The Swedish Drug Development Pipeline. December & overview of Swedish companies with R&D activities in Sweden
|
|
|
- Vivien Wilcox
- 8 years ago
- Views:
Transcription
1 The Swedish Drug Development Pipeline & overview of Swedish companies with R&D activities in Sweden December 2013 This report is compiled by SwedenBIO with support from VINNOVA and Business Sweden
2 Table of Content Introduction Key Findings... 4 Analysis results 1 Pipeline AstraZeneca Orphan Drugs Companies with R&D Function in Sweden Material & Methods Revised version
3 Introduction This report provide facts and figures about the current Swedish drug development pipeline. The report has been published annually since 2006 and is compiled by SwedenBIO, the Swedish Life Science Industry Organization ( with support from VINNOVA, the Swedish Governmental Agency for Innovation Systems ( and Business Sweden, the Swedish Trade & Invest Council ( The report focuses on Swedish companies with R&D in Sweden and their drug pipeline. The projects being analyzed are product for human use that reached clinical development, Phase I-III. Data and information in this report originates from a web-based survey and public information. The report serves as a quantitative indicator of the progress of the Swedish drug pipeline compounds, projects and their characteristics. The report is used to promote Swedish drug development. The report also includes information about the pipeline of AstraZeneca. Due to the large pipeline and that the research portfolio is not divided in different national platforms. In addition, the report includes information about orphan drugs designations received by Swedish companies and a overview of biotech/pharma companies with R&D function in Sweden. SwedenBIO, Busines Sweden and VINNOVA would like to thank all companies participating in the survey. The Swedish Drug Development Pipeline
4 Key Findings About 100 drug developing Swedish biotech and pharma companies have R&D activities in Sweden. About 50% of these companies have ongoing clinical trials. Development projects are distributed across many therapeutic areas, including cancer, infection, CNS and dermatological deceases. Development of new cancer drugs shows the greatest number of both preclinical and clinical projects. The drug development pipeline contain nearly 70 products in clinical development (i.e. molecules which have advanced to clinical testing in human volunteers, but have not yet been launched). Taking into account that a product may be undergoing clinical trials in more than one indication, there are currently about 80 projects in clinical development (that is, unique molecule-indication combination). 14 of the projects are in Phase III. 7 of the projects reported last year progressed to next phase this year. The Swedish Drug Development Pipeline
5 1 Pipeline 2013 The current Swedish drug pipeline include at least 81 projects that reached clinical development stages, Phase I- III. The projects are developed by 49 companies. This year the web based survey was complemented with public information which may explain the increased number of both companies and projects No of projects No of companies CHAPTER 1 The Swedish Drug Development Pipeline
6 Products and Projects The current Swedish drug pipeline include at least 67 unique products, i.e. small and large molecules, that are being evaluated for various indications in 81 ongoing clinical trials, i.e. number of projects in Phase I-III. 11 of the 67 products are undergoing clinical trials in more than one indication e.g., a particular drug in clinical trials for use in Alzheimer s disease and schizophrenia would be counted as one product and two projects. 67 products 81 ongoing clinical trials 1 The Swedish Drug Development Pipeline
7 Projects by Phase and Progress from Last Year Of the 81 ongoing clinical trials identified, most projects are in Phase II. Seven of the projects reported last year progressed to next phase this year. One Phase III project reported last year was filed for registration. 15 of the companies participating in the survey reported that they received clinical trial authorization during 2012 for Phase I-III studies. 50 No of projects Phase I Phase II Phase III The Swedish Drug Development Pipeline
8 Small and Large Molecules Small molecules make up the majority of drugs on the market today. Large molecules* are getting increased attention due to the great potential they have for diseases that still have high unmet medical needs. 36 of the 67 products are small molecules and 31 are large molecules. The group of large molecules include: antibodies, therapeutic vaccines, cell therapies, hormones and peptides No of projects small molecules large molecules *) also known as biologics or protein-based molecules, here also including the hybrid class of peptides) The Swedish Drug Development Pipeline
9 Choice of Country for Clinical Trial Swedish patients are included in two thirds of the ongoing clinical trials. This result is similar to last years report. More than 50% of companies chose Sweden and Swedish subjects/ patients for Phase I and II trials and about 50 % include Swedish patients in their Phase III trial (response rate 85%). 50 No of projects Abroad Sweden & Abroad Sweden Phase I Phase II Phase III 1 The Swedish Drug Development Pipeline
10 Therapeutic Areas Most projects currently in clinical development, Phase I-III, are in the area of cancer, followed by infection. The category Other include projects in the area of e.g. osteoporosis, transplantation and obstetrics. Cancer Infection CNS Gastro-Intestinal Diabetic/Metabolism Pain Inflammation Endocrinology Obstetrics Cardiovascular Dermatology Other No of projects 1 The Swedish Drug Development Pipeline
11 2 AstraZeneca AstraZeneca is currently the only global pharmaceutical company with R&D function in Sweden. AstraZeneca has a strong presence in Sweden, with approximately employees (end of 2012) working with research, production and marketing. After several years of cutbacks, Mölndal in western Sweden remains the only research site in Sweden. The site is one of three global strategic research sites with research on heart / cardiovascular, metabolic, respiratory, inflammation and autoimmunity. AstraZeneca was formed by the merger of Swedish Astra and UK firm Zeneca in Astra was at that time one of the oldest Swedish pharmaceutical companies, founded in After the merge, AstraZeneca moved the HQ from Stockholm to London. The Swedish R&D teams at Astra/AstraZeneca have developed world-leading products such as: Xylocain, Bricanyl, Pulmicort, Losec and Nexium. AstraZenecas pipeline are presented on page 12 in this report. Note that AstraZeneca does not divide their research portfolio in different national platforms. Phase III projects also include projects ready for registration. Data presented at the next page was obtained from AstraZenecas annual report presenting the pipeline status end of CHAPTER 2 The Swedish Drug Development Pipeline
12 AstraZeneca, Drug Development Pipeline 2012 Globally, AstraZeneca reported 65 projects in Phase I-III end of projects are developed in collaboration with a partner and 25 projects are developed without a partner. Most projects are in the therapeutic area of cancer and respiratory/ inflammation. AstraZeneca reported an additional 19 products in clinical trials that are line extensions No of projects AstraZeneca Pipeline Swedish Drug Development Pipeline Source: AstraZeneca annual report 2012 phase I phase II phase III 2 The Swedish Drug Development Pipeline
13 3 Orphan Drugs New treatments of rare diseases is an emerging trend in drug development. A rare disease, or orphan disease, is any disease that affects a small percentage of the population. Since development of pharmaceuticals is expensive, special incentives have been created to encourage pharma and biotech companies to conduct research on these diseases No of projects EMA, number of orphan designations FDA, number of orphan designations Companies may apply for orphan drug designation, meaning that the sponsor qualifies for certain benefits, such as protocol assistance and reduced taxes from the federal government. The market for orphan drugs is relatively small due to the low number of patients. However, total market value is estimated to $50 billion dollars end of 2011* *) The Economic Power of Orphan Drugs, 2012, Thomson Reuters Source: FDAs and EMAs Webpages CHAPTER 3 The Swedish Drug Development Pipeline
