Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial
|
|
|
- Vincent Garrett
- 8 years ago
- Views:
Transcription
1 Br. J. Cancer (1989), 59, (B8 The Macmillan Press Ltd., 1989 Duration of chemotherapy in small cell lung cancer: a Cancer Research Campaign trial S.G. Spiro1, R.L. Souhami2, D.M. Geddes3, C.M. Ash2, H. Quinn2, P. G. Harper4, J.S. Tobias2, M. Partridge5, & D. Eraut6 1Brompton Hospital, London SW3; 2Department of Radiotherapy and Oncology, University College Hospital, London WCIE; 3London Chest Hospital, London E2; 4Department of Oncology, Guy's Hospital, London SE]; 5Department of Thoracic Medicine, Whipps Cross Hospital, London El]; and 6Department of Thoracic Medicine, Southend Hospital, Essex SS ORY, U.K. Summary A total of 61 patients with small cell lung cancer were entered into a randomised trial designed to assess the effect of duration of initial chemotherapy on survival. Patients were randomised to receive either four or eight courses of cytotoxic chemotherapy with cyclophosphamide, vincristine and etoposide and also randomised to receive, on disease progression, either second line chemotherapy (methotrexate and doxorubicin) or symptomatic treatment only. In the whole study 196 (32.1%) had limited disease and 414 (67.9%) extensive disease. During initial chemotherapy the response rate (complete and partial responses) after four courses of treatment was 61% with no significant increase in patients receiving eight courses (63%). In those randomised to receive relapse chemotherapy the response rate was improved slightly for those who had originally received four courses of chemotherapy (25.6%) over those receiving eight (18.7%). The overall results show that of the four possible treatment randomisations, four courses of chemotherapy alone is inferior in terms of overall survival (3 weeks median survival) to the other three treatment options (39 weeks median survival, P<.1). In patients responding to initial chemotherapy the disadvantage of four courses of chemotherapy alone was apparent (median survival of 4 weeks versus 49 weeks, P=.3) but not if drug treatment was given on relapse. The study shows that limiting treatment to four courses of chemotherapy alone is associated with inferior survival, but this is not the case if chemotherapy is given at relapse. Although combination chemotherapy has improved median survival in small cell lung cancer (SCLC) it is increasingly clear that long-term survival is confined to those with both limited disease and good performance status (PS) who constitute approximately 2% of all cases (Osterlind & Andersen, 1986; Souhami et al., 1985). Intensive or prolonged therapy would be justifiable for these good prognosis patients if it improved survival, but most patients present with extensive disease, poor performance status or advanced age. For these patients, chemotherapy is palliative. The optimal duration of chemotherapy has not been established with certainty. Cullen et al. (1986) administered six courses of chemotherapy and the patients with no unequivocal residual disease were randomised to either symptomatic treatment or a further eight courses of maintenance chemotherapy. A survival advantage was shown for those with extensive disease receiving maintenance therapy. An EORTC study (Splinter et al., 1986) with a similar design showed no advantage for 12 courses of monthly maintenance therapy. Stopping chemotherapy early may improve the quality of life of the patients by minimising toxicity, but in responding patients may diminish survival unless further chemotherapy is effective on relapse. Although response to chemotherapy at relapse is usually clinically disappointing, Evans et al. (1985) recorded a 55% response rate with cisplatin and etoposide after previous treatment with cyclophosphamide, adriamycin and vincristine. We report here a large scale randomised trial designed to assess the effects of duration of chemotherapy on survival in patients with SCLC. The trial evaluates the effect of either 3 or 6 months' chemotherapy and then, at relapse, the effects of further chemotherapy compared with symptomatic treatment alone. Patients and methods During the period February 1982 to September 1985, 616 patients were entered into the study from the participating Correspondence: R.L. Souhami. Received 25 June 1988, and in revised form, 4 October hospitals. All patients had SCLC diagnosed by histology (from bronchial biopsy, lymph node biopsy or biopsy of a metastasis), by cytology from bronchial brushings at bronchoscopy or from three specimens of sputum. Patients were required to be below 75 years, with no vascular, renal or neurological disease which would preclude chemotherapy and no previous malignancy during the preceding 5 years. No exclusion was made on the basis of performance status. Patients were not entered if they had received prior chemotherapy. Eight patients had had a previous lobectomy or pneumonectomy for SCLC but had developed further disease, and 17 had received emergency radiotherapy to the chest, spine, brain or to painful bone deposits. Patients were staged by chest radiography, full blood count, urea, electrolytes, liver function tests, proteins and calcium estimations. Bone scans were routinely performed but liver isotope or ultrasound scans ordered only if clinically indicated by abnormal liver function tests or hepatomegaly. CT or isotope brain scans were not performed in the absence of clinical suspicion of CNS metastasis. Bone marrow aspiration was performed only when indicated by abnormal blood counts. Limited disease was defined as disease confined to one hemithorax or ipsilateral supraclavicular nodes. Extensive disease was more widespread intrathoracic disease, pleural effusions or metastases. Informed consent was obtained according to the practices laid down by the individual ethical committee of the participating hospitals. At diagnosis there was a double randomisation of treatment. The randomisations were stratified by stage of disease (limited or extensive). The first randomisation was to receive either four or eight courses of initial cytotoxic chemotherapy (short course or long course respectively). The treatment regimen was cyclophosphamide I gm-2 i.v.; vincristine 1.4 mgm -2 i.v. (maximum dose 2 mg) both on day 1 and etoposide capsules 1mg orally eight hourly on days 1-3 (total dose 9mg). Each treatment was given every 21 days provided that the total white cell count on the day of treatment was equal to or greater than 3,5 mm3 and the platelet count equal to, or greater than 1, mm3. If not, treatment dosage was reduced according to the following schedule. If the total white cell count was 3,499-3,, 75% of cyclophosphamide and 7mg etoposide was given, and if
