerant of, or have a contraindication to the combination of, metformin and a sulfonylurea. 24,28 How does it compare? There are no other PBS-listed FDC products currently available for treating type 2 diabetes and hyperlipidaemia. Juvicor compared with sitagliptin and simvastatin taken concomitantly There is no evidence of additional clinical benefit using the FDC formulation over concomitant use of sitagliptin and simvastatin taken separately. Bioequivalence studies established that the Juvicor 100 mg/10 mg, 100 mg/20 mg or 100 mg/40 mg taken once daily are bioequivalent with the corresponding strengths of sitagliptin and simvastatin taken concomitantly once daily. 1 Reducing microvascular complications and safety profile There are no long-term data on the effects of gliptins on microvascular complications and mortality in people with type 2 diabetes In comparison there is evidence that metformin, sulfonylureas and insulin reduce the incidence of microvascular complications 21,22 and, although they each have a range of possible adverse effects, their long-term safety profile is better understood than that of sitagliptin. 35 A systematic assessment of cardiovascular adverse events reported in 19 clinical trials of sitagliptin, clinical trials of vildagliptin 37 and eight clinical trials of saxagliptin 38 found no increased rate of cardiovascular events in people treated for type 2 diabetes, although these were post-hoc analyses of adverse effects in trials with different comparators, and patients were only followed for up to 104 weeks.
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
The submission positioned dimethyl fumarate as a first-line treatment option.
 Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Escitalopram (Lexapro) for major depressive disorder
 Escitalopram (Lexapro) for major depressive disorder Summary PBS listing Reason for listing Place in therapy Restricted benefit: Major depressive disorders. Escitalopram was listed on the basis of cost-minimisation
Escitalopram (Lexapro) for major depressive disorder Summary PBS listing Reason for listing Place in therapy Restricted benefit: Major depressive disorders. Escitalopram was listed on the basis of cost-minimisation
Medications for chronic pain
 Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance
 Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION
 MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Clinical evaluation D The basic
MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Clinical evaluation D The basic
The Pharmacological Management of Cancer Pain in Adults. Clinical Audit Tool
 The Pharmacological Management of Cancer Pain in Adults Clinical Audit Tool 2015 This clinical audit tool accompanies the Pharmacological Management of Cancer Pain in Adults NCEC National Clinical Guideline
The Pharmacological Management of Cancer Pain in Adults Clinical Audit Tool 2015 This clinical audit tool accompanies the Pharmacological Management of Cancer Pain in Adults NCEC National Clinical Guideline
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN
 MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
MOH CLINICAL PRACTICE GUIDELINES 2/2008 Prescribing of Benzodiazepines
 MOH CLINICL PRCTICE GUIELINES 2/2008 Prescribing of Benzodiazepines College of Family Physicians, Singapore cademy of Medicine, Singapore Executive summary of recommendations etails of recommendations
MOH CLINICL PRCTICE GUIELINES 2/2008 Prescribing of Benzodiazepines College of Family Physicians, Singapore cademy of Medicine, Singapore Executive summary of recommendations etails of recommendations
Abstral Prescriber and Pharmacist Guide
 Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Drug Class Review Neuropathic Pain
 Drug Class Review Neuropathic Pain Final Update 1 Report June 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of the is to summarize key information
Drug Class Review Neuropathic Pain Final Update 1 Report June 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of the is to summarize key information
Opioid Prescribing for Chronic Pain: Guidelines for Marin County Clinicians
 Opioid Prescribing for Chronic Pain: Guidelines for Marin County Clinicians Although prescription pain medications are intended to improve the lives of people with pain, their increased use and misuse
Opioid Prescribing for Chronic Pain: Guidelines for Marin County Clinicians Although prescription pain medications are intended to improve the lives of people with pain, their increased use and misuse
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Drug treatments for neuropathic pain
 Understanding NICE guidance Information for people who use NHS services Drug treatments for neuropathic pain NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases
Understanding NICE guidance Information for people who use NHS services Drug treatments for neuropathic pain NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases
2. Background This indication of rivaroxaban had not previously been considered by the PBAC.
 PUBLIC SUMMARY DOCUMENT Product: Rivaroxaban, tablets, 15mg and 20mg, Xarelto Sponsor: Bayer Australia Ltd Date of PBAC Consideration: March 2013 1. Purpose of Application The application requested the
PUBLIC SUMMARY DOCUMENT Product: Rivaroxaban, tablets, 15mg and 20mg, Xarelto Sponsor: Bayer Australia Ltd Date of PBAC Consideration: March 2013 1. Purpose of Application The application requested the
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
Guidelines for the management of basal cell carcinoma N.R. Telfer, G.B. Colver* and C.A. Morton
 GUIDELINES DOI 10.1111/j.1365-2133.2008.08666.x Guidelines for the management of basal cell carcinoma N.R. Telfer, G.B. Colver* and C.A. Morton Dermatology Centre, Salford Royal Hospitals NHS Foundation
GUIDELINES DOI 10.1111/j.1365-2133.2008.08666.x Guidelines for the management of basal cell carcinoma N.R. Telfer, G.B. Colver* and C.A. Morton Dermatology Centre, Salford Royal Hospitals NHS Foundation
POST-TEST Pain Resource Professional Training Program University of Wisconsin Hospital & Clinics
 POST-TEST University of Wisconsin Hospital & Clinics True/False/Don't Know - Circle the correct answer T F D 1. Changes in vital signs are reliable indicators of pain severity. T F D 2. Because of an underdeveloped
POST-TEST University of Wisconsin Hospital & Clinics True/False/Don't Know - Circle the correct answer T F D 1. Changes in vital signs are reliable indicators of pain severity. T F D 2. Because of an underdeveloped
Questions and answers on breast cancer Guideline 10: The management of persistent pain after breast cancer treatment
 Questions and answers on breast cancer Guideline 10: The management of persistent pain after breast cancer treatment I ve had breast cancer treatment, and now I m having pain. Does this mean the cancer
Questions and answers on breast cancer Guideline 10: The management of persistent pain after breast cancer treatment I ve had breast cancer treatment, and now I m having pain. Does this mean the cancer
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Rivaroxaban (Xarelto) for preventing venous thromboembolism after hip or knee replacement surgery
 for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
for preventing venous thromboembolism after hip or knee replacement surgery (riv-ah-rocks-ah-ban) Summary Rivaroxaban is an oral anticoagulant and the first direct factor Xa inhibitor. Rivaroxaban has
Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Fentanyl patches (Durogesic) for chronic pain
 Fentanyl patches (Durogesic) for chronic pain FENT-a-nil Summary Oral morphine is preferred when an opioid is required for severe chronic pain, because of its familiarity, availability and the ease of
Fentanyl patches (Durogesic) for chronic pain FENT-a-nil Summary Oral morphine is preferred when an opioid is required for severe chronic pain, because of its familiarity, availability and the ease of
m y f o u n d a t i o n i n f o s h e e t
 Pain and Myeloma m y f o u n d a t i o n i n f o s h e e t Pain is the most common symptom of myeloma and can greatly affect all areas of your life, especially if it is untreated or poorly managed. This
Pain and Myeloma m y f o u n d a t i o n i n f o s h e e t Pain is the most common symptom of myeloma and can greatly affect all areas of your life, especially if it is untreated or poorly managed. This
A Guide to pain relief medicines For patients receiving Palliative Care
 A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE
 1 DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE ASSESSMENT/PROBLEM RECOGNITION 1. Did the staff and physician seek and document risk factors for depression and any history of depression? 2. Did staff
1 DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE ASSESSMENT/PROBLEM RECOGNITION 1. Did the staff and physician seek and document risk factors for depression and any history of depression? 2. Did staff
Test Content Outline Effective Date: June 9, 2014. Pain Management Nursing Board Certification Examination
 Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
Summary 1. Comparative-effectiveness
 Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Medical marijuana for pain and anxiety: A primer for methadone physicians. Meldon Kahan MD CPSO Methadone Prescribers Conference November 6, 2015
 Medical marijuana for pain and anxiety: A primer for methadone physicians Meldon Kahan MD CPSO Methadone Prescribers Conference November 6, 2015 Conflict of interest statement No conflict of interest to
Medical marijuana for pain and anxiety: A primer for methadone physicians Meldon Kahan MD CPSO Methadone Prescribers Conference November 6, 2015 Conflict of interest statement No conflict of interest to
Facts About Chickenpox and Shingles for Adults
 Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Care Manager Resources: Common Questions & Answers about Treatments for Depression
 Care Manager Resources: Common Questions & Answers about Treatments for Depression Questions about Medications 1. How do antidepressants work? Antidepressants help restore the correct balance of certain