14 Orphan Drug Designations by Swedish companies Since the year 2000 Swedish companies have received orphan drug designations from EMA and/or FDA for nearly 50 drugs. During 2012 the following Swedish companies got orphan drug designations granted from EMA and/or FDA: Albireo, Axcentua Pharmaceuticals, Cortendo and Premacure. According to answers from the drug development pipeline survey this year, 20% of the projects in Phase I-III are projects intending to apply for orphan drug designation or has already received it (response rate 74%) No of projects EMA, Swedish companies FDA, Swedish companies Source: FDAs and EMAs Webpages 3 The Swedish Drug Development Pipeline
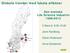
15 4 Biotech & Pharma Companies with R&D Activities in Sweden A total of 99 Swedish biotech and pharmaceutical companies, engaged in research and development in Sweden, were identified to be actively working with drug development during spring The companies are centered to four regions of the country and the majority of companies (62%) are localized in the Stockholm- Uppsala region, followed by the Malmö-Lund region, Gothenburg and Umeå. Umeå, 5% Stockholm/ Uppsala, 62% Gothenburg, 13% Malmö/Lund, 20% CHAPTER 4 The Swedish Drug Development Pipeline
16 Size of Swedish Biotech & Pharma Companies with R&D Activities in Sweden Most companies are so called micro-sized companies with 10 or fewer employees (81%). Only one company, Swedish Orphan Biovitrum is large, i.e. with more than 250 employees. 6% 1% 12% Micro (0-10 FTE) Small (11-50 FTE) Midsize ( FTE) Large (+250 FTE) 81% Source: Information is from The Swedish Drug Development Pipeline
17 Top 10 List of largest companies by Number of Employees Company FTE 2012 Turnover 2012 (TSEK) Head office Therapeutic area Founded Swedish Orphan Biovitrum Stockholm Rare diseases: inflammation, genetics & metabolism. Medivir Stockholm Infectious diseases: hepatitis C Orexo Uppsala Specialty pharma and drug delivery technology Active Biotech Lund Immunology: multiple sclerosis and cancer Oasmia Pharmaceutical Uppsala Nanoparticle formulations and drug-delivery systems based on well-established cytostatics BioInvent International Lund Antibody therapeutics: treatment of cancer Karo Bio Stockholm Nuclear receptors: neuropsychiatry, inflammation, autoimmune diseases and cancer. Camurus Lund Drug-delivery systems for development of highvalue therapeutics. Bioarctic Neuroscience Stockholm Neurodegenerative diseases (alzheimer) 2000 Alligator Bioscience Lund Immunotherapy of cancer Source: Note: Information about Oasmia Pharmaceutical is from The Swedish Drug Development Pipeline
18 Top 10 List of largest companies by Turnover Company FTE 2012 Turnover 2012 (TSEK) Head office Therapeutic area Founded Swedish Orphan Biovitrum Stockholm Rare diseases: inflammation, genetics & metabolism. Medivir Stockholm Infectious diseases: hepatitis C Albireo Göteborg Gastrointestinal diseases: e.g. chronic constipation and IBS Orexo Uppsala Specialty pharma and drug delivery technology Active Biotech Lund Immunology: multiple sclerosis and cancer Moberg Derma Stockholm Skin diseases 2006 Oasmia Pharmaceutical Uppsala Nanoparticle formulations and drug-delivery systems based on well-established cytostatics Camurus Lund Drug-delivery systems for development of highvalue therapeutics. BioInvent International Lund Antibody therapeutics: treatment of cancer Affibody Stockholm Next generation biopharmaceuticals based on its technology platforms Source: Note: Information about Oasmia Pharmaceutical is from The Swedish Drug Development Pipeline
19 Swedish Biotech & Pharma Companies with R&D Activities in Sweden A1M Pharma BioInvent International Hansa Medical Neurosearch Sweden Redoxis Active Biotech Camurus Helicure NeuroVive Pharmaceutical Respiratorius AcuCort Acure Pharma Adenovir Pharma Affibody Akinion Pharmaceuticals Albireo Alligator Bioscience Alzacure Alzinova Anamar Apodemus Aprea Athera Biotechnologies Axcentua Pharmaceuticals Axelar Betagenon Bioarctic Neuroscience BioChromix Pharma BioCrine Cantargia Cardoz Cebix Chemilia ChronTech Pharma ClanoTech Cortendo Creative Antibiotics Sweden DermaGen Dextech Medical Diamyd Medical Dilafor Dilaforette Duecom Plus-A Edio HealthCare Eurocine Vaccines Glactone Pharma GliGene Glucox Biotech Hyron BioMedical Immun System I.M.S. Immunicum InDex Pharmaceuticals Isifer AB Isofol Medical Kancera Karo Bio KDev Exploratory KDev Oncology LIDDS Lipidor Lipigon Pharmaceuticals Lipopeptide Medivir Metakalinin Moberg Derma Molecules of Man Neuronova Northern Light Pharmaceuticals NovaSaid Oasmia Pharmaceutical Omnio Healer Umeå Oncopeptides Oncorena Orexo OxThera Pep-Tonic Medical Pergamum Pharmalink Pharmalundensis Pharmanest PledPharma Premacure Premune Recopharma Red Glead Discovery Scandinavian Biopharma Strongbone Swedish Orphan Biovitrum Synphora TikoMed Toleranzia Umecrine Cognition Umecrine Mood Velit Biologics Vicore Pharma WilsonTherapeutics Vivolux WntResearch XImmune The Swedish Drug Development Pipeline
20 Material & Method The survey investigates drug projects for human use developed by Swedish biotech and pharmaceutical companies. The focus of the survey are finding projects in clinical development, Phase I-III. Note that a product may be tested for several indications and each indication reported is referred to as a project. Each project may eventually lead to an approval. Initially, a list of companies with potentially relevant projects was compiled. The list included companies that answered the survey last year and in addition VINNOVA provided a list of drug development companies from their report "Life Science companies in Sweden" (VA 2011:03). Further, regional bioorganizations, science parks, incubators etc. were contacted for information about companies fulfilling the requirements. Companies on the list were invited to answer the survey (webbased questionnaire) upon invitation by and subsequent phone calls. SwedenBIO also informed about the survey in a weekly newsletter and on the web site. In total, 39 companies answered the survey, of which 34 reported projects in Phase I-III. For the remaining companies, pipeline information was collected from company web sites and In parallel a company survey was done. Publicdata was obtained from The result from this survey is presented on page AstraZeneca does not divide their research portfolio in different national platforms and as the portfolio alone is approximately the same size as the whole Swedish drug development the pipeline it is shown separately at page 12. Data from previous years drug development pipeline reports, i.e. -06, -07, -08, -09, -10, -11 and -12 were included for comparison when relevant. All reports may be downloaded from the website of SwedenBIO: The Swedish Drug Development Pipeline
21 Contact SwedenBIO - the Swedish Life Science Industry Organization Wallingatan 24, Stockholm info@swedenbio.com Current and previous pipeline reports may be downloaded from:
The Swedish Drug Development Pipeline 2014
 The Swedish Drug Development Pipeline 2014 and an overview of swedish r&d companies 2013 december 2014 this report is compiled by swedenbio with support from vinnova 2 the swedish drug development pipeline
The Swedish Drug Development Pipeline 2014 and an overview of swedish r&d companies 2013 december 2014 this report is compiled by swedenbio with support from vinnova 2 the swedish drug development pipeline
The Swedish Drug Development Pipeline. Dec. 2013. Sara Gunnerås, Senior Manager Science & IP, SwedenBIO SwedenBIO CEO Summit 2013, AstraZenaca Mölndal
