Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences)
|
|
|
- Branden Barrett
- 8 years ago
- Views:
Transcription
1 ! M Mertiva!! Pressmeddelande!den!24!september!2015! Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein(Sciences(Corporation((Protein(Sciences)(har(distribuerat(ett(informationsbrev(till(sina( aktieägare(( Shareholder(Letter(September(2015 ).(Protein(Sciences(har(godkänt(att(Mertiva( offentliggör(detta(informationsbrev.(informationsbrevet(innehåller(en(redogörelse(för(de(viktigaste( händelserna(i(bolaget(samt(oreviderade(siffror(för(första(halvåret(2015(och(är(bifogat(till(detta( pressmeddelande.( Informationsbrevet!innehåller!även!pressmeddelanden,!vilka!tidigare!publicerats!på!Protein! Sciences!hemsida.! Protein!Sciences!avser!att!distribuera!informationsbrev!kvartalsvis!till!sina!aktieägare.!När!Mertiva! erhåller!sådana!informationsbrev!avser!mertiva!publicera!dessa.! För$ytterligare$information,$vänligen$kontakta:$ Andreas!Bergsten,!VD!Mertiva!AB! info@mertiva.se! 070E !!! Om$Mertiva$ Mertiva!AB!är!ett!investeringsföretag!som!i!huvudsak!består!av!innehav!i!Protein!Sciences! Corporation!och!Mercodia!AB.!! MertivaEaktien!är!listad!på!NGM:s!handelsplats!Nordic!MTF!(kortnamn:!MERT!MTF).!! Mer!information!finns!på! Denna( information( offentliggörs( enligt( lagen( om( värdepappersmarknaden,( lagen( om( handel( med( finansiella(instrument(eller(krav(ställda(i(noteringsavtal.! Mertiva!AB!(publ.)! Upplandsgatan!67,!113!28!Stockholm,!Sverige! EEpost:!info@mertiva.se! Org.!Nr:!556530E1420!
2 To Our Shareholders: September 2015 The Company s business remains positive as we head into the 2015/16-influenza season. This quarter we received excellent results from our large clinical trial of Flublok Quadrivalent in comparison to a traditional egg-based inactivated quadrivalent vaccine in adults 50 years and older. The study showed that people vaccinated with Flublok were ~45% less likely to get confirmed flu than people that received the traditional vaccine. Protection against the dangerous H3N2 virus was even better more than 50% better than the egg-based vaccine. No other influenza vaccine has ever shown superiority results like these. In June, we hosted our quarterly Board of Directors meeting and our annual shareholder meeting at our Pearl River facility. Following the shareholder meeting, we held a press conference where we were joined by BARDA officials and local political and economic development leaders to announce the FDA approval of the facility, the top line results from our recent clinical trials and the FDA s grant of 12 years exclusivity for Flublok. You can view the complete press conference at the following link: In August, we convened a meeting in New York City of Key Opinion Leaders in the influenza field. The experts discussed the clinical data from our most recent Flublok study, strategies for building awareness, future directions for Flublok and new product opportunities. Overall, the participants were very enthusiastic about Flublok and agreed that its purity and superior efficacy are true game changers. Priorities are being placed on publishing the paper on the quadrivalent clinical study in a leading medical journal and creating strong messaging for various audiences. We have implemented a radio campaign in selected cities to drive pull-through of Flublok at stores in which it is stocked. We had commitments in place to produce approximately 1.2 million doses of Flublok this year (12 lots at Hospira and 6 lots at MassBio) and are on track to successfully complete production of projected materials. One lot (~25,000 doses) will be provided to our Mexican partners, Liomont, whose application for licensure of Flublok was accepted by Mexican authorities. Approval is expected later this fall. The Board of Directors approved the promotion of Mireli Fino to Senior Vice President of Manufacturing and Tim Fields to Senior Vice President of Quality. Mireli and Tim have both made very significant contributions to the Company, most recently playing a major role in securing FDA licensure of the Pearl River facility. Flublok: Pre-booking has concluded with approximately 160,000 doses pre-booked (this includes a 90,000 dose order from Target; approximately 10,000 from TOPCO Supermarkets; and hospitals nationwide). In-season sales have begun and we expect these to be filled primarily by independent pharmacies, doctor s offices, our clinic initiatives and re-orders from stores carrying our vaccine. Our contracts with the Connecticut State Government and the CDC also allow for the purchase of over 300,000 doses of Flublok. Flublok will be available at Passport Health Clinics
3 and other national access points. It will also be widely available at regional supermarkets and independent pharmacies including, Roundys and Mariano s in the Chicago area, Brookshires in Texas and Price Chopper in the Northeast. We are focusing our efforts on all of our access points to drive pull-through of the product. This includes furnishing marketing and promotional materials and development and implementation of a carefully targeted radio campaign in collaboration with iheart Media. We are running a national advertising campaign this fall directed at independent pharmacies in partnership with the National Community Pharmacists Association (NCPA) as we have found independent pharmacies to be strong proponents of Flublok. In addition, we are attending several pharmacy and healthcare professional annual meetings. We continue to develop and leverage our relationships with our four distributors: FFF Enterprises, McKesson, Cardinal Health and Henry Schein. We have met with them in person, conducted comprehensive training of their sales teams on the advantages of Flublok and how to position it in the marketplace and we are working with them to develop creative approaches to drive demand for the product. We have also become licensed to direct ship from our facilities in Connecticut. Our mass immunization initiative to offer workplace and community Flublok clinics in Connecticut and the surrounding regions is off to a great start. Health-At-Work ( is a partnership with physicians from Velocity Urgent Care that is a full service vaccination provider that allows employers to customize vaccination clinics for their employees. We hosted three breakfast events on site and partnered with Goodwin College on a fourth breakfast event to promote Flublok to local employers and generate clinics for Health-at-Work. Those efforts have yielded increased interest and repeat and new business. The Healthy Choices Mobile Vaccination collaboration with Hunters Ambulance, Hartford Healthcare at Home and the Meriden Housing Authority has a new name: FastVax! A bus has been wrapped to showcase Flublok and the logos of each of the partners. We added Hancock Pharmacy as a partner in the initiative that will further increase our opportunity to bill insurance companies. The initiative is growing and the community s response has been great. Clinical Trials: Our large clinical trials of Flublok Quadrivalent were completed in late May and the data are now available. PSC12 assessed the protective efficacy of Flublok Quadrivalent in comparison to a licensed, inactivated egg-grown quadrivalent vaccine in ~9,000 adults 50 years of age and older. This trial was designed to support full, traditional approval (as opposed to accelerated approval) of trivalent Flublok in this age group and to support traditional approval of the quadrivalent vaccine in older adults. The exciting news is that the study met all endpoints and that Flublok demonstrated superiority over the traditional vaccine. People that received Flublok were ~45% less likely to get flu than those that received the egg-grown vaccine. Moreover, the flu season was a particularly severe one caused largely by the H3N2 strain that is often responsible for severe complications from influenza. Flublok recipients were >50% less likely to get H3N2 influenza than the egg-grown vaccine recipients. These data have been accepted for presentation at the American Society for Microbiology s Interscience 2
4 Conference of Antimicrobial Agents and Chemotherapy (ICAAC) (the largest infectious diseases meeting in the world annually) in September. The other clinical trial, PSC16, studied the safety and immunogenicity of Flublok Quadrivalent in 1,350 adults years of age. The results of this trial will support approval of the quadrivalent formulation in the younger adult age group, for whom Flublok already has full, traditional approval. The results were positive and contribute to the provision of a consistent story of the significant improvement that Flublok brings to the armamentarium of influenza vaccines. We are preparing to conduct a large post-marketing study this season with Kaiser Permanente to satisfy FDA commitments. This study that will include approximately 50,000 subjects is expected to generate important health outcome data. Manufacturing: The manufacturing teams in Pearl River and Meriden continued production of Flublok drug substance during the second quarter. We experienced higher than expected productivity that offset production delays that occurred in the first quarter. This allowed us to maintain a 10-day cadence in Pearl River and slow down the projected staff growth for With the early start in 2014, we were able to begin filling drug product at MassBio and Hospira in mid-july. This quarter we initiated two new projects in manufacturing in parallel with commercial Flublok production. The first project allows us to increase the number of times we can reuse one of our most expensive reagents and, therefore, reduce the cost of producing Flublok. We received FDA approval for this change. The second project supports our Pandemic Vaccine. The Meriden team successfully implemented a new aseptic filling team and completed production of vials for the Pandemic clinical trial PSC26. This allowed PSC26 to be fully enrolled in August BARDA: We are pleased to report continued progress on the milestones that were established under the second option of the BARDA contract that extends through December Milestone 11D-2: Perform a clinical study to support Emergency Use Authorization (EUA) of Panblok in case of an influenza pandemic. Status: Patient enrollment for Stage 1 was completed on August 4 th. 2. Milestone 11D-3a and b: Approval of seasonal Flublok Quadrivalent Status: Completed necessary clinical trials; met criteria for primary endpoint of noninferiority and exploratory endpoint of superiority. BARDA Stock Pile Contract: We anticipate award of a new contract from BARDA relating to stock piling: however, award of this contract has been delayed until January Collaborations: We attended the BIO 2015 International Convention in Philadelphia in June where we had multiple productive meetings centered around our platform technology and license opportunities. The companies we met with ranged in size and were interested in either licensing our cell line for development of new products or licensing Flublok for new 3