Care Manager Resources: Common Questions & Answers about Treatments for Depression Questions about Medications 1. How do antidepressants work? Antidepressants help restore the correct balance of certain
MULTIPLE SCLEROSIS AUSTRALIA MULTIPLE SCLEROSIS RESEARCH AUSTRALIA
 MULTIPLE SCLEROSIS AUSTRALIA MULTIPLE SCLEROSIS RESEARCH AUSTRALIA Submission to the ACT Legislative Assembly Health, Ageing, Community and Social Services Inquiry into the exposure draft of the Drugs
MULTIPLE SCLEROSIS AUSTRALIA MULTIPLE SCLEROSIS RESEARCH AUSTRALIA Submission to the ACT Legislative Assembly Health, Ageing, Community and Social Services Inquiry into the exposure draft of the Drugs
NICE Clinical guideline 23
 NICE Clinical guideline 23 Depression Management of depression in primary and secondary care Consultation on amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised
NICE Clinical guideline 23 Depression Management of depression in primary and secondary care Consultation on amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised
Review of Pharmacological Pain Management
 Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Summary of the risk management plan (RMP) for Cerdelga (eliglustat)
 EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
EMA/743948/2014 Summary of the risk management plan (RMP) for Cerdelga (eliglustat) This is a summary of the risk management plan (RMP) for Cerdelga, which details the measures to be taken in order to
Nalmefene for reducing alcohol consumption in people with alcohol dependence
 Nalmefene for reducing alcohol consumption in people with alcohol dependence Issued: November 2014 guidance.nice.org.uk/ta325 NICE has accredited the process used by the Centre for Health Technology Evaluation
Nalmefene for reducing alcohol consumption in people with alcohol dependence Issued: November 2014 guidance.nice.org.uk/ta325 NICE has accredited the process used by the Centre for Health Technology Evaluation
Basal Cell Carcinoma Affecting the Eye Your Treatment Explained
 Basal Cell Carcinoma Affecting the Eye Your Treatment Explained Patient Information Introduction This booklet is designed to give you information about having a Basal Cell Carcinoma near your eye and the
Basal Cell Carcinoma Affecting the Eye Your Treatment Explained Patient Information Introduction This booklet is designed to give you information about having a Basal Cell Carcinoma near your eye and the
NeuroStar TMS Therapy Patient Guide for Treating Depression
 NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
NeuroStar TMS Therapy Patient Guide for Treating Depression This NeuroStar TMS Therapy Patient Guide for Treating Depression provides important safety and use information for you to consider about treating
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Antipsychotic drug prescription for patients with dementia in long-term care. A practice guideline for physicians and caregivers
 SUPPLEMENT 1: (Supplementary Material for online publication) Antipsychotic drug prescription for patients with dementia in long-term care. A practice guideline for physicians and caregivers About this
SUPPLEMENT 1: (Supplementary Material for online publication) Antipsychotic drug prescription for patients with dementia in long-term care. A practice guideline for physicians and caregivers About this
Summary of the risk management plan (RMP) for Ofev (nintedanib)
 EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
Antidepressants and suicidal thoughts and behaviour. Pharmacovigilance Working Party. January 2008
 Antidepressants and suicidal thoughts and behaviour Pharmacovigilance Working Party January 2008 PhVWP PAR January 2008 Page 1/15 1. Introduction The Pharmacovigilance Working Party has on a number of
Antidepressants and suicidal thoughts and behaviour Pharmacovigilance Working Party January 2008 PhVWP PAR January 2008 Page 1/15 1. Introduction The Pharmacovigilance Working Party has on a number of
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment
 Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Understanding your Tecfidera treatment Information for patients who have been prescribed treatment with Tecfidera. (dimethyl fumarate) Contents About Multiple Sclerosis (MS) What is MS? Symptoms of MS
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Michigan Board of Nursing Guidelines for the Use of Controlled Substances for the Treatment of Pain
 JENNIFER M. GRANHOLM GOVERNOR STATE OF MICHIGAN DEPARTMENT OF COMMUNITY HEALTH LANSING JANET OLSZEWSKI DIRECTOR Michigan Board of Nursing Guidelines for the Use of Controlled Substances for the Treatment
JENNIFER M. GRANHOLM GOVERNOR STATE OF MICHIGAN DEPARTMENT OF COMMUNITY HEALTH LANSING JANET OLSZEWSKI DIRECTOR Michigan Board of Nursing Guidelines for the Use of Controlled Substances for the Treatment
KENTUCKY ADMINISTRATIVE REGULATIONS TITLE 201. GENERAL GOVERNMENT CABINET CHAPTER 9. BOARD OF MEDICAL LICENSURE
 KENTUCKY ADMINISTRATIVE REGULATIONS TITLE 201. GENERAL GOVERNMENT CABINET CHAPTER 9. BOARD OF MEDICAL LICENSURE 201 KAR 9:260. Professional standards for prescribing and dispensing controlled substances.