 The Swedish Drug Development Pipeline & overview of companies with R&D activities in Sweden Dec. 2013 Sara Gunnerås, Senior Manager Science & IP, SwedenBIO SwedenBIO CEO Summit 2013, AstraZenaca Mölndal
The Swedish Drug Development Pipeline & overview of companies with R&D activities in Sweden Dec. 2013 Sara Gunnerås, Senior Manager Science & IP, SwedenBIO SwedenBIO CEO Summit 2013, AstraZenaca Mölndal
The Swedish Drug Development Pipeline June 2012. A survey conducted by:
 The Swedish Drug Development Pipeline June 2012 A survey conducted by: Key findings More companies report clinical trials There has been a increase in number of companies contributing with information
The Swedish Drug Development Pipeline June 2012 A survey conducted by: Key findings More companies report clinical trials There has been a increase in number of companies contributing with information
A survey conducted by: The Swedish Drug Development Pipeline May 2011
 A survey conducted by: The Swedish Drug Development Pipeline May 2011 The survey In February March 2011 a survey of the Swedish Drug Development Pipeline was conducted by SwedenBIO in cooperation with
A survey conducted by: The Swedish Drug Development Pipeline May 2011 The survey In February March 2011 a survey of the Swedish Drug Development Pipeline was conducted by SwedenBIO in cooperation with
U.S. Contract Research Outsourcing Market: Trends, Challenges and Competition in the New Decade. N8B7-52 December 2010
 U.S. Contract Research Outsourcing Market: Trends, Challenges and Competition in the New Decade December 2010 Table of Contents Notes on Methodology 8 Market Introduction and Segmentation Introduction
U.S. Contract Research Outsourcing Market: Trends, Challenges and Competition in the New Decade December 2010 Table of Contents Notes on Methodology 8 Market Introduction and Segmentation Introduction
JOHN REID PhD, MBA. (302) 544-1743 entrepidbd@gmail.com http://www.linkedin.com/in/jdreid1
 JOHN REID PhD, MBA (302) 544-1743 entrepidbd@gmail.com http://www.linkedin.com/in/jdreid1 PROFILE Senior-level business development and licensing executive with a well-honed ability to translate scientific
JOHN REID PhD, MBA (302) 544-1743 entrepidbd@gmail.com http://www.linkedin.com/in/jdreid1 PROFILE Senior-level business development and licensing executive with a well-honed ability to translate scientific
Orphan Pharma: pathfinders for an increasingly specialised industry
 Orphan Pharma: pathfinders for an increasingly specialised industry Foreword We are continuing our series by profiling the R&D and Clinical Development leadership of European and North American specialty
Orphan Pharma: pathfinders for an increasingly specialised industry Foreword We are continuing our series by profiling the R&D and Clinical Development leadership of European and North American specialty
Life Science Sector Opportunities Northern Ireland. Clinical Trials. investni.com
 Life Science Sector Opportunities Northern Ireland investni.com Introduction Clinical trials commonly refer to testing the effectiveness of experimental drugs and are typically categorised into four phases;
Life Science Sector Opportunities Northern Ireland investni.com Introduction Clinical trials commonly refer to testing the effectiveness of experimental drugs and are typically categorised into four phases;
Join our scientific talent community
 Join our scientific talent community There has never been a better time to be a part of Janssen Research & Development. We are at the forefront of healthcare leading, evolving and transforming it into
Join our scientific talent community There has never been a better time to be a part of Janssen Research & Development. We are at the forefront of healthcare leading, evolving and transforming it into
Groundbreaking Collaborative Clinical Trial Launched
 Groundbreaking Collaborative Clinical Trial Launched For immediate release Media Contacts: June 16, 2014 Richard Folkers Alison Hendrie 9:00 a.m., EDT Foundation for the NIH Rubenstein Communications (301)
Groundbreaking Collaborative Clinical Trial Launched For immediate release Media Contacts: June 16, 2014 Richard Folkers Alison Hendrie 9:00 a.m., EDT Foundation for the NIH Rubenstein Communications (301)
Knowledge Synergies The New Paradigm of Innovation. Israel Makov
 Knowledge Synergies The New Paradigm of Innovation Israel Makov New England-Israel Life Sciences Summit - October 26, 2009 Pharma Industry Challenges and Pressures At an All-Time High Historically Low
Knowledge Synergies The New Paradigm of Innovation Israel Makov New England-Israel Life Sciences Summit - October 26, 2009 Pharma Industry Challenges and Pressures At an All-Time High Historically Low
Winter 2013. Changing landscapes, pipeline products and plan sponsor impact
 Winter 2013 Changing landscapes, pipeline products and plan sponsor impact Changing landscapes, pipeline products and plan sponsor impact The pharmaceutical landscape is changing as is the profile of blockbuster
Winter 2013 Changing landscapes, pipeline products and plan sponsor impact Changing landscapes, pipeline products and plan sponsor impact The pharmaceutical landscape is changing as is the profile of blockbuster
THE VIRAL HEPATITIS CONGRESS 2015. 10 12 September 2015, Kap Europa, Frankfurt, Germany THE VIRAL HEPATITIS CONGRESS. Scientific Programme
 THE VIRAL HEPATITIS CONGRESS THE VIRAL HEPATITIS CONGRESS 2015 10 12 September 2015, Kap Europa, Frankfurt, Germany Scientific Programme PRE-CONGRESS WORKSHOP SPONSOR ACHILLION Achillion is seeking to
THE VIRAL HEPATITIS CONGRESS THE VIRAL HEPATITIS CONGRESS 2015 10 12 September 2015, Kap Europa, Frankfurt, Germany Scientific Programme PRE-CONGRESS WORKSHOP SPONSOR ACHILLION Achillion is seeking to
Tech transfer The Swedish Context. Academia-industry collaboration. .. Created the largest selling drug, LOSEC, followed by Nexium
 Tech transfer The Swedish Context Claes Post, May 21 2010, Brussels Academia-industry collaboration Scand J Gastroenterol Suppl. 1989;166:3-11.The gastric H+,K+- ATPase: the site of action of omeprazole.sachs
Tech transfer The Swedish Context Claes Post, May 21 2010, Brussels Academia-industry collaboration Scand J Gastroenterol Suppl. 1989;166:3-11.The gastric H+,K+- ATPase: the site of action of omeprazole.sachs
Globala trender med lokala effekter
 Globala trender med lokala effekter Den svenska Life Science industrin 1998-212 5 Mars kl. 8.3-1. Jenni Nordborg Göran Andersson Anna Sandström Life Sciences evolutionary and iterative innovation processes
Globala trender med lokala effekter Den svenska Life Science industrin 1998-212 5 Mars kl. 8.3-1. Jenni Nordborg Göran Andersson Anna Sandström Life Sciences evolutionary and iterative innovation processes
Roche Position on Human Stem Cells
 Roche Position on Human Stem Cells Background Stem cells and treating diseases. Stem cells and their applications offer an enormous potential for the treatment and even the cure of diseases, along with
Roche Position on Human Stem Cells Background Stem cells and treating diseases. Stem cells and their applications offer an enormous potential for the treatment and even the cure of diseases, along with
What Lies Ahead? Trends to Watch: Health Care Product Development in North America
 What Lies Ahead? Trends to Watch: Health Care Product Development in North America What Lies Ahead? for 2015 DIA has released its third annual What Lies Ahead? report, providing experts insights into the
What Lies Ahead? Trends to Watch: Health Care Product Development in North America What Lies Ahead? for 2015 DIA has released its third annual What Lies Ahead? report, providing experts insights into the
How To Compare Sweden To Danesland
 Life science companies in Sweden Including a comparison with Denmark addendi Life science companies in Sweden - Including a comparison with Denmark Anna Sandström, VINNOVA Tage Dolk och Benny Dolk, Addendi
Life science companies in Sweden Including a comparison with Denmark addendi Life science companies in Sweden - Including a comparison with Denmark Anna Sandström, VINNOVA Tage Dolk och Benny Dolk, Addendi
CAN-FITE BIOPHARMA LTD.