5 territories. We also had a Flublok exhibit where we created broad awareness and generated vaccine sales leads. Liomont, our Flublok licensee in Mexico, submitted its application for licensure of Flublok in Mexico to the Mexican regulatory authorities. Approval is expected this fall. Liomont is purchasing one lot of Flublok this season to test market in Mexico following licensure. We will receive a milestone payment when the vaccine is approved. Progress continues to be made in Japan as our Flublok licensee, UMN Pharma, which is partnered with Astellas, works with Japanese regulatory authorities to secure licensure. The license application has been submitted and approval is expected later this year. We will receive a significant milestone payment upon licensure. Our SBIR grant from the National Cancer Institute that funds development work on the production of vault nanoparticles for treatment of lung cancer in collaboration with UCLA and Vault Nano, Inc. was funded for a second year. Process development work is nearing completion and material will soon be produced for preclinical testing. We were also awarded a Phase I SBIR grant sponsored by the Department of Defense for development of an adenovirus vaccine. Financial Results: For the six months ended June 30, 2015 revenues increased 37% to $19.4 million from $14.2 million in This increase reflects an increase in the contribution from our BARDA contract that accounted for approximately 89% of revenues, up from 73% in the comparable period in 2014 primarily as a result of the initiation of two large clinical trials that are funded by BARDA. Product sales increased 28% to $723,000 but our Collaborative Agreements and Technology Licenses that together accounted for 7% of revenues were down 21% and 79%, respectively to $1 million and $398,000, respectively. As a reminder, revenues from Collaborative Agreements and Technology Licenses are based on success in product development by our customers and, therefore, significantly increase or decrease on a quarterly basis. Operating expenses increased by 74% to $19.6 million from $11.3 million in 2014 primarily because of clinical trial expenses covered under the BARDA contract. We had an operating and net loss before tax expense and benefit of $250,000 and $245,000, respectively, compared to an operating and net profit before tax expense and benefit of $2.9 million in The difference results primarily from the sale of a portion of the UMN Pharma shares we own in Cash and receivables net of payables were $9.8 million - $625,000 less than at the end of Q1 and $7.7 million less than at the end of 2014 reflecting the seasonal impact of our flu business where almost all expenses are incurred in Qs 1, 2 and 3 and most revenues received in Q4 and the following Q1. Now that we are manufacturing for commercial sale the impact is much clearer than in previous years. We closed a $10 million banking working capital line to assist in covering seasonal cash flow shortfall. 4
6
7 Protein Sciences Corporation Balance Sheets (Unaudited) June 30, December 31, ASSETS CURRENT ASSETS: Cash and cash equivalents $ 5,193,526 $ 5,818,164 Short term investment 3,446,206 3,630,057 BARDA funds receivable -- including unbilled of $823,839 and $4,738,666, respectively 6,175,597 13,428,471 Accounts receivable -- including unbilled of $52,961 and $245,818, respectively and net of allowance for doubtful accounts of $50,000 and $50,000, respectively 400,684 2,279,502 Inventory 6,893,402 2,207,079 Deferred tax asset 3,168,463 2,974,473 Other current assets 584, ,319 Total current assets 25,862,740 31,265,065 PROPERTY, PLANT AND EQUIPMENT -- Net 3,811,047 4,020,026 Long term deferred tax asset 4,960,017 4,960,017 Restricted cash 638, ,258 Other assets 384, ,859 TOTAL ASSETS $ 35,656,742 $ 41,234,225 LIABILITIES AND STOCKHOLDERS' EQUITY CURRENT LIABILITIES: Accounts payable $ 5,394,555 $ 7,627,970 Accrued expenses 2,483,491 4,904,104 Deferred revenue, current portion 598,779 1,076,578 Other current liabilities 147, ,290 Total current liabilities 8,624,116 13,755,942 LONG TERM LIABILITIES: Deferred revenue 717, ,555 Other liabilities 270, ,677 Total long term liabilities 987,198 1,300,232 TOTAL LIABILITIES 9,611,314 15,056,175 STOCKHOLDERS' EQUITY Common Stock, $0.001 par value; 150,000,000 shares authorized; 77,606,902 and 77,549,402 shares issued and outstanding at June 30, 2015 and December 31, 2014, respectively 77,607 77,549 Additional paid-in capital 63,691,497 63,519,642 Accumulated other comprehensive income, net of tax 487, ,955 Accumulated deficit (38,211,391) (38,061,095) Total stockholders' equity 26,045,428 26,178,051 TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY $ 35,656,742 $ 41,234,225
8 Six Months Ending June 30, Percent 2015 Percent Change of Revenue REVENUES: BARDA contract $ 17,252,242 $ 10,419,271 66% 88.9% Collaborative agreements 1,023,077 1,299,835-21% 5.3% Technology licenses 398,189 1,881,362-79% 2.1% Product sales 723, ,780 28% 3.7% Total revenues 19,396,772 14,164,249 37% 100.0% OPERATING EXPENSES: Protein Sciences Corporation Statements of Operations (Unaudited) Research and development 16,540,216 8,734,522 89% 85.3% Cost of goods sold 291, ,450 93% 1.5% General and administrative 2,814,178 2,399,328 17% 14.5% Total operating expenses 19,646,249 11,285,300 74% 101.3% INCOME FROM OPERATIONS (249,477) 2,878, % -1.3% (INCOME) OTHER EXPENSE: Interest expense % 0.0% Interest income (6,203) (4,673) 33% 0.0% Other income/expense 1,286 1,182 Total other (income) expense (4,917) (3,366) 46% 0.0% Net income before tax expense and benefit (244,560) 2,882, % -1.3% Tax (expense) benefit 94, ,620 0% 0.5% Net Income $ (150,297) $ 3,103, % -0.8%
9 Protein Sciences Corporation Statements of Cash Flow (Unaudited) Six Months Ending June 30, CASH FLOWS FROM OPERATING ACTIVITIES: Net income $ (150,297) $ 3,103,934 Adjustments to reconcile net income to net cash provided by operating activities: Realized gain on short term investment - - Depreciation and amortization 287, ,864 Share-based compensation 157, ,791 Tax Impact on unrealized loss on S/T investment 29,612 - Excess tax benefit from stock compensation - - Loss on disposal of assets 1,494 - Deferred taxes (193,991) - Changes in operating assets and liabilities: Inventory (4,686,323) (2,694,000) Accounts receivable 1,878,818 1,109,107 BARDA funds receivable 7,252,874 1,832,263 Restricted cash - - Other assets 308,637 (46,770) Accounts payable and accrued expenses (4,654,027) (1,841,746) Other liabilities (73,645) (365,394) Deferred accounts (717,188) (366,460) Net cash (used in) provided by operating activities (559,006) 1,647,589 CASH FLOWS FROM INVESTING ACTIVITIES: Proceed from sale of short term investment - - Purchase of investments - - Purchases of property and equipment (80,007) (145,997) Net cash (used in) investing activities (80,007) (145,997) CASH FLOWS FROM FINANCING ACTIVITIES: Proceeds from exercise of stock options 14, Payments of capital lease obligations - - Net cash (used in) provided by financing activities 14, NET INCREASE IN CASH AND CASH EQUIVALENTS (624,638) 1,502,049 CASH AND CASH EQUIVALENTS - Beginning of period 5,818,164 4,936,355 CASH AND CASH EQUIVALENTS - End of period $ 5,193,526 $ 6,438,404 SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: Cash paid for interest $ - $ 124 Cash paid for taxes $ 51,115 $ 70,129
10 Protein Sciences Corporation Statements of Comprehensive Income (Unaudited) Six Months Ending June 30, Net Income $ (150,297) 3,103,934 Other comprehensive income: Net change in unrealized gain in investments $ (154,239) 52,280 Total Comprehensive Income $ (304,537) $ 3,156,214
11 Flublok Influenza Vaccine is now Nationwide The Pure Choice for Adults 18 and Older For Immediate Release September 22, 2015 Contact: Courtney Goodwin Communications Associate Phone: (203) ext. 301 Meriden, CT Protein Sciences Corporation announced today that Flublok Influenza Vaccine, that was shown to provide better protection against the flu than a traditional flu vaccine in a recent clinical study, is now available nationwide. Not only does Flublok work well, it is the only flu vaccine that is free of all of the following undesirable ingredients: formaldehyde, antibiotics, gluten, gelatin, egg protein, latex, thimerosal and other preservatives. This makes it the pure choice for flu protection for adults 18 and older. Getting Flublok has never been easier. National retailers Target and Walmart are offering Flublok. Flublok is also available in many regional supermarkets and independent pharmacies, including Mariano s and Roundy s in the Chicago area, Brookshire s in Texas, and Price Chopper in the Northeast. Passport Health Clinics will once again offer Flublok at its sites nationwide. Passport patients can even book their vaccines online. We are pleased to partner with leading pharmacies to greatly expand access to our vaccine, said Manon Cox, President and CEO of Protein Sciences Corporation. Anyone over 18 years of age will be able to walk into Target, Mariano s, Roundy s or Brookshire s nationwide and receive Flublok. At other stores, people are encouraged to call the pharmacy in advance to make sure Flublok is in stock when they come in for vaccination. Nearly all insurance providers cover Flublok so most people can get Flublok free of charge. Flublok has found special appeal among health conscious individuals that prefer its purity and its popularity is growing among people focused on better protection. In a recent clinical study of the quadrivalent version of Flublok in approximately 9,000 adults 50 years and older, Flublok recipients were 43% less likely to develop culture confirmed influenza than people who received a licensed quadrivalent egg-based influenza vaccine. For more information about Flublok and where to get it, please visit About Protein Sciences Protein Sciences specializes in vaccine development and protein production. Our mission is our inspiration: to save lives and improve health through the creation of innovative vaccines and biopharmaceuticals. FB14052