KENTUCKY ADMINISTRATIVE REGULATIONS TITLE 201. GENERAL GOVERNMENT CABINET CHAPTER 9. BOARD OF MEDICAL LICENSURE 201 KAR 9:260. Professional standards for prescribing and dispensing controlled substances.
Low back pain. Quick reference guide. Issue date: May 2009. Early management of persistent non-specific low back pain
 Issue date: May 2009 Low back pain Early management of persistent non-specific low back pain Developed by the National Collaborating Centre for Primary Care About this booklet This is a quick reference
Issue date: May 2009 Low back pain Early management of persistent non-specific low back pain Developed by the National Collaborating Centre for Primary Care About this booklet This is a quick reference
Informed Consent For Laser Hair Removal
 Informed Consent For Laser Hair Removal INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedures, its risks, as well as alternative treatment(s). It is important
Informed Consent For Laser Hair Removal INSTRUCTIONS This informed-consent document has been prepared to help inform you about laser procedures, its risks, as well as alternative treatment(s). It is important
Getting the best result from Opioid medicine. in the management of chronic pain
 Getting the best result from Opioid medicine in the management of chronic pain Your doctor has prescribed you opioid medicine to help you manage your chronic pain. This patient information leaflet gives
Getting the best result from Opioid medicine in the management of chronic pain Your doctor has prescribed you opioid medicine to help you manage your chronic pain. This patient information leaflet gives
ADVISORY OPINION THE USE OF CONTROLLED SUBSTANCES FOR THE TREATMENT OF CHRONIC PAIN
 Janice K. Brewer Governor Arizona State Board of Nursing 4747 North 7 th Street, Suite 200 Phoenix, AZ 85014-3655 Phone (602) 889-5150 Fax - (602) 889-5155 E-Mail: arizona@azbn.gov Home Page: http://www.azbn.gov
Janice K. Brewer Governor Arizona State Board of Nursing 4747 North 7 th Street, Suite 200 Phoenix, AZ 85014-3655 Phone (602) 889-5150 Fax - (602) 889-5155 E-Mail: arizona@azbn.gov Home Page: http://www.azbn.gov
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below
 Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
Name: generic (trade) Rivaroxaban (Xarelto ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) RIVAROXABAN RECOMMENDED see specific recommendations for licensed indications below What it is Indications
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain
 Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended)
 Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
Intravenous Methyl Prednisolone in Multiple Sclerosis
 Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
(a) New Oral Anticoagulants & (b) Neuropathic Pain
 Evidence Updates: (a) New Oral Anticoagulants & (b) Neuropathic Pain Brendalynn Ens, RN, MN, CCN(c) CADTH-Saskatchewan Gaetanne Murphy, BSc Pharm CADTH-Edmonton CADTH s Programs HTA CDR Health Technology
Evidence Updates: (a) New Oral Anticoagulants & (b) Neuropathic Pain Brendalynn Ens, RN, MN, CCN(c) CADTH-Saskatchewan Gaetanne Murphy, BSc Pharm CADTH-Edmonton CADTH s Programs HTA CDR Health Technology
medicineupdate Tramadol for pain Asking the right questions about new medicines Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1
 medicineupdate Asking the right questions about new medicines Tramadol for pain Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1 Section 3: Who can take tramadol 2 Section 4: What does
medicineupdate Asking the right questions about new medicines Tramadol for pain Page Section 1: What tramadol is 1 Section 2: What tramadol is for 1 Section 3: Who can take tramadol 2 Section 4: What does
Teriflunomide (Aubagio) 14mg once daily tablet
 Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Guidance on competencies for management of Cancer Pain in adults
 Guidance on competencies for management of Cancer Pain in adults Endorsed by: Contents Introduction A: Core competencies for practitioners in Pain Medicine B: Competencies for practitioners in Pain Medicine
Guidance on competencies for management of Cancer Pain in adults Endorsed by: Contents Introduction A: Core competencies for practitioners in Pain Medicine B: Competencies for practitioners in Pain Medicine
Amitriptyline. Drug information Amitriptyline
 Drug information Amitriptyline Amitriptyline This leaflet provides information on amitriptyline and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Drug information Amitriptyline Amitriptyline This leaflet provides information on amitriptyline and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Version History. Previous Versions. Drugs for MS.Drug facts box fampridine Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
What alternatives are there to the use of opioid analgesics in the treatment of chronic pain in light of existing evidence and its limitations?