2 DURATION OF CHEMOTHERAPY 579 less than 3,, the treatment was omitted and the blood count repeated a week later. If the platelet count was 75,-99,999, the etoposide dose was reduced to 5%. Any further decrease in platelet count caused the treatment to be delayed with the blood count repeated a week later. These dose reductions were carried over to subsequent chemotherapy cycles. Patients who achieved a complete or partial response to chemotherapy after four cycles were offered prophylactic cranial irradiation (PCI) 2Gy in five fractions over seven days. In the short arm 77% of eligible patients received the assigned PCI and 72% in the long arm. The second randomisation was to receive, on disease progression, either second-line chemotherapy or symptomatic treatment. Relapse chemotherapy consisted of methotrexate 5 mgm 2 i.v. and doxorubicin 5 mgm-2 i.v. Folinic acid 15 mg p.o. 6 hourly for four doses was given 24 h after chemotherapy to prevent methotrexate toxicity. This schedule was repeated every three weeks up to a maximum of nine courses provided there was no evidence of disease progression. Before each course of chemotherapy the total white cell count had to be equal to, or greater than 3,5 and the platelets equal to or greater than 1,, or treatment was postponed for one week or until the counts were satisfactory. Patients randomised to receive symptomatic treatment on relapse were followed as outpatients every three weeks with clinical examination, chest X-ray and blood investigations in the same way as those on the active relapse treatment arm. Treatment consisted of palliative irradiation at a dose chosen by each treatment centre for indications including bone pain, cerebral metastases, haemoptysis, dyspnoea, superior vena caval obstruction or large airway obstruction. Analgesics and corticosteroids were administered as necessary. No cytotoxic chemotherapy was given to patients in the symptomatic arm of the study following initial relapse. Response criteria All patients had radiologically visible disease. Response was assessed clinically, radiologically and biochemically before each chemotherapy cycle. After four and eight cycles, restaging with bone and liver scans, but not with bronchoscopy, was carried out if clinically indicated. A complete response was defined as complete radiological clearing of the chest X- ray abnormality seen at diagnosis and all symptoms and signs and biochemical abnormalities indicating metastatic disease should have resolved completely. Fibreoptic bronchoscopy was not routinely used to assess response. Bone scans which were abnormal at presentation were not systematically repeated since they are an unreliable indicator of short-term response. A partial response was a 5% or greater reduction in tumour area as measured by two straight lines drawn across the tumour at right angles to each other. Both complete and partial responses had to be maintained for at least three weeks. Stable disease was any response less than 5% and progressive disease (or relapse) was recorded if the tumour mass enlarged or reappeared three weeks after the last course of chemotherapy, or during the follow-up period, or if a new metastasis appeared. CNS relapse was confirmed by CT brain scan and liver relapse by isotope or ultrasound scans with deteriorating liver function tests. If biochemical deterioration in liver function tests was detected as an isolated feature, this was only judged to be due to metastatic disease if the abnormality was sustained or increased at next planned visit. The development of changes in urea and electrolytes possibly attributable to inappropriate secretion of ADH was not interpreted as relapse if it occurred in isolation. The development of bone pain was interpreted as due to metastatic disease if associated either with appropriate X-ray changes, or a positive bone scan; or an elevated alkaline phosphatase on two consecutive visits with no concomitant rise in the other liver enzymes. Relapse in lymph nodes or skin lesions was confirmed by biopsy or cytology only if there was doubt as to their nature. the After completion of short or long course chemotherapy the patient attended every three weeks when a chest X-ray was taken and full blood count, urea, electrolytes, liver function tests and protein estimations and performance status were measured. If there was evidence that relapse had occurred, either during initial chemotherapy or on 3-weekly follow-up, the patient was treated according to the second randomisation. The criteria for response and progression with the relapse chemotherapy regimen were the same as for initial chemotherapy. The maximal response at any stage in treatment was recorded. At second relapse, chemotherapy was discontinued and the patient seen every three weeks as an outpatient and treated palliatively without further cytotoxic drugs. Statistical methods On entry to the study patients were randomised to short or long chemotherapy and to the relapse treatment. Stratification was for disease extent only. While it is desirable, in trials with a double randomisation, to perform the second randomisation at the point where the treatment policy is changed, this creates problems in a multicentre trial in small cell lung cancer, especially for patients with extensive disease. In the first three months of this study the second randomisation was delayed in this way, but 26 patients were not randomised on relapse because of clinicians' reluctance to offer further chemotherapy due to the poor quality of health of the patient or because of refusal by the patient to accept second-line chemotherapy. This would have created a bias in selection of better performance status patients for the second randomisation which would be likely to operate unequally in the short and long initial treatment arms (patients relapsing after prolonged treatment being more likely to refuse further treatment). Since the aim of the trial is to assess different treatment policies, for the rest of the trial both randomisations were made at entry and analyses are presented according to treatment intention. All eligible patients are analysed according to initial chemotherapy but 26 patients are excluded from the sub-analysis of the effects of symptomatic or second-line chemotherapy. The number of patients required was determined before the trial began and was based on the ability to detect a 1% difference in overall survival from 2 to 3% at one year. This required 551 patients to be randomised using.5 and.9 as the type I and II errors respectively (Freedman, 1982). The estimation was based on survival data in previous studies with a 25% survival at one year (Souhami et al., 1984). It was assumed that the survival may be less with fewer courses of initial chemotherapy. Survival curves were constructed according to the method of Kaplan & Meier (1958) and statistical significance evaluated by the log-rank test (Peto et al., 1977). To ensure that where no difference was seen between two curves the result was likely to be a true negative the power of the test was computed according to Hughes (1981). The probability that the result is not a false negative is given, in addition to the usual P value. Results Of the 616 patients entering the study between February 1982 and September 1985, six were subsequently excluded. These exclusions were due to wrong diagnosis (four) and previous malignancy within the preceding 5 years (two). Thus 61 patients were available for analysis. The characteristics of the 61 patients allocated at entry to each treatment are shown in Table I. In the whole study the median age was 62 years; 68.4% were male and 31.6% female; 196 (32.1%) had limited disease and 414 (67.9%) extensive disease. The response rate (complete and partial) to the initial chemotherapy is shown in Table II. These response rates are given after four courses of chemotherapy in both groups and after a further four cycles in the long course group. After four courses of chemotherapy the response rates in the short