12 Flublok, the world s first recombinant protein-based vaccine for the prevention of seasonal influenza disease, was approved by FDA in January Flublok is the only flu vaccine made in a 100% egg-free system using modern cell culture technology, making it unnecessary to use an infectious influenza virus or antibiotics in manufacturing. Flublok is highly purified and does not contain any preservatives (e.g., thimerosal, a mercury derivative), egg proteins, gelatin or latex. In addition, Flublok contains three times more antigen than traditional flu vaccines (3x45mcg hemagglutinin protein versus 3x15mcg hemagglutinin protein)*. Flublok is a perfect copy of the virus coat and is not subject to the egg-adapted mutations associated with low vaccine effectiveness (see Skowronski et al. (2014) PLOS ONE 9(3), e92153). Healthcare professionals wishing to order Flublok should contact one of the following distributors: FFF Enterprises: Cardinal Health: McKesson: 877-MCK-4FLU mms.mckesson.com Henry Schein: Learn more at and Flublok Safety Information Flublok is approved for people 18 and older to prevent influenza disease. The most common side effect from Flublok is pain at the site of injection. Headache, fatigue or muscle ache may occur. Tell the doctor if you have ever experienced Guillain-Barré syndrome (severe muscle weakness) or have had a severe allergic reaction to any component of Flublok vaccine. Vaccination with Flublok may not protect all individuals. Clinical effectiveness in adults 50 and older is based on the immune response elicited by Flublok and not on demonstration of decreased influenza disease. Please see the complete Package Insert available at or call for more information. *Flublok demonstrated a higher antibody response to the A strains during 2 clinical trials in adults 50 years old. The B strain antibody response was comparable to traditional trivalent vaccines. ### FB14052
13 Protein Sciences Promotes Vice Presidents Mireli Fino and Tim Fields to Senior Vice President For Immediate Release July 10, 2015 Contact: Courtney Goodwin Communications Associate Phone: (203) ext. 301 Meriden, CT Protein Sciences Corporation announced that the Board of Directors has approved the promotion of Mireli Fino, Vice President, Manufacturing Operations to Senior Vice President, Manufacturing Operations and Tim Fields, Vice President, Quality to Senior Vice President, Quality. Ms. Fino joined Protein Sciences in 2012 as VP, Manufacturing Operations. She came from Wyeth (now Pfizer) where she spent 20 years in various roles in vaccine development and commercial manufacturing. Most recently, she served as Director Manufacturing Sciences and Technology - Drug Substance. She played a key role in the successful development and launch of Prevnar13 pneumococcal 13- valent conjugate vaccine, considered one of the most complex biologics licensed to date. In addition, Ms. Fino led global initiatives implementing process and yield improvements based on the successful applications of risk management and Quality by Design (QbD) and Process Analytical Technology (PAT) concepts. She has a B.S. in Biochemical Engineering from the University of Aguascalientes in Mexico. Tim Fields joined Protein Sciences in 2010 as Director of Compliance and Training. Subsequently, he served as Senior Director of Quality Operations before becoming VP of Quality and Validation in He has more than 30 years of experience in the pharmaceutical industry, including more than 13 years at Pfizer and 16 years as a GMP compliance consultant. Mr. Fields has experience in a variety of compliance areas, including quality systems, validation, aseptic processing, computerized system validation, GMP training, 21CFR Part 11, document management and auditing. He is a member of PDA and ISPE and is on the editorial review board for the Journal of Validation and Journal of GXP Compliance. Previously, he was a member of DIA, RAPS and ASQC and was an adjunct instructor at the Community College of Rhode Island. He FB14052
14 has a B.A. in Biology from Indiana University and an M.A. in Life Sciences from Indiana State University. Mireli and Tim have played critical leadership roles within the organization most recently to secure FDA licensure of the Pearl River manufacturing facility, said Manon Cox, President and CEO of Protein Sciences. Licensure of this facility allows us to scale up our commercial manufacturing of Flublok fivefold. I am delighted that the Board has recognized their extraordinary contributions. About Protein Sciences Protein Sciences specializes in vaccine development and protein production. Our mission is our inspiration: to save lives and improve health through the creation of innovative vaccines and biopharmaceuticals. Flublok, the world s first recombinant protein-based vaccine for the prevention of seasonal influenza disease, was approved by FDA in January Flublok is the only flu vaccine made in a 100% egg-free system using modern cell culture technology, making it unnecessary to use an infectious influenza virus or antibiotics in manufacturing. Flublok is highly purified and does not contain any preservatives (e.g., thimerosal, a mercury derivative), egg proteins, gelatin or latex. In addition, Flublok contains three times more antigen than traditional flu vaccines (3x45mcg hemagglutinin protein versus 3x15mcg hemagglutinin protein)*. Flublok is a perfect copy of the virus coat and is not subject to the egg-adapted mutations associated with low vaccine effectiveness (see Skowronski et al. (2014) PLOS ONE 9(3), e92153). Healthcare professionals wishing to pre-order Flublok should contact one of the following distributors: FFF Enterprises: Cardinal Health: McKesson: 877-MCK-4FLU mms.mckesson.com Henry Schein Medical: Learn more at and Flublok Safety Information Flublok is approved for people 18 and older to prevent influenza disease. The most common side effect from Flublok is pain at the site of injection. Headache, fatigue or muscle ache may occur. Tell the doctor if you have ever experienced Guillain-Barré syndrome (severe muscle weakness) or have had a severe allergic reaction to any component of Flublok vaccine. Vaccination with Flublok may not protect all individuals. Clinical effectiveness of trivalent Flublok in adults 50 and older is based on the immune response elicited by trivalent Flublok and not on demonstration of decreased influenza disease. FB14052
15 Please see the complete Package Insert available at or call for more information. *Trivalent Flublok demonstrated a higher antibody response to the A strains during 2 clinical trials in adults 50 years old. The B strain antibody response was comparable to traditional trivalent vaccines. ### FB14052
16 New Clinical Study of Flublok Influenza Vaccine Shows That Groundbreaking Flublok Is More Effective Than the Traditional Flu Shot For Immediate Release June 25, 2015 Contact: Courtney Goodwin Communications Associate Phone: (203) Meriden, CT Protein Sciences Corporation announced today topline data showing that Flublok Quadrivalent the quadrivalent version of FDA-approved trivalent Flublok influenza vaccine outperformed a traditional influenza vaccine last season and was better at preventing the flu. The company presented the results of a clinical trial comparing Flublok Quadrivalent to a traditional egg-based quadrivalent inactivated vaccine. The data demonstrate superior performance of Flublok based on a significantly lower number of people contracting the flu after vaccination with Flublok Quadrivalent. In this study of approximately 9,000 subjects aged 50 and older, half received Flublok Quadrivalent influenza vaccine and half received quadrivalent inactivated influenza vaccine produced in eggs. The results show that 31% more people were protected by Flublok than by the egg-derived vaccine. This study points out the advantages of using modern technology to overcome problems with traditional influenza vaccine manufacturing, said Manon Cox, President and CEO of Protein Sciences. Ten years ago we had the first evidence in clinical trial PSC01 that more antigen was beneficial even for healthy adults. Today with support from the Biomedical Advanced Research and Development Authority (BARDA) we were able to show statistically significant better performance than traditional egg-produced vaccines." Flublok is the only flu vaccine made without the use of eggs and therefore is not subject to the mutations that are sometimes introduced into the vaccine during the process of egg adaptation that can cause the traditional vaccines to be ineffective (see Skowronski et al. (2014) PLOS ONE 9(3), e92153). Flublok contains three times more active ingredients than traditional vaccines (3 or 4x45mcg hemagglutinin protein versus 3 or 4x15mcg hemagglutinin protein for trivalent or quadrivalent vaccine, respectively) and produced significantly higher immune responses to the A strains of influenza (especially H3N2) in the Flublok Quadrivalent study.* Flublok is highly purified and does not contain influenza virus, antibiotics, formaldehyde, preservatives, latex, gluten or gelatin unlike other flu vaccines. FB14052
17 This project has been funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Department of Health and Human Services, under Contract No. HHSO C. For more information about Flublok, please visit About Protein Sciences Protein Sciences specializes in vaccine development and protein production. Our mission is our inspiration: to save lives and improve health through the creation of innovative vaccines and biopharmaceuticals. Flublok, the world s first recombinant protein-based vaccine for the prevention of seasonal influenza disease, was approved by FDA in January Flublok is the only flu vaccine made in a 100% egg-free system using modern cell culture technology, making it unnecessary to use an infectious influenza virus or antibiotics in manufacturing. Healthcare professionals wishing to pre-order Flublok should contact one of the following distributors: FFF Enterprises: Cardinal Health: McKesson: 877-MCK-4FLU mms.mckesson.com Henry Schein Medical: Learn more at and Flublok Safety Information Trivalent Flublok is approved for people 18 and older to prevent influenza disease. The most common side effect from Flublok is pain at the site of injection. Headache, fatigue or muscle ache may occur. Tell the doctor if you have ever experienced Guillain-Barré syndrome (severe muscle weakness) or have had a severe allergic reaction to any component of Flublok vaccine. Vaccination with Flublok may not protect all individuals. Clinical effectiveness of trivalent Flublok in adults 50 and older is based on the immune response elicited by trivalent Flublok and not on demonstration of decreased influenza disease. Please see the complete Package Insert available at or call for more information. *Trivalent Flublok demonstrated a higher antibody response to the A strains during 2 clinical trials in adults 50 years old. The B strain antibody response was comparable to traditional trivalent vaccines. FB14052
Informationsbrev.till.aktieägarna.från.Protein.Sciences,.december.2015.