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 Under the Securities Exchange Act of 1934 For the Month
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 Under the Securities Exchange Act of 1934 For the Month
Domanda di innovazione nel mondo industriale. Modalità di interazione tra pubblico e privato
 Domanda di innovazione nel mondo industriale. Modalità di interazione tra pubblico e privato Ennio Ongini Nicox Consiglio Nazionale delle Ricerche Quarta Giornata Informativa "Dalla ricerca al Trasferimento
Domanda di innovazione nel mondo industriale. Modalità di interazione tra pubblico e privato Ennio Ongini Nicox Consiglio Nazionale delle Ricerche Quarta Giornata Informativa "Dalla ricerca al Trasferimento
CCS net sales increased to SEK 42.1 (35.0) m. Profit after financial items was SEK 8.8 (3.1) m.
 PRESS RELEASE, 29 April INTERIM REPORT, 1 January - 31 March MIV-210 producing positive results in ongoing phase I trial. More opportunities for Cathepsin S inhibitor. Successes in the explorative activities
PRESS RELEASE, 29 April INTERIM REPORT, 1 January - 31 March MIV-210 producing positive results in ongoing phase I trial. More opportunities for Cathepsin S inhibitor. Successes in the explorative activities
Projects by area. Projects by development phase. Med Tech Drug Formulation. Market introduction. Women s Health. Discovery-Lead optimization/prototype
 Annual Report 2011 Content 4 Significant events during the financial year 4 Significant events after the balance sheet date 5 Messsage from the CEO 6 Our strengths 8 Commercialization in three steps 10
Annual Report 2011 Content 4 Significant events during the financial year 4 Significant events after the balance sheet date 5 Messsage from the CEO 6 Our strengths 8 Commercialization in three steps 10
Company Profile // May 2012
 Company Profile // May 2012 1 Disclaimer This presentation has been prepared for the group of companies held by Clal Biotechnology Industries Ltd. (CBI or the Group ) and is provided to you solely for
Company Profile // May 2012 1 Disclaimer This presentation has been prepared for the group of companies held by Clal Biotechnology Industries Ltd. (CBI or the Group ) and is provided to you solely for
THE BIOTECH & PHARMACEUTICAL INDUSTRY
 THE BIOTECH & PHARMACEUTICAL INDUSTRY ESSENTIAL CAREERS INFORMATION CALUM LECKIE KATIE BISARO CAREERS CONSULTANTS What we will cover Sector overview Types of role Graduate recruitment trends and issues
THE BIOTECH & PHARMACEUTICAL INDUSTRY ESSENTIAL CAREERS INFORMATION CALUM LECKIE KATIE BISARO CAREERS CONSULTANTS What we will cover Sector overview Types of role Graduate recruitment trends and issues
EVT Execute & EVT Innovate World-class drug discovery
 EVT Execute & EVT Innovate World-class drug discovery Evotec AG, First Quarter Report 2015, 12 May 2015 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
EVT Execute & EVT Innovate World-class drug discovery Evotec AG, First Quarter Report 2015, 12 May 2015 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
INTERIM REPORT, 1 January - 30 June 2003
 PRESS RELEASE, 9 July INTERIM REPORT, 1 January - 30 June In May, Medivir reached a licensing agreement on MIV-210 with GlaxoSmithKline; EUR 6 m was received upon signing and up to EUR 86 m on the achievement
PRESS RELEASE, 9 July INTERIM REPORT, 1 January - 30 June In May, Medivir reached a licensing agreement on MIV-210 with GlaxoSmithKline; EUR 6 m was received upon signing and up to EUR 86 m on the achievement
Annual Report on Form 20-F
 Annual Report on Form 20-F 2 Teva at a Glance Founded in 1901 by three young pharmacists, today Teva Pharmaceutical Industries Ltd. is a leader in the global pharmaceutical industry, providing medicines
Annual Report on Form 20-F 2 Teva at a Glance Founded in 1901 by three young pharmacists, today Teva Pharmaceutical Industries Ltd. is a leader in the global pharmaceutical industry, providing medicines
The Cell Therapy Catapult UK Clinical Trials Database
 May 2015 The UK Clinical Trials Database covers cell therapy clinical trial activity that the Cell Therapy Catapult believes to be ongoing in the UK as of May 2015. It supersedes the database of April
May 2015 The UK Clinical Trials Database covers cell therapy clinical trial activity that the Cell Therapy Catapult believes to be ongoing in the UK as of May 2015. It supersedes the database of April
October 13, 2014. Signature Programs
 Signature Programs October 13, 2014 Neuroscience The goal of the neuroscience initiative is to create a nationally recognized center of research into the biological bases of brain function and dysfunction
Signature Programs October 13, 2014 Neuroscience The goal of the neuroscience initiative is to create a nationally recognized center of research into the biological bases of brain function and dysfunction
E- MARKETPLACES IN THE BIOTECH INDUSTRY
 E- MARKETPLACES IN THE BIOTECH INDUSTRY By Jonas Bygdeson, Head of emarket Services Swedish Trade Council www.emarketservices.com May 2004 Abstract The purpose of this report is to study how electronic
E- MARKETPLACES IN THE BIOTECH INDUSTRY By Jonas Bygdeson, Head of emarket Services Swedish Trade Council www.emarketservices.com May 2004 Abstract The purpose of this report is to study how electronic
Daiichi Sankyo to Acquire Ambit Biosciences
 For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Joji Nakayama, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please
For Immediate Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Joji Nakayama, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please
Recruitment Start date: April 2010 End date: Recruitment will continue until enrolment is fully completed
 Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
We set things in motion and keep them moving. Metronomia Clinical Research Services
 We set things in motion and keep them moving Metronomia Clinical Research Services Our customers love the fact that we keep up with their individual tempos. Dorothea Wessiepe, Director Biostatistics Metronomia:
We set things in motion and keep them moving Metronomia Clinical Research Services Our customers love the fact that we keep up with their individual tempos. Dorothea Wessiepe, Director Biostatistics Metronomia:
Biotech Short Profile
 The Company X is leading the development of next generation biological therapies and production systems. The Company is in two pivotal Phase III clinical studies in colorectal cancer for a unique anticancer
The Company X is leading the development of next generation biological therapies and production systems. The Company is in two pivotal Phase III clinical studies in colorectal cancer for a unique anticancer
Innovation in the Biopharmaceutical Pipeline: A Multidimensional View
 Innovation in the Biopharmaceutical Pipeline: A Multidimensional View Innovation in the Biopharmaceutical Pipeline: A Multidimensional View Authors: Genia Long Analysis Group, Inc. 111 Huntington Avenue,
Innovation in the Biopharmaceutical Pipeline: A Multidimensional View Innovation in the Biopharmaceutical Pipeline: A Multidimensional View Authors: Genia Long Analysis Group, Inc. 111 Huntington Avenue,
MRC Technology Centre for Therapeutics Discovery
 MRC Technology Centre for Therapeutics Discovery fast-tracking discovery and development of novel drugs from academia Dr Duncan Young Business Development Manager UbiFrance Symposium, October 2012 established
MRC Technology Centre for Therapeutics Discovery fast-tracking discovery and development of novel drugs from academia Dr Duncan Young Business Development Manager UbiFrance Symposium, October 2012 established
Bringing knowledge to life
 Bringing knowledge to life High-impact content, flexible delivery informahealthcare.com Committed to Quality Content At Informa Healthcare, our mission is to connect scientists, researchers, and clinicians
Bringing knowledge to life High-impact content, flexible delivery informahealthcare.com Committed to Quality Content At Informa Healthcare, our mission is to connect scientists, researchers, and clinicians
Pharmacology skills for drug discovery. Why is pharmacology important?
 skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
BioWales 2015 Pharmaceutical Supply Chain Showcase. 04 March 2015
 BioWales 2015 Pharmaceutical Supply Chain Showcase 04 March 2015 SIMBEC RESEARCH 40 YEARS EXPERIENCE IN EARLY STAGE CLINICAL DRUG DEVELOPMENT BACKGROUND Over 1,200 clinical studies safely and successfully
BioWales 2015 Pharmaceutical Supply Chain Showcase 04 March 2015 SIMBEC RESEARCH 40 YEARS EXPERIENCE IN EARLY STAGE CLINICAL DRUG DEVELOPMENT BACKGROUND Over 1,200 clinical studies safely and successfully
Karriärdagar Uppsala - Vad händer inom bioteknikbranschen?
 The Swedish Biotechnology Industry Organization Karriärdagar Uppsala - Vad händer inom bioteknikbranschen? Uppsala karriärdagar March 15th, 2006 Per-Erik Sandlund SwedenBIO Wallingatan 24 SE-111 24 Stockholm
The Swedish Biotechnology Industry Organization Karriärdagar Uppsala - Vad händer inom bioteknikbranschen? Uppsala karriärdagar March 15th, 2006 Per-Erik Sandlund SwedenBIO Wallingatan 24 SE-111 24 Stockholm
STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016
 STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments, as well as negations of
STRATEGY PROGRAM NEXT LEVEL BERLIN, 9 JUNE 2016 Disclaimer Certain statements in this presentation contain formulations or terms referring to the future or future developments, as well as negations of
EVT Execute & EVT Innovate Innovation Efficiency
 EVT Execute & EVT Innovate Innovation Efficiency Evotec AG, H1 2015 Interim Report, 12 August 2015 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
EVT Execute & EVT Innovate Innovation Efficiency Evotec AG, H1 2015 Interim Report, 12 August 2015 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
How To Improve Project Management
 PRTM Global Management Consultants Successful Portfolio and Resource Management Practices for Pharmaceutical & Biopharmaceutical Companies Industry Benchmarking Report For further information, contact:
PRTM Global Management Consultants Successful Portfolio and Resource Management Practices for Pharmaceutical & Biopharmaceutical Companies Industry Benchmarking Report For further information, contact:
2013-03-04. Kurt Strömgren. Business coach - Uminova Innovation Manager Biotech Umeå. Ideas become business.
 Kurt Strömgren Business coach - Uminova Innovation Manager Biotech Umeå Ideas become business. 1 The Umeå support system for innovations IDEA GROW EXPAND Innovationsslussen Entrepreneur center Uminova
Kurt Strömgren Business coach - Uminova Innovation Manager Biotech Umeå Ideas become business. 1 The Umeå support system for innovations IDEA GROW EXPAND Innovationsslussen Entrepreneur center Uminova
To Our Shareholders. 2013: Keeping Our Commitments, Continuing Our Momentum. Continued Transformation of Our Innovative Core. Annual Review 2013
 To Our Shareholders In 2013, Pfizer achieved solid results for patients, consumers, and shareholders by maintaining our focus on developing new therapies, driving growth, and delivering value. Ian C. Read
To Our Shareholders In 2013, Pfizer achieved solid results for patients, consumers, and shareholders by maintaining our focus on developing new therapies, driving growth, and delivering value. Ian C. Read
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO
 Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
Q4 2015 PRESENTATION February 9, 2016 Per Walday, CEO Ronny Skuggedal, CFO 1 PCI BIOTECH Important notice and disclaimer This presentation may contain certain forward-looking statements and forecasts based
LIFE SCIENCES QUEENSLAND LIMITED (LSQ) Mario Pennisi CEO. @LSQld
 LIFE SCIENCES QUEENSLAND LIMITED (LSQ) Mario Pennisi CEO @LSQld LEADING DESTINATION FOR CLINICAL RESEARCH INVESTORS IN QLD INCLUDE: LIFE SCIENCES IN QLD ~300 companies in life sciences ~$5B invested by
LIFE SCIENCES QUEENSLAND LIMITED (LSQ) Mario Pennisi CEO @LSQld LEADING DESTINATION FOR CLINICAL RESEARCH INVESTORS IN QLD INCLUDE: LIFE SCIENCES IN QLD ~300 companies in life sciences ~$5B invested by
Leading Innovation Efficiency Evotec Company Overview
 Leading Innovation Efficiency Evotec Company Overview Evotec AG, Company presentation January 2016 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
Leading Innovation Efficiency Evotec Company Overview Evotec AG, Company presentation January 2016 Forward-looking statements Information set forth in this presentation contains forward-looking statements,
Building innovative drug discovery alliances. Profitable. Growth Go
 Building innovative drug discovery alliances Innovation n & Profitable Growth Go Evotec AG, H1 Presentation, 8 th August 2012 Forward-looking statements Information set forth in this presentation contains
Building innovative drug discovery alliances Innovation n & Profitable Growth Go Evotec AG, H1 Presentation, 8 th August 2012 Forward-looking statements Information set forth in this presentation contains
Automating Cell Biology Annual general meeting, September 7, 2015. Phase Holographic Imaging www.phiab.se
 Automating Cell Biology Annual general meeting, September 7, 2015 www.phiab.se 1 PHASE HOLOGRAPHIC IMAGING (PHI) Began as a research project at Lund University, Sweden, in 2000 Founded in 2004 Sales in
Automating Cell Biology Annual general meeting, September 7, 2015 www.phiab.se 1 PHASE HOLOGRAPHIC IMAGING (PHI) Began as a research project at Lund University, Sweden, in 2000 Founded in 2004 Sales in
Navigating England s clinical research infrastructure. How NOCRI helps simplify access to accelerate discoveries
 Navigating England s clinical research infrastructure How NOCRI helps simplify access to accelerate discoveries 2» The National Institute for Health Research (NIHR) Clinical Research Network recruited
Navigating England s clinical research infrastructure How NOCRI helps simplify access to accelerate discoveries 2» The National Institute for Health Research (NIHR) Clinical Research Network recruited
Specialty Pharmacy. Oncology
 Specialty Pharmacy Oncology Background What are specialty drugs? Not an FDA designation No standard industry definition Categorized as: High cost (>$1,200 / month) Require special handling and administration