 What alternatives are there to the use of opioid analgesics in the treatment of chronic pain in light of existing evidence and its limitations? Michael C. Rowbotham, MD Scientific Director California Pacific
What alternatives are there to the use of opioid analgesics in the treatment of chronic pain in light of existing evidence and its limitations? Michael C. Rowbotham, MD Scientific Director California Pacific
BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU)
 BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU) What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinoma in situ (Bowen s disease). It
BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU) What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinoma in situ (Bowen s disease). It
Sativex. Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353
 Sativex Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Sativex Date of issue: July 2010 Review date: July 2011 Contents 1. Introduction 1 2. What is Sativex?
Sativex Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Sativex Date of issue: July 2010 Review date: July 2011 Contents 1. Introduction 1 2. What is Sativex?
How To Treat An Elderly Patient
 1. Introduction/ Getting to know our Seniors a. Identify common concepts and key terms used when discussing geriatrics b. Distinguish between different venues of senior residence c. Advocate the necessity
1. Introduction/ Getting to know our Seniors a. Identify common concepts and key terms used when discussing geriatrics b. Distinguish between different venues of senior residence c. Advocate the necessity
Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU
 FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
Varenicline (Champix)
 1 for smoking cessation (VA-ren-i-kleen) Consider medical and psychiatric history before prescribing Key points Varenicline is a non-nicotine drug for smoking cessation It has a different mechanism of
1 for smoking cessation (VA-ren-i-kleen) Consider medical and psychiatric history before prescribing Key points Varenicline is a non-nicotine drug for smoking cessation It has a different mechanism of
TREATING MAJOR DEPRESSIVE DISORDER
 TREATING MAJOR DEPRESSIVE DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Second Edition, originally published in April 2000.
TREATING MAJOR DEPRESSIVE DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Second Edition, originally published in April 2000.
DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource
 E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
MQAC Rules for the Management of Chronic Non-Cancer Pain
 MQAC Rules for the Management of Chronic Non-Cancer Pain Effective January 2, 2012 246-919-850 Pain management Intent. These rules govern the use of opioids in the treatment of patients for chronic noncancer
MQAC Rules for the Management of Chronic Non-Cancer Pain Effective January 2, 2012 246-919-850 Pain management Intent. These rules govern the use of opioids in the treatment of patients for chronic noncancer
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Vildagliptin (Galvus) for type 2 diabetes
 Vildagliptin (Galvus) for type 2 diabetes This Medicine Update is for people who are taking, or thinking about taking, vildagliptin. Summary Vildagliptin (brand name Galvus) is a new medicine. It is a
Vildagliptin (Galvus) for type 2 diabetes This Medicine Update is for people who are taking, or thinking about taking, vildagliptin. Summary Vildagliptin (brand name Galvus) is a new medicine. It is a
Elements for a public summary. VI.2.1 Overview of disease epidemiology. VI.2.2 Summary of treatment benefits
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Pain is one of the most common reasons for a patient to seek medical attention. Moderate or severe intensity pain can be acute
STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative treatment
 STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative Pregnancy Chlamydia Chancroid Epdidymitis Gonorrhea: Uncomplicated (cx, urethra, and rectum):
STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative Pregnancy Chlamydia Chancroid Epdidymitis Gonorrhea: Uncomplicated (cx, urethra, and rectum):
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules
 Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Policy for the issue of permits to prescribe Schedule 8 poisons
 Policy for the issue of permits to prescribe Schedule 8 poisons May 2011 Introduction The Victorian Drugs, Poisons and Controlled Substances (DPCS) legislation sets out certain circumstances when a medical
Policy for the issue of permits to prescribe Schedule 8 poisons May 2011 Introduction The Victorian Drugs, Poisons and Controlled Substances (DPCS) legislation sets out certain circumstances when a medical
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Chemotherapy The literal meaning of the term chemotherapy is to treat with a chemical agent, but the term generally refers
Best Practices Treatment Guideline for Major Depression
 Best Practices Treatment Guideline for Major Depression Special Report on New Depression Treatment Technology Based on 2010 APA Practice Guidelines Best Practices Guideline for the Treatment of Patients