 ! M Mertiva!! Pressmeddelande!den!28!december!2015! Informationsbrev.till.aktieägarna.från.Protein.Sciences,.december.2015. Protein(Sciences(Corporation((Protein(Sciences)(har(distribuerat(ett(nytt(informationsbrev(till(sina(
! M Mertiva!! Pressmeddelande!den!28!december!2015! Informationsbrev.till.aktieägarna.från.Protein.Sciences,.december.2015. Protein(Sciences(Corporation((Protein(Sciences)(har(distribuerat(ett(nytt(informationsbrev(till(sina(
Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences)
 ! M Mertiva!! Pressmeddelande den 1 januari 2015 Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein Sciences Corporation (Protein Sciences) har distribuerat ett informationsbrev
! M Mertiva!! Pressmeddelande den 1 januari 2015 Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein Sciences Corporation (Protein Sciences) har distribuerat ett informationsbrev
Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences)
 ! M Mertiva!! Pressmeddelande den 4 april 2015 Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein Sciences Corporation (Protein Sciences) har distribuerat ett informationsbrev
! M Mertiva!! Pressmeddelande den 4 april 2015 Kvartalets)informationsbrev)till)aktieägarna)från)Protein)Sciences) Protein Sciences Corporation (Protein Sciences) har distribuerat ett informationsbrev
SPHERIX INCORPORATED (Exact name of registrant as specified in its charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results
 China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
China Pharma Holdings, Inc. Reports Second Quarter 2010 Financial Results HAIKOU CITY, China, August 10, 2010 China Pharma Holdings, Inc. (NYSE AMEX: CPHI) ( China Pharma or the Company ), a leading fully
HSA Consumer Guide. Understanding Vaccines, Vaccine Development and Production. www.hsa.gov.sg November 2009. How a Vaccine Works.
 November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
November 2009 Understanding Vaccines, Vaccine Development and Production Vaccines, in general, help protect people from harmful infections before they come in contact with the disease. Vaccines may also
NORWEGIAN CRUISE LINE HOLDINGS LTD. CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data)
 CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data) Revenue Passenger ticket $ 583,923 $ 490,322 $ 1,400,470 $ 1,257,871 Onboard and other 213,962 184,089 569,479
CONSOLIDATED STATEMENTS OF OPERATIONS (unaudited, in thousands, except share and per share data) Revenue Passenger ticket $ 583,923 $ 490,322 $ 1,400,470 $ 1,257,871 Onboard and other 213,962 184,089 569,479
Inventory Management Tracking, Distribution and Delivery
 Inventory Management Tracking, Distribution and Delivery Ben Erickson Public Health Analyst Office of Public Health Preparedness and Response Division of Strategic National Stockpile Agenda Inventory Management
Inventory Management Tracking, Distribution and Delivery Ben Erickson Public Health Analyst Office of Public Health Preparedness and Response Division of Strategic National Stockpile Agenda Inventory Management
PROMETIC LIFE SCIENCES INC.
 PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
AMAZON.COM, INC. CONSOLIDATED STATEMENTS OF CASH FLOWS (in millions)
 CONSOLIDATED STATEMENTS OF CASH FLOWS CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD $ 8,084 $ 5,269 $ 3,777 OPERATING ACTIVITIES: Net income (loss) 274 (39) 631 Adjustments to reconcile net income (loss)
CONSOLIDATED STATEMENTS OF CASH FLOWS CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD $ 8,084 $ 5,269 $ 3,777 OPERATING ACTIVITIES: Net income (loss) 274 (39) 631 Adjustments to reconcile net income (loss)
China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results
 China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results - Revenue Up 13.5% Year-Over-Year to $24.4 Million, Exceeding Guidance - Cash Receipts From Online Course
China Distance Education Holdings Limited Reports First Quarter Fiscal Year 2016 Financial Results - Revenue Up 13.5% Year-Over-Year to $24.4 Million, Exceeding Guidance - Cash Receipts From Online Course
Third Quarter 2015 Highlights:
 OxySure (OXYS) Reports Third Quarter 2015 Financial Results with Annual Run Rate Approaching $5 Million Company on Track to Reach $10 Million Run Rate and Breakeven in 2016 Frisco, Texas, November 17,
OxySure (OXYS) Reports Third Quarter 2015 Financial Results with Annual Run Rate Approaching $5 Million Company on Track to Reach $10 Million Run Rate and Breakeven in 2016 Frisco, Texas, November 17,
GeckoSystems International Corporation. Financial Statements (For Period Ending June 30, 2013)
 Financial Statements (For Period Ending June 30, 2013) Balance Sheet As of June 30, 2013 (Unaudited) Assets Current assets: Cash $ 18,245 Cash equivalents 525,000 Supply inventory (frames, motors, electronics,
Financial Statements (For Period Ending June 30, 2013) Balance Sheet As of June 30, 2013 (Unaudited) Assets Current assets: Cash $ 18,245 Cash equivalents 525,000 Supply inventory (frames, motors, electronics,
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 6-K SINOVAC BIOTECH LTD.
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month
Cytec Announces First Quarter 2010 Results. As-Adjusted EPS of $0.66, Significantly Above Prior Year As-Adjusted EPS of $0.06
 cytec News & Information Cytec Industries Inc. Five Garret Mountain Plaza Woodland Park, New Jersey 07424 www.cytec.com Contact: Jodi Allen (Investor Relations) (973) 357-3283 Release Date: Immediate Cytec
cytec News & Information Cytec Industries Inc. Five Garret Mountain Plaza Woodland Park, New Jersey 07424 www.cytec.com Contact: Jodi Allen (Investor Relations) (973) 357-3283 Release Date: Immediate Cytec
H1N1 Flu Vaccine Available to All Virginia Beach City Public Schools Students
 V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
V i r g i n i a B e a c h C i t y P u b l i c S c h o o l s apple-a-day F o r O u r F a m i l y o f I n t e r e s t e d C i t i z e n s Special Edition H1N1 Flu Vaccine Available to All Virginia Beach
FOR INFORMATION CONTACT:
 NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
NEWS RELEASE FOR INFORMATION CONTACT: Caroline Calderone Baisley Deborah C. Travers Director of Health Director of Family Health Tel [203] 622-7836 Tel [203] 622-7854 September 10, 2014 For Immediate Release
EVERYDAY HEALTH, INC.