Specialty Pharmacy Oncology Background What are specialty drugs? Not an FDA designation No standard industry definition Categorized as: High cost (>$1,200 / month) Require special handling and administration
Graduate and Postdoctoral Affairs School of Biomedical Sciences College of Medicine. Graduate Certificate. Metabolic & Nutritional Medicine
 Graduate and Postdoctoral Affairs School of Biomedical Sciences College of Medicine Graduate Certificate in Metabolic & Nutritional Medicine Graduate Certificate Metabolic & Nutritional Medicine Purpose
Graduate and Postdoctoral Affairs School of Biomedical Sciences College of Medicine Graduate Certificate in Metabolic & Nutritional Medicine Graduate Certificate Metabolic & Nutritional Medicine Purpose
New York Bio Conference 2016. Mark J. Alles Chief Executive Officer
 New York Bio Conference 2016 Mark J. Alles Chief Executive Officer Great Progress AGE 72 World Life Expectancy 2005-2014* 17 new drugs for rare diseases 71.5 71 Two new MS drugs First drug to target root
New York Bio Conference 2016 Mark J. Alles Chief Executive Officer Great Progress AGE 72 World Life Expectancy 2005-2014* 17 new drugs for rare diseases 71.5 71 Two new MS drugs First drug to target root
Needs, Providing Solutions
 Identifying Needs, Providing Solutions 1 I n d u s t r y The growth of medical research and the countless innovations coming from the pharmaceutical, biotechnology and medical device industry, has improved
Identifying Needs, Providing Solutions 1 I n d u s t r y The growth of medical research and the countless innovations coming from the pharmaceutical, biotechnology and medical device industry, has improved
Partnering for scientific leadership
 Partnering for scientific leadership AstraZeneca is a global, innovation-driven biopharmaceutical business that focuses on the discovery, development and commercialisation of prescription medicines, primarily
Partnering for scientific leadership AstraZeneca is a global, innovation-driven biopharmaceutical business that focuses on the discovery, development and commercialisation of prescription medicines, primarily
Global Monoclonal Antibodies Pipeline Insight 2015
 Brochure More information from http://www.researchandmarkets.com/reports/3113388/ Global Monoclonal Antibodies Pipeline Insight 2015 Description: Ever since the Nobel Prize was bestowed on the person who
Brochure More information from http://www.researchandmarkets.com/reports/3113388/ Global Monoclonal Antibodies Pipeline Insight 2015 Description: Ever since the Nobel Prize was bestowed on the person who
TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet
 TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet Dalvir Gill, PhD - Chief Executive Officer 17 October, 2014 Presentation Objectives + TransCelerate History + Participating
TransCelerate's Role in Transforming Pharmaceutical Trials Presentation to PCORNet Dalvir Gill, PhD - Chief Executive Officer 17 October, 2014 Presentation Objectives + TransCelerate History + Participating
Healthcare services requiring prior authorisation
 Annex 2 Healthcare requiring prior authorisation This list does not include organ transplants and does also not apply to long-term care and the primary purpose of which is to support people in need of
Annex 2 Healthcare requiring prior authorisation This list does not include organ transplants and does also not apply to long-term care and the primary purpose of which is to support people in need of
Biologics: Specific Drug Safety Challenges. Violetta B. Kyburz
 Biologics: Specific Drug Safety Challenges Violetta B. Kyburz 2012 2013 2014 Biologics: Specific Drug Safety Challenges Topics for discussion Particular issues in the preclinical development of biologics
Biologics: Specific Drug Safety Challenges Violetta B. Kyburz 2012 2013 2014 Biologics: Specific Drug Safety Challenges Topics for discussion Particular issues in the preclinical development of biologics
Infoset builds software and services to advantage business operations and improve patient s life
 Infoset builds software and services to advantage business operations and improve patient s life Clinical Data Management ecrf & EDC Patient Support Programs Medication Adherence Mobile e-health Big Data
Infoset builds software and services to advantage business operations and improve patient s life Clinical Data Management ecrf & EDC Patient Support Programs Medication Adherence Mobile e-health Big Data
EVT Execute & EVT Innovate Leading drug discovery
 EVT Execute & EVT Innovate Leading drug discovery Evotec AG, Nine-month 2015 Interim Report, 10 November 2015 Forward-looking statements Information set forth in this presentation contains forward-looking
EVT Execute & EVT Innovate Leading drug discovery Evotec AG, Nine-month 2015 Interim Report, 10 November 2015 Forward-looking statements Information set forth in this presentation contains forward-looking
TOP 10 TRENDS IN HEALTHCARE, MEDICAL. HJ ABDUL AZIZ ABDUL RAHMAN Chief Executive Officer
 TOP 10 TRENDS IN HEALTHCARE, MEDICAL AND PHARMACEUTICAL INDUSTRY HJ ABDUL AZIZ ABDUL RAHMAN Chief Executive Officer KPJSeremban Specialist Hospital KPJ SEREMBAN SPECIALIST HOSPITAL Presented by : Hj Abd
TOP 10 TRENDS IN HEALTHCARE, MEDICAL AND PHARMACEUTICAL INDUSTRY HJ ABDUL AZIZ ABDUL RAHMAN Chief Executive Officer KPJSeremban Specialist Hospital KPJ SEREMBAN SPECIALIST HOSPITAL Presented by : Hj Abd
For personal use only
 6 July 2015 KEY ADVANCEMENT TOWARDS COMMENCEMENT OF PHASE 1 CLINICAL STUDY OF ORAL FORMULATIONS Highlights PhytoTech Medical has submitted key documents to the Institutional Review Board (IRB or Helsinki
6 July 2015 KEY ADVANCEMENT TOWARDS COMMENCEMENT OF PHASE 1 CLINICAL STUDY OF ORAL FORMULATIONS Highlights PhytoTech Medical has submitted key documents to the Institutional Review Board (IRB or Helsinki
Medivir s financial reports are available from its Website, www.medivir.se from these dates, under the Financial Information heading.