Best Practices Treatment Guideline for Major Depression Special Report on New Depression Treatment Technology Based on 2010 APA Practice Guidelines Best Practices Guideline for the Treatment of Patients
APPROVAL OF SATIVEX WITH CONDITIONS
 Health Canada posts safety alerts, public health advisories, press releases and other notices from industry as a service to health professionals, consumers and other interested parties. Although Health
Health Canada posts safety alerts, public health advisories, press releases and other notices from industry as a service to health professionals, consumers and other interested parties. Although Health
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: spinal_cord_stimulation 3/1980 10/2015 10/2016 10/2015 Description of Procedure or Service Spinal cord stimulation
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
PAIN MANAGEMENT. Louise Smith Clinical Nurse Specialist
 PAIN MANAGEMENT Louise Smith Clinical Nurse Specialist Objectives To understand:- The concept of Total Pain The types of Physical Pain Holistic assessment Pain management; pharmaceutical & non pharmaceutical
PAIN MANAGEMENT Louise Smith Clinical Nurse Specialist Objectives To understand:- The concept of Total Pain The types of Physical Pain Holistic assessment Pain management; pharmaceutical & non pharmaceutical
GMMMG Interface Prescribing Subgroup. Shared Care Template
 GMMMG Interface Prescribing Subgroup Shared Care Template Shared Care Guideline for Selective Serotonin Reuptake Inhibitors (SSRIs) for the treatment of Obsessive Compulsive Disorder (OCD) and Body Dysmorphic
GMMMG Interface Prescribing Subgroup Shared Care Template Shared Care Guideline for Selective Serotonin Reuptake Inhibitors (SSRIs) for the treatment of Obsessive Compulsive Disorder (OCD) and Body Dysmorphic
Saxagliptin (Onglyza) for type 2 diabetes
 Saxagliptin (Onglyza) for type 2 diabetes This Medicine Update is for people who are taking, or thinking about taking, saxagliptin. Summary Saxagliptin (brand name Onglyza) is a tablet that can be used
Saxagliptin (Onglyza) for type 2 diabetes This Medicine Update is for people who are taking, or thinking about taking, saxagliptin. Summary Saxagliptin (brand name Onglyza) is a tablet that can be used
Skin cancer Patient information
 Skin cancer Patient information What is cancer? The human body is made up of billions of cells. In healthy people, cells grow, divide and die. New cells constantly replace old ones in an orderly way. This
Skin cancer Patient information What is cancer? The human body is made up of billions of cells. In healthy people, cells grow, divide and die. New cells constantly replace old ones in an orderly way. This
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
What s new, and why, in Neurology 4?
 What s new, and why, in Neurology 4? All topics in Neurology 4 have been extensively reviewed and updated by the expert writing group, to provide concise evidence-based advice for the busy practitioner.
What s new, and why, in Neurology 4? All topics in Neurology 4 have been extensively reviewed and updated by the expert writing group, to provide concise evidence-based advice for the busy practitioner.
MEDICATION GUIDE. These serious side effects are described below:
 MEDICATION GUIDE LYRICA (LEER-i-kah) (pregabalin) Capsules and Oral Solution, CV Read this Medication Guide before you start taking LYRICA and each time you get a refill. There may be new information.
MEDICATION GUIDE LYRICA (LEER-i-kah) (pregabalin) Capsules and Oral Solution, CV Read this Medication Guide before you start taking LYRICA and each time you get a refill. There may be new information.
Major Depression. What is major depression?
 Major Depression What is major depression? Major depression is a serious medical illness affecting 9.9 million American adults, or approximately 5 percent of the adult population in a given year. Unlike
Major Depression What is major depression? Major depression is a serious medical illness affecting 9.9 million American adults, or approximately 5 percent of the adult population in a given year. Unlike
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa )
 Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
SHINGLES (Herpes zoster infection)
 SHINGLES (Herpes zoster infection) What are the aims of this leaflet? This leaflet has been written to help you understand more about shingles. It will tell you what it is, what causes it, what can be
SHINGLES (Herpes zoster infection) What are the aims of this leaflet? This leaflet has been written to help you understand more about shingles. It will tell you what it is, what causes it, what can be
Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery
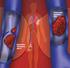 medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
TUE Physician Guidelines Medical Information to Support the Decisions of TUE Committees Neuropathic Pain. Neuropathic Pain
 1. Medical Condition Neuropathic pain is defined as pain arising from a lesion or any disease that causes dysfunction of the somatosensory system. Neuropathic pain is frequently difficult to treat, and
1. Medical Condition Neuropathic pain is defined as pain arising from a lesion or any disease that causes dysfunction of the somatosensory system. Neuropathic pain is frequently difficult to treat, and