 EVERYDAY HEALTH, INC. FORM 8-K (Current report filing) Filed 05/11/15 for the Period Ending 05/11/15 Address 345 HUDSON STREET 16TH FLOOR NEW YORK, NY 10014 Telephone 718-797-0722 CIK 0001358483 Symbol
EVERYDAY HEALTH, INC. FORM 8-K (Current report filing) Filed 05/11/15 for the Period Ending 05/11/15 Address 345 HUDSON STREET 16TH FLOOR NEW YORK, NY 10014 Telephone 718-797-0722 CIK 0001358483 Symbol
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington D.C. 20549
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington D.C. 20549 REPORT OF FOREIGN ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934 For the month of April, 2009 Commission
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington D.C. 20549 REPORT OF FOREIGN ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934 For the month of April, 2009 Commission
China Distance Education Holdings Limited Reports Financial Results for the First Quarter of Fiscal 2014
 China Distance Education Holdings Limited Reports Financial Results for the First Quarter of Fiscal 2014 Revenue Up 43.7% Year-over-year to $18.7 Million Net Income Up 131.9% Year-over-year to $3.4 Million
China Distance Education Holdings Limited Reports Financial Results for the First Quarter of Fiscal 2014 Revenue Up 43.7% Year-over-year to $18.7 Million Net Income Up 131.9% Year-over-year to $3.4 Million
MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial Results
 MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial
MGC Diagnostics Corporation 350 Oak Grove Parkway Saint Paul, MN 55127 Telephone: (651) 484-4874 Facsimile: (651) 484-4826 FOR IMMEDIATE RELEASE MGC Diagnostics Corporation Reports Fiscal Year 2015 Financial
ACCESS TO THE SEASONAL FLU VACCINE IN CANADA. How the flu shot makes its way from the laboratory to the doctor s office.
 ACCESS TO THE SEASONAL FLU VACCINE IN CANADA How the flu shot makes its way from the laboratory to the doctor s office. Health Canada is the federal department responsible for helping Canadians maintain
ACCESS TO THE SEASONAL FLU VACCINE IN CANADA How the flu shot makes its way from the laboratory to the doctor s office. Health Canada is the federal department responsible for helping Canadians maintain
Boss Holdings, Inc. 2015 Fiscal Year End Report
 Boss Holdings, Inc. 1221 Page Street Kewanee, IL 61443 PH: 800.447.4581 F: 309.852.0848 2015 Fiscal Year End Report Consolidated revenues of Boss Holdings, Inc. (the Company ) for the fourth quarter of
Boss Holdings, Inc. 1221 Page Street Kewanee, IL 61443 PH: 800.447.4581 F: 309.852.0848 2015 Fiscal Year End Report Consolidated revenues of Boss Holdings, Inc. (the Company ) for the fourth quarter of
Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law
 March 18, 2015 TRADING SYMBOL: The Toronto Stock Exchange: Village Farms International, Inc. VFF Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law Vancouver,
March 18, 2015 TRADING SYMBOL: The Toronto Stock Exchange: Village Farms International, Inc. VFF Village Farms International Announces Year End 2014 Results and Adoption of Advance Notice By-Law Vancouver,
Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, 2015 (408) 432-1900 NATIONAL DISTRIBUTION
 e News Release Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS INCREASES IN REVENUE AND NET INCOME OVER
e News Release Contact: Don Zerio 5:00 EDT Vice President, Finance, Chief Financial Officer July 21, (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS INCREASES IN REVENUE AND NET INCOME OVER
Zebra Technologies Announces Record Sales for Second Quarter of 2006
 FOR IMMEDIATE RELEASE Zebra Technologies Announces Record Sales for Second Quarter of 2006 Vernon Hills, IL, July 26, 2006 Zebra Technologies Corporation (NASDAQ: ZBRA) today announced that net income
FOR IMMEDIATE RELEASE Zebra Technologies Announces Record Sales for Second Quarter of 2006 Vernon Hills, IL, July 26, 2006 Zebra Technologies Corporation (NASDAQ: ZBRA) today announced that net income
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
XENOPORT INC FORM 8-K. (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14
 XENOPORT INC FORM 8-K (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14 Address 3410 CENTRAL EXPRESSWAY SANTA CLARA, CA 95051 Telephone 4086167200 CIK 0001130591 Symbol XNPT SIC Code
XENOPORT INC FORM 8-K (Current report filing) Filed 05/08/14 for the Period Ending 05/08/14 Address 3410 CENTRAL EXPRESSWAY SANTA CLARA, CA 95051 Telephone 4086167200 CIK 0001130591 Symbol XNPT SIC Code
Vaccine Production Strategies: Ensuring Alignment and Sustainability
 Vaccine Production Strategies: Ensuring Alignment and Sustainability Second WHO Consultation on Global Action Plan for Influenza Vaccines (GAP-II) Robert W Malone, MD, MS Consulting, Vaccines and Biologicals
Vaccine Production Strategies: Ensuring Alignment and Sustainability Second WHO Consultation on Global Action Plan for Influenza Vaccines (GAP-II) Robert W Malone, MD, MS Consulting, Vaccines and Biologicals
Consolidated Balance Sheets
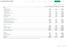 Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Influenza Vaccine Frequently Asked Questions. Influenza Control Program
 Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
Vice President, Finance, Chief Financial Officer April 19, 2016 (408) 432-1900 NATIONAL DISTRIBUTION
 News Release Contact: Donald P. Zerio Vice President, Finance, Chief Financial Officer April 19, 2016 (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS SEQUENTIAL INCREASES IN REVENUE, NET
News Release Contact: Donald P. Zerio Vice President, Finance, Chief Financial Officer April 19, 2016 (408) 432-1900 NATIONAL DISTRIBUTION LINEAR TECHNOLOGY REPORTS SEQUENTIAL INCREASES IN REVENUE, NET
TransUnion Reports Third Quarter 2014 Results
 TransUnion Reports Third Quarter 2014 Results Revenue of $338 million, an increase of 13 percent on a GAAP basis (14 percent on a constant currency basis) compared with the third quarter of 2013 Adjusted
TransUnion Reports Third Quarter 2014 Results Revenue of $338 million, an increase of 13 percent on a GAAP basis (14 percent on a constant currency basis) compared with the third quarter of 2013 Adjusted
of Non-GAAP Financial Measures below. 1 For more information about the non-gaap financial measures contained in this press release, please see Use
 China Distance Education Holdings Limited Reports Financial Results for the First Quarter of Fiscal 2015, and Announces Changes in Management Revenue Up 14.9% Year-over-year to $21.5 Million, Exceeding
China Distance Education Holdings Limited Reports Financial Results for the First Quarter of Fiscal 2015, and Announces Changes in Management Revenue Up 14.9% Year-over-year to $21.5 Million, Exceeding
Winland Reports Q1 2013 Financial Results, Increased Sales
 Winland Reports Q1 2013 Financial Results, Increased Sales Company s transition to providing real-time environmental monitoring services on SaaS platform on track to launch as scheduled MANKATO, Minn.,
Winland Reports Q1 2013 Financial Results, Increased Sales Company s transition to providing real-time environmental monitoring services on SaaS platform on track to launch as scheduled MANKATO, Minn.,
BMC Software Announces Fiscal 2008 First Quarter Results
 Corporate Communications: Investor Relations: Mark Stouse Derrick Vializ 713-918-2714 713-918-1805 mark_stouse@bmc.com derrick_vializ@bmc.com BMC Software Announces Fiscal 2008 First Quarter Results GAAP
Corporate Communications: Investor Relations: Mark Stouse Derrick Vializ 713-918-2714 713-918-1805 mark_stouse@bmc.com derrick_vializ@bmc.com BMC Software Announces Fiscal 2008 First Quarter Results GAAP
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications
 FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
FDA Update on the H1N1 Flu Vaccine and Antiviral Medications Beth Fabian Fritsch, R.Ph., M.B.A. Commander, U.S. Public Health Service Health Programs Coordinator Office of Special Health Issues Food and
2-8. Identify whether each of the following items increases or decreases cash flow:
 Problems 2-8. Identify whether each of the following items increases or decreases cash flow: Increase in accounts receivable Increase in notes payable Depreciation expense Increase in investments Decrease
Problems 2-8. Identify whether each of the following items increases or decreases cash flow: Increase in accounts receivable Increase in notes payable Depreciation expense Increase in investments Decrease
Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets
 Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets ASSETS March 31, December 2014 31, 2013 ------- --------- Current Assets Cash and cash equivalents $ 39 $ 39 --------
Cynk Technology Corp. (A Development Stage Company) (formerly Introbuzz) Balance Sheets ASSETS March 31, December 2014 31, 2013 ------- --------- Current Assets Cash and cash equivalents $ 39 $ 39 --------
CARDIOME PHARMA CORP.