 PRESS RELEASE, 23 October INTERIM REPORT, 1 January - 30 September In July, Medivir entered a licensing agreement with Boehringer Ingelheim (BI) on MIV-310. CCS group divested to the Segulah II L.P. investment
PRESS RELEASE, 23 October INTERIM REPORT, 1 January - 30 September In July, Medivir entered a licensing agreement with Boehringer Ingelheim (BI) on MIV-310. CCS group divested to the Segulah II L.P. investment
CORPORATE PRESENTATION
 CORPORATE PRESENTATION 1537 NW 65th Avenue Plantation, FL 33313 USA Phone: (954) 321-8988 Fax: (954) 321-9778 info@receptopharm.com www.receptopharm.com OVERVIEW ReceptoPharm is an innovative biopharmaceutical
CORPORATE PRESENTATION 1537 NW 65th Avenue Plantation, FL 33313 USA Phone: (954) 321-8988 Fax: (954) 321-9778 info@receptopharm.com www.receptopharm.com OVERVIEW ReceptoPharm is an innovative biopharmaceutical
August 28, 2012. Company Update Commerzbank Sector Conference Week
 August 28, 2012 Company Update Commerzbank Sector Conference Week Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included in the forward-looking
August 28, 2012 Company Update Commerzbank Sector Conference Week Safe Harbour This presentation includes forward-looking statements. Actual results could differ materially from those included in the forward-looking
Gene Therapy. The use of DNA as a drug. Edited by Gavin Brooks. BPharm, PhD, MRPharmS (PP) Pharmaceutical Press
 Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
Gene Therapy The use of DNA as a drug Edited by Gavin Brooks BPharm, PhD, MRPharmS (PP) Pharmaceutical Press Contents Preface xiii Acknowledgements xv About the editor xvi Contributors xvii An introduction
Agenda Item 3. Discovery to Product Accelerator
 Agenda Item 3 Discovery to Product Accelerator BOARD OF REGENTS SUMMARY OF ITEM FOR ACTION INFORMATION OR DISCUSSION TOPIC: Discovery to Product Accelerator (information item) COMMITTEE: Economic Development
Agenda Item 3 Discovery to Product Accelerator BOARD OF REGENTS SUMMARY OF ITEM FOR ACTION INFORMATION OR DISCUSSION TOPIC: Discovery to Product Accelerator (information item) COMMITTEE: Economic Development
Abt Associates Inc. Abt Associates Clinical Trials Comprehensive clinical research services
 Abt Associates Inc. Abt Associates Clinical Trials Comprehensive clinical research services Providing a wide range of clinical research services Abt Associates Clinical Trials (AACT) is dedicated to providing
Abt Associates Inc. Abt Associates Clinical Trials Comprehensive clinical research services Providing a wide range of clinical research services Abt Associates Clinical Trials (AACT) is dedicated to providing
Core therapeutic areas
 Core therapeutic areas Platforms for growth Luke Miels, Executive Vice President, Global Portfolio & Product Strategy and Corporate Affairs Current business is led by core therapeutic areas Sales 9M 2014
Core therapeutic areas Platforms for growth Luke Miels, Executive Vice President, Global Portfolio & Product Strategy and Corporate Affairs Current business is led by core therapeutic areas Sales 9M 2014
The MBBS/BSc programme of study is an integrated programme extending over 6 years.
 Regulations for the award of the Degrees of MBBS/BSc 1 General Information 1.1 The degrees of Bachelor of Medicine, Bachelor of Surgery and Bachelor of Science in Medical Sciences will be awarded to any
Regulations for the award of the Degrees of MBBS/BSc 1 General Information 1.1 The degrees of Bachelor of Medicine, Bachelor of Surgery and Bachelor of Science in Medical Sciences will be awarded to any
BioPharmaceutical Royalty Rates & Deal Terms Report. Licensing Executives Society (U.S.A. & Canada), Inc.
 BioPharmaceutical Royalty Rates & Deal Terms Report Licensing Executives Society (U.S.A. & Canada), Inc. June 2008 Table of Contents Introductory Letter 1 LES Survey Committee 2 Introduction 3 Report Highlights
BioPharmaceutical Royalty Rates & Deal Terms Report Licensing Executives Society (U.S.A. & Canada), Inc. June 2008 Table of Contents Introductory Letter 1 LES Survey Committee 2 Introduction 3 Report Highlights
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Testimony of Timothy Coetzee, Ph.D. Representative of a Patient Advocacy Organization President, Fast Forward LLC
 Testimony of Timothy Coetzee, Ph.D. Representative of a Patient Advocacy Organization President, Fast Forward LLC Committee on Oversight and Government Reform Domestic Policy Subcommittee U.S. House of
Testimony of Timothy Coetzee, Ph.D. Representative of a Patient Advocacy Organization President, Fast Forward LLC Committee on Oversight and Government Reform Domestic Policy Subcommittee U.S. House of
Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation
 Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation Hans S. Keirstead President, NeoStem Oncology NeoStem Presented by GTC, a conference production company 626-256-6405
Novel Targeted Immunotherapy Approach for Metastatic Melanoma Under Phase 3 Investigation Hans S. Keirstead President, NeoStem Oncology NeoStem Presented by GTC, a conference production company 626-256-6405
The Active Biotech Group Half-Year Accounts January June 2000
 The Active Biotech Group Half-Year Accounts January June 2000 Results according to schedule, MSEK -59 (-75 previous year) Vaccine sales increase by 20% Continued positive development for Dukoral +42% Aventis
The Active Biotech Group Half-Year Accounts January June 2000 Results according to schedule, MSEK -59 (-75 previous year) Vaccine sales increase by 20% Continued positive development for Dukoral +42% Aventis
Career Opportunities within the French Alliance for Life and Health Sciences
 1 Career Opportunities within the French Alliance for Life and Health Sciences Mireille Guyader, PhD Director, Inserm-USA Office INSERM KEY FIGURES (Year 2013) Budget: 970 M (~ $1.2 Billion) Human resources:
1 Career Opportunities within the French Alliance for Life and Health Sciences Mireille Guyader, PhD Director, Inserm-USA Office INSERM KEY FIGURES (Year 2013) Budget: 970 M (~ $1.2 Billion) Human resources:
Active Biotech Group Interim Report 1 January 30 September 1999
 Active Biotech Group Interim Report 1 January 30 September 1999 The new annual forecast shows a considerably better result. Sale of the property in Solna expected to be completed before the end of the
Active Biotech Group Interim Report 1 January 30 September 1999 The new annual forecast shows a considerably better result. Sale of the property in Solna expected to be completed before the end of the
IMMUNOMEDICS, 300 The American Road, Morris Plains, New Jersey 07950 (973) 605-8200 Fax (973) 605-8282
 IMMUNOMEDICS, INC. 300 The American Road, Morris Plains, New Jersey 07950 (973) 605-8200 Fax (973) 605-8282 IMMUNOMEDICS ANNOUNCES FIRST QUARTER FISCAL 2014 RESULTS AND CLINICAL PROGRAM DEVELOPMENTS Morris
IMMUNOMEDICS, INC. 300 The American Road, Morris Plains, New Jersey 07950 (973) 605-8200 Fax (973) 605-8282 IMMUNOMEDICS ANNOUNCES FIRST QUARTER FISCAL 2014 RESULTS AND CLINICAL PROGRAM DEVELOPMENTS Morris
The Active Biotech Group Quarterly Report January - March 1999
 1 The Active Biotech Group Quarterly Report January - March 1999 (all figures refer exclusively to biotechnology, excluding Wilh. Sonesson) SAIK project ahead of schedule ETEC - Given top priority by SmithKline
1 The Active Biotech Group Quarterly Report January - March 1999 (all figures refer exclusively to biotechnology, excluding Wilh. Sonesson) SAIK project ahead of schedule ETEC - Given top priority by SmithKline
Taking Strategic Partnerships to the Next Level: An Alternative Approach to Licensing Your Development Asset
 Taking Strategic Partnerships to the Next Level: An Alternative Approach to Licensing Your Development Asset Introduction In this era of strategic development deals, inventiv Health has significantly broadened