3 58 S.G. SPIRO et al. Table I Patients characteristics at presentation according to assigned treatment According to 2nd According to 2nd randomisation randomisation At presentation (total) Chemo. Sympt. At presentation Chemo. Sympt. n a 145a 35 l oa 145a Lim/ext (%) 31/69 34/66 32/68 33/67 35/65 32/68 PS and 1 71% 75% 69% 77% 78% 77% % 25% 31% 23% 22% 23% Age (years) Median range (31-74) (34-74) (31-74) (34-74) (34-74) (34-74) M/F(%) 67/33 65/35 69/31 7/3 73/27 68/32 athe total of patients who received a second randomisation is 584. This excludes 26 patients who were not randomised at the time of relapse, from the analysis of survival after relapse (see statistical methods). Table II Response rates to initial chemotherapy; the reponse is recorded as the best response achieved during treatment Response by Response by Response by 4 courses 4 courses 8 courses n % n % n % Complete response % 61% 63% Partial response Stable disease Progressive disease (including deaths Inevaluable Total and long course chemotherapy groups were similar, and comparable numbers of patients showed progression of disease during chemotherapy. After eight cycles the overall response had not significantly increased but there was a small increase in proportion of complete responders. Eighty-eight (29%) patients did not complete the four allocated courses of chemotherapy in the short course group and 152 (5%) patients failed to complete long course treatment. The reasons for failure are summarised in Table III. The resulting distribution of numbers of courses actually received for each intended randomisation is shown in Table IV. During the second randomisation, 54 patients allocated to receive relapse chemotherapy after short course treatment failed to receive it and 7 patients similarly failed to receive allocated relapse chemotherapy after long course chemotherapy. The reasons are summarised in Table V. Ninety subjects went on to relapse chemotherapy after short course treatment and 8 did so following long course chemotherapy. Responses to relapse chemotherapy were low, with a total of 22.3% of patients showing a complete or partial response. There was no statistical difference in the response rate in the two groups, although the response rate was slightly higher for those who had received short course chemotherapy (P=.37; Table VI) before their relapse chemotherapy. The overall survival for all patients, based on the initial randomisation, is shown in Figure 1. There was no significant difference between the two survival curves (P=.85), but median survival in the patients randomised to long course chemotherapy was 39 weeks, compared with 32 weeks in those receiving short course treatment (false negative P =.7). The combined results (Figure 2) show that of the four treatment policies, four courses of chemotherapy alone gives inferior survival to the other three treatments, which are equivalent in outcome. Thus, if chemotherapy is given on relapse, there is no survival disadvantage when initial treatment is stopped after four cycles. In contrast, in those Table III Reasons for not completing assigned initial chemotherapy (n) (n) Withdrawals Disease progression (including deaths) during chemotherapy Medical complications 1 5 Errors 6 5 Died before course 1 given 2 - Given radiotherapy instead of 1st course (29%) 152 (5%) Table IV The number (and %) of patients stopping initial chemotherapy at each point in the assigned chemotherapy programme (n = 35) (n = 35) n % n % oa oa b a 1.3 arandomised but not treated. btwo patients treated in error. patients receiving eight cycles of initial treatment, chemotherapy on relapse does not significantly improve survival compared with symptomatic treatment. The progression-free interval was significantly longer for patients allocated to receive long course chemotherapy
4 DURATION OF CHEMOTHERAPY 581 Table V Reasons for patients not receiving assigned relapse chemotherapy course course Deaths during initial chemotherapy Patient refusals Medical contraindications 8 13 Emergency radiotherapy 3 7 Died in remission 1 Not yet relapsed 4 3 Reasons not known 5 7 Protocol violation 3 54/144 (37.5%) 7/15(47%) Table VI Response to relapse chemotherapy following short or long course initial chemotherapy (short vs long, P =.37) n % n % Complete response Partial response Stable disease Progressive disease Inevaluable a) C U) ) E U3 l 15 Figure 2 Overall survival for all patients according to the four treatment intentions. (A) initial treatment, symptomatic relapse treatment (n = 145, MS = 3 weeks); (B) short initial treatment, chemotherapy on relapse (n = 144, MS = 38 weeks); (C) long initial treatment, symptomatic relapse treatment (n = 145, MS = 38 weeks); (D) long initial treatment, chemotherapy on relapse (n=15, MS 42 weeks). A vs. B P=.4; A vs. C P=.5; B vs. D P=.84 (false negative P<.1). ) ) Co - Co 4- E Z B E5 - B Figure 1 Overall survival for all patients based on the initial randomisation. (A) course chemotherapy (n=35, MS 32 weeks); (B) long course chemotherapy (n=35, MS 39 weeks) (p=.85, false negative P=.7). (Figure 3). During the initial three months of the study, when all patients were receiving chemotherapy, the rates of relapse were similar but the rate of progression increased in the short course group once chemotherapy had ceased. The median progression-free intervals were 23 and 31 weeks for short and long course patients respectively (P<.1). Figure 4 shows the survival curves from relapse to death. For patients treated with short or long course chemotherapy, who were randomised to receive symptomatic treatment only at relapse, the curves are identical with median survivals of 11 and 12 weeks. In the patients randomised to further chemotherapy at relapse, survival from relapse was better than with symptomatic treatment. Median survival was 15 weeks for those allocated to long course chemotherapy, and 2 weeks for those allocated to receive short course treatment. Comparing the four curves in Figure 4, survival from relapse was longer for those patients receiving short course and allocated to relapse chemotherapy. When the effects of the treatment policies are separated according to disease extent the results are as shown in Table VII. Survival was adversely affected in patients with extensive disease who were treated with four cycles of initial chemotherapy only. A similar trend was observed for limited stage patients but was not statistically significant. In the BJC-D 2 25 Figure 3 The progression free interval, for patients with stable and responding disease, following initial chemotherapy. Median interval following short (A) 23 weeks, n = 25; long (B) 31 weeks, n=254; P<.1. (Vertical bars indicate patients in whom the exact date of progression was not known.) CY) 2._ U) l Figure 4 The survival curves for relapse to death comparing short symptomatic (A) (n = 16, MS-l 1 weeks), long and symptomatic (B) (n= 112, MS= 12 weeks), long and relapse chemotherapy (C) (n= 114, MS= 15 weeks), and short and relapse chemotherapy (D) (n= 15, MS=2 weeks). A vs. D P<.1. B vs. C P=.16.