 Consolidated Financial Statements (Expressed in thousands of United States (U.S.) dollars) (Prepared in accordance with generally accepted accounting principles used in the United States of America (U.S.
Consolidated Financial Statements (Expressed in thousands of United States (U.S.) dollars) (Prepared in accordance with generally accepted accounting principles used in the United States of America (U.S.
Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results
 FOR IMMEDIATE RELEASE Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results Annual revenues up 15.6% to a record $214.8 million Annual net income increases 30.4% to a record $33.3
FOR IMMEDIATE RELEASE Diodes Incorporated Reports Record Fourth Quarter and Full Year 2005 Results Annual revenues up 15.6% to a record $214.8 million Annual net income increases 30.4% to a record $33.3
APPLE INC FORM 8-K. (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15
 APPLE INC FORM 8-K (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15 Address ONE INFINITE LOOP CUPERTINO, CA 95014 Telephone (408) 996-1010 CIK 0000320193 Symbol AAPL SIC Code 3571
APPLE INC FORM 8-K (Current report filing) Filed 10/27/15 for the Period Ending 10/27/15 Address ONE INFINITE LOOP CUPERTINO, CA 95014 Telephone (408) 996-1010 CIK 0000320193 Symbol AAPL SIC Code 3571
WILLIAMS-SONOMA, INC.
 WILLIAMS-SONOMA, INC. 3250 Van Ness Avenue San Francisco, CA 94109 CONT: Julie P. Whalen EVP, Chief Financial Officer (415) 616-8524 Gabrielle L. Rabinovitch Vice President, Investor Relations (415) 616-7727
WILLIAMS-SONOMA, INC. 3250 Van Ness Avenue San Francisco, CA 94109 CONT: Julie P. Whalen EVP, Chief Financial Officer (415) 616-8524 Gabrielle L. Rabinovitch Vice President, Investor Relations (415) 616-7727
ACXIOM ANNOUNCES FIRST QUARTER RESULTS. Audience Operating System to Launch September 24 at AdWeek
 For more information, contact: Katharine Boyce Acxiom Investor Relations 501-342-1321 investor.relations@acxiom.com EACXM ACXIOM ANNOUNCES FIRST QUARTER RESULTS Audience Operating System to Launch September
For more information, contact: Katharine Boyce Acxiom Investor Relations 501-342-1321 investor.relations@acxiom.com EACXM ACXIOM ANNOUNCES FIRST QUARTER RESULTS Audience Operating System to Launch September
NETFLIX REPORTS PRO-FORMA NET INCOME ON RECORD 4 th QUARTER 2002 REVENUE
 FOR RELEASE AT 1:02 PM PDT IR CONTACT: Barry McCarthy Wednesday, January 15, 2003 CFO 408 399-3740 PR CONTACT: Lynn Brinton Director of Corporate Communications 408 399-3726 NETFLIX REPORTS PRO-FORMA NET
FOR RELEASE AT 1:02 PM PDT IR CONTACT: Barry McCarthy Wednesday, January 15, 2003 CFO 408 399-3740 PR CONTACT: Lynn Brinton Director of Corporate Communications 408 399-3726 NETFLIX REPORTS PRO-FORMA NET
N E W S R E L E A S E
 N E W S R E L E A S E Investors: Brett Manderfeld John S. Penshorn G. Mike Mikan Vice President Senior Vice President Chief Financial Officer 952-936-7216 952-936-7214 952-936-7374 Media: Don Nathan Senior
N E W S R E L E A S E Investors: Brett Manderfeld John S. Penshorn G. Mike Mikan Vice President Senior Vice President Chief Financial Officer 952-936-7216 952-936-7214 952-936-7374 Media: Don Nathan Senior
ico Therapeutics Inc. Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 (in Canadian dollars)
 Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event
HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth
 HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth CAMBRIDGE, MA (February 11, 2015) HubSpot, Inc. (NYSE: HUBS), a leading inbound marketing and sales software company,
HubSpot's Momentum Accelerates in Q4 2014 with 53% Revenue Growth and 35% Customer Growth CAMBRIDGE, MA (February 11, 2015) HubSpot, Inc. (NYSE: HUBS), a leading inbound marketing and sales software company,
Intel Reports Second-Quarter Results
 Intel Corporation 2200 Mission College Blvd. Santa Clara, CA 95054-1549 CONTACTS: Mark Henninger Amy Kircos Investor Relations Media Relations 408-653-9944 480-552-8803 mark.h.henninger@intel.com amy.kircos@intel.com
Intel Corporation 2200 Mission College Blvd. Santa Clara, CA 95054-1549 CONTACTS: Mark Henninger Amy Kircos Investor Relations Media Relations 408-653-9944 480-552-8803 mark.h.henninger@intel.com amy.kircos@intel.com
LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results
 For Immediate Release LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results Continued Momentum for Global Sales of Specialty and Infrared Products ORLANDO,
For Immediate Release LightPath Technologies Reports 61% Revenue Increase with Fiscal 2016 First Quarter Financial Results Continued Momentum for Global Sales of Specialty and Infrared Products ORLANDO,
U. S. Securities and Exchange Commission Washington, D. C. 20549 FORM 10-QSB
 U. S. Securities and Exchange Commission Washington, D. C. 20549 FORM 10-QSB [X] QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended September
U. S. Securities and Exchange Commission Washington, D. C. 20549 FORM 10-QSB [X] QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended September
James L. Dunn, Jr. Senior Vice President and Chief Financial Officer (602) 952-1200
 Contact: James M. Powers, Jr. President and Chief Executive Officer (602) 952-1200 James L. Dunn, Jr. Senior Vice President and Chief Financial Officer (602) 952-1200 ilinc COMMUNICATIONS ANNOUNCES FISCAL
Contact: James M. Powers, Jr. President and Chief Executive Officer (602) 952-1200 James L. Dunn, Jr. Senior Vice President and Chief Financial Officer (602) 952-1200 ilinc COMMUNICATIONS ANNOUNCES FISCAL
Numerex Reports First Quarter 2015 Financial Results
 May 11, 2015 Numerex Reports First Quarter 2015 Financial Results ATLANTA, May 11, 2015 (GLOBE NEWSWIRE) -- Numerex Corp (Nasdaq:NMRX), a leading provider of on-demand and interactive machine-to-machine
May 11, 2015 Numerex Reports First Quarter 2015 Financial Results ATLANTA, May 11, 2015 (GLOBE NEWSWIRE) -- Numerex Corp (Nasdaq:NMRX), a leading provider of on-demand and interactive machine-to-machine
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
About TurboChef. Forward-Looking Statements
 TurboChef Reports Results for Second Quarter 2006 Cites Strong Momentum in Commercial Oven Business, Sees Increasing Revenues in Q3 and Q4; Significant Progress Cited Regarding Residential Oven Atlanta,
TurboChef Reports Results for Second Quarter 2006 Cites Strong Momentum in Commercial Oven Business, Sees Increasing Revenues in Q3 and Q4; Significant Progress Cited Regarding Residential Oven Atlanta,
Tower International Reports Solid Third Quarter And Raises Full Year Outlook
 FOR IMMEDIATE RELEASE Tower International Reports Solid Third Quarter And Raises Full Year Outlook LIVONIA, Mich., November 3, 2011 Tower International, Inc. [NYSE: TOWR], a leading integrated global manufacturer
FOR IMMEDIATE RELEASE Tower International Reports Solid Third Quarter And Raises Full Year Outlook LIVONIA, Mich., November 3, 2011 Tower International, Inc. [NYSE: TOWR], a leading integrated global manufacturer
MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS
 Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Government of the Republic of Trinidad and Tobago MINISTRY OF HEALTH PANDEMIC INFLUENZA A / H1N1 2009 VACCINE FREQUENTLY ASKED QUESTIONS Influenza vaccines are one of the most effective ways to protect
Half Year 2015 Results
 Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
Half Year 2015 Results Letter to shareholders LifeWatch First Half Highlights Revenue growth of 9.1% to USD 52.5 million Above-market growth of over 12% in core monitoring services resulting in market
Performance Food Group Company Reports First-Quarter Fiscal 2016 Earnings
 NEWS RELEASE For Immediate Release November 4, 2015 Investors: Michael D. Neese VP, Investor Relations (804) 287-8126 michael.neese@pfgc.com Media: Joe Vagi Manager, Corporate Communications (804) 484-7737
NEWS RELEASE For Immediate Release November 4, 2015 Investors: Michael D. Neese VP, Investor Relations (804) 287-8126 michael.neese@pfgc.com Media: Joe Vagi Manager, Corporate Communications (804) 484-7737