Taking Strategic Partnerships to the Next Level: An Alternative Approach to Licensing Your Development Asset Introduction In this era of strategic development deals, inventiv Health has significantly broadened
Profile of Biomedical Research and Biotechnology Commercialization. San Francisco Oakland San Jose Consolidated Metropolitan Statistical Area
 Profile of Biomedical Research and Biotechnology Commercialization San Oakland San Jose Consolidated Metropolitan Statistical Area Overview and History of Biotechnology in San The San Bay area is in many
Profile of Biomedical Research and Biotechnology Commercialization San Oakland San Jose Consolidated Metropolitan Statistical Area Overview and History of Biotechnology in San The San Bay area is in many
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market
 A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
A Product and Pipeline Analysis of the Multiple Sclerosis Therapeutics Market Launch of Several Pipeline Oral Products Could Diminish the Market for Injectable Therapies NEC3-52 November 2014 Contents
PERSPECTIVE Drug Discovery Today Volume 17, Numbers 13/14 July 2012
 drug development: an economically viable strategy for biopharma R&D feature Kiran N. Meekings 1, kiran.meekings@thomsonreuters.com, Cory S.M. Williams 2 and John E. Arrowsmith 1 drug incentives have stimulated
drug development: an economically viable strategy for biopharma R&D feature Kiran N. Meekings 1, kiran.meekings@thomsonreuters.com, Cory S.M. Williams 2 and John E. Arrowsmith 1 drug incentives have stimulated
Data include post-hoc assessments of controlled studies in relapsing MS regarding evolution of
 September 10, 2014 Contact: Shikha Virdi 905-919-0200 ext. 5504 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data
September 10, 2014 Contact: Shikha Virdi 905-919-0200 ext. 5504 EMD Serono Presents New Data on Rebif (Interferon beta-1a) and Multiple Sclerosis Pipeline at Joint ACTRIMS-ECTRIMS Meeting in Boston Data
Life science companies in Sweden Including a comparison with Denmark
 VINNOVA ANALYSIS VA 2011:03 Life science companies in Sweden Including a comparison with Denmark Life science companies in Sweden Including a comparison with Denmark 0 addendi addendi Title: Life science
VINNOVA ANALYSIS VA 2011:03 Life science companies in Sweden Including a comparison with Denmark Life science companies in Sweden Including a comparison with Denmark 0 addendi addendi Title: Life science
News Release. Merck KGaA, Darmstadt, Germany, and Pfizer Receive FDA Breakthrough Therapy Designation for Avelumab in Metastatic Merkel Cell Carcinoma
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Markus Talanow +49 6151 72 7144 Investor Relations +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor
Master of Physician Assistant Studies Course Descriptions for Year I
 FALL TERM COURSES: Master of Physician Assistant Studies Course Descriptions for Year I PHAC 7230 Fundamentals in Pharmacology for Health Care I Credit Hrs: 3 This course will build on foundational knowledge
FALL TERM COURSES: Master of Physician Assistant Studies Course Descriptions for Year I PHAC 7230 Fundamentals in Pharmacology for Health Care I Credit Hrs: 3 This course will build on foundational knowledge
A Texas Life Science Perspective. Thomas R. Kowalski President Texas Healthcare and Bioscience Institute
 A Texas Life Science Perspective Thomas R. Kowalski President Texas Healthcare and Bioscience Institute 1 What is THBI? The Texas Healthcare and Bioscience Institute (THBI) is a non-profit, public policy
A Texas Life Science Perspective Thomas R. Kowalski President Texas Healthcare and Bioscience Institute 1 What is THBI? The Texas Healthcare and Bioscience Institute (THBI) is a non-profit, public policy
Building A Focused Oncology Business
 Building A Focused Oncology Business David Meek President, Oncology Our Strategic Priority: To be at the forefront of patient-centric innovation, bringing life-changing cancer therapies to patients with
Building A Focused Oncology Business David Meek President, Oncology Our Strategic Priority: To be at the forefront of patient-centric innovation, bringing life-changing cancer therapies to patients with
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN
 FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
FACT SHEET TESTETROL, A NOVEL ORALLY BIOACTIVE ANDROGEN General Pantarhei Bioscience B.V. is an emerging specialty pharmaceutical company with a creative approach towards drug development. The Company
Healthcare Innovation in the UK - A Royal Society of Chemistry Position Paper
 Healthcare Innovation in the UK - A Royal Society of Chemistry Position Paper Introduction The pharmaceutical industry (Pharma) has made important contributions to quality of life, longevity, economic
Healthcare Innovation in the UK - A Royal Society of Chemistry Position Paper Introduction The pharmaceutical industry (Pharma) has made important contributions to quality of life, longevity, economic
Building innovative drug discovery alliances. Addressing causes not. symptoms in diabetes
 Building innovative drug discovery alliances Addressing causes not symptoms in diabetes Evotec AG, Analyst information on CureBeta Alliance with Janssen, Hamburg, 2012 Forward-looking statements Information
Building innovative drug discovery alliances Addressing causes not symptoms in diabetes Evotec AG, Analyst information on CureBeta Alliance with Janssen, Hamburg, 2012 Forward-looking statements Information
Venture Funding of Therapeutic Innovation
 Venture Funding of Therapeutic Innovation A Comprehensive Look at a Decade of Venture Funding of Drug R&D by David Thomas, CFA and Chad Wessel BIO INDUSTRY ANALYSIS February 2015 A Letter from The Honorable
Venture Funding of Therapeutic Innovation A Comprehensive Look at a Decade of Venture Funding of Drug R&D by David Thomas, CFA and Chad Wessel BIO INDUSTRY ANALYSIS February 2015 A Letter from The Honorable
SIPBS Portfolio. 2012-13 Entry
 SIPBS Portfolio 2012-13 Entry MSc Programmes Existing: MSc Pharmaceutical Analysis MSc Analysis of Medicines (distance learning) MSc Clinical Pharmacy New Programmes just launched for Sept 2012 entry:
SIPBS Portfolio 2012-13 Entry MSc Programmes Existing: MSc Pharmaceutical Analysis MSc Analysis of Medicines (distance learning) MSc Clinical Pharmacy New Programmes just launched for Sept 2012 entry:
NEWS FROM THERAPEUTIC AREAS
 HIGHLIGHTS IN MEDICINE IN 2014 NEWS FROM THERAPEUTIC AREAS Research, development and medicine at Boehringer Ingelheim can look back on yet another very successful year. Research, development and medicine
HIGHLIGHTS IN MEDICINE IN 2014 NEWS FROM THERAPEUTIC AREAS Research, development and medicine at Boehringer Ingelheim can look back on yet another very successful year. Research, development and medicine
Monoclonal antibody (mab) products are currently a fast
 Antibody Monoclonal Therapeutics Janice M. Reichert, Ph.D. Monoclonal antibody (mab) products are currently a fast growing category of drugs. Like antibodies produced naturally by the human immune system,
Antibody Monoclonal Therapeutics Janice M. Reichert, Ph.D. Monoclonal antibody (mab) products are currently a fast growing category of drugs. Like antibodies produced naturally by the human immune system,
The Future of Monoclonal Antibodies Therapeutics: Innovation in Antibody Engineering, Key Growth Strategies and Forecasts to 2011
 Brochure More information from http://www.researchandmarkets.com/reports/344881/ The Future of Monoclonal Antibodies Therapeutics: Innovation in Antibody Engineering, Key Growth Strategies and Forecasts
Brochure More information from http://www.researchandmarkets.com/reports/344881/ The Future of Monoclonal Antibodies Therapeutics: Innovation in Antibody Engineering, Key Growth Strategies and Forecasts