5 582 S.G. SPIRO et al. patients with limited disease the proportion of 24-month survivors was lowest in those treated with four chemotherapy cycles alone (although this was less apparent at 36 months). If the study is analysed further to include only the responding population, the disadvantage of giving four courses of chemotherapy alone becomes more apparent. Figure 5 shows the survival curves for the responding population randomised to receive short and symptomatic treatment versus long and symptomatic. The survival curve for the long treatment arm is significantly better (P=.1) than for the short and a difference is still apparent at two years. The other two randomisations (Figure 6) show no difference for the responders receiving short and relapse or long and relapse chemotherapy (false negative P=.2). Toxicity data included all deaths considered to be directly attributable to drug toxicity. There were 18 in the short course and 11 in the long course chemotherapy arms. However, the death rate in early cycles is not randomly distributed during the inter-cycle period and we have shown, and will report separately, that it is likely that chemotherapy induced toxicity contributes to early deat-h in patients with extensive disease. The number of reported episodes of serious infection (WHO grade 2) was 45 during short course chemotherapy and 7 during long. Recorded episodes of total white cell count falling below 3, or a neutropenia of Table VII Median and 2-year survival related to disease extent presentation Median survival % 2-year (weeks) survival Limited disease relapse CT symptomatic relapse CT symptomatic Extensive disease relapse CT symptomatic relapse symptomatic In limited disease patients: short and symptomatic vs short and relapse CT P=.2; vs long and symptomatic P=.1; vs long and relapse CT P=.726. In extensive disease patients: short and symptomatic vs short and relapse CT P=.11; vs long and symptomatic P=.224; vs long and relapse CT P=.1. C) c UL)._ 4-) 4_ Figure 5 Overall survival of responding population receiving symptomatic treatment only at relapse. (A=short and symptomatic (n=89, MS=4 weeks); B=long and symptomatic (n=91, MS=49 weeks); P =.1). C) C U, (-) Figure 6 Overall survival of responding population receiving relapse chemotherapy. (A = short and relapse (n =95, MS = 48 weeks); B=long and relapse (n=97, MS=51 weeks); P=.42). 1, before a course of chemotherapy was 55 during short course and 182 during the long course treatment. Courses were delayed a total of 71 and 217 times during short and long course treatment respectively. Activity, mood, pain, nausea and vomiting were assessed in detail using daily diary cards as part of a study on quality of life which will be reported separately. In summary, mild nausea and vomiting occurred in almost all patients but continued longer in those receiving eight cycles of treatment. Hair loss was universal. Severe (WHO grade 2) mucositis occurred in 16 and 35 patients respectively, and neuropathy on four and 15 occasions during the short and long courses. Discussion This is one of the largest studies to address the effects of length of chemotherapy in patients with SCLC. The major referring centres within the multicentre group enter all patients presenting with the disease who are judged likely to survive a minimum of three weeks if left untreated, and the patients are therefore representative of the disease pattern within the community. Since the overall prognosis of SCLC is poor (Davis et al., 1985) and many patients are elderly and of poor performance status, the duration of chemotherapy is an important practical consideration. There have been few studies addressing this question. Cullen et al. (1986) evaluated maintenance chemotherapy versus no treatment in patients with no unequivocal residual disease after induction therapy and found a 16-week advantage for continuing chemotherapy. In the EORTC study (Splinter et al., 1986) five initial courses of chemotherapy were followed by a randomisation in the responding population either to a further seven courses of drug treatment or symptomatic treatment. There was no survival advantage for further chemotherapy in limited disease patients but a small advantage for extensive disease patients. There has been no study evaluating different durations of initial chemotherapy and the effect of further chemotherapy after relapse. The UK MRC Lung Cancer Working Party have compared six and 12 courses of chemotherapy and found no difference in median or 3-year survival (D. Girling, personal communication). In our study, where treatment was stopped after only 12 weeks of chemotherapy in half the patients, the issue of what to do at relapse was important because the early cessation of therapy, especially in patients responding to treatment, may result in an unacceptably worse survival. For this reason we adopted a second randomisation which posed the question of the possible benefits of second line chemotherapy. The initial chemotherapy used widely accepted drugs active in small cell lung cancer (Hansen & Rrth, 1979). The second line drugs, 1