Heat Biologics, Inc. (Exact name of registrant as specified in charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (date of earliest event
Scancell Holdings plc ( Scancell Holdings or the Company )
 Release Date: 29 January 2010 GB00B39J5N63 Scancell Holdings plc ( Scancell Holdings or the Company ) Interim Results for the six month period to 31 Scancell (SCLP.PL), the developer of therapeutic cancer
Release Date: 29 January 2010 GB00B39J5N63 Scancell Holdings plc ( Scancell Holdings or the Company ) Interim Results for the six month period to 31 Scancell (SCLP.PL), the developer of therapeutic cancer
Great Basin Reports 2015 Second Quarter Results and Business Update
 Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Great Basin Reports 2015 Second Quarter Results and Business Update Company Reports 122 Revenue-Generating Customers, Reaffirms Guidance of 170-180 Customers by Year End SALT LAKE CITY, August 12, 2015
Flamel Technologies Announces Second Quarter Results of Fiscal Year 2015
 Flamel Technologies Announces Second Quarter Results of Fiscal Year 2015 Product revenue guidance for 2015 of $170-$185 million reaffirmed Conference call with management to take place at 10:00 am ET on
Flamel Technologies Announces Second Quarter Results of Fiscal Year 2015 Product revenue guidance for 2015 of $170-$185 million reaffirmed Conference call with management to take place at 10:00 am ET on
Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings
 Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings 1.0 PURPOSE The purpose of this policy is to minimize transmission of the influenza virus in the workplace by
Sample: Policy for the Administration of Influenza Vaccine Employees in Health Care Settings 1.0 PURPOSE The purpose of this policy is to minimize transmission of the influenza virus in the workplace by
Kansas City 1Life Insurance Company
 Kansas City 1Life Insurance Company 2011 First Quarter Report Includes our subsidiaries: Sunset Life Insurance Company of America Old American Insurance Company Sunset Financial Services, Inc. Post Office
Kansas City 1Life Insurance Company 2011 First Quarter Report Includes our subsidiaries: Sunset Life Insurance Company of America Old American Insurance Company Sunset Financial Services, Inc. Post Office
LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS
 FOR IMMEDIATE RELEASE January 28, 2016 SYMBOL: LANC TRADED: Nasdaq LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS COLUMBUS, Ohio, January 28 - Lancaster Colony Corporation (Nasdaq: LANC) today
FOR IMMEDIATE RELEASE January 28, 2016 SYMBOL: LANC TRADED: Nasdaq LANCASTER COLONY REPORTS SECOND QUARTER SALES AND EARNINGS COLUMBUS, Ohio, January 28 - Lancaster Colony Corporation (Nasdaq: LANC) today
Recombinant protein vaccines produced in insect cells
 Recombinant protein vaccines produced in insect cells Manon M.J. Cox Hong Kong 25JAN2013 Flublok BREAKING NEWS 16JAN2013 FDA approves Flublok for the prevention of influenza in adults 18-49 years old The
Recombinant protein vaccines produced in insect cells Manon M.J. Cox Hong Kong 25JAN2013 Flublok BREAKING NEWS 16JAN2013 FDA approves Flublok for the prevention of influenza in adults 18-49 years old The
SanDisk Corporation Preliminary Condensed Consolidated Statements of Operations (in thousands, except per share amounts, unaudited)
 Preliminary Condensed Consolidated Statements of Operations (in thousands, except per share amounts, unaudited) Revenue $ 1,634,011 $ 1,476,263 $ 3,145,956 $ 2,816,992 Cost of revenue 854,640 789,614 1,595,679
Preliminary Condensed Consolidated Statements of Operations (in thousands, except per share amounts, unaudited) Revenue $ 1,634,011 $ 1,476,263 $ 3,145,956 $ 2,816,992 Cost of revenue 854,640 789,614 1,595,679
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q (Mark One) QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q (Mark One) QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period
Pursuit Management, Inc. dba: MobileSpike Technologies, Inc. Financial Statements and Independent Accountants Review Report December 31, 2015 and
 Financial Statements and Independent Accountants Review Report December 31, 2015 and 2014 Financial Statements Contents INDEPENDENT ACCOUNTANTS REVIEW REPORT 1 Page FINANCIAL STATEMENTS: Balance sheets
Financial Statements and Independent Accountants Review Report December 31, 2015 and 2014 Financial Statements Contents INDEPENDENT ACCOUNTANTS REVIEW REPORT 1 Page FINANCIAL STATEMENTS: Balance sheets
SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH QUARTER AND FISCAL YEAR 2007
 PRESS RELEASE INVESTOR CONTACT: Lisa L. Ewbank Synopsys, Inc. 650-584-1901 EDITORIAL CONTACT: Yvette Huygen Synopsys, Inc. 650-584-4547 yvetteh@synopsys.com SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH
PRESS RELEASE INVESTOR CONTACT: Lisa L. Ewbank Synopsys, Inc. 650-584-1901 EDITORIAL CONTACT: Yvette Huygen Synopsys, Inc. 650-584-4547 yvetteh@synopsys.com SYNOPSYS POSTS FINANCIAL RESULTS FOR FOURTH
THIRD QUARTER 2015 RECORD RESULTS REPORTED BY AMPHENOL CORPORATION
 Amphenol News Release World Headquarters 358 Hall Avenue P. O. Box 5030 Wallingford, CT 06492-7530 Telephone (203) 265-8900 FOR IMMEDIATE RELEASE For Further Information: Craig A. Lampo Senior Vice President
Amphenol News Release World Headquarters 358 Hall Avenue P. O. Box 5030 Wallingford, CT 06492-7530 Telephone (203) 265-8900 FOR IMMEDIATE RELEASE For Further Information: Craig A. Lampo Senior Vice President
Valneva Reports Preliminary FY 2013 Revenues of EUR 36.0m and Cash Position of EUR 40.2m at End of 2013
 Valneva Reports Preliminary FY 2013 Revenues of EUR 36.0m and Cash Position of EUR 40.2m at End of 2013 Revenue Ahead of Guidance and Cash Target Met in First year Following Merger Total revenues and grants
Valneva Reports Preliminary FY 2013 Revenues of EUR 36.0m and Cash Position of EUR 40.2m at End of 2013 Revenue Ahead of Guidance and Cash Target Met in First year Following Merger Total revenues and grants
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K. GEOVAX LABS, INC. (Exact name of registrant as specified in its charter)
 SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event reported):
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event reported):
Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc.
 Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc. Horsham, PA December 23, 2014 Capmark Financial Group Inc.
Capmark Financial Group Inc. Announces Stand Alone Third Quarter 2014 Earnings Results for its Wholly Owned Subsidiary, Bluestem Brands, Inc. Horsham, PA December 23, 2014 Capmark Financial Group Inc.
SuccessFactors Announces Record First Quarter Fiscal 2009 Results
 CONTACTS: Dominic Paschel SuccessFactors, Inc. Public & Investor Relations 415-262-4641 dpaschel@successfactors.com SuccessFactors Announces Record First Quarter Fiscal 2009 Results Revenues Grow 50%,
CONTACTS: Dominic Paschel SuccessFactors, Inc. Public & Investor Relations 415-262-4641 dpaschel@successfactors.com SuccessFactors Announces Record First Quarter Fiscal 2009 Results Revenues Grow 50%,
Understanding the Rules for Creating CVX and MVX Codes
 Understanding the Rules for Creating CVX and MVX Codes Objective: The objective of this document is to explain the HL7 s needed to identify the vaccine used in an immunization. The document will: Provide
Understanding the Rules for Creating CVX and MVX Codes Objective: The objective of this document is to explain the HL7 s needed to identify the vaccine used in an immunization. The document will: Provide
News Release INVESTOR AND MEDIA CONTACT: George R. Kirkland Senior Vice President and Treasurer Phone: (229) 873-3830 gkirkland@sgfc.
 Southwest Georgia Financial Corporation 201 First Street S.E. Moultrie, GA 31768 PH: (229) 985-1120 FX: (229) 985-0251 News Release INVESTOR AND MEDIA CONTACT: George R. Kirkland Senior Vice President
Southwest Georgia Financial Corporation 201 First Street S.E. Moultrie, GA 31768 PH: (229) 985-1120 FX: (229) 985-0251 News Release INVESTOR AND MEDIA CONTACT: George R. Kirkland Senior Vice President
FLEET MANAGEMENT SOLUTIONS INC.