6 DURATION OF CHEMOTHERAPY 583 methotrexate and doxorubicin, were of different classes, modes of action and are also active against the disease. The results of this study show that continuing initial chemotherapy for eight courses does not greatly increase the response rate compared with four courses. However, stopping chemotherapy after four cycles resulted in earlier disease progression. At progression, there was a clear survival advantage for second line chemotherapy in those previously receiving short course chemotherapy, but this was less apparent in those given eight cycles. The overall study design therefore showed that four cycles of chemotherapy alone resulted in worse survival, but any of the other treatment policies equal. This disadvantage of short course chemotherapy alone was particularly obvious in the responding population both in median and 2-year survival. The study analysis is based on intention to treat but it must be noted that many patients failed during initial chemotherapy and that the clinicians in charge sometimes felt that an individual was too ill to undergo relapse chemotherapy. Furthermore, some patients refused chemotherapy on relapse. Patients dropping out of the study are particularly likely to be patients with poor performance status and/or extensive disease. The study has shown that intention to treat with chemotherapy at relapse was harder to realise with long course chemotherapy. Less than 5% of patients allocated to relapse chemotherapy following long course treatment were either willing or able to receive it. It can be concluded from this study that the policy of stopping treatment early will lead to earlier relapse. In the group as a whole no survival disadvantage results provided chemotherapy is given on relapse, and clinicians might judge that in some circumstances this may be a convenient treatment policy; for example in treating elderly and poor prognosis patients or in those with particularly severe side-effects of treatment. However, taken together with the other studies (Cullen et al., 1986; Evans et al., 1985; Splinter et al., 1986) the present results show that there is a limit to the degree to which chemotherapy can be reduced without a deterioration in survival especially in those responding to treatment. The data from this study and from the MRC trial indicate that, for the majority of patients, six cycles of chemotherapy represents adequate treatment with combination chemotherapy programmes similar to those used in these two studies. Further improvements await new drug combinations and schedules for good prognosis limited disease patients and better palliative regimens for the others. This study was supported by the Cancer Research Campaign. Walter Gregory gave invaluable help in statistical analysis. The computing facilities were made available by the Imperial Cancer Research Fund. Miss Angela Betchley and Miss Piera Cassettari typed the manuscript with great care. We would like to thank the following physicians for referring cases to this trial: Dr J.R. Govan, Dr G.H. Wiggins, Dr D. Phillips, Dr A. Willis, Dr C. Trask, Dr R. Weatherstone, Dr D.W. Empey, Dr G.W. Bradley, Dr J. Millard, Dr P. Cole, Dr J.V. Collins, Dr R.K. Knight, Dr M. Apps, Dr R.A. Storring, Dr J. Utting, Dr J. Warren, Dr P.R. Studdy, Dr J. Riordan, Dr M.W. McNicol, Dr J. Meadway, Dr M. Harrier, Dr W.A.C. McAllister, Dr M.E. Hodson, Dr A. Newman-Taylor, Dr R.A. Banks, Dr L.R. Bagg, Dr W.P.U. Kennedy, Dr I. Williams, Dr. McCarthy, Dr N. Eiser, Dr G.M. Cochrane, Dr S. Steel, Dr M.R. Hetzel, Dr J. Milledge, Dr J. Waller, Dr M.E. Turner- Warwick, Dr M. Green and Dr A.H. Diamond. References CULLEN, M. MORGAN, D., GREGORY, W. & 11 others (1986). Maintenance chemotherapy for anaplastic small cell carcinoma of the bronchus: a randomised controlled trial. Cancer Chem. Plharniacol., 17, 157. DAVIS, S., WRIGHT, P.W., SCHULMAN, S.F., SCHOLES, D., THORNING, D. & HAMMAR S. (1985). -term survival in small-cell carcinoma of the lung: a population experience. J. Clin. Oncol., 3, 8. EVANS, W.K., OSOBA, D., FELD, R., SHEPHERD, F.A., BAZOS, M.J. & DEBOER, G. (1985). Etoposide (VP-16) and cisplatin: an effective treatment for relapse in small cell lung cancer. J. Clin. Oncol., 3, 65. FREEDMAN, L.S. (1982). Tables of the number of patients required in clinical trials using the Logrank test. Stat. Med., 1, 121. HANSEN, H.H. & RRTH, M. (1979). Lung cancer. In Cancer Chemotherapy, Pinedo, H.M. (ed.) p Excerpta Medica: Amsterdam. HUGHES, D.J. (1981). Approximate error probabilities for the log rank test. Working paper of Research Centre for Mathematical Modelling of Clinical Trials, University of Warwick. KAPLAN, E.L. & MEIER, P. (1958). Non parametric estimation from incomplete observations. J. Am. Stat. Assoc., 53, 457. OSTERLIND, K. & ANDERSEN, P.K. (1986). A model for survival in small cell lung cancer. A study of prognostic factors in 874 patients treated with chemotherapy with or without irradiation. Cancer Res., 46, PETO, R., PIKE, M.C., ARMITAGE, P. & 7 others (1977). Design and analysis of randomised clinical trials requiring prolonged observation of each patient. Br. J. Cancer, 35, 1. SOUHAMI, R.L., BRADBURY, I., GEDDES, D.M., SPIRO, S.G., HARPER, P.G. & TOBIAS, J.S. (1985). The prognostic significance of laboratory parameters measured at di4gnosis in small cell carcinoma of the lung. Cancer Res., 45, SOUHAMI, R.L., GEDDES, D.M., SPIRO, S.G. & 5 others (1984). Radiotherapy in small cell cancer of the lung treated with combination chemotherapy: a controlled trial. Br. Med. J., 288, SPLINTER, T., McVIE, J.G., DELASSIO,. & 2 others (1986). Induction versus induction plus maintenance chemotherapy in small cell lung cancer. Am. Soc. Clin. Oncol., C188.
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
Small Cell Lung Cancer
 Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Small Cell Lung Cancer Types of Lung Cancer Non-small cell carcinoma (NSCC) (87%) Adenocarcinoma (38%) Squamous cell (20%) Large cell (5%) Small cell carcinoma (13%) Small cell lung cancer is virtually
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof.
 Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Corso Integrato di Clinica Medica ONCOLOGIA MEDICA AA 2010-2011 LUNG CANCER. VIII. THERAPY. V. SMALL CELL LUNG CANCER Prof. Alberto Riccardi SMALL CELL LUNG CARCINOMA Summary of treatment approach * limited
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians
 Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Treatment Algorithms for the Management of Lung Cancer in NSW Guide for Clinicians Background The Cancer Institute New South Wales Oncology Group Lung (NSWOG Lung) identified the need for the development
Small Cell Lung Cancer
 Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Small Cell Lung Cancer Lung Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Approval Date: April 2007 Revised: November 2008 This guideline is a statement of consensus of the Thoracic Disease Site Team
Combination Chemotherapy for Small Cell Lung Cancer
 ORIGINAL ARTICLE Combination Chemotherapy for Small Cell Lung Cancer A W. Sufarlan, MRCP B.M.Z. Zainudin, MRCP Respiratory Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia,
ORIGINAL ARTICLE Combination Chemotherapy for Small Cell Lung Cancer A W. Sufarlan, MRCP B.M.Z. Zainudin, MRCP Respiratory Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia,
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
In the mid 1950s Roger Altounyan began work Small cell lung cancer
 598 Thorax 1997;52:598 Altounyan address Clinical trials in lung cancer: nihilism versus enthusiasm Stephen G Spiro In the mid 1950s Roger Altounyan began work Small cell lung cancer on a natural compound,
598 Thorax 1997;52:598 Altounyan address Clinical trials in lung cancer: nihilism versus enthusiasm Stephen G Spiro In the mid 1950s Roger Altounyan began work Small cell lung cancer on a natural compound,
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Radiation Therapy in the Treatment of
 Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
Lung Cancer Radiation Therapy in the Treatment of Lung Cancer JMAJ 46(12): 537 541, 2003 Kazushige HAYAKAWA Professor and Chairman, Department of Radiology, Kitasato University School of Medicine Abstract:
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER
 CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
CHAPTER 6: TREATMENT FOR SMALL CELL LUNG CANCER INTRODUCTION This chapter provides an overview of treatment for small cell lung cancer (SCLC). Treatment options are presented based on the extent of disease.
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Radiotherapy in locally advanced & metastatic NSC lung cancer
 Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Radiotherapy in locally advanced & metastatic NSC lung cancer Dr Raj Hegde. MD. FRANZCR Consultant Radiation Oncologist. William Buckland Radiotherapy Centre. Latrobe Regional Hospital. Locally advanced
Hodgkin Lymphoma Disease Specific Biology and Treatment Options. John Kuruvilla
 Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
Hodgkin Lymphoma Disease Specific Biology and Treatment Options John Kuruvilla My Disclaimer This is where I work Objectives Pathobiology what makes HL different Diagnosis Staging Treatment Philosophy
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
Stage I, II Non Small Cell Lung Cancer
 Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
Stage I, II Non Small Cell Lung Cancer Best Results T1 (less 3 cm) N0 80% 5 year survival No Role Adjuvant Chemotherapy Radiation Therapy Reduces Local Recurrence No Improvement in Survival 1 Staging Mediastinal
3.0 With final Comments for presentation at Sub Group Meeting 24. 24.11.10
 Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Guideline for the Treatment of Lung Cancer Version History 2.0 Endorsed by the Governance Committee as treatment of lung cancer 27.07.09 with radiotherapy and chemotherapy. 2.1 Re-written to include the
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Breast cancer Faculty of Clinical Radiology www.rcr.ac.uk Contents Breast cancer 2 Clinical background 2 Who
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer
 How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer Jeffrey S Tobias, Jayant S Vaidya, Frederik Wenz and Michael Baum, University College Hospital, London, UK - on behalf
How TARGIT Intra-operative Radiotherapy can help Older Patients with Breast cancer Jeffrey S Tobias, Jayant S Vaidya, Frederik Wenz and Michael Baum, University College Hospital, London, UK - on behalf
Update on Small Cell Lung Cancer
 Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Welcome to Master Class for Oncologists Session 3: 2:45 PM - 3:30 PM Washington, DC March 28, 2009 Small Cell Lung Cancer: Best Practices & Recent Advances Speaker: Bruce E. Johnson, MD Professor of Medicine,
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including anaplastic, gliosarcoma and glioblastoma multiforme)
 Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Canine Lymphoma Frequently Asked Questions by Pet Owners
 Canine Lymphoma Frequently Asked Questions by Pet Owners What is lymphoma? The term lymphoma describes a diverse group of cancers in dogs that are derived from white blood cells called lymphocytes. Lymphocytes
Canine Lymphoma Frequently Asked Questions by Pet Owners What is lymphoma? The term lymphoma describes a diverse group of cancers in dogs that are derived from white blood cells called lymphocytes. Lymphocytes
Management of low grade glioma s: update on recent trials
 Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Lung Cancer. This reference summary will help you better understand lung cancer and the treatment options that are available.
 Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
Lung Cancer Introduction Lung cancer is the number one cancer killer of men and women. Over 165,000 people die of lung cancer every year in the United States. Most cases of lung cancer are related to cigarette
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Lung cancer forms in tissues of the lung, usually in the cells lining air passages.
 Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
SMALL. 1-800-298-2436 LungCancerAlliance.org
 Understanding series SMALL CELL LUNG CANCER 1-800-298-2436 LungCancerAlliance.org A guide for the patient I TABLE OF CONTENTS ANATOMY OF THE LUNGS The following image shows different parts that make up
Understanding series SMALL CELL LUNG CANCER 1-800-298-2436 LungCancerAlliance.org A guide for the patient I TABLE OF CONTENTS ANATOMY OF THE LUNGS The following image shows different parts that make up
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
LYMPHOMA IN DOGS. Diagnosis/Initial evaluation. Treatment and Prognosis
 LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
LYMPHOMA IN DOGS Lymphoma is a relatively common cancer in dogs. It is a cancer of lymphocytes (a type of white blood cell) and lymphoid tissues. Lymphoid tissue is normally present in many places in the
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
REPORT ASCO 1998 LOS ANGELES : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group Educational session Treatment of stage III non-small cell lung cancer (NSCLC) in
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
Small-Cell Lung Cancer: Is There a Standard Therapy?
 Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Small-Cell Lung Cancer: Is There a Standard Therapy? Review Article [1] January 02, 1998 By Pieter E. Postmus, MD, PhD [2] and Egbert F. Smit, MD [3] For more than 25 years, chemotherapy has been the cornerstone
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
National Clinical Guideline. Diagnosis, staging and Treatment of Gestational Trophoblastic Disease. Literature Search Strategies
 National Clinical Guideline Diagnosis, staging and Treatment of Gestational Trophoblastic Disease Literature Search Strategies NCCP 1 Diagnosis Question 1 Clinical Question Should all women undergoing
National Clinical Guideline Diagnosis, staging and Treatment of Gestational Trophoblastic Disease Literature Search Strategies NCCP 1 Diagnosis Question 1 Clinical Question Should all women undergoing
Pulmonary function. Is patient potentially operable? Yes. tests 3. Yes. Pulmonary function. tests 3, if clinically indicated. Yes
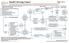 INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
INITIAL EVALUATION Pathology consistent with small cell lung cancer History and physical Chest X-ray Laboratory studies to include: hematological and full chemistry panels CT chest and upper abdomen Pet
95% of childhood kidney cancer cases are Wilms tumours. Childhood kidney cancer is extremely rare, with only 90 cases a year in
 James Whale Fund for Kidney Cancer Childhood kidney cancer factsheet Kidney cancer rarely afflicts children and about 90 paediatric cases are diagnosed in the UK each year. About 75% of childhood kidney
James Whale Fund for Kidney Cancer Childhood kidney cancer factsheet Kidney cancer rarely afflicts children and about 90 paediatric cases are diagnosed in the UK each year. About 75% of childhood kidney
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Principal Investigator: Valerie W. Rusch, MD, FACS, Chief, Thoracic Surgery Memorial Sloan-Kettering Cancer Center
 Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Protocol 1101-1088 Phase I study of intra-pleural administration of GL-ONC1 in patients with malignant pleural effusion: primary, metastases and mesothelioma Principal Investigator: Valerie W. Rusch, MD,
Lung Cancer. Public Outcomes Report. Submitted by Omar A. Majid, MD
 Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Public Outcomes Report Lung Cancer Submitted by Omar A. Majid, MD Lung cancer is the most common cancer-related cause of death among men and women. It has been estimated that there will be 226,1 new cases
Radiotherapy in Plasmacytoma and Myeloma. David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015
 Radiotherapy in Plasmacytoma and Myeloma David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015 Contents Indications for radiotherapy: Palliation in Multiple Myeloma Solitary Bone Plasmacytoma
Radiotherapy in Plasmacytoma and Myeloma David Cutter Multiple Myeloma NSSG Annual Meeting 14 th September 2015 Contents Indications for radiotherapy: Palliation in Multiple Myeloma Solitary Bone Plasmacytoma
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Pediatric Oncology for Otolaryngologists
 Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
Pediatric Oncology for Otolaryngologists Frederick S. Huang, M.D. Division of Hematology/Oncology Department of Pediatrics The University of Texas Medical Branch Grand Rounds Presentation to Department
A912: Kidney, Renal cell carcinoma
 A912: Kidney, Renal cell carcinoma General facts of kidney cancer Renal cell carcinoma, a form of kidney cancer that involves cancerous changes in the cells of the renal tubule, is the most common type
A912: Kidney, Renal cell carcinoma General facts of kidney cancer Renal cell carcinoma, a form of kidney cancer that involves cancerous changes in the cells of the renal tubule, is the most common type
The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options
 Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer
 CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
Managing Lymphoma. Professor Clare Knottenbelt BVSc MSc DSAM MRCVS
 Managing Lymphoma Professor Clare Knottenbelt BVSc MSc DSAM MRCVS Lymphoma Common cancer (18% of dog cancers) DOGS: Multicentric CATS: Alimentary Presentation varies with site of LSA and paraneoplastic
Managing Lymphoma Professor Clare Knottenbelt BVSc MSc DSAM MRCVS Lymphoma Common cancer (18% of dog cancers) DOGS: Multicentric CATS: Alimentary Presentation varies with site of LSA and paraneoplastic
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin: Glossary of key terms
 Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Avastin: Glossary of key terms Adenocarcinoma Adenoma Adjuvant therapy Angiogenesis Anti-angiogenics Antibody Antigen Avastin (bevacizumab) Benign A form of carcinoma that originates in glandular tissue.
Feline Lymphoma Chemotherapy and Chemotherapy Protocols
 Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
Feline Lymphoma Chemotherapy and Chemotherapy Protocols If you have reached this page, your cat probably has a definite diagnosis of feline lymphoma from your veterinarian. The information below is not
ACUTE MYELOID LEUKEMIA (AML),
 1 ACUTE MYELOID LEUKEMIA (AML), ALSO KNOWN AS ACUTE MYELOGENOUS LEUKEMIA WHAT IS CANCER? The body is made up of hundreds of millions of living cells. Normal body cells grow, divide, and die in an orderly
1 ACUTE MYELOID LEUKEMIA (AML), ALSO KNOWN AS ACUTE MYELOGENOUS LEUKEMIA WHAT IS CANCER? The body is made up of hundreds of millions of living cells. Normal body cells grow, divide, and die in an orderly
ADJUVANT TREATMENT CLINICAL EVALUATION NEOADJUVANT TREATMENT
 te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
te: Consider Clinical Trials as treatment options for eligible patients. Referral to a center with both pediatric oncology and orthopedic surgery is essential. CLINICAL EVALUATION This practice algorithm
The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in Breast Cancer
 BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
BIT's 4th World Cancer Congress 2011 People s Republic of China Dalian The Di Bella Method (DBM) improves Survival, Objective Response and Performance Status in treated with DBM therapy Retrospective observational
Summary of treatment benefits
 Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Lung Cancer - Combination Chemotherapy and the Risk of Failure
 Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
Evidence-based Series 7-13-1 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) The Role of Combination Chemotherapy in the Initial Management of Limited-Stage
FOLLOW UP OF TREATED NON-SMALL CELL LUNG CANCER PATIENTS
 FOLLOW UP OF TREATED NON-SMALL CELL LUNG CANCER PATIENTS THE ART AND THE SCIENCE GIOVANNI BATTISTA MORGAGNI 1682-1771 ITALIAN ANATOMIST FATHER OF PATHOLOGIC ANATOMY 1 ST DESCRIPTION OF LUNG CANCER IN 1761
FOLLOW UP OF TREATED NON-SMALL CELL LUNG CANCER PATIENTS THE ART AND THE SCIENCE GIOVANNI BATTISTA MORGAGNI 1682-1771 ITALIAN ANATOMIST FATHER OF PATHOLOGIC ANATOMY 1 ST DESCRIPTION OF LUNG CANCER IN 1761
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Epidemiology, Staging and Treatment of Lung Cancer. Mark A. Socinski, MD
 Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Epidemiology, Staging and Treatment of Lung Cancer Mark A. Socinski, MD Associate Professor of Medicine Multidisciplinary Thoracic Oncology Program Lineberger Comprehensive Cancer Center University of
Medical Oncology. Rotation Goals & Objectives for rotating residents. General Objectives THE UNIVERSITY OF BRITISH COLUMBIA
 THE UNIVERSITY OF BRITISH COLUMBIA Department of Urologic Sciences Faculty of Medicine Gordon & Leslie Diamond Health Care Centre Level 6, 2775 Laurel Street Vancouver, BC, Canada V5Z 1M9 Tel: (604) 875-4301
THE UNIVERSITY OF BRITISH COLUMBIA Department of Urologic Sciences Faculty of Medicine Gordon & Leslie Diamond Health Care Centre Level 6, 2775 Laurel Street Vancouver, BC, Canada V5Z 1M9 Tel: (604) 875-4301
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Lung Cancer and Mesothelioma
 Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Lung Cancer and Mesothelioma Robert Kratzke, M.D. John C. Skoglund Professor of Lung Cancer Research Section of Heme/Onc/Transplant Department of Medicine University of Minnesota Medical School Malignant
Recruitment Start date: April 2010 End date: Recruitment will continue until enrolment is fully completed
 Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
Apitope study The study drug (ATX-MS-1467) is a new investigational drug being tested as a potential treatment for relapsing forms of multiple sclerosis (RMS). The term investigational drug means it has
Management of spinal cord compression
 Management of spinal cord compression (SUMMARY) Main points a) On diagnosis, all patients should receive dexamethasone 10mg IV one dose, then 4mg every 6h. then switched to oral dose and tapered as tolerated
Management of spinal cord compression (SUMMARY) Main points a) On diagnosis, all patients should receive dexamethasone 10mg IV one dose, then 4mg every 6h. then switched to oral dose and tapered as tolerated
Small cell lung cancer
 Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Current Status and Perspectives of Radiation Therapy for Breast Cancer
 Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
Breast Cancer Current Status and Perspectives of Radiation Therapy for Breast Cancer JMAJ 45(10): 434 439, 2002 Masahiro HIRAOKA, Masaki KOKUBO, Chikako YAMAMOTO and Michihide MITSUMORI Department of Therapeutic
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW. FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012
 Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Active centers: 2. Number of patients/subjects: Planned: 20 Randomized: Treated: 20 Evaluated: Efficacy: 13 Safety: 20
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50
 General Data Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50 The vast majority of the patients in this study were diagnosed
General Data Seton Medical Center Hepatocellular Carcinoma Patterns of Care Study Rate of Treatment with Chemoembolization 2007 2012 N = 50 The vast majority of the patients in this study were diagnosed
Mesothelioma. Malignant Pleural Mesothelioma
 Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Mesothelioma William G. Richards, PhD Brigham and Women s Hospital Malignant Pleural Mesothelioma 2,000-3,000 cases per year (USA) Increasing incidence Asbestos (50-80%, decreasing) 30-40 year latency
Before, Frank's immune cells could
 Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Before, Frank's immune cells could barely recognize a prostate cancer cell. Now, they are focused on it. Stimulate an immune response against advanced prostate cancer Extend median survival beyond 2 years
Lung cancer case study
 Change Presentation title and date in Footer dd.mm.yyyy Lung cancer case study Dr Jaishree Bhosle Consultant Medical Oncologist Change Presentation title and date in Footer dd.mm.yyyy 1 2 Part One Initial
Change Presentation title and date in Footer dd.mm.yyyy Lung cancer case study Dr Jaishree Bhosle Consultant Medical Oncologist Change Presentation title and date in Footer dd.mm.yyyy 1 2 Part One Initial
Results of Surgical Treatment for Small Cell Lung Cancers
 NAOSITE: Nagasaki University's Ac Title Author(s) Results of Surgical Treatment for S Ayabe, Hiroyoshi; Nakamura, Akihiro Hara, Shinsuke; Tagawa, Yutaka; Kaw Citation Acta medica Nagasakiensia. 1994, 39
NAOSITE: Nagasaki University's Ac Title Author(s) Results of Surgical Treatment for S Ayabe, Hiroyoshi; Nakamura, Akihiro Hara, Shinsuke; Tagawa, Yutaka; Kaw Citation Acta medica Nagasakiensia. 1994, 39
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER
 GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
Treatment of Small Cell Lung Cancer
 Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Chapter 5 Treatment of Small Cell Lung Cancer Ariel Lopez-Chavez MD, MS Introduction There are two main types of lung cancer, non-small cell lung cancer and small cell lung cancer. 1 It is important that
Bendamustine for the fourth-line treatment of multiple myeloma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine for the fourth-line treatment of multiple myeloma Contents Summary 1 Background 2 Epidemiology 3 Cost 6 References 7 Summary There is no standard