 FLEET MANAGEMENT SOLUTIONS INC. (Formerly: Silverton Mining Corp.) CONSOLIDATED FINANCIAL STATEMENTS (unaudited prepared by management) March 31, 2013 (Expressed in US Dollars) 1 FLEET MANAGEMENT SOLUTIONS
FLEET MANAGEMENT SOLUTIONS INC. (Formerly: Silverton Mining Corp.) CONSOLIDATED FINANCIAL STATEMENTS (unaudited prepared by management) March 31, 2013 (Expressed in US Dollars) 1 FLEET MANAGEMENT SOLUTIONS
Intuit Reports Third-Quarter Results; Total Revenue Increases 13 Percent
 May 21, 2013 Intuit Reports Third-Quarter Results; Total Revenue Increases 13 Percent Small Business Group Grows Revenue 17 Percent MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)-- Intuit Inc. (Nasdaq: INTU) today
May 21, 2013 Intuit Reports Third-Quarter Results; Total Revenue Increases 13 Percent Small Business Group Grows Revenue 17 Percent MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)-- Intuit Inc. (Nasdaq: INTU) today
FORM 8-K. ELECTROMED, INC. (Exact Name of Registrant as Specified in Its Charter)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of report (Date of earliest event
Thomas A. Bessant, Jr. (817) 335-1100
 Additional Information: Thomas A. Bessant, Jr. (817) 335-1100 For Immediate Release ********************************************************************************** CASH AMERICA FIRST QUARTER NET INCOME
Additional Information: Thomas A. Bessant, Jr. (817) 335-1100 For Immediate Release ********************************************************************************** CASH AMERICA FIRST QUARTER NET INCOME
AAA PUBLIC ADJUSTING GROUP, INC. (EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q [ X] QUARTERLY REPORT PURSUANT TO SECTION 13 OF 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q [ X] QUARTERLY REPORT PURSUANT TO SECTION 13 OF 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended
ACER INCORPORATED AND SUBSIDIARIES. Consolidated Balance Sheets
 Consolidated Balance Sheets June 30, 2015, December 31, 2014, and (June 30, 2015 and 2014 are reviewed, not audited) Assets 2015.6.30 2014.12.31 2014.6.30 Current assets: Cash and cash equivalents $ 36,400,657
Consolidated Balance Sheets June 30, 2015, December 31, 2014, and (June 30, 2015 and 2014 are reviewed, not audited) Assets 2015.6.30 2014.12.31 2014.6.30 Current assets: Cash and cash equivalents $ 36,400,657
Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance
 News Release Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance Englewood, CO May 10, 2016 - Westmoreland Coal Company (NasdaqGM:WLB) today reported financial results for the
News Release Westmoreland Reports First Quarter 2016 Results and Affirms Full-year Guidance Englewood, CO May 10, 2016 - Westmoreland Coal Company (NasdaqGM:WLB) today reported financial results for the
Diluted EPS of. $0.94 and. return on. were lower. modestly
 PRIMERICA REPORTS SECOND QUARTER 2015 RESULTS 14% growth in life insurance policies issued 9% growth in Investment and Savings Products saless 5% increase in life insurance licensed representatives to
PRIMERICA REPORTS SECOND QUARTER 2015 RESULTS 14% growth in life insurance policies issued 9% growth in Investment and Savings Products saless 5% increase in life insurance licensed representatives to
NOVAVAX INC FORM 424B5. (Prospectus filed pursuant to Rule 424(b)(5)) Filed 03/26/15
 NOVAVAX INC FORM 424B5 (Prospectus filed pursuant to Rule 424(b)(5)) Filed 03/26/15 Address 20 FIRSTFIELD ROAD GAITHERSBURG, MD 20878 Telephone 240-268-2000 CIK 0001000694 Symbol NVAX SIC Code 2836 - Biological
NOVAVAX INC FORM 424B5 (Prospectus filed pursuant to Rule 424(b)(5)) Filed 03/26/15 Address 20 FIRSTFIELD ROAD GAITHERSBURG, MD 20878 Telephone 240-268-2000 CIK 0001000694 Symbol NVAX SIC Code 2836 - Biological
TripAdvisor Reports Fourth Quarter and Full Year 2013 Financial Results
 TripAdvisor Reports Fourth Quarter and Full Year 2013 Financial Results NEWTON, MA, February 11, 2014 -- TripAdvisor, Inc. (NASDAQ: TRIP), the world s largest travel website*, today announced financial
TripAdvisor Reports Fourth Quarter and Full Year 2013 Financial Results NEWTON, MA, February 11, 2014 -- TripAdvisor, Inc. (NASDAQ: TRIP), the world s largest travel website*, today announced financial
NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS
 Condensed Interim Consolidated Financial Statements of THE BRICK LTD. For the three months ended March 31, 2013 NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
Condensed Interim Consolidated Financial Statements of THE BRICK LTD. For the three months ended March 31, 2013 NOTICE OF NO AUDITOR REVIEW OF INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
FOR IMMEDIATE RELEASE
 FOR IMMEDIATE RELEASE March 19, 2014 NEWS NYSE MKT: GORO GOLD RESOURCE CORPORATION REPORTS $16.2M NET INCOME AND RECORD ANNUAL METAL PRODUCTION FOR 2014; PROVIDES 2015 PRODUCTION OUTLOOK COLORADO SPRINGS
FOR IMMEDIATE RELEASE March 19, 2014 NEWS NYSE MKT: GORO GOLD RESOURCE CORPORATION REPORTS $16.2M NET INCOME AND RECORD ANNUAL METAL PRODUCTION FOR 2014; PROVIDES 2015 PRODUCTION OUTLOOK COLORADO SPRINGS
American Electric Technologies Inc
 SECURITIES & EXCHANGE COMMISSION EDGAR FILING American Electric Technologies Inc Form: 8-K Date Filed: 2015-11-16 Corporate Issuer CIK: 1043186 Copyright 2015, Issuer Direct Corporation. All Right Reserved.
SECURITIES & EXCHANGE COMMISSION EDGAR FILING American Electric Technologies Inc Form: 8-K Date Filed: 2015-11-16 Corporate Issuer CIK: 1043186 Copyright 2015, Issuer Direct Corporation. All Right Reserved.
Key Facts about Influenza (Flu) & Flu Vaccine
 Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
Key Facts about Influenza (Flu) & Flu Vaccine mouths or noses of people who are nearby. Less often, a person might also get flu by touching a surface or object that has flu virus on it and then touching
From Research Services and Process Development to GMP Manufacturing
 From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
From Research Services and Process Development to GMP Manufacturing P a r ag o n B i o s e r v i c e s, I n c. A contract research and GMP manufacturing organization (CMO) with a focus on the development
SUPPLEMENTAL INVESTOR INFORMATION. Fourth Quarter 2012
 SUPPLEMENTAL INVESTOR INFORMATION Fourth Quarter 2012 Kevin Bryant Tony Carreño VP Investor Relations and Strategic Director Investor Relations Planning & Treasurer 816-556-2782 816-654-1763 anthony.carreno@kcpl.com
SUPPLEMENTAL INVESTOR INFORMATION Fourth Quarter 2012 Kevin Bryant Tony Carreño VP Investor Relations and Strategic Director Investor Relations Planning & Treasurer 816-556-2782 816-654-1763 anthony.carreno@kcpl.com
WuXi PharmaTech Announces Second-Quarter 2014 Results
 WuXi PharmaTech Announces Second-Quarter 2014 Results SHANGHAI, August 13, 2014 /Xinhua-PRNewswire/ -- WuXi PharmaTech (Cayman) Inc. (NYSE: WX), a leading research and development services company serving
WuXi PharmaTech Announces Second-Quarter 2014 Results SHANGHAI, August 13, 2014 /Xinhua-PRNewswire/ -- WuXi PharmaTech (Cayman) Inc. (NYSE: WX), a leading research and development services company serving
WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/
 WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/ FOR IMMEDIATE RELEASE Investor Relations Contacts Investor Relations 479-273-8446 Carol Schumacher 479-277-1498 Pauline Tureman 479-277-9558
WAL-MART STORES, INC. 800-331-0085 www.walmartstores.com/news/ FOR IMMEDIATE RELEASE Investor Relations Contacts Investor Relations 479-273-8446 Carol Schumacher 479-277-1498 Pauline Tureman 479-277-9558
Walmart reports Q3 FY 16 EPS of $1.03, Walmart U.S. added $2.7 billion in sales, comp sales of 1.5%
 Walmart reports Q3 FY 6 EPS of.03, Walmart U.S. added 2.7 billion in sales, comp sales of.5% Q3 diluted EPS from continuing operations was.03, benefited by approximately 0.04 from an adjustment for certain
Walmart reports Q3 FY 6 EPS of.03, Walmart U.S. added 2.7 billion in sales, comp sales of.5% Q3 diluted EPS from continuing operations was.03, benefited by approximately 0.04 from an adjustment for certain
Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011
 Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011 1 MEXICAN RESTAURANTS, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (unaudited) 7/3/2011 1/2/2011 ASSETS Current assets:
Unaudited Interim Consolidated Financial Statements and Footnotes July 3, 2011 1 MEXICAN RESTAURANTS, INC. AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (unaudited) 7/3/2011 1/2/2011 ASSETS Current assets:
